Abstract
Diabetes is associated with cardiac inflammation and impaired endothelium-dependent coronary vasodilation, but molecular mechanisms involved in this dysfunction remain unclear. We examined contributions of inflammatory molecules lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), stress-activated kinases (c-Jun N-terminal kinase [JNK] and p38), arginase, and reactive oxygen species to coronary arteriolar dysfunction in a porcine model of type 1 diabetes. Coronary arterioles were isolated from streptozocin-induced diabetic pigs and control pigs for vasoreactivity and molecular/biochemical studies. Endothelium-dependent nitric oxide (NO)-mediated vasodilation to serotonin was diminished after 2 weeks of diabetes, without altering endothelium-independent vasodilation to sodium nitroprusside. Superoxide scavenger TEMPOL, NO precursor L-arginine, arginase inhibitor nor-NOHA, anti-LOX-1 antibody or JNK inhibitors SP600125 and BI-78D3 improved dilation of diabetic vessels to serotonin. However, hydrogen peroxide scavenger catalase, anti-IgG antibody or p38 kinase inhibitor SB203580 had no effect. Combined inhibition of arginase and superoxide levels did not further improve vasodilation. Arginase-I mRNA expression, LOX-1 and JNK protein expression, and superoxide levels were elevated in diabetic arterioles. In conclusion, sequential activation of LOX-1, JNK, and L-arginine consuming enzyme arginase-I in diabetes elicits superoxide-dependent oxidative stress and impairs endothelial NO-mediated dilation in coronary arterioles. Therapeutic targeting of these adverse vascular molecules may improve coronary arteriolar function during diabetes.
Keywords: Vasodilation, Nitric Oxide, Endothelial Dysfunction, Oxidative Stress, Microvascular Complications
1. Introduction
Type 1 diabetes mellitus is a major risk factor for the development of cardiomyopathy, a leading cause of heart failure and mortality in diabetic patients [1]. The pathogenesis of diabetic cardiomyopathy leading to ventricular dysfunction remains unclear, but reduction in coronary blood flow, in the absence of large artery obstruction, may be involved [2]. Regulation of vascular tone in the coronary microcirculation at the level of resistance arterioles plays a major role in adjusting coronary blood flow to match the metabolic demand of the myocardium for oxygen and nutrients [3]. When metabolic activity of the myocardium is increased, the dilation of coronary arterioles leads to reduction of coronary vascular resistance and thus increases blood flow. This coronary flow reserve capacity is reduced in diabetic animals [4–6] and human patients [2, 7–10], suggesting that the vasomotor regulation of the coronary arterioles is compromised in diabetes. Additional evidence has shown decreased dilation of coronary arterioles to endothelium-dependent agonists during diabetes [11–13]. One of the major endothelial factors contributing to the dilation of coronary arterioles under normal healthy conditions is nitric oxide (NO) [14, 15]. Clinical and experimental investigations indicate that NO contributes to coronary vasodilation in response to increased metabolic demand [16] and lumenal shear stress/flow [17, 18], to some endothelium-dependent vasodilators released from the myocardium [19, 20] and circulating blood cells [15, 18], and to neurotransmitters [21]. Therefore, diminished production of the potent vasodilator NO by the coronary arteriolar endothelium may contribute to myocardial ischemia and pathogenesis of diabetic cardiomyopathy leading to ventricular dysfunction [2]. However, the precise mechanisms contributing to diabetes-induced impairment of endothelium-dependent NO-mediated dilation of coronary arterioles are largely unknown.
Local cardiac inflammation and oxidative stress via reactive oxygen species have been implicated in the development of cardiac damage associated with diabetes [22, 23]. The expression of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), a proinflammatory signaling molecule, has been shown to be elevated in diabetic rat aorta [24] and in cultured human aortic endothelial cells after exposure to high glucose [25]. Activation of LOX-1 triggers signaling pathways via stress-activated kinases, c-Jun N-terminal kinase (JNK) and p38 kinase, in cardiomyocytes [26] and endothelial cells [27, 28]. However, the contribution of LOX-1 and stress-activated kinases to vasomotor dysfunction during diabetes has not been evaluated. Additional signaling molecules that have been shown to be influenced downstream from LOX-1 activation are superoxide-generating oxidant enzymes [29] and arginase [30]. It is well established that superoxide is a potent NO scavenger and its excess production could have a detrimental impact on endothelium-dependent NO-mediated coronary arteriolar dilation [15, 29, 31, 32]. In the vascular endothelium, arginase limits the availability of the precursor L-arginine for endothelial NO synthase (eNOS) to produce NO [33–35]. Although diabetes has been show to elevate vascular arginase expression and blunt endothelium-dependent vasorelaxation [12, 36, 37], its signaling related to stress-activated kinases in coronary vasomotor dysfunction remains elusive. Therefore, the objective of the present study was to assess whether activation of LOX-1, stress-activated kinases, arginase, and reactive oxygen species (superoxide anion and hydrogen peroxide) contribute to the impairment of endothelium-dependent NO-mediated dilation of coronary arterioles in pigs exhibiting type 1 diabetes [38]. We used an isolated vessel approach in vitro, which excludes confounding influences from hemodynamic and humoral factors associated with in vivo preparations, to evaluate vasodilator function of coronary arterioles. Pharmacological tools and biochemical assays were performed to detect LOX-1, JNK and arginase isoform expression, superoxide production, and molecular signaling events in coronary arterioles.
2. Materials and Methods
2.1. Porcine Diabetes Model
All animal procedures were approved by the Baylor Scott & White Institutional Animal Care and Use Committee. Domestic (Yorkshire) male pigs (8–12 weeks old, 8–15 kg) were purchased from Real Farms (San Antonio, TX, USA). Type 1 diabetes was induced by selective ablation of pancreatic ß-cells with intravenous injection of streptozocin (STZ, Zanosar®, 150–200 mg/kg in saline) via an ear vein (47 pigs), as described in detail in our previous study [38]. The control group was intravenously injected with saline (40 pigs). The pigs were maintained for a period of 2 weeks. The general condition, body weight, and the level of blood glucose were closely monitored in all pigs. Fasting blood glucose levels were obtained every other day in the morning using a Bayer Contour glucometer. Pigs were treated with insulin (Humulin® 70/30, 2–8 units; Lilly; Indianapolis, IN, USA) if the fasting blood glucose was sustained above 600 mg/dl to keep the level between 250 and 600 mg/dl, but it was not given 48 hours prior to terminal surgery. After the 2-week time period, pigs were sedated with Telazol (4–8 mg/kg, intramuscularly), anesthetized with 2% to 5% isoflurane, and intubated. The heart was removed and immediately placed on iced (5°C) saline.
