Abstract
Atypical ulcers show atypical clinical features, histology, localization, and resistance to standard therapies. The persistence of a chronic ulcer despite treatment with standard therapies requires a more specific diagnostic investigation. Diagnosis involves obtaining the history and performing clinical examination and additional tests. A skin biopsy is frequently used to confirm unclear diagnosis. In difficult cases, microbiological and immunohistochemical examinations, laboratory blood tests, or instrumental tests should be evaluated. The treatment of atypical wounds is characterized by local systemic therapy and pain control. Our results highlight the need for early diagnosis, and standardized and targeted management by a multidisciplinary wound healing center.
Keywords: atypical ulcers, wound healing, wound bed preparation, wound management
Introduction
Typical cutaneous chronic ulcers result from venous insufficiency, arterial insufficiency, neuropathy, diabetes and external pressure on body prominences.1 Venous ulcers tipically occur around the medial malleolus. Wound bed is generally covered by a fibrinous layer mixed with granulation tissue and it shows irregular edges. Perilesional skin is often characterized by atrophie blanche, lipodermatosclerosis and leg edema. The classification of venous leg ulcers is based on clinical aspects, etiologic factors, anatomic localizations and pathophysiologic findings (CEAP classification).2 Arterial ulcers are often necrotic, well defined ulcers and they are localized on the dorsum of the foot and on distal locations. Pain occurs with leg elevation.3
Diabetic foot ulcers are defined as a foot wound in a patient with diabetes, neuropathy and/or peripheral arterial disease. Diabetic foot ulcers can be classified based on anatomical wound characteristic, presence of infection and ischemia.4 Pressure ulcers occur typically over bones prominences as a result of pressure in combination with shearing forces. The Braden and Norton scales are commonly used for pressure ulcer risk assessment. The most commonly used staging system for pressure ulcers is the European Pressure Ulcer Advisory Panel (EPUAP) staging system.5
Atypical cutaneous ulcers are caused by inflammatory, neoplastic, vasculopathic, hematological, infectious and drug-induced etiologies.6,7 Approximately 20% of ulcers are caused by rare etiologies.8
Atypical ulcers show atypical clinical features, histology, localization and resistance to standard therapies and diagnosis is often delayed. Pyoderma gangrenosum (PG) and vasculitis are the most frequent inflammatory ulcers, which are associated with autoimmune intestinal, rheumatological, neurological inflammatory diseases and solid and hematological tumors.9
Vasculopathies may develop due to a variety of factors, especially coagulation disorders or kidney failure.10
Neoplastic ulcers are classified as primitive ulcerated skin cancers and metastatic secondary skin cancers. The most frequent primitive skin cancer is basal cell carcinoma, followed by squamous cell carcinoma, other non-melanocytic skin tumors, melanocytes and cutaneous lymphomas.11 Lung, breast and head-neck cancers develop most frequent cutaneous metastasis.12
Marjolin described the evolution of chronic ulcers, scars, burns, radiodermatitis in neoplastic ulcers. Approximately 1.7% of chronic cutaneous ulcers have a neoplastic transformation, particularly in squamous cell carcinoma.13,14 Hematologic ulcers tend to occur in patients with inherited hemoglobin anomalies.15 Infection is another etiologic factor which occurs most commonly after primary inoculation. Bacteria (Gram negative and Gram positive), mycobacteria (Mycobacterium tubercolosis), yeast (Candida albicans), mycetes (Sporotricosis), protozoa (Leishmania), skin parasites (Conus, Tunga) and arthropod bites (Entomodermatosis) are some of the different etiological pathogens of infectious ulcers. Infectious ulcers often have an endemic distribution or may be associated with outdoor activities.16–18 Hydroxyurea ulcers affect patients with hematological disorders.19 Heroin extravasation and secondary ulcers are typical in patients with heroin addiction.20
Clinical Features
