Abstract
Growth hormone receptor knockout mice (GHRKO) have reduced body size and increased insulin sensitivity. These mice are known for having extended lifespan, healthspan and female reproductive longevity. Seventeen α-estradiol (17α-E2) is reported to increase insulin sensitivity and extend lifespan in male mice, with less robust effects in female mice. The aim of this study was to evaluate the ovarian reserve in wild type and GHRKO mice treated with 17α-E2. The mice were divided into four groups, GHRKO mice receiving a standard chow diet, GHRKO mice treated 17α-E2, wild type mice receiving a standard chow diet and WT mice treated with 17α-E2. 17α-E2 was provided in the diet for four months. IGF1 plasma concentrations and changes in body weight were assessed. Histological slides were prepared from the ovaries and the number of follicles were counted. GHRKO mice receiving the control diet had a greater number of primordial follicles and lower numbers of primary follicles compared to the other groups (p<0.05). 17α-E2 treatment decreased the number of primordial follicles in GHRKO mice (p<0.05), however had no effect in wild type mice. Treatment with 17α-E2 had no significant effect on the change in body weight during the experiment (p=0.75). Plasma IGF1 concentrations were significantly lower in GHRKO mice as compared to wild type. In conclusion, we found that GHRKO mice displayed lesser primordial follicle activation as compared to wild type mice, but this phenotype was reversed by 17α-E2 administration, suggesting that ovarian aging is increased by 17α-E2 in long-living mice with extended reproductive longevity.
Keywords: follicles, ovarian aging, ovarian reserve, reproductive lifespan
1. Introduction
Mice with a disruption of the growth hormone receptor (GHR)/GH binding protein gene (GHRKO) are characterized by a severe reduction in hepatic insulin-like growth factor (IGF1) production and reduced adult body size (Dominici et al., 2000). Interestingly, these mice exhibit an extended lifespan (Coschigano et al., 2003), resulting from lower morbidity and disease-related mortality (Ikeno et al., 2009). Growth hormone receptor knockout mice have increased percentage of body fat with a disproportionate amount of fat deposition in the subcutaneous white adipose tissue depot (Berryman et al., 2010, Masternak et al., 2012). GHRKO mice also have increased insulin sensitivity (Junnila et al., 2016) which is believed to be associated with their extended longevity phenotype (Masternak et al., 2009).
Many characteristics of GHRKO mice are similar to animals subjected to caloric restriction (CR), including reduced body mass, decreased IGF1 production, robust metabolic plasticity, and extended lifespan (Li et al., 2011). However, it seems unlikely that CR will ever become a long-term interventional strategy in humans due to adverse effects associated with thermoregulation, libido, and mood (Dirks and Leeuwenburgh, 2006). As such, pharmacological interventions that mitigate age-related morbidities may prove to be appropriate alternative strategies. Recent studies have shown that 17α-estradiol (17α-E2) extends lifespan in male mice similarly to CR (Harrison et al., 2014). 17α-E2 is a naturally occurring enantiomer of 17β-estradiol (17β-E2), although it is considered a nonfeminizing estrogen due to its minimal activation of classical estrogen receptors (Anstead et al., 1997). The physiological functions of endogenous 17α-E2 are unclear, even though it is found in several tissues from both sexes in mammals (Dykens et al., 2005, Toran-Allerand, 2004). Treatment with 17α-E2 reduces body mass, visceral adiposity, and ectopic lipid deposition without decreasing lean mass only in males, suggesting a sexually dimorphic regulation (Stout et al., 2017, Miller et al., 2019, Garratt et al., 2019). These changes were associated with reductions in energy intake due to the activation of hypothalamic anorexigenic pathways, thereby reducing food intake (Steyn et al., 2018, Garratt et al., 2019).
The reproductive lifespan of a female depends on the initial size of the ovarian primordial follicle reserve and the depletion rate of these follicles (te Velde et al., 1998). The continuous activation and recruitment of primordial follicles during the female lifespan eventually leads to depletion of this reserve, resulting in a natural sterility, which in humans is known as menopause (Richardson et al., 2014). However, even before complete depletion of the follicular reserve, fertility starts to decline as the size of the follicular reserve is reduced and oocyte quality is diminished (Richardson et al., 2014). Compared to WT mice, young GHRKO females have a larger primordial follicle reserve, but when treated with exogenous IGF1 they demonstrate a significant decrease in the number of primordial follicles (Slot et al., 2006). Similarly, while GH-deficient Ames dwarf mice (df/df) have a larger ovarian primordial follicle reserve (Saccon et al., 2017), transgenic mice overexpressing GH have a smaller primordial follicle reserve (Saccon et al., 2017). Preservation of the primordial follicle reserve is also observed in rats undergoing CR (Li et al., 2011, Li et al., 2015, Xiang et al., 2012, Garcia et al., 2019). Since the lack of GH signaling and CR are related to both aging and ovarian reserve depletion, drugs that can increase longevity may also decrease primordial follicles activation. One of these drugs is rapamycin, which increases longevity in mice (Anisimov et al., 2011, Harrison et al., 2009) and also reduces depletion of primordial follicles (Zhou et al., 2017, Garcia et al., 2019). Collectively, these studies suggest that different lifespan-extending treatments also prolong fertility and decrease ovarian activation of primordial follicles.