2.2. Isolation and Cannulation of Coronary Arterioles
Individual subepicardial arterioles (≈1 mm in length; ~20–60 μm in situ diameter) were dissected from the surrounding cardiac tissue [39]. Vessels were then cannulated on each end with glass micropipettes containing a physiological saline solution (PSS)-albumin (1%) and pressurized to 60 cmH2O intraluminal pressure without flow by two independent pressure reservoir systems [17]. The inner diameter of coronary arterioles was recorded using videomicroscopic techniques throughout the experiments, as we described previously [17, 39].
2.3. Study of Vasomotor Function
Cannulated, pressurized arterioles were bathed in PSS-albumin at 36 to 37°C to allow the development of basal tone (stable within 60 minutes). To evaluate the effect of diabetes on vasomotor function, endothelium-dependent NO-mediated vasodilation to serotonin (0.1 nM to 0.1 μM) [18, 33, 40] and endothelium-independent vasodilation to sodium nitroprusside (1 nM to 10 μM) were established in vessels isolated from the diabetic and saline-control pigs. To assess contributions of LOX-1, arginase, and superoxide in the diabetes-induced effect, the vasodilator responses were examined following intraluminal treatment of vessels with anti-LOX-1 antibody (1 μg/ml; Catalog #ab60178, Abcam; Cambridge, MA, USA) [29], arginase inhibitor nor-NOHA (0.1 mM; Cayman Chemical; Ann Arbor, MI, USA) [35, 41, 42], and superoxide dismutase mimetic TEMPOL (1 mM) [29, 31] for 90 minutes. The impact of superoxide-derived hydrogen peroxide was examined after incubation of vessels with hydrogen peroxide scavenger catalase (1000 U/ml) for 30 minutes, as established in our previous study for preventing hydrogen peroxide-induced dysfunction of coronary arterioles [42]. To assess the contribution of L-arginine deficiency, the serotonin-induced dilation of diabetic vessels was examined after treating the vessels with L-arginine (3 mM) for 30 minutes [15, 42]. For some vessels, involvement of NO in the vasodilation to serotonin was evaluated in the presence of NO synthase (NOS) inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 10 μM, 30-minute incubation) [29, 40] with or without the anti-LOX-1 antibody or nor-NOHA. The impact of stress-activated kinases on serotonin-induced vasodilation was evaluated after incubation with JNK inhibitors SP600125 (3 μM) [31, 43] or BI-78D3 (3 μM) [44, 45], or p38 inhibitor SB203580 (0.1 μM; EMD Millipore; Billerica, MA, USA) [46–48] for 90 minutes. In another cohort of vessels, the potential nonspecific effect of antibody treatment was assessed after intraluminal incubation with an anti-IgG antibody (1 μg/ml; Catalog #ab176094, Abcam) for 90 minutes.
2.4. RNA Isolation and Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was isolated from coronary arterioles (3–4 vessels per sample pooled from one heart) of similar size to those used for functional studies, as we described previously [33]. Sets of primers specific for arginase-I, arginase-II, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes were engineered (Sigma-Genosys, The Woodlands, TX, USA), as we reported previously [33]. Using 0.5 μg of total RNA per sample, RT-PCR was conducted, as we described previously [33]. The PCR reaction was optimized and run for 35 cycles for all genes. The level of expression of arginase isoform transcripts was normalized to that of GAPDH transcripts.
2.5. Western Blot Analysis
Total protein samples were extracted from coronary arterioles (4–6 vessels per sample pooled from one heart) of similar size to those used for functional studies, as we described previously [29]. Proteins (2.5–10 μg) were separated by 4–15% SDS-PAGE (Bio-Rad, Hercules, CA, USA), transferred onto nitrocellulose membrane, and then incubated with rabbit anti-LOX-1 polyclonal antibody (1:1000 dilution; Catalog #ab60178, Abcam), rabbit anti-JNK polyclonal antibody (1:1000 dilution; Catalog #9252, Cell Signaling Technology, Danvers, MA), or mouse anti-eNOS monoclonal antibody (1:500: Catalog #610297, BD Transduction Laboratories, San Jose, CA, USA). After incubation with an appropriate secondary antibody (anti-rabbit or anti-mouse IgG, 1:1000; Catalog # 7074s and 7076s, Cell Signaling Technology, Danvers, MA, USA), the membranes were washed and developed by enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL, USA). The membranes were stripped and re-probed with rabbit anti-α-smooth muscle actin antibody (1:1000 to 1:5000; Catalog #A2547, Sigma, St. Louis, MO, USA). Densitometric analyses of immunoblots were performed by NIH ImageJ software. Results for total LOX-1, JNK, and eNOS in vessel samples were normalized to total α-smooth muscle actin.
2.6. Detection of Superoxide
Isolated control and diabetic coronary arterioles (40 to 100 μm in diameter and 1.5 mm in length) were embedded in OCT compound (Tissue-Tek) and cut into 12-μm-thick sections using a Leica CM1850 cryostat (Leica, Germany), as we described previously [49]. Sections were stained with dihydroethidium (DHE, 4 μM) for 30 minutes to detect superoxide. Images were taken using an Axiovert 200 microscope (Zeiss, Germany) and fluorescence intensities of DHE staining were analyzed by AxioVision software (Zeiss). Settings for image acquisition were identical for both samples.
2.7. Chemicals
All drugs were obtained from Sigma-Aldrich (St. Louis, MO, USA) except as specifically stated. SP600125, BI-78D3, and SB203580 were dissolved at 10 mM in dimethyl sulfoxide, with subsequent concentrations diluted in PSS. All other drugs were dissolved in PSS. The final concentration of dimethyl sulfoxide in the vessel bath did not exceed 0.03% by volume. The 0.03% dimethyl sulfoxide had no significant effect on vessel viability, vasodilator responses, or maintenance of tone (data not shown).
2.8. Data Analysis
At the end of each experiment, the maximum diameter of the vessels was obtained at 0.1 mM sodium nitroprusside in the presence of calcium-free PSS with EDTA (1 mM) [46]. Diameter changes in response to vasodilator agonists were normalized to this maximum vasodilation and expressed as % maximum dilation. Data are reported as mean ± SEM and n value represents the number of vessels (1 per pig per treatment group) studied for functional studies or number of pigs used for molecular/biochemical studies. Student’s t-test or ANOVA followed by Bonferroni multiple-range test was used to determine the significance of experimental interventions, as appropriate. A value of P < 0.05 was considered significant.
3. Results
3.1. Animal Model of Diabetes Mellitus
Two weeks after STZ injection, blood glucose in pigs elevated from 93 ± 8 mg/dl to 494 ± 16 mg/dl. Pigs injected with saline (control) had comparable blood glucose levels after 2 weeks (92 ± 3 mg/dl vs. 95 ± 4 mg/dl after saline injection; P = 0.42). The body weight gain was less for diabetic pigs (before STZ injection: 11.9 ± 0.4 kg; 2 weeks after STZ: 14.2 ± 0.5 kg) than for control pigs (before saline injection: 12.6 ± 0.4 kg; 2 weeks after saline: 22.1 ± 0.8 kg).
