Abstract
Objective:
Five biologic therapies have FDA-approved indications for difficult-to-control asthma. The clinical trials that proved the efficacy and safety of these biologics were often similar in their inclusion criteria, study designs, and endpoints. Many of these trials have been re-analyzed post-hoc to identify subsets of subjects considered to be enhanced responders. As a result, it has become increasingly difficult to keep up with the literature and decide on the most appropriate biologic for our patients. This review summarizes and compares trial designs, patient cohorts and study results of the major trials involving these therapies.
Data Sources:
Included are basic science articles, online FDA applications, and all the published reports of phase II and phase III clinical trials for FDA-approved asthma biologics.
Study Selections:
Included are the major phase II and phase III clinical trials of 5 asthma biologics.
Results:
Due to variations in inclusion criteria and natural variations in enrolled cohorts, the baseline clinical traits and severity of study populations in asthma biologic trials differed significantly, which is important since baseline annualized exacerbation rates and blood eosinophilia are both strong predictors of a biologic’s success. Notwithstanding, the trial results, when considered together, can help guide care providers in choosing the most appropriate biologic for our patients.
Conclusion:
Understanding the details and differences in asthma biologic trial designs, patient cohorts, and in study results will help care providers make more informed decisions when choosing a biologic. We are hopeful this review will serve as reference to care providers for this purpose.
INTRODUCTION
Affecting roughly 8% of the U.S. population, asthma costs an estimated $82 billion annually related to medication use, missed work and school days, urgent healthcare utilization, and early deaths [1–3]. Severe asthma (SA) is defined as asthma requiring high dose inhaled corticosteroids (ICS) plus a second maintenance medication and/or systemic steroids to prevent becoming uncontrolled or remaining uncontrolled despite this therapy [4]. SA affects 10% of asthma patients, but accounts for 50% of all healthcare costs [5]. In children, poor asthma control increases the risk of hospitalization, ED visit, or systemic steroid burst by six-fold [6].
Asthma is diagnosed by a clinical history of cough, shortness of breath, and chest tightness with confirmatory pulmonary function tests showing increased airway hyper-reactivity and/or reversible airflow limitation. There exists a diverse array of asthma phenotypes [7], although it is common practice to characterize asthma patients by the presence or absence of Type 2 (T2) inflammation in the airways. T2 inflammation is driven by cytokines IL-4, IL-5 and IL-13, which are secreted by CD4+ Type 2 (Th2) lymphocytes and innate lymphoid type 2 (ILC2s) cells [8]. The presence of T2 inflammation in the airways can be identified by a specific airway epithelial cell (AEC) mRNA gene expression signature [9–11], which when detected, correlates clinically with atopy, eosinophilia, and high fractional exhaled nitric oxide (FeNO) [10, 11]. This T2 gene expression signature was identified by measuring global mRNA gene expression changes in AECs (in an air liquid interface cell culture) following in vitro IL-13 stimulation [9, 10]. The signature was then validated in ex vivo AECs derived from the lower airways of asthmatic and healthy control subjects [9, 10]. Subsequent studies, although performed primarily in cross-section, have indicated that roughly 50% of asthma patients have T2 ‘high’ disease [9–11].
T2 inflammation and eosinophilia are highly connected. A gene included in the AEC T2 gene signature is eotaxin-3 (CCL26), a major eosinophil chemokine [11]. Its presence indicates that IL-13 upregulates CCL26 gene expression in AECs, which ultimately increases eotaxin-3 protein secretion and trafficking of eosinophils into the airways. In agreement, treatment of AECs in vitro with IL-13 has shown to induce eotaxin-3 protein expression [12]. IL-5, which is required for eosinophil maturation and their release from the bone marrow, also connects T2 and eosinophilic inflammation [13]. Eosinophils amplify airway inflammation, worsen exacerbations, and likely contribute to the chronic airway changes in asthma [14]. Approximately 50% of patients with severe asthma have persistent airway tissue eosinophils [4, 15].
The disease mechanisms in T2 ‘low’ asthma are largely unknown. Some proposed drivers of inflammation in T2 low asthma include innate cytokines (IL-1, IL-6, IL-8), interferons, TNF-α [16, 17], thymic stromal lymphopoietin (TSLP) [18–20], IL-25 [20, 21], and IL-33 [20, 22]. TSLP and IL-33 likely also promote T2 inflammatory responses [23, 24].
Five biologic therapies have FDA-approved indications for moderate-to-severe allergic asthma (omalizumab), moderate-to-severe asthma with an eosinophilic phenotype or with oral corticosteroid dependent asthma (dupilumab), or severe eosinophilic asthma (mepolizumab, reslizumab, benralizumab). These biologics target either T2- or eosinophilic-driven inflammation (See Table 1), which as mentioned, are often connected. The clinical trials that proved the efficacy and safety of these biologics were similar in their inclusion criteria, study protocols, and measured outcomes (i.e. annualized exacerbation rates, forced expiratory volume in 1 second (FEV1), standardized asthma symptoms scores, and ability to wean systemic steroids). Initial trial results are now being re-analyzed and re-interpreted in subsets of asthma patients that demonstrate enhanced responses. As a result, it has become increasingly difficult to keep up with the growing body of literature surrounding asthma biologic therapy. The aim of this review is to summarize and compare trial designs, patient cohorts and the study results of all major trials involving asthma biologics. To maintain a clinical focus, we limit our discussion to FDA-approved therapies only, and unless specified, only statistically significant study results are reported in our review.
Table 1.
Indications and Dosing for FDA-Approved Asthma Biologies
| DRUG | INDICATION | DOSING |
|---|---|---|
| OMALIZUMAB | Add-on maintenance treatment for moderate-to-severe persistent asthma in those aged 6 years or older with a positive skin test or in vitro reactivity to a perennial aeroallergen, and symptoms that are inadequately controlled with inhaled corticosteroids. | 75 to 375 mg SC every 2 or 4 weeks* |
| MEPOLIZUMAB | Add-on maintenance treatment of patients with severe eosinophilic asthma, and for the treatment of adult patients with eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome). | 100 mg SC every 4 weeks (bodyweight ≥ 40 kg); 40 mg SC every 4 weeks (bodyweight < 40 kg) |
| Newly approved as an add-on maintenance treatment of patients with severe eosinophilic asthma aged 6 years or older. | ||
| RESLIZUMAB | Add-on maintenance treatment of patients with severe asthma, aged 18 years and older and with an eosinophilic phenotype. | 3 mg/kg IV every 4 weeks |
| BENRALIZUMAB | Add-on maintenance treatment of patients with severe asthma aged 12 years and older, and with an eosinophilic phenotype. | 30 mg SC every 4 weeks X 3 doses, then every 8 weeks |
| DUPILUMAB | Add-on maintenance treatment in patients with moderate-to-severe asthma aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid dependent asthma. | 400 or 600* mg (two injections) SC followed by 200 or 300** mg every other week |
Dose and frequency is determined by serum total IgE level (lU/mL) pre-treatment & body weight (kg).
300 mg (600 mg initial) dose is indicated for those with oral corticosteroid dependent asthma or with atopic dermatitis. Abbreviations: IV: intravenous, kg: kilogram, mg: milligram, SC: subcutaneous
BODY
Mechanisms of Action (See Figure 1)
Figure 1. Mechanisms of FDA-Approved Asthma Biologics.
Illustrative representation of the pathways targeted by all five asthma biologics.
Anti-IgE therapy (omalizumab):
Omalizumab is a recombinant, DNA-derived humanized IgG1 κ antibody that binds to the Fc region of IgE and blocks its binding to the high-affinity IgE receptor, FcεR1 [25]. Omalizumab reduces free IgE by 96% [26, 27], although total IgE levels increase after the first injection due to the formation of omalizumab:IgE complexes. In a Type 1 allergic reaction, allergens cross link IgE bound to surface FcεR1 receptors on mast cells, which in turn triggers their degranulation and the release of potent preformed mediators such as histamine [28]. Following degranulation, activated mast cells continue to secrete pro-inflammatory cytokines and arachidonic acid metabolites [28, 29]. Omalizumab downregulates the FcεR1 receptor on mast cells and basophils, reducing their ability to degranulate [30]. As a result, omalizumab reduces wheal and flare reactions (i.e. mast cell degranulation) as well as late allergic asthma responses [31].
Omalizumab also reduces fall season asthma exacerbations in children, which are more likely to be driven by respiratory viral infections [32]. Discussed in further detail below, the PROSE study showed that starting omalizumab in inner-city children and adolescents prior to starting school prevented fall asthma exacerbations [32]. One hypothesis, supported by in vitro studies, is that IgE receptor activation decreases the generation of IFN-α from plasmacytoid dendritic cells, thereby increasing host susceptibility to viruses [33].
Anti-IL-5 therapy (mepolizumab, reslizumab, benralizumab):
The IL-5 cytokine has several known, and likely yet to be elucidated, effects on the airway [34]. IL-5 secretion by Th2 lymphocytes, ILC2s, and AECs induces airway inflammation and mediates recruitment, activation, and maturation of eosinophils [35]. Monoclonal antibodies mepolizumab and reslizumab bind and systemically deplete the IL-5 cytokine, which reduces peripheral eosinophil counts and tempers airway inflammation through mechanisms not entirely understood [36]. Alternatively, benralizumab irreversibly binds to the IL-5 receptor alpha chain (IL-5Rα), which triggers NK cell cytotoxic killing of eosinophils. Benralizumab treatment results in nearly complete systemic depletion of the body’s eosinophils [37, 38]. Interestingly, baseline blood eosinophilia prior to treatment predicts a positive response to IL-5-blocking antibodies better than sputum eosinophilia [39].
