Abstract
Pain is a subjective, multidimensional experience that is distinct from nociception. A large body of work has focused on whether pain processing is supported by specific, dedicated brain circuits. Despite advances in human neuroscience and neuroimaging analysis, dissociating acute pain from other sensations has been challenging since both pain and non-pain stimuli evoke salience and arousal responses throughout the body and in overlapping brain circuits. In this review, we discuss these challenges and propose that brain-body interactions in pain can be leveraged in order to improve tests for pain specificity. We review brain and bodily responses to pain and nociception and extant efforts toward identifying pain-specific brain networks. We propose that autonomic nervous system activity should be used as a surrogate measure of salience and arousal to improve these efforts and enable researchers to parse out pain-specific responses in the brain, and demonstrate the feasibility of this approach using example fMRI data from a thermal pain paradigm. This new approach will improve the accuracy and specificity of functional neuroimaging analyses and help to overcome current difficulties in assessing pain specific responses in the human brain.
Keywords: brain-body, pain specificity, salience, arousal, functional neuroimaging, autonomic nervous system
Introduction
Pain is fundamental to survival. It motivates us to escape danger, seek care, and learn to avoid harm in the future. Successful identification of pain-specific brain patterns could enhance diagnosis and prognosis of pain in the clinic, as well as reveal fundamental truths about the nature of pain as a percept and how it is instantiated in the brain. Yet despite decades of efforts, it is still unclear whether pain is dissociable from other sensations at the level of the central nervous system, i.e., whether pain is a special type of percept mediated by unique, dedicated neural circuits (Craig, 2003; Perl, 2007), or if it is a sensation experienced in response to any extremely salient sensory stimulus (Legrain et al., 2011). Pain is a complex, subjective phenomenon defined as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’ (IASP, 1994). These sensory and emotional components of pain distinguish it from nociception, which is defined as the neural process of encoding stimuli that damage or threaten to damage tissue (Merskey, 1994), yet they also highlight the link between pain and salience. Salience is defined as a characteristic of a stimulus that makes it perceived as distinct from its surroundings (Itti and Koch, 2001; Menon and Uddin, 2010; Uddin, 2015). Regardless of stimulus modality, salient stimuli capture our attention, cause autonomic and emotional responses, and modify our behavior, similar to acute pain (Bernstein, 1969; Bradley, 2009; Lang, 2014; Liberati et al., 2016; Menon and Uddin, 2010; Mouraux et al., 2011; Parr and Friston, 2018). Acute pain also automatically captures our attention (Iannetti and Mouraux, 2010; Legrain et al., 2009; Legrain et al., 2011) to generate a wide range of adaptive behaviors including withdrawal from the stimulus, seeking relief, and collecting information to predict future damage (Eccleston and Crombez, 1999; Fields, 2018). Thus, determining if and how pain can be distinguished from nociception and salience is an area of active research, and the question of whether there may be pain-specific brain responses is still a controversial issue in need of additional empirical data (Berkley and Hubscher, 1995; Moayedi and Davis, 2013; Mouraux and Iannetti, 2018).
We propose that that there may be unique pain-related processes that do not overlap with other sensory processes in the brain. Yet because pain shares characteristics with other salient somatosensory and non-somatosensory stimuli across modalities, perhaps difficulty in controlling for the non-specific features of pain (i.e., features of pain that are shared with non-pain stimuli) has restricted our ability to acquire the necessary empirical data to resolve this debate. New approaches are necessary to distinguish pain from other sensations. We propose that measuring and accounting for brain responses associated with autonomic nervous system (ANS) arousal in the periphery will help identify and rule out acute pain-related brain processes that are related to salience and arousal and thereby isolate pain-specific brain processes. Though machine learning classifiers and multivariate analyses have achieved recent success in identifying pain-specific patterns of brain activity (Marquand et al., 2010; Schulz et al., 2012; Wager et al., 2013), accounting for salience and arousal has the potential to enhance the accuracy of such classifiers and further our understanding of the brain response specific to pain. In line with the approach and theory we outline, two recent empirical studies demonstrate promise for accounting for salience using either subjective or peripheral ANS measures to evaluate pain-specific brain responses (Horing et al., 2019; Liang et al., 2019). Here, we review why accounting for ANS activity holds such promise for the important test of whether pain-specific processes exist in the human brain.
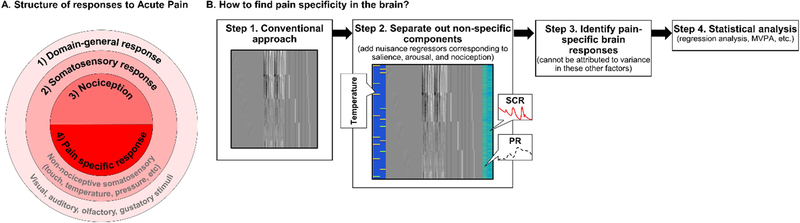
As illustrated in Figure 1A, we assume that the brain response to pain is comprised of: 1) nonspecific processes that are shared between pain and other sensations (e.g., salience and arousal); 2) features shared by somatosensory sensations (e.g. intensity estimation and location processing); 3) responses that differentiate between innocuous and noxious stimuli, irrespective of subjective experience (i.e., nociception); and 4) pain-specific processes. Thus, we define pain-specific processes as brain responses that are uniquely associated with subjective pain and are not involved in the processing of stimuli of other modalities. To be pain-specific, associations with pain must remain after accounting for non-specific and domain-general responses, somatosensory responses, and the effects of nociceptive inputs (Figure 1B). By studying bodily responses alongside brain responses to pain and non-pain control stimuli, we can account for the non-specific factors of salience and arousal. We believe that this approach may also have utility in the future for accounting for other non-specific factors, though they will not be discussed further here.
Figure 1. Hierarchy of specific and non-specific components of the brain response to acute pain and approach for identifying pain-specific responses.
A. Brain responses to pain are composed of four hierarchical components of processing, illustrated in concentric rings from most general (outer pink circle) to most specific (inner red semi-circle): 1) domain-general responses to any sensations regardless of the sensory modality of the stimulus (e.g. arousal, attention, salience, valence), 2) processing of somatosensory information regardless of nociception (e.g. intensity, location, and modality), 3) processing of nociceptive information, regardless of pain perception; and 4) pain-specific responses (i.e. those that track the subjective pain experience).
B. Here we illustrate a potential fMRI analysis pipeline that leverages autonomic nervous system (ANS) isolate pain-specific BOLD responses. Step 1 depicts a conventional fMRI analysis that models only standard nuisance regressors (e.g., motion, global signal, spikes, intercepts). If correlations between BOLD responses and subjective pain are evaluated, results are unlikely to be specific to pain. Step 2 depicts a design matrix that incorporates additional regressors (in color) corresponding to temperature effects (left, blue) and the timeseries of recorded autonomic measures (right, green), such as skin conductance (SCR) or pupillary response (PR), alongside nuisance regressors. This design matrix thereby accounts for differences in arousal (non-specific response) and nociception. Residual associations between brain activity and subjective pain can therefore be evaluated (Steps 3 and 4), which are more likely to be specific to pain.
This review is comprised of two parts; first, we evaluate the current knowledge on pain and its specificity and work demonstrating that pain shares general features with other non-painful stimuli that influence the brain’s response to pain, confounding our current ability to detect pain-specific brain responses. We then describe a new approach to link arousal patterns in the brain and periphery in service of parsing out similarities between pain, salience, and arousal to determine a pain-specific brain response. Finally, we demonstrate the feasibility of this approach using example data from an fMRI study of acute pain. We focus here on stimulus-evoked pain (e.g., pain elicited by noxious heat, pressure, visceral, electrical stimulation), though this approach has the promise to inform the investigation of the specificity of other types of pain in the future.
Distinguishing pain and nociception using responses in the brain and body
As mentioned earlier, pain is distinct from nociception, the neural process of encoding and processing noxious stimuli (IASP, 1994). Functional neuroimaging and electrophysiological studies in humans and animals have revealed a wide range of brain regions consistently modulated by noxious mechanical, thermal, and chemical stimuli including the primary and secondary somatosensory cortex, anterior cingulate cortex (ACC), insula, thalamus, prefrontal cortex (PFC), inferior/posterior parietal cortex, and periaqueductal gray (PAG) (Apkarian et al., 2005; Davis and Moayedi, 2013; Derbyshire et al., 1997; Duerden and Albanese, 2013; Iadarola et al., 1998; Melzack, 1999; Talbot et al., 1991) (see Figure 2A). The term ‘pain matrix’ has been used widely to describe this set of brain regions involved in human nociceptive processing (Garcia-Larrea and Peyron, 2013; Iannetti and Mouraux, 2010). However, there is mixed processing of pain and nociception in the pain matrix, and many of these regions may not be specific to pain. Pain and nociception are distinct processes that can, and should, be distinguished (Garland, 2012). Pain can exist in the absence of nociceptive input (e.g., the experience of pain from amputated limb (Melzack, 1990)) and nociceptive input does not always produce pain (e.g., battlefield analgesia (Beecher, 1946, 1956); attentional modulation of pain (Torta et al., 2017)). Thus, one continued thread of research on pain-specific processing focuses on distinguishing pain from nociception.
