Abstract
Background
Few population-based studies have evaluated the association between location of care, complications with induction therapy and early mortality in acute myeloid leukemia (AML) patients.
Methods
Using linked data from the California Cancer Registry and Patient Discharge Dataset (1999–2014), we identified adult AML patients (≥18 years) who received inpatient treatment within 30 days of diagnosis. A propensity score was created for treatment at an NCI-CC. Inverse probability-weighted, multivariable logistic regression models were used to determine associations between location of care, complications and early mortality (death ≤ 60 days from diagnosis).
Results
Of the 7007 patients with AML, 1762 (25%) were treated at a NCI-CC. AML patients treated at NCI-CCs were more likely to be ≤65 years of age, live in higher socioeconomic status neighborhoods, have fewer comorbidities and have public health insurance. Patients treated at NCI-CCs had higher rates of renal failure (23% vs 20%, P=0.010) and lower rates of respiratory failure (11% vs 14%, P=0.003) and cardiac arrest (1% vs 2%, P=0.014). After adjustment for baseline characteristics, treatment at a NCI-CC was associated with lower early mortality (OR 0.46, CI 0.38–0.57). The impact of complications on early mortality did not differ by location of care except for higher early mortality in patients with respiratory failure treated at non-NCI-CC.
Conclusions
Initial treatment of adult AML at NCI-CCs is associated with a 53% reduction in the odds of early mortality compared with treatment at non-NCI-CCs. Lower early mortality may result from differences in hospital or provider experience and supportive care.
Condensed abstract:
In this observational cohort study that included 7007 adult acute myeloid leukemia patients hospitalized in California, patients treated at National Cancer Institute (NCI) designated cancer centers had a 53% reduction in the odds of death at 60 days of diagnosis compared to those treated elsewhere. Patients treated at NCI-CCs were more likely to be younger, live in more affluent neighborhoods, have fewer comorbidities and have public health insurance.
INTRODUCTION
Acute myeloid leukemia (AML) is the one of the most common leukemias in adults and is associated with a poor overall prognosis.1 Initial standard treatment of AML consists of induction chemotherapy that usually requires an inpatient hospitalization of at least one month, a period that is associated with a high early mortality of 12–26% due to the underlying disease and complications of treatment.2–4 Early mortality, or death within 30–60 days of diagnosis, has improved over the last 40 years largely due to advances in supportive care, including treatment of infections and rigorous transfusions5,6, but there continue to be disparities in outcomes between specific groups7,8. We previously observed that race/ethnicity, neighborhood socioeconomic status, marital status and location of care impacted early mortality in AML patients.9.
Recent research among patients with solid tumors has highlighted the impact of the cancer care delivery setting on patient outcomes. In patients with lung, prostate, breast and colorectal cancer, treatment at specialty cancer hospitals compared to community centers was associated with improved 1-year mortality after adjustment for cancer stage.10 In addition, patients undergoing cancer surgery for lung, gastrointestinal and bladder cancers have been shown to experience reduced surgical and late mortality rates when treated at National Cancer Institute designated cancer centers (NCI-CC) rather than community hospitals.11–13 Few studies have evaluated the association between location of care and outcomes in patients with hematological malignancies, including AML. One recent study showed that adolescents and young adults with acute lymphoblastic leukemia and acute myeloid leukemia treated at NCI-CCs or Children’s Oncology Group sites had better survival compared to those treated elsewhere. This study, however, was limited to facilities in Los Angeles county and did not consider early mortality.14
In a previous report, we showed that early complications and early mortality were lower for AML patients treated at NCI-CC; however, that report did not examine potential reasons for this disparity nor utilize more robust analytical methods to mitigate the selection bias inherent in which patients receive treatment at an NCI-CC.9 In this present study, we examine differences in sociodemographic and clinical characteristics of AML patients treated at NCI versus non-NCI-CCs. We also evaluate the impact of hospital type on early mortality while controlling for these differences and examine whether complications during initial therapy by location of care impact early mortality. We hypothesized that AML patients treated at NCI-CCs would have lower rates of complications related to induction therapy and lower early mortality compared to those treated elsewhere.