3.2. Effect of Diabetes on NOS-Mediated Vasodilation
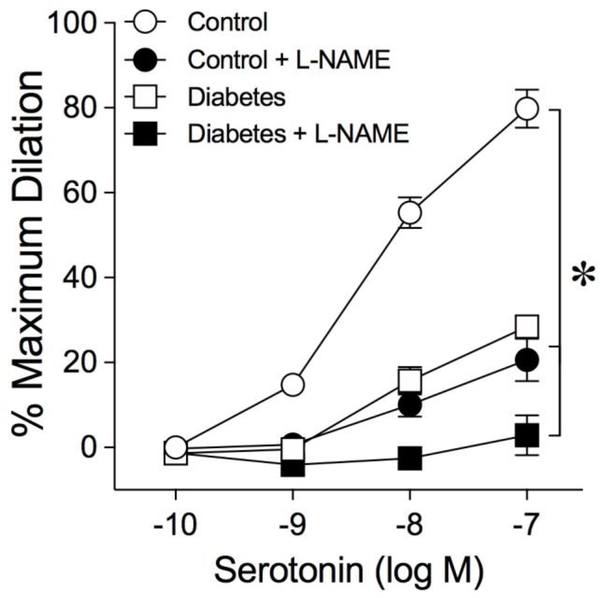
Coronary arterioles from control (n = 40) and diabetic (n = 47) pigs developed a comparable level of basal tone (Control: 58 ± 1% of 96 ± 2 μm maximum diameter vs. Diabetes: 56 ± 1% of 89 ± 2 μm maximum diameter; P = 0.12). In one cohort, coronary arterioles from control pigs dilated concentration-dependently to serotonin with maximum dilation of about 80% at 0.1 μM (Fig. 1). The serotonin-induced vasodilation was significantly reduced after treating the control vessels with NOS inhibitor L-NAME (Fig. 1), while basal tone (Control: 62 ± 1% of maximum diameter vs. Control + L-NAME: 56 ± 2% of maximum diameter) was unaltered (P = 0.10). After 2 weeks of diabetes, the dilation of coronary arterioles to serotonin was significantly reduced with maximum dilation of about 25% at 0.1 μM (Fig. 1). This residual vasodilation to serotonin was slightly reduced further in the presence of L-NAME (Fig. 1).
Fig. 1.
Diabetes impairs NOS-mediated dilation of coronary arterioles. Concentration-dependent dilation of isolated and pressurized porcine coronary arterioles to serotonin was examined in the absence or presence of NOS inhibitor L-NAME after 2 weeks of euglycemia (Control; n = 6) or hyperglycemia (Diabetes; n = 5). *P<0.05 vs. Control, two-way ANOVA with Bonferroni multiple-range test.
3.3. Roles of LOX-1 and Stress-Activated Kinases in Diabetes-Induced Vascular Dysfunction
To determine whether LOX-1, JNK and p38 are involved in the reduction of serotonin-induced vasodilation in diabetic pigs, coronary arterioles were treated with LOX-1 blocking antibody and specific kinase inhibitors. Incubation of diabetic vessels with a LOX-1 antibody did not alter basal tone (Diabetes: 55 ± 2% of maximum diameter vs. Diabetes + LOX-1 antibody: 59 ± 1% of maximum diameter, P = 0.08) but improved the vasodilation to serotonin (Fig. 2), in a manner sensitive to L-NAME treatment (Fig. 2). In contrast, an anti-IgG antibody did not alter the resting tone of diabetic vessels (Diabetes: 62 ± 2% of maximum diameter vs. Diabetes + IgG antibody: 59 ± 4% of maximum diameter, P = 0.60) or the response to serotonin (Fig. 2). For control vessels, the LOX-1 antibody treatment did not affect the serotonin-induced vasodilation (data not shown). Incubation of diabetic vessels with JNK inhibitor SP600125 slightly reduced basal tone (Diabetes: 51 ± 3% of maximum diameter vs. Diabetes + SP600125: 59 ± 2% of maximum diameter, P = 0.03) and increased the vasodilator response to serotonin (Fig. 3). An additional JNK inhibitor, BI-78D3, had a similar effect on basal tone and improvement of vasodilation to serotonin (Fig. 3). In contrast, p38 kinase inhibitor SB203580 did not alter the resting vessel tone or the dilation of diabetic vessels to serotonin (Fig. 3). For control vessels, SP600125, BI-78D3, and SB203580 did not alter basal tone or serotonin-induced vasodilation (data not shown).
Fig. 2.
Blockade of LOX-1 activation improves serotonin-induced dilation of coronary arterioles isolated from diabetic pigs. Dilation of isolated and pressurized porcine coronary arterioles to serotonin was examined after 2 weeks of euglycemia (Control; n = 14) or hyperglycemia (Diabetes; n = 14). In another cohort of diabetic vessels, vasodilation to serotonin was examined in the presence of a LOX-1 antibody (n = 11) before and after treatment with L-NAME (n = 4) or the presence of an IgG antibody (n = 6). *P<0.05 vs. Control, #P<0.05 vs. Diabetes, two-way ANOVA with Bonferroni multiple-range test.
Fig. 3.
Blockade of JNK activation improves serotonin-induced dilation of coronary arterioles isolated from diabetic pigs. Dilation of isolated and pressurized porcine coronary arterioles to serotonin was examined after 2 weeks of euglycemia (Control; n = 8) or hyperglycemia (Diabetes; n = 9). In another cohort of diabetic vessels, vasodilation to serotonin was examined in the presence of JNK inhibitors SP600125 (n = 6) or BI-78D3 (n = 3), or p38 kinase inhibitor SB203580 (n = 6). *P<0.05 vs. Control, #P<0.05 vs. Diabetes, two-way ANOVA with Bonferroni multiple-range test.
3.4. Roles of Arginase, L-arginine, and Reactive Oxygen Species in Diabetes-Induced Vasodilator Dysfunction
In another cohort, the relative contributions of arginase, L-arginine deficiency, and reactive oxygen species (superoxide and hydrogen peroxide) to the diabetes-induced impairment of vasodilation to serotonin were assessed by treating vessels with arginase inhibitor nor-NOHA, excess L-arginine, superoxide dismutase mimetic TEMPOL, and hydrogen peroxide scavenger catalase. Intraluminal treatment with nor-NOHA did not alter basal tone (Diabetes: 55 ± 2% of maximum diameter vs. Diabetes + nor-NOHA: 58 ± 2% of maximum diameter; P = 0.20) but significantly increased the dilation of diabetic coronary arterioles to serotonin (Fig. 4A). In the diabetic vessels treated with nor-NOHA, the vasodilator responses to serotonin were significantly reduced by L-NAME (Fig. 4A). The dilation of diabetic vessels to serotonin was also increased in a similar manner as nor-NOHA after addition of L-arginine (Fig. 4A) or treatment with TEMPOL (Fig. 4B). However, combination of TEMPOL and nor-NOHA did not further improve the serotonin-induced vasodilation (Fig. 4B). Catalase did not alter the dilation of diabetic vessels to serotonin (Fig. 4B). Administration of nor-NOHA, excess L-arginine, or TEMPOL did not affect serotonin-induced dilations in control vessels (data not shown).