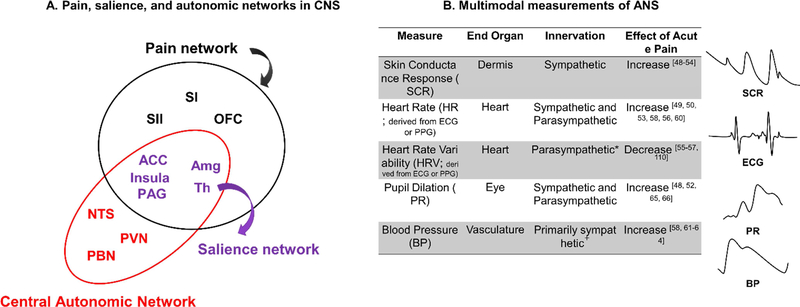
Figure 2. Measurements of brain and bodily responses to pain.
A. Anatomical overlap between pain (black and purple regions), salience (purple regions), and autonomic networks (red and purple regions) in central nervous system. Amg: amygdala; ACC: anterior cingulate cortex; NTS: nucleus tractus solitarius of the medulla; OFC: orbitofrontal cortex; PAG: periacqueductal gray; PBN: parabrachial nuclei; PVN: paraventricular nucleus of the hypothalamus; SI: primary somatosensory cortex; SII: secondary somatosensory cortex; Th: thalamus.
B. Various ANS measurements (e.g., skin conductance response, heart rate, heart rate variability, pupil dilation, blood pressure) across different end-organs are related to pain. Because these end-organs are innervated by different branches of the autonomic nervous system and influenced by different mechanisms in the periphery, they provide non-overlapping information about arousal, and are best used in combination. *Many measures of HRV are parasympathetically mediated, but some measures are jointly influenced by both the sympathetic and parasympathetic nervous system. +Blood pressure in the vasculature is primarily controlled by the sympathetic nervous system, but parasympathetic control of the heart also influences blood pressure.
Indeed, much research has been devoted to identifying brain regions that uniquely process nociceptive information or pain and how nociceptive information is translated into the experience of pain (Apkarian et al., 1999; Atlas et al., 2014; Baliki et al., 2009; Buchel et al., 2002; Craig et al., 2000). For instance, in one of the first functional magnetic resonance imaging (fMRI) investigations of acute thermal pain, Apkarian et al. (1999) differentiated between brain activity related to stimulus intensity (variations in temperature over time) and pain (variations in perception over time) (Apkarian et al., 1999). A functional gradient separated nociception and pain: anterior regions (e.g., insula) were more related to the time course of the stimulus, while posterior regions (e.g., BA 5/7, i.e., the somatosensory association cortex) were more related to the time course of perceived pain. Buchel et al. (2002) reported similarly that anterior portions of the dorsal posterior ACC (dpACC) were linked to stimulus intensity regardless of subjective pain (Buchel et al., 2002) and discriminated noxious and innocuous stimulation, while posterior portions of the dpACC tracked subjective pain. A more recent fMRI study (Atlas et al., 2014) also observed similar gradients of sensitivity to temperature and pain using voxel-wise multilevel mediation analysis. The left insula and the cingulate cortex both exhibited gradients such that anterior portions were related to temperature (and not pain), posterior portions were related to pain (and not temperature), and middle portions formally mediated temperature effects on subjective pain (Atlas et al., 2014). These results suggest that pain is subserved by not only pain-specific and temperature-specific processes, but also key brain networks that link or convert nociceptive inputs (e.g., stimulus intensity) into pain.
Machine learning and multi-voxel pattern analyses (MVPA) have also been used to determine whether patterns of activity can discriminate painful and non-painful stimuli above and beyond the effects of temperature (Woo et al., 2017b). The stimulus intensity independent pain signature-1 (SIIPS1) is a brain-based pattern that is sensitive to pain, but independent of the stimulus intensity that elicits the pain (Woo et al., 2017b). The SIIPS1 pattern of predictive weights includes portions of regions within the so-called “pain matrix” (e.g., insula, ACC, and thalamus) that were activated by noxious stimulation but also predicted pain above and beyond the effect of temperature, as well as regions in the PFC and striatum, which are not classic targets of ascending nociceptive pathways, that predicted variations in pain but were not influenced by differences in temperature (Woo et al., 2017b). This classification approach demonstrates considerable success in distinguishing pain from non-pain, suggesting that some brain responses may be sensitive and specific to pain, rather than nociception.
Painful and noxious stimuli also reliably modulate responses in the ANS (for a review, see Kyle and McNeil (2014) (Kyle and McNeil, 2014)), consistent with pain’s relevance for survival. The ANS is comprised of both a sympathetic and a parasympathetic branch, which together innervate end organs in the periphery in accordance with changing physiological and psychological demands. Noxious stimuli elicit robust ANS responses across multiple end organs (for a brief synopsis of peripheral ANS measures and pain, see Figure 2B). Specifically, noxious stimuli can enhance sympathetic activity, resulting in enhanced SCR (Geuter et al., 2014; Hampf, 1990; Loggia et al., 2011; Mischkowski et al., 2019; Nickel et al., 2017; Paine et al., 2009; Schestatsky et al., 2007), and elicit parasympathetic withdrawal, as indexed by reductions of vagally-mediated heart rate variability (HRV) during pain (Bendixen et al., 2012; Pollatos et al., 2012; Sclocco et al., 2016a; Treister et al., 2012b). Some measures (e.g. increases in heart rate (HR) (Hampf, 1990; Loggia et al., 2011; Moltner et al., 1990; Paine et al., 2009; Treister et al., 2012a; Victor et al., 1987), blood pressure (Chalaye et al., 2013; Kregel et al., 1992; Reyes del Paso et al., 2011; Reyes Del Paso et al., 2014; Victor et al., 1987), pupil diameter (Eisenach et al., 2017; Geuter et al., 2014; Höfle et al., 2008; Mischkowski et al., 2019), and some measures of heart rate variability (Aslaksen et al., 2007; Chouchou et al., 2011; Terkelsen et al., 2005) are likely to reflect activity in both branches of the ANS, which can operate reciprocally, in parallel, or independently (Berntson et al., 1991; Berntson et al., 1994).
Recently, pain researchers have begun assessing whether peripheral ANS responses may be specific to pain or nociception. In one study, ten minutes of tonic heat pain were administered to 39 healthy volunteers while they rated their subjective pain continuously. Researchers found that SCR was more related to changes in stimulus intensity than subjective pain (Nickel et al., 2017). However, opposing conclusions were found in a series of acute pain studies that included a larger range of temperatures across both innocuous and noxious ranges (Mischkowski et al., 2019). In those studies, temperature effects on SCR and pupil dilation were mediated by subjective pain, and simply labeling a noxious stimulus as painful increased the stimulus-evoked SCR response, even in response to the same objective temperature. Since combining multiple metrics of the ANS response across different end organs best explains variance in pain intensity in both regression (Geuter et al., 2014) and multivariate classifier models (Donaldson et al., 2003), understanding the sensitivity of different ANS measures to pain and nociception will be an important direction for future work.
Together, this work indicates that it is possible to distinguish pain from nociception at the level of the central and peripheral nervous system and that portions of both the “pain matrix” and the ANS process both nociceptive and nociceptive-independent pain information. However, in the interest of evaluating pain specificity, an important limitation of this body of work is that studies that focus entirely on dissociating pain from nociception rarely compare pain and nociception with other stimulus modalities. Though pain intensity may scale with nociceptive input, it may also be related to non-specific features of the stimulus, such as salience (Wiech et al., 2010), aversiveness/unpleasantness (Bushnell et al., 2013), and/or endogenous fluctuations in arousal and attention (Ploner et al., 2010). This begs the question of whether pain-related processes identified in these studies are specific to the subjective sensation of pain elicited by noxious stimulation, or whether they might be associated more broadly with non-specific processes that are shared between pain and other sensory modalities. In the next section, we discuss two primary domain-general processes that may account for a large portion of the non-specific brain response to pain: salience processing and arousal.
What are salience and arousal, and how are they represented in the brain and body?
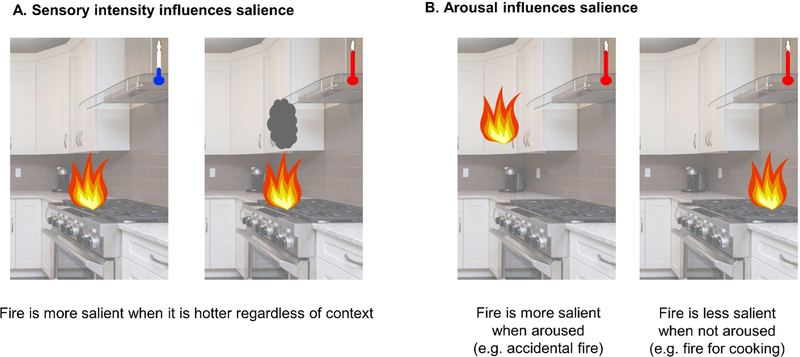
Salience is a perceived characteristic of a stimulus that makes it is more likely to be distinct from other stimuli (Itti and Koch, 2001; Uddin, 2015; Yantis and Hillstrom, 1994). Salience is one of the most universal and non-specific factors in a large variety of exteroceptive sensations, including acute pain (Craig, 2009). The perceived salience of a stimulus is different from the sensory intensity of a stimulus, similar to the distinction between pain and nociception, as salience is affected by interoceptive and environmental factors (Borsook et al., 2013). For example, a fire might have a certain stimulus intensity (e.g., temperature, luminance), but the perceived salience of the fire may vary depending on psychological factors (e.g., attention) and environmental factors (e.g., context). A fire in one’s kitchen cabinet is likely more salient than a fire on the stovetop or in a fireplace (Figure 3).
