Abstract
Activation of renin-angiotensin- system, nitric oxide (NO•) bioavailability and subsequent sympathoexcitation plays a pivotal role in the pathogenesis of many cardiovascular diseases, including hypertension. Previously we have shown increased protein expression of PIN (a protein inhibitor of nNOS: neuronal nitric oxide synthase, known to dissociate nNOS dimers into monomers) with concomitantly reduced levels of catalytically active dimers of nNOS in the PVN of rats with heart failure. To elucidate the molecular mechanism by which Angiotensin II (Ang II) increases PIN expression, we used Sprague-Dawley rats (250–300 g) subjected to intracerebroventricular infusion of Ang II (20 ng/min, 0.5 μl/h) or saline as vehicle (Veh) for 14 days through osmotic mini-pumps and NG108-15 hybrid neuronal cell line treated with Ang II as an in vitro model. Ang II infusion significantly increased baseline renal sympathetic nerve activity and mean arterial pressure. Ang II infusion increased the expression of PIN (1.24 ± 0.04* Ang II vs. 0.65 ± 0.07 Veh) with a concomitant 50% decrease in dimeric nNOS and PIN-Ub conjugates (0.73 ± 0.04* Ang II vs. 1.00 ± 0.03 Veh) in the PVN. Substrate-dependent ligase assay in cells transfected with pCMV-(HA-Ub)8 vector revealed a reduction of HA-Ub-PIN conjugates after Ang II and a proteasome inhibitor, Lactacystin (LC), treatment (4.5 ± 0.7* LC Ang II vs. 9.2 ± 2.5 LC). TUBE (Tandem Ubiquitin-Binding Entities) assay showed decrease PIN-Ub conjugates in Ang II-treated cells (0.82 ± 0.12* LC Ang II vs. 1.21 ± 0.06 LC) while AT1R blocker, Losartan (Los) treatment diminished the Ang II-mediated stabilization of PIN (1.21 ± 0.07 LC Los vs. 1.16 ± 0.04* LC Ang II Los). Taken together, our studies suggest that increased central levels of Ang II contribute to the enhanced expression of PIN leading to reduced expression of the dimeric form of nNOS, thus diminishing the inhibitory action of NO• on pre-autonomic neurons in the PVN resulting in increased sympathetic outflow.
Keywords: nNOS, Angiotensin II, Paraventricular nucleus, Neurogenic hypertension
1. Introduction
Reduced bioavailability of nitric oxide (NO•) concomitant with increased sympathetic outflow is implicated in the pathogenesis of many cardiovascular diseases, including hypertension, heart failure, and diabetes [1-5]. Further, hypertensive patients and animal models of hypertension have demonstrated increased sympathetic nerve activity (SNA), accompanied by alterations in body fluid homeostasis [6]. The NO•-pathway contributes to the blood pressure-lowering effect of many commonly used therapeutic agents and emerging basic science research on this pathway is elucidating potential new antihypertensive drug targets. NO• is a gaseous paracrine molecule produced by a family of NADPH-dependent enzymes named nitric oxide synthases (NOS) [7-9]. NO• acts as a pivotal signaling molecule that regulates blood flow and tissue oxygenation by activating soluble guanylate cyclase in the vascular smooth muscle [10]. NO• produced by the neuronal isoform of NOS (nNOS) plays an essential role in the paraventricular nucleus (PVN) via acting as a retrograde inhibitory neurotransmitter that regulates synaptic efficacy and modulates neuronal activity leading to changes in SNA and consequently neurogenic control of blood pressure [11-13]. Of the three NOS isoforms, nNOS is the largest, due to a 300-amino-acid PDZ (PSD-95/Discs large/zona occludens-1) domain insertion at the N-terminus targeting nNOS to the postsynaptic density (PSD) protein in the synaptic spines of neurons [14]. The catalytic activity of nNOS is regulated by several other scaffolding proteins such as syntrophin, PSD93, PSD95 and CAPON (carboxy-terminal PDZ ligand of nNOS) which have been identified to interact with nNOS in neurons and modify its activity. In neurons, another nNOS-interacting protein the dynein light chain, LC8 (DYNLL1) or PIN (protein inhibitor of nNOS) physically interacts with nNOS and inhibits its activity [15]. PIN is a highly conserved, small homodimer cytosolic protein that is reported to bind to a 17-residue peptide fragment from Met-228 to His-244 of nNOS [16] and destabilizes the nNOS homodimer [15], a conformation necessary for the catalytic activity of the enzyme. Monomerization of nNOS promotes ubiquitination and degradation of nNOS by the proteasomal pathway [17,18]. Previously, we have shown that there is a decrease in nNOS expression [19] and enhanced expression of CAPON [20] and PIN [4] in the PVN of rats with heart failure. Moreover, Losartan (Los) an AT1R blocker treatment restores the elevated SNA and normalizes the changes in expression of nNOS, CAPON, and PIN suggesting that the AT1 receptor activation may be linked to the reciprocal changes in these regulators(CAPON and PIN) and nNOS in the PVN [4,20]. Furthermore, we described the in vitro molecular mechanism of downregulation of nNOS involving interactions between nNOS and PIN through an Ang II-mediated signaling pathway(s) and suggested that post-translational processes, such as protein degradation/stabilization are involved in Ang II-dependent downregulation of nNOS [4]. To further understand how Ang II to PIN link, regulates nNOS, we dissect the molecular mechanism of increased PIN expression in a well-established model of neurogenic hypertension produced by intracerebroventricular infusion of Ang II for two weeks.
2. Methods
2.1. Animal model
Male Sprague-Dawley rats weighing between 250 and 280 g (Sasco Breeding Laboratories, Omaha, NE) were fed and housed according to institutional guidelines. The University of Nebraska Medical Center Institutional Animal Care and Use Committee approved all protocols (IACUC protocol # 14-036-07-FC) which adhere to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, Eighth Edition (National Academic Press; 2011).
2.2. Intracerebroventricular (ICV) infusion of Ang II
Rats were randomly assigned to one of two groups: vehicle or Ang II and were anesthetized with a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg) given intraperitoneally, and then subjected to stereotaxic implantation of a cannula directed to the lateral ventricle. The stereotaxic coordinates for the placement of the tip of the cannula were, 0.8mm caudal to bregma, 1.5mm lateral to the midline and 4.0mm ventral to the dorsal surface of the skull, according to the atlas of Paxinos & Watson [21]. The cannula was connected to an osmotic minipump (Alzet, model 2002) for ICV infusion of Ang II (20 ng/min, 0.5 μl/h) or isotonic sterile saline as vehicle control.
2.3. Arterial blood pressure, heart rate, and renal sympathetic nerve activity (RSNA) recording
Experiments were performed at the end of 14 days of ICV Ang II infusion. Rats were anesthetized using urethane (0.75 g/kg ip) and α-chloralose (70 mg/kg ip). The left femoral vein was cannulated with polyethylene tubing (PE-50) for the injection of supplemental anesthesia. The left femoral artery was cannulated and connected via a pressure transducer (Gould P23 1D) to a computer-based data-recording and -analyzing program (Power Lab) to record mean arterial pressure and heart rate (MAP and HR). The left kidney was exposed through a retroperitoneal flank incision, and RSNA was recorded according to previously described method [20,22,23]. After completion of the RSNA recording, rats were injected with KCl (75–150 mg/kg, iv) and then background noise was determined. The level of RSNA was calculated by subtracting the background noise from the actual recorded value and normalized by the maximal RSNA as calculated previously [20,23].