Anti-IL-4 and IL-13 therapy (dupilumab):
The dupilumab monoclonal antibody binds the IL-4 receptor a chain (IL-4Rα), blocking both I L-4 and IL-13 receptors and their signaling [40]. The inhibition of either IL-4 or IL-13 cytokines alone was proven to be ineffectual in asthma, likely due to the biological redundancy of these two signaling pathways [40–42]. IL-4 and IL-13 are major drivers of airway inflammation in asthma with multiple effects on the airway. These effects include epithelium thickening, goblet cell hyperplasia with mucous overproduction, subepithelial fibrosis, smooth muscle hypertrophy with increased contractility, B cell isotype switching with increased IgE production, and increased eosinophil trafficking into the airways [43].
Reduction in Exacerbation Rates Across Biologics
Omalizumab:
In two seminal phase III trials, omalizumab was evaluated in asthma subjects aged 12 to 75 years with symptomatic asthma despite treatment with medium-to-high dose ICS therapy [31, 44]. To determine the ability of omalizumab to reduce ICS use, both studies included a 4-month steroid- stable phase followed by a 2 to 3-month steroid-reduction phase. In study 1 with 525 subjects, omalizumab enabled a reduction in ICS dose, but also reduced the number of exacerbations by 48% and 41% relative to placebo during the steroid stable and reduction phases, respectively. In study 2 with 546 subjects, exacerbation reductions were 58% and 52% relative to placebo during the steroid-stable and reduction phases, respectively, and a similar reduction in ICS use as study 1. Baseline prebronchodilator FEV1 % predicted was reported at ~68% and ~70% among the treatment arms of study 1 and study 2, respectively. No patients were using daily oral steroids. A third phase III trial (study 3), which was reported in the FDA drug application, had a comparable study design as trials 1 and 2 but also included patients on long-acting beta agonists (LABAs), a subset of patients on oral steroids (~27%), and subjects with a baseline prebronchodilator FEV1 predicted > 80%. Study 3 found that exacerbation rates were similar between the omalizumab-treated and placebo treatment arms [45]. A lack of efficacy in study 3 was likely due to the inclusion of subjects with a prebronchodilator FEV1 > 80% predicted.
A recent post-hoc analysis including trials 1 and 2 showed that omalizumab reduced asthma exacerbations by 67% and 74% relative to placebo in subgroups of subjects with a baseline eosinophil count ≥ 300 and ≥ 400 cells/μl, respectively [46]. Enhanced responses to omalizumab were also seen in patients using higher doses of ICS (defined as beclomethasone ≥600 μg/day) or a long-acting beta agonist (LABA), and in those with a history of hospitalization, a history of ED visit, or FEV1 < 65% predicted.
In disagreement, a real-world observational study (PROSPERO) comparing pre- vs. post-treatment exacerbation rates found that blood eosinophils, fractional exhaled nitric oxide (FeNO) and total IgE levels did not predict a positive response to omalizumab [47]. In PROSPERO, the exacerbation rate improved from a mean of 3.0 in the 12 months before treatment to 0.8 through month 12 of treatment (relative reduction = 74%). Roughly 24% (191 of 801) of patients had a baseline total IgE outside the lower (< 30 IU/mL) or upper limit (> 700 IU/mL) of the dosing table. Most of these patients (n=120; 63%) had an IgE greater than 700 IU/mL. In subjects with an IgE above the standard dosing table, exacerbation rates were similarly decreased from 3.2 at baseline to 0.7 post-treatment (a 78% relative reduction). Another real-world retrospective study performed in Europe (STELLAIR) found that omalizumab’s effectiveness, which was determined by a physician’s evaluation or a ≥ 40% improvement in annualized exacerbation rates, was similar in eosinophil high (≥ 300 cells/uL) and eosinophil low (< 300 cells/uL) subgroups of subjects [48].
In another phase III clinical trial (INNOVATE), omalizumab was evaluated as an add-on therapy in 419 patients with difficult-to-control SA, defined as ≥2 exacerbations requiring steroids despite the use of a high dose ICS and a LABA [49]. Omalizumab reduced exacerbation rates by 26% relative to placebo during a relatively short (7-month) intervention period, although the detection of this improvement required a post hoc adjustment for differences in the baseline exacerbation history among treatment arms. Specifically, the authors made an adjustment to the primary efficacy variable (i.e. annualized exacerbation rate) based on an unexpected between-group difference in pretreatment exacerbation history. In comparison to trials 1 and 2 mentioned above, the INNOVATE cohort was arguably more severe, with a lower FEV1 at 61 to 62% predicted, 2.1 asthma exacerbations in the prior year, and with 20 to 23% of subjects using daily oral steroids.
In the EXTRA trial, omalizumab was evaluated as an add-on therapy in 850 patients who had ≥ 1 exacerbation in the past year and uncontrolled asthma symptoms despite the use of a high dose ICS and a LABA [50]. Like INNOVATE, there was a ~25% reduction in exacerbation rates relative to placebo with omalizumab treatment. A follow-up reanalysis of the EXTRA data showed that reductions in asthma exacerbations were enhanced for subgroups of patients with high FeNO measurements (53% reduction in FeNO high subgroup versus 16% in FeNO low) and high blood eosinophils (32% in eosinophil high versus 9% in eosinophil low) [51]. The EXTRA cohort had a FEV1 of 64 to 65% predicted, 1.9 to 2.0 exacerbations in the prior year, and 16 to 17% of subjects were on oral steroids. Including adults and children with moderate asthma, a Cochrane review (n= 25 trials) showed that 16 out of 100 people on omalizumab versus 26 out of 100 people on placebo were likely to have an asthma exacerbation, a 38% relative reduction [52].
In the PROSE trial, children and adolescents aged 6 to 17 years were enrolled to receive placebo, ICS boost treatments (definition: effectively doubled ICS dose at 100 or 250 μg fluticasone propionate twice daily) or omalizumab therapy starting before the fall season [32]. After a 4- to 9-month run-in period, the intervention period started 4 to 6 weeks before, and ended 90 days after, the start of school. Among participants with an asthma exacerbation during the 4- to 9-month run-in phase, omalizumab was significantly more effective than both placebo (6.4 vs 36.3%) and ICS boost (2.0 vs 27.8%) at preventing asthma exacerbations.
Mepolizumab:
In a phase II trial (DREAM), mepolizumab was evaluated at three intravenous (IV) doses (75, 250, and 750 mg) in 621 patients aged 12 to 74 years with ≥2 exacerbations in the previous year despite high dose ICS use and one or more of the following: sputum eosinophil ≥3%, FeNO ≥50 ppb, blood eosinophils ≥300 cells/μL, or deterioration of asthma control after a ≤25% reduction in maintenance inhaled or oral steroids [53]. The 3 mepolizumab-treated arms demonstrated reductions in asthma exacerbations by 48%, 39%, and 52%, respectively, relative to placebo. Both baseline eosinophil counts and the number of exacerbations in the prior year associated with a positive response to mepolizumab. Among the 4 study arms, baseline prebronchodilator FEV1 % predicted ranged from 59% to 61%, annualized exacerbation rates were high at 3.4 to 3.7, 29 to 33% used oral steroids daily, and the geometric mean on loge scale of blood eosinophils varied from 230 to 280 cells/μL.
In a subsequent phase III trial (MENSA), mepolizumab was evaluated at two doses (75 mg IV or 100 mg subcutaneous (SC)) in 576 subjects aged 12 to 82 years with a baseline peripheral eosinophil count ≥ 150 cells/μL, or ≥300 cells/μL at some point in the year prior, and ≥2 exacerbations in the year prior despite the use of a high dose ICS [54]. Over a 32-week treatment phase and 8-week follow-up safety phase, mepolizumab SC and IV doses reduced annualized exacerbation rates by 47% and 53%, respectively, relative to placebo. The SC dose reduced hospitalizations by 69% and a cumulative endpoint including ED visits and hospital admissions by 61%. In patients with a peripheral eosinophil count ≥500 cells/μL, mepolizumab reduced exacerbation rates by 74 and 79% relative to placebo with SC and IV doses, respectively. Similar to DREAM, baseline prebronchodilator FEV1 % predicted ranged from 59 to 62%, annualized exacerbation rates were high at 3.5 to 3.8, and the geometric mean on loge scale of blood eosinophils varied from 280 to 320 cells/μL across treatment arms. In comparison to DREAM, a higher percentage of subjects were using daily oral steroids at 44 to 52%.
In a small cohort of 135 patients with uncontrolled eosinophilic asthma despite the use of maintenance oral steroids (SIRIUS trial), as a secondary outcome, annual asthma exacerbation rates were lowered by 32% relative to placebo despite a higher reduction of steroids in the treatment arm [55]. This high severity cohort had a baseline prebronchodilator FEV1 of 58 to 60% predicted, at least 6 months of oral corticosteroid maintenance therapy, and the geometric mean on loge scale of blood eosinophils varied from 230 to 250 cells/μL.
A 2015 Cochrane review of 8 trials compared mepolizumab’s efficacy to placebo and included 1,707 participants in the analysis [56]. There was a wide variance in the severity of asthma among participants, which ranged from mild atopic to SA subjects with recurrent exacerbations. In those with eosinophilic asthma (2 studies), a significant reduction of exacerbation rates was confirmed (risk ratio=0.52, n=690). In 4 studies with non-eosinophilic asthma, no statistically significant reduction was found in exacerbations (n=468).
Reslizumab:
Reslizumab was evaluated in two independent phase III trials with a total of 953 subjects aged 12 to 75 years with a blood eosinophil count ≥400 cells/μL and ≥1 exacerbation in the year prior despite medium-to-high doses of ICS [57]. Over a 1-year treatment period, reslizumab reduced annualized asthma exacerbation rates by 50% (study 1) and 59% (study 2) relative to placebo. It was particularly effective in subjects with high blood eosinophils despite the use of oral steroids, who had a relative reduction of 68% compared to the placebo. Reductions of 34% (study 1) and 31% (study 2) were seen in a cumulative endpoint including hospital admissions and ED visits, but neither were statistically significant. Among all four treatment arms, the baseline prebronchodilator FEV1 ranged from 64 to 70% predicted, annualized exacerbations were moderate at 1.9 to 2.1, and the mean blood eosinophil counts was 610 to 696 cells/μL. Subjects receiving oral corticosteroid at enrollment ranged from 12 to 19%.
