Abstract
Alterations of energy metabolism and of astrocyte number/function in ventral anterior cingulate cortex (vACC) have been reported in major depressive disorder (MDD) patients and may contribute to MDD pathophysiology. We recently developed a mouse model of MDD mimicking these alterations. We knocked down the astroglial glutamate transporters GLAST and GLT-1 in infralimbic cortex (IL, rodent equivalent of vACC) using small interfering RNA (siRNA). GLAST and GLT-1 siRNA microinfusion in IL evoked a depressive-like phenotype, associated with a reduced serotonergic function and reduced forebrain BDNF expression. Neither effect occurred after siRNA application in the adjacent prelimbic cortex (PrL), thus emphasizing the critical role of vACC/IL in MDD pathogenesis. Here we examined the cellular/network basis of the changes induced in IL using intracellular recordings of layer V pyramidal neurons from mice microinjected with siRNA 24 h before. We analyzed i) the electrophysiological characteristics of neurons; ii) the synaptic transmission properties, by monitoring miniature, spontaneous and evoked EPSCs, and iii) the gliotransmission, by monitoring slow inward currents (SICs), mediated by astrocytic glutamate release and activation of extra-synaptic NMDA receptors. GLT-1 and GLAST knockdown led to a more depolarized membrane potential and increased action potential firing rate of layer V pyramidal neurons, and enhanced excitatory synaptic transmission, as shown by the enhanced amplitude/frequency of spontaneous EPSCs. Gliotransmission was also increased, as indicated by the enhanced SIC amplitude/frequency. Hence, the depressive-like phenotype is associated with IL hyperactivity, likely leading to an excessive top-down inhibitory control of serotonergic activity through IL-midbrain descending pathways.
Keywords: GLAST, GLT-1, Infralimbic cortex, layer V pyramidal neurons, excitatory synapses
1. INTRODUCTION
Major depressive disorder (MDD) is a severe mental disorder with a very large socioeconomic impact worldwide (Vos et al., 2016). This situation is attributable to the high prevalence of MDD and to the slow clinical action and limited efficacy of monoamine-based antidepressants.
Although the pathophysiology of MDD remains largely unknown, neuroimaging studies reported an enhanced energy metabolism in ventral areas of the anterior cingulate (vACC) of MDD patients (Mayberg, 2009; Savitz and Drevets, 2009). This area shows a large reciprocal connectivity with many other cortical and subcortical areas, including the brainstem monoamine cell groups (Croenewegen and Uylings, 2000). In particular, layer V pyramidal neurons in the medial prefrontal cortex (mPFC) project extensively to the serotonergic dorsal raphe nucleus (DR) (Gabbott et al., 2005), and their stimulation at physiological rates markedly inhibits the activity of most serotonergic neurons via local GABAA inputs and 5-HT1A autoreceptors (Celada et al., 2001). Therefore, an enhanced vACC activity may induce downstream activity changes of subcortical areas involved in emotional processing, including DR, leading to MDD symptoms (Artigas, 2015). Interestingly, fast-acting antidepressant strategies such as ketamine and deep brain stimulation regulate neuronal activity in vACC (Fuchikami et al., 2015; Mayberg 2009).
Astrocytes are emerging as key players in synaptic function, controlling extracellular levels of ions and neurotransmitters, responding to them, and releasing gliotransmittters that regulate synaptic transmission, plasticity (Perea and Araque 2007; Perea et al., 2014), and animal behavior (Oliveira et al., 2015). The astroglial glutamate transporters GLT-1 and GLAST take up most synaptic glutamate from central excitatory synapses (Danbolt et al., 1992; Petr et al., 2015), thereby exerting a tight direct control of neuronal excitability. Astrocytes have been proposed as an early contributor to the underlying pathogenesis of MDD (Sanacora and Banasr 2013) and alterations in astrocyte number/function have been reported in MDD patients and suicide victims (Banasr and Duman, 2008; Rajkowska and Stockmeier, 2013). Hence, the functional hyperactivity observed in the vACC of MDD patients may result from a disturbed astrocyte-based glutamate homeostasis.