Figure 3. How sensory intensity and arousal influence perception in the environment.
Salience, or the perception that a stimulus is distinct from its surroundings, is influenced by a number of factors including (A) objective properties of the stimulus (e.g., its intensity) and (B) properties of the perceiver that influence the perceiver’s perception of the stimulus (e.g., whether the perceiver is aroused). The temperature of a fire is an objective feature of the stimulus (e.g., its intensity) that will affect how salient it is. If a perceiver is more aroused (e.g., because they reached toward a cabinet and learned it was on fire), the fire would be perceived as more salient than if a perceiver were less aroused (e.g., because they intentionally start a fire on their gas stovetop to prepare a meal).
The salience network is comprised of brain regions that process salient environmental changes regardless of stimulus modality. It has been postulated that the anterior insula and the dorsal ACC – two key regions of the pain matrix discussed above - are hubs of the salience network (Downar et al., 2003; Seeley et al., 2007; Taylor et al., 2009), which also includes the amygdala, ventral tegmental area, thalamus, hypothalamus, and PAG (Menon and Uddin, 2010; Seeley et al., 2007) (purple regions in Figure 2A). A wide range of functions has been ascribed to these regions: They are involved in interoceptive processing (Seeley et al., 2007), respond to emotion (Critchley, 2009), reward (Menon and Levitin, 2005), and threats to homeostatic regulation (Peyron et al., 2000), are activated by a variety of exteroceptive sensations (Craig, 2009; Critchley, 2009), show intrinsic connectivity during resting state (Seeley et al., 2007), and facilitate network switching (Goulden et al., 2014; Sridharan et al., 2008). For extensive discussions on salience and the salience network, please see Menon and Uddin (Menon and Uddin, 2010) and Uddin (Uddin, 2015).
One factor that can render stimuli salient is arousal (Mather and Sutherland, 2011; Sutherland and Mather, 2018). Arousal is a state of heightened awareness of and sensitivity to the environment (Critchley et al., 2013), and can influence which stimuli in an environment are most salient (e.g., Figure 3). A state of arousal enables an organism to act upon its environment by engaging motoric, emotional, and motivational systems and enhancing sensitivity to sensory input (Calderon et al., 2016; Pfaff et al., 2012). Arousal is a heterogenous construct, and the term has been used variously to describe multiple concepts, including physiological (autonomic) arousal, affective (i.e., subjective) arousal, and wakefulness (Satpute et al., 2018). Here we focus on autonomic arousal to index the state of being aware of and sensitive to one’s environment. Arousal is usually associated with both sympathetic nervous system outflow (e.g., increased HR or SCR) and parasympathetic nervous system withdrawal (e.g., decreased vagally mediated HRV; Figure 2). Though it is tempting to use a single measure of autonomic arousal as a proxy for the psychological state of being aroused, activity at different end organs does not always correspond in a one-to-one fashion (Berntson and Cacioppo, 2007; Cacioppo and Tassinary, 1990; Norman, 2016), as autonomic output results not only from brain processes but also mechanisms in the periphery (e.g., the sinoatrial node of the heart) and spinal reflexes. Thus, combining autonomic metrics across many endorgans permits a more precise characterization of physiological arousal.
Physiological arousal is thought to be driven by activity in a network of regions that is referred to as the central autonomic network (CAN in Figure 2A (Benarroch, 1993; Berntson et al., 1993; Cacioppo et al., 2007)). A great deal of animal work (Jhamandas and Renaud, 1987; Jordan and Spyer, 1986; Sawchenko and Swanson, 1981; Smith et al., 1991; Strack et al., 1989), as well as post-mortem human work (Arango et al., 1988) and work with direct stimulation of cortical tissue in humans (Chouchou et al., 2019; Oppenheimer et al., 1992), has linked ANS responses in the periphery with a network of regions in the brain, including cortical structures such as the anterior and posterior insular cortex and ACC, midbrain structures such as the thalamus and amygdala, and brainstem structures such as the PAG, the nucleus tractus solitarius of the medulla (NTS), the paraventricular nucleus of the hypothalamus (PVN), and the parabrachial nuclei (PBN) (red and purple regions in Figure 2A). In fMRI studies, it is also widely established that activity in regions of the CAN corresponds with ANS activity (Critchley et al., 2000; Eisenbarth et al., 2016; Napadow et al., 2008; Sclocco et al., 2016b; Thayer et al., 2012) (for a meta-analysis, see (Beissner et al., 2013)). However, widespread cortical activation has been found to correlate with autonomic parameters in fMRI (e.g., Valenza et al., 2019); thus, we focus only on regions that are corroborated by both fMRI studies as well as studies using animals, electrical stimulation, lesions, or post-mortem tissues. The regions that have the strongest convergent evidence are the ACC, insula, PAG, amygdala, and thalamus (see Figure 2A). The CAN is responsible for coordinating organized ANS responses across multiple end-organs that together enable the body to effectively minimize or escape potential bodily threat, and activity in the CAN reflects both efferent and afferent control of autonomic functions, as well as both sympathetic and parasympathetic peripheral activity. For instance, activity in the posterior insula, which is the target of many ascending projections from the periphery via the lamina I spinothalamocortical tract (Craig, 2002; Craig, 2009; Damasio and Carvalho, 2013; Dum et al., 2009), tends to be involved in afferent control of cardiac function, whereas activity in the anterior insula tends to be involved in efferent control of cardiac function (Cechetto, 2014; Macey et al., 2012; Palma and Benarroch, 2014). Further, electrical stimulation has suggested functional dissociations between the middle and posterior human insular cortex in the control of the two branches of the ANS (at least with respect to the heart). Posterior stimulation is associated with tachycardia, corresponding to sympathetic control, while more anterior stimulation is associated with bradycardia, corresponding to parasympathetic control (Chouchou et al., 2019), consistent with animal work (Marins et al., 2016; Oppenheimer & Cechetto, 1990).
Emerging evidence suggests that physiological arousal may also be related to activity and connectivity within the salience network (Schneider et al., 2016; Xia et al., 2017; Young et al., 2017). Indeed, interoceptive ascending inputs are delivered to the thalamus by autonomic afferent nuclei in the CAN network (e.g., NTS and PBN) and integrated in the posterior insula and ACC, and the salience network projects the information to autonomic efferent nuclei and the dorsal motor nucleus to activate a viscero-motor response (Uddin, 2015). Further, stimuli that exhibit characteristics that could make them perceived as more salient, such as those that are more intense (e.g., louder sounds, brighter lights, and images with greater emotional valence) or more distinct from their surroundings, elicit greater physiological arousal responses (Barry and Furedy, 1993; Beissner et al., 2013; Bull and Lang, 1972; Gatchel and Lang, 1973; Napadow et al., 2008; Simons and Lang, 1976; Smith and Strawbridge, 1969; Turpin and Siddle, 1979; Uno and Grings, 1965). We therefore assume that more salient stimuli evoke greater physiological responses.
Given the strong relationship between pain, salience, and arousal, we propose that researchers in search of pain-specific brain processes should isolate and account for variations in salience by statistically controlling for physiological arousal. Doing so will enhance identification of pain-specific processes. In the following sections, we evaluate evidence of functional and anatomical overlap between salience, arousal, and pain, and propose an approach by which to use this overlap to isolate and evaluate pain-specific brain processes.
Relationships between pain, salience, and physiological arousal
As mentioned earlier, few studies that distinguish pain from nociception in the interest of isolating pain-specific processes have accounted for non-specific processes such as salience and arousal. An active debate over whether there are pain-specific brain responses (Berkley and Hubscher, 1995; Hu and Iannetti, 2016a; Moayedi and Davis, 2013; Mouraux and Iannetti, 2018; Zunhammer et al., 2016) persists in large part because painful stimuli 1) are salient and automatically capture our attention; 2) elicit physiological arousal across multiple end organs (Chalaye et al., 2013; Eisenach et al., 2017; Geuter et al., 2014; Hampf, 1990; Höfle et al., 2008; Kregel et al., 1992; Loggia et al., 2011; Mischkowski et al., 2019; Moltner et al., 1990; Nickel et al., 2017; Paine et al., 2009; Reyes del Paso et al., 2011; Reyes Del Paso et al., 2014; Schestatsky et al., 2007; Treister et al., 2012a; Victor et al., 1987); and 3) recruit much of the same neural circuitry as salience and arousal. Further, many of the brain regions within the pain matrix are associated with processing stimulus-general effects across sensory modalities. The insula, for instance, is the key integration region in many theories of cortical pain perception (reviewed in Moayedi, 2014), and electrical stimulation and lesion of the posterior insula produces pain in patient populations (Garcia-Larrea et al., 2010; Mazzola et al., 2009; Montavont et al., 2015). Yet the insula has also been variously associated with disgust (Wicker et al., 2003), pleasantness (Koelsch et al., 2006), and awareness (Craig, 2009), and is activated by noxious stimulation even in patients with congenital insensitivity to pain (Salomons et al., 2016). Moreover, insula lesions do not abolish pain processing (Baier et al., 2014; Feinstein et al., 2016; Starr et al., 2009). Similarly, the dorsal ACC (dACC) contains nociceptive neurons (Hutchison et al., 1999) and was one of the earliest loci of pain modulation (Rainville et al., 1997), yet pain can persist after cingulotomies (Yen et al., 2005), and heated debate concerns the dACC’s role in conflict (Kerns et al., 2004), foraging (Kolling et al., 2012), and response inhibition (Braver et al., 2001; Garavan et al., 2002) – non-specific functions that may be linked with pain, but certainly are not specific to pain. These two regions are widely considered to act as hubs of the salience network (Downar et al., 2003; Seeley et al., 2007; Taylor et al., 2009), as mentioned above, and have been proposed to make up a “task-set network” (Dosenbach et al., 2006) based on the fact that they are co-activated by nearly all cognitive and affective tasks (Dosenbach et al., 2006; Yarkoni et al., 2011). Most importantly, in direct comparisons, these regions and others in the so-called pain matrix all show considerable activity in response to not only painful stimulation, but also olfactory stimuli (Lotsch et al., 2012), non-painful pressure (Wey et al., 2014), and auditory, visual, and non-nociceptive somatosensory stimuli (Lui et al., 2008; Mouraux et al., 2011).