METHODS
Databases
This study used a linked database between the California Cancer Registry (CCR) and the California Office of Statewide Health Planning and Development Patient Discharge Database (PDD). The CCR contains sociodemographic, clinical, and pathologic information on nearly all patients diagnosed with cancer in California. Reporting is mandatory and completeness of cases is at least 98%.15 From the CCR, information on age at diagnosis, race/ethnicity, year of diagnosis, gender, marital status, neighborhood socioeconomic status (SES), health insurance at diagnosis or initial treatment, date of initial chemotherapy and vital status complete through 2014 was obtained.1
The PDD contains information about all patients hospitalized in the California, except patients admitted to one of 14 Federal hospitals (12 Veterans Affairs hospitals and two military hospitals). Serial records from a single person are linked using an encrypted form of the social-security number, called the record linkage number.16,17 PDD records include a principal medical diagnosis, up to 24 additional ‘secondary’ diagnoses, and a principal and up to 20 secondary procedures coded using International Classification of Diseases, 9th Revision, Clinical Modification codes (ICD-9-CM). From the PDD, we obtained information on chemotherapy administration, leukapheresis (a procedure used as a surrogate for a diagnosis of hyperleukocytosis; ICD-9, 99.72), and comorbidities up to 2 years prior to or at AML first admission using the Elixhauser index.18 We were also able to obtain information on complications which were included if they occurred within any hospitalization from the time of diagnosis to 60 days, or death. Complications determined included: major bleeding, sepsis, venous thrombosis, renal failure, liver dysfunction, respiratory failure, or cardiac arrest (ICD-9-CM codes in Supplementary Table 1). These complications were chosen as they have been previously identified as being common complications during AML induction treatment.3 The database also includes a hospital identifier. From this list of hospitals, we were able to classify hospitals into those associated with one of the eight NCI-CCs in California. All other hospitals were classified as non-NCI-CCs.
Study Population
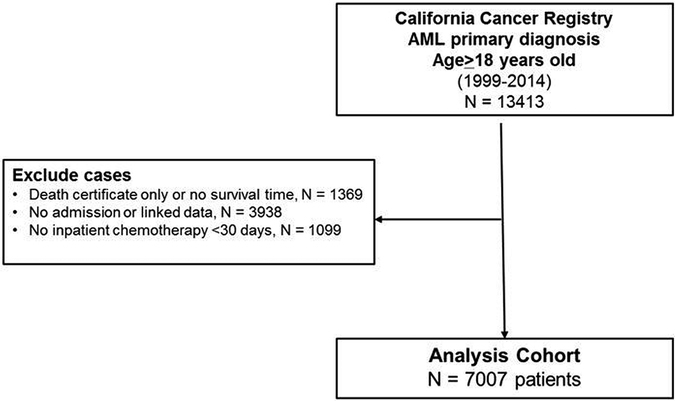
Adult patients (≥18 years of age) diagnosed with a first primary AML and treated at a hospital with chemotherapy in California from 1999–2014 were eligible for the study. To identify cases of AML, we used the following morphology codes from the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) (World Health Organization, 2000): 9840, 9861, 9865 9867, 9869–9874, 9891, 9895–9898, 9910, 9911, 9920, and 9931. We excluded patients with a diagnosis of acute promyelocytic leukemia because the treatment and management differs from AML. In addition, we excluded patients without a record linkage number to hospital data; patients with an AML diagnosis at autopsy or death certificate only; patients who did not receive chemotherapy within 30 days of diagnosis; and those without an inpatient hospitalization or known hospital type (Figure 1).
Figure 1.
Analysis cohort of patients with first primary acute myeloid leukemia in California.
Statistical Analysis
The differences in baseline characteristics and complications by location of care (NCI-CC vs non-NCI-CC facilities) were assessed by Chi-square tests. Propensity score methodology was used to balance the baseline covariates between patients treated at an NCI-CC and those treated at non-NCI-CC facilities.
Multivariable logistic regression was used to estimate propensity scores for the variable location of care (NCI-CC/non-NCI-CC facilities), predicted from baseline characteristics: age, sex, race/ethnicity, year of diagnosis, marital status, neighborhood socioeconomic status, health insurance type and medical comorbidities. (Supplementary Figure 1). To obtain groups similar in baseline characteristics between those treated at NCI and non-NCI cancer centers, inverse probability weighting was used in the multivariable models for mortality. The quality of the propensity scores estimated are evaluated using two types of comparisons: comparing the distributions of propensity scores across the two groups (NCI-CC/non-NCI-CC facilities) and comparing the distribution of each covariate across the two groups. Furthermore, the standardized mean differences in baseline characteristics between the NCI-CC and non-NCI-CC groups were used to determine the effectiveness of the propensity score adjustment.