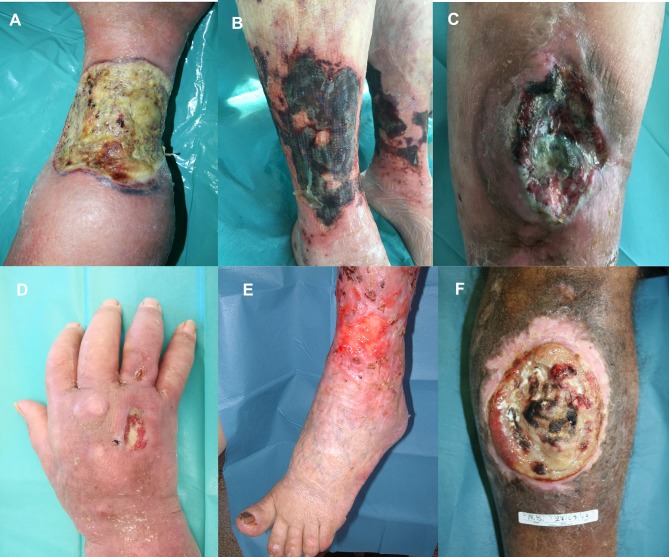
Atypical ulcers are characterized by an atypical wound bed, edges and perilesional skin. The clinical aspects are correlated with different etiologies (Figure 1). The wound bed is often exuberant or vegetative, with hyper-granulation tissue or necrotic tissue. Wound edges are undermined or exuberant. Perilesional skin may present with inflammation or satellite lesions.6–8
Figure 1.
(A) Pyoderma gangrenosum (Inflammatory ulcer), (B) Calciphylaxis (Vasculopathy), (C) Adamantinoma (Neoplastic ulcer), (D) Mycobatteriosis (Infectious ulcer), (E) Hydroxyurea-induced ulcer (F) Heroin induced ulcer.
Inflammatory ulcers are extremely painful. The wound bed is necrotic and fibrinous and the perilesional skin is inflamed. Perilesional skin in PG appears with a characteristic lilac ring. Vasculitis presents painful ulcers, purpuras, petechiae and blisters and other polymorphic lesions.9 Vasculopathies are multiple, painful ulcers and present a necrotic wound bed.10
Neoplastic ulcers vary from nodular ulcerated lesions, vegetative lesions, ulcerated plaques to chronic ulcers with exuberant granulation tissue and pseudoepithelium.11–13
Hematological ulcers occur with a fibrinous wound bed, irregular edges and purpuric lesions on perilesional skin.15 Infectious ulcers differ clinically with different morphological forms. The early lesions typically evolve from multiple nodules to ulcerative necrotic or fibrinous lesions.16,17
The cutaneous side effects of hydroxyurea treatment include hyperpigmentation, alopecia, melanonychia and painful ulcers. These ulcers have a well-defined and adherent fibrinous wound bed and often appear at the same sites of a previous trauma.19 Heroin induced ulcers show an irregular wound bed with necrotic fibrinous areas and frequent colonization. The perilesional skin presents numerous post-injection scars.20
Diagnosis
Wounds assessment is crucial to determine the appropriate plan of care. Location, stage, undermining, measurement, tissue type, odor, anatomical structures, surrounding skin and pain are critical steps of wounds assessment. All observations and assessments, management plan, rationale, and schedule for reassessment should be documented to aid monitoring and facilitate communication between wounds experts. Medical, travel and occupational histories and physical examination suggest additional investigations. In order to diagnose an atypical ulcer, exclusion of typical etiologies is the first step. The ankle brachial pressure index (ABI) is the ratio of systolic blood pressure (BP) in the ankle to systolic BP in upper arm. ABI can be performed with a handheld Doppler unit to categorized venous, mixed and arterial ulcers. Venous leg ulcers have an ABI greater than 0.8. Mixed ulcer result with an ABI between 0.6 e 0.8. Abi below 0.6 need a vascular surgeon assessment. Colour Doppler ultrasound (CDU) provides accurate anatomical and functional information in venous and arterial insufficiency. Arterial and venous CDU findings can be used to identify patients eligible for the surgery.21
The persistence of a chronic ulcer despite treatment with standard therapies required a more specific diagnostic investigation. Diagnosis involves performing a history, clinical examination and additional tests. Skin biopsy is frequently used to confirm an unclear diagnosis.22 The best practice is to perform an incisional biopsy of the border of the ulcer and perilesional skin. Multiple biopsies are helpful in difficult diagnoses. In the case of polymorphic lesions, the lesion to be biopsied should be early and untreated (Figure 2). Punch biopsy is the preferred sampling method after local anaesthetic injection. For histopathological assessments, a 4 mm diameter punch, including the deeper layers (dermis and partial hypodermis) provides an adequate tissue sample. In the case of a suspected infectious ulcer a biopsy of > 5 mm is appropriate by dividing the biopsy into two parts for histological and microbiological evaluations.23 In many conditions the histopathological features are pathognomonic, in other conditions microbiological and immunohistochemical information, laboratory blood tests or instrumental tests are essential.24
Figure 2.