Similarly, while individual studies may measure whether stimulus-evoked ANS responses are more related to nociception or pain (Mischkowski et al., 2019; Nickel et al., 2017), none of these studies have compared ANS responses to pain with other arousing modalities. ANS responses are linked to subjective experience across many different domains (e.g., auditory, visual, and emotional stimuli elicit ANS responses (Beissner et al., 2013; Bradley et al., 2001; Napadow et al., 2008; Smith and Strawbridge, 1969; Turpin and Siddle, 1979; Uno and Grings, 1965)). The CAN also overlaps to a large extent with pain matrix (including the insula, ACC, and PAG; Figure 2A). Activation in brain regions typically active during pain, including the dACC, thalamus, anterior insula, and amygdala, is associated with greater skin conductance responses (i.e., greater sympathetic arousal) to painful versus innocuous stimuli (Dubé et al., 2009; Piché et al., 2010). Furthermore, much (though not all) of the brain’s response to noxious, relative to innocuous, stimuli overlaps with a network that may be involved in central regulation of peripheral blood flow via sympathetic afferents (Maihöfner et al., 2011)., suggesting that this network may be involved in modulation of arousal-related processes in the periphery during pain. Thus, activation of what is sometimes called the pain matrix may be explained in large part by CAN activity corresponding with evoked autonomic arousal, a process that is not specific to pain.
The above evidence is consistent with the argument that no brain regions or responses are specific to pain (Carmon et al., 1976; Iannetti et al., 2008; Iannetti and Mouraux, 2010; Legrain et al., 2011). However, there is an alternative interpretation of this work: pain-specific brain responses may exist, but these responses may be obfuscated by domain-general brain responses that are sensitive to features of sensory stimuli across all modalities. These domain-general processes must be appropriately accounted for in order to identify pain-specific brain responses.
State of the field: Evidence of pain specificity
To evaluate the role of non-specific processes such as salience, Mouraux et al. (2011) tested various sensory stimuli, including painful heat, non-nociceptive electrical stimuli, and visual and auditory stimuli. Across participants, average salience ratings were correlated with the magnitude of stimulus-evoked blood oxygenation level dependent (BOLD) response in most regions of the pain matrix, even within non-painful sensory modalities. That is, even highly salient auditory and visual stimuli caused brain responses in insula, ACC, and S2 similar to pain (Mouraux et al., 2011). Yet these results do not conclusively suggest a lack of pain-specific responses in the brain since analyses did not account for within-subjects variations in salience. A follow-up analysis by Liang and colleagues (2019) (Liang et al., 2019) used the same data (as well as a second similar dataset) but matched painful and non-painful sensory stimuli within subjects using trial-by-trial salience ratings. Pain-specific brain responses (i.e. those that differed from equally salient innocuous touch, visual stimuli, and auditory stimuli) were indeed present when stimuli were matched based on perceived salience, specifically within the insula and secondary somatosensory cortex (SII), as well as when the authors used MVPA. Successful pain classification was observed when MVPA was restricted to either pain matrix regions or non-pain matrix regions. These results indicate that painspecific patterns of activation indeed exist, but it is critical to account for dynamic variations in salience specificity to pain. This work, however, did not measure relationships with subjective pain, but instead treated pain as a categorical percept, which was not distinguished from nociception. A remaining question is whether brain responses can specifically predict the subjective experience of pain, above and beyond both salience and nociception, which is critical to the search for brain-based biomarkers of subjective pain (Mouraux and Iannetti, 2018; Poldrack, 2011; Robinson et al., 2013; Woo et al., 2017a).
Two promising candidate regions for pain-specificity are the dorsal posterior insula (dpIns) and SII. Electrical stimulation of the dpIns can produce pain (Mazzola et al., 2009; Montavont et al., 2015) and one ASL study (Segerdahl et al., 2015) found that the dpIns uniquely correlated with pain reports during tonic capsaicin-induced pain, but was insensitive to vibratory stimulation. However, dpIns stimulation also increases physiological arousal (Chouchou et al., 2019) and the ASL study could not conclusively rule out the possibility that the dpIns also plays a role in non-specific processes (Davis et al., 2015), in part because non-somatosensory stimuli were not presented. A recent study by Horing & colleagues took a definitive step towards identifying pain-specific brain processes. They accounted for both salience and modality-specificity by comparing painful heat with auditory stimuli and matching stimuli on the basis of stimulus-evoked physiological arousal (i.e., SCR). SII and dpIns both showed greater activation to heat (both painful and non-painful) compared to sound, and the activity in the SII and dorsal anterior insula were significantly related to perceived intensity rating (Horing et al., 2019). However, only SII showed both specificity to nociception (heat vs. sound, noxious vs innocuous heat) and pain (correlation with subjective ratings). This is an important advance toward evidence of specificity, although a complete demonstration will require additional control modalities.
Pain-specific patterns of activity may also be distributed, rather than isolated within specific regions, since individual brain regions are often insufficient for the production of pain (Penfield and Boldrey, 1937; Feinstein et al., 2016; Foltz and White, 1962; Geschwind, 2010; though see Mazzola et al., 2009 & Montavont et al., 2015). As alluded to earlier, an increasing number of neuroimaging studies have applied multivariate analyses such as MVPA and machine learning classifiers to extract latent information from neuroimaging data across brain regions (Kragel et al., 2018). Multivariate patterns of brain activation have shown considerable sensitivity and specificity to acute pain in healthy individuals (Cecchi et al., 2012; Marquand et al., 2010; Schulz et al., 2012; Wager et al., 2013) and have revealed significant pain-related patterns in patients with chronic pain (Callan et al., 2014; Lopez-Sola et al., 2017). The Neurologic Pain Signature, for example, is a physical pain-specific pattern in the brain that predicts subjective pain and is driven by activation in the PAG, thalamus, secondary somatosensory cortex, posterior and anterior insula, and ACC (Wager et al., 2013). It discriminates heat/mechanical/electrical/visceral pain from warmth and the anticipation of pain, social, and vicarious pain (Krishnan et al., 2016; Wager et al., 2013; Woo et al., 2017a; Woo et al., 2014). In addition, MVPA applied to electroencephalography signals in response to repeated pain stimuli predicted individuals’ pain sensitivity (with an accuracy of 83%) (Schulz et al., 2012) and a machine learning algorithm on patterns of whole-brain fMRI BOLD signals distinguished between painful and non-painful thermal stimuli (with an accuracy of 81–84%) (Brown et al., 2011).
While extremely promising and productive, to our knowledge most of these patterns have not yet fully accounted for salience, although the recent work by Liang et al. (Liang et al., 2019) is a promising step in this direction. When studies do not formally account for salience using subjective or physiological measures, differences attributed to pain may simply reflect the fact that pain is more salient and arousing than most non-painful stimuli. Thus, what has been observed to be a pain-specific pattern could still be partially related to pain-related salience and arousal, leaving open the question of whether purported pain-specific brain processes described above are indeed specific to pain (Hu and Iannetti, 2016b; Salomons et al., 2016). In the next section, we outline an approach that builds on the work of Horing et al. (Horing et al., 2019) and uses peripheral signals of autonomic arousal to account for salience and arousal processing in the brain, rather than experimentally matching the salience of stimuli based on subjective ratings. This approach circumvents assumptions that painful and non-painful stimuli must be matched on these attributes, as arousal and salience responses might be different in each individual (e.g., phobia-related visual cues could produce an attentional bias and increase arousal in a person with phobia more than in non-fearful participants (Aue et al., 2013; Ohman and Soares, 1994)) and are also affected by experimental context and stimulus novelty (Foley et al., 2014; Kalat, 1974).
How can we use brain-body connections to determine pain specificity in the brain?