In males, 17α-E2 has similar effects to CR and GH signaling deficiency (Strong et al., 2016, Stout et al., 2017). Despite the fact that female mice appear less responsive to 17α-E2 treatment (Garratt et al., 2017, Garratt et al., 2018, Garratt et al., 2019), it still seems reasonable to hypothesize that 17α-E2 treatment could reduce ovarian primordial follicle depletion. Therefore, the aim of this study was to evaluate the ovarian reserve of normal and GHRKO mice treated with 17α-E2.
2. Material and Methods
2.1. Animals and treatment
Six-month-old WT (n=10) and GHRKO (n=10) mice were individually housed at 24 ± 0.5°C on a 12:12-hour light-dark cycle. Unless otherwise noted, mice had ad libitum access to food and water throughout the experiment. LabDiet® 58YP (66.4% carbohydrate, 20.5% protein, 13.1% fat) was utilized for the control diet and was supplemented with 17α-E2 (14.4ppm; Steraloids, Newport, RI) for the treatment diet (TestDiet; Richmond, IN, USA). The treatment was provided during four months.
At the end of treatment, when mice were 10-month-old, mice were anesthetized with isoflurane and euthanized by cervical dislocation prior to dissection. Blood was collected into EDTA-lined tubes by cardiac puncture, and plasma was collected and frozen for IGF1 assessment. Tissues were excised, weighed, flash frozen, and stored at −80°C unless otherwise noted. The ovaries were excised and placed in 10% buffered formaldehyde for histological analyses. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committees at the Southern Illinois University.
2.2. Plasma Analytes
Plasma IGF1 concentrations were evaluated using ELISA kits from R&D Systems Inc. (Minneapolis, MN) as previously described (Chen et al., 2016).
2.3. Ovarian histological analysis
For histological analysis ovaries were removed from formaldehyde, dehydrated in alcohol, cleared in xylol and embebed in Paraplast Plus® (Sigma Chemical Company®, St. Louis, MO, USA). The ovaries embebed in Paraplast Plus® were sequentially cut at 5 μm in a microtome (Model RM2245, Leica Biosystems Newcastle Ltd, Newcastle Upon Tyne, UK). One section every six cuts was placed on standard histological slides. The entire ovary was serially cut and used. The slides, after drying in the oven at 56°C for 24 hours, were stained with hematoxylin-eosin and mounted with coverslips and synthetic resin (Sigma Chemical Company®, St. Louis, MO, USA). Images of the ovarian sections were captured by a camera coupled to a microscope using the software TC Capture (Tucsen Photomics Co.) with the 10x and 40x lenses. The counted follicles were those with clearly visible oocyte nuclei and their quantity was multiplied by six, to account for the sampling method, and by two, to account for the two ovaries of the female, as previously published (Saccon et al., 2017, Schneider et al., 2014).
Follicles were classified as primordial when surrounded by a single layer of flattened granulosa cells, as primary when surrounded by a single layer of cuboid granulosa and as transition follicle when surrounded by both flattened and cuboid cells. Secondary follicles were surrounded by more than one layer of cuboid granulosa cells, with no visible antrum and when the follicle had a clearly defined antral space and a layer of cumulus granulosa cells around the oocyte, it was classified as a tertiary follicle (Li et al., 2015).
To access follicle and oocyte size, three follicles from each stage were randomly selected from each mouse and the widest cross section for nuclei, oocyte and follicle was measured by the software Motic Image Plus 2.0 (Motic®, Hong Kong, China). The number of granulosa cells surrounding primordial, transition and primary follicles were also counted.
2.4. Statistical analysis
Statistical analysis was performed using the software GraphPad Prism 6. A Two-way ANOVA was performed to compare the number of follicles and percentage of follicles in each phase; nucleus, oocyte, and follicle size, IGF1 plasma concentrations and percentage of body weight gain. The analysis considered the effect of the genotype (GHRKO vs WT), treatment (E2 vs CONT) and their interaction. When the interaction was significant, means were compared among groups using the Tukey post-hoc test. A value of P lower or equal to 0.05 was considered significant.