In an attempt to mimic glial dysfunction in MDD, we developed a mouse model of MDD through the acute RNAi-induced knockdown of GLT-1 or GLAST expression in infralimbic cortex (IL, rodent equivalent of vACC). The local microinfusion of small interfering RNA (siRNA) targeting GLT-1 or GLAST into mouse IL evoked a moderate, long-lasting (≥7 days) decrease of GLAST/GLT-1 expression and induced a robust depressive-like phenotype as soon as 1 day after siRNA administration, associated to a reduced 5-HT release and a reduced BDMF expression (Fullana et al., 2019). The behavioral phenotype was reversed by classical (citalopram) and fast-acting (ketamine) antidepressant treatments. Remarkably, no behavioral/neurochemical effects were observed when GLT-1 or GLAST were knocked down in the adjacent prelimbic cortex (PrL) (Fullana et al., 2019), which emphasizes the critical role of IL-based circuits in emotional control and stress resilience.
We carried out the present electrophysiological study in order to better understand the cellular basis of the model described above, under the working hypothesis that GLAST/GLT-1 knockdown in IL would enhance excitatory activity in layer V pyramidal neurons, that project extensively to raphe serotonergic neurons (Gabbott et al., 2005)and that markedly inhibit their activity (Celada et al., 2001).
2. EXPERIMENTAL PROCEDURES
2.1. Ethics statement
All procedures for handling and sacrificing animals were approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC) in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals (#1701A34507).
2.2. Animals
Male and female C57BL/6J mice were housed under controlled conditions (22±1°C; 12 h light/dark cycle) with food and water available ad libitum and up to five animals per cage. Cortical slices were obtained from male and female C57BL/6J (6–8 weeks old), 24 h after siRNA application.
2.3. Drugs and chemicals
(2S)3-[(1S)1-(3,4-Dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphonic acid hydrochloride (CGP 55845) was purchased from Tocris Cookson (Bristol, UK) and picrotoxin from Indofine Chemical Company (Hillsborough, NJ). All other drugs were purchased from Sigma.
2.4. iRNA synthesis and treatments
Synthesis and purification of siRNA targeting GLAST and GLT-1 (GLAST siRNA: GenBank accession NM_148938 and GLT-1 siRNA: GenBank accession NM_001077514) were performed by nLife Therapeutics S.L. (Granada, Spain). Stock solutions were prepared in RNAse-free water and stored at −20°C until use.
Mice were anesthetized with ketamine + xylacine (80–120 mg/kg i.p. + 5–16 mg/kg i.p., respectively) and were placed in the stereotaxic frame. Mice were treated unilaterally with 1 μl of artificial cerebrospinal fluid (aCSF) or a pool of three different siRNAs targeting GLAST or GLT-1 at 60 μg/μl (4.2 nmol in total) into IL cortex (AP+2, ML-0.2, DV-3.3 in mm, Franklin and Paxinos, 2008) as described elsewhere (Fullana et al., 2019). siRNA microinfusion was performed using a glass capillary (240 μm-outer diameter, 110 μm-inner diameter) fitted to a 10 μl Hamilton syringe connected to a precision minipump (KDS 310 plus nano legacy syringe pump, Kd Scientific) at a rate of 0.5 μl/min.
2.5. Cortical slice preparation
Mice were anaesthetized and decapitated 24h after siRNA microinfusion. The brain was rapidly removed and placed in ice-cold aCSF. Slices 300 μm thick were made and incubated (>30 min) at room temperature in aCSF containing (in mM): NaCl 124, KCl 5, NaH2PO4 1.25, MgSO4 2, NaHCO3 26, CaCl2 2, and 10% glucose, gassed with 95% O2/5% CO2 (pH = 7.3–7.4). Slices were transferred to an immersion recording chamber, superfused at 2 ml/min with gassed aCSF and visualized under an Olympus BX50WI microscope (Olympus Optical, Japan).