A proposal to use autonomic activity to isolate non-specific salience and arousal in responses to pain
We propose that we must consider the role of nonspecific processes and related responses in the brain and body to isolate and understand pain-specific responses. We suggest that researchers investigating pain-specific brain responses measure and statistically control for the brain signals associated with salience and arousal by including continuous physiological measures of arousal (down-sampled to the timing of the BOLD signal) in their FMRI analyses as nuisance regressors of no interest. One or more measures of physiological arousal should be used (such as SCR, HR, HRV, BP, or pupil dilation), although integrating across physiological measures is likely to be best, as discussed below. To the extent that the brain response to pain is non-specific, brain activity may correspond temporally with physiological arousal not only during pain but also during endogenous fluctuations in arousal between stimuli. If a non-painful control condition is also assessed (e.g. warm temperature), this brain activity should correspond temporally to non-painful stimulus evoked arousal as well. The residualized BOLD signal associated with painful stimuli, compared to non-pain control stimuli, that remains after removing variance associated with ANS-related arousal should be less confounded by non-specific features of pain, and thus arguably more specific to pain. Though this approach would primarily account for stimulus-related salience and fluctuations in endogenous arousal, it is also possible that it accounts at least partially for other non-specific factors, such as fear, anxiety, or threat-processing, since salience and arousal are also related to emotion and motivation. It should be noted that raw physiological signals measured during the scan should be preprocessed and converted into informative variables, if necessary, prior to downsampling to the timing of the BOLD signal. For example, in our initial feasibility application described below, we computed a continuous timeseries of respiration flow rate, the integral of respiratory excursion per each cycle period (Bach et al. 2016), from the raw respiration signal, and included it as a regressor, rather than using the raw respiration trace. On the other hand, since electrodermal activity is directly linked to physiological arousal, the SCR signal can be used as a regressor for our approach after appropriate preprocessing (e.g. filtering, smoothing, down-sampling).
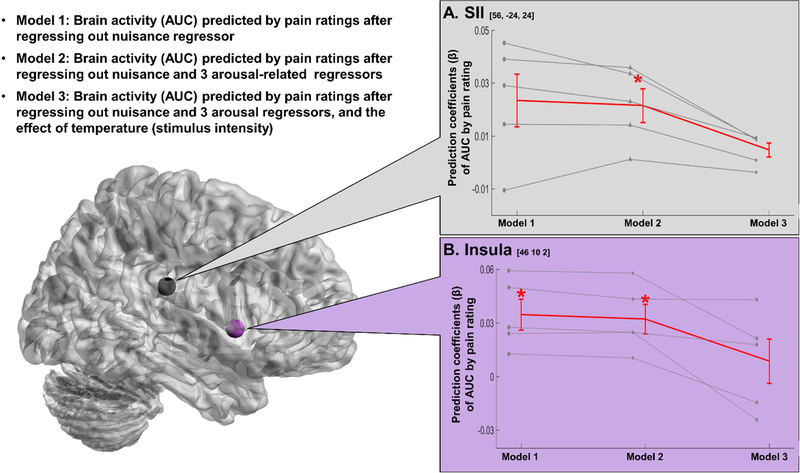
To test the feasibility of our approach, we turned to empirical data from a subset of participants from a previously published study (for more details, please see Atlas et al., 2014. In brief, participants received 4 different intensities of thermal stimuli (non-painful warm, low pain, medium pain, and high pain) and provided pain ratings on every trial. SCR, photoplethysmography (PPG), and respiration were measured continuously during scanning and preprocessed using the PsPM toolbox, then downsampled to the resolution of the TR (2s; 0.5Hz). For SCR, we applied low-pass filter of 1Hz, and smoothed using a moving average of each second. We converted PPG signal (obtained by peripheral pulse oximeter) into heart beat using the pspm_ppu2hb function and calculated heart rate per each TR. Respiration flow rate (integral of respiratory excursion per each respiration cycle period) was computed from raw respiration signal in PsPM using pspm_resp_pp function. To evaluate our approach, we extracted data from SII and insula and used single trial analysis (Atlas et al., 2014; Mumford et al., 2014; Rissman et al., 2010) to fit heat-evoked area under the curve (AUC) estimates for each trial in three models (see Figure 4). Model 1 estimated AUC after regressing out standard nuisance regressors (head motion and spikes), identical to our original processing pipeline (Atlas et al., 2014). Model 2 included standard nuisance regressors (motion, spikes) as well as regressors for physiological arousal (SCR, HR) and noise (respiration). Note that though respiration could contain some arousal-related signal (e.g., if a participant holds their breath in anticipation of or during pain), respiratory fluctuations also introduce noise into both the BOLD timeseries and the timeseries of other peripheral autonomic measures. Model 3 included the same physiological regressors as Model 2, but measured relationships between AUC and pain while controlling for stimulus intensity (i.e. nociception). We tested each model using the general linear model, in which subjective pain was the independent variable and AUC for each ROI was the dependent variable. Preliminary results are illustrated in Figure 4. The residual brain activity in the right insula, but not in the SII, is significantly predicted by pain ratings after regressing out nuisance variables (Model 1), while the residual brain activity in both regions is significantly predicted by pain ratings after regressing out the effect of arousal as well as nuisance (Model 2). However, after regressing out the effect of nociception from the brain activity in Model 3, pain ratings do not predict brain activity in either region, suggesting responses in these five subjects were driven by nociception, rather than subjective pain. Of course, given the small sample size and the fact that this study was not designed to evaluate pain specificity (i.e. other stimulus modalities were not presented), conclusions from these findings pertain to the utility of this recommended analytical approach, rather than speaking empirically to presence of pain-specific brain responses. However, we believe this preliminary example provides promising support for the argument that regressing out the effect of arousal using ANS activity will improve tests of pain specificity.
Figure 4. Preliminary result of our approach to using autonomic activity to isolate non-specific salience and arousal in the brain response to pain.
Here we illustrate our approach by applying it to a subset of fMRI data (n = 5) acquired during an acute thermal pain task (Atlas et al., 2014). To test the effect of regressing out physiological arousal using autonomic nervous system (ANS) activity, we evaluated three regression models that measured subjective pain as a function of brain activity. We extracted data from right secondary somatosensory cortex (SII) and right middle insula, two ROIs previously associated with subjective pain (Atlas et al., 2014), and used single trial analysis (Atlas et al., 2014; Mumford et al., 2014; Rissman et al., 2010) to generate heat-evoked area under the curve (AUC) estimates for each trial based on three models. Model 1 included only traditional nuisance regressors (motion, spikes, and intercepts). Model 2 included nuisance regressors and regressors for physiological responses (skin conductance response, respiration, and heart rate). Model 3 included nuisance regressors, regressors for physiological responses, and the effect of stimulus intensities (4 levels; warm, low, medium, and high pain) to account for nociceptive processing. We used linear regression to evaluate the link between subjective pain and AUC estimates generated by each model and tested effects across participants.
Caveats
Our approach is not without limitations. For one, our approach advises measuring the construct of ‘arousal’, but to do so most precisely, it may be necessary to combine autonomic measurements (e.g., SCR, pupil diameter, HR, HRV, BP) across different end-organs (e.g., dermis, pupil, heart, and vasculature, respectively; (Norman, 2016)). Indeed, identifying brain responses commonly associated with physiological arousal across many different end-organs may elucidate brain processes that are involved in the central regulation of peripheral arousal more broadly, rather than processes that are specific to one autonomic system or end-organ. Given that ANS metrics are influenced by factors outside the brain, including spinal reflexes (Pickering and Paton, 2006) and cells in the periphery (such as the sinoatrial node, which serves as a pacemaker to modulate intrinsic heart rate in the absence of central autonomic influence; Jose and Collison, 1970; Opthof, 2000; Mangoni and Nargeot, 2008), brain correlates that are common to multiple ANS responses might better correspond to central regulation of peripheral arousal than regions that exhibit different patterns of activation for ANS metrics from different end-organs. Furthermore, combinations of ANS measurements across many end-organs better predicts graded differences in pain intensity (Donaldson et al., 2003; Geuter et al., 2014; Jiang et al., 2018; Matthewson et al., 2019; Treister et al., 2012a), demonstrating the utility of studying the arousal response as a whole for capturing the pain percept. However, this can be a constraint of our method, as multimodal measurement of the ANS response can be costly and can demand intensive processing resources. Techniques such as principal component analysis will help reduce the dimensionality of autonomic time series from multiple end-organs and identify shared variance across peripheral signals. Another potential caveat to our approach is that it makes no strong assumptions on the direction of brain-body interactions (e.g., whether pain-evoked ascending ANS activity elicits a brain response, or the brain sends descending signals to regulate the ANS response to pain, or both), but rather argues that central activity related to arousal (either efferent or afferent) is a process that is not unique to pain and therefore should be accounted for to better identify pain-specific brain responses. Whether correlations between BOLD activity and autonomic metrics reflect efferent, versus afferent, brain activity is not well established (see Napadow (2008) (Napadow et al., 2008) and Maihöfner et al. (2011) (Maihöfner et al., 2011) for a similar concern). Further, best practices for accounting for differences in the time course of the autonomic signal, relative to the BOLD signal, have yet to be determined (though see Iacovella & Hasson, 2011 (Iacovella and Hasson, 2011) for a thoughtful discussion on decisions regarding removing ANS correlates from fMRI data). Further, though the vast majority of studies that have examined brain correlates of autonomic measures have directly correlated the autonomic time series with voxel time series (Beissner et al., 2013), it is likely that the temporal correspondence between autonomic measures and BOLD activity is heterogenous throughout the brain (Chang and Glover, 2009; Chang et al., 2008; Chen et al., 2019). In longer block designs, timing may not matter as much, since signals are sustained over time allowing researchers to decouple autonomic activity regardless of slight shifts in timing. For example, one study suggested that autonomic signals can be shifted up to 30 seconds relative to the BOLD signal with minimal impact on observed BOLD-autonomic correlations (Eisenbarth et al., 2016). However, in event-related designs where precise timing is more important, conclusions may be affected if the temporal correspondence between the BOLD and autonomic time series is incorrectly specified. Thus, this approach might be best suited for tonic pain stimuli, or for stimuli that last several seconds rather than brief transient stimuli such as shocks, although researchers can explore approaches such as shifting the ANS regressors in time to capture potential delays when longer stimuli are not possible. With these design caveats in mind, concerns regarding the direction and timing of brain-body interactions are unlikely to limit the promise of our approach for identifying pain specificity, as whether brain activity is afferent versus efferent should not affect how specific it is to pain.