The primary outcome was death ≤60 days (early mortality) from AML diagnosis. Inverse probability weighted multivariable logistic regression models were used to determine associations between location of care and complications with early mortality, adjusting for baseline patient characteristics. Interactions of complications and location of care with early mortality were also determined for each covariate in the model. Analyses were performed using SAS® (9.4) and a two-sided p-value <0.05 was considered statistically significant, including interactions.
RESULTS
From a total of 13413 adult patients with first primary AML, we identified 7007 patients that fulfilled our inclusion criteria (Figure 1). Of these, 1762 (25.1%) were treated at an NCI-CC. The median number of new AML patients per year at a NCI-CC was 13.5 (range 0,43) compared to a median of 2 patients per year (range 1, 17) at non-NCI-CCs who admitted at least one AML patient. More than half of non-NCI-CCs had a median of zero new AML patients per year. By univariate analysis and chi-square tests, patients treated at a NCI-CC were more likely to be ≤65 years of age (73.9% vs. 60.1%), live in higher socioeconomic status neighborhoods (46.9% vs. 44.1%) and have public insurance (16.9% vs. 11.5%) (Table 1). Patients treated at a NCI-CC also had less comorbidities compared to those treated elsewhere (79.7% with 0–2 comorbidities at NCI-CC vs. 59.2% with 0–2 comorbidities at non-NCI-CC, p<0.001).
Table 1.
Sociodemographic and clinical characteristics of hospitalized acute myeloid leukemia patients receiving chemotherapy by location of care, California, 1999–2014.
| Total | NCI | non NCI | ||
|---|---|---|---|---|
| N = 7007 | N = 1762 | N = 5245 | P-value* | |
| Age | ||||
| 18–39 | 1071 (15.3%) | 330 (18.7%) | 741 (14.1%) | |
| 40–54 | 1624 (23.2%) | 475 (27.0%) | 1149 (21.9%) | |
| 55–65 | 1761 (25.1%) | 497 (28.2%) | 1264 (24.1%) | |
| ≥66 | 2551 (36.4%) | 460 (26.1%) | 2091 (39.9%) | <.0001 |
| Gender | ||||
| Male | 3874 (55.3%) | 972 (55.2%) | 2902 (55.3%) | |
| Female | 3133 (44.7%) | 790 (44.8%) | 2343 (44.7%) | 0.9045 |
| Race/Ethnicity | ||||
| White | 4292 (61.3%) | 1098 (62.3%) | 3194 (60.9%) | |
| African American | 366 (5.2%) | 71 (4.0%) | 295 (5.6%) | |
| Hispanic | 1381 (19.7%) | 330 (18.7%) | 1051 (20.0%) | |
| Asian | 927 (13.2%) | 253 (14.4%) | 674 (12.9%) | |
| Other/unknown | 41 (0.6%) | 10 (0.6%) | 31 (0.6%) | 0.0359 |
| Year of diagnosis | ||||
| 1999–2002 | 1745 (24.9%) | 400 (22.7%) | 1345 (25.6%) | |
| 2003–2006 | 1628 (23.2%) | 424 (24.1%) | 1204 (23.0%) | |
| 2007–2010 | 1816 (25.9%) | 489 (27.8%) | 1327 (25.3%) | |
| 2011–2014 | 1818 (25.9%) | 449 (25.5%) | 1369 (26.1%) | 0.0361 |
| Marital status at diagnosis | ||||
| Married | 4182 (59.7%) | 1055 (59.9%) | 3127 (59.6%) | |
| Not married | 2700 (38.5%) | 693 (39.3%) | 2007 (38.3%) | |
| Unknown | 125 (1.8%) | 14 (0.8%) | 111 (2.1%) | 0.0098 |
| Neighborhood Socioeconomic status (SES) | ||||
| Low SES | 3698 (52.8%) | 874 (49.6%) | 2824 (53.8%) | |
| High SES | 3139 (44.8%) | 827 (46.9%) | 2312 (44.1%) | |
| Unknown | 170 (2.4%) | 61 (3.5%) | 109 (2.1%) | <.0001 |
| Health insurance status | ||||
| Public insurance | 902 (12.9%) | 298 (16.9%) | 604 (11.5%) | |
| Private insurance | 3513 (50.1%) | 814 (46.2%) | 2699 (51.5%) | |
| Medicare | 2011 (28.7%) | 467 (26.5%) | 1544 (29.4%) | |
| Self-pay | 116 (1.7%) | 35 (2.0%) | 81 (1.5%) | |
| Unknown | 465 (6.6%) | 148 (8.4%) | 317 (6.0%) | <.0001 |
| Comorbidities | ||||
| 0 comorbidities | 1419 (20.3%) | 408 (23.2%) | 1011 (19.3%) | |
| 1–2 comorbidities | 2912 (41.6%) | 819 (46.5%) | 2093 (39.9%) | |
| 3+ comorbidities | 2676 (38.2%) | 535 (30.4%) | 2141 (40.8%) | <.0001 |
Chi-square test
In the multivariable model, several sociodemographic and clinical factors were associated with treatment at a NCI-CC (Table 2). Age ≤65 years and being diagnosed after 2002 was significantly associated with treatment at a NCI-CC. Having Medicare insurance (OR 1.89, CI 1.58–2.24) or other public insurance (OR 1.71, CI 1.44–2.03) was associated with higher odds of treatment at a NCI-CC when compared to private insurance. Patients who were Hispanic (OR 0.79, CI 0.68–0.92), African American (OR 0.66, CI 0.50–0.87), lived in low socioeconomic status neighborhoods (OR 0.84, CI 0.75–0.95) and had more than 3 comorbidities (0.67, CI 0.57–0.78) were less likely to receive treatment at a NCI-CC.