2.4. Micro punch of the PVN area
The brain was removed from the euthanized animal and quickly frozen on dry ice. Six serial coronal sections (100 μm/section) were cut through the hypothalamus at the level of the PVN with a cryostat. The PVN tissue was obtained using the micro-punch method of Palkovit and Brownstein [24] as described previously [3,20,22] using a diethylpyrocarbonate-treated blunt 18-gauge needle attached to a syringe.
2.5. Cell culture model
NG108-15 cells (neuroblastoma X glioma) (ATCC HB-12317) cells (passage 10–15) were grown in Dulbecco's modified Eagle's medium (DMEM) without pyruvate, supplemented with 0.1mM hypoxanthine, 400 nM aminopterin, 0.016mM thymidine, 1.5 g/L sodium bicarbonate, 10% fetal bovine serum, 10% streptomycin, and penicillin solution (GIBCO). The cultures were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37 °C.
2.6. Immunoblotting
Western blotting was performed on lysates of PVN punches or cell culture samples. After the animal was euthanized by an overdose of pentobarbital (65 mg/kg/ip), the brain was removed and quickly frozen on dry ice. Total protein in PVN lysates was extracted with a radioimmunoprecipitation assay buffer (10mM Tris, 1mM EDTA, 1% SDS, 0.1% Triton X-100) supplemented with protease inhibitors. The protein lysates (20–30 μg), mixed with SDS-PAGE buffer were fractionated on 12% polyacrylamide gel and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore). Non-fat dry milk (5% w/v) in TBST (10mM Tris, 150mM NaCl, 0.05% Tween-20) was used to block membrane at ambient temperature for 1 h. Then the membrane was incubated with the appropriate primary antibody overnight, anti-PIN (rabbit) (Santa Cruz; sc-13969) and anti-actin (rabbit) (Sigma; A-2066), followed by the corresponding peroxidaseconjugated secondary antibody for 1 h. An enhanced chemiluminescence substrate (Pierce Chemical, Rockford, IL) was used to visualize the signals, which were detected by Worklab digital image system. Image J (NIH) was used to quantify the signal.
2.7. Assay of nNOS dimers
Dimeric nNOS (active form) was determined using Low-Temperature Polyacrylamide Gel Electrophoresis (LT-PAGE) following the Klatt et al. [25] technique and as described previously [4,22]. The procedure was performed as mentioned above using a 5% separating gel except that the electrophoresis was done in a cold room at 25 V, and gel and buffers were pre-equilibrated to 4 °C. After LT-PAGE, the gel was transferred to PVDF membrane overnight in cold room at 30 V and probed with nNOS specific antibody. Tubulin was used as a housekeeping gene for this experiment.
2.8. TUBE (Tandem Ubiquitin-Binding Entities) assays
TUBE assays using Agarose TUBE (UM1401, Life Sensors) were performed on PVN lysates or NG108 cells treated with proteasome inhibitor Lactacystin (LC, 10 mM) alone or with Ang II or Ang II and Los as per the manufacturer instructions. TUBE allow poly-ubiquitylated proteins to be efficiently purified from cell extracts in native conditions protecting from proteasomal degradation and de-ubiquitination [26]. Briefly, punched PVN tissues or NG108 cells were sonicated in 100 μl of lysis buffer (50mM Tris-HCL, pH 7.5, 0.15M NaCl, 1mM EDTA, 1% 1% Nonidet P-40,10% glycerol) supplemented with complete protease inhibitor cocktail, 10mM sodium orthovanadate, 5mM N-ethylmaleimide, 10mM PR-619 and 1,10-phenanthroline to pull down polyubiquitinated proteins. The cell lysates (30–40 μg) were precleared by centrifugation, and the supernatants normalized for protein content were incubated with 20 μl of pre-equilibrated TUBE2-agarose beads in 20mM Tris (pH 8.0), 0.15M NaCl, and 0.1% Tween-20 with rotation overnight at 4 °C. The immune complexes were washed twice with TBST, eluted with 25 μl 2X Laemmeli buffer, and subjected to SDS PAGE and immunoblot analysis.
2.9. In vivo substrate-dependent ubiquitin ligase assay
This protocol was done according to the method of Furukawa [27] using NG108 cells transiently transfected with pCMV-(HA-Ub)8 using Lipofectamine 3000 (Invitrogen, Carlsbad, CA) according to the manufacturer instructions. Twenty-four-hour post-transfection, cells were treated with 100 μM Ang II for 18 h followed by LC (10 μM) for an additional 6 h to accumulate the high molecular weight polyubiquitinated forms. Cells were sonicated in 100 μl of lysis buffer, as mentioned in section 2.8. Immunoprecipitation using PIN antibody was carried out on total cell lysates (400 μg) [4] followed by immunoblotting with HA antibodies to detect PIN-ubiquitinated species.
2.10. Statistical analyses
Data were expressed as mean ± SEM, and statistical significance was determined at p ≤ 0.05. Statistical comparisons of the groups were made using a Student's t-test, one- or two-way analysis of variance (ANOVA) followed by Bonferroni's test for posthoc analysis using the Prism 7; GraphPad Software.
3. Results
In the present study, we used the ICV infusion of Ang II as a model of hypertension, which has been previously used by numerous investigators to produce hypertension with evidence for a strong contribution of the neurogenic component [28-31]. As anticipated, ICV infusion of Ang II-induced an increase in mean arterial pressure compared to vehicle group (126 ± 9 Ang II vs. 84 ± 4mmHg) and increased baseline RSNA (20.5 ± 2.3 Ang II vs. 6.4 ± 1.9% n=5 and 4, respectively of Maximal activity) characteristic of neurogenic hypertension.