Future Application of this Approach
As pain is a complex phenomenon, it is interesting and also complicated to postulate which components of pain would be left after regressing out the non-specific effects. The ability to shift our attention to painful stimuli and process pain-related information (i.e., the source of the pain, where the body is affected, how likely the stimulus is to damage tissue) is crucial for survival of an organism. Acute pain strongly motivates prediction, decision, and actions (e.g., one takes one’s hand away from boiling water to prevent future tissue damage; (Van Damme et al., 2010)) and some have argued that pain has a higher priority to be processed than other non-painful but salient and arousing sensations (Wiech and Tracey, 2013). Thus, pain may be distinguished from other percepts based on its function to motivate defensive behaviors and enable survival. Work on the neural circuitry of survival and threat processing may indicate potential circuitry that could persist accounting for the non-specific effects of pain (for a review on threat and neural survival circuits, please see LeDoux and Daw (2018) (LeDoux and Daw, 2018)). In addition, subjective pain experience related signals in the OFC, ventro-medial PFC, ACC, and insula (Atlas et al., 2014) and distinguishable patterns of sensory modality in the primary sensory cortices (e.g., pain versus touch, pain versus auditory stimuli) (Liang et al., 2013) could survive as a pain-specific brain response even after regressing out non-specific components. It is important here to note that the presence of pain-specific brain responses does not necessarily suggest that overall processing of pain is entirely different from the processing of other sensations in the brain, but rather that some components may be pain-specific, even if they co-exist with other brain processes that are related to pain but also shared with other sensations. For example, though expectations can modulate pain through domain-specific modulatory mechanisms (for instance, within the insular cortex (Fazeli and Büchel, 2018; Sharvit et al., 2015)), expectancy effects on pain are also related to activity in the ventrolateral prefrontal cortex (vlPFC) in a domain-general fashion (Petrovic et al., 2010). Indeed, most modulatory mechanisms are likely to involve both domain-general and domain-specific processes (Atlas and Wager, 2013).
Of course, it is also possible that statistically controlling for non-specific effects of pain does not leave any brain response that is specific to pain. Indeed, in our preliminary application of this approach, two regions that have been implicated as pain-related, the insula and SII, no longer exhibited correlations with pain when we accounted for both physiological arousal and nociception (Figure 4). Does this suggest that pain is not a unique percept? On the contrary, it is possible that pain may be distinct from other sensations not in the brain processes involved but in the relative contribution of each of those constituent processes. Throughout this review, we have argued that it is important to account for brain processes that pain may share with other sensations, namely salience processing and arousal. But we remark here that if accounting for these non-specific processes leaves no ‘pain-specific’ pattern of brain activation, that does not necessarily imply that the brain response to pain cannot be distinguished from the brain’s responses to other stimuli. Rather, the relative contribution of each of these constituent non-specific processes - that is how salience and arousal processing work in tandem during pain perception, as well as how they work with nociception and other processes non-specific to pain that were not discussed here (e.g., motivation to survive, fear or threat processing) – may be what distinguishes the brain’s response to the pain from its response to other percepts. In other words, the relative balance and interacting contributions between arousal, salience, nociception, and other non-specific factors in explaining the brain’s response to pain may be unique from the relative weights or contributions in any other percept. For example, pain may differ from other salient and threatening stimuli in terms of the relative contribution of the amygdalar evaluation of threat, hippocampal processing of contextual information, prefrontal switching of amgydalar threat signals into behavioral response, and motor cortex preparation and execution of behavior, all processes involved in survival and threat processing (LeDoux and Daw, 2018). Empirical questions remain open, and application of our approach is a promising direction for answering these questions and identifying what remains of the brain response to pain after accounting for non-specific processes.
We have focused presently primarily on experimental research using neuroimaging to measure the brain response to acute pain. To advance the scientific endeavor of identifying pain specificity in the brain, it will be necessary to accumulate converging evidence from various approaches, spanning not only neuroimaging data but also direct brain stimulation/inhibition and lesion studies in animals. Our approach has the potential to fill a much-needed role in the pursuit of techniques that can help inform clinical assessment and treatment of pain. In patients with chronic pain, complementing brain data with ANS measures has already enhanced differentiation of clinical pain states. One recent study combined brain data (cerebral blood flow and functional connectivity) with pulse rate variability in patients with chronic low back pain to distinguish between high and low clinical pain states with over 90% accuracy (Lee et al., 2018). This study demonstrates that meaningful variance in chronic pain states can be explained by ANS arousal. To the extent that we can account for brain activity associated with those physiological responses, we can rule out non-specific variance in the brain’s response to pain and begin to identify more pain-specific responses.
If we successfully identify pain-specific brain patterns that cannot be explained by salience and arousal, this work could have great potential for use in clinics as a diagnostic and prognostic biomarker for pain. Despite decades of efforts of clinicians and researchers, we still rely on subjective reporting to characterize pain, which has a risk of being biased by self-presentation concerns and has limitations in the clinic, especially when trying to understand the pain of infants and patients with cognitive impairments or without an ability to make conscious reports (i.e., patients in a coma). The need to evaluate pain in these patient populations has hastened the search for biomarkers of pain. Yet it has also increased the potential for ethical dilemmas (Davis, 2016; Robinson et al., 2013), as these patients can neither confirm nor deny if the pain biomarker maps onto their subjective pain. Thus, it is critical that scientists use caution when searching for neuroimaging pain biomarkers, using the approach recommended here or others. Salience circuits have been implicated in chronic pain (Borsook et al., 2013), but our goal in the present review was to suggest an approach that can determine whether there are circuits that are specific to acute pain, above and beyond the effects of salience. Further testing will be necessary to validate whether our recommended approach generalizes to identification of pain-specific brain processes in patients populations, especially because there may be potential differences in arousal-related autonomic processing in patient populations, particularly comatose patients. For an in-depth discussion on ethical issues of neuroimaging pain biomarker, please see Davis (2016) (Davis, 2016).
Conclusion
In this review, we suggest that pain is a complex phenomenon composed of non-specific factors and a potentially pain-specific response that distinguishes pain from other sensations. Though current work identifying a truly pain-specific brain response remains elusive, we have argued that parsing out non-specific responses to pain by studying brain-body interactions can improve the specificity of analyses and may uncover pain-specific neural patterns and brain responses in the future. To achieve this goal, it is necessary to measure brain processes associated with nociception as well as stimulus salience and arousal. We recommend multimodal data acquisition through the brain (CNS) and body (ANS) and that this approach be applied to multivariate data analysis that has achieved recent success in beginning to identify pain-specific neural signatures. The brain responses that are uniquely specific to pain, and not to stimulus intensity, salience, or arousal, will guide us to better measurements and improve our ability to characterize pain in research and in the clinic.
Acknowledgement
The authors would like to thank Qingbao Yu for advice on data analysis, and Troy Dildine, Ian Lyons, Shara Grant, Carolyn Amir, Margaret Rose-McCandlish, and Olga Oretsky for their helpful comments and discussion.
Funding source
This work was supported by the Intramural Research Program of the National Center for Complementary and Integrative Health, National Institutes of Health.
Footnotes
Data and code availability statement
Data available on request from the authors.