Benralizumab:
In a phase IIb trial, benralizumab was evaluated in 609 subjects aged 18 to 75 years with 2 to 6 exacerbations in the prior year despite the use of medium-to-high dose ICS and LABA therapy [58]. In eosinophilic individuals, annual exacerbation rate reduction for patients receiving benralizumab 2 mg, 20 mg, and 100 mg were −9% (p=0.78), 36% (p=0.17), and 41% (p=0.096), respectively, compared to placebo. In participants with a baseline blood eosinophil cutoff ≥300 cells/μL, exacerbation rates were significantly reduced by 57% and 43% for the benralizumab 20 mg and 100 mg groups, respectively, in comparison to placebo. A dose of 30mg SC was chosen for subsequent phase III studies.
Two phase III trials (CALIMA and SIROCCO) subsequently evaluated benralizumab in both eosinophil high (≥300 cells/μL) and eosinophil low (<300 cells/μL) asthma patients [59, 60]. In CALIMA, benralizumab treatment was studied over 56-weeks in 1,306 patients aged 12 to 75 years with ≥2 exacerbations in the last year despite high dose ICS and LABA therapy. Benralizumab reduced asthma exacerbations by 36% and 28% relative to placebo in the eosinophil high population with every 4-week (q4w) and 8-week (q8w) dosing, respectively. SIROCCO reported stronger results. In SIROCCO, benralizumab therapy in 1,205 adolescents and adults reduced exacerbation rates by 45% and 51% compared to placebo in the eosinophil high population with q4w and q8w dosing, respectively. In the eosinophil low population, benralizumab reduced exacerbation rates by only 17% relative to placebo with the q8w dosing.
The study populations for these two trials were very similar. Baseline prebronchodilator FEV1 % was low and ranged from ~56 to 59% among the treatment arms. Exacerbations in the past year were moderately high at ~2.6 to 3.1, and the median blood eosinophil counts were high at ~370 to 400 cells/μL for all participants, and ~500 cells/mL for the eosinophil high groups. Oral steroid use was 13 to 16%.
In a smaller cohort of 220 patients with uncontrolled eosinophilic asthma despite maintenance oral steroids (ZONDA trial), as a secondary endpoint, there was a 70% reduction in asthma exacerbations relative to placebo with q8w dosing despite a higher reduction of steroids in the treatment arm [61]. This high severity cohort had a baseline prebronchodilator FEV1 of 57 to 62% predicted, at least 6 months of oral corticosteroid maintenance therapy, and a median blood eosinophil count of 440 to 540 cells/μL.
A 2017 Cochrane review evaluated 13 randomized trials including 6,000 participants comparing the efficacy of mepolizumab, reslizumab, and benralizumab to placebo. An exacerbation was defined as worsening asthma symptoms that required treatment with systemic corticosteroids for 3 days or more. The review concluded that all of the anti-IL5 treatments reduced rates of exacerbation by approximately half in participants with severe eosinophilic asthma [62].
Dupilumab:
In a phase IIa study, dupilumab was evaluated in 104 adults aged 18 to 65 years with blood eosinophils levels ≥ 300 cells/μL or sputum eosinophil levels ≥ 3% and at least one exacerbation within 2 years despite medium-to-high dose ICS and LABA therapy [63]. In an innovative study design, subjects were instructed to discontinue LABA therapy at week 4 and then taper inhaled steroids in weeks 6 to 9. Thus, moderate-to-severe asthma subjects were tapered off maintenance medications, increasing the likelihood of asthma exacerbations in a short period of time. Over a 12-week treatment and 8-week follow period, dupilumab treatment resulted in an 87% relative reduction in asthma exacerbation rates relative to placebo.
In a subsequent phase III trial (QUEST), dupilumab was evaluated at two SC doses every 2 weeks (200 mg and 300 mg) in 1,902 patients aged 12 years of older with ≥1 exacerbation in the last year despite medium-to-high dose ICS and up to 2 additional controllers (e.g. LABA and leukotriene receptor antagonists), regardless of blood eosinophil count [64]. In subjects with higher eosinophils (≥300 cells/μL), there were 66% and 67% reductions in exacerbation rates relative to placebo with the 200mg and 300mg doses, respectively. In the eosinophil-medium (150 to 300 cells/μL) group, there was a significant reduction only in the 300mg dosage group at 44.3% relative to placebo. In this large cohort, FEV1 % predicted was 58% among treatment arms, with 2.1 exacerbations in the past year, and a mean of blood eosinophils of 360 cells/μL (median = 255 cells/μL). As an exclusion criterion, no oral steroids were allowed for 1 month prior to the screening visit.
In a study of 210 patients that used dupilumab at 300 mg to reduce oral steroid use (LIBERTY ASTHMA VENTURE), as a secondary endpoint, there was a reduction in severe exacerbation rates by 59% relative to placebo [65]. Among patients with blood eosinophils ≥300 cells/μl, there was a 71% lower rate of exacerbations compared to placebo. This cohort had 2.1 exacerbations in the past year, FEV1 % predicted of 52%, and a mean blood eosinophil count of 350 cells/μL.
Improvements in Lung Function and Symptom Scores
Omalizumab: In both study 1 and 2 mentioned above, FEV1 improved from baseline in both the omalizumab-treated and placebo groups [31, 44]. The treatment groups of both studies had slightly better improvements from baseline in FEV1 % predicted in comparison to placebo, as FEV1 improved from 68 to 72.5% in the treatment group vs. 68 to 69% in the placebo group [44]. Using 2 customized scores to assess daytime and nighttime asthma symptoms (scale 1 range: 0–4; scale 2 range: 0–9), both trials demonstrated a small, but statistically significant (~0.5 point) differential improvement with treatment compared to placebo [31].
In the PROSPERO observational study, lung function remained relatively unchanged over the study period, although a sub-analysis of adolescents showed a 170 mL improvement in FEV1 from baseline, which was not reported as statistically significant. Overall, ACT scores improved by ~4 points from baseline during the study period with treatment, whereas a 3-point change is considered the minimally important difference (MID).
In INNOVATE, FEV1 showed a differential improvement from baseline by ~100 mL in the omalizumab-treated patients compared to placebo (190 vs 96 mL) [49]. Omalizumab-treated patients showed a 0.45 differential improvement in Juniper AQLQ compared to placebo (0.91 vs 0.46), with 60.8% vs. 47.8% (p = 0.008) achieving a clinically meaningful ≥0.5-point improvement from baseline for treatment and placebo arms, respectfully [49].
In EXTRA, it was not reported whether or not there were changes in lung function with treatment [50]. There were small but significant differential improvements in AQLQ (+0.29) and total asthma symptom scale (TASS; −0.26) in the treatment group compared to placebo. The 2014 Cochrane review for omalizumab versus placebo demonstrated a significant improvement in asthma symptoms and patients’ health-related quality of life with treatment [52].
Mepolizumab:
In MENSA, SC and IV treatment groups demonstrated prebronchodilator FEV1 improvements from baseline of +98 mL and +100 mL, respectively, in comparison to placebo [54]. SC and IV groups had a stronger improvement in postbronchodilator FEV1, which improved in comparison to placebo by +146 mL and +138 mL, respectively. Results were better in patients with an eosinophil count ≥500 cells/μL, which improved by +185 and +132 mL (prebronchodilator), and by +318 and +222 mL (postbronchodilator) relative to placebo.
In DREAM, there were nonsignificant increases in prebronchodilator FEV1 relative to placebo of +61 mL, +80 mL, and +55 mL with the 75 mg, 250 mg and 750 mg doses, respectively [53]. In SIRIUS, there was a nonsignificant trend toward improvement in the FEV1 before and after bronchodilation in the mepolizumab group, with differences of +114 (p=0.15) and +128 mL (p=0.06), respectively, compared to placebo. ACQ-5 scores improved by ~0.4 points (MID=0.5 points) in both treatment arms relative to placebo. St. George’s Respiratory Questionnaire (SGRQ; quality of life) scores showed 7.0 and 6.4 point (MID=4 points) reductions (i.e. improvements in quality of life) in comparison to placebo [54].
The MUSCA trial assessed the change in quality of life with mepolizumab therapy as the primary endpoint [54]. This randomized, placebo-controlled trial included 551 patients 12 years and older and measured the change in the St. George’s Respiratory Questionnaire (SGRQ) score after 24 weeks of therapy. As a secondary endpoint, there was an improvement of +120 mL from baseline in prebronchodilator FEV1 relative to placebo at week 24. Mepolizumab treatment significantly improved SGRQ total score from baseline compared to placebo at week 24 from baseline (−15.6 vs. −7.9, a treatment difference of −7.7; minimal clinically important difference (MCID) = 4 points)).
Reslizumab:
In its two major phase III trials, reslizumab treatment improved prebronchodilator FEV1 from baseline in comparison to placebo by +126 mL for study 1, +90 mL for study 2, and +100 mL for the pooled data [57]. In a subgroup analysis, there were increasing FEV1 improvements that tracked with worsening disease severity on the basis of background medication, with the highest improvement seen in those on maintenance oral steroids. Pooled data of studies 1 and 2 showed changes of +0.23 in the AQLQ, −0.25 in the ACQ-7, and +0.05 in the asthma symptoms utility index (ASUI; MID=0.09) relative to placebo. In a separate phase III trial featuring 492 total adult patients, reslizumab showed no statistical improvement in FEV1 compared to placebo in a population unselected for blood eosinophils. A post-hoc analysis attempted to detect any further improvement in the eosinophil-high group (≥ 400 cells/μL), but was not sufficiently powered [66].
Benralizumab:
In the CALIMA trial, the eosinophil-high group demonstrated prebronchodilator FEV1 improvements of +125 and +116 mL with the q4w and q8w dosing regimens, respectfully, relative to placebo [59]. The eosinophil-high group showed a TASS reduction of −0.23 with the q8w dose in relation to placebo. In SIROCCO, the eosinophil-high patients had prebronchodilator FEV1 increases of +106 and +159 mL in the q4w and q8w regimens, respectfully, compared to placebo [60].