Table 2.
Multivariable model of the relationship of sociodemographic and clinical factors to treatment at a National Cancer Institute (NCI)-designated cancer center (versus non-NCI designated cancer center) in hospitalized acute myeloid leukemia patients receiving chemotherapy, California 1999–2014.
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Age (vs ≥66) | ||
| 18–39 | 2.58 (2.09, 3.20) | <.001 |
| 40–54 | 2.46 (2.03, 2.98) | <.001 |
| 55–65 | 2.29 (1.92, 2.74) | <.001 |
| Gender (vs Male) | ||
| Female | 0.98 (0.88, 1.10) | 0.749 |
| Race/Ethnicity (vs White) | ||
| Asian | 0.99 (0.84, 1.17) | 0.920 |
| Hispanic | 0.79 (0.68, 0.92) | 0.003 |
| African American | 0.66 (0.50, 0.87) | 0.004 |
| Other/unknown | 0.92 (0.44, 1.92) | 0.819 |
| Year of diagnosis (vs 1999–2002) | ||
| 2003–2006 | 1.19 (1.01, 1.40) | 0.034 |
| 2007–2010 | 1.28 (1.09, 1.50) | 0.002 |
| 2011–2014 | 1.21 (1.03, 1.42) | 0.024 |
| Marital status at diagnosis (vs Married) | ||
| Not married | 0.99 (0.88, 1.12) | 0.876 |
| Unknown | 0.40 (0.23, 0.70) | 0.002 |
| Neighborhood Socioeconomic status (vs High) | ||
| Low SES | 0.84 (0.75, 0.95) | 0.004 |
| Unknown | 1.53 (1.10, 2.14) | 0.013 |
| Health insurance status (vs Private insurance) | ||
| Medicare | 1.89 (1.58, 2.24) | <.001 |
| Public insurance | 1.71 (1.44, 2.03) | <.001 |
| Uninsured | 1.49 (0.99, 2.26) | 0.057 |
| Unknown | 1.62 (1.31, 2.02) | <.001 |
| Comorbidities (vs 0 comorbidities) | ||
| 1–2 comorbidities | 0.99 (0.86, 1.15) | 0.906 |
| 3+ comorbidities | 0.67 (0.57, 0.78) | <.001 |
Adjust for all the variables in the table (age, sex, year of diagnosis, marital status, insurance and comorbidities)
Differences in complication rates within 60 days of AML diagnosis between NCI-CC and non-NCI-CCs are described in Table 3. Leukapheresis occurred more frequently amongst patients treated at a NCI-CC (5.5% vs 2.7%, p<0.001). Patients treated at NCI–CCs had higher rates of renal failure (22.8% vs 19.9%, p=0.010), but lower rates of respiratory failure (11.6% vs 14.3%, p=0.003) and cardiac arrest (1.1% vs 2.0%, p=0.014) than patients treated at non-NCI-CCs. At 60 days after diagnosis, more patients treated at NCI-CC were alive (88.0% vs 76.3%, p<0.001). Other complications did not significantly differ by location of care.
Table 3.