Fig. 4.
Blockade of arginase activation, addition of L-arginine, and scavenging of superoxide improve serotonin-induced dilation of coronary arterioles isolated from diabetic pigs. (A) The serotonin-induced dilation was compared between euglycemic control (n = 13) and diabetic (n = 13) vessels. In another cohort of diabetic vessels, vasodilation to serotonin was examined in the presence of arginase inhibitor nor-NOHA before (n = 10) and after (n = 6) treatment with L-NAME, or after administration of L-arginine (n = 3). (B) The serotonin-induced dilation was compared between euglycemic control (n = 19) and diabetic (n = 20) vessels. In another cohort of diabetic vessels, vasodilation to serotonin was examined in the presence of superoxide dismutase mimetic TEMPOL alone (n = 9) or combined with arginase inhibitor nor-NOHA (n = 7), or after administration of hydrogen peroxide scavenger catalase (n = 5). *P<0.05 vs. Control, #P<0.05 vs. Diabetes, two-way ANOVA with Bonferroni multiple-range test.
3.5. Vasodilation to Sodium Nitroprusside After Diabetes
Control and diabetic coronary arterioles dilated in a comparable manner to endothelium-independent NO donor sodium nitroprusside with maximum dilation of about 95% at 10 μM for both groups of vessels (Supplementary Fig. 1).
3.6. Effect of Diabetes on Vascular Arginase mRNA Expression
Arginase-I mRNA expression was significantly greater in the diabetic coronary arterioles than the control vessels (Fig. 5). The mRNA expression of arginase-II was not detected in the vessel samples from control or diabetic pigs (data not shown).
Fig. 5.
Diabetes increases arginase-I mRNA expression in coronary arterioles from diabetic pigs. Upper Panel: The mRNA expression of arginase-I in isolated porcine coronary arterioles after 2 weeks of euglycemia (Control; n = 3) or hyperglycemia (Diabetes; n = 3) was determined by RT-PCR analysis. The expression of GAPDH was used as an internal control. Lower Panel: The quantitative results of three independent experiments. *P<0.05 vs. Control, t-test.
3.7. Effect of Diabetes on Vascular LOX-1, JNK, and eNOS Protein Expression
Protein expression of LOX-1 (Fig. 6A) and JNK isoform p46 but not p54 (Fig. 6B) was significantly greater in diabetic coronary arterioles than in control vessels. The eNOS protein level in coronary arterioles isolated from control and diabetic pigs was comparable (Supplementary Fig. 2).
Fig. 6.
Diabetes increases LOX-1 and p46 JNK protein expression in coronary arterioles from diabetic pigs. Protein expression of (A) LOX-1 and (B) JNK splicing isoforms p46 and p54 in isolated porcine coronary arterioles after 2 weeks of euglycemia (Control) or hyperglycemia (Diabetes) was determined by Western blot analysis. The expression of α-smooth muscle actin (SMA) was used as an internal control and used to normalize the LOX-1 and JNK expression from (A) four and (B) three independent experiments. *P<0.05 vs. Control, t-test.
3.8. Effect of Diabetes on Vascular Superoxide Levels
The effect of diabetes on superoxide levels in isolated coronary arterioles was determined by histochemical staining for superoxide. In the control vessels, DHE fluorescence revealed sparse levels of superoxide (Fig. 7). In contrast, 2 weeks of diabetes markedly increased superoxide in the vessel wall (Fig. 7).
Fig. 7.
Diabetes increases superoxide levels in coronary arterioles from diabetic pigs. Upper Panel: Superoxide levels in isolated porcine coronary arterioles after 2 weeks of euglycemia (Control) or hyperglycemia (Diabetes) was determined by DHE fluorescence imaging. Scale bar: 50 μm. Lower Panel: The quantitative results of five independent experiments. *P<0.05 vs. Control, t-test.
4. Discussion
Although the phenomenon of reduction in endothelium-dependent dilation of coronary arterioles has been observed in animals and patients with type 1 diabetes [11–13], there is sparse information on the molecular mechanisms contributing to the damage of these major resistance vessels that regulate myocardial blood flow. In this study we demonstrate that activation of LOX-1, JNK, and arginase signaling events, linked to L-arginine deficiency and superoxide-dependent oxidative stress, impairs endothelium-dependent NO-mediated dilation of coronary arterioles in type 1 diabetic pigs. The proposed signaling pathway for this vasodilator dysfunction is delineated in Figure 8 and is discussed below based on our present findings and other reports. These findings provide the first evidence for a functional impact of LOX-1 and JNK in diminishing coronary vasodilator function in early diabetes.
Fig. 8.
Proposed signaling sequence for the development of coronary arteriolar dysfunction by diabetes/hyperglycemia. Under normal conditions, serotonin activates eNOS in the endothelium of coronary arterioles leading to the production of NO from the substrate L-arginine and subsequent dilation. During prolonged hyperglycemia of type 1 diabetes, endothelial LOX-1 in coronary arterioles is activated with subsequent downstream signaling via JNK and arginase-I causing a reduction in L-arginine availability for eNOS. In the absence of sufficient levels of L-arginine, eNOS produces superoxide anions (O2•−), which scavenge NO and reduce endothelium-dependent vasodilation (represented by dashed lines).
Previous experimental evidence has shown a relatively rapid onset of endothelial vasodilator dysfunction of coronary arterioles in type 1 diabetes models [11, 13]. In these studies, coronary arteriolar dilation to topical administration of endothelial agonist acetylcholine in vivo was diminished after 1 week of hyperglycemia/diabetes in dogs [11, 13]. However, these earlier reports did not assess whether impairment of endothelium-dependent vasodilation was a result of intrinsic vascular damage or diminished NOS function. In the present study, we evaluated the vasomotor function of isolated coronary arterioles in vitro with nonselective NOS inhibitor L-NAME after chronic hyperglycemia in an established type 1 diabetic pig model [38, 44]. We observed early development of diminished endothelium-dependent dilation to serotonin within 2 weeks of sustained hyperglycemia and L-NAME treatment nearly abolished the remaining vasodilation to serotonin (Fig. 1). Because L-NAME also nearly abolished the dilation of coronary arterioles from 2-week control pigs, it is likely that diabetes compromises the endothelium-dependent NO bioavailability by reducing NO production from NOS and/or enhancing the NO degradation. In our study, diabetes appeared not to affect eNOS protein expression and function in coronary arterioles because the eNOS protein level was maintained (Supplementary Fig. 2) and the impaired endothelium-dependent NO-mediated dilation was readily reversible. Furthermore, the vascular smooth muscle reactivity in response to NO was not affected by diabetic insult because the vasodilation to endothelium-independent NO donor sodium nitroprusside remained intact (Supplementary Fig. 1). These findings are consistent with the preserved coronary vasodilation to sodium nitroprusside in vivo in diabetic dogs [11, 13] and rats [5]. Collectively, our findings suggest that the impairment of endothelium-dependent NO-mediated dilation of coronary arterioles within 2 weeks of type 1 diabetes unlikely resulted from eNOS protein downregulation or vascular smooth muscle dysfunction. However, we have not ruled out the possibility that phosphorylation-dependent activation of eNOS [50] is also influenced by diabetes, as has been reported for skeletal muscle arterioles exposed to hyperglycemia [51]. Because all treatments in our study did not fully restore endothelium-dependent NO-mediated dilation of diabetic coronary arterioles, the phosphorylation of eNOS activation sites is an additional mechanism worthy of future molecular studies in this diabetes model.