3.1. Expression of PIN within the PVN
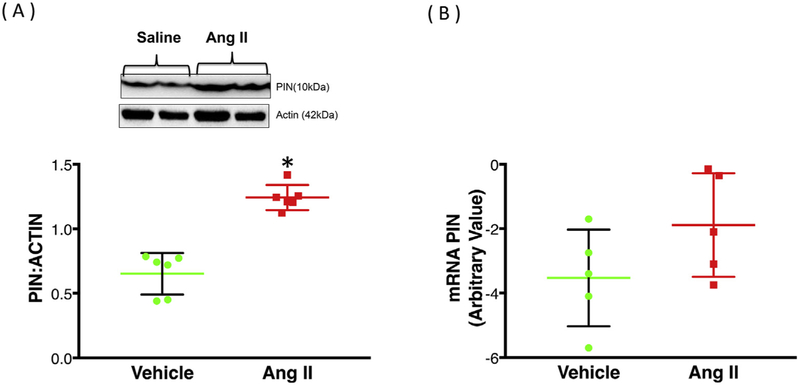
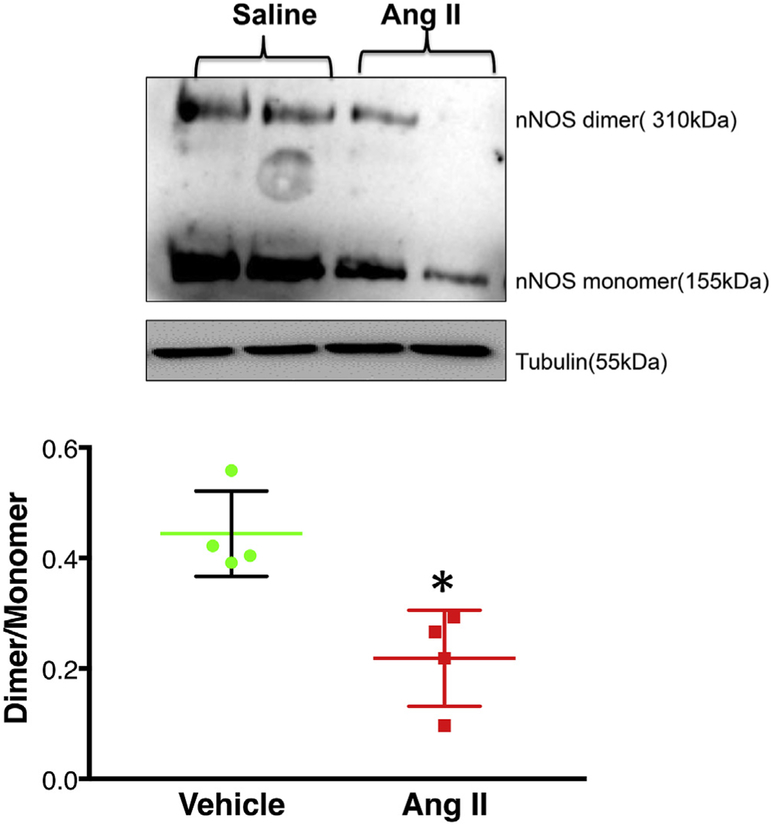
PIN protein expression was significantly increased (~47%) in the PVN of Ang II-infused rats compared to vehicle controls (1.28 ± 0.06 Ang II vs. 0.71 ± 0.05 Veh) (Fig. 1A). Interestingly, the fold changes in PIN mRNA levels as determined by RT-PCR were not significantly different in the PVN of Ang II-infused rats compared to vehicle-treated controls (1.00 ± 0.67 Veh vs. 0.76 ± 0.72 Ang II) (Fig. 1B). Next, we examined the level of monomeric and dimeric forms of nNOS in PVN lysates using LT-PAGE so that the SDS-resistant dimeric form of nNOS could be measured (Fig. 2). In the vehicle group, baseline nNOS levels both monomeric, as well as dimeric, are high possibly due to moderate levels of PIN. However, we observed a significant decrease in dimer/ monomer ratio of nNOS in the PVN of Ang II-infused rats compared to vehicle controls (0.44 ± 0.04 Veh vs. 0.22 ± 0.05 Ang II).
Fig. 1.
Chronic icv Ang II infusion increases PIN expression post-transcriptionally. (A) Western blot analysis of PIN in the PVN of vehicle and Ang II-infused group. Top: a representative Western blot, bottom: densitometric analyses of PIN level normalized to Actin. (B) PIN mRNA expression in the PVN of vehicle and Ang II-infused group. Values are mean ± SEM (n = 5–6 rats per group). *P < 0.05 vs. vehicle.
Fig. 2.
Chronic icv Ang II infusion destabilizes nNOS dimers in the PVN. Top panel: Representative LT-PAGE blot showing dimer and monomer of nNOS, bottom panel: densitometry analyses of monomer and dimer nNOS levels represented as a ratio of the dimer to the monomer. Values are mean ± SEM (n = 4 rats per group). *P < 0.05 vs. vehicle.
3.2. PIN undergoes ubiquitination
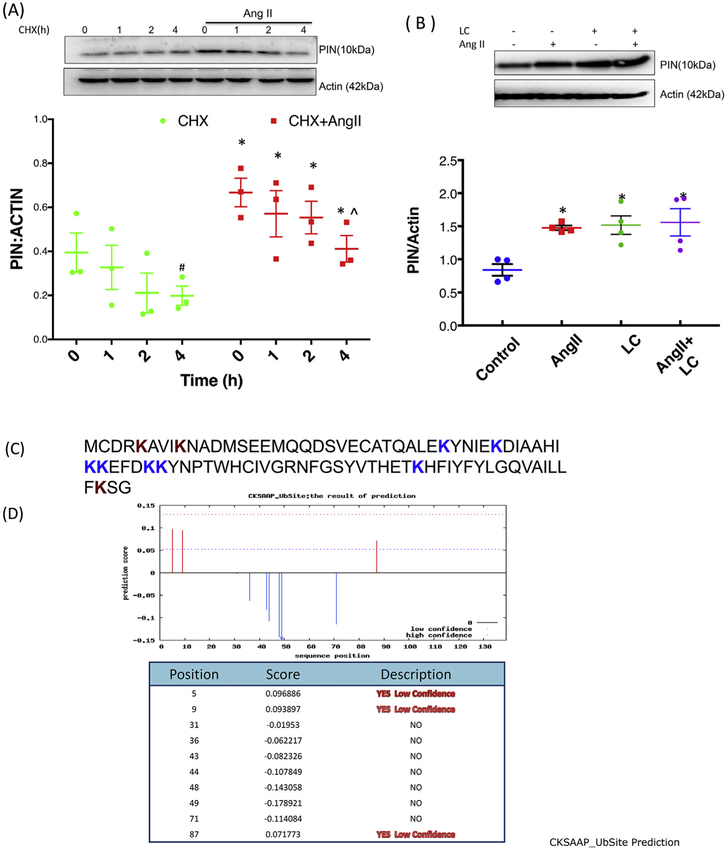
NG108 cells treated with different doses of Ang II showed no significant changes in PIN mRNA levels while there was significant upregulation of protein expression [4]. To determine whether de novo protein synthesis was required for Ang II-mediated upregulation of PIN, we performed a pulse-chase experiment in NG108 cells using cycloheximide (CHX) a protein synthesis inhibitor which prevents translational elongation. NG108 cells were treated with Ang II for 20 h, followed by treatment with CHX (10 μM) for different durations, as shown in Fig. 3A. The cells were collected immediately after the addition of CHX(0 h) and at specific time points following treatment. A significant gradual decline was observed in PIN expression after CHX treatment for 4 h in both groups (CHX alone and CHX + Ang II treated). The rate and extent of downregulation with CHX alone were similar to those in CHX + Ang II-treated samples as evident with no change in the slope of the relationship between the groups (p = 0.681). Further, LC (a cellpermeable inhibitor that specifically blocks the activity of the 26S proteasome) treatment dramatically elevated endogenous PIN protein levels suggesting a major role of proteasome system in the turnover of PIN (Fig. 3B). Thus, LC causes accumulation of ubiquitinated proteins destined to be degraded in the proteasome (0.79 ± 0.08 Control vs. 1.44 ± 0.16* LC). Interestingly, Ang II-mediated increase in PIN expression (0.79 ± 0.08 Control vs. 1.40 ± 0.03* Ang II) was almost similar to LC-mediated accumulation of PIN levels and was not increased further with combined stimulation of Ang II and LC, suggesting that Ang II-mediated upregulation of PIN is via posttranslational stabilization of PIN. The stabilizing effect of the proteasomal inhibitor on PIN lead us to explore further whether the PIN is a direct substrate for ubiquitination. The PIN protein sequence (Fig. 3C) was uploaded into multiple ubiquitination prediction software to predict the possible lysine residues targeted for ubiquitination. The example of CKSAAP_UbSite Webserver (cksaap_ubsite/index.php) prediction analysis was shown in Fig. 3D, which suggest three putative lysine residues at position 5, 9, and 87 with ubiquitination prediction scores.