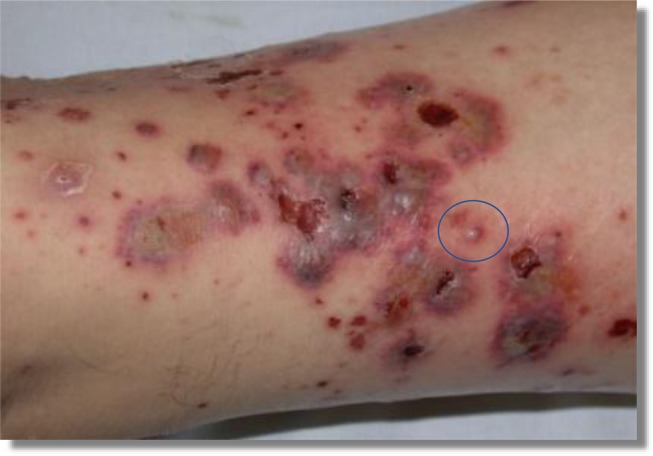
Leg vasculitis: The blue ring indicates the selected lesion for the biopsy because of an early and untreated lesion.
Histological evaluation differentiates between neoplastic, vascular or inflammatory ulcers. Vasculitis is defined as inflammation and necrosis of blood vessels. PG is often a diagnosis of exclusion and the histological examination typically shows a dermal neutrophilic infiltrate. Chronic wounds contain different biomarkers such as proinflammatory cytokines, metalloproteases (MMP), and metalloproteases inhibitors (TIMPs). Immunohistochemical evaluation reflects the wound status and provides a valuable basis for possible targeted therapies.25
Neoplastic wounds are characterized by atypical malignant cells. Immunohistochemistry is an important tool to differentiate the neoplastic ulcers.26 Periodic acid shiff (PAS) or Ziehl- Neelsen stain (ZN stain) are staining methods used to detect the presence of specific microorganisms.27
Blood screening with complete blood count (CBC) renal and liver function test, lipid profile, serum electrolytes, serum electrophoresis, antibody profile (Antinuclear antibody and anti-neutrophil cytoplasmic antibody), complement, cryoglobulins, erythrocyte sedimentation rate (ESR), C-reactive protein (CPR), serology for rheumatoid factor, thyroid function test, coagulation tests, blood glucose evaluation, and paraneoplastic screen tests are routine tests performed in vasculitis and pyoderma gangrenosum. Imaging techniques such as chest x-ray, abdominal ultrasound can help to determine if others organs are affected.
These tests look for signs of inflammation, anaemia, liver or kidney disfunction, associated cancers or different antibodies that can help with diagnosis of inflammatory ulcers or associated comorbidities.28,29
Management
High compression therapy is the standard therapy of venous leg ulcers. In some case surgical management may be considered. Mixed and arterial ulcers are treated by a team of interventional radiologists, vascular surgeons and dermatologist. Arteriography is the standard test prior the intervention. Diabetic foot ulcer treatment includes debridement of the wound, control of infection, revascularization procedures when indicated, and off-loading.3
Ulcers assessment, use of a specialized support surface, repositioning, mobilization, avoiding friction, and management of nutrition and moisture are associated with standard of care in pressure leg ulcers.5
Atypical ulcers require a complex therapeutic approach. Local and systemic therapies are specific to each etiology (Table 1).