LOX-1 was initially identified as a vascular endothelial receptor for oxidized low-density lipoprotein and has been implicated in the pathogenesis of cardiovascular disease [52, 53]. Subsequent studies have revealed that additional ligands, such as C-reactive protein (CRP), polyanionic compounds, oxidized low-density lipoprotein, and advanced glycation end products are capable of binding to LOX-1 [54]. Our previous study provided evidence of endothelial expression of LOX-1 in coronary arterioles and the adverse role of CRP-dependent activation of LOX-1 in mediating endothelial vasodilator dysfunction [29]. Although LOX-1 expression was elevated in the endothelial layer of diabetic rat aorta [24] and in cultured human aortic endothelial cells after treatment with high glucose [25], it remains unknown whether changes in LOX-1 expression/activation contribute to vascular endothelial vasodilator dysfunction during diabetes. In the current study, we showed that functional blockade of LOX-1 with an anti-LOX-1 antibody improved endothelium-dependent NO-mediated dilation of coronary arterioles during diabetes (Fig. 2). This LOX-1 antibody has been shown to detect the knockdown of LOX-1 protein in human coronary artery endothelial cells subjected to the treatment with LOX-1 siRNA, indicating the specificity and potency of this antibody [29]. The lack of an effect of the anti-LOX-1 antibody on the dilation of control vessels and the inability of an anti-IgG antibody to improve dilation of diabetic vessels also support the functional specificity of the LOX-1 antibody in blocking LOX-1 activation in the diabetic vessels. The LOX-1 protein expression in coronary arterioles was confirmed in the current study, and it appears that diabetes elevates LOX-1 expression in these microvessels (Fig. 6A). It is reasonable to speculate that a possible ligand for activating LOX-1 and regulating its expression during diabetes is high glucose per se and/or advanced glycation end products derived from hyperglycemia [25, 55]. Future studies are needed to investigate this possible metabolic link to LOX-1 activation in diabetic coronary arterioles. Nonetheless, our molecular and functional findings support the idea that increased expression/activation of LOX-1 contributes to the endothelial vasodilator dysfunction of coronary arterioles during diabetes (Fig. 8).
Cardiac dysfunction in type 1 diabetes is associated with local inflammation in the heart, including activation of stress-activated kinases JNK and p38 [56]. It is important to address the potential contribution of these kinases to coronary arteriolar vasomotor dysfunction during diabetes because p38 [46] and JNK [31] have been shown to mediate coronary arteriolar dysfunction elicited by the inflammatory molecules CRP and tumor necrosis factor-α, respectively. In the present study, we found that treatment of diabetic vessels with JNK inhibitor SP600125, but not p38 kinase inhibitor SB203580, improved the dilation to serotonin in diabetic vessels (Fig. 3), suggesting the activation of JNK signaling contributes to coronary endothelial dysfunction during the early onset of diabetes. The concentration of SB203580 (0.1 μM) used in the present study was adequate because its efficacy has been demonstrated in preventing endothelial vasodilator dysfunction in coronary arterioles induced by CRP [46] or endothelin-1 [47]. Treatment with another JNK inhibitor, BI-78D3, also augmented the NO-mediated dilation of coronary arterioles from diabetic pigs to serotonin (Fig. 3). Whereas SP600125 directly inhibits the activity of JNK [43], the structurally distinct BI-78D3 [44, 45] interferes with the binding of JNK to JNK-interacting protein-1, a scaffolding protein that facilitates sequential kinase activation in the JNK signaling pathway [57]. The reversal effect of BI-78D3 further supports the JNK activation in diabetes-induced coronary arteriolar dysfunction. There are 2 JNK splicing isoforms, p46 and p54, which may contribute to the observed coronary arteriolar dysfunction [58]. The increase of p46 but not p54 JNK protein expression in coronary arterioles (Fig. 6B) indicates potential selective activation of JNK signaling in diabetes. Because LOX-1 activation can upregulate JNK protein expression in cultured human umbilical vein endothelial cells [27], it is possible that activation of LOX-1-dependent signaling in diabetes regulates JNK expression in coronary arterioles in our study. Notably, we were unable to reliably detect phosphorylation of JNK isoforms. This limitation may have resulted from insufficient protein from small coronary arterioles or the lack of robust antibody for porcine vessels. The potential molecular interaction of LOX-1 and JNK in coronary arterioles leading to endothelial vasodilator dysfunction during diabetes warrants evaluation in future studies. We have previously shown that JNK activation can diminish endothelial NO-mediated dilation of coronary arterioles by increasing superoxide production [31], which could have contributed to the vascular impairment observed in the present study (Fig. 8).
Elevation of superoxide anions in blood vessels can interact with NO and reduce its bioavailability. An earlier work showed that topical application of superoxide dismutase and hydrogen peroxide scavenger catalase to the epicardial arterioles in dogs partially restored the suppressed acetylcholine-mediated coronary arteriolar dilation by 1 week of diabetes [13]. Because both of these oxidant scavengers were simultaneously applied to the surface of the heart in this in vivo study, it is not clear whether superoxide anions and/or hydrogen peroxide were involved and whether myocardial or vascular sources of oxidants contributed to the vascular dysfunction. We found that treatment of the isolated vessels with superoxide dismutase mimetic TEMPOL but not catalase improved the endothelium-dependent dilation of coronary arterioles isolated from diabetic pigs (Fig. 4B), suggesting the critical role of vascular superoxide in this setting. This notion is further supported by the histochemical detection of increased superoxide levels in the diabetic coronary arterioles (Fig. 7). It should be noted that our study does not exclude the adverse role of myocardial source of oxidants in influencing coronary arteriolar function, although the vascular production of superoxide is apparently sufficient to exert the observed endothelial dysfunction.