Fig. 3.
Ang II stabilizes PIN expression post-translationally. (A) Effect of translation inhibitor CHX (10 μM) on Ang II-mediated upregulation of PIN. Top: a representative Western blot. Bottom: densitometry analyses of PIN protein expression normalized to Actin. Data were represented as mean ± SEM from three independent experiments. *P < 0.05 vs. CHX, #P < 0.05 vs. CHX 0 h and ^P < 0.05 vs. CHX-Ang II 0 h. (B) Effect of proteasomal inhibitor LC (10 μM) on Ang II-mediated upregulation of PIN. Top: a representative Western blot. Bottom: densitometry analyses of PIN protein expression normalized to Actin. Data were represented as mean ± SEM from four independent experiments. *P < 0.05 vs vs. control. (C) The protein sequence of PIN (NP_445771.1). (D) In silico analysis of PIN protein sequence for prediction of ubiquitination sites. The PIN amino acid sequence was analyzed using the bioinformatics CKSAAP_UbSite Webserver (cksaap ubsite/index.php) to predict ubiquitination potential sites in this protein. The brown color “K” (lysine) residues at positions 5, 9, and 87 were predicted to undergo ubiquitination. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Ubiquitination of PIN in NG108 cells treated with Ang II
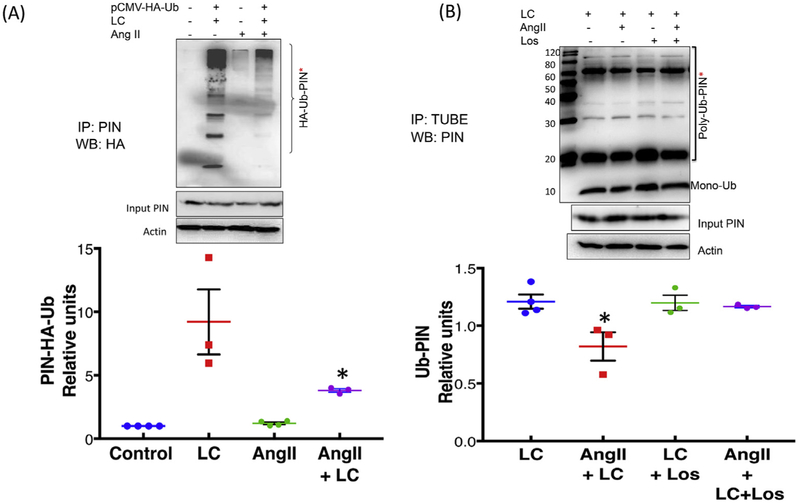
To further confirm whether Ang II-mediated upregulation of PIN is via stabilizing the posttranslational levels of PIN, we performed substrate-dependent ubiquitin ligase assay in NG108 cells transfected with pCMV-(HA-Ub)8 vector to exogenously incorporate ubiquitin in the presence of proteasomal inhibitor. The cell lysates from four groups of cells were subjected to immunoprecipitation with an anti-PIN antibody followed by Western blotting with an HA antibody to detect higher molecular weight HA-Ub-PIN conjugates. Fig. 3A demonstrates that proteasomal inhibition of NG108 cells with LC dramatically elevated high molecular weight ubiquitinated PIN bands implicating a role for the proteasomal system in fast turnover of PIN. These intense higher molecular weight bands were not detected in cells that were treated with Ang II along with LC (cf. lane 2 with lane 4). We observed an approximately 2-fold reduction of higher molecular weight bands of PIN when cells were treated with Ang II and LC together compared to LC alone (4.47 ± 0.71* LC Ang II vs. 9.20 ± 2.56 LC). These results suggest that the effect of Ang II on stabilization of PIN is likely to be mediated through inhibition of ubiquitination of PIN. Furthermore, we performed experiment in NG108 cells treated with Ang II and AT1R blocker Los in the presence of LC (Fig. 4B). TUBE assay and subsequent Western blotting with anti-PIN IgG revealed a decreased PIN-Ub conjugates in Ang II-treated cells compared to LC treated controls (cf. lane 2 with lane 1) (0.82 ± 0.12 LC Ang II vs. 1.21 ± 0.06) suggesting that Ang II treatment decreases ubiquitination of PIN. As expected Los treatment ameliorated the Ang II-dependent decrease in PIN-Ub levels (cf. lane 2 with lane 4) (1.17 ± 0.01 LC Ang II Los vs. 0.82 ± 0.12 LC Ang II) suggesting the involvement of AT1R in Ang II-mediated upregulation of PIN via decreasing the ubiquitination.
Fig. 4.
PIN- ubiquitination decreases with Ang II treatment in NG108 cells. (A) Substrate-dependent ubiquitin ligase assay. NG108 cells were transfected with pCMV-HA-Ub plasmid or lipofectamine alone as a control. Twenty-four-hour post-transfection, cells were treated with 100 μM Ang II for 18 h followed by LC (10 mM) for an additional 6 h to accumulate the high molecular weight polyubiquitinated forms. The immunoprecipitation of the PIN with an anti-PIN antibody was performed in four groups of cell lysates (1) Control, 2) pCMV-HA-Ub + LC, 3) Ang II, 4) pCMV-HA-Ub + LC + Ang II followed by a Western blot with anti-HA. Top: a representative Western blot, bottom: densitometry analyses of HA-Ub-PIN bands. A representative experiment distinctive of 3–4 independent experiments is shown. Values were mean ± SEM. *P < 0.05 vs. LC. (B) TUBE assay from four groups of cells treated with 1) LC, 2) Ang II and LC, 3) Los and LC, and 4) Ang II, Los, and LC followed by Western blot with the anti-PIN antibody. Top: a representative Western blot, bottom: densitometry analyses of Ub-PIN bands. Values were plotted as mean ± SEM from three independent experiments. *Indicates P < 0.05 vs. LC.
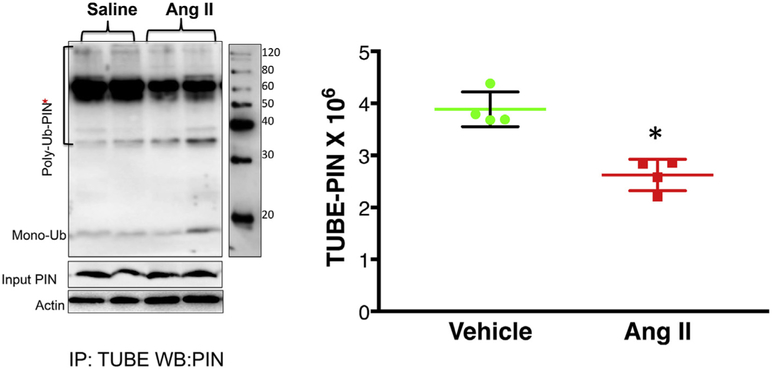
3.4. Ubiquitination of PIN in the PVN
After establishing that PIN is a target for post-translational regulation by ubiquitination, and Ang II inhibits this process, we want to examine whether Ang II alters the ubiquitination of PIN in the PVN. To probe for the ubiquitinated, high molecular weight forms of the PIN; PVN lysates from vehicle or Ang II-infused rats were immunoprecipitated with TUBE and then immunoblotted with a monoclonal antibody against the PIN. As shown in Fig. 4A, there was diminished accumulation of higher molecular weight PIN-ubiquitin conjugates in the PVN of Ang II-infused rats compared to vehicle (0.73 ± 0.04* Ang II vs. 1 ± 0.03 Veh) (Fig. 5), thereby increasing the expression of PIN (1.36 ± 0.04* Ang II vs. 0.81 ± 0.02 Veh) in the PVN (Fig. 1A) asserting that increased central levels of Ang II may contribute to the upregulation of PIN expression in the PVN via decreasing the ubiquitination.
