Abstract
Uterine cancer is the 6th leading cause of cancer death amongst American women. Most uterine cancers are endometrial carcinomas (ECs), which are classified into histological subtypes including endometrioid, serous, and clear cell ECs. Somatic copy number alterations (SCNAs) are frequent in serous EC, infrequent in endometrioid ECs, and poorly defined in clear cell ECs. The purpose of this study was to evaluate the occurrence of SCNAs in clinically diagnosed clear cell ECs. Paired tumor-normal DNAs for 51 ECs were hybridized to Illumina Infinium HumanHap650Y or Human660W-Quad Beadchips. Copy number calls were made using the Hidden Markov Model based SNP-FASST2 segmentation algorithm within Nexus Copy Number software (v.6.1). High-level SCNAs were defined as gain of ≥5 copies or homozygous deletion, both <10Mb. GISTIC 1.0, in Nexus, was used to identify statistically significant SCNAs, corrected for multiple testing. One or more high-level SCNAs were detected in 50% of 6 clear cell ECs, 78.6% of 28 serous ECs, and 17.6% of 17 endometrioid ECs. A positive association was found between high-level SCNAs and TP53 mutation across ECs (two-tailed p value<0.0001). Classifying tumors according to POLE, MSI, and TP53 status yielded four molecular subgroups; copy number altered tumors were more frequent in the TP53-mutated subgroup (95.8%) than in the unspecified subgroup (22.2%), and absent from the POLE and MSI subgroups. In conclusion, our study provides evidence of inter-tumor heterogeneity in the extent to which SCNAs occur in clinically diagnosed clear cell EC, and across molecular subgroups of EC. The co-occurrence of high-level SCNAs and TP53 mutations in some clear cell ECs is consistent with the view that a subset of clinically diagnosed clear cell ECs have molecular similarities to serous ECs.
Keywords: Endometrial, uterine, cancer, clear cell, copy number
INTRODUCTION
Cancers of the uterine corpus are estimated to account for 11,350 deaths among American women in 2018 [1]. Endometrial carcinomas (ECs) make up the majority of uterine corpus tumors (reviewed in [2]) and are classified into a number of histopathologic subtypes including endometrioid, serous, and clear cell ECs. Endometrioid tumors constitute approximately 87–90% of newly diagnosed ECs, whereas serous and clear cell tumors, which by definition are high-grade tumors, account for approximately 3–10% and 1–3% of such cases [2, 3]. Compared with endometrioid ECs, serous and clear cell tumors have generally less favorable outcomes. For example, within the SEER registry (1988–2001), five-year relative survival rates were 91.2% for endometrioid, 44.7% for serous, and 64.8% for clear cell ECs [4], considering all stages and grades. For all histologies, increasing stage is associated with decreased outcomes [5, 6]. For endometrioid ECs, increasing grade is also associated with reduced outcomes [5, 6]. This fact is illustrated by a 5-year disease-specific survival rate of only 77% for grade 3-endometrioid ECs (all stages) within a study of cases in the SEER database [7], and by 5-year overall survival rates for grade 3 endometrioid EC ranging from 77.5% at stage I to 49.6% at stage III for cases within the National Cancer Database [6].
Endometrioid and serous ECs have been relatively well-studied at the molecular level (reviewed in [8, 9]), and were the focus of an integrated genomic analysis by The Cancer Genome Atlas (TCGA) [10]. They exhibit a number of differences in their molecular pathogenesis. The endometrioid subtype is typified by frequent aberrations in the PI3K-AKT-mTOR pathway, the WNT/β-catenin pathway, the RAS-RAF-MAPK-ERK pathway, and ARID1A, as well as frequent microsatellite instability (MSI) and relatively frequent somatic mutations affecting the exonuclease domain of POLE (reviewed in [11]). In contrast to endometrioid EC, serous EC frequently exhibits TP53 mutation and/or p53 stabilization, PPP2R1A mutation, ERBB2 amplification, and high-level somatic copy number alterations (SCNAs) (Reviewed in [12]).
The mutational profiles of some clear cell tumors resemble those of either serous or endometrioid ECs [13–21]. However, the extent to which genome-wide SCNAs occur in this subtype remains poorly understood. Very few clear cell ECs have been karyotyped or analyzed by chromosomal comparative genomic hybridization (cCGH) [22–24]. Moreover, high-resolution SNP array based genome-wide copy number analysis has, to our knowledge, only been reported for two primary clear cell ECs both of which were relatively copy number quiet [25].
Here, we used high-density, SNP array analysis to determine the incidence of SCNAs in six clinically diagnosed clear cell ECs, and, for comparison on the same platform, 28 serous ECs and 17 endometrioid ECs. Focal high-level SCNAs were detected in 50% of clear cell ECs, compared with 78.6% of serous, and 17.6% of endometrioid ECs. Stratifying tumors into “TCGA-like” molecular subgroups, agnostic of histology, revealed a significant association between copy number alterations and TP53 mutations.
MATERIALS AND METHODS
Clinical material
The NIH Office of Human Subjects Research Protection determined that the research using the specimens in this study was not “human subjects research” per the Common Rule (45 CFR 46) and was exempt from IRB review. Snap-frozen primary tumor tissues, matched non-tumor tissues, and corresponding hematoxylin and eosin (H&E)-stained tumor sections were acquired as de-identified material from the Cooperative Human Tissue Network (CHTN), which is funded by the National Cancer Institute. Matched tumor and normal DNAs for four cases were purchased from Oncomatrix Inc (San Marcos, CA). Tumor specimens were collected at surgical resection prior to treatment. A histological classification was rendered based upon the entire specimen at time of diagnosis; this classification is used herein. For fresh-frozen tumor specimens received from CHTN, a single gynecologic pathologist reviewed an H&E section of this tumor tissue to identify regions of high (>70%) neoplastic cellularity for macrodissection and DNA isolation. Matched normal samples were from uninvolved reproductive tissue (n=49), buffy coat (n=1), or whole blood (n=1).
DNA extraction
Genomic DNA was isolated from regions of macrodissected tissue comprised of >70% neoplastic cells, and from normal tissues, using the PUREGENE kit (Qiagen) as described elsewhere [16, 17]. DNA yield was quantified using the Qubit fluorometer (Invitrogen).
Identity testing
To confirm that tumor-normal pairs were derived from the same individual, paired DNAs were typed using the Coriell Identity Mapping kit (Coriell) as previously described [16]. Genotyping fragments were resolved on an ABI-3730xl DNA analyzer (Applied Biosystems) and scored using GeneMapper software.
SNP genotyping
Genomic DNA from each tumor and normal sample was hybridized either to an Illumina Infinium HumanHap650Y Genotyping BeadChip (660,919 probes) by the NHGRI Genomics Core, or to an Illumina Infinium Human660W-Quad BeadChip (657,367 probes) by the Johns Hopkins University SNP Center. Hybridizations, as well as quantification of signal intensities and allele calls, were performed according to standard procedures. SNP positions and downstream analyses were based on the hg18/NCBI Build 36 of the human genome.