A long-term extension trial featuring participants of CALIMA and SIROCCO, the BORA trial, showed that improvements in prebronchodilator FEV1 were roughly maintained after 1 year [67]. The eosinophil-high group showed a comparable improvement of −0.25 relative to placebo with the 8-week dose on the TASS.
In the ZONDA trial, as a secondary outcome, prebronchodilator FEV1 improved by +256 and +222 mL relative to placebo with the q4w and q8w dosing regimens, respectively, at week 20. Yet, by week 28 there was no significant difference between treatment and placebo groups [61]. In the q8w treatment arm, ACQ-6 and AQLQ improved by −0.55 and +0.45 points, respectively, in relation to placebo.
In a 2017 Cochrane review looking at all three anti-IL5 therapies, the health-related quality of life in non-eosinophilic participants treated with benralizumab was not statistically significant. In patients with severe eosinophilic asthma, all the anti-IL5 agents showed some improvement in the health-related quality of life scores [62].
Dupilumab:
In the phase 2A study, dupilumab improved prebronchodilator FEV1 by +270 mL and reduced ACQ-5 by 0.73 points, both relative to placebo [63]. The Sino-Nasal Outcome test (SNOT-22) showed an −8.5-point change relative to placebo (MCID = −8.9; higher scores indicate poorer outcome) [68].
In QUEST, prebronchodilator FEV1 improved by +140 and +130 mL compared to placebo in the low and high doses, respectively [64]. Results were better in those with blood eosinophil counts ≥300 cells/μL, which demonstrated +210 and +240 mL improvements with the low and high doses, respectively. Patients with the higher baseline FeNO (≥ 25 to < 50 ppb or ≥ 50 ppb) showed a further beneficial effect in FEV1 improvement relative to the overall study population. In LIBERTY ASTHMA VENTURE, FEV1 was +220 mL higher in the dupilumab group than in placebo at week 24. Compared to placebo, dupilumab-treated patients showed improvements in AQLQ (+1.3, +1.3) and ACQ-5 (−0.15, −0,16) at week 52 in the low and high dose treatment arms, respectively [64].
Ability to Reduce Steroids with Biologics
Omalizumab:
Both studies 1 and 2 showed an increased ability to stop ICS use in those on omalizumab relative to placebo (Study 1: 40 vs. 19%; Study 2: 43 vs. 19%) [31, 44]. Reductions in ICS dose were 38 to 75% in treatment groups vs. 13 to 25% in placebo. A Cochrane meta-analysis showed that omalizumab treatment resulted in a significant reduction of ICS use, with improved ability to completely withdraw ICS (OR 2.50) [52].
Mepolizumab:
Bel et al. showed in 135 patients on maintenance oral steroids (SIRIUS trial) that mepolizumab enabled a 50% reduction in oral steroids compared to no change in placebo [55]. According to the dose titration schedule, patients receiving doses of ≤20 mg/day of prednisone at the end of the run-in/optimization phase were eligible to wean completely off steroids. A higher percentage on mepolizumab had a 90 to 100% dose reduction in comparison to placebo (23% vs. 11%). Participants were required to have a blood eosinophil level ≥150 cells/μl during the optimization phase (or ≥300 cells/μl in the last year), to be treated with a high dose ICS plus another controller, and at least 6 months of oral steroids use prior to enrollment. FEV1s ranged from 58 to 60% predicted, annualized exacerbation rates 2.9 to 3.3, and the geometric mean on loge scale of blood eosinophils varied from 230 to 250 cells/μl. The median oral steroid dose was 10 and 12.5 mg/day during the optimization phase for the placebo and mepolizumab arms, respectively.
Benralizumab:
Nair et al. showed in 220 patients on oral steroids (ZONDA trial) that benralizumab enabled a 75% reduction in oral steroids compared to 25% in the placebo group [61]. In comparison to 19% in the placebo group, 56% and 52% of patients on benralizumab therapy, on q4w and q8w doses, respectively, had 100% reductions in oral steroid use. According to the dose titration schedule, only patients receiving doses of ≤ 12.5 mg/day of prednisone at the end of the run-in/optimization phase were eligible to wean completely off steroids. Subjects were required to have a blood eosinophil level ≥150 cells/μl and to be treated with high dose ICS plus LABA plus oral steroids for 6 months. FEV1s were 59 to 62% predicted, annualized exacerbation rates 2.5 to 3.1, and median blood eosinophils were 437 to 535 cells/μl. The median oral steroid dose following the run-in phase was ~10 mg/day for all arms of the study.
Dupilumab:
Rabe et al. showed in 210 patients on oral steroids that dupilumab (300 mg subcutaneously every 2 weeks) enabled a 70.1% reduction in oral steroids compared to 41.9% in placebo [65]. In comparison to 25% on placebo, 48% of patients on treatment discontinued oral steroid use altogether. According to the dose titration protocol, patients receiving doses of ≤30 mg/day of prednisone at the end of the run-in/optimization phase were eligible to wean completely off steroids Subjects were required to be treated with high dose ICS plus LABA plus 1 to 2 controllers for 3 months and oral steroids for 6 months. FEV1s were 52 to 53% predicted, annualized exacerbation rates ~2, and mean blood eosinophils ranged from 325 to 370 cells/μl. The median oral steroid dose following the run-in phase was ~11 to 12 mg/day for all arms of the study.
Biomarkers that Predict Response or Change with Biologics
Omalizumab:
Omalizumab reduces free IgE by 89–99% [31, 44], but pre-treatment total IgE levels do not necessarily predict a response to omalizumab. A comparable positive response to omalizumab has been demonstrated in patients with a baseline total IgE levels outside the dosing table [47]—i.e. patients with a low baseline IgE (< 30 IU/ml) and very high IgE (> 700 IU/ml) level. High exhaled nitric oxide (eNO; > 19.5 to 25 ppb) and a high blood eosinophils (> 260 to 300 cells/μL) likely predict a likely positive response to omalizumab [47, 50].
Mepolizumab and Reslizumab:
In DREAM (mepolizumab), FeNO levels did not change with treatment or predict response to therapy [53]. Similarly, neither total IgE nor atopic status (defined as a positive radioallergosorbent test for any of four specified aeroallergens) was associated with a positive response to mepolizumab. The MENSA trial showed that blood eosinophil counts decrease 83 to 86% on mepolizumab [54]. Haldar et al. subsequently showed that blood eosinophils return to pre-treatment levels after discontinuing therapy, along with exacerbation rates [69]. Similarly, reslizumab reduced eosinophils ~93% on therapy [57]. As expected, pre-treatment blood eosinophil counts predict a positive response to both anti-IL-5 agents.
Benralizumab:
A post hoc analysis including pooled data from CALIMA and SIROCCO showed that maintenance oral steroid use, nasal polyposis, prebronchodilator FEV1 <65% predicted, ≥3 exacerbations in year prior to enrollment, and age of onset ≥18 years all associated with enhanced responsiveness to benralizumab (in patients with elevated blood eosinophils) [70]. Benralizumab reduces blood eosinophil counts to nearly zero [62].
Dupilumab:
In the phase Ila trial, there were decreases in eoxtaxin-3 (eosinophil chemokine), total IgE and FeNO on treatment [63]. Four of 52 subjects on treatment experienced increases in blood eosinophils. In QUEST, reductions were again seen with eotaxin-3, IgE and FeNO [64]. A small percentage of patients showed a transient increase in blood eosinophils. A sub-analysis showed a greater treatment benefit in those with blood eosinophils ≥150 cells/μL and FeNO ≥25 ppb.
Differences in Safety Concerns
Omalizumab:
In study 1 and study 2, omalizumab-treated subjects had higher incidences of local injection site reactions (9 to 12% treatment vs. 7 to 8% in control in the 2 studies) [31, 44]. Omalizumab caused anaphylaxis at a frequency of 0.1% (1:1000). Initial reports suggested a slight increase in malignancy (0.5 vs. 0.2%), although a 5-year observational study found that rates on treatment were similar to placebo [71]. The same observational safety study found a slight increased risk of cardiovascular and cerebrovascular events (13.4 vs. 8.1 events/1000 patient-years), but there was a disparity in both asthma severity and cardiovascular disease at baseline. The long-term safety data in omalizumab has been reassuring [71].
Mepolizumab:
Similar to omalizumab, mepolizumab-treated patients had higher incidences of local injection site reactions (8% vs. 3%). In a total of 1,327 subjects evaluated in phase III trials, 2 mepolizumab treated patients, while no placebo treated patients, developed herpes zoster. In the 2017 Cochrane review looking at all anti-IL5 therapies, there was a reduction of adverse effects with mepolizumab that was considered possible due to the beneficial effect on asthma-related adverse effects [62].
In children ages 6 to 11 with severe eosinophilic asthma, mepolizumab SC at 40 or 100mg doses (based on a body weight <40 kg or ≥40 kg, respectively) was used for 12 weeks and no new safety concerns were observed [72]. Mepolizumab drug exposures were higher and apparent clearance was lower than predicted. A positive clinical effect was observed, although the study was not designed to evaluate efficacy outcomes.
The long-term safety data for mepolizumab has been reported on 347 participants enrolled for an average of 3.5 years (COLUMBA study) [73]. The safety profile of long-term SC mepolizumab treatment was similar to that seen in the previous randomized placebo-controlled trials. The most common on-treatment adverse events (AEs) were respiratory tract infections (67%), headache (29%) and asthma worsening (27%). The most common serious AEs included asthma worsening (10%) and pneumonia (2%). Eight subjects (2%) experienced allergic/hypersensitivity reactions, although none met diagnostic criteria for anaphylaxis. Eight subjects (17 events per 1000 patient-years) experienced a herpes zoster infection. No parasitic infections were reported. Given the concern for herpes zoster, care providers are advised to consider vaccination when appropriate. For all the new biologics, there is a theoretical risk of helminth infections. The advice given for all biologics is to treat any pre-existing helminth infections prior to therapy.