Complications in hospitalized acute myeloid leukemia patients receiving chemotherapy by location of care, California, 1999–2014.
| Total | NCI-CC | non-NCI-CC | P-value | |
|---|---|---|---|---|
| N = 7007 | N = 1762 | N = 5245 | ||
| Leukapheresis | 236 (3.4%) | 97 (5.5%) | 139 (2.7%) | <.001 |
| Sepsis | 2,497 (35.6%) | 594 (33.7%) | 1,903 (36.3%) | 0.051 |
| Major bleeding | 869 (12.4%) | 211 (12.0%) | 658 (12.5%) | 0.530 |
| Thrombosis | 136 (1.9%) | 41 (2.3%) | 95 (1.8%) | 0.175 |
| Renal failure | 1,445 (20.6%) | 401 (22.8%) | 1,044 (19.9%) | 0.010 |
| Liver failure | 105 (1.5%) | 21 (1.2%) | 84 (1.6%) | 0.221 |
| Respiratory failure | 956 (13.6%) | 204 (11.6%) | 752 (14.3%) | 0.004 |
| Cardiac arrest | 127 (1.8%) | 20 (1.1%) | 107 (2.0%) | 0.014 |
| Death | 1,454 (20.8%) | 212 (12.0%) | 1,242 (23.7%) | <.001 |
NCI-CC = National Cancer Institute designated cancer center
Chi-square test
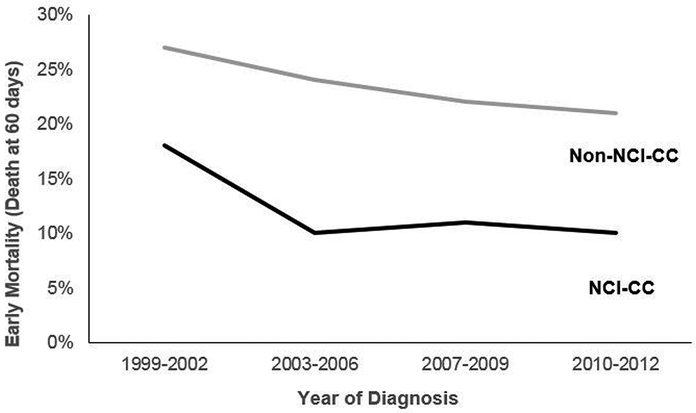
Early mortality amongst AML patients improved over time at both NCI-CCs and non-NCI-CCs (Figure 2). However, throughout the study period, patients treated at NCI-CCs had a persistently lower early mortality (average 12%) relative to those treated at non-NCI-CC (average 24%).
Figure 2.
60-day Mortality in hospitalized acute myeloid leukemia patients receiving chemotherapy by location of care, California, 1999–2014.
*NCI-CC = National Cancer Institute designated cancer center
After inverse probability weighting and adjustment for sociodemographic factors, comorbidities, and complications, treatment at an NCI-CC was associated with significantly lower early mortality compared with treatment at a non-NCI-CC (OR 0.46, CI 0.40–0.54) (Table 4). Complications associated with increased early mortality included major bleeding, liver, renal and respiratory failure, and cardiac arrest. The impact of complications on early mortality did not differ by location of care with the exception of respiratory failure (P for interaction=0.009) and thrombosis (P for interaction=0.034). AML patients with respiratory failure had higher odds of early mortality when treated at non-NCI-CCs (OR 9.48, CI 7.06–12.74) versus NCI-CCs (OR 4.20, CI 2.61–6.78). Though the association between thrombosis and early mortality differed between NCI-CCs and non-NCI-CCs, neither association reached statistical significance (Table 4). Similar results were seen in the traditional multivariate model (Supplementary Table 2).
Table 4.