In addition to superoxide scavenging NO, bioavailability of NO may be limited by increased activity of arginase. Vascular arginase in the endothelium can compete with eNOS for their common substrate L-arginine and reduce the availability of this amino acid for the production of NO [33–35]. An increase in arginase-I expression in coronary arterioles isolated from human patients with type 1 and type 2 diabetes has been suggested to play a role in blunting vasodilation to acetylcholine [12]. However, the direct role of arginase in this clinical study is unclear because these diabetic patients also exhibited multiple cardiovascular stresses, including hypertension, hypercholesterolemia, myocardial infarction and various coronary artery diseases, which are known to activate arginase [34, 35, 41, 59]. The observed increase in arginase-I mRNA expression in diabetic coronary arterioles (Fig. 5) suggests that arginase activity may be influenced at least at the transcriptional level in these vessels in early diabetes before the development of apparent cardiovascular disease. As demonstrated in our previous study, we were unable to detect arginase-II mRNA in isolated porcine coronary arterioles [33]. It is possible that JNK-dependent signaling contributes to the upregulation of arginase-I mRNA as has been shown in cultured rat aortic endothelial cells [60] and in the heart [61] in response to thrombin and hypobaric hypoxia, respectively. Both of these studies found that recruitment of transcription factor c-Jun, a downstream target of JNK, to the promoter of the arginase-I gene is required for upregulation of this arginase isoform.
Interestingly, our current findings showed that pharmacologic blockade of arginase with specific arginase inhibitor nor-NOHA [41, 42] improved endothelium-dependent dilation of coronary arterioles from diabetic pigs, in a manner sensitive to L-NAME treatment (Fig. 4A). These observations indicate that arginase inhibition is able to provide sufficient NO for coronary arteriolar dilation during eNOS stimulation. It appears that both arginase activation and superoxide generation are through a similar pathway to reduce NO bioavailability, because nor-NOHA and TEMPOL improved vasodilations similarly and combination of nor-NOHA and TEMPOL did not provide additional improvement of dilation of diabetic vessels (Fig. 4B). It is possible that oxidative stress, i.e., superoxide-derived hydrogen peroxide, is upstream of arginase and can activate this enzyme to reduce L-arginine availability and diminish endothelial NO-mediated vasodilation as previously demonstrated [42]. However, catalase did not improve the dilation of diabetic coronary arterioles in the present study (Fig. 4B). Thus, superoxide signaling, corresponding to coronary arteriolar dysfunction, is unlikely upstream of arginase during diabetic insult. Alternatively, oxidative stress is downstream of arginase because enhanced vascular arginase activity can uncouple eNOS due to L-arginine deficiency and consequently lead to production of superoxide instead of NO [62]. This notion is supported by the improved dilation of diabetic vessels treated with excess L-arginine in a similar manner as treatment with nor-NOHA (Fig. 4A) or TEMPOL (Fig. 4B). These data suggest a correlative link between arginase activation (i.e., L-arginine deficiency) and superoxide production. Interestingly, low-density lipoprotein has been shown to activate arginase and subsequently promote superoxide production and compromise endothelial NO bioavailability for dilation in coronary arterioles [35], a pathway that can be mediated by LOX-1 activation in endothelial cells [30]. Collectively, our current findings bolster the idea that reduced L-arginine availability for eNOS due to increased LOX-1-dependent arginase activity promotes superoxide production from eNOS and subsequently scavenges NO and reduces endothelium-dependent dilation of coronary arterioles during diabetes (Fig. 8). To confirm the interactions and links between arginase, superoxide, and LOX-1 in vasomotor function under hyperglycemic and diabetic conditions, future molecular study on endothelial cells will be required.
5. Conclusions
Our study demonstrates that endothelium-dependent NO-mediated dilation of coronary arterioles is impaired in pigs with type 1 diabetes. It appears that LOX-1 signaling linked to JNK and arginase-I contributes to coronary endothelial vasodilator dysfunction by decreasing L-arginine availability and promoting superoxide production. The ability of pharmacologic blockade of LOX-1, JNK, and arginase-I to improve arteriolar vasodilator function after 2 weeks of hyperglycemia suggests that these specific signaling molecules may be beneficial clinical targets for treatment of coronary deficiency in association with early diabetes.
Supplementary Material
Highlights.
Diabetes impairs endothelial NO vasodilator function of porcine coronary arterioles
Blockade of LOX-1, JNK, or arginase improves dilation of diabetic vessels
NO precursor L-arginine or superoxide scavenger improves vasodilation
Arginase-I mRNA expression and superoxide levels are elevated in diabetic vessels
LOX-1 and JNK protein expressions are elevated in diabetic vessels
Acknowledgments
We are grateful to Angie Hitt and the animal facility staff in the Department of Comparative Medicine at Baylor Scott & White Health for their assistance with animal care.
Funding
This work was supported by the National Institutes of Health [R01EY023335 and R01EY024624] to T.H. and the Baylor Scott & White Kruse Chair Endowment Fund to L.K.
Abbreviations
- CRP
C-reactive protein
- DHE
dihydroethidium
- JNK
c-Jun N-terminal kinase
- L-NAME
Nω-nitro-L-arginine methyl ester
- LOX-1
lectin-like oxidized low-density lipoprotein receptor-1
- NO
nitric oxide
- NOS
nitric oxide synthase
Footnotes
Disclosures
The authors report no commercial or proprietary interest in any product or concept discussed in this article.