Reslizumab:
Two patients (0.3%) in phase III trials across both studies had anaphylactic reactions attributed to reslizumab [57, 66]. In general, IV administration of medications increases the risk of anaphylaxis. Per prescribing information, 6/1028 (0.6%) patients receiving reslizumab had at least 1 malignant neoplasm reported compared to 2/730 (0.3%) patients in the placebo group. The long-term safety data has been reported on 1,051 patients that received > 1 reslizumab dose at 3.0 mg/kg intravenously every 4 weeks for up to 24 months (median exposure = ~330 days) [74]. The most common AEs included worsening asthma (29%), nasopharyngitis (14%), respiratory tract infections (10%), and headache (7%). The incidence of serious AEs was 8%. There were 2 drug allergy/hypersensitivity reactions, but no cases of anaphylaxis. Three patients were diagnosed with skin basal cell carcinomas. Twelve other patients were diagnosed with a malignancy, although 5 of these subjects had a history of malignancy prior to enrollment. No parasitic or suspected opportunistic infections were reported.
Benralizumab:
Hypersensitivity reactions have occurred with benralizumab. Injection site reactions have been low at 2.2% compared to 1.9% in placebo. There has been a recent case report of disseminated herpes zoster on benralizumab [75]. Significantly more patients discontinued benralizumab than placebo when comparing adverse effects leading to discontinuation, but the absolute numbers for this finding were small (36/1599 benralizumab versus 9/998 placebo) [62].
The long-term safety has been recently reported on 1,576 patients that received benralizumab q4w and q8w for up to 2 years (BORA study). An additional 633 subjects that received placebo during the clinical trials were randomly reassigned to receive benralizumab q4w and q8w in BORA. The safety profile of benralizumab was similar to that seen in the previous clinical trials. The most common on-treatment AEs were viral respiratory tract infection (14 to 16% among all groups), worsening asthma (8 to 10%), upper respiratory tract infection (6 to 8%), and headache (3 to 6%). The most common serious AEs included worsening asthma (3 to 4%) and pneumonia (~1%). One patient had an anaphylactic reaction. No parasitic or suspected opportunistic infections reported, including no reports of herpes zoster.
Dupilumab:
Compared to the other biologics, injection site reactions were relatively higher with dupilumab, reported in 15 to 18% of treated subjects compared to 5 to 10% in placebo [64]. Eosinophilia (>3,000 cells/μL) occurred in 4.1% of dupilumab patients compared to 0.6% in placebo. In QUEST, the mean percent change in blood eosinophilia from baseline peaked at week 20. It returned to normal and showed no difference between treatment arms by week 52. There was no significant difference in the median percent change in blood eosinophilia between treatment arms throughout the trial. A blood eosinophil >1,500 cells/μL at baseline was an exclusion criterion for both dupilumab phase III trials. Of 2,888 total subjects in dupilumab trials, there were a few cases of blood eosinophilia associated with symptoms. A 50-year-old male had an atypical hypersensitivity reaction several hours after his second scheduled dose, with symptoms including chills, fever, myalgia, arthralgia and slight worsening of asthma. Subsequently, he had an eosinophil count of 10,280 cells/μL and discontinued treatment. Other diagnoses or symptoms associated with high blood eosinophilia included chronic eosinophilic pneumonia, myositis plus radiculopathy, pneumonitis of moderate intensity, and a likely case of eosinophilic granulomatosis with polyangiitis (EGPA).
In the smaller steroid-sparing trial, 13% on treatment vs. 1% in placebo developed significant peripheral eosinophilia, but none developed any associated symptoms. In summary, care providers should be wary of patients presenting with a vasculitic-appearing rash, worsening pulmonary or cardiac symptoms, and/or neuropathy with peripheral eosinophilia. Closer monitoring may be warranted in those with an eosinophil count that’s high at baseline, especially during the first 5 to 6 months of treatment.
A Descriptive Comparison of Baseline Characteristics in Phase III Trials (See Table 2).
Table 2.
Comparison of Biologics by Seminal Studies
| BIOLOGIC AGENT | POPULATION | EXACERBATION RATES |
LUNG FUNCTION |
SYMPTOM SCORES |
REF. |
|---|---|---|---|---|---|
| OMALIZUMAB | Severe, allergic asthma Size: 525 Age, mean: 39.0–39.3; range: 12–74 Pre-treatment FEV1: 67.7–68.2% Time: 28 weeks |
Exacerbations per patient (during trial) SSP: −48.1% SRP: −40.9% |
Pre-BDR FEV1 (% predicted) +2.93% |
N/A |
Busse et al Phase III[31] |
| OMALIZUMAB | Stable, moderate-severe allergic asthma Size: 546 Age, mean: 39.0–40.0;range: 12–76 Pre-treatment FEV1: 69.8–69.9% Time: 28 weeks |
Exacerbations per patient (during trial) SSP: −57.6% SRP: −52.0% |
N/A | N/A | Solèr et al. [44] |
| OMALIZUMAB | Severe, poorly controlled asthma Size: 419 Age, mean: 43.3–43.4:range: 12–75 Pre-treatment FEV1:61.0–61.6% Time: 28 weeks |
Rate of exacerbations (during trial) −26%* Rate of severe exacerbations (during trial) −50% |
Pre-BDR FEV1 +0.094 FEV1 % predicted +2.8% |
AQLQ +0.45 |
INNOVATE Phase III [49] |
| OMALIZUMAB | Severe, poorly controlled, allergic asthma Size: 850 Age, mean: 43.7–45.3; range: 12–75 Pre-treatment FEV1: 64.4–65.4% Time: 48 weeks |
Rate of exacerbations (during trial) −25% |
N/A |
AQLQ(S) +0.29 Asthma Symptom Score −0.26 |
EXTRA [50] |
| MEPOLIZUMAB | Severe, uncontrolled, eosinophilic asthma Size: 616 Age, mean: 46.4–50.2, range: 12–74 Initial FEV1: 59–61% Time: 52 weeks |
Exacerbations (per patient per year) 75mg: −48% 250mg: −39% 750mg:−52% |
Pre-BDR FEV1 75mg: +0.061L 250mg: +0.081L 750mg: +0.056L |
ACQ-6 75mg: −0.16 250mg: −0.27 750mg: −0.20 AQLQ 75mg: +0.08 250mg: +0.05 750mg: +0.22 |
DREAM Phase II [53] |
| MEPOLIZUMAB | Severe, uncontrolled, eosinophilic asthma Size: 576 Age, mean: 49–51 years, range: 12–82 years Initial FEV1: 59–62% Time: 32 weeks |
Exacerbations (per patient per year) IV: −47% SC: −53% |
Pre-BDR FEV1 IV: +0.100L SC: +0.098L Post-BDR FEV1 IV: +0.146L SC: +0.138L |
ACQ-5 IV: −0.42 SC: −0.44 SGRQ IV: −6.4 SC: −7.0 |
MENSA Phase III[54] |
| RESLIZUMAB | Moderate-severe, uncontrolled, eosinophilic asthma Size: 953 Age: median: 48–49, range: 12–75 Pre-treatment FEV1: 63.6–70.4% Time: 52 weeks |
Exacerbations (per patient per year) Study 1: −50% Study 2: −59% Pooled Data: −54% |
Pre-BDR FEV1** Study 1: +0.126L Study 2: +0.090L Pooled Data: +0.11L |
AQLQ +0.23 ACQ-7 −0.25 ASUI +0.05 Pooled Data** |
Castro et al. Phase III [57] |
| BENRALIZUMAB+ | Severe, uncontrolled, eosinophilic asthma Size: 1306 Age, mean: 48.8–50.0; range 12–75 Pre-treatment FEV1: 57.7–58.9% Time: 56 weeks |
Annual Rate Ratio Q4w: −36% Q8w: −28% |
Pre-BDR FEV1** Q4w: +0.125L Q8w: +0.116L |
Total Asthma Symptom Score
Q4w: −0.12 Q8w: −0.23 ACQ-6 Scores** Q4w: −0.19 Q8w: −0.25 AQLQ(S) +12 Scores** Q4w: +0.16 Q8w: +0.24 |
CALIMA Phase III[59] |
| BENRALIZUMAB+ | Severe, uncontrolled, eosinophilic asthma Size: 1205 Age, mean: 47.6–50.1; range: 12–75 Pre-treatment FEV1: 56.1–57.4% Time: 48 weeks |
Annual Rate Ratio
Q4w: −45% Q8w: −51% |
Pre-BDR FEV1** Q4w: +0.106L Q8w: +0.159L |
Total Asthma Symptom Score**
Q4w: −0.08 Q8w: −0.25 ACQ-6** Q4w: −0.15 Q8w: −0.29 AQLQ[S]+12** Q4w: +0.18 Q8w: +0.30 |
SIROCCO Phase III [60] |
| BENRALIZUMAB | Severe, uncontrolled, eosinophilic and oral glucocorticoid- dependent asthma Size: 220 Age, mean 49.9–52.9; range: n/a Pre-treatment FEV1: 57.4–62.0% Time: 28 weeks |
Annual Exacerbation Rate Q4w: −55% Q8w: −70% |
Pre-BDR FEV1*** (week 20) Q4w: +0.256L Q8w: +0.222L |
ACQ-6
Q4w: −0.24 Q8w: −0.55 AQLQ(S)+12 Q4w: +0.23 Q8w: +0.45 |
ZONDA [61] |
| DUPILUMAB | Persistent, moderate-severe, eosinophilic asthma Size: 104 Age: mean: 37.8–41.6, range: 18–65 Pre-treatment FEV1: 72.0% Time: 12 weeks |
Occurrence of asthma exacerbation (during trial) −87% |
Pre-BDR FEV1 +0.270L |
ACQ-5 −0.73 Asthma symptom scores −0.7 SNOT-22 −8.49 |
Wenzel et al. Phase IIA [63] |
| DUPILUMAB | Uncontrolled,moderate-to-severe asthma Size: 1902 Age: mean: 47.9, range: 12+ Pre-treatment FEV1: 58.43% Time: 52 weeks |
Adjusted annualized rate of severe exacerbations Overall: 200mg: −47.7% 300mg: −46.0% Eos-high: 200mg: −65.8% 300mg: −67.4% |
Pre-BDR FEV1 Overall: 200mg: +0.14L 300mg: +0.13L Eos-high: 200mg: +0.21L 300mg: +0.24L |
AQLQ
200mg: +1.28 300mg: +1.29 ACQ-5 200mg: −0.15 300mg: −0.16 |
Castro et al. Phase 3[64] |
| DUPILUMAB | Severe, oral glucocorticoid-dependent asthma Size: 210 Age: mean: 50.7–51.9, range: >12 Pre-treatment FEV1: 51.64–52.69% Time: 24 weeks |
Annualized rate of severe exacerbation events Overall: −59% Eos-high: −71% Eos-low: −60% |
Pre-BDR FEV1** Overall: +0.22L Eos-high: +0.32L Eos-low: +0.24L |
ACQ-5** Overall: −0.47 |
Rabe et al.[65]. |
Index
Abbreviations: SSP: steroid-stable phase, SRP: steroid-reduction phase, IV: intravenous, SC: subcutaneous, Exac.: exacerbation, Sx: symptoms, Dx: diagnosis, OGCs: oral glucocorticoids, FOE: fluticasone or equivalent, BPD: beclomethasone dipropionate, HD: high dose, mo: months, adoles: adolescents, maint: maintenance, Q4w: Q4 weeks, Q8w: Q8 weeks. Eosinophil-high: ≥300 cells/μL. Eosinophil-low: <150 cells/μL. Exacerbation rate column: values are reported as “rate reduction versus placebo”, unless otherwise noted. Lung function and symptom column: values are reported as “difference from placebo”, unless otherwise noted. Un-bolded values demarcate results that did not meet statistical significance.