Fig. 5.
Ang II decreases the accumulation of higher molecular weight forms of the immuno-detectable PIN in the PVN of ICV-Ang II-infused rats. The TUBE assay was performed on PVN lysates of Ang II and vehicle infused rats as outlined in the method section followed by Western blotting with PIN antibody. Left panel: Representative blot of TUBE assay of the PIN from Ang II- and vehicle-infused rats and right panel: densitometric analyses of Ub- PIN bands. Values are mean ± SEM from 4 rats/group. *P < 0.05 vs. vehicle.
4. Discussion
The salient findings of this study are that central infusion of Ang II; 1) increases RSNA and MAP, 2) increases PIN expression, and decreases the ubiquitination of PIN within the PVN, and 3) decreases the dimeric level of nNOS and hence decrease NO• bioavailability. Further, data showing no effects of Ang II on mRNA expression and new protein synthesis of PIN indicate that protein degradation may be the primary mechanism for the upregulation of PIN by Ang II. These results suggest that central Ang II stabilized the levels of PIN within the PVN, thereby decreasing the active dimeric form of nNOS resulting in an increased sympathetic outflow and robust hypertensive response.
The ICV-Ang II-infusion model is extensively used as a model of increased sympathoexcitation and arterial blood pressure in numerous studies [28-30]. To substantiate our in vivo whole animal results, we have also used NG108 cell line for in vitro studies which have neuronal properties and endogenous expression of the renin-angiotensin system (RAS) viz. renin, angiotensinogen, angiotensin-converting enzyme 1 and 2, as well as AT1 and AT2 receptor subtypes [32]. These cells also have a robust expression of nNOS and PIN, therefore serve as a suitable model for studying Ang II-mediated regulatory mechanisms of nNOS [4,20].
Exaggerated sympathetic tone and elevated arterial blood pressure are associated with increased central Ang II signaling in cardiovascular disease states such as hypertension, diabetes, and chronic heart failure. Numerous studies have identified the PVN of the hypothalamus as being an essential integration site for the regulation of SNA [33,34]. The PVN plays an important role in integrating signals/inputs from circumventricular organs and other brain areas involved in cardiovascular regulation and generating an output to the rostroventrolateral medulla and other downstream areas to influence overall sympathoexcitation [35]. Multiple previous studies from our laboratory [11,20,36] have explored the association between Ang II and decreased NO• bioavailability within the PVN, which may eventually contribute to enhanced sympathoexcitation. However, the precise molecular mechanism/s involved in Ang II-mediated increase in sympathoexcitation has not yet been established.
PIN, an 89-amino acid, 10-kDa protein, was so named because it was thought to regulate nNOS activity and hence NO• generation, negatively. The amino acid 228–244 in the exon 2 region of nNOS binds to PIN [37]. The studies by Hemmens et al. using purified nNOS and GST tagged PIN showed concentration-dependent inhibition of nNOS with 50% nNOS inhibition observed with 290 fold molar excess of the PIN [38]. We have demonstrated previously that Ang II treatment of neurons results in increased accumulation of PIN and increased PIN-nNOS binding in vitro, suggesting a relevant functional interaction between these two proteins [4]. Furthermore, this effect is associated with the decreased expression and active catalytic dimers of nNOS, which would be expected to result in reduced bioavailability of NO•. In our studies we have observed approximately 2-fold higher levels of PIN (1.24 ± 0.04* Ang II vs. 0.65 ± 0.07 Veh) are required to decrease the dimer to monomer ratio by 50%(0.22 ± 0.04* Ang II vs. 0.44 ± 0.07 Veh). The central Ang II increased the levels of PIN via decreasing the ubiquitination within the PVN, thereby reducing the active dimeric form of nNOS, which consequently results in an increased sympathetic outflow and a robust hypertensive response. NO• bioavailability plays an essential role in the regulation of blood pressure peripherally as well as centrally [13,39,40]. NO• modulates the release of several neurotransmitters, such as acetylcholine, catecholamine, and excitatory and inhibitory amino acids (i.e., glutamate and γ-aminobutyric acid) to influence neuronal function [2,11,41-43]. nNOS is the primary source of nitric NO• in the brain [44]. The regulation of nNOS activity is highly controlled physiologically to perform a variety of functions because the upregulated/downregulated release of NO• production can give rise to pathological conditions [45-48]. nNOS activity is subject to transcriptional, translational, and post-translational regulation, which dictate the specificity of NO• signaling and limit NO• toxicity [49]. The post-translational controls include lipid modifications, phosphorylation events, and protein-protein interactions [48]. A large number of protein partners for nNOS, including PIN, have been identified and described in our published review [48]. The catalytic activity of nNOS is regulated by different mechanisms such as Ca2+-calmodulin binding and phosphorylation [49]. Numerous scaffolding proteins, such as syntrophin, PSD93, and PSD95 have been identified to interact with nNOS in neurons and modify its activity. The NMDAR (N-methyl-d-aspartic acid receptor) and nNOS are linked through intermediary adaptor protein PSD95 for NMDAR-mediated calcium influx and nNOS activation in the PSD of synaptic spines [14]. In the brain, CAPON competes with PSD95 for interaction with nNOS, therefore acting as a negative regulator of NO production and its downstream signaling effects [14,50]. In synaptic spines, another nNOS-interacting protein (NOSIP) interacts with nNOS and inhibits its activity [14,51].
The PIN binding regions are unique to nNOS and located within the specific amino-terminal sequence. The PIN binding was initially reported to destabilize the nNOS dimer [15]. In our previous studies, we reported that nNOS expression in the PVN is decreased in CHF [19,52] via a PIN-mediated molecular mechanism [4] where we observed increased PIN protein expression along with a decrease in the nNOS dimer/monomer ratio and increased proteolytic degradation of nNOS. Our in vitro study with knockdown of PIN in neuronal cells also resulted in an accumulation of nNOS as well as increase in dimer/monomer ratio with increased NO• production, which supports the observation that PIN affects nNOS expression and catalytic activity [4], therefore, offering a new target for the treatment of pathologies associated with decreased expression of nNOS centrally.
Ubiquitination has emerged as a major regulatory mechanism in controlling the levels and function of several neuronal proteins. The stability of many proteins at the presynaptic and post-synaptic density is regulated by the ubiquitin-proteasome system (UPS) [53]. The UPS is the most crucial mechanism controlling protein degradation of the majority of intracellular protein in eukaryotic cells while extracellular proteins and some cell surface proteins are endocytosed and degraded within lysosomes [54]. The UPS consists of concerted actions of three enzymes that link Ub to protein lysine residues, usually Lys48, to mark them for degradation. E1 (Ub-activating enzyme) and E2s (Ub-carrier or conjugating proteins) prepare Ub for conjugation, and the key enzyme in the process is the E3 (Ub-protein ligase) which provides specificity to the reaction [55]. This tagging process leads to the recognition of Ub-tagged protein by the 26S proteasome, an intracellular complex that degrades polyubiquitin-conjugated protein to small peptides. Studies have shown that in prolonged but not in short-term obesity, the UPS system fails to maintain an adequate rate of protein recycling in the hypothalamus, leading to the accumulation of ubiquitinated proteins that could contribute to the continuous deterioration of the hypothalamic neurons that regulate body energy homeostasis [56]. In neurons, the precise regulation of protein turnover is also essential for synaptic plasticity and cell viability [57]. In neurodegenerative conditions such as Parkinson's and Alzheimer's diseases, the impairment of this process leads to the accumulation of protein aggregates in the cells [58,59]. The immunoproteasome has been observed in neurons in three human neurodegenerative diseases: Huntington's Disease [60], Amyotropic Lateral Sclerosis [61], and Alzheimer's Disease [62]. Previously we have established the post-translational mechanism of increased ubiquitination-mediated degradation of nNOS in the PVN of CHF rats, which leads to enhanced sympathoexcitation.