Appendix. Supplementary Data
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Boudina S, Abel ED, Diabetic cardiomyopathy revisited, Circulation 115 (25) (2007) 3213–3223. [DOI] [PubMed] [Google Scholar]
- [2].Strauer BE, Motz W, Vogt M, Schwartzkopff B, Evidence for reduced coronary flow reserve in patients with insulin-dependent diabetes. A possible cause for diabetic heart disease in man, Exp Clin Endocrinol Diabetes 105 (1) (1997) 15–20. [DOI] [PubMed] [Google Scholar]
- [3].Kuo L, Davis MJ, Chilian WM, Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation, Circulation 92 (3) (1995) 518–525. [DOI] [PubMed] [Google Scholar]
- [4].Kersten JR, Brooks LA, Dellsperger KC, Impaired microvascular response to graded coronary occlusion in diabetic and hyperglycemic dogs, Am J Physiol 268 (4 Pt 2) (1995) H1667–H1674. [DOI] [PubMed] [Google Scholar]
- [5].Durante W, Sunahara FA, Sen AK, Effect of diabetes on metabolic coronary dilatation in the rat, Cardiovasc Res 23 (1) (1989) 40–45. [DOI] [PubMed] [Google Scholar]
- [6].Tune JD, Yeh C, Setty S, Zong P, Downey HF, Coronary blood flow control is impaired at rest and during exercise in conscious diabetic dogs, Basic Res Cardiol 97 (3) (2002) 248–257. [DOI] [PubMed] [Google Scholar]
- [7].Pitkanen OP, Nuutila P, Raitakari OT, Ronnemaa T, Koskinen PJ, Iida H, Lehtimaki TJ, Laine HK, Takala T, Viikari JS, Knuuti J, Coronary flow reserve is reduced in young men with IDDM, Diabetes 47 (2) (1998) 248–254. [DOI] [PubMed] [Google Scholar]
- [8].Atar AI, Altuner TK, Bozbas H, Korkmaz ME, Coronary flow reserve in patients with diabetes mellitus and prediabetes, Echocardiography 29 (6) (2012) 634–640. [DOI] [PubMed] [Google Scholar]
- [9].Nitenberg A, Valensi P, Sachs R, Dali M, Aptecar E, Attali JR, Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function, Diabetes 42 (7) (1993) 1017–1025. [DOI] [PubMed] [Google Scholar]
- [10].Nahser PJ Jr., Brown RE, Oskarsson H, Winniford MD, Rossen JD, Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus, Circulation 91 (3) (1995) 635–640. [DOI] [PubMed] [Google Scholar]
- [11].Ammar RF Jr., Gutterman DD, Brooks LA, Dellsperger KC, Impaired dilation of coronary arterioles during increases in myocardial O2 consumption with hyperglycemia, Am J Physiol Endocrinol Metab 279 (4) (2000) E868–E874. [DOI] [PubMed] [Google Scholar]
- [12].Beleznai T, Feher A, Spielvogel D, Lansman SL, Bagi Z, Arginase 1 contributes to diminished coronary arteriolar dilation in patients with diabetes, Am J Physiol Heart Circ Physiol 300 (3) (2011) H777–H783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ammar RF Jr., Gutterman DD, Brooks LA, Dellsperger KC, Free radicals mediate endothelial dysfunction of coronary arterioles in diabetes, Cardiovasc Res 47 (3) (2000) 595–601. [DOI] [PubMed] [Google Scholar]
- [14].Chilian WM, Kuo L, DeFily DV, Jones CJH, Davis MJ, Endothelial regulation of coronary microvascular tone under physiological and pathophysiological conditions, Eur Heart J 14 (Suppl I) (1993) 55–59. [PubMed] [Google Scholar]
- [15].Hein TW, Kuo L, LDLs impair vasomotor function of the coronary microcirculation: Role of superoxide anions, Circ Res 83 (4) (1998) 404–414. [DOI] [PubMed] [Google Scholar]
- [16].Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO 3rd, Contribution of nitric oxide to metabolic coronary vasodilation in the human heart, Circulation 92 (3) (1995) 320–326. [DOI] [PubMed] [Google Scholar]
- [17].Kuo L, Davis MJ, Chilian WM, Endothelium-dependent, flow-induced dilation of isolated coronary arterioles, Am J Physiol 259 (4 Pt 2) (1990) H1063–H1070. [DOI] [PubMed] [Google Scholar]
- [18].Kuo L, Davis MJ, Cannon MS, Chilian WM, Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation: Restoration of endothelium-dependent responses by L-arginine, Circ Res 70 (3) (1992) 465–476. [DOI] [PubMed] [Google Scholar]
- [19].Hein TW, Kuo L, cAMP-independent dilation of coronary arterioles to adenosine: role of nitric oxide, G proteins, and KATP channels, Circ Res. 85 (7) (1999) 634–642. [DOI] [PubMed] [Google Scholar]
- [20].Fogarty JA, Muller-Delp JM, Delp MD, Mattox ML, Laughlin MH, Parker JL, Exercise training enhances vasodilation responses to vascular endothelial growth factor in porcine coronary arterioles exposed to chronic coronary occlusion, Circulation 109 (5) (2004) 664–670. [DOI] [PubMed] [Google Scholar]
- [21].Komaru T, Lamping KG, Eastham CL, Harrison DG, Marcus ML, Dellsperger KC, Effect of an arginine analogue on acetylcholine-induced coronary microvascular dilatation in dogs, Am J Physiol Heart Circ Physiol 261 (6 Pt 2) (1991) H2001–H2007. [DOI] [PubMed] [Google Scholar]
- [22].Gupta SK, Dongare S, Mathur R, Mohanty IR, Srivastava S, Mathur S, Nag TC, Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats, Mol Cell Biochem 408 (1–2) (2015) 63–72. [DOI] [PubMed] [Google Scholar]
- [23].Faria A, Persaud SJ, Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential, Pharmacol Ther 172 (2017) 50–62. [DOI] [PubMed] [Google Scholar]
- [24].Chen M, Nagase M, Fujita T, Narumiya S, Masaki T, Sawamura T, Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: Possible role of LOX-1 ligand and AGE, Biochem Biophys Res Commun 287 (4) (2001) 962–968. [DOI] [PubMed] [Google Scholar]
- [25].Li L, Sawamura T, Renier G, Glucose enhances endothelial LOX-1 expression: Role for LOX-1 in glucose-induced human monocyte adhesion to endothelium, Diabetes 52 (7) (2003) 1843–1850. [DOI] [PubMed] [Google Scholar]
- [26].Liu T, Zhou Y, Liu YC, Wang JY, Su Q, Tang ZL, Li L, Coronary microembolization induces cardiomyocyte apoptosis through the LOX-1-dependent endoplasmic reticulum stress pathway involving JNK/p38 MAPK, Can J Cardiol 31 (10) (2015) 1272–1281. [DOI] [PubMed] [Google Scholar]
- [27].Hong D, Bai YP, Gao HC, Wang X, Li LF, Zhang GG, Hu CP, Ox-LDL induces endothelial cell apoptosis via the LOX-1-dependent endoplasmic reticulum stress pathway, Atherosclerosis 235 (2) (2014) 310–317. [DOI] [PubMed] [Google Scholar]
- [28].Zhang L, Jia YH, Zhao XS, Zhou FH, Pan YY, Wan Q, Cui XB, Sun XG, Chen YY, Zhang Y, Cheng SB, Trichosanatine alleviates oxidized low-density lipoprotein induced endothelial cells injury via inhibiting the LOX-1/p38 MAPK pathway, Am J Transl Res 8 (12) (2016) 5455–5464. [PMC free article] [PubMed] [Google Scholar]
- [29].Hein TW, Qamirani E, Ren Y, Xu X, Thengchaisri N, Kuo L, Selective activation of lectin-like oxidized low-density lipoprotein receptor-1 mediates C-reactive protein-evoked endothelial vasodilator dysfunction in coronary arterioles, Circ Res 114 (1) (2014) 92–100. [DOI] [PubMed] [Google Scholar]
- [30].Ryoo S, Bhunia A, Chang F, Shoukas A, Berkowitz DE, Romer LH, OxLDL-dependent activation of arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling, Atherosclerosis 214 (2) (2011) 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang C, Hein TW, Wang W, Ren Y, Shipley RD, Kuo L, Activation of JNK and xanthine oxidase by TNF-α impairs nitric oxide-mediated dilation of coronary arterioles, J Mol Cell Cardiol 40 (2) (2006) 247–257. [DOI] [PubMed] [Google Scholar]