Inverse probability weighted multivariable model of the relationship of location of care and complications with 60-day mortality in hospitalized acute myeloid leukemia patients receiving chemotherapy, California 1999–2014**
| Variable | OR (95% Cl) | P-value |
|---|---|---|
| NCI-CC vs non-NCI-CC | 0.46 (0.38, 0.57) | <.001 |
| Complications | ||
| Major bleeding | 1.79(1.39,2.31) | <.001 |
| Sepsis | 1.12(0.92, 1.37) | 0.263 |
| Thrombosis* | 0.63(0.37, 1.09) | 0.100 |
| NCI-CC | 0.12(0.01, 1.07) | |
| non-NCI-CC | 1.06(0.49,2.28) | |
| Liver failure | 1.95(0.96, 3.99) | 0.066 |
| Renal failure | 2.33(1.86,2.91) | <.001 |
| Respiratory failure* | 6.46(5.01,8.34) | <.001 |
| NCI-CC | 4.20(2.61,6.78)) | |
| non-NCI-CC | 9.48(7.06, 12.74) | |
| Cardiac arrest | 13.33(5.50,32.32) | <.001 |
| Leukapheresis (vs none) | 1.51 (0.95,2.39) | 0.085 |
interaction OR are from stratified models
adjusted for age, sex, year of diagnosis, marital status, insurance, comorbidities
DISCUSSION
In our analysis using a large and diverse cohort of hospitalized AML patients receiving initial chemotherapy, treatment at NCI–CCs was associated with a 53% reduction in the odds of early mortality compared with treatment at non-NCI-CCs. This association persisted in propensity-weighted analyses adjusted for sociodemographic factors, comorbidities, and complications. We did not find substantial differences in the rates of complications by location of care, except that patients treated at NCI-CCs had higher rates of renal failure and lower rates of respiratory failure and sepsis. While most complications were associated with increased early mortality, patients with respiratory failure had worse outcomes when treated at a non-NCI-CC. This study adds to the growing body of research that suggests that access to, and type of, hospital may impact cancer outcomes.19,20
While there have been many successful advances in the care and support of AML patients, our findings of such striking variation in early mortality outcomes by cancer care setting suggest that these advances may not have disseminated across all treatment settings. This is supported by the conclusion of a recent Institute of Medicine report that the cancer care system is in crisis with inconsistency in the quality of care being delivered to patients.21 Further research should evaluate specific differences in the care provided to AML patients hospitalized at NCI-CCs compared to other facilities in order to implement policies and practices that will ensure that all patients receive high-value and effective care. The NCI cancer center designation specifically requires depth and breadth in clinical and basic science research and population sciences, cancer prevention programs, and wide-ranging clinical resources. Recent research has suggested that the designation may also serve as a benchmark to assess the quality of cancer care.10,11,22,23
There are many potential reasons to explain the decreased early mortality seen for patients treated at NCI-CCs. It has been reported that high volume centers such as NCI-designated cancer centers may have better expertise at performing specialized care than low volume non-NCI designated facilities.13,24–26 In this study, NCI-designated cancer centers saw a median of 13 AML patients annually while non-NCI cancer centers saw a median of only 2 patients. A recent study showed reduced inpatient mortality rates in AML patients treated at high versus low volume centers.27 High volume centers, defined as those in the highest quartile of annual number of AML patients admitted for chemotherapy, had an inpatient mortality rate of 1.59% compared to 4.97% in low volume centers (those in the lowest quartile). High volume centers may have greater hospital resources, including advanced intensive care units, lower nursing staffing ratios and more diagnostic capabilities, factors that have been speculated to account for part of the mortality differences seen in surgical procedures.28,29 Differences in health care delivery practices between institutions may also play a role in outcomes. Prior studies have noted substantial hospital variation in adherence to diagnosis, treatment and follow-up guidelines for several malignancies due to both patient and hospital specific characteristics including cancer type, availability of multidisciplinary consultation, and hospital region.30–33
Because the NCI cancer center designation requires a robust research program, AML patients treated at NCI designated cancer centers may have increased access to clinical trials with novel agents beyond the standard of care. Prior studies that have evaluated the impact of clinical trial enrollment on mortality in cancer found an improvement in lower overall- and cancer-specific mortality among common cancer sites.34–36 This access to clinical trials may contribute to the improved outcomes seen in AML patients treated at NCI-designated cancer centers. This may be especially relevant in AML where molecular discoveries and the development of targeted therapies have led to recent approvals of several new drugs based on survival improvements demonstrated in clinical trials.37
Patients treated at NCI-CCs had higher rates of renal failure and lower rates of respiratory failure and sepsis. Prior studies have noted renal failure as a known complication in acute leukemias.38 We speculate that patients at NCI-CCs had higher rates of renal failure due to potential administration of nephrotoxic drugs, such as novel agents or antimicrobials. The higher rates of leukapheresis we observed at NCI-CCs suggests that patients treated at NCI-CCs may have higher white blood cell counts, which is known to be a risk factor for kidney dysfunction.39 Renal failure was associated with higher early mortality, but associations did not differ by location of care. Patients treated at NCI-CCs who had respiratory failure, however, did have lower early mortality than those with respiratory failure treated at non-NCI-CCs. The higher patient volume at NCI-CCs may result in improved recognition and management of common clinical sequelae of AML treatment such as renal and respiratory failure.