3. Results
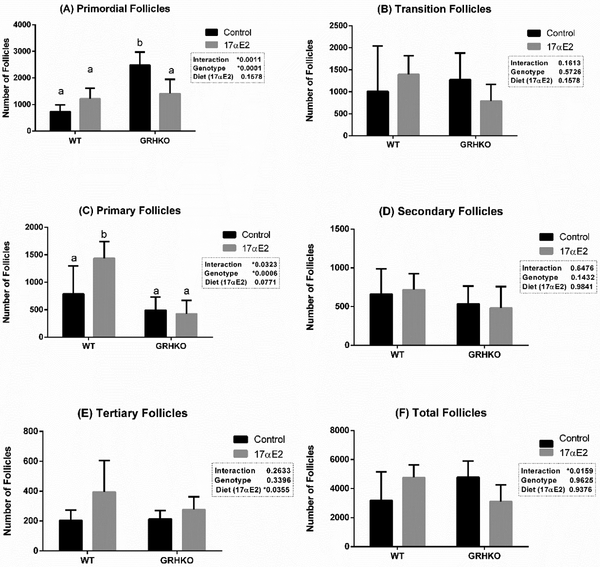
The number of primordial, transition, primary, secondary, tertiary and total follicles is presented on Figure 1. The number of primordial follicles was the highest in GHRKO mice (2479 ± 221) compared to other groups. No significant difference was detected among WT (724 ± 117), WT-17αE2 (1214 ± 179) and GHRKO-17αE2 (1411 ± 239) mice for the number of primordial follicles (Fig. 1A). For transition follicles no effects of the genotype (P=0.228), diet (P=0.332) or interaction (P=0.161) were observed (Fig. 1B).
Figure 1 -.
Number of primordial (A), transition (B), primary (C), secondary (D), tertiary (E) and total (F) follicles from wild-type (WT) and growth hormone receptor knockout (GHRKO) mice treated with 17α estradiol (17αE2) or not (Control). *Different letters indicate statistical difference (p<0.05)
For primary follicles, the WT-17αE2 group had the greatest number of primary follicles (1435 ± 136) compared to WT (787 ± 227), GHRKO (494 ± 104) and GHRKO-17αE2 groups (424 ± 111) (Fig. 1C). For secondary follicles, no effects of genotype, diet or interaction were observed (p>0.05) (Fig. 1D). For tertiary follicles, only the diet had an effect (p=0.035), with more tertiary follicles observed for 17αE2 treated groups (Fig. 1E). An effect of the diet by genotype interaction was found for total follicles (p=0.012), but no significant differences among individual groups were found (Fig. 1F).
Follicle, oocyte and nuclei size is presented in Table 1. GHRKO mice had slightly smaller primordial follicles when compared to WT mice (p=0.005). Regarding transition follicles, GHRKO mice also had smaller nuclei and oocytes (p=0.012 and 0.032, respectively). GHRKO control mice had smaller transition follicles than the other groups (p=0.005). However, oocytes of primary follicles where smaller in WT-E2 mice.
Table 1 -.
Diameter of the nucleus, oocyte and follicle and number of granulosa cells from wild-type (WT) and growth hormone receptor knockout (GHRKO) mice treated with 17α estradiol (17αE2) or not (Control).
| Wild-Type | GHRKO | P value | |||||
|---|---|---|---|---|---|---|---|
| - | Control | 17αE2 | Control | 17αE2 | Genotype | 17αE2 | Interact. |
| Primordial | |||||||
| Nucleus | 6.5 ± 0.1 | 6.6 ± 0.1 | 6.39 ± 0.1 | 6.82 ± 0.2 | 0.141 | 0.323 | 0.323 |
| Oocyte | 11.0 ± 0.4 | 11.0 ± 0.4 | 10.18 ± 0.3 | 11.54 ± 0.3 | 0.095 | 0.701 | 0.089 |
| Follicle | 15.6 ± 0.7 | 16.2 ± 0.3 | 13.92 ± 0.4 | 16.31 ± 0.4 | 0.005 | 0.127 | 0.089 |
| Cells | 6.0 ± 0.3 | 6.8 ± 0.3 | 6.06 ± 0.4 | 5.73 ± 0.3 | 0.489 | 0.169 | 0.123 |
| Transition | |||||||
| Nucleus | 6.8 ± 0.1 | 7.2 ± 0.3 | 6.41 ± 0.1 | 7.09 ± 0.2 | 0.012 | 0.223 | 0.596 |
| Oocyte | 11.7 ± 0.3 | 12.4 ± 0.7 | 10.26 ± 0.3 | 11.71 ± 0.3 | 0.032 | 0.025 | 0.440 |
| Follicle | 20.3 ± 0.5 a | 19.9 ± 0.7 a | 15.66 ± 0.6 b | 18.96 ± 0.5 a | 0.023 | <0.0001 | 0.005 |
| Cells | 7.8 ± 0.4 | 7.2 ± 0.5 | 7.13 ± 0.4 | 7.2 ± 0.4 | 0.531 | 0.444 | 0.444 |
| Primary | |||||||
| Nucleus | 9.3 ± 0.4 | 9.1 ± 0.5 | 9.49 ± 0.4 | 8.99 ± 0.4 | 0.768 | 0.972 | 0.768 |
| Oocyte | 22.9 ± 1.4 a | 18.3 ± 1.4 b | 21.58 ± 1.4 a | 22.05 ± 1.5 a | 0.0006 | <0.0001 | 0.0018 |
| Follicle | 36.3 ± 2.0 | 32.2 ± 1.8 | 34.30 ± 2.1 | 34.53 ± 1.9 | 0.306 | 0.954 | 0.253 |
| Cells | 18.5 ± 1.5 | 14.4 ± 1.1 | 17.33 ± 1.5 | 15.20 ± 1.0 | 0.014 | 0.872 | 0.423 |