- [32].Kang LS, Chen B, Reyes RA, Leblanc AJ, Teng B, Mustafa SJ, Muller-Delp JM, Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles, Am J Physiol Heart Circ Physiol 300 (6) (2011) H2105–H2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang C, Hein TW, Wang W, Chang CI, Kuo L, Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function, FASEB J 15 (7) (2001) 1264–1266. [DOI] [PubMed] [Google Scholar]
- [34].Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L, Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: Counteracting role of arginase, FASEB J 17 (15) (2003) 2328–2330. [DOI] [PubMed] [Google Scholar]
- [35].Wang W, Hein TW, Zhang C, Zawieja DC, Liao JC, Kuo L, Oxidized low-density lipoprotein inhibits nitric oxide-mediated coronary arteriolar dilation by up-regulating endothelial arginase I, Microcirculation 18 (1) (2011) 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW, Diabetes-induced coronary vascular dysfunction involves increased arginase activity, Circ Res 102 (1) (2008) 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Romero MJ, Iddings JA, Platt DH, Ali MI, Cederbaum SD, Stepp DW, Caldwell RB, Caldwell RW, Diabetes-induced vascular dysfunction involves arginase I, Am J Physiol Heart Circ Physiol 302 (1) (2012) H159–H166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hein TW, Potts LB, Xu W, Yuen JZ, Kuo L, Temporal development of retinal arteriolar endothelial dysfunction in porcine type 1 diabetes, Invest Ophthalmol Vis Sci 53 (13) (2012) 7943–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kuo L, Davis MJ, Chilian WM, Myogenic activity in isolated subepicardial and subendocardial coronary arterioles, Am J Physiol Heart Circ Physiol 255 (6 Pt 2) (1988) H1558–H1562. [DOI] [PubMed] [Google Scholar]
- [40].Hein TW, Qamirani E, Ren Y, Kuo L, C-reactive protein impairs coronary arteriolar dilation to prostacyclin synthase activation: Role of peroxynitrite, J Mol Cell Cardiol 47 (2) (2009) 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, Humphrey JD, Kuo L, Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles, Hypertension 44 (6) (2004) 935–943. [DOI] [PubMed] [Google Scholar]
- [42].Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, Kuo L, Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles, Arterioscler Thromb Vasc Biol 26 (9) (2006) 2035–2042. [DOI] [PubMed] [Google Scholar]
- [43].Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW, SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase, Proc Natl Acad Sci U S A 98 (24) (2001) 13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hein TW, Xu W, Xu X, Kuo L, Acute and chronic hyperglycemia elicit JIP1/JNK-mediated endothelial vasodilator dysfunction of retinal arterioles, Invest Ophthalmol Vis Sci 57 (10) (2016) 4333–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stebbins JL, De SK, Machleidt T, Becattini B, Vazquez J, Kuntzen C, Chen LH, Cellitti JF, Riel-Mehan M, Emdadi A, Solinas G, Karin M, Pellecchia M, Identification of a new JNK inhibitor targeting the JNK-JIP interaction site, Proc Natl Acad Sci U S A 105 (43) (2008) 16809–16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Qamirani E, Ren Y, Kuo L, Hein TW, C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase, Arterioscler Thromb Vasc Biol 25 (5) (2005) 995–1001. [DOI] [PubMed] [Google Scholar]
- [47].Thengchaisri N, Hein TW, Ren Y, Kuo L, Endothelin-1 impairs coronary arteriolar dilation: Role of p38 kinase-mediated superoxide production from NADPH oxidase, J Mol Cell Cardiol 86 (2015) 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cirillo PF, Pargellis C, Regan J, The non-diaryl heterocycle classes of p38 MAP kinase inhibitors, Curr Top Med Chem 2 (9) (2002) 1021–1035. [DOI] [PubMed] [Google Scholar]
- [49].Zhang C, Hein TW, Wang W, Kuo L, Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function, Circ Res 92 (3) (2003) 322–329. [DOI] [PubMed] [Google Scholar]
- [50].Heiss EH, Dirsch VM, Regulation of eNOS enzyme activity by posttranslational modification, Curr Pharm Des 20 (22) (2014) 3503–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Beleznai T, Bagi Z, Activation of hexosamine pathway impairs nitric oxide (NO)-dependent arteriolar dilations by increased protein O-GlcNAcylation, Vascul Pharmacol 56 (3–4) (2012) 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Stancel N, Chen CC, Ke LY, Chu CS, Lu J, Sawamura T, Chen CH, Interplay between CRP, atherogenic LDL, and LOX-1 and its potential role in the pathogenesis of atherosclerosis, Clin Chem 62 (2) (2016) 320–327. [DOI] [PubMed] [Google Scholar]
- [53].Chistiakov DA, Orekhov AN, Bobryshev YV, LOX-1-mediated effects on vascular cells in atherosclerosis, Cell Physiol Biochem 38 (5) (2016) 1851–1859. [DOI] [PubMed] [Google Scholar]
- [54].Horiuchi S, Sakamoto Y, Sakai M, Scavenger receptors for oxidized and glycated proteins, Amino Acids 25 (3–4) (2003) 283–292. [DOI] [PubMed] [Google Scholar]
- [55].Chen X, Zhang T, Du G, Advanced glycation end products serve as ligands for lectin-like oxidized low-density lipoprotein receptor-1(LOX-1): biochemical and binding characterizations assay, Cell Biochem Funct 26 (7) (2008) 760–770. [DOI] [PubMed] [Google Scholar]
- [56].Zuo G, Ren X, Qian X, Ye P, Luo J, Gao X, Zhang J, Chen S, Inhibition of JNK and p38 MAPK-mediated inflammation and apoptosis by ivabradine improves cardiac function in streptozotocin-induced diabetic cardiomyopathy, J Cell Physiol 234 (2) (2018) 1925–1936. [DOI] [PubMed] [Google Scholar]
- [57].Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ, MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines, Genes Dev 15 (11) (2001) 1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Davis RJ, Signal transduction by the JNK group of MAP kinases, Cell 103 (2) (2000) 239–252. [DOI] [PubMed] [Google Scholar]
- [59].Jung C, Gonon AT, Sjoquist PO, Lundberg JO, Pernow J, Arginase inhibition mediates cardioprotection during ischaemia-reperfusion, Cardiovasc Res 85 (1) (2010) 147–154. [DOI] [PubMed] [Google Scholar]
- [60].Zhu W, Chandrasekharan UM, Bandyopadhyay S, Morris SM Jr., DiCorleto PE, Kashyap VS, Thrombin induces endothelial arginase through AP-1 activation, Am J Physiol Cell Physiol 298 (4) (2010) C952–C960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Singh M, Padhy G, Vats P, Bhargava K, Sethy NK, Hypobaric hypoxia induced arginase expression limits nitric oxide availability and signaling in rodent heart, Biochim Biophys Acta 1840 (6) (2014) 1817–1824. [DOI] [PubMed] [Google Scholar]
- [62].Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, Santhanam L, Webb A, Camara A, Sikka G, Nyhan D, Shoukas AA, Ilies M, Christianson DW, Champion HC, Berkowitz DE, Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats, J Appl Physiol 107 (4) (2009) 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.