Interestingly, the present data showed that Ang II infusion in the rats does not alter the PIN transcript levels while PIN protein expression augmented with a concurrent decrease in PIN-Ub conjugates in the PVN. Ang II reduces protein degradation and prolongs the half-life of PIN, possibly by interfering with the UPS degradation pathway, which leads to its intracellular accumulation. Ang II treatment may regulate the expression or activity of E3 Ub-ligases or deubiquitinases, thus altering the functional half-life of the PIN protein rather than changing the levels of transcriptional factors involved in transcription of PIN. Previous studies by Yang et al. [63] have also confirmed that PIN is targeted for rapid degradation by the UPS. IFN-γ treatment of neurons increases degradation of PIN via ubiquitination leading to increased nNOS levels and production of NO• by neuronal cells, which are correlated with an increased antiviral effect of IFN-γ.
To study the ubiquitination of nNOS, we used TUBE: tandem, ubiquitin-binding entities rather than simple immunoprecipitation using Ub antibody. Detection of polyubiquitinated protein has always been a difficult task due to the labile nature of these post-translational modifications. TUBE not only increases the affinity for poly-ubiquitin chains up to a thousand times but also protects ubiquitylated proteins from the action of the proteasome and de-ubiquitylating enzymes [64].
The role of the renal PIN in the regulation of nNOS and hence, blood pressure regulation has been examined in pre-hypertensive and hypertensive stages in SHR rats [65]. The authors demonstrated that inhibition of PIN expression by siRNA attenuates the development of hypertension in SHRs at 12 weeks of age. PIN can inhibit nNOS-α activity through disruption of nNOS dimerization to reduce NO• production while another β form of nNOS, which lacks exon 2 and hence PIN binding domain, is insensitive to PIN mediated inactivation [65]. Increased PIN expression and decreased nNOS dimers are also reported in the rostral ventrolateral medulla of high-fructose diet-fed rats contributing to increased sympathoexcitation and hypertension [66]. The results of the present studies provide a potential explanation that PIN levels within the PVN are post-translationally regulated by Ang II-mediated-ubiquitination, as summarized in Fig. 6. Circulating levels of Ang II are reported to be higher in patients with hypertension as compared to normotensive subjects [67]. During hypertension, circulating Ang II evokes increased BBB permeability, facilitating, in turn, its passage to critical brain regions associated with blood pressure regulation [68]. In addition to circulating Ang II, local production of brain Ang II also contributes to hypertension development as all components of the RAS system are present within the CNS [69]. Therefore, we expect that perhaps the increased levels of Ang II is increasing the expression of PIN post-translationally in specific areas of the brain, such as the PVN. Reduced levels of active nNOS dimers due to increased levels of PIN leads to the reduction of NO• centrally within the PVN, resulting in reduced inhibitory influences to neurons, and thereby, enhanced sympathoexcitation observed in cardiovascular diseases including hypertension and heart failure. The bioavailability of NO• can also be limited by an increase in reactive oxygen species(ROS) in the PVN due to increased activation of the RAS system. The increase in ROS limits the availability of NO due to the formation of peroxynitrite. Further, there is evidence that supports that destabilization of eNOS dimers uncouples the enzyme machinery to produce superoxide rather than NO [70]. Perhaps the same mechanism is present for nNOS, and PIN is playing an essential role in uncoupling of nNOS to produce ROS, which remains to be explored. Taken together, these observations provide a unique insight into the possible mechanism(s) within the PVN that contribute to the over-activation of sympathetic drive and offer a new target for the treatment of enhanced sympathoexcitation in various cardiovascular diseases.
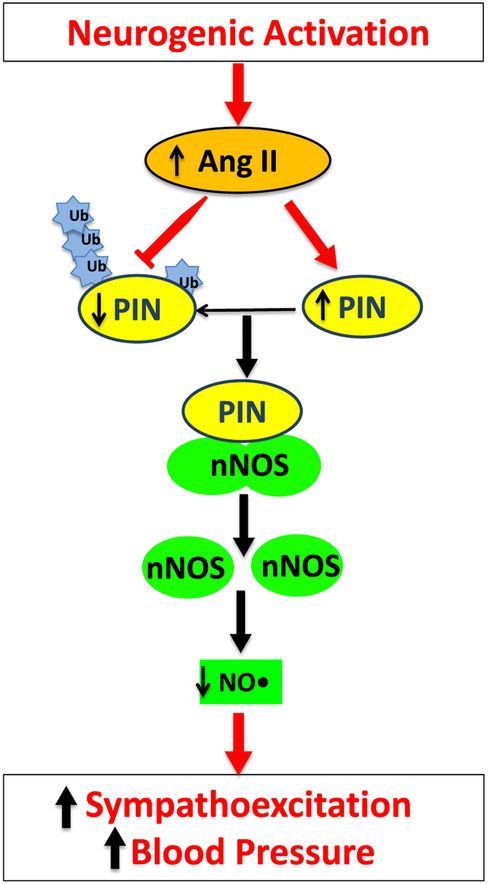
Fig. 6.
Proposed model for the up-regulation of the PIN by post-translational regulation in the PVN. Elevated central Ang II levels via AT1 receptors in the PVN increases the expression of PIN via decreasing the ubiquitination. Increased expression of PIN destabilizes nNOS dimers, which renders nNOS catalytically inactive, either by interfering with the assembly or dimer stability. A reduced level of functional nNOS reduces NO• production in the PVN, causing an increase in sympathoexcitation and associated blood pressure.
Acknowledgments
Funding from American Heart Association National Center grant 14SDG19980007(N. Sharma) and National Institutes of Health, Heart, Lung, & Blood Institute, Grant R01-DK-114663, HL62222 and McIntyre Professorship (K.P. Patel) supported this work.
Footnotes
Declaration of competing interest
The authors declare no potential conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.niox.2019.10.007.