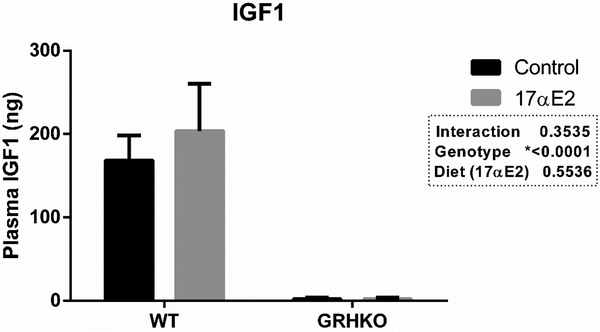
Treatment with 17α-E2 had no effect on the percentage of weight gain during the experiment (p=0.752). There was also no effect of genotype (p=0.722) or the diet by genotype interaction (p=0.752). Control WT and GHRKO females gained 5.91% and 5.50% of the initial weight during the experiment, while treated WT and GHRKO females lost 5.90 and 0.13% of their initial weight during the experiment. Plasma IGF1 concentrations were 3-fold lower in GHRKO mice (p<0.0001), as expected, however, treatment with 17α-E2 did not affect IGF1 plasma concentrations (Figure 2).
Figure 2 -.
IGF1 plasma concentration wild-type (WT) and GHRKO mice treated or not with 17α estradiol (17αE2) for 4 months. *Different letters indicate statistical difference on the multiple comparisons test (p<0.05).
4. Discussion
As previously reported, GHRKO mice have dramatically lower plasma IGF1 concentrations than WT littermates (Junnila et al., 2016, Duran-Ortiz et al., 2018). This condition was accompanied by decreased activation of primordial follicles as compared to WT mice. The reduced activation is suggested by the increased number of primordial and decreased number of primary follicles in ovaries from GHRKO mice. Others have previously reported an increased number of primordial follicles in GHRKO mice (Bachelot et al., 2002, Slot et al., 2006, Zaczek et al., 2002). We found an overall lower number of follicles than previously observed, which can be due the fact we used older mice (10 months old), while Slot et al. (2006) used young mice (9-weeks-old), and it is known that the primordial follicle reserve is depleted as the mouse ages (Kevenaar et al., 2006). The increased number of primordial follicles in GHRKO mice, even with advanced age, is further evidence of the interplay between somatic and gonadal aging. This was also evidenced by a previous report of a 24-month-old GHRKO female having different follicle categories and corpora lutea while WT mice had no evidence of ovarian structures (Sluczanowska-Glabowska et al., 2012). Our group has previously shown that GH deficient df/df mice have more primordial follicles than normal mice at 12 (Schneider et al., 2014) and 18 months of age (Saccon et al., 2017), further emphasizing the role of the GH/IGF1 axis in ovarian aging.
The GH/IGF1 axis is important for the activation of primordial follicles to start their growth, mostly through phosphorylation of FOXO3a protein (Castrillon et al., 2003, Saccon et al., 2017, Schneider et al., 2014). It is suggested that a local functional GHR may not be needed for ovarian IGF1 production, as there is ovarian expression of IGF1 mRNA in GHRKO mice, similar to the levels found in WT mice (Schneider et al., 2014). In addition, when GHRKO animals where treated with exogenous IGF1 for 14 days, the number of primordial follicles dropped drastically to a level similar to WT mice (Slot et al., 2006), suggesting a primary role of circulating IGF1 in follicle activation. Similarly, when exogenous GH was given to GH-deficient df/df mice the number of primordial follicles was reduced, followed by increased number of primary follicles (Saccon et al., 2017), suggesting increasing activation of the primordial follicle reserve stimulated by the GH/IGF1 axis.