2.6. Electrophysiology
Electrophysiological recordings from layer V pyramidal cortical neurons were made in whole-cell configuration of the patch-clamp technique. Patch electrodes had impedances of 3–10 MΩ when filled with the internal solution containing (in mM): KMeSO4 135, KCl 10, HEPES 10, NaCl 5, ATP-Mg2+ 2.5, and GTP-Na+ 0.3 (pH = 7.3), except for the analysis of slow inward currents (SIC), in which a free- Mg2+ internal solution was used. Recordings were obtained with PC-ONE amplifiers (Dagan Instruments, MN, US). I/V curves were set at the steady state. Membrane potential was held at −70 mV to record excitatory postsynaptic currents (EPSCs) and SICs. EPSCs were recorded in the presence of 50 μM picrotoxin and 1 μM CGP45626 to block GABAA and GABAB receptors, respectively. For SICs recordings, aCSF was adjusted with 4 mM CaCl2, 10 μM glycine, 0 mM magnesium and 1 μM tetrodotoxin (TTX). Signals were fed into a Pentium-based PC through a DigiData 1440A interface board. Signals were filtered at 1 KHz and acquired at 10 KHz sampling rate. The pCLAMP 10.4 (Axon instruments) software was used for stimulus generation, data display, acquisition and storage.
2.7. Statistical Analysis
Data are expressed as mean±SEM of N≥7 mice per group and experimental condition, unless otherwise indicated. Sample size was based on values previously found sufficient to detect significant changes in cortical synaptic parameters in previous studies from the laboratory. One-way ANOVA followed by post hoc Tukey’s test was used for multiple comparisons. Statistical significance was established at p<0.05 (two-tailed).
3. RESULTS
3.1. Increased excitability of layer V pyramidal neurons in IL of siRNA treated mice
We analyzed: 1) the electrophysiological characteristics of neurons; 2) the synaptic transmission properties, by monitoring miniature, spontaneous and evoked EPSCs, and 3) the gliotransmission, as assessed by monitoring slow inward currents (SICs), which are known to be mediated by glutamate release form astrocytes and the subsequent activation of extrasynaptic NMDA receptors (Fellin et al., 2004; Perea and Araque, 2005). Recordings were made in layer V pyramidal neurons of the IL in aCSF- and siRNA-treated mice 24 post-siRNA microinfusion, a time at which siRNA-treated mice exhibited a marked depressive-like behavioral phenotype (Fullana et al., 2019).
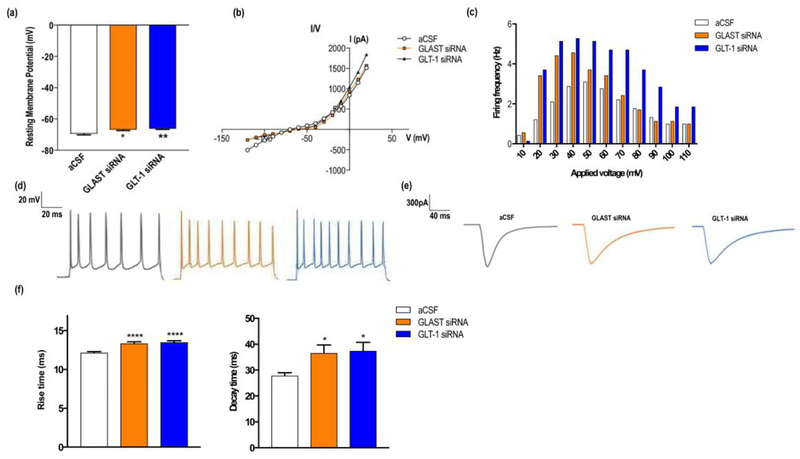
Recordings of the resting membrane potential showed significant differences between groups, being more depolarized in siRNA-treated mice (aCSF: −69.4±2.4; GLAST siRNA: −66.8±2.0; GLT-1 siRNA: −66.2±1.6, in mV) (Fig. 1a) [F(2,27)=6.97, p<0.005; significant group effect, one-way ANOVA]. I/V curves did not reveal alterations in conductance (Fig. 1b), yet an increased discharge rate was found in siRNA-treated mice, compared to controls, when increasing current pulses were applied (Fig. 1c, 1d) [F(10,100)=11.25, p<0.0001; significant group effect, one-way ANOVA].