Table 1.
Systemic and Local Therapies for Atypical Wounds
| Etiologies | Local Therapies | Systemic Therapies |
|---|---|---|
| Inflammatory ulcers | Non-adherent dressings, autolytic and enzymatic debridement, absorbent dressings, anesthetic cream, compression bandaging | Corticosteroids, immunosuppressants, biological therapies |
| Vasculopathies | Non-adherent dressings, autolytic and enzymatic debridement, absorbent dressings, compression bandaging | Anticoagulants, antiplatelets, pentoxifylline |
| Neoplastic ulcers | Surgery, radiotherapy, electrochemiotherapy, palliative dressings based on WBP* | Adjuvant and neoadjuvant therapies |
| Hematological ulcers | Non-adherent dressings, enzymatic and autolytic debridement, absorbent dressings | Hydroxyurea, blood transfusion, hyperbaric therapy |
| Infectious ulcers | Silver dressings, PHMB** dressings, iodine dressings, binding bacteria dressings, secondary dressings |
Antibiotics, antimycotic, antiparasitic drugs |
| Hydroxyurea- induced ulcers | Dressings based on WBP*, compression bandaging | Drug suspension or replacement, prostaglandinE1, pentoxifylline |
| Heroin induced ulcers | Silver dressings, PHMB** dressings, iodine, binding bacteria dressings, compression bandaging | Antibiotics |
Notes: *Wound bed preparation. **Polyhexamethylene biguanide.
Patients condition and environment and wound severity influence monitoring and treatment frequency. Twice a week monitoring and regular assessment (at least weekly) are generally recommended in chronic wounds.
Local Treatment
Wound cleansing helps optimize the healing environment and decreases the potential for infection. Saline is the preferred cleanser for most wounds. Most commercial wound cleansers control the growth of bacteria and fungi and are preferred in colonized and infected wounds.30
The local approach is based on Wound Bed Preparation principles (WBP). The suitable dressing depends on the tissue and edges features, exudation, infection or inflammation. Protecting the perilesional skin against damage is an important component of wound care.31
Etiological Treatment
Dressing changes can cause moderate to severe pain particularly in inflammatory ulcers, vasculopathies and hematological ulcers. Non-adherent dressings, local anesthetic, painkiller and local anti-inflammatory creams may improve the pain control and the patient’s quality of life.32,33 Autolytic or enzymatic debridement reduces fibrin in an atraumatic way.31 The aim of neoplastic wound palliative management is to control disease progression and optimise quality of life by alleviating exudate, malodour, pain and bleeding through the selection of an appropriate dressing.34
Topical antimicrobial dressings, including those that contain silver, iodine or polyhexamethylene biguanide (PHMB), and bacterial binding dressings are used locally to control or manage infection.31,35
Hematologic ulcers are often resistant to standard treatments. Autolytic and enzymatic debridement and non-adherent dressings are better tolerated.31,36 The treatment of choice for hydroxyurea-induced leg ulcers is discontinuation or replacement of the drug. These ulcers may remain stable for about 6 months after the suspension.19
The use of atraumatic debridement, biological dressings, skin substitutes and systemic prostaglandin E1 and pentoxifylline are reported in the literature.31,37 In heroin ulcers antimicrobial dressings are preferred to prevent bacterial superinfection.31
Compression bandaging increases healing in inflammatory ulcers, vasculopathies and heroin ulcers and controls the underlying vascular damage. Compression also can help to reduce a prolonged therapy with systemic corticosteroids.38,39
Surgical Treatment
Surgery is the first choice in neoplastic ulcers, followed by chemotherapy, radiotherapy, electrochemotherapy or other types of adjuvant or neoadjuvant therapies.11–13
Systemic Treatment
Systemic corticosteroids, systemic immunosuppressants, and biological drugs are used in inflammatory ulcers in addition to the local approach.39,40
The systemic treatment of vasculopathies is based on antithrombotics, anti-platelets, pentoxifylline and anticoagulant drugs.10 In infectious ulcers it is very important to identify the pathogens and to select appropriate systemic antimicrobial, antimycotic or antiparasitic drugs.35 Transfusions, hydroxyurea or hyperbaric therapy are often used in hematologic ulcers.36 A systemic treatment must be organized and prescribed according to a comprehensive holistic patient assessment, considering comorbidities and concomitant therapies.