Interestingly, treatment with 17α-E2 produced a diet by genotype interaction effect on the number of primordial follicles. 17α-E2 reduced the number of primordial follicles in GHRKO mice, however had no effect on primordial follicle activation in WT mice. It is interesting to note that this was not followed by increased number of primary or secondary follicles suggesting that there was no increased activation of the primordial reserve. Treatment with 17α-E2 had no effect on body weight in this study. Previous reports indicate that weight loss due to 17α-E2 is predominantly sexually dimorphic, with more weight loss occurring in males, while little or no effects are observed in females (Harrison et al., 2014, Garratt et al., 2019), in agreement with our observations. Treatment with 17α-E2 produced CR like effects in previous studies by stimulating the anorexigenic POMC neurons within the ARC to reduce feed intake (Steyn et al., 2018). 17α-E2 treated mice had reduced body weight, fat mass and increased insulin sensitivity (Stout et al., 2017). These effects are very similar to the phenotype of GHRKO mice (List et al., 2011). Therefore, we speculated that 17α-E2 treatment would elicit similar effects on ovarian primordial follicle activation. Our group and others have shown that CR decreases the activation of primordial follicles in the ovary (Liu et al., 2015, Xiang et al., 2012, Garcia et al., 2019). However, we observed that 17α-E2 did not slow ovarian aging as observed for other life extension therapies. This is consistent with previous work showing that female mice longevity is less affected by 17α-E2 treatment (Garratt et al., 2017, Garratt et al., 2018, Garratt et al., 2019). Therefore, additional studies that provide mechanistic insight into the interplay of germline and somatic aging are greatly needed. In the current study, despite no significant effects were observed, mice treated with 17α-E2 did not increased body weight during the experiment, while control treated mice had a small increase. However, it should be taken into account the low number of mice used in this study.
As 17α-E2 treatment reduced the primordial follicle reserve only in GHRKO mice, it is possible that it had a local, direct ovarian effect, increasing follicle activation. However, previous evidences suggest otherwise. The effects of estradiol (E2) on follicular development and oocyte maturation are mediated through interaction with specific estradiol receptors (ER) (Britt and Findlay, 2002). ERα, is expressed in cumulus cells, germinal epithelium, interstitial cells and thecal cells, while ERß, is expressed in oocytes, cumulus cells, and granulosa cells of primary, secondary, and mature follicles (Britt and Findlay, 2002, Couse et al., 2005, Drummond et al., 2002). The presence of ER within developing ovaries, which possess only primordial and primary follicles, suggests that E2 may have a role in follicle activation (Montano et al., 1995), however a role for E2 in primordial follicle formation and activation in rodents is not yet well established. A previous study has shown that ovariectomized female mice receiving 17α-E2 displayed increased uterine mass, similar to that of intact controls (Strong et al., 2016), suggesting 17α-E2 may also have mild effects through classical estrogen receptors resulting in divergent effects in male and female aging.
Most models with ER deficiency have normal follicle development until the antral stage (Couse and Korach, 1999) but no quantitative measures of preantral follicle numbers have been reported in these models. In aromatase knockout (ArKO) mice, which are deficient in 17ßE2, a decreased number of primordial and primary follicle number compared with WT mice was reported (Britt et al., 2004). Additionally, the oocyte diameter in primordial follicles was larger in ArKO mice (Britt et al., 2004), while we observed that 17α-E2 increased oocyte diameter of transition follicles. Primordial oocyte diameter is believed to be associated to the activation and transition to primary stage (Lintern-Moore and Moore, 1979). This suggest that despite the beneficial metabolic effects of 17α-E2, at the local ovarian level 17α-E2 increased oocyte growth which may be related to increased follicle activation, especially in GHRKO mice, who possess inherent follicular reserve. Traditionally, ERß is considered the main ER in the ovaries (Lee et al., 2009). Disruption of the gene that codes for the ERß, however, caused no significant fertility problems (Schomberg, et al. 1999). Knockout of the ERα gene (Esr1) in mice, on the other hand, resulted in infertile females (Schomberg et al., 1999). In humans, defects in the Esr1 gene have been associated with premature depletion of ovarian reserve (Bretherick et al., 2008). Recent studies with disruption of the Esr1 gene in zebra fish reported enhanced fertility in the mutant females during young ages, with greater egg production (Chen et al., 2018). However, the fertility of these females decreased with age, and ovarian reserve depletion was reached much earlier than in WT females (Chen et al., 2018), suggesting that Esr1 deficiency is associated with increased ovarian primordial reserve activation.
To summarize, our results do not confirm our hypothesis that 17α-E2 would preserve ovarian primordial follicle reserve. CR-like effects and direct effects of 17α-E2 on the ovaries appear to be not additive. In this sense, previous studies suggest that 17α-E2 is able to increase median lifespan in males, but not in females (Harrison et al., 2014). Furthermore, evidence suggests 17α-E2 has direct effects on reproductive tissues of females (Strong et al., 2016), which could explain the lack of effect of 17α-E2 on longevity in females. The effects of 17α-E2 treatment on metabolic profile are stronger in males and decrease upon castration, while in females the effects where milder and in general did not change after ovariectomy (Garratt et al., 2018). Further studies addressing 17α-E2 effects on gonadal activity must be undertaken to better understand the association between somatic and germline aging and how the lack of effect on the ovaries can contribute to the lack of effect of 17α-E2 on somatic aging.
In conclusion, we observed that GHRKO mice had lower activation of primordial follicles, preserving the primordial reserve. However, 17α-E2 had no effect on the ovarian reserve of WT mice and decreased the number of primordial follicles in GHRKO mice.
Highlights.