Figure 1.
GLAST or GLT-1 knockdown in mouse infralimbic cortex (IL) altered electrophysiological properties of layer V pyramidal neurons. (a) Bar graphs showing a significant reduction of the resting membrane potential (RMP) in siRNA-treated mice compared to controls. (b) I/V curves showed no changes in neuronal membrane conductance between groups. (c) Representative examples of action potential firing evoked by a depolarizing pulse (0.1–10 mA, 2 ms) recorded in current-clamp conditions in each group. (d) Bar graph showing the firing rate after applying increasing depolarizing current pulses to layer V neurons, indicating a higher excitability in siRNA-treated mice. (e) Representative traces of evoked EPSCs in aCSF, GLAST and GLT-1 siRNA-treated mice, showing significant changes in EPSC waveform. Note that the stimulation artifact has been removed from the traces. (f) 10–90% rise and decay times of evoked EPSCs showing a significant slowing-down in GLAST and GLT-1 knockdown mice. Data are presented as mean±SEM. P<0.05 (*), P<0.01 (**), and P<0.001 (***) vs controls, post-hoc Tukey’s test after one-way ANOVA.
Previous studies showed that glutamate uptake inhibitors modulate EPSC waveform and frequency (Marcaggi et al, 2003; Takayasu et al, 2004). Therefore, we examined the effect of the reduced GLT-1 and GLAST expression on the time course of EPSCs evoked by electrical stimulation of afferent fibers with a bipolar electrode located in cortical layers II–III. Synaptic stimulation revealed important differences in the time course of evoked EPSCs in GLAST/GLT-1 siRNA-treated mice, showing a significantly larger duration in the analyzed basic components of the evoked EPSCs compared to controls, as follows (in ms): i) 10–90% rise time: aCSF: 12.15±0.74; GLAST siRNA: 13.35±0.98; GLT-1 siRNA: 13.5±0.92; [F(2,63)=15.33, p<0.0001; significant group effect, one-way ANOVA]; ii) 10–90% decay time: aCSF: 27.81±4.60; GLAST siRNA: 36.54±12.56; GLT-1 siRNA: 37.43±13.07; [F(2,45)=3.88, p<0.003; significant group effect, one-way ANOVA] (Fig. 1e, f).
3.2. Decreased GLAST and GLT-1 function changes baseline activity, synaptic transmission and evokes neuronal hyperactivity
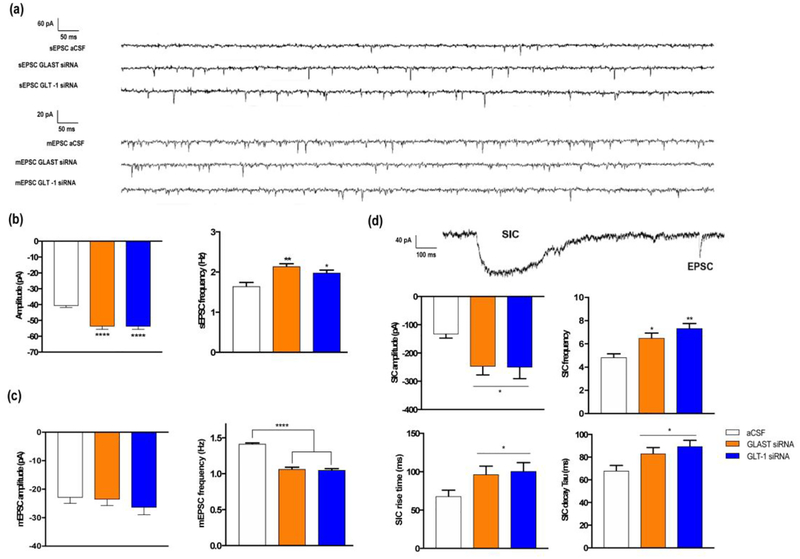
In siRNA-treated mice, 10-min baseline free recordings revealed a significant enhancement of both amplitude and frequency of spontaneous EPSCs (sEPSCs, in pA), as follows: i) sEPSC amplitude: aCSF: −40.8±11.0; GLAST siRNA: −53.8±20.6; GLT-1 siRNA: −53.9±20.6; [F(2,383)=21.14, p<0.0001; significant group effect, one-way ANOVA]; ii) sEPSC frequency (Hz): aCSF: 1.65±0.24; GLAST siRNA: 2.14±0.17; GLT-1 siRNA: 1.981±0,17 [F(2,15)=10.22, p<0.002; significant group effect, one-way ANOVA] (Fig. 2a, 2b). These results, which are in agreement with the observed resting membrane potential depolarization, suggest an increase in the spontaneous action potential-evoked synaptic transmission and/or an increase in the presynaptic transmitter release.