SNP data were visualized and preprocessed using GenomeStudio (v2011.1) (Illumina Inc), including implementation of the updated cluster generation protocol for SNPs on the X chromosome. LogR intensity ratios and B allele frequencies for all probes were exported from GenomeStudio and imported into Nexus Copy Number™ software (v.6.1) (BioDiscovery). Within Nexus, a customized linear systematic probe correction was applied to correct for waviness in the data, and to exclude data from probes that were unique to either BeadChip; only data from the 549,242 probes present on both Beadchips was retained. Next, for each tumor-normal pair, the datasets were re-centered to user-defined regions of balanced heterozygosity that had similar Log R ratios across multiple chromosomes within the tumor sample. The copy number changes reported herein represent a derivation from this baseline of balanced heterozygosity, but it should not be assumed that the baseline represents a diploid state.
Copy number calls and allelic status calls were made using the Hidden Markov Model based SNP-FASST2 segmentation algorithm within Nexus Copy Number™ (v.6.1). For data generated on the HumanHap650Y Genotyping BeadChip, settings used in the SNP-FASST2 segmentation algorithm included a significance threshold of 1.0E-6, a maximum contiguous probe spacing of 1000kb, a minimum of 5 probes per segment, a homozygous frequency threshold of 0.85, a homozygous value threshold of 0.8, a heterozygous imbalance threshold of 0.4, a minimum LOH length of 500kb, a high gain of 0.6, a gain of 0.18, a loss of −0.18 and a big loss of −1.0. For data generated on the Human660W-Quad BeadChip, the settings used in the SNP-FASST2 segmentation algorithm were identical to those for the HumanHap650Y Genotyping BeadChip with the exception of the high gain and gain which were set at 0.38 and 0.1 respectively. We also used a setting of 3% for the removal of outliers in the Nexus calculation of probe-to-probe variance, or quality. Tumor-normal pairs for which the tumor and/or normal data had a Nexus quality score >0.1 after removing the top and bottom 1.5% of outlier probes, were considered to have poor quality data and were excluded from further analysis. Copy number alterations of the same type and at the same loci in tumor-normal pairs, were considered germline, and were manually removed from each tumor profile. Only somatic copy number alterations (SCNAs) were considered in subsequent analyses. We defined “focal high-level SCNAs” as gains of ≥5 copies or homozygous deletions, both <10Mb. Genomic co-ordinates of “high-level SCNAs” were converted from hg18 to hg19 using the UCSC LiftOver utility, and queried against “gold standard variants” in the Database of Genomic Variants (DGV) (URL:http://dgv.tcag.ca/dgv/app/home); regions that had at least 75% overlap with one or more “DGV Gold” variants were filtered out.
Regions of statistically significant copy number alteration (Q-bound value <= 0.25 and G-score cut-off ≥1.0) were identified within Nexus Copy Number (v.6.1) using GISTIC (Genomic Identification of Significant Targets in Cancer) 1.0 [26, 27]. Extended GISTIC regions of statistically significant SCNA and corresponding peak GISTIC regions were identified separately for tumor and normal datasets. Extended and peak regions present in both the combined tumor and combined normal datasets were manually excluded because they may represent germline CNVs. Extended and peak GISTIC regions that had at least 75% overlap with one or more “DGV Gold” variants were filtered out. Cancer genes in peak GISTIC regions were annotated by querying the Cancer Gene Census (COSMICv90) (URL: http://cancer.sanger.ac.uk/census/).
RESULTS
Somatic copy number status varies according to EC histotypes
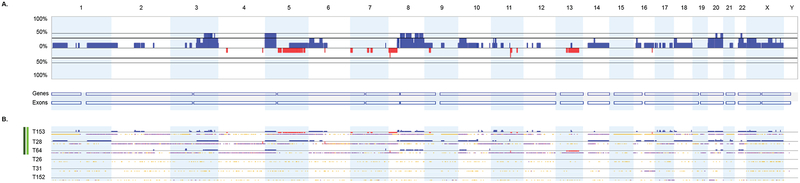
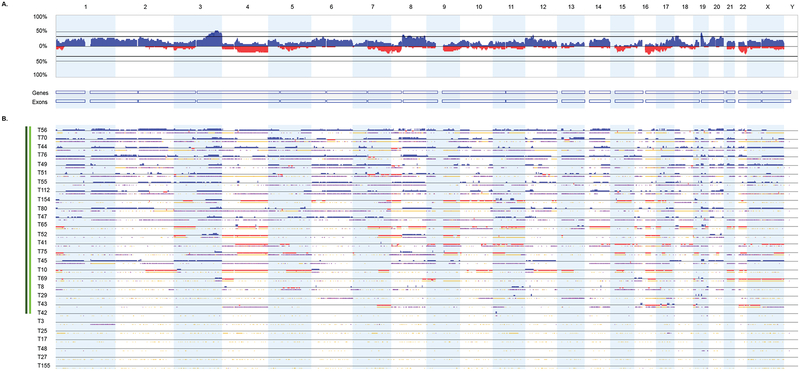
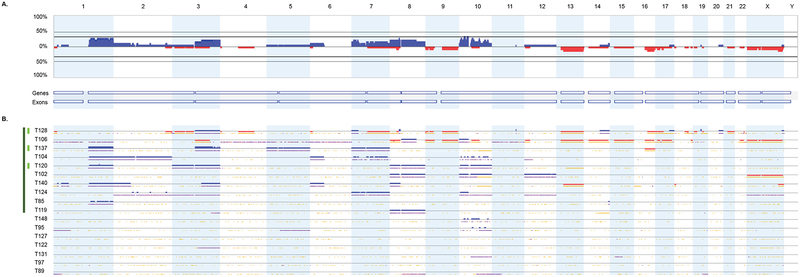
We detected one or more SCNAs in 50.0% (3 of 6) of clear cell, 82.1% (23 of 28) serous, and 70.5% (12 of 17) of endometrioid ECs (Figure 1, Figure 2, Figure 3). Gains involving 3q, 5p, 8q, 20q, and 22q were the most frequent SCNAs amongst clear cell tumors (Figure 1, Supplementary Figure 1). In serous tumors, SCNAs occurred throughout the genome with gains often involving chromosomes 1p, 1q, 2p, 2q, 3q, 5p, 6p, 6q, 8q, 10p, 12p, 14q, 17q, 18p, 19q, 20p, and 20q, and losses affecting 4q, 7q, 8p, 9q, 15q, 16q, and 22q (Figure 2, Supplementary Figure 2). Gains involving chromosomes 1q, 3q, 7p, 8p, 8q, 10p, 10q, and losses involving 13q and 16q were the most common SCNAs among endometrioid ECs (Figure 3, Supplementary Figure 3). One or more focal high-level SCNAs were detected in 50% (3 of 6) of clear cell, 78.5% (22 of 28) of serous, and 17.6% (3 of 17) of endometrioid ECs (Supplementary Table 1, Supplementary Table 2, Supplementary Table 3, Supplementary Table 4). Consensus cancer genes residing within focal high-level SCNAs in clear cell EC included KAT6B (within a chr10q22.2-q22.3 gain in T28) and MECOM (within a chr3q26.1-q26.31 gain in T153) (Supplementary Table 2). Five genes (ERBB2, CDK12, FGFR1, INPP4A, and PRKAR1A) within focal high-level SCNAs in a clear cell EC (T153), have also been reported, via next generation sequencing, to be amplified among clear cell ECs [28].