GHRKO mice have increased reserve of ovarian primordial follicles
17αE2 increased activation of primordial follicles in GHRKO mice but did not affect wild-type mice
17αE2 did not affect weight gain or IGF-I concentration for GHRKO and wild-type female mice
6. Funding
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), The National Institutes of Health (R00 AG51661 to M.B.S., R15 AG059190 to M.M.M., R03 AG059846 to M.M.M., and R21 AG051869 to A.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflicts of interests.
7. References
- ANISIMOV VN, ZABEZHINSKI MA, POPOVICH IG, PISKUNOVA TS, SEMENCHENKO AV, TYNDYK ML, YUROVA MN, ROSENFELD SV & BLAGOSKLONNY MV 2011. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle, 10, 4230–6. [DOI] [PubMed] [Google Scholar]
- ANSTEAD GM, CARLSON KE & KATZENELLENBOGEN JA 1997. The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids, 62, 268–303. [DOI] [PubMed] [Google Scholar]
- BACHELOT A, MONGET P, IMBERT-BOLLORE P, COSHIGANO K, KOPCHICK JJ, KELLY PA & BINART N 2002. Growth hormone is required for ovarian follicular growth. Endocrinology, 143, 4104–12. [DOI] [PubMed] [Google Scholar]
- BERRYMAN DE, LIST EO, PALMER AJ, CHUNG MY, WRIGHT-PIEKARSKI J, LUBBERS E, O’CONNOR P, OKADA S & KOPCHICK JJ 2010. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci, 65, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRETHERICK KL, HANNA CW, CURRIE LM, FLUKER MR, HAMMOND GL & ROBINSON WP 2008. Estrogen receptor alpha gene polymorphisms are associated with idiopathic premature ovarian failure. Fertil Steril, 89, 318–24. [DOI] [PubMed] [Google Scholar]
- BRITT KL & FINDLAY JK 2002. Estrogen actions in the ovary revisited. J Endocrinol, 175, 269–76. [DOI] [PubMed] [Google Scholar]
- BRITT KL, SAUNDERS PK, MCPHERSON SJ, MISSO ML, SIMPSON ER & FINDLAY JK 2004. Estrogen actions on follicle formation and early follicle development. Biol Reprod, 71, 1712–23. [DOI] [PubMed] [Google Scholar]
- CASTRILLON DH, MIAO L, KOLLIPARA R, HORNER JW & DEPINHO RA 2003. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science, 301, 215–8. [DOI] [PubMed] [Google Scholar]
- CHEN VP, GAO Y, GENG L, STOUT MB, JENSEN MD & BRIMIJOIN S 2016. Butyrylcholinesterase Deficiency Promotes Adipose Tissue Growth and Hepatic Lipid Accumulation in Male Mice on High-Fat Diet. Endocrinology, 157, 3086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Y, TANG H, WANG L, HE J, GUO Y, LIU Y, LIU X & LIN H 2018. Fertility Enhancement but Premature Ovarian Failure in esr1-Deficient Female Zebrafish . Front Endocrinol (Lausanne), 9, 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSCHIGANO KT, HOLLAND AN, RIDERS ME, LIST EO, FLYVBJERG A & KOPCHICK JJ 2003. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology, 144, 3799–810. [DOI] [PubMed] [Google Scholar]
- COUSE JF & KORACH KS 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev, 20, 358–417. [DOI] [PubMed] [Google Scholar]
- COUSE JF, YATES MM, DEROO BJ & KORACH KS 2005. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology, 146, 3247–62. [DOI] [PubMed] [Google Scholar]
- DIRKS AJ & LEEUWENBURGH C 2006. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev, 127, 1–7. [DOI] [PubMed] [Google Scholar]
- DOMINICI FP, AROSTEGUI DIAZ G, BARTKE A, KOPCHICK JJ & TURYN D 2000. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J Endocrinol, 166, 579–90. [DOI] [PubMed] [Google Scholar]
- DRUMMOND AE, BRITT KL, DYSON M, JONES ME, KERR JB, O’DONNELL L, SIMPSON ER & FINDLAY JK 2002. Ovarian steroid receptors and their role in ovarian function. Mol Cell Endocrinol, 191, 27–33. [DOI] [PubMed] [Google Scholar]
- DURAN-ORTIZ S, NOBOA V & KOPCHICK JJ 2018. Disruption of the GH receptor gene in adult mice and in insulin sensitive tissues. Growth Horm IGF Res, 38, 3–7. [DOI] [PubMed] [Google Scholar]
- DYKENS JA, MOOS WH & HOWELL N 2005. Development of 17alpha-estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann N Y Acad Sci, 1052, 116–35. [DOI] [PubMed] [Google Scholar]