Discussion
Wound healing is a complex process and direct and indirect comorbidities are involved. Direct factors include venous and arterial insufficiency, diabetes and trauma. Indirect factors are dementia, immobility, malnutrition and neuropathy. Within the multidisciplinary team approach nurses, physicians and surgeons contribute with other disciplines to address wounds education, prevention, assessment, and treatment. In a Wound Healing Center wound specialists should provide wound evidence-based assessment protocols and cost-effective treatments. New therapies for chronic wounds have led to improvements in lesion management and in the quality of care provided by medical and nursing staff, but lesion diagnostics and monitoring methodologies have not kept pace with this progress particularly in atypical wounds. The constant improvement of diagnostic and therapeutic procedures, together with the increase of life lasting, results in a higher frequency of patients suffering from chronic cutaneous ulcers. Due to the high costs these pathologies imply for treatments and often poor outcomes in terms of quality of life, a decrease in patients’ hospitalization, without a corresponding worsening of the quality of therapy, would provide important benefit. Moreover, since the healing process is remarkably slow, the clinical perception of the phases that lead a chronic wound to complete restoration is often penalized: this effect is dramatically amplified in those cases in which the patient is followed by more than one operator. Therefore, the study of wound healing pathophysiology and the development of new tools for the monitoring of the healing process may represent a possible optimization of the treatment efficacy for these lesions.
The morphologic features of an ulcerative cutaneous lesion can be substantially analyzed according to two distinguished modalities: the quantification of the loss of substance such as the extension and depth of the lesion, characteristics of the edges, and the qualitative discrimination of the several areas of the wound bed like the presence of necrosis, fibrin, fluid, extension of the surrounding skin inflammation. The use of wound and skin biomarkers will provide substantial information in the near future to better understand the non healing wounds process.
Conclusions
The diagnosis and management of atypical ulcers is challenging for physicians. Skin biopsy is recommended in the case of refractory wounds or when wounds present with atypical features. In difficult cases microbiological and immunohistochemical examinations, laboratory blood tests or instrumental tests should be evaluated. The treatment of atypical wounds is characterized by local, systemic therapy and control of pain. We believe that there is a fundamental need for earlier diagnosis, standardized and targeted management by a multidisciplinary wound healing center.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol. 2019;29:8–15. doi: 10.1016/j.annepidem.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 2.Carman TL, Al-Omari A. Evaluation and management of chronic venous disease using the foundation of CEAP. Curr Cardiol Rep. 2019;21(10):114. doi: 10.1007/s11886-019-1201-1 [DOI] [PubMed] [Google Scholar]
- 3.Greer N, Foman NA, MacDonald R, et al. Advanced wound care therapies for nonhealing diabetic, venous, and arterial ulcers: a systematic review. Ann Intern Med. 2013;159(8):532–542. doi: 10.7326/0003-4819-159-8-201310150-00006 [DOI] [PubMed] [Google Scholar]