There are several limitations to our findings. Selection bias was introduced because we included only those patients who received chemotherapy and did not include patients who received treatment only in the outpatient setting. We did not have information on the specific type of chemotherapy given or whether patients were treated on clinical trial protocols at NCI-CCs. While this may have contributed to the differences in outcomes seen, we speculate that the majority of patients were treated similarly, as induction chemotherapy for AML had not significantly changed for the last 40 years despite more recent trends in the use of hypomethylating agents for older patients.40 We did not have details on important prognostic and predictive factors, including laboratory and molecular data, to consider in our early mortality and propensity analyses. As a result, there is likely to be some residual confounding from the imbalance in baseline characteristics among patients treated at NCI-CCs versus non-NCI-CCs. Similar to prior studies41,42, we did observe differences in baseline characteristics of patients treated at NCI-CCs: specifically, that they were younger, White or Asian race, lived in more affluent neighborhoods and had less comorbidities. However, after using propensity score methodology which reduced the standardized mean differences to <10% for most variables, the early mortality benefit associated with NCI designation persisted. Therefore, it is less likely that the differences in these patient characteristics could solely account for the difference noted in outcomes.
Despite these limitations, our study includes a large and diverse patient population with findings that are representative of contemporary treatment and health care delivery of AML patients at the population-level. The use of these large administrative databases provided the statistical power to identify disparities in early mortality that could have implications for cancer care and delivery.
In conclusion, this large population-based study in adult hospitalized patients with AML, we found a significant reduction in early mortality associated with care at an NCI-designated cancer center. This difference persisted even after consideration for differences in rates of and outcomes after complications and sociodemographic factors. This finding suggests potential disparities in the effectiveness of care for patients with AML across treatment facilities, and reinforces the need to further evaluate and measure how care is delivered in order to improve outcomes in all care settings.
Supplementary Material
Acknowledgements:
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
BAJ is supported by the National Cancer Institute of the National Institutes of Health under Award Number K12CA138464. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
TW is supported by UL1 TR001860, NCATS, NIH.
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Deschler B, Lubbert M: Acute myeloid leukemia: epidemiology and etiology. Cancer 107:2099–107, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Walter RB, Othus M, Borthakur G, et al. : Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol 29:4417–23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara F, Schiffer CA: Acute myeloid leukaemia in adults. Lancet 381:484–95, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Hahn A, Giri S, Yaghmour G, et al. : Early mortality in acute myeloid leukemia. Leuk Res 39:505–9, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Higby DJ, Cohen E, Holland JF, et al. : The prophylactic treatment of thrombocytopenic leukemic patients with platelets: a double blind study. Transfusion 14:440–6, 1974 [DOI] [PubMed] [Google Scholar]
- 6.Cornely OA, Maertens J, Winston DJ, et al. : Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356:348–59, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Kristinsson SY, Derolf AR, Edgren G, et al. : Socioeconomic differences in patient survival are increasing for acute myeloid leukemia and multiple myeloma in sweden. J Clin Oncol 27:2073–80, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Bierenbaum J, Davidoff AJ, Ning Y, et al. : Racial differences in presentation, referral and treatment patterns and survival in adult patients with acute myeloid leukemia: a single-institution experience. Leuk Res 36:140–5, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho G, Jonas B., Li Q., Brunson A., Wun Ann., Keegan T.: Early mortality and complications in hospitalized adult Californians with acute myeloid leukaemia. British Journal of Hematology In Press, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfister DG, Rubin DM, Elkin EB, et al. : Risk Adjusting Survival Outcomes in Hospitals That Treat Patients With Cancer Without Information on Cancer Stage. JAMA Oncol 1:1303–10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birkmeyer NJ, Goodney PP, Stukel TA, et al. : Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer 103:435–41, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Friese CR, Earle CC, Silber JH, et al. : Hospital characteristics, clinical severity, and outcomes for surgical oncology patients. Surgery 147:602–9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etzioni DA, Young-Fadok TM, Cima RR, et al. : Patient survival after surgical treatment of rectal cancer: impact of surgeon and hospital characteristics. Cancer 120:2472–81, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Wolfson J, Sun CL, Wyatt L, et al. : Adolescents and Young Adults with Acute Lymphoblastic Leukemia and Acute Myeloid Leukemia: Impact of Care at Specialized Cancer Centers on Survival Outcome. Cancer Epidemiol Biomarkers Prev, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiatt RA, Tai CG, Blayney DW, et al. : Leveraging state cancer registries to measure and improve the quality of cancer care: a potential strategy for California and beyond. J Natl Cancer Inst 107, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Grannis SJ, Overhage JM, McDonald CJ: Analysis of identifier performance using a deterministic linkage algorithm. Proc AMIA Symp:305–9, 2002 [PMC free article] [PubMed] [Google Scholar]