- GARCIA DN, SACCON TD, PRADIEE J, RINCON JAA, ANDRADE KRS, ROVANI MT, MONDADORI RG, CRUZ LAX, BARROS CC, MASTERNAK MM, BARTKE A, MASON JB & SCHNEIDER A 2019. Effect of caloric restriction and rapamycin on ovarian aging in mice. Geroscience. 10.1007/s11357-019-00087-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARRATT M, BOWER B, GARCIA GG & MILLER RA 2017. Sex differences in lifespan extension with acarbose and 17-alpha estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell, 16, 1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARRATT M, LAGERBORG KA, TSAI YM, GALECKI A, JAIN M & MILLER RA 2018. Male lifespan extension with 17-alpha estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice. Aging Cell, 17, e12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARRATT M, LEANDER D, PIFER K, BOWER B, HERRERA JJ, DAY SM, FIEHN O, BROOKS SV & MILLER RA 2019. 17-alpha estradiol ameliorates age-associated sarcopenia and improves late-life physical function in male mice but not in females or castrated males. Aging Cell, 18, e12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON DE, STRONG R, ALLISON DB, AMES BN, ASTLE CM, ATAMNA H, FERNANDEZ E, FLURKEY K, JAVORS MA, NADON NL, NELSON JF, PLETCHER S, SIMPKINS JW, SMITH D, WILKINSON JE & MILLER RA 2014. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell, 13, 273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON DE, STRONG R, SHARP ZD, NELSON JF, ASTLE CM, FLURKEY K, NADON NL, WILKINSON JE, FRENKEL K, CARTER CS, PAHOR M, JAVORS MA, FERNANDEZ E & MILLER RA 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460, 392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKENO Y, HUBBARD GB, LEE S, CORTEZ LA, LEW CM, WEBB CR, BERRYMAN DE, LIST EO, KOPCHICK JJ & BARTKE A 2009. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci, 64, 522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNNILA RK, DURAN-ORTIZ S, SUER O, SUSTARSIC EG, BERRYMAN DE, LIST EO & KOPCHICK JJ 2016. Disruption of the GH Receptor Gene in Adult Mice Increases Maximal Lifespan in Females. Endocrinology, 157, 4502–4513. [DOI] [PubMed] [Google Scholar]
- KEVENAAR ME, MEERASAHIB MF, KRAMER P, VAN DE LANG-BORN BM, DE JONG FH, GROOME NP, THEMMEN AP & VISSER JA 2006. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology, 147, 3228–34. [DOI] [PubMed] [Google Scholar]
- LEE S, KANG DW, HUDGINS-SPIVEY S, KRUST A, LEE EY, KOO Y, CHEON Y, GYE MC, CHAMBON P & KO C 2009. Theca-specific estrogen receptor-alpha knockout mice lose fertility prematurely. Endocrinology, 150, 3855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI L, FU YC, XU JJ, CHEN XC, LIN XH & LUO LL 2011. Caloric restriction promotes the reproductive capacity of female rats via modulating the level of insulin-like growth factor-1 (IGF-1). Gen Comp Endocrinol, 174, 232–7. [DOI] [PubMed] [Google Scholar]
- LI L, FU YC, XU JJ, LIN XH, CHEN XC, ZHANG XM & LUO LL 2015. Caloric restriction promotes the reserve of follicle pool in adult female rats by inhibiting the activation of Mammalian target of rapamycin signaling. Reprod Sci, 22, 60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINTERN-MOORE S & MOORE GP 1979. The initiation of follicle and oocyte growth in the mouse ovary. Biol Reprod, 20, 773–8. [DOI] [PubMed] [Google Scholar]
- LIST EO, SACKMANN-SALA L, BERRYMAN DE, FUNK K, KELDER B, GOSNEY ES, OKADA S, DING J, CRUZ-TOPETE D & KOPCHICK JJ 2011. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR−/−) mouse. Endocr Rev, 32, 356–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU W-J, ZHANG X-M, WANG N, ZHOU X-L, FU Y-C & LUO L-L 2015. Calorie restriction inhibits ovarian follicle development and follicle loss through activating SIRT1 signaling in mice. European Journal of Medical Research, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTERNAK MM, BARTKE A, WANG F, SPONG A, GESING A, FANG Y, SALMON AB, HUGHES LF, LIBERATI T, BOPARAI R, KOPCHICK JJ & WESTBROOK R 2012. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell, 11, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTERNAK MM, PANICI JA, BONKOWSKI MS, HUGHES LF & BARTKE A 2009. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci, 64, 516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER BF, PHARAOH GA, HAMILTON KL, PEELOR FF, KIRKLAND JL, FREEMAN WM, MANN SN, KINTER M, PRICE JC & STOUT MB 2019. Short-term calorie restriction and 17alpha-estradiol administration elicit divergent effects on proteostatic processes and protein content in metabolically active tissues. J Gerontol A Biol Sci Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTANO MM, WELSHONS WV & VOM SAAL FS 1995. Free estradiol in serum and brain uptake of estradiol during fetal and neonatal sexual differentiation in female rats. Biol Reprod, 53, 1198–207. [DOI] [PubMed] [Google Scholar]