- 4.Boulton AJ. Diabetic neuropathy and foot complications. Handb Clin Neurol. 2014;126:97–107. [DOI] [PubMed] [Google Scholar]
- 5.Jaul E. Assessment and management of pressure ulcers in the elderly: current strategies. Drugs Aging. 2010;27(4):311–325. doi: 10.2165/11318340-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 6.Hofman MD. Atypical ulcers. Dermatol Ther. 2013;26:222–223. doi: 10.1111/dth.12048 [DOI] [PubMed] [Google Scholar]
- 7.Shelling ML, Federman DG, Kirsner RS. Clinical approach to atypical wounds with a new model for understanding hypertensive ulcers. Arch Dermatol. 2010;146:1026–1029. doi: 10.1001/archdermatol.2010.213 [DOI] [PubMed] [Google Scholar]
- 8.Isoherranen K, O’Brien JJ, Barker J, et al. Atypical wounds. Best clinical practice and challenges. J Wound Care. 2019;28(Sup6):S1–S92. doi: 10.12968/jowc.2019.28.Sup6.S1 [DOI] [PubMed] [Google Scholar]
- 9.Shanmugam VK, Angra D, Rahimi H, et al. Vasculitic and autoimmune wounds. J Vasc Surg Venous Lymphat Disord. 2017;5(2):280–292. doi: 10.1016/j.jvsv.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Mera C, Fraga J, Capusan TM, et al. Vasculopathies, cutaneous necrosis and emergency in dermatology. G Ital Dermatol Venereol. 2017;152(6):615–637. [DOI] [PubMed] [Google Scholar]
- 11.Linares MA, Zakaria A, Nizran P. Skin cancer. Prim Care. 2015;42(4):645–659. doi: 10.1016/j.pop.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 12.Hussein MR. Skin metastasis: a pathologist’s perspective. J Cutan Pathol. 2010;37(9):e1–e20. doi: 10.1111/j.1600-0560.2009.01469.x [DOI] [PubMed] [Google Scholar]
- 13.Trent JT, Kirsner RS. Wounds and malignancy. Adv Skin Wound Care. 2003;16(1):31–34. doi: 10.1097/00129334-200301000-00014 [DOI] [PubMed] [Google Scholar]
- 14.Bazaliński D, Przybek-Mita J, Barańska B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol. 2017;21(3):197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaney KM, Axelrod KC, Buscetta A, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. 2013;37(4):325–332. doi: 10.3109/03630269.2013.789968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toutous Trellu L, Nkemenang P, Comte E, et al. Differential diagnosis of skin ulcers in a mycobacterium ulcerans endemic area: data from a prospective study in Cameroon. PLoS Negl Trop Dis. 2016;10(4):e0004385. doi: 10.1371/journal.pntd.0004385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clebak KT, Malone MA. Skin infections. Prim Care. 2018;45(3):433–454. doi: 10.1016/j.pop.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 18.Oranges T, Janowska A, Tonini A, et al. Necrotoxic spider bite: a successful noninvasive wound management. Int J Dermatol. 2019;58(7):e128–e130. doi: 10.1111/ijd.2019.58.issue-7 [DOI] [PubMed] [Google Scholar]
- 19.Quattrone F, Dini V, Barbanera S, et al. Cutaneous ulcers associated with hydroxyurea therapy. J Tissue Viability. 2013;22(4):112–121. doi: 10.1016/j.jtv.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 20.Onesti MG, Fioramonti P, Fino P, et al. Skin ulcer caused by venous extravasation of heroin. Int Wound J. 2014;11(4):409–411. doi: 10.1111/iwj.2014.11.issue-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and management of lower-extremity ulcers. N Engl J Med. 2017;377(16):1559–1567. doi: 10.1056/NEJMra1615243 [DOI] [PubMed] [Google Scholar]
- 22.Stevenson P, Rodins K. Improving diagnostic accuracy of skin biopsies. Aust J Gen Pract. 2018;47(4):216–220. doi: 10.31128/AJGP [DOI] [PubMed] [Google Scholar]