Key:
Post-hoc analysis
LS mean ∂ vs placebo
No significant difference by week 28 (end of study)
For almost all biologics, the number of baseline exacerbation rates in the months to years prior to enrollment correlate proportionally to the clinical response to therapy. High eosinophil counts prior to treatment also correlate strongly with positive clinical responses. Other likely predictors of positive response include lower baseline lung function, oral steroid use, and nasal polyposis. Thus, the severity of the cohort prior to enrollment should be considered during our interpretation of the trial results involving asthma biologics.
Of note, it is difficult to compare baseline eosinophil counts across these study trials since they were reported differently. Blood eosinophils were reported according to the geometric mean on loge scale (mepolizumab studies), mean (reslizumab), and median (benralizumab). QUEST reported the baseline eosinophils by mean and median, but only included values for those with a high blood eosinophil counts (≥ 300 cells/μL). It can be said that reslizumab had the highest blood eosinophils since an eosinophil count ≥ 400 cells/μL was an inclusion criterion, which is above the geometric mean, mean or median of the other biologic trials.
The omalizumab trials 1 and 2 had likely the least severe cohorts of any phase III biologic trial, with relatively high lung function (FEV1 = ~68% and ~70% in studies 1 and 2, respectively) and with no LABA or oral steroid use. The INNOVATE and EXTRA cohorts were more severe with lower lung functions (FEV1 = 61 to 62% and 64 to 65%, respectively) and more oral steroid use (20 to 23% and 16 to 17%, respectively). Exacerbation rates were similar at ~1.9 to 2.1 per year. Eosinophil counts were not reported.
Excluding the three steroid-sparing trials, the MENSA trial (mepolizumab) had arguably the most severe cohort, marked by the highest baseline exacerbation rates of any trial (3.5 to 3.8), low lung function (FEV1 = 59 to 62% predicted), and the highest percentage using daily oral steroids at 44 to 52%. According to the inclusion criteria, the CALIMA and SIROCCO eosinophil high cohorts should have had comparable baseline characteristics to MENSA, as these studies all required ≥ 2 exacerbations in the year prior, treatment with high dose ICS, and high blood eosinophilia. Although the lung function was slightly lower (FEV1s = 55 to 60% predicted), both severe exacerbation rates (2.7) and oral steroid use were notably lower (13–16%). Although the reslizumab trials had the highest blood eosinophils at baseline (~600 to 700 cells/μL), they also had relatively high lung function (FEV1 = 64 to 70%), relatively low oral steroid use (12 to 19%), and the lowest exacerbation rates (1.9).
The QUEST (dupilumab) trial had the largest cohort (n=1902), was similar to SIROCCO’s (benralizumab) and MENSA’s (mepolizumab) in lung function (FEV1 = ~58%), but none of the patients were on oral steroids. While QUEST enrolled participants regardless of eosinophil count, the primary outcomes focused on those with a high blood eosinophil counts (≥ 300 cells/μL). Since we do not have a report of baseline exacerbations per year or blood eosinophils in the eosinophil high group, a descriptive comparison with other biologic studies is more difficult. For all subjects, including eosinophil high or low subsets, there were relatively lower exacerbations per year (2.0 to 2.3) and eosinophils were moderate (mean = 349 to 391 cells/μL) at baseline.
When to Start a Biology and Which One to Choose
Biologic therapy is started when symptoms remain uncontrolled and/or exacerbation risk is high despite Global Initiative for Asthma (GINA) Step 4 therapy [76]. The GINA 2019 guidelines provide basic guidance regarding the appropriate initiation of a biologic therapy [76]. First, any patient on maintenance oral steroids for asthma should be considered for dupilumab, mepolizumab, or benralizumab therapy. Inclusion criteria for biologic studies have typically included a) prebronchodilator FEV1 between 40 to 80 % predicted, and b) ≥1 exacerbation in the year prior despite treatment with a moderate-to-high dose ICS. Thus, ensuring compliance with Step 4 therapy, obtaining full lung function testing, and verifying a course of oral steroid use (≥ 3 days) in the past year are important initial steps. Identification and treatment of comorbid factors, particularly gastroesophageal reflux and sinus disease, is necessary. It is also important to consider or rule out other conditions that may mimic asthma, such as vocal cord dysfunction, chronic obstructive pulmonary disease, bronchiectasis, tracheobronchomalacia, atypical or “walking” pneumonia, or bronchiolitis obliterans.
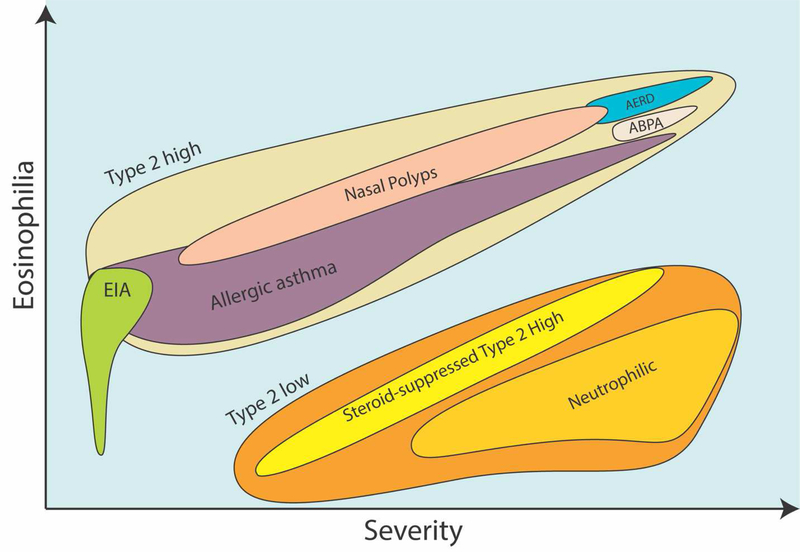
Choosing the most appropriate biologic for an individual is a topic of debate that lacks head-to-head comparisons. An objective for this review was to provide the reader with the tools to weigh and consider the evidence without our biases, over-interpretation and speculation. Nonetheless, we will provide our general thoughts and guidance when choosing a biologic while aiming for objectivity. To start, please see Figure 2 for our general conceptualization of asthma phenotypes as they relate to severity, eosinophilia and Type 2 inflammation.
Figure 2.
Relationships of Common Asthma Phenotypes to Severity and Eosinophilia.
For children under 12 years, omalizumab and mepolizumab are the only biologics that are FDA-approved. Unfortunately, there have been limited pediatric patients enrolled in asthma biologic trials, underscoring the need for new studies and careful post-marketing safety monitoring in this population. Omalizumab has the most safety data in children, although recent safety data of mepolizumab in children 6 to 11 has also been reassuring [72, 77]. Both therapies have demonstrated a strong ability to reduce exacerbations with lesser effects on lung function observed. Omalizumab has shown to reduce inhaled ICS doses. Mepolizumab has demonstrated an ability to reduce oral steroid use in adults, although chronic oral steroid use for asthma in children is rare.
For adolescents and adults without peripheral eosinophilia and not on treatment with maintenance steroids, omalizumab is likely the first choice, particularly if there has been ≥1 severe exacerbation in the last 8 months or exacerbations are related to viral infections. As indicated by the PROSPERO study, omalizumab should be considered even if IgE levels are less than 30 or greater 700 IU/ml [47]. Although, it must be stated that there is little data to support the use of omalizumab if total IgE is low and there is no sensitization to perennial allergens. The omalizumab data indicates that these patients should have a reduction in the number of clinically significant exacerbations and an improvement in quality of life. Omalizumab has the most long-term safety data and is typically less expensive than the newer agents [78, 79].
For adolescents and adults with blood eosinophilia, any of the biologics are reasonable choices, noting that reslizumab is FDA-approved only for adults older than 18 years of age. If a care provider’s aim is to reduce clinically significant exacerbations, it is our opinion that dupilumab, mepolizumab and reslizumab have the strongest supporting data. Mepolizumab has also demonstrated an ability to reduce ED visits and hospitalizations.