Figure 2.
Recordings in layer V pyramidal neurons (IL cortex) of aCSF, GLAST and GLT-1 siRNA treated mice show significant changes in synaptic properties. (a) Representative traces of spontaneous (sEPSC) and miniature (mEPSC) EPSCs in mice of each group. (b) sEPSCs analysis revealed an increase in both amplitude and frequency in mice with reduced expression of glutamate transporters compared to control mice. (c) Bar graph showing no changes in mEPSC amplitude, but a lower frequency in siRNA-treated mice. (d) Representative trace showing the difference between a SIC and an EPSC shape in the different experimental groups. Analysis of SICs revealed a significant increase in both amplitude and frequency, as well as longer SIC time courses (a higher 10–90% rise and decay time of GLAST and GLT-1 knockdown mice compared to controls. Data are presented as the mean±SEM. P<0.05 (*), P<0.01 (**), and P<0.001 (***).
To test the latter possibility we examined the effects of the impaired glutamate reuptake on the miniature EPSCs (mEPSCs). 10-min free recordings in the presence of tetrodotoxin (TTX) showed significant reductions in mEPSCs frequency in GLAST/GLT-1 siRNA-treated mice compared to control mice, without altering mEPSCs amplitude, as follows: i) mEPSC amplitude (pA): aCSF: −23.0±18.9; GLAST siRNA: −23.6±17.9; GLT-1 siRNA: −26.4±21.2, n.s., one-way ANOVA; ii) mEPSC frequency (Hz): aCSF: 1.41±0.04; GLAST siRNA: 1.06±0.06; GLT-1 siRNA: 1.05±0.05 [F(2,15)=97.26, p<0.0001; significant group effect, one-way ANOVA] (Fig. 2a,2c). These results suggest that impairment of glutamate uptake reduces presynaptically the probability of neurotransmitter release without modifying the quantal content.
Glutamate released by astrocytes induces slow inward currents (SICs) in postsynaptic neurons through activation of extra-synaptic NMDA receptors containing the GluN2B subunit (Fellin et al. 2004; Perea and Araque 2005; Kozlov et al. 2006; Shigetomi et al. 2008). Next, we examined the occurrence of SICs in 10-min recordings of layer V pyramidal whole-cell currents. Spontaneous SICs were found in all groups, with significant differences in amplitude and frequency, as well as in SIC waveform (rise and decay time courses), as follows: i) SIC amplitude (pA): aCSF: −133.6±34.4; GLAST siRNA: −247.4±84.1; GLT-1 siRNA: −250.9±119.2 [F(2,20)=3.51, p<0.05; significant group effect, one-way ANOVA], ii) SIC Frequency/10 min: aCSF: 4.83±0.8; GLAST siRNA group: 6.5±1.1; GLT-1 siRNA group: 7.333±1.033 [F(2,15)=10,67, p<0.0015; significant group effect, one-way ANOVA], iii) SIC 10–90% rise time (ms): aCSF: 67.9±55.7; GLAST siRNA group: 100.6±74.5; GLT-1 siRNA group: 96.4±69.7 [F(2,131)=3.32, p<0.05; significant group effect, one-way ANOVA], and iv) SIC 10–90% decay time (ms): aCSF: 68.0±32.8; GLAST siRNA: 83.1±35.9; GLT-1 siRNA: 89.5±37.0 [F(2,141)=4.73, p<0.015, significant group effect, one-way ANOVA] (Fig. 2d). These results suggest that impairing glutamate uptake leads to the enhancement of gliotransmission.