Figure 1.
Distribution of SCNAs among six clear cell ECs. (A) Genome-wide frequency plots of SCNAs for all cases combined. The frequency plot shows the percentage of tumors with somatic copy number gains (dark blue) and losses (red) above and below baseline (0%). (B) Profiles of SCNAs within each individual tumor (T). Tumors with at least one SCNA are indicated by the dark green column (left); tumors with at least one focal high-level SCNA are indicated by the light green column (left). Chromosomes 1 through 22 and the sex chromosomes are displayed sequentially from left to right.
Figure 2.
Distribution of SCNAs among 28 serous ECs. (A) Genome-wide frequency plots of SCNAs for all cases combined. The frequency plot shows the percentage of tumors with somatic copy number gains (dark blue) and losses (red) above and below baseline (0%). (B) Profiles of SCNAs within each individual tumor (T). Tumors with at least one SCNA are indicated by the dark green column (left); tumors with at least one focal high-level SCNA are indicated by the light green column (left). Chromosomes 1 through 22 and the sex chromosomes are displayed sequentially from left to right.
Figure 3.
Distribution of SCNAs among 17 endometrioid ECs. (A) Genome-wide frequency plots of SCNAs for all cases combined. The frequency plot shows the percentage of tumors with somatic copy number gains (dark blue) and losses (red) above and below baseline (0%). (B) Profiles of SCNAs within each individual tumor (T). Tumors with at least one SCNA are indicated by the dark green column (left); tumors with at least one focal high-level SCNA are indicated by the light green column (left). Chromosomes 1 through 22 and the sex chromosomes are displayed sequentially from left to right.
Statistically significant SCNAs identified by GISTIC 1.0 analysis in histological subtypes of EC
GISTIC analysis identified one peak region of SCNA, involving chr4q34.3 loss, in clear cell EC (Supplementary Table 1); this region was devoid of protein-encoding genes but encompassed a long non-coding RNA gene (LINC02500) (Table 1). In contrast, there were 41 peak GISTIC regions of SCNA among serous tumors (Table 2). Genes in individual high level SCNAs in clear cell EC, and within GISTIC peaks in serous EC were KAT6B, MECOM-ACTRT3-MYNN-LRRC34-LRR1Q4, ZMAT4, LINC02548-LINC02545, RNU6–2; LINC02082 was located within an individual high level SCNA in clear cell EC and in an endometrioid EC GISTIC peak. Consensus cancer genes in peak regions of serous EC GISTIC gain were MECOM, and KAT6B. Consensus cancer genes in peak regions of GISTIC loss in serous EC were LRP1B, MAP2K4, ERBB4, and PTEN. Eighteen protein-encoding genes in peak GISTIC regions in our serous ECs were also located in peak GISTIC regions in serous ECs and/or copy number cluster 4 ECs in TCGA [10] (Table 1, Table 2). Genes (and chromosomal locations) identified by both studies in peak GISTIC gains were EVI1/MECOM/MDS1 and MYNN (both 3q26.2); TERC (3q26.2); NEDD9 (6p24.2); ZMAT4 (8p11.21); PSD4 (2q13); and KAT6B (10q22.2). Genes identified by both studies in regions of peak GISTIC loss were LRP1B (2q22.1-q22.2); WASF2 (1p36.11); PTEN (10q23.31); AHDC1 (1p36.11-p35.3); PDE4D (5q11.2-q12.1); DNAH9, MAP2K4, and ZNF18 (all 17p12); ARAP2 (4p14); TUSC1 (9p21.2); and LHX1 and AATF (both 17q12). Several of these genes are proposed driver genes in statistically significant recurring SCNAs in the TCGA Pan-Gyn cohort specifically: MECOM, NEDD9, and KAT6B in GISTIC gains; LRP1B, PDE4D, PTEN, and MAP2K4 in GISTIC losses [29].
Table 1.
Extended and peak GISTIC regions identified among clear cell ECs
| SCNA Type | Genomic Coordinates (hg18) of Extended GISTIC Region | Chromosomal Location of Extended GISTIC Region | Genomic Coordinates (hg18) of Peak GISTIC Region | Q-bound for Peak Region | G-Score for Peak Region | Genes in Peak GISTIC Region |
|---|---|---|---|---|---|---|
| Loss | chr4:97,886,832-191,273,063 | chr4:182,415,084-182,433,256 | 0.24887827 | 1.17969014 | LINC02500 |
Table 2.
Extended and peak GISTIC regions identified among serous ECs
| SCNA Type | Genomic Coordinates (hg18) of Extended GISTIC Region | Chromosomal Location of Extended GISTIC Region | Genomic Coordinates (hg18) of Peak GISTIC Region | Q-bound for Peak Region | G-Score for Peak Region | Genes in Peak GISTIC Region |
|---|---|---|---|---|---|---|
| Gain | chr1:176,942,142-190,068,868 | 1q25.2-q31.2 | chr1:181,211,397-181,376,609 | 5.04E-3 | 4.568 | LAMC1, LAMC1-AS1 |
| Gain | chr1:42,689,950-49,840,316 | 1p34.2-p33 | chr1:44,358,961-44,455,683 | 7.26E-2 | 3.842 | KLF17, DMAP1 |
| Gain | chr10:76,048,493-86,655,854 | 10q22.2-q23.1 | chr10:76,285,006-76,292,848 | 8.97E-2 | 3.789 | KAT6B (MYST4)^ |
| Gain | chr11:13,107,660-17,245,167 | 11p15.2-p15.1 | chr11:13,807,145-13,855,429 | 1.72E-1 | 3.553 | LINC02548, LINC02545 |
| Gain | chr12:24,710,025-25,457,711 | 12p12.1 | chr12:24,983,158-25,022,328 | 6.31E-05 | 5.447 | BCAT1 |
| Gain | chr13:91,908,665-92,569,702 | 13q31.3 | chr13:92,353,870-92,391,097 | 9.63E-2 | 3.764 | / |
| Gain | chr14:42,169,974-85,498,415 | 14q21.2-31.3 | chr14:45,216,232-45,273,870 | 9.97E-2 | 3.757 | LINC02303 |