If the goal is to improve lung function, dupilumab therapy has demonstrated the largest numeric improvements in prebronchodilator FEV1 % predicted compared to placebo, with consistent results across all phase II and phase III trials, including the steroid-sparing trial (QUEST LIBERTY VENTURE). As discussed above, this is likely because of the known structural changes in the airway due to chronic IL-4 and IL-13 stimulation. Benralizumab also demonstrated improvements in lung function in two phase III trials (SIROCCO, CALIMA) and its long-term safety trial (BORA). In the benralizumab steroid-sparing trial (ZONDA), there was an improvement in FEV1 at week 20, but by week 28 there was no significant difference between treatment and placebo groups. Reslizumab showed improvements in lung function in both of phase III trials. Mepolizumab showed improvements in lung function in MENSA, but not DREAM or SIRIUS.
If the objective is to reduce daily oral steroids, benralizumab and dupilumab have the strongest supporting data, with roughly 50% of subjects in both trials demonstrating an ability to stop steroids altogether. Of note, there was a strong difference across studies in the ability to reduce steroids among the placebo groups, which makes comparisons between these three steroid-sparing trials challenging. There were also differences, according to protocol, in the percent of patients that were eligible to be completely weaned off steroids. E.g. in the benralizumab study, only patients on ≤12.5 mg/day of prednisone at the end of the run-in/optimization phase were eligible to wean completely off steroids vs. 30 mg in the dupilumab study.
It is our preference that patients with aspirin exacerbated respiratory disease (AERD) or atopic dermatitis (AD) are treated with dupilumab, which is likely to treat each of these comorbid conditions. AERD is known for markedly high Type 2 inflammation in upper and lower airways (See Figure 2), while dupilumab has demonstrated an ability to improve nasal congestion and symptom scores, reduce polyp size, reduce steroid use and surgery in patients with chronic rhinosinusitis with nasal polyps (CRSwNP; SINUS-24 trial phase III trial) [80]. Patients with atopic dermatitis and asthma should be treated on the 300 mg dose of dupilumab, which is the approved treatment dose for AD. Although dupilumab is the only biologic that is currently FDA-approved for the treatment of nasal polyposis, two recent phase II studies showed that mepolizumab therapy reduced nasal polyp score and the need for surgery in subjects with severe bilateral nasal polyposis [81, 82]. Although the results of phase III studies of mepolizumab in nasal polyposis are pending, it is likely that either mepolizumab or dupilumab are reasonable choices for these patients.
Benralizumab, dosed SC only every two months after an initial loading phase, has the easiest dosage regimen, followed by mepolizumab (SC monthly), dupilumab (SC twice monthly), and reslizumab (IV monthly). Dupilumab appears to cause more injection site reactions. For patients with very high severity and inadequate response to one biologic, some physicians are trialing combinations of biologics, such as a Type 2 (e.g. dupilumab) plus one of the IL-5 pathway agents. This practice is without supporting evidence or safety data. For obese patients, a common clinical practice is to consider reslizumab in place of the other I L-5 agents since it is dosed based on weight. This practice is also speculative at this point.
CONCLUSION
Understanding the details and differences in asthma biologic trial designs, patient cohorts, and in study results will help care providers make more informed decisions when choosing a biologic. We are hopeful this review will serve as reference to care providers for this purpose.
Key Messages.
While five biologic therapies have FDA-approved indications for difficult-to-control asthma, the clinical trials that proved the efficacy and safety of these biologics were similar in their inclusion criteria, study protocols, and measured outcomes. Initial trial results are now being re-analyzed and re-interpreted in subsets of asthma patients that demonstrate enhanced responses. As a result, it has become increasingly difficult to keep up with the growing body of literature surrounding asthma biologic therapy. This review summarizes and compares trial designs, patient cohorts and study results of the major trials involving these therapies.
Due to variations in inclusion criteria and natural variations in enrolled cohorts, the baseline clinical traits and severity of study populations in asthma biologic trials differed significantly. E.g. baseline annualized exacerbation rates in the year prior to enrollment and blood eosinophilia, which are both strong predictors of a biologic’s success, differed strongly among populations.
Early omalizumab efficacy trials did not stratify subjects by blood eosinophils or include patients on long-acting beta agonists or oral steroids but showed relative reductions in exacerbation rates comparable to the newer asthma biologics among less severe cohorts.
If a care provider’s aim is to reduce clinically significant exacerbations, it is our opinion that dupilumab, mepolizumab and reslizumab have the strongest supporting data. Mepolizumab has also demonstrated an ability to reduce ED visits and hospitalizations.
If the goal is to improve lung function, dupilumab therapy has demonstrated the largest numeric improvements in prebronchodilator FEV1 % predicted compared to placebo, with consistent results across all phase II and phase III trials, including its steroid-sparing trial (QUEST LIBERTY VENTURE). Benralizumab also demonstrated improvements in lung function in two phase III trials (SIROCCO, CALIMA) and its long-term safety trial (BORA).
If the objective is to reduce daily oral steroids, benralizumab and dupilumab have the strongest supporting data, with roughly 50% of subjects in both trials demonstrating an ability to stop steroids altogether. Of note, there was a strong difference across studies in the ability to reduce steroids among the placebo groups, which makes comparisons between these three steroid-sparing trials challenging.
Acknowledgments
Funding sources: K23 HL144418 funding through NHLBI provided for Brian Modena.
Footnotes
Author Disclosures: Brian Modena receives research support from the NIH and Industry (Self Care Catalysts, Propeller Health). He has participated in advisory boards and/or speaker bureaus for the following companies: AstraZeneca, Circassia, Teva, Regeneron, and Sanofi. All other authors have nothing relevant to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loftus PA and Wise SK, Epidemiology of asthma. Curr Opin Otolaryngol Head Neck Surg, 2016. 24(3): p. 245–9. [DOI] [PubMed] [Google Scholar]
- 2.Loftus PA and Wise SK, Epidemiology and economic burden of asthma. Int Forum Allergy Rhinol, 2015. 5 Suppl 1: p. S7–10. [DOI] [PubMed] [Google Scholar]
- 3.Nurmagambetov T, Kuwahara R, and Garbe P, The Economic Burden of Asthma in the United States, 2008–2013. Annals of the American Thoracic Society, 2018. 15(3): p. 348–356. [DOI] [PubMed] [Google Scholar]
- 4.Chung KF, et al. , International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. European Respiratory Journal, 2014. 43(2): p. 343. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan SD, et al. , Extent, patterns, and burden of uncontrolled disease in severe or difficult-to-treat asthma. Allergy, 2007. 62(2): p. 126–33. [DOI] [PubMed] [Google Scholar]
- 6.Haselkorn T, et al. , Consistently very poorly controlled asthma, as defined by the impairment domain of the Expert Panel Report 3 guidelines, increases risk for future severe asthma exacerbations in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol, 2009. 124(5): p. 895–902.e1–4. [DOI] [PubMed] [Google Scholar]
- 7.Wenzel SE, Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med, 2012. 18(5): p. 716–25. [DOI] [PubMed] [Google Scholar]
- 8.Doherty TA and Broide DH, Group 2 innate lymphoid cells: new players in human allergic diseases. J Investig Allergol Clin Immunol, 2015. 25(1): p. 1–11; quiz 2p following 11. [PMC free article] [PubMed] [Google Scholar]
- 9.Woodruff PG, et al. , Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A, 2007. 104(40): p. 15858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodruff PG, et al. , T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med, 2009. 180(5): p. 388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modena BD, et al. , Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am J Respir Crit Care Med, 2014. 190(12): p. 1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larose MC, et al. , Correlation between CCL26 production by human bronchial epithelial cells and airway eosinophils: Involvement in patients with severe eosinophilic asthma. J Allergy Clin Immunol, 2015. 136(4): p. 904–13. [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg ME and Hogan SP, The eosinophil. Annu Rev Immunol, 2006. 24: p. 147–74. [DOI] [PubMed] [Google Scholar]
- 14.Fehrenbach H, Wagner C, and Wegmann M, Airway remodeling in asthma: what really matters. Cell and tissue research, 2017. 367(3): p. 551–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenzel S, Severe asthma in adults. Am J Respir Crit Care Med, 2005. 172(2): p. 149–60. [DOI] [PubMed] [Google Scholar]
- 16.Berry M, et al. , TNF-alpha in asthma. Curr Opin Pharmacol, 2007. 7(3): p. 279–82. [DOI] [PubMed] [Google Scholar]
- 17.Brightling C, Berry M, and Amrani Y, Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol, 2008. 121(1): p. 5–10; quiz 11–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allakhverdi Z, et al. , Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med, 2007. 204(2): p. 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsilingiri K, Fornasa G, and Rescigno M, Thymic Stromal Lymphopoietin: To Cut a Long Story Short. Cell Mol Gastroenterol Hepatol, 2017. 3(2): p. 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Divekar R. and Kita H, Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr Opin Allergy Clin Immunol, 2015. 15(1): p. 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouzaki H, et al. , Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol, 2013. 49(5): p. 741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prefontaine D, et al. , Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol, 2010. 125(3): p. 752–4. [DOI] [PubMed] [Google Scholar]