4. DISCUSSION
The present study indicates that the siRNA-induced decrease in GLT-1 and GLAST expression enhances excitatory neurotransmission and cortical circuit excitability in mouse IL. In particular, the reduced GLT-1 or GLAST expression in IL results in i) more depolarized resting membrane potentials, ii) changes in in miniature, spontaneous and evoked EPSCs, and iii) enhanced gliotransmission, as shown by the increase in slow inward currents (SICs), a variable sensitive to the activation of extra-synaptic NMDA-R expressing the GluN2B subunit. The present data are in line with previous reports showing a critical role of glial glutamate transporters expression in the control of excitatory synapses (Takayasu et al. 2006; Petr et al., 2015). However, to our knowledge, no previous study used RNAi strategies to modulate GLAST/GLT-1 expression in vivo. This approach allows us to examine acute changes of expression/function, avoiding long-term adaptive processes associated to mouse generic models. Likewise, given that the observed synaptic changes occurred in layer V pyramidal neurons projecting to the DR, they may partly explain the decreased serotonergic activity in mice subjected to identical GLAST/GLT-1 knock down in IL (Fullana et al., 2019).
Micro-infusion of GLT-1 or GLAST siRNAs decreased the frequency of mEPSCs, without altering their amplitude, indicating a reduction of the presynaptic transmitter release and an unchanged quantal content. In contrast, both the frequency and amplitude of sEPSCs were increased, thus suggesting an enhancement of action potential-mediated synaptic transmission. This is consistent with an increased excitability of postsynaptic neurons in treated mice, as indicated by the observed depolarization of the resting membrane potential and increased action potential firing rate. These results suggest a higher excitability of the neuronal cortical circuit, in line with the increased c-fos expression observed in the IL of mice treated with GLT-1 or GLAST siRNAs (Fullana et al., 2019).
Additionally, we found that gliotransmission was enhanced in GLT-1 or GLAST siRNA-injected mice as we observed that SIC frequency and amplitude were increased. The impaired glutamate uptake may account for the increase of SIC amplitude as well as the rise and decay time courses. These observations are in line with other studies showing that SIC frequency is increased in the presence of glutamate uptake blockade (Nie et al., 2010). In addition, a higher astrocyte activity induced by the enhanced synaptic transmission may also contribute to this effect. Since SICs have been shown to increase neuronal excitability and synchronization (Fellin et al., 2004; Perea and Araque, 2005), the effects of GLT-1 or GLAST siRNAs on SIC frequency, amplitude and time course may further bolster the enhanced neuronal excitability. The reduced GLT1 and GLAST expression was associated to a reduced density/activity of astroglial markers (Fullana et al., 2019). Hence, siRNA directed against both transporters may have affected the release of a pool of gliotransmitters, thus additionally contributing to modify the electrophysiological variables reported herein.
In summary, given i) the large population of layer V pyramidal neurons in the mPFC -including IL- projecting to the dorsal raphe nucleus, and ii) the tight inhibitory control of serotonergic activity by the mPFC (see Introduction), the enhanced excitatory inputs onto layer V IL pyramidal neurons may account for the observed reduction in serotonergic activity, and the subsequent depressive-like phenotype in mice with GLAST/GLT-1 knockdown. Further studies will extend the present observations by examining synaptic and circuit alterations responsible for the depressive-like phenotype in mice with GLAST/GLT-1 knockdown.
Funding and Disclosure
This work was supported by the Spanish Ministry of Economy and Competitiveness (grant numbers SAF2015-68346 to F.A., SAF2016-75797-R to A.B.), co-financed by European Regional Development Fund (ERDF) and NIH-NINDS (R01NS097312-01) and Human Frontier Science Program (Research Grant RGP0036/2014) to AA. The Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM) and CERCA Programme/Generalitat de Catalunya are also acknowledged. N.F. is a recipient of a fellowship from Spanish Ministry of Education, Culture and Sport. The sponsors had no further role in the design of the review, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The data contained in the manuscript are fully original and have not been submitted for publication elsewhere. All authors revised critically the manuscript and they gave final approval. We thank María Jaramillo for secretarial assistance.