| Gain | chr16:29,245,504-32,045,466 | 16p11.2 | chr16:30,279,779-30,400,867 | 1.20E-1 | 3.681 | TBC1D10B, MYLPF, SEPTIN1, ZNF48, ZNF771, DCTPP1, SEPHS2, ITGAL, SNORA80C |
| Gain | chr18:4,158,565-16,100,000 | 18p11.31-p11.1 | chr18:10,911,569-11,090,060 | 1.49E-2 | 4.287 | PIEZO2 |
| Gain | chr19:22,755,564-24,191,440 | 19p12 | chr19:22,831,403-22,857,036 | 2.56E-3 | 4.717 | ZNF723 |
| Gain | chr19:34,477,100-37,581,903 | 19q12-q13.11 | chr19:34,839,864-34,892,756 | 5.73E-14 | 10.488 | PLEKHF1, C19orf12 |
| Gain | chr2:94,793,151-118,939,207 | 2q11.1-q14.2 | chr2:113,590,192-113,656,137 | 2.69E-2 | 4.124 | IL1RN, PSD4^ |
| Gain | chr20:14,929,825-17,414,153 | 20p12.1 | chr20:15,505,853-15,529,115 | 9.40E-3 | 4.408 | MACROD2 |
| Gain | chr20:51,602,600-51,797,590 | 20q13.2 | chr20:51,722,390-51,726,725 | 9.44E-08 | 6.587 | / |
| Gain | chr21:28,466,487-46,944,323 | 21q21.3-q22.3 | chr21:43,661,758-43,750,095 | 1.40E-1 | 3.632 | SIK1, SIK1B, LINC00319, LINC01669 |
| Gain | chr3:169,808,488-171,669,472 | 3q26.2 | chr3:170,858,670-171,032,269 | 4.45E-11 | 7.895 | MECOM^, ACTRT3, MYNN^, LRRC34, LRRIQ4, TERC ** |
| Gain | chr4:61,820,279-64,360,471 | 4q13.1 | chr4:64,148,793-64,360,471 | 1.54E-2 | 4.275 | / |
| Gain | chr6:11,306,574-11,952,750 | 6p24.1 | chr6:11,470,627-11,545,036 | 1.53E-3 | 4.823 | NEDD9^ |
| Gain | chr7:37,375,580-57,890,326 | 7p14.2-p11.1 | chr7:53,114,918-53,194,448 | 3.20E-2 | 4.070 | / |
| Gain | chr8:129,370,592-132,726,767 | 8q24.21-q24.22 | chr8:129,601,251-129,636,803 | 1.49E-2 | 4.283 | LINC00824 |
| Gain | chr8:40,154,687-43,910,848 | 8p11.21-p11.1 | chr8:40,583,025-40,670,377 | 1.94E-2 | 4.214 | ZMAT4** |
| Gain | chrX:61,845,481-69,098,104 | Xq11.1-q13.1 | chrX:61,845,481-62,571,001 | 2.01E-1 | 3.495 | SPIN4, LINC01278 |
| Loss | chr1:16,797,436-35,789,123 | 1p36.13-p34.3 | chr1:27,616,247-27,756,094 | 6.82E-2 | 4.597 | WASF2^, AHDC1^ |
| Loss | chr10:89,251,828-90,869,249 | 10q23.2-q23.31 | chr10:89,620,956-89,745,318 | 9.04E-2 | 2.421 | PTEN* |
| Loss | chr16:33,744,011-34,319,481 | 16p11.2 | chr16:33,744,011-34,311,321 | 6.82E-2 | 3.473 | LOC112268173, MIR9901 |
| Loss | chr16:45,079,464-45,425,095 | 16q11.2 | chr16:45,096,893-45,329,968 | 6.82E-2 | 3.188 | SHCBP1, VPS35,ORC6,MYLK3 |
| Loss | chr17:10,300,648-16,935,194 | 17p13.1-p11.2 | chr17:11,807,637-11,917,951 | 6.82E-2 | 3.290 | DNAH9^, ZNF18^,MAP2K4^ |
| Loss | chr17:30,019,514-33,321,712 | 17q12 | chr17:32,373,680-32,486,434 | 9.04E-2 | 2.426 | LHX1^, AATF^, MIR2909 |
| Loss | chr19:5,166,212-8,072,557 | 19p13.3-p13.2 | chr19:7,120,029-7,174,340 | 1.80E-1 | 2.069 | INSR |
| Loss | chr19:0-1,654,336 | 19p13-3 | chr19:839,331-876,857 | 6.82E-2 | 5.197 | MED16, R3HDM4, KISS1R, RNU6-2 |
| Loss | chr2:141,290,607-142,091,845 | 2q22.1 | chr2:141,514,130-141,549,533 | 6.82E-2 | 3.629 | LRP1B^ |
| Loss | chr2:212,586,754-213,027,807 | 2q34 | chr2:212,761,893-212,772,982 | 8.48E-2 | 2.477 | ERBB4 |
| Loss | chr22:14,884,399-20,930,009 | 22q11.1-q11.22 | chr22:15,869,733-15,915,743 | 1.72E-1 | 2.089 | CECR7 |
| Loss | chr22:45,341,436-49,691,432 | 22q13.31-q13.33 | chr22:47,505,660-47,562,070 | 6.82E-2 | 3.612 | TAFA5 |
| Loss | chr4:115,451,731-140,666,120 | 4q26-q31.1 | chr4:129,663,522-129,691,216 | 6.95E-2 | 2.722 | / |
| Loss | chr4:181,771,427-182,320,436 | 4q34.3 | chr4:182,084,359-182,181,085 | 6.82E-2 | 3.525 | / |
| Loss | chr4:35,651,979-48,132,950 | 4p14-p12 | chr4:35,780,455-35,831,233 | 6.82E-2 | 3.158 | ARAP2^ |
| Loss | chr5:56,081,575-63,823,550 | 5q11.2-q12.3 | chr5:59,205,723-59,266,038 | 6.82E-2 | 3.602 | PDE4D^ |
| Loss | chr9:74,060,683-90,190,867 | 9q21.13-q22.1 | chr9:80,427,442-80,485,053 | 6.90E-2 | 2.785 | / |
| Loss | chrX:121,101,286-121,546,546 | Xq25 | chrX:121,162,142-121,262,032 | 6.82E-2 | 4.580 | / |
To determine whether SCNA of genes in peak GISTIC regions in our serous EC cohort is associated with survival, we queried these genes in the TCGA serous EC (n=108) data [29], using the cBIOPortal [30, 31]. This analysis showed that LAMC1 gain was statistically significantly associated with decreased overall survival (OS) (median OS 14.7 months versus 106.9 months; Logrank Test P-value 5.659e-4) and shorter disease-free survival (DFS) (median disease-free interval 14.0 months versus NA; Logrank Test P-value 1.244e-4) for serous EC patients (Supplementary Figure 4). SPIN4 gain was statistically significantly associated with reduced OS (Logrank Test P-value 2.828e-3) (Supplementary Figure 4), although this finding is based on a single patient with amplification.
GISTIC analysis of endometrioid ECs identified six significant peak regions of gain and one significant peak region of loss (Table 3). The consensus cancer gene MRTFA/MKL1 was located within a peak region of GISTIC loss among endometrioid ECs. There was no overlap between the genes located in peak GISTIC regions in endometrioid ECs in this study and those in TCGA EC copy number cluster-2 or cluster-3, which were predominated by endometrioid ECs [10]. ZMAT4 (Zinc finger matrin type 4) was located within a peak region of gain in both serous and endometrioid ECs.
Table 3.