Declarations of interest: none
References
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK, 2005. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9, 463–484. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Darbar A, Krauss BR, Gelnar PA, Szeverenyi NM, 1999. Differentiating cortical areas related to pain perception from stimulus identification: temporal analysis of fMRI activity. J Neurophysiol 81, 2956–2963. [DOI] [PubMed] [Google Scholar]
- Arango V, Ruggiero DA, Callaway JL, Anwar M, Mann JJ, Reis DJ, 1988. Catecholaminergic neurons in the ventrolateral medulla and nucleus of the solitary tract in the human. J Comp Neurol 273, 224–240. [DOI] [PubMed] [Google Scholar]
- Aslaksen PM, Myrbakk IN, Hoifodt RS, Flaten MA, 2007. The effect of experimenter gender on autonomic and subjective responses to pain stimuli. Pain 129, 260–268. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Lindquist MA, Bolger N, Wager TD, 2014. Brain mediators of the effects of noxious heat on pain. Pain 155, 1632–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Wager TD, 2013. Expectancies and beliefs: insights from cognitive neuroscience. The Oxford handbook of cognitive neuroscience 2, 359–381. [Google Scholar]
- Aue T, Guex R, Chauvigne LA, Okon-Singer H, 2013. Varying expectancies and attention bias in phobic and non-phobic individuals. Front Hum Neurosci 7, 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier B, zu Eulenburg P., Geber C, Rohde F, Rolke R, Maihofner C, Birklein F, Dieterich M, 2014. Insula and sensory insular cortex and somatosensory control in patients with insular stroke. Eur J Pain 18, 1385–1393. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, 2009. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol 101, 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, Furedy JJ, 1993. Stimulus intensity and novelty interact in elicitation of the phasic electrodermal orienting response. Int J Psychophysiol 14, 249–254. [DOI] [PubMed] [Google Scholar]
- Beecher HK, 1946. Pain in Men Wounded in Battle. Ann Surg 123, 96–105. [PMC free article] [PubMed] [Google Scholar]
- Beecher HK, 1956. Relationship of significance of wound to pain experienced. J Am Med Assoc 161, 1609–1613. [DOI] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar K-J, Napadow V, 2013. The Autonomic Brain: An Activation Likelihood Estimation Meta-Analysis for Central Processing of Autonomic Function. Journal of Neuroscience 33, 10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza G, Sclocco R, Duggento A, Passamonti L, Napadow V, Barbieri R, Toschi N, 2019. The central autonomic network at rest: Uncovering functional MRI correlates of time-varying autonomic out flow. NeuroImage, 197, 383–390. [DOI] [PubMed] [Google Scholar]
- Benarroch EE, 1993. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 68, 988–1001. [DOI] [PubMed] [Google Scholar]
- Bendixen KH, Terkelsen AJ, Baad-Hansen L, Cairns BE, Svensson P, 2012. Experimental stressors alter hypertonic saline-evoked masseter muscle pain and autonomic response. J Orofac Pain 26, 191–205. [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH, 1995. Are there separate central nervous system pathways for touch and pain? Nat Med 1, 766–773. [DOI] [PubMed] [Google Scholar]
- Bernstein AS, 1969. To what does the orienting response respond? Psychophysiology 6, 338–351. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, 2007. Integrative Physiology: Homeostasis, Allostasis, and the Orchestration of Systemic Physiology In: Cacioppo JT, Tassinary LG, Berntson GG (Eds.), Handbook of Psychophysiology. Cambridge University Press, Cambridge, UK, pp. 433–452. [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS, 1991. Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev 98, 459–487. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS, 1993. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology 30, 183–196. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS, Fabro VT, 1994. Autonomic space and psychophysiological response. Psychophysiology 31, 44–61. [DOI] [PubMed] [Google Scholar]
- Borsook D, Edwards R, Elman I, Becerra L, Levine J, 2013. Pain and analgesia: The value of salience circuits. Progress in Neurobiology 104, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, 2009. Natural selective attention: Orienting and emotion. Psychophysiology 46, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ, 2001. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion 1, 276–298. [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A, 2001. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex 11, 825–836. [DOI] [PubMed] [Google Scholar]
- Brown JE, Chatterjee N, Younger J, Mackey S, 2011. Towards a physiology-based measure of pain: patterns of human brain activity distinguish painful from non-painful thermal stimulation. PLoS One 6, e24124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, 2002. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study. J Neurosci 22, 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull K, Lang PJ, 1972. Intensity judgments and physiological response amplitude. Psychophysiology 9, 428–436. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, Low LA, 2013. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG, 1990. Inferring psychological significance from physiological signals. The American psychologist 45, 16–28. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG, Berntson GG, 2007. Psychophysiological Science: Interdisciplinary Approaches to Classic Questions About the Mind. Handbook of Psychophysiology, pp. 1–16. [Google Scholar]
- Calderon DP, Kilinc M, Maritan A, Banavar JR, Pfaff D, 2016. Generalized CNS arousal: An elementary force within the vertebrate nervous system. Neuroscience and Biobehavioral Reviews 68, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan D, Mills L, Nott C, England R, England S, 2014. A tool for classifying individuals with chronic back pain: using multivariate pattern analysis with functional magnetic resonance imaging data. PLoS One 9, e98007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon A, Mor J, Goldberg J, 1976. Evoked cerebral responses to noxious thermal stimuli in humans. Exp Brain Res 25, 103–107. [DOI] [PubMed] [Google Scholar]
- Cecchi GA, Huang L, Hashmi JA, Baliki M, Centeno MV, Rish I, Apkarian AV, 2012. Predictive dynamics of human pain perception. PLoS Comput Biol 8, e1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF, 2014. Cortical control of the autonomic nervous system. Experimental physiology 99, 326–331. [DOI] [PubMed] [Google Scholar]
- Chalaye P, Devoize L, Lafrenaye S, Dallel R, Marchand S, 2013. Cardiovascular influences on conditioned pain modulation. Pain 154, 1377–1382. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH, 2009. Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI. Neuroimage 47, 1381–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Thomason ME, Glover GH, 2008. Mapping and correction of vascular hemodynamic latency in the BOLD signal. Neuroimage 43, 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JE, Lewis LD, Chang C, Fultz NE, Ohringer NA, Rosen BR, Polimeni JR, 2019. Resting-state “Physiological Networks”. BioRxiv, 660787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchou F, Mauguiere F, Vallayer O, Catenoix H, Isnard J, Montavont A, Jung J, Pichot V, Rheims S, Mazzola L, 2019. How the insula speaks to the heart: Cardiac responses to insular stimulation in humans. Hum Brain Mapp 40, 2611–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchou F, Pichot V, Perchet C, Legrain V, Garcia-Larrea L, Roche F, Bastuji H, 2011. Autonomic pain responses during sleep: a study of heart rate variability. Eur J Pain 15, 554–560. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews neuroscience 3, 655. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2003. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci 26, 1–30. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2009. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM, 2000. Thermosensory activation of insular cortex. Nat Neurosci 3, 184–190. [DOI] [PubMed] [Google Scholar]
- Critchley HD, 2009. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. International Journal of Psychophysiology 73, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Eccles J, Garfinkel SN, 2013. Interaction between cognition, emotion, and the autonomic nervous system. Handb Clin Neurol 117, 59–77. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ, 2000. Neural Activity Relating to Generation and Representation of Galvanic Skin Conductance Responses : A Functional Magnetic Resonance Imaging Study. Journal of Neuroscience 20, 3033–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A, Carvalho GB, 2013. The nature of feelings: evolutionary and neurobiological origins. Nature reviews neuroscience 14, 143. [DOI] [PubMed] [Google Scholar]
- Davis KD, 2016. Legal and ethical issues of using brain imaging to diagnose pain. Pain Reports 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Bushnell MC, Iannetti GD, Lawrence KS, Coghill R, 2015. Evidence against pain specificity in the dorsal posterior insula. F1000Research 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Moayedi M, 2013. Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol 8, 518–534. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL, 1997. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain 73, 431–445. [DOI] [PubMed] [Google Scholar]
- Donaldson GW, Chapman CR, Nakamura Y, Bradshaw DH, Jacobson RC, Chapman CN, 2003. Pain and the defense response: structural equation modeling reveals a coordinated psychophysiological response to increasing painful stimulation. Pain 102, 97–108. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE, 2006. A core system for the implementation of task sets. Neuron 50, 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Mikulis DJ, Davis KD, 2003. Neural correlates of the prolonged salience of painful stimulation. Neuroimage 20, 1540–1551. [DOI] [PubMed] [Google Scholar]