References
- [1].Perin PC, Maule S, Quadri R, Sympathetic nervous system, diabetes, and hypertension, Clin. Exp. Hypertens 23 (2001) 45–55. [DOI] [PubMed] [Google Scholar]
- [2].Zucker IH, Wang W, Pliquett RU, Liu JL, Patel KP, The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training, Ann. N. Y. Acad. Sci 940 (2001) 431–443. [PubMed] [Google Scholar]
- [3].Zheng H, Sharma NM, Liu X, Patel KP, Exercise training normalizes enhanced sympathetic activation from the paraventricular nucleus in chronic heart failure: role of angiotensin II, Am. J. Physiol. Regul. Integr. Comp. Physiol 303 (2012) R387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sharma NM, Llewellyn TL, Zheng H, Patel KP, Angiotensin II-mediated posttranslational modification of nNOS in the PVN of rats with CHF: role for PIN, Am. J. Physiol. Heart Circ. Physiol 305 (2013) H843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fyhrquist F, Metsarinne K, Tikkanen I, Role of angiotensin II in blood pressure regulation and in the pathophysiology of cardiovascular disorders, J. Hum. Hypertens 9 (Suppl 5) (1995) S19–24. [PubMed] [Google Scholar]
- [6].Palatini P, Julius S, The role of cardiac autonomic function in hypertension and cardiovascular disease, Curr. Hypertens. Rep 11 (2009) 199–205. [DOI] [PubMed] [Google Scholar]
- [7].Gorren AC, Mayer B, The versatile and complex enzymology of nitric oxide synthase. Biochemistry (Mosc.) 63 (1998) 734–743. [PubMed] [Google Scholar]
- [8].Stuehr DJ, Mammalian nitric oxide synthases, Biochim. Biophys. Acta 1411 (1999) 217–230. [DOI] [PubMed] [Google Scholar]
- [9].Werner ER, Werner-Felmayer G, Mayer B, Tetrahydrobiopterin, cytokines, and nitric oxide synthesis, Proc. Soc. Exp. Biol. Med 219 (1998) 171–182. [DOI] [PubMed] [Google Scholar]
- [10].Chen K, Pittman RN, Popel AS, Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective, Antioxidants Redox Signal. 10 (2008) 1185–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang K, Mayhan WG, Patel KP, Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity, Am. J. Physiol 273 (1997) R864–R872. [DOI] [PubMed] [Google Scholar]
- [12].Zhang K, Patel KP, Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge:role of GABA, Am. J. Physiol 275 (1998) R728–R734. [DOI] [PubMed] [Google Scholar]
- [13].McBryde FD, Liu BH, Roloff EV, Kasparov S, Paton JFR, Hypothalamic paraventricular nucleus neuronal nitric oxide synthase activity is a major determinant of renal sympathetic discharge in conscious Wistar rats, Exp. Physiol 103 (2018) 419–428. [DOI] [PubMed] [Google Scholar]
- [14].Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH, CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95, Neuron 20 (1998) 115–124. [DOI] [PubMed] [Google Scholar]
- [15].Jaffrey SR, Snyder SH, PIN: an associated protein inhibitor of neuronal nitric oxide synthase, Science 274 (1996) 774–777. [DOI] [PubMed] [Google Scholar]
- [16].Fan JS, Zhang Q, Li M, Tochio H, Yamazaki T, Shimizu M, et al. , Protein inhibitor of neuronal nitric-oxide synthase, PIN, binds to a 17-amino acid residue fragment of the enzyme, J. Biol. Chem 273 (1998) 33472–33481. [DOI] [PubMed] [Google Scholar]
- [17].Bender AT, Demady DR, Osawa Y, Ubiquitination of neuronal nitric oxide synthase in vitro and in vivo, J. Biol. Chem 275 (2000) 17407–17411. [DOI] [PubMed] [Google Scholar]
- [18].Dunbar AY, Kamada Y, Jenkins GJ, Lowe ER, Billecke SS, Osawa Y, Ubiquitination and degradation of neuronal nitric-oxide synthase in vitro: dimer stabilization protects the enzyme from proteolysis, Mol. Pharmacol 66 (2004) 964–969. [DOI] [PubMed] [Google Scholar]
- [19].Li Y-F, Patel KP, Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: altered inhibtory mechanisms, Acta Physiol. Scand 177 (2003) 17–26. [DOI] [PubMed] [Google Scholar]
- [20].Sharma NM, Zheng H, Mehta PP, Li YF, Patel KP, Decreased nNOS in the PVN leads to increased sympathoexcitation in chronic heart failure: role for CAPON and Ang II, Cardiovasc. Res. 92 (2011) 342–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paxinos G, Watson C, The Rat Brain in Stereotaxic Coordinates, Academic Press, Orlando, 1986. [Google Scholar]
- [22].Sharma NM, Liu X, Llewellyn TL, Katsurada K, Patel KP, Exercise training augments neuronal nitric oxide synthase dimerization in the paraventricular nucleus of rats with chronic heart failure, Nitric Oxide 87 (2019) 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li YF, Wang W, Mayhan WG, Patel KP, Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide, Am. J. Physiol. Regul. Integr. Comp. Physiol 290 (2006) R1035–1043. [DOI] [PubMed] [Google Scholar]
- [24].Palkovits M, Brownstein M, Brain microdissection techniques, in: Cuello AE(Ed.), Brain Microdissection Techniques, John Wiley & Sons, Chichester, 1983. [Google Scholar]
- [25].Klatt P, Schmidt K, Lehner D, Glatter O, Bachinger HP, Mayer B, Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and L-arginine in the formation of an SDS-resistant dimer, EMBO J. 14 (1995) 3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hjerpe R, Aillet F, Lopitz-Otsoa F, Lang V, England P, Rodriguez MS, Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities, EMBO Rep. 10 (2009) 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Furukawa M, Andrews PS, Xiong Y, Ubiquitin proteosome protocols, Methods Mol. Biol 301 (2005) 37–46. [DOI] [PubMed] [Google Scholar]
- [28].Lohmeier TE, Angiotensin II infusion model of hypertension: is there an important sympathetic component? Hypertension 59 (2012) 539–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J, Angiotensin II-induced hypertension is modulated by nuclear factor-kappaBin the paraventricular nucleus, Hypertension 59 (2012) 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Collister JP, Taylor-Smith H, Drebes D, Nahey D, Tian J, Zimmerman MC, Angiotensin II-induced hypertension is attenuated by overexpressing copper/zinc superoxide dismutase in the brain organum vasculosum of the lamina terminalis, Oxid Med Cell Longev 2016 (2016) 3959087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].King AJ, Fink GD, Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms, Hypertension 48 (2006) 927–933. [DOI] [PubMed] [Google Scholar]
- [32].Fishman MC, Zimmerman EA, Slater EE, Renin and angiotensin: the complete system within the neuroblastoma x glioma cell, Science 214 (1981) 921–923. [DOI] [PubMed] [Google Scholar]
- [33].Hirooka Y, Kishi T, Sakai K, Takeshita A, Sunagawa K, Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension, Am. J. Physiol. Regul. Integr. Comp. Physiol 300 (2011) R818–826. [DOI] [PubMed] [Google Scholar]
- [34].Gao HL, Yu XJ, Liu KL, Shi XL, Qi J, Chen YM, et al. , PVN blockade of p44/42 MAPK pathway attenuates salt-induced hypertension through modulating neurotransmitters and attenuating oxidative stress, Sci. Rep 7 (2017) 43038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guyenet PG, The sympathetic control of blood pressure, Nat. Rev. Neurosci 7 (2006) 335–346. [DOI] [PubMed] [Google Scholar]