Extended and peak GISTIC regions identified among endometrioid ECs
| SCNA Type | Genomic Coordinates (hg18) of Extended GISTIC Region | Chromosomal Location of Extended GISTIC Region | Genomic Coordinates (hg18) of Peak GISTIC Region | Q-bound for Peak Region | G-Score for Peak Region | Genes in Peak GISTIC Region |
|---|---|---|---|---|---|---|
| Gain | chr1:147,305,744-247,249,719 | 1q21.1-q44 | chr1:189,156,518-189,322,948 | 4.13E-03 | 1.669 | / |
| Gain | chr10:11,552,951-30,309,068 | 10p14-p11.23 | chr10:23,244,490-23,432,842 | 4.75E-02 | 1.099 | ARMC3 |
| Gain | chr10:41,756,307-45,536,421 | 10q11.1-q11.21 | chr10:42,305,364-42,587,946 | 1.85E-02 | 1.194 | ZNF33B, LINC008039, LINC01518,LOC283028,LOC105378269 |
| Gain | chr3:121,385,071-192,547,654 | 3q13.33-q28 | chr3:170,041,278-170,109,492 | 9.99E-02 | 1.255 | LINC02082 |
| Gain | chr7:109,318,773-109,646,296 | 7q31.1 | chr7:109,318,773-109,412,379 | 7.37E-02 | 1.024 | / |
| Gain | chr8:37,345,764-43,910,848 | 8p12-p11.1 | chr8:40,608,933-40,638,501 | 5.90E-03 | 1.319 | ZMAT4 |
| Loss | chr22:14,884,399-49,691,432 | 22q11.1-q13.33 | chr22:39,148,396-39,289,536 | 6.26E-02 | 1.875 | MRTFA/MKL1 |
| Loss | chrX:61,845,481-148,937,305 | Xq11.1-q28 | chrX:93,562,756-93,703,848 | 6.26E-03 | 1.001 | PLCXD1, GTPBP6, PPP2R3B, SHOX |
Consensus cancer genes (* Tier-1 or ** Tier 2; COSMIC v90) are shown in bold
Correlation of SCNA status with TP53 mutational status, MSI, and POLE mutational status
We previously reported the incidence of TP53 somatic mutations (all coding exons) and microsatellite instability (MSI) for the 51 tumors in this study [18, 19]. Here, we compared the SCNA status and TP53 status of tumors (Supplementary Table 5). We observed a statistically significant positive correlation between TP53 mutation and the presence of focal high-level SCNAs across ECs: 85.7% (24 of 28) of tumors with focal high-level SCNAs were TP53-mutated compared with 4.3% (1 of 23) of tumors without focal high-level SCNAs (two-tailed p value<0.0001). With respect to histologic subtype, all (3 of 3) clear cell tumors with focal high-level SCNAs were TP53-mutated versus 0% (0 of 3) of clear cell tumors without focal high-level SCNAs, a difference that was not statistically significant; 90.9% (20 of 22) of serous tumors with focal high-level SCNAs were TP53-mutated compared with 0% (0 of 6) of serous tumors without focal high-level SCNAs (two-tailed p value<0.0001); and 33.3% (1 of 3) of endometrioid tumors with focal high-level SCNAs were TP53-mutated versus 7.1% (1 of 14) of tumors without focal high-level SCNAs, a difference that was not statistically significant.
No statistically significant associations were found between the presence of focal high-level SCNAs and MSI in any histotype. MSI was not detected in any clear cell or serous ECs in this study. For endometrioid ECs, 0% (0 of 3) of tumors with focal high-level SCNAs had MSI compared with 28.6% (4 of 14) of tumors without high-level SCNAs, a difference that was not statistically significant.
The POLE (exon 3–13) mutation status of 49 tumors in this study was reported elsewhere [32]. POLE hotspot mutations (Supplementary Table 5) were present in one endometrioid EC, and in two of eight (25%) TP53 wildtype serous tumors versus 0 of 20 (0%) TP53 mutant serous tumors (a difference that was not statistically significant) (Supplementary Table 5). Focal high-level SCNAs were not detected in either of the POLE-mutated tumors or in MSI tumors.
Distribution of SCNAs and GISTIC regions in “TCGA-like” molecular subtypes of EC
MSI status, the mutational status of the POLE exonuclease domain (ED)(exons 9–13), and the mutational status of TP53 can be used to approximate the TCGA molecular subtypes of EC [33]. We therefore stratified the 49 tumors previously typed for all three markers into the following molecular groups: POLE-ED-mutated (n=3), MSI (n=4), TP53-mutated (n=24), and unspecified (MSS/POLE-ED-wildtype/TP53-wildtype) (n=18) (Supplementary Table 5). Tumors with any level of SCNA were more common in the TP53-mutated group (100%, 24 of 24) than in the unspecified group (50%, 9 of 18) and were absent from the POLE-mutated (0 of 3) and MSI-high (0 of 4) groups. Tumors with focal high-level SCNAs (gains of ≥5 copies or homozygous deletions, both <10Mb) were more frequent in the TP53-mutated group (95.8%, 23 of 24) than in the unspecified group (22.2%, 4 of 18), a difference that was statistically significant (two-tailed p value<0.0001). No GISTIC regions of SCNA were detected in the POLE-ED-mutated or MSI groups whereas the TP53-mutated and unspecified groups had 41 and 8 GISTIC regions of SCNA respectively (Table 4 and Table 5). Among tumors within the TP53-mutated group, consensus cancer genes KAT6B, MECOM, ERBB2, PTEN and LRP1B were within peak GISTIC regions (Table 4). No consensus cancer genes were identified among tumors within the unspecified molecular subgroup (Table 5).
Table 4.
Extended and peak GISTIC regions identified among the TP53-mutated molecular subgroup of EC
| SCNA Type | Genomic Coordinates (hg18) of Extended GISTIC Region | Location of Extended GISTIC Region | Genomic Coordinates (hg18) of Peak GISTIC Region | Q-bound for Peak Region | G-Score for Peak Region | Genes in Peak GISTIC Region |
|---|---|---|---|---|---|---|
| Gain | chr1:114027311-121013322 | 1p13.2-p11.2 | chr1:115921462-116005785 | 3.96E-2 | 4.374 | VANGL1 |
| Gain | chr1:176942142-207275546 | 1q25.2-q32.2 | chr1:181211397-181376609 | 2.00E-3 | 5.110 | LAMC1, LAMC1-AS1 |
| Gain | chr10:76097779-76809750 | 10q22.2 | chr10:76285006-76292848 | 3.32E-3 | 4.996 | KAT6B |
| Gain | chr12:24710025-25457711 | 12p12.1 | chr12:24983158-25022328 | 1.57E-4 | 5.674 | BCAT1 |
| Gain | chr13:91908665-92569702 | 13q31.3 | chr13:92353870-92391097 | 2.25E-1 | 3.713 | / |
| Gain | chr14:73019559-85498415 | 14q24.3-q31.3 | chr14:73276000-73364208 | 2.60E-2 | 4.491 | ELMSAN1, MIR4505, LOC100506476, LINC02274 |
| Gain | chr17:34942209-35561769 | 17q12-q21.1 | chr17:35024060-35199745 | 2.70E-2 | 4.482 | PPP1R1B, STARD3, TCAP, PNMT, PGAP3, ERBB2, MIEN1, GRB7, IKZF3, MIR4728 |