- 23.Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74(1):1–16. doi: 10.1016/j.jaad.2015.06.033 [DOI] [PubMed] [Google Scholar]
- 24.Tang JC, Vivas A, Rey A, et al. Atypical ulcers: wound biopsy results from a university wound pathology service FEFF. Ostomy Wound Manage. 2012;58(6):20–2, 24, 26–29. [PubMed] [Google Scholar]
- 25.Patel S, Maheshwari A, Chandra A. Biomarkers for wound healing and their evaluation. J Wound Care. 2016;25(1):46–55. doi: 10.12968/jowc.2016.25.1.46 [DOI] [PubMed] [Google Scholar]
- 26.Compton LA, Murphy GF, Lian CG. Diagnostic immunohistochemistry in cutaneous neoplasia: an update. Dermatopathology (Basel). 2015;2(1):15–42. doi: 10.1159/000377698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kain R. Histopathology. Methods Mol Biol. 2017;1508:185–193. [DOI] [PubMed] [Google Scholar]
- 28.Shavit E, Alavi A, Sibbald RG. Vasculitis-what do we have to know? A review of literature. Int J Low Extrem Wounds. 2018;17(4):218–226. doi: 10.1177/1534734618804982 [DOI] [PubMed] [Google Scholar]
- 29.Riyaz N, Mary V, Sasidharanpillai S, et al. Pyoderma gangrenosum: a clinico-epidemiological study. Indian J Dermatol Venereol Leprol. 2017;83(1):33–39. doi: 10.4103/0378-6323.188654 [DOI] [PubMed] [Google Scholar]
- 30.Pilcher M. Wound cleansing: a key player in the implementation of the TIME paradigm. J Wound Care. 2016;25(3 Suppl):S7–S9. doi: 10.12968/jowc.2016.25.Sup3.S7 [DOI] [PubMed] [Google Scholar]
- 31.Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(Suppl 1):S1–S28. [Review]. doi: 10.1046/j.1524-475X.11.s2.1.x [DOI] [PubMed] [Google Scholar]
- 32.Boateng J, Catanzano O. Advanced therapeutic dressings for effective wound healing–A review. J Pharm Sci. 2015;104(11):3653–3680. doi: 10.1002/jps.24610 [DOI] [PubMed] [Google Scholar]
- 33.Beiteke U, Bigge S, Reichenberger C, et al. Pain and pain management in dermatology. J Dtsch Dermatol Ges. 2015;13(10):967–987. [DOI] [PubMed] [Google Scholar]
- 34.Adderley UJ, Holt IG. Topical agents and dressings for fungating wounds. Cochrane Database Syst Rev. 2014;15(5):CD003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González del Castillo J, Isernia V, Candel FJ, Martín-Sánchez FJ. Approach for initial treatment of skin and soft tissue infection. Clin Infect Dis. 2015;60(1):169–171. doi: 10.1093/cid/ciu776 [DOI] [PubMed] [Google Scholar]
- 36.Minniti CP, Kato GJ. Critical reviews: how we treat sickle cell patients with leg ulcers. Am J Hematol. 2016;91(1):22–30. doi: 10.1002/ajh.24134 [DOI] [PubMed] [Google Scholar]
- 37.Dissemond J, Hoeft D, Knab J, Franckson T, Kroger K, Goos M. Leg ulcer in a patient associated with hydroxyurea therapy. Int J Dermatol. 2006;45(2):158–160. doi: 10.1111/ijd.2006.45.issue-2 [DOI] [PubMed] [Google Scholar]
- 38.Sunderkötter C, Bonsmann G, Sindrilaru A, et al. Management of leukocytoclastic vasculitis. J Dermatolog Treat. 2005;16(4):193–206. doi: 10.1080/09546630500277971 [DOI] [PubMed] [Google Scholar]
- 39.Blättler W, Zimmet SE. Compression therapy in venous disease. Phlebology. 2008;23(5):203–205. doi: 10.1258/phleb.2008.081004 [DOI] [PubMed] [Google Scholar]
- 40.Dini V, Romanelli M, Bertone M, et al. Improvement of idiopathic pyoderma gangrenosum during treatment with anti-tumor necrosis factor alfa monoclonal antibody. Int J Low Extrem Wounds. 2007;6:108–113. doi: 10.1177/1534734607300912 [DOI] [PubMed] [Google Scholar]