- 23.Chan BCL, et al. , IL33: Roles in Allergic Inflammation and Therapeutic Perspectives. Frontiers in Immunology, 2019. 10(364). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito T, et al. , TSLP-activated dendritic cells induce an inflammatory Thelper type 2 cell response through OX40 ligand. J Exp Med, 2005. 202(9): p. 1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker S, et al. , Anti-IgE for chronic asthma. Cochrane Database Syst Rev, 2003(3): p. CD003559. [DOI] [PubMed] [Google Scholar]
- 26.Hochhaus G, et al. , Pharmacodynamics of omalizumab: implications for optimised dosing strategies and clinical efficacy in the treatment of allergic asthma. Curr Med Res Opin, 2003. 19(6): p. 491–8. [DOI] [PubMed] [Google Scholar]
- 27.Casale TB, et al. , Use of an anti-IgE humanized monoclonal antibody in ragweed- induced allergic rhinitis. J Allergy Clin Immunol, 1997. 100(1): p. 110–21. [DOI] [PubMed] [Google Scholar]
- 28.Galli SJ and Tsai M, IgE and mast cells in allergic disease. Nat Med, 2012. 18(5): p. 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modena BD, Dazy K, and White AA, Emerging concepts: mast cell involvement in allergic diseases. Transl Res, 2016. 174: p. 98–121. [DOI] [PubMed] [Google Scholar]
- 30.Pelaia G, et al. , Update on optimal use of omalizumab in management of asthma. J Asthma Allergy, 2011. 4: p. 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busse W, et al. , Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol, 2001. 108(2): p. 184–90. [DOI] [PubMed] [Google Scholar]
- 32.Teach SJ, et al. , Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. The Journal of allergy and clinical immunology, 2015. 136(6): p. 1476–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durrani SR, et al. , Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol, 2012. 130(2): p. 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorman SC, et al. , Sputum CD34+IL-5Ralpha+ cells increase after allergen: evidence for in situ eosinophilopoiesis. Am J Respir Crit Care Med, 2004. 169(5): p. 573–7. [DOI] [PubMed] [Google Scholar]
- 35.Lampinen M, et al. , Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy, 2004. 59(8): p. 793–805. [DOI] [PubMed] [Google Scholar]
- 36.Tan LD, et al. , Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy, 2016. 9: p. 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolbeck R, et al. , MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol, 2010. 125(6): p. 1344–1353.e2. [DOI] [PubMed] [Google Scholar]
- 38.Kotsimbos AT and Hamid Q, IL-5 and IL-5 receptor in asthma. Mem Inst Oswaldo Cruz, 1997. 92 Suppl 2: p. 75–91. [DOI] [PubMed] [Google Scholar]
- 39.Rothenberg ME, Eosinophilia. N Engl J Med, 1998. 338(22): p. 1592–600. [DOI] [PubMed] [Google Scholar]
- 40.Kau AL and Korenblat PE, Anti-interleukin 4 and 13 for asthma treatment in the era of endotypes. Curr Opin Allergy Clin Immunol, 2014. 14(6): p. 570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akdis CA, Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med, 2012. 18(5): p. 736–49. [DOI] [PubMed] [Google Scholar]
- 42.Bagnasco D, et al. , A Critical Evaluation of Anti-IL-13 and Anti-IL-4 Strategies in Severe Asthma. Int Arch Allergy Immunol, 2016. 170(2): p. 122–31. [DOI] [PubMed] [Google Scholar]
- 43.Gandhi NA, et al. , Targeting key proximal drivers of type 2 inflammation in disease. Nature Reviews Drug Discovery, 2015. 15: p. 35. [DOI] [PubMed] [Google Scholar]
- 44.Solèr M, et al. , The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. 2001. [DOI] [PubMed] [Google Scholar]
- 45.Cantero D, et al. , Staphylococcus aureus biofilm activates the nucleotide-binding oligomerization domain containing 2 (Nod2) pathway and proinflammatory factors on a human sinonasal explant model. Int Forum Allergy Rhinol, 2013. 3(11): p. 877–84. [DOI] [PubMed] [Google Scholar]
- 46.Casale TB, et al. , Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy, 2018. 73(2): p. 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casale TB, et al. , Omalizumab Effectiveness by Biomarker Status in Patients with Asthma: Evidence From PROSPERO, A Prospective Real-World Study. J Allergy Clin Immunol Pract, 2019. 7(1): p. 156–164.e1. [DOI] [PubMed] [Google Scholar]
- 48.Humbert M, et al. , Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. The European respiratory journal, 2018. 51(5): p. 1702523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humbert M, et al. , Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy, 2005. 60(3): p. 309–16. [DOI] [PubMed] [Google Scholar]
- 50.Hanania NA, et al. , Omalizumab in Severe Allergic Asthma Inadequately Controlled With Standard Therapy: A Randomized Trial. Annals of Internal Medicine, 2011. 154(9): p. 573–582. [DOI] [PubMed] [Google Scholar]
- 51.Hanania NA, et al. , Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med, 2013. 187(8): p. 804–11. [DOI] [PubMed] [Google Scholar]
- 52.Omalizumab for asthma in adults and children - Normansell, R - 2014 | Cochrane Library. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pavord ID, et al. , Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. The Lancet, 2012. 380(9842): p. 651–659. [DOI] [PubMed] [Google Scholar]
- 54.Ortega HG, et al. , Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med, 2014. 371(13): p. 1198–207. [DOI] [PubMed] [Google Scholar]
- 55.Bel EH, et al. , Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med, 2014. 371(13): p. 1189–97. [DOI] [PubMed] [Google Scholar]
- 56.Powell C, et al. , Mepolizumab versus placebo for asthma. Cochrane Database Syst Rev, 2015(7): p. Cd010834. [DOI] [PubMed] [Google Scholar]
- 57.Castro M, et al. , Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med, 2015. 3(5): p. 355–66. [DOI] [PubMed] [Google Scholar]
- 58.Castro M, et al. , Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med, 2014. 2(11): p. 879–890. [DOI] [PubMed] [Google Scholar]
- 59.FitzGerald JM, et al. , Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet, 2016. 388(10056): p. 2128–2141. [DOI] [PubMed] [Google Scholar]
- 60.Bleecker ER, et al. , Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet, 2016. 388(10056): p. 2115–2127. [DOI] [PubMed] [Google Scholar]
- 61.Nair P, et al. , Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. N Engl J Med, 2017. 376(25): p. 2448–2458. [DOI] [PubMed] [Google Scholar]
- 62.Farne HA, et al. , Anti-IL5 therapies for asthma. Cochrane Database Syst Rev, 2017. 9: p. Cd010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wenzel S, et al. , Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med, 2013. 368(26): p. 2455–66. [DOI] [PubMed] [Google Scholar]
- 64.Castro M, et al. , Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med, 2018. 378(26): p. 2486–2496. [DOI] [PubMed] [Google Scholar]
- 65.Rabe KF, et al. , Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N Engl J Med, 2018. 378(26): p. 2475–2485. [DOI] [PubMed] [Google Scholar]
- 66.Corren J, et al. , Phase 3 Study of Reslizumab in Patients With Poorly Controlled Asthma: Effects Across a Broad Range of Eosinophil Counts. Chest, 2016. 150(4): p. 799–810. [DOI] [PubMed] [Google Scholar]
- 67.Busse WW, et al. , Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med, 2019. 7(1): p. 46–59. [DOI] [PubMed] [Google Scholar]
- 68.Hopkins C, Rudmik L, and Lund VJ, The predictive value of the preoperative Sinonasal Outcome Test-22 score in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope, 2015. 125(8): p. 1779–84. [DOI] [PubMed] [Google Scholar]
- 69.Haldar P, et al. , Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J Allergy Clin Immunol, 2014. 133(3): p. 921–3. [DOI] [PubMed] [Google Scholar]
- 70.FitzGerald JM, et al. , Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med, 2018. 6(1): p. 51–64. [DOI] [PubMed] [Google Scholar]
- 71.FDA Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. 2019. [Google Scholar]
- 72.Gupta A, et al. , Subcutaneous mepolizumab in children aged 6 to 11 years with severe eosinophilic asthma. Pediatr Pulmonol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khatri S, et al. , Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol, 2019. 143(5): p. 1742–1751.e7. [DOI] [PubMed] [Google Scholar]
- 74.Murphy K, et al. , Long-term Safety and Efficacy of Reslizumab in Patients with Eosinophilic Asthma. J Allergy Clin Immunol Pract, 2017. 5(6): p. 1572–1581.e3. [DOI] [PubMed] [Google Scholar]
- 75.Mishra AK, Sahu KK, and James A, Disseminated herpes zoster following treatment with benralizumab. Clin Respir J, 2019. 13(3): p. 189–191. [DOI] [PubMed] [Google Scholar]
- 76.The Global Asthma Report 2014. 2014, Global Asthma Network: Auckland, New Zealand. [Google Scholar]
- 77.Gupta A, et al. , Long-term safety and pharmacodynamics of mepolizumab in children with severe asthma with an eosinophilic phenotype. Journal of Allergy and Clinical Immunology. [DOI] [PubMed] [Google Scholar]
- 78.Zhou B, et al. , Tolerability, pharmacokinetics and pharmacodynamics of CMAB007, a humanized anti-immunoglobulin E monoclonal antibody, in healthy Chinese subjects, in MAbs. 2012. p. 110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jacobs I, et al. , Monoclonal Antibody and Fusion Protein Biosimilars Across Therapeutic Areas: A Systematic Review of Published Evidence. BioDrugs, 2016. 30(6): p. 489–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han JK, Bachert Claus, Desrosiers Martin, Laidlaw Tanya M., Hopkins Claire, Fokkens Wytske J., Paggiaro Pierluigi, Seong Ho Cho Heidi Olze, Greos Leon S., Zhang Mei, Fan Chunpeng, Draikiwicz Steven, Amin Nikhil, Kamat Siddhesh, Khan Asif, Pirozzi Gianluca, Graham Neil M. H., Ruddy Marcella, Staudinger Heribert, Mannent Leda P., Efficacy and Safety of Dupilumab in Patients with Chronic Rhinosinusitis with Nasal Polyps: Results from the Randomized Phase 3 Sinus-24 Study. Journal of Allergy and Clinical Immunology, 2019. 143(2): p. AB422. [Google Scholar]
- 81.Gevaert P, et al. , Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. Journal of Allergy and Clinical Immunology, 2011. 128(5): p. 989–995.e8. [DOI] [PubMed] [Google Scholar]
- 82.Bachert C, et al. , Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. Journal of Allergy and Clinical Immunology, 2017. 140(4): p. 1024–031.e14. [DOI] [PubMed] [Google Scholar]