| Gain | chr18:0-16100000 | 18p11.32-p11.1 | chr18:10911569-11090060 | 1.37E-2 | 4.658 | PIEZO2 |
| Gain | chr19:22755564-24191440 | 19p12 | chr19:22831403-22857036 | 1.18E-2 | 4.692 | ZNF723 |
| Gain | chr19:34477100-37895554 | 19q12-q13.11 | chr19:34839864-34892756 | 7.50E-14 | 10.887 | PLEKHF1, C19orf12 |
| Gain | chr2:94793151-118939207 | 2q11.1-q14.2 | chr2:95017289-95050247 | 2.00E-2 | 4.561 | / |
| Gain | chr20:14929825-17414153 | 20p12.1 | chr20:15505853-15529115 | 2.46E-3 | 5.069 | MACROD2 |
| Gain | chr20:51602600-51797590 | 20q13.2 | chr20:51722390-51726725 | 1.26E-8 | 7.356 | / |
| Gain | chr21:28466487-46944323 | 21q21.3-q22.3 | chr21:43661758-43718330 | 1.27E-1 | 3.991 | SIK1, SIK1B, LINC00319, LINC01669, LINC00313 |
| Gain | chr3:169808488-171669472 | 3q26.2 | chr3:170858670-171032269 | 7.64E-14 | 8.985 | MECOM, ACTRT3, MYNN, LRRC34, LRRIQ4, TERC |
| Gain | chr4:61820279-64360471 | 4q13.1 | chr4:64148793-64360471 | 4.12E-2 | 4.362 | / |
| Gain | chr6:11306574-11952750 | 6p24.1 | chr6:11470627-11545036 | 7.64E-4 | 5.340 | NEDD9 |
| Gain | chr6:71020557-74065543 | 6q13 | chr6:72383552-72520206 | 1.88E-1 | 3.803 | / |
| Gain | chr7:37375580-57890326 | 7p14.2-p11.1 | chr7:53114918-53194448 | 3.47E-2 | 4.410 | / |
| Gain | chr8:129370592-132726767 | 8q24.21-q24.22 | chr8:129601251-129636803 | 7.64E-3 | 4.792 | LINC00824 |
| Gain | chr8:40154687-43910848 | 8p11.21-p11.1 | chr8:40608933-40638501 | 6.65E-4 | 5.365 | ZMAT4 |
| Gain | chrX:61845481-67200492 | Xq11.1-q12 | chrX:61845481-62571001 | 2.22E-1 | 3.729 | SPIN4, LINC01278 |
| Loss | chr1:16797436-35789123 | 1p36.13-p34.3 | chr1:27616247-27756094 | 8.88E-2 | 4.704 | WASF2, AHDC1 |
| Loss | chr10:89251828-90869249 | 10q23.2-q23.31 | chr10:89620956-89745318 | 1.03E-1 | 2.504 | PTEN |
| Loss | chr16:33744011-34459363 | 16p11.2-p11.1 | chr16:33744011-34319481 | 8.88E-2 | 2.860 | MIR9901, LOC112268173 |
| Loss | chr16:45205840-66994888 | 16q11.2-q22.1 | chr16:58316118-58346135 | 8.88E-2 | 3.042 | / |
| Loss | chr17:18083937-18771584 | 17p11.2 | chr17:18558072-18591780 | 8.88E-2 | 3.474 | TRIM16L, FBXW10 |
| Loss | chr19:5166212-8072557 | 19p13.3-p13.2 | chr19:7120029-7174340 | 1.46E-1 | 2.318 | INSR |
| Loss | chr19:0-1654336 | 19p13.3 | chr19:839331-876857 | 8.88E-2 | 4.636 | MED16, R3HDM4, KISS1R, RNU6-2 |
| Loss | chr2:141290607-142091845 | 2q22.1 | chr2:141512334-141549533 | 2.41E-1 | 2.074 | LRP1B |
| Loss | chr21:16104648-46944323 | 21q21.1-q22.3 | chr21:25436494-25569678 | 2.04E-1 | 2.160 | / |
| Loss | chr22:45341436-49106708 | 22q13.31-q13.33 | chr22:48742080-48975222 | 8.88E-2 | 3.386 | PIM3, IL17REL, TTLL8, MLC1, MOV10L1, PANX2, TRABD, MIR6821* |
| Loss | chr4:115451731-141479193 | 4q13.31-q13.33 | chr4:129663522-129691216 | 1.01E-1 | 2.528 | / |
| Loss | chr4:181771427-182518809 | 4q34.3 | chr4:182415084-182433256 | 8.88E-2 | 4.764 | LINC02500 |
| Loss | chr4:35651979-40878942 | 4p14 | chr4:35780455-35831233 | 8.88E-2 | 3.203 | ARAP2 |
| Loss | chr5:59205723-59759773 | 5q12.1 | chr5:59288399-59292832 | 8.88E-2 | 4.161 | PDE4D |
| Loss | chr7:109360477-116122766 | 7q31.1-q31.2 | chr7:109663496-109845497 | 8.88E-2 | 2.990 | / |
| Loss | chr8:24314997-29040226 | 8p21.2-p21.1 | chr8:26258130-26349606 | 1.19E-1 | 2.419 | PPP2R2A, BNIP3L |
| Loss | chr9:25609706-28594341 | 9p21.2-p21.1 | chr9:25926637-26889573 | 8.88E-2 | 3.058 | LOC100506422, CAAP1 |
| Loss | chr9:74060683-90190867 | 9q21.13-q22.1 | chr9:78714346-78849837 | 8.88E-2 | 3.134 | FOXB2 |
| Loss | chrX:121101286-121546546 | Xq25 | chrX:121162142-121262032 | 8.88E-2 | 4.675 | / |
Consensus cancer genes (* Tier-1 COSMIC v90) are shown in bold
Table 5.
Extended and peak GISTIC regions identified among the “unspecified” molecular subgroup of EC
| SCNA Type | Genomic Coordinates (hg18) of Extended GISTIC Region | Location of Extended GISTIC Region | Genomic Coordinates (hg18) of Peak GISTIC Region | Q-bound for Peak Region | G-Score for Peak Region | Genes in Peak GISTIC Region |
|---|---|---|---|---|---|---|
| Gain | chr1:176889044-247249719 | 1q25.2-q44 | chr1:226520130-226684198 | 1.201E-2 | 1.625 | OBSCN, TRIM11, TRIM17, HIST3H3, MIR6742 |
| Gain | chr11:0-2278975 | 11p15.5 | chr11:2262382-2277501 | 1.302E-2 | 1.174 | C11orf21 |
| Gain | chr16:25589827-32045466 | 16p12.1-p11.2 | chr16:30005992-30178440 | 2.767E-2 | 1.076 | TBX6,YPEL3,GDPD3,MAPK3,CORO1A, BOLA2B,BOLA2, SLX1A, SLX1B, SULT1A3, SULT1A4, NPIPB13, NPIPB12, SLX1B-SULTA4, SLX1BA-SULT1A3 |
| Gain | chr17:46022175-71330793 | 17q21.33-q25.1 | chr17:49051857-49099570 | 2.574E-2 | 1.083 | / |
| Gain | chr19:32615675-37581903 | 19q12-q13.11 | chr19:33868381-33998333 | 1.265E-2 | 1.180 | / |
| Loss | chr16:34584567-35106851 | 16p11.1 | chr16:34911034-35106851 | 1.563E-1 | 1.356 | / |
| Loss | chr5:70715382-98608326 | 5q13.2-21.1 | chr5:98552049-98608326 | 1.563E-1 | 1.100 | / |
| Loss | chrX:13889477-58363397 | Xp22.2-p11.1 | chrX:29494339-29543664 | 1.563E-1 | 1.092 | IL1RAPL1, MIR4666B |
DISCUSSION
Clear cell EC is a rare histological subtype of uterine cancer that is associated with relatively poor clinical outcomes [4, 34] and to date is lacking the genomic characterization to which other EC histologic subtypes have been subjected. Here we provide high-resolution copy number analysis of 6 clinically diagnosed clear cell ECs and show that half of these tumors have acquired one or more SCNAs; the most frequent SCNAs involved gains of 3q, 5p, 8q, 20q, and 22q. Our findings are consistent with a comparative genomic hybridization (CGH) study by Micci et al., which reported genomic imbalances in 75% (3 of 4) of clear cell ECs, with gains involving 3q and 8q occurring in all chromosomally unstable tumors [23]. Compared to gains, we found that genomic losses in copy number altered clear cell ECs were relatively rare, which is also consistent with previous observations [23]. Although we observed no statistically significant association between TP53 mutation status and copy number status in clear cell EC, it is noteworthy that TP53 mutations were restricted to tumors with focal high-level SCNAs.