- 17.Hser YI, Evans E: Cross-system data linkage for treatment outcome evaluation: lessons learned from the California Treatment Outcome Project. Eval Program Plann 31:125–35, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoenman JA, Sutton JP, Elixhauser A, et al. : Understanding and enhancing the value of hospital discharge data. Med Care Res Rev 64:449–68, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Onega T, Duell EJ, Shi X, et al. : Geographic access to cancer care in the U.S. Cancer 112:909–18, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Onega T, Duell EJ, Shi X, et al. : Race versus place of service in mortality among medicare beneficiaries with cancer. Cancer 116:2698–706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Press IoMWDNA: Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis, 2013 [PubMed]
- 22.Wolfson JA, Sun CL, Wyatt LP, et al. : Impact of care at comprehensive cancer centers on outcome: Results from a population-based study. Cancer 121:3885–93, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulson EC, Mitra N, Sonnad S, et al. : National Cancer Institute designation predicts improved outcomes in colorectal cancer surgery. Ann Surg 248:675–86, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Begg CB, Cramer LD, Hoskins WJ, et al. : Impact of hospital volume on operative mortality for major cancer surgery. JAMA 280:1747–51, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Halm EA, Lee C, Chassin MR: Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med 137:511–20, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Bilimoria KY, Talamonti MS, Wayne JD, et al. : Effect of hospital type and volume on lymph node evaluation for gastric and pancreatic cancer. Arch Surg 143:671–8; discussion 678, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Giri S, Pathak R, Aryal MR, et al. : Impact of hospital volume on outcomes of patients undergoing chemotherapy for acute myeloid leukemia: a matched cohort study. Blood 125:3359–60, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Billingsley KG, Morris AM, Dominitz JA, et al. : Surgeon and hospital characteristics as predictors of major adverse outcomes following colon cancer surgery: understanding the volume-outcome relationship. Arch Surg 142:23–31; discussion 32, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Aiken L, Kesselring A, Schubert M: [Job satisfaction and treatment outcomes. Support is vital for nursing quality]. Krankenpfl Soins Infirm 96:10–2, 62–3, 2003 [PubMed] [Google Scholar]
- 30.Stienen JJ, Hermens RP, Wennekes L, et al. : Variation in guideline adherence in non-Hodgkin’s lymphoma care: impact of patient and hospital characteristics. BMC Cancer 15:578, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weeks JC, Uno H, Taback N, et al. : Interinstitutional variation in management decisions for treatment of 4 common types of cancer: A multi-institutional cohort study. Ann Intern Med 161:20–30, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monson JR, Probst CP, Wexner SD, et al. : Failure of evidence-based cancer care in the United States: the association between rectal cancer treatment, cancer center volume, and geography. Ann Surg 260:625–31; discussion 631–2, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Charlton ME, Hrabe JE, Wright KB, et al. : Hospital Characteristics Associated with Stage II/III Rectal Cancer Guideline Concordant Care: Analysis of Surveillance, Epidemiology and End Results-Medicare Data. J Gastrointest Surg 20:1002–11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow CJ, Habermann EB, Abraham A, et al. : Does enrollment in cancer trials improve survival? J Am Coll Surg 216:774–80; discussion 780–1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unger JM, Barlow WE, Martin DP, et al. : Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst 106:dju002, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unger JM, LeBlanc M, Blanke CD: The Effect of Positive SWOG Treatment Trials on Survival of Patients With Cancer in the US Population. JAMA Oncol 3:1345–1351, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saygin C, Carraway HE: Emerging therapies for acute myeloid leukemia. J Hematol Oncol 10:93, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keating MJ, Smith TL, Gehan EA, et al. : A prognostic factor analysis for use in development of predictive models for response in adult acute leukemia. Cancer 50:457–65, 1982 [DOI] [PubMed] [Google Scholar]
- 39.Munker R, Hill U, Jehn U, et al. : Renal complications in acute leukemias. Haematologica 83:416–21, 1998 [PubMed] [Google Scholar]
- 40.Medeiros BC, Satram-Hoang S, Hurst D, et al. : Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol 94:1127–38, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gage-Bouchard EA, Rodriguez EM, Saad-Harfouche FG, et al. : Factors influencing patient pathways for receipt of cancer care at an NCI-designated comprehensive cancer center. PLoS One 9:e110649, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballard DJ, Bryant SC, O’Brien PC, et al. : Referral selection bias in the Medicare hospital mortality prediction model: are centers of referral for Medicare beneficiaries necessarily centers of excellence? Health Serv Res 28:771–84, 1994 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.