F.A. has received lecture fees from Lundbeck and he is PI and grant from Lundbeck. He is also member of the scientific advisory board of Neurolixis. F.A. and A.B. are co-inventors of a patent on conjugated RNAi molecules. The rest of authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
F.A. has received consulting honoraria from Lundbeck and he is PI and grant from Lundbeck. He is also member of the scientific advisory board of Neurolixis. F.A. and A.B. are co-inventors of a patent on conjugated RNAi molecules. The rest of authors declare no conflict of interest.
Reference list
- Artigas F, 2015. Developments in the field of antidepressants, where do we go now? Eur Neuropsychopharmacol; 25(5):657–70. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS, 2008. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry; 64: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F, 2001. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci;21(24):9917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC, Storm-Mathisen J, Kanner BI, 1992. An [Na+ + K+]coupled L-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience; 51(2):295–310. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G, 2004. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron;43(5):729–43. [DOI] [PubMed] [Google Scholar]
- Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, Aghajanian GK, Duman RS, 2015. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci USA; June 30;112(26):8106–11. doi: 10.1073/pnas.1414728112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullana MN, Ruiz-Bronchal E, Ferrés-Coy A, Juárez-Escoto E, Artigas F, Bortolozzi A, 2019. Regionally selective knockdown of astroglial glutamate transporters in infralimbic cortex induces a depressive phenotype in mice. Glia 67(6):1122–1137 [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ, 2005. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol;492(2):145–77. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Uylings HB, 2000. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. [DOI] [PubMed] [Google Scholar]
- Kozlov AS, Angulo MC, Audinat E, Charpak S, 2006. Target cell-specific modulation of neuronal activity by astrocytes. Proc Natl Acad Sci USA;103(26):10058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Zhang HJ, Wang HR., 2010. Bidirectional neuron-glia interactions triggered by deficiency of glutamate uptake at spinal sensory synapses. J Neurophysiol 104(2), 713–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JF, Sardinha VM, Guerra-Gomes S, Araque A, Sousa N, 2015. Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci; 38(9):535–49. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A, 2005. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci;25(9):2192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A, 2007. Astrocytes potentiate transmitter release at single hippocampal synapses. Science; 317(5841):1083–6. [DOI] [PubMed] [Google Scholar]
- Perea G, Sur M, Araque A, 2014. Neuron-glia networks: integral gear of brain function. Front Cell Neurosci; 8:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petr GT, Sun Y, Frederick NM, Zhou Y, Dhamne SC, Hameed MQ, Miranda C, Bedoya EA, Fischer KD, Armsen W, Wang J, Danbolt NC, Rotenberg A, Aoki CJ, Rosenberg PA, 2015. Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes. J Neurosci; 35(13):5187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaggi P, Billups D, Attwell D, 2003. The role of glial glutamate transporters in maintaining the independent operation of juvenile mouse cerebellar parallel fibre synapses. J Physiol;552(Pt 1):89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg H, 2009. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest; 119(4): 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Stockmeier CA, 2013. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14: 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Banasr M, 2013. From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry 73: 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB and Drevets WC, 2009. Imaging Phenotypes of Major Depressive Disorder: Genetic Correlates. Neuroscience; 164(1): 300–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS, 2008. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci;28(26):6659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayasu Y, Iino M, Ozawa S, 2004. Roles of glutamate transporters in shaping excitatory synaptic currents in cerebellar Purkinje cells. Eur J Neurosci;19(5):1285–95. [DOI] [PubMed] [Google Scholar]
- Takayasu Y, Iino M, Shimamoto K, Tanaka K, Ozawa S. 2006. Glial glutamate transporters maintain one-to-one relationship at the climbing fiber-Purkinje cell synapse by preventing glutamate spillover. J Neurosci 26(24):6563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Allen C, Arora M. et al. 2016. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet; 388(10053); 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]