- RICHARDSON MC, GUO M, FAUSER BC & MACKLON NS 2014. Environmental and developmental origins of ovarian reserve. Hum Reprod Update, 20, 353–69. [DOI] [PubMed] [Google Scholar]
- SACCON TD, MOREIRA F, CRUZ LA, MONDADORI RG, FANG Y, BARROS CC, SPINEL L, BARTKE A, MASTERNAK MM & SCHNEIDER A 2017. Ovarian aging and the activation of the primordial follicle reserve in the long-lived Ames dwarf and the short-lived bGH transgenic mice. Mol Cell Endocrinol, 455, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER A, ZHI X, MOREIRA F, LUCIA T JR., MONDADORI RG & MASTERNAK MM 2014. Primordial follicle activation in the ovary of Ames dwarf mice. J Ovarian Res, 7, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOMBERG DW, COUSE JF, MUKHERJEE A, LUBAHN DB, SAR M, MAYO KE & KORACH KS 1999. Targeted disruption of the estrogen receptor-alpha gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology, 140, 2733–44. [DOI] [PubMed] [Google Scholar]
- SLOT KA, KASTELIJN J, BACHELOT A, KELLY PA, BINART N & TEERDS KJ 2006. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction, 131, 525–32. [DOI] [PubMed] [Google Scholar]
- SLUCZANOWSKA-GLABOWSKA S, LASZCZYNSKA M, PIOTROWSKA K, GLABOWSKI W, KOPCHICK JJ, BARTKE A, KUCIA M & RATAJCZAK MZ 2012. Morphology of ovaries in laron dwarf mice, with low circulating plasma levels of insulin-like growth factor-1 (IGF-1), and in bovine GH-transgenic mice, with high circulating plasma levels of IGF-1. J Ovarian Res, 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEYN FJ, NGO ST, CHEN VP, BAILEY-DOWNS LC, XIE TY, GHADAMI M, BRIMIJOIN S, FREEMAN WM, RUBINSTEIN M, LOW MJ & STOUT MB 2018. 17alpha-estradiol acts through hypothalamic pro-opiomelanocortin expressing neurons to reduce feeding behavior. Aging Cell, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOUT MB, STEYN FJ, JURCZAK MJ, CAMPOREZ JG, ZHU Y, HAWSE JR, JURK D, PALMER AK, XU M, PIRTSKHALAVA T, EVANS GL, DE SOUZA SANTOS R, FRANK AP, WHITE TA, MONROE DG, SINGH RJ, CASACLANG-VERZOSA G, MILLER JD, CLEGG DJ, LEBRASSEUR NK, VON ZGLINICKI T, SHULMAN GI, TCHKONIA T & KIRKLAND JL 2017. 17alpha-Estradiol Alleviates Age-related Metabolic and Inflammatory Dysfunction in Male Mice Without Inducing Feminization. J Gerontol A Biol Sci Med Sci, 72, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRONG R, MILLER RA, ANTEBI A, ASTLE CM, BOGUE M, DENZEL MS, FERNANDEZ E, FLURKEY K, HAMILTON KL, LAMMING DW, JAVORS MA, DE MAGALHAES JP, MARTINEZ PA, MCCORD JM, MILLER BF, MULLER M, NELSON JF, NDUKUM J, RAINGER GE, RICHARDSON A, SABATINI DM, SALMON AB, SIMPKINS JW, STEEGENGA WT, NADON NL & HARRISON DE 2016. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell, 15, 872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TE VELDE ER, SCHEFFER GJ, DORLAND M, BROEKMANS FJ & FAUSER BC 1998. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol, 145, 67–73. [DOI] [PubMed] [Google Scholar]
- TORAN-ALLERAND CD 2004. Estrogen and the brain: beyond ER-alpha and ER-beta. Exp Gerontol, 39, 1579–86. [DOI] [PubMed] [Google Scholar]
- XIANG Y, XU J, LI L, LIN X, CHEN X, ZHANG X, FU Y & LUO L 2012. Calorie restriction increases primordial follicle reserve in mature female chemotherapy-treated rats. Gene, 493, 77–82. [DOI] [PubMed] [Google Scholar]
- ZACZEK D, HAMMOND J, SUEN L, WANDJI S, SERVICE D, BARTKE A, CHANDRASHEKAR V, COSCHIGANO K & KOPCHICK J 2002. Impact of growth hormone resistance on female reproductive function: new insights from growth hormone receptor knockout mice. Biol Reprod, 67, 1115–24. [DOI] [PubMed] [Google Scholar]
- ZHOU L, XIE Y, LI S, LIANG Y, QIU Q, LIN H & ZHANG Q 2017. Rapamycin Prevents cyclophosphamide-induced Over-activation of Primordial Follicle pool through PI3K/Akt/mTOR Signaling Pathway in vivo. J Ovarian Res, 10, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]