By comparison to clear cell ECs, 82.1% of serous and 70.5% of endometrioid ECs in our study had at least one SCNA. The fraction of tumors with focal high-level SCNAs was greater for serous than endometrioid ECs (78.6% versus 17.6% respectively). Our observations that SCNAs in serous tumors were widely distributed throughout the genome and consisted of gains and losses, whereas SCNAs in endometrioid tumors often involved chromosomes 1, 3, 7, 8 and 10 and tended to be gains, is in keeping with the distribution of SCNAs observed by TCGA in these histotypes [10] and in an early CGH study by Pere et al [35].
We noted a positive correlation between TP53 mutation and the presence of focal high-level SCNA in ECs (two-tailed p value<0.0001). Although the overall frequency of TP53 mutations in our serous EC cohort (71%) is relatively low, it is within the range (59%-93%, mean 81.7%) reported by others [10, 28, 36–40]. Inter-study variability in TP53 mutation rate may reflect differences in the extent of pathological review of tumors; studies based on clinical diagnoses, such as ours, will inevitably reflect the challenges in reliably determining the histology of some high-grade endometrial tumors [41]. Because of the well-known challenges associated with reproducible histopathologic diagnoses of a subset of ECs [42], we stratified our entire tumor cohort into “TCGA-like” molecular subgroups based on POLE (exonuclease domain) mutation status, MSI status, and TP53 mutation status. Clear cell ECs were equally distributed among the TP53 mutated and unspecified groups. Copy number altered tumors were more common in the TP53-mutated group than in the unspecified group and were absent from the POLE-mutated or MSI-high groups. The most statistically significant peak GISTIC regions in the TP53-mutated group were gains involving 3q26.2 (MECOM, ACTRT3, MYNN, LRRC34, LRRIQ4, TERC), 19q12-q13.11 (PLEKHF1, C19orf12), and 20q13.2. Although the peak GISTIC region on 20q13.2 did not encompass any gene, the corresponding extended GISTIC region included ZNF217, a putative oncogene that is amplified and/or overexpressed in a variety of tumor types [43]. Of note, MECOM, TERC, and ZNF217 are also in significantly recurring regions of amplification in TCGA copy number cluster 4 tumors. Other genes in peak GISTIC regions in the TP53-mutated group and in significantly recurring regions of SCNA in TCGA copy number cluster 4 tumors are NEDD9, ERBB2, and LRP1B.
While our study provides insights into the occurrence of SCNAs in clear cell ECs, it has several limitations. Our analysis was restricted to a small number of clinically diagnosed clear cell ECs. Because clear cell ECs and a subset of serous ECs are recognized as being difficult to reproducibly classify, even by multiple gynecologic pathologists [41, 44–48], our findings should be viewed in the context of clinically diagnosed cases, not consensus (pure) cases. As such, our study was not designed to reliably evaluate certain genotype-phenotype correlations such as the issue of whether TP53-mutant clear cell ECs are really serous carcinomas with clear cell-like morphology. Studies to address such questions are important future directions for the field. Finally, in the absence of orthogonal validation, the copy number alterations reported herein should be regarded as putative alterations.
In conclusion, using high resolution SNP-based array analysis, we show that some clinically diagnosed clear cell ECs have high-level copy number alterations and are TP53-mutated whereas others are relatively copy number quiet and TP53-nonmutated, a finding that corroborates previous reports of genomic heterogeneity in clear cell ECs with respect to mutations [13–21]. Our observations that SCNAs are more frequent in serous EC than in low- and intermediate-grade endometrioid ECs also confirms previous observations [10]. To our knowledge this is only the second series of primary clear cell ECs analyzed genome-wide for somatic copy number alterations using high-resolution SNP arrays. Our findings add to the current understanding of the molecular etiology of this rare but often clinically aggressive histological subtype of EC and provide impetus for future studies.
Supplementary Material
ACKNOWLEDGEMENTS
Our sincere thanks to Dr. Mary Ellen Urick for critical and insightful comments on the manuscript. We thank Settara Chandrasekharappa, Ph.D., MaryPat Sussex Jones, M.S., and Ursula Harper M.S., of the NHGRI Genomics Core, and Kim Doheny, Ph.D., Roxann Ashworth, M.H.S., and Michelle Zilka, M.S., at the Center for Inherited Disease Research (CIDR) at Johns Hopkins University for technical assistance. Data have been deposited into dbGaP (phs001690.v1.p1) with controlled-access.