- [36].Zheng H, Liu X, Li Y, Sharma NM, Patel KP, Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure, Hypertension 58 (2011) 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chaudhury A, Rao YM, Goyal RK, PIN/LC8 is associated with cytosolic but not membrane-bound nNOS in the nitrergic varicosities of mice gut: implications for nitrergic neurotransmission, Am. J. Physiol. Gastrointest. Liver Physiol 295 (2008) G442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hemmens B, Woschitz S, Pitters E, Klosch B, Volker C, Schmidt K, et al. , The protein inhibitor of neuronal nitric oxide synthase (PIN): characterization of its action on pure nitric oxide synthases, FEBS Lett. 430 (1998) 397–400. [DOI] [PubMed] [Google Scholar]
- [39].Hermann M, Flammer A, Luscher TF, Nitric oxide in hypertension, J. Clin. Hypertens 8 (2006) 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Puzserova A, Bernatova I, Blood pressure regulation in stress: focus on nitric oxide-dependent mechanisms, Physiol. Res 65 (2016) S309–S342. [DOI] [PubMed] [Google Scholar]
- [41].Schuman EM, Madison DV, Nitric oxide and synaptic function, Annu. Rev. Neurosci 17 (1994) 153–183. [DOI] [PubMed] [Google Scholar]
- [42].Patel KP, Li YF, Hirooka Y, Role of nitric oxide in central sympathetic outflow, Proc. Soc. Exp. Biol. Med 226 (2001) 814–824. [DOI] [PubMed] [Google Scholar]
- [43].Liu JL, Zucker IH, Regulation of sympathetic nerve activity in heart failure: a role for nitric oxide and angiotensin II, Circ. Res 84 (1999) 417–423. [DOI] [PubMed] [Google Scholar]
- [44].Guix FX, Uribesalgo I, Coma M, Munoz FJ, The physiology and pathophysiology of nitric oxide in the brain, Prog. Neurobiol 76 (2005) 126–152. [DOI] [PubMed] [Google Scholar]
- [45].Whittle BJ, Nitric oxide in physiology and pathology, Histochem. J 27 (1995) 727–737. [PubMed] [Google Scholar]
- [46].Zhang K, Li YF, Patel KP, Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure, Am. J. Physiol. Heart Circ. Physiol 281 (2001) H995–H1004. [DOI] [PubMed] [Google Scholar]
- [47].Zheng H, Mayhan WG, Bidasee KR, Patel KP, Blunted nitric oxide-mediated inhibition of sympathetic nerve activity within the paraventricular nucleus in diabetic rats, Am. J. Physiol. Regul. Integr. Comp. Physiol 290 (2006) R992–R1002. [DOI] [PubMed] [Google Scholar]
- [48].Sharma NM, Patel KP, Post-translational regulation of neuronal nitric oxide synthase: implications for sympathoexcitatory states, Expert Opin. Ther. Targets (2016) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Alderton WK, Cooper CE, Knowles RG, Nitric oxide synthases: structure, function and inhibition, Biochem. J 357 (2001) 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jaffrey SR, Benfenati F, Snowman AM, Czernik AJ, Snyder SH, Neuronal nitric-oxide synthase localization mediated by a ternary complex with synapsin and CAPON, Proc. Natl. Acad. Sci 99 (2002) 3199–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dreyer J, Schleicher M, Tappe A, Schilling K, Kuner T, Kusumawidijaja G, et al. , Nitric oxide synthase (NOS)-interacting protein interacts with neuronal NOS and regulates its distribution and activity, J. Neurosci 24 (2004) 10454–10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP, Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure, Am. J. Physiol. Heart Circ. Physiol 288 (2005) H2332–2341. [DOI] [PubMed] [Google Scholar]
- [53].Rinetti GV, Schweizer FE, Ubiquitination acutely regulates presynaptic neurotransmitter release in mammalian neurons, J. Neurosci 30 (2010) 3157–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nath D, Shadan S, The ubiquitin system, Nature 458 (2009) 421. [DOI] [PubMed] [Google Scholar]
- [55].Lecker SH, Goldberg AL, Mitch WE, Protein degradation by the ubiquitin-proteasome pathway in normal and disease states, J. Am. Soc. Nephrol 17 (2006) 1807–1819. [DOI] [PubMed] [Google Scholar]
- [56].Ignacio-Souza LM, Bombassaro B, Pascoal LB, Portovedo MA, Razolli DS, Coope A, et al. , Defective regulation of the ubiquitin/proteasome system in the hypothalamus of obese male mice, Endocrinology 155 (2014) 2831–2844. [DOI] [PubMed] [Google Scholar]
- [57].Cajigas IJ, Will T, Schuman EM, Protein homeostasis and synaptic plasticity, EMBO J. 29 (2010) 2746–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Almeida CG, Takahashi RH, Gouras GK, Beta-amyloid accumulation impairs multivesicular body sorting by inhibiting the ubiquitin-proteasome system, J. Neurosci 26 (2006) 4277–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bedford L, Hay D, Devoy A, Paine S, Powe DG, Seth R, et al. , Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies, J. Neurosci 28 (2008) 8189–8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Diaz-Hernandez M, Hernandez F, Martin-Aparicio E, Gomez-Ramos P, Moran MA, Castano JG, et al. , Neuronal induction of the immunoproteasome in Huntington's disease, J. Neurosci 23 (2003) 11653–11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cheroni C, Peviani M, Cascio P, Debiasi S, Monti C, Bendotti C, Accumulation of human SOD1 and ubiquitinated deposits in the spinal cord of SOD1G93A mice during motor neuron disease progression correlates with a decrease of proteasome, Neurobiol. Dis 18 (2005) 509–522. [DOI] [PubMed] [Google Scholar]
- [62].Mishto M, Bellavista E, Santoro A, Stolzing A, Ligorio C, Nacmias B, et al. , Immunoproteasome and LMP2 polymorphism in aged and Alzheimer's disease brains, Neurobiol. Aging 27 (2006) 54–66. [DOI] [PubMed] [Google Scholar]
- [63].Yang J, Tugal D, Reiss CS, The role of the proteasome-ubiquitin pathway in regulation of the IFN-gamma mediated anti-VSV response in neurons, J. Neuroimmunol 181 (2006) 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Aillet F, Lopitz-Otsoa F, Hjerpe R, Torres-Ramos M, Lang V, Rodriguez MS, Isolation of ubiquitylated proteins using tandem ubiquitin-binding entities, Methods Mol. Biol 832 (2012) 173–183. [DOI] [PubMed] [Google Scholar]
- [65].Wang SC, Lin KM, Chien SJ, Huang LT, Hsu CN, Tain YL, RNA silencing targeting PIN (protein inhibitor of neuronal nitric oxide synthase) attenuates the development of hypertension in young spontaneously hypertensive rats, J Am Soc Hypertens 8 (2014) 5–13. [DOI] [PubMed] [Google Scholar]
- [66].Wu KL, Chao YM, Tsay SJ, Chen CH, Chan SH, Dovinova I, et al. , Role of nitric oxide synthase uncoupling at rostral ventrolateral medulla in redox-sensitive hypertension associated with metabolic syndrome, Hypertension 64 (2014) 815–824. [DOI] [PubMed] [Google Scholar]
- [67].Catt KJ, Cran E, Zimmet PZ, Best JB, Cain MD, Coghlan JP, Angiotensin II blood-levels in human hypertension, Lancet 1 (1971) 459–464. [DOI] [PubMed] [Google Scholar]
- [68].Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE, Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier, Hypertension 63 (2014) 572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Grobe JL, Xu D, Sigmund CD, An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy, Physiology (Bethesda) 23 (2008) 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bendall JK, Alp NJ, Warrick N, Cai S, Adlam D, Rockett K, et al. , Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression, Circ. Res 97 (2005) 864–871. [DOI] [PubMed] [Google Scholar]