FUNDING
This work was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Footnotes
Declaration of interest: Dr. Daphne W. Bell receives royalty income from the licensing of US patent No. 7,294,468 “Method to determine responsiveness of cancer to epidermal growth factor receptor targeting treatments”
REFERENCES
- [1].American Cancer Society. Cancer Facts and Figures. American Cancer Society; 2018;1:1–76. [Google Scholar]
- [2].Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol 2011;8:261–71. [DOI] [PubMed] [Google Scholar]
- [3].Clement PB, Young RH. Non-endometrioid carcinomas of the uterine corpus: a review of their pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol 2004;11:117–42. [DOI] [PubMed] [Google Scholar]
- [4].Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001 Patient and tumor characteristics. National Cancer Institute, SEER Program, NIH Pub No 07–6215, Bethesda, MD, 2007. [Google Scholar]
- [5].Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 2006;95 Suppl 1:S105–43. [DOI] [PubMed] [Google Scholar]
- [6].McGunigal M, Liu J, Kalir T, Chadha M, Gupta V. Survival differences among uterine papillary serous, clear cell and grade 3 endometrioid adenocarcinoma endometrial cancers: a national cancer database analysis. Int J Gynecol Cancer 2017;27:85–92. [DOI] [PubMed] [Google Scholar]
- [7].Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer 2006;94:642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Walker CJ, Goodfellow PJ. Traditional approaches to molecular genetic analysis. Adv Exp Med Biol 2017;943:99–118. [DOI] [PubMed] [Google Scholar]
- [9].Le Gallo M, Lozy F, Bell DW. Next-generation sequencing. Adv Exp Med Biol 2017;943:119–48. [DOI] [PubMed] [Google Scholar]
- [10].Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol 2014;15:e268–78. [DOI] [PubMed] [Google Scholar]
- [12].Urick ME, Rudd ML, Bell DW. Molecular pathology of serous carcinoma In: Deavers MT, Coffey D, editors. Precision molecular pathology of uterine cancer: Springer, 2017;87–122. [Google Scholar]
- [13].An HJ, Logani S, Isacson C, Ellenson LH. Molecular characterization of uterine clear cell carcinoma. Mod Pathol 2004;17:530–7. [DOI] [PubMed] [Google Scholar]
- [14].Huang HN, Chiang YC, Cheng WF, Chen CA, Lin MC, Kuo KT. Molecular alterations in endometrial and ovarian clear cell carcinomas: clinical impacts of telomerase reverse transcriptase promoter mutation. Mod Pathol 2015;28:303–11. [DOI] [PubMed] [Google Scholar]
- [15].Hoang LN, McConechy MK, Meng B, McIntyre JB, Ewanowich C, Gilks CB, et al. Targeted mutation analysis of endometrial clear cell carcinoma. Histopathology 2015;40:166–80. [DOI] [PubMed] [Google Scholar]
- [16].Rudd ML, Price JC, Fogoros S, Godwin AK, Sgroi DC, Merino MJ, et al. A unique spectrum of somatic PIK3CA (p110alpha) mutations within primary endometrial carcinomas. Clin Cancer Res 2011;17:1331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Urick ME, Rudd ML, Godwin AK, Sgroi D, Merino M, Bell DW. PIK3R1 (p85alpha) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res 2011;71:4061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Le Gallo M, O’Hara AJ, Rudd ML, Urick ME, Hansen NF, O’Neil NJ, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet 2012;44:1310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Le Gallo M, Rudd ML, Urick ME, Hansen NF, Zhang S, NISC Comparative Sequencing Program, et al. Somatic mutation profiles of clear cell endometrial tumors revealed by whole exome and targeted gene sequencing. Cancer 2017;123:3261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].DeLair DF, Burke KA, Selenica P, Lim RS, Scott SN, Middha S, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol 2017;243:230–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stelloo E, Bosse T, Nout RA, MacKay HJ, Church DN, Nijman HW, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol 2015;28:836–44. [DOI] [PubMed] [Google Scholar]
- [22].Suzuki A, Fukushige S, Nagase S, Ohuchi N, Satomi S, Horii A. Frequent gains on chromosome arms 1q and/or 8q in human endometrial cancer. Hum Genet 1997;100:629–36. [DOI] [PubMed] [Google Scholar]
- [23].Micci F, Teixeira MR, Haugom L, Kristensen G, Abeler VM, Heim S. Genomic aberrations in carcinomas of the uterine corpus. Genes Chromosomes Cancer 2004;40:229–46. [DOI] [PubMed] [Google Scholar]
- [24].Bardi G, Pandis N, Schousboe K, Holund B, Heim S. Near-diploid karyotypes with recurrent chromosome abnormalities characterize early-stage endometrial cancer. Cancer Genet Cytogenet 1995;80:110–4. [DOI] [PubMed] [Google Scholar]
- [25].Gibson WJ, Hoivik EA, Halle MK, Taylor-Weiner A, Cherniack AD, Berg A, et al. The genomic landscape and evolution of endometrial carcinoma progression and abdominopelvic metastasis. Nat Genet 2016;48:848–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Beroukhim R, Getz G, Nghiemphu L, Barretina J, Hsueh T, Linhart D, et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci U S A 2007;104:20007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Petrini I, Meltzer PS, Kim IK, Lucchi M, Park KS, Fontanini G, et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat Genet 2014;46:844–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Soumerai TE, Donoghue MTA, Bandlamudi C, Srinivasan P, Chang MT, Zamarin D, et al. Clinical utility of prospective molecular characterization in advanced endometrial cancer. Clin Cancer Res 2018;24:5939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Berger AC, Korkut A, Kanchi RS, Hegde AM, Lenoir W, Liu W, et al. A comprehensive pan-cancer molecular study of gynecologic and breast cancers. Cancer Cell 2018;33:690–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rudd ML, Mohamed H, Price JC, O’Hara AJ, Le Gallo M, Urick ME, et al. Mutational analysis of the tyrosine kinome in serous and clear cell endometrial cancer uncovers rare somatic mutations in TNK2 and DDR1. BMC Cancer 2014;14:884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017;123:802–13. [DOI] [PubMed] [Google Scholar]
- [34].Hasegawa K, Nagao S, Yasuda M, Millan D, Viswanathan AN, Glasspool RM, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the uterine corpus and cervix. Int J Gynecol Cancer 2014;24:S90–5. [DOI] [PubMed] [Google Scholar]
- [35].Pere H, Tapper J, Wahlstrom T, Knuutila S, Butzow R. Distinct chromosomal imbalances in uterine serous and endometrioid carcinomas. Cancer Res 1998;58:892–5. [PubMed] [Google Scholar]
- [36].Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U.S.A. 2013;110:2916–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McConechy MK, Ding J, Cheang MC, Wiegand K, Senz J, Tone A, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol 2012;228:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kuhn E, Wu RC, Guan B, Wu G, Zhang J, Wang Y, et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J Natl Cancer Inst 2012;104:1503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tashiro H, Isacson C, Levine R, Kurman RJ, Cho KR, Hedrick L. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol 1997;150:177–85. [PMC free article] [PubMed] [Google Scholar]
- [40].Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 2000;88:814–24. [PubMed] [Google Scholar]
- [41].Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol 2013;37:874–81. [DOI] [PubMed] [Google Scholar]
- [42].Murali R, Davidson B, Fadare O, Carlson JA, Crum CP, Gilks CB, et al. High-grade endometrial carcinomas: morphologic and immunohistochemical features, diagnostic challenges and recommendations. Int J Gynecol Pathol 2019;38 Suppl 1:S40–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rahman MT, Nakayama K, Rahman M, Nakayama N, Ishikawa M, Katagiri A, et al. Prognostic and therapeutic impact of the chromosome 20q13.2 ZNF217 locus amplification in ovarian clear cell carcinoma. Cancer 2012;118:2846–57. [DOI] [PubMed] [Google Scholar]
- [44].Thomas S, Hussein Y, Bandyopadhyay S, Cote M, Hassan O, Abdulfatah E, et al. Interobserver variability in the diagnosis of uterine high-grade endometrioid carcinoma. Arch Pathol Lab Med 2016;140:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Han G, Soslow RA, Wethington S, Levine DA, Bogomolniy F, Clement P, et al. Endometrial carcinomas with clear cells: a study of a heterogeneous group of tumors including interobserver variability, mutation analysis, and immunohistochemistry with HNF-1beta. Int J Gynecol Pathol 2015;34:323–33. [DOI] [PubMed] [Google Scholar]
- [46].Fadare O, Parkash V, Dupont WD, Acs G, Atkins KA, Irving JA, et al. The diagnosis of endometrial carcinomas with clear cells by gynecologic pathologists: an assessment of interobserver variability and associated morphologic features. Am J Surg Pathol 2012;36:1107–18. [DOI] [PubMed] [Google Scholar]
- [47].Han G, Sidhu D, Duggan MA, Arseneau J, Cesari M, Clement PB, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol 2013;26:1594–604. [DOI] [PubMed] [Google Scholar]
- [48].Clarke BA, Gilks CB. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol 2010;63:410–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.