- Dubé AA, Duquette M, Roy M, Lepore F, Duncan G, Rainville P, 2009. Brain activity associated with the electrodermal reactivity to acute heat pain. Neuroimage 45, 169–180. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Albanese MC, 2013. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp 34, 109–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL, 2009. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. Journal of Neuroscience 29, 14223–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Crombez G, 1999. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull 125, 356–366. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, Curry R, Aschenbrenner CA, Coghill RC, Houle TT, 2017. Pupil responses and pain ratings to heat stimuli: Reliability and effects of expectations and a conditioning pain stimulus. J Neurosci Methods 279, 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth H, Chang LJ, Wager TD, 2016. Multivariate Brain Prediction of Heart Rate and Skin Conductance Responses to Social Threat. Journal of Neuroscience 36, 11987–11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli S, Büchel C, 2018. Pain-related expectation and prediction error signals in the anterior insula are not related to aversiveness. Journal of Neuroscience 38, 6461–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Khalsa SS, Salomons TV, Prkachin KM, Frey-Law LA, Lee JE, Tranel D, Rudrauf D, 2016. Preserved emotional awareness of pain in a patient with extensive bilateral damage to the insula, anterior cingulate, and amygdala. Brain Struct Funct 221, 1499–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, 2018. How expectations influence pain. Pain 159 Suppl 1, S3–S10. [DOI] [PubMed] [Google Scholar]
- Foley NC, Jangraw DC, Peck C, Gottlieb J, 2014. Novelty enhances visual salience independently of reward in the parietal lobe. J Neurosci 34, 7947–7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz EL, White LE, 1962. Pain “relief” by frontal cingulumotomy. Journal of neurosurgery 19, 89–100. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA, 2002. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage 17, 1820–1829. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Perchet C, Creac’h C, Convers P, Peyron R, Laurent B, Mauguiere F, Magnin M, 2010. Operculo-insular pain (parasylvian pain): a distinct central pain syndrome. Brain 133, 2528–2539. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Peyron R, 2013. Pain matrices and neuropathic pain matrices: a review. Pain 154 Suppl 1, S29–43. [DOI] [PubMed] [Google Scholar]
- Garland EL, 2012. Pain processing in the human nervous system: a selective review of nociceptive and biobehavioral pathways. Prim Care 39, 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel RJ, Lang PJ, 1973. Accuracy of psychophysical judgments and physiological response amplitude. J Exp Psychol 98, 175–183. [DOI] [PubMed] [Google Scholar]
- Geschwind N, 2010. Disconnexion syndromes in animals and man: Part I. 1965. Neuropsychology review 20, 128. [DOI] [PubMed] [Google Scholar]
- Geuter S, Gamer M, Onat S, Büchel C, 2014. Parametric trial-by-trial prediction of pain by easily available physiological measures. Pain 155, 994–1001. [DOI] [PubMed] [Google Scholar]
- Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG, 2014. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage 99, 180–190. [DOI] [PubMed] [Google Scholar]
- Hampf G, 1990. Influence of Cold Pain in the Hand on Skin Impedance, Heart Rate and Skin Temperature. Physiology & behavior 47, 217–218. [DOI] [PubMed] [Google Scholar]
- Höfle M, Kenntner-Mabiala R, Pauli P, Alpers GW, 2008. You can see pain in the eye: Pupillometry as an index of pain intensity under different luminance conditions. International Journal of Psychophysiology 70, 171–175. [DOI] [PubMed] [Google Scholar]
- Horing B, Sprenger C, Büchel C, 2019. The parietal operculum preferentially encodes heat pain and not salience. BioRxiv, 581504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Iannetti GD, 2016a. Painful issues in pain prediction. Trends in neurosciences 39, 212–220. [DOI] [PubMed] [Google Scholar]
- Hu L, Iannetti GD, 2016b. Painful Issues in Pain Prediction. Trends Neurosci 39, 212–220. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO, 1999. Pain-related neurons in the human cingulate cortex. Nat Neurosci 2, 403–405. [DOI] [PubMed] [Google Scholar]
- Iacovella V, Hasson U, 2011. The relationship between BOLD signal and autonomic nervous system functions: implications for processing of “physiological noise”. Magn Reson Imaging 29, 1338–1345. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG, Gracely RH, Max MB, Bennett GJ, 1998. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain 121 (Pt 5), 931–947. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Hughes NP, Lee MC, Mouraux A, 2008. Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J Neurophysiol 100, 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A, 2010. From the neuromatrix to the pain matrix (and back). Exp Brain Res 205, 1–12. [DOI] [PubMed] [Google Scholar]
- IASP, 1994. Classification of Chronic Pain “Part III: Pain Terms, A Current List with Definitions and Notes on Usage” second edition ed. IASP Press, Seattle. [Google Scholar]
- Itti L, Koch C, 2001. Computational modelling of visual attention. Nat Rev Neurosci 2, 194–203. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Renaud LP, 1987. Neurophysiology of a central baroreceptor pathway projecting to hypothalamic vasopressin neurons. Can J Neurol Sci 14, 17–24. [DOI] [PubMed] [Google Scholar]
- Jiang M, Mieronkoski R, Syrjala E, Anzanpour A, Terava V, Rahmani AM, Salantera S, Aantaa R, Hagelberg N, Liljeberg P, 2018. Acute pain intensity monitoring with the classification of multiple physiological parameters. Journal of Clinical Monitoring and Computing, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D, Spyer KM, 1986. Brainstem integration of cardiovascular and pulmonary afferent activity. Prog Brain Res 67, 295–314. [DOI] [PubMed] [Google Scholar]
- Jose AD, Collison D, 1970. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res 4, 160–167. [DOI] [PubMed] [Google Scholar]
- Kalat JW, 1974. Taste salience depends on novelty, not concentration, in taste-aversion learning in the rat. J Comp Physiol Psychol 86, 47–50. [DOI] [PubMed] [Google Scholar]
- Bach DR, Gerster S, Tzovara A and Castegnetti G, 2016. A linear model for event-related respiration responses. Journal of Neuroscience Methods, 270, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS, 2004. Anterior cingulate conflict monitoring and adjustments in control. Science 303, 1023–1026. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Fritz T, DY VC, Muller K, Friederici AD, 2006. Investigating emotion with music: an fMRI study. Hum Brain Mapp 27, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Behrens TE, Mars RB, Rushworth MF, 2012. Neural mechanisms of foraging. Science 336, 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel PA, Koban L, Barrett LF, Wager TD, 2018. Representation, Pattern Information, and Brain Signatures: From Neurons to Neuroimaging. Neuron 99, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel BYKC, Seals DR, Callister R, 1992. Sympathetic nervous system activity during skin cooling in humans: Relationship to stimulus intensity and pain sensation. Journal of Physiology 454, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Woo CW, Chang LJ, Ruzic L, Gu X, Lopez-Sola M, Jackson PL, Pujol J, Fan J, Wager TD, 2016. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle BN, McNeil DW, 2014. Autonomic Arousal And Experimentally Induced Pain: A Critical Review of the Literature. Pain Research and Management 19, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, 2014. Emotion’s Response Patterns: The Brain and the Autonomic Nervous System. Emotion Review 6, 93–99. [Google Scholar]
- LeDoux J, Daw ND, 2018. Surviving threats: neural circuit and computational implications of a new taxonomy of defensive behaviour. Nat Rev Neurosci 19, 269–282. [DOI] [PubMed] [Google Scholar]
- Lee J, Mawla I, Kim J, Loggia ML, Ortiz A, Jung C, Chan S-T, Gerber J, Schmithorst VJ, Edwards RR, Wasan AD, Berna C, Kong J, Kaptchuk TJ, Gollub RL, Rosen BR, Napadow V, 2018. Machine learning-based prediction of clinical pain using multimodal neuroimaging and autonomic metrics. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Damme SV, Eccleston C, Davis KD, Seminowicz DA, Crombez G, 2009. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain 144, 230–232. [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A, 2011. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol 93, 111–124. [DOI] [PubMed] [Google Scholar]
- Liang M, Mouraux A, Hu L, Iannetti GD, 2013. Primary sensory cortices contain distinguishable spatial patterns of activity for each sense. Nat Commun 4, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Su Q, Mouraux A, Iannetti GD, 2019. Spatial Patterns of Brain Activity Preferentially Reflecting Transient Pain and Stimulus Intensity. Cereb Cortex 29, 2211–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati G, Klocker A, Safronova MM, Ferrao Santos S, Ribeiro Vaz JG, Raftopoulos C, Mouraux A, 2016. Nociceptive Local Field Potentials Recorded from the Human Insula Are Not Specific for Nociception. PLoS Biol 14, e1002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Juneau M, Bushnell MC, 2011. Autonomic responses to heat pain: Heart rate, skin conductance, and their relation to verbal ratings and stimulus intensity. Pain 152, 592–598. [DOI] [PubMed] [Google Scholar]
- Lopez-Sola M, Woo CW, Pujol J, Deus J, Harrison BJ, Monfort J, Wager TD, 2017. Towards a neurophysiological signature for fibromyalgia. Pain 158, 34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotsch J, Walter C, Felden L, Noth U, Deichmann R, Oertel BG, 2012. The human operculo-insular cortex is pain-preferentially but not pain-exclusively activated by trigeminal and olfactory stimuli. PLoS One 7, e34798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui F, Duzzi D, Corradini M, Serafini M, Baraldi P, Porro CA, 2008. Touch or pain? Spatio-temporal patterns of cortical fMRI activity following brief mechanical stimuli. Pain 138, 362–374. [DOI] [PubMed] [Google Scholar]
- Macey PM, Wu P, Kumar R, Ogren JA, Richardson HL, Woo MA, Harper RM, 2012. Differential responses of the insular cortex gyri to autonomic challenges. Autonomic Neuroscience 168, 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maihöfner C, Seifert F, DeCol R, 2011. Activation of central sympathetic networks during innocuous and noxious somatosensory stimulation. Neuroimage 55, 216–224. [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Nargeot J, 2008. Genesis and regulation of the heart automaticity. Physiol Rev 88, 919–982. [DOI] [PubMed] [Google Scholar]
- Marquand A, Howard M, Brammer M, Chu C, Coen S, Mourao-Miranda J, 2010. Quantitative prediction of subjective pain intensity from whole-brain fMRI data using Gaussian processes. Neuroimage 49, 2178–2189. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR, 2011. Arousal-Biased Competition in Perception and Memory. Perspect Psychol Sci 6, 114–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthewson G, Woo CW, Reddan MC, Wager TD, 2019. Cognitive self-regulation influences pain-related physiology. Pain. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Peyron R, Guenot M, Mauguiere F, 2009. Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain 146, 99–104. [DOI] [PubMed] [Google Scholar]
- Melzack R, 1990. Phantom limbs and the concept of a neuromatrix. Trends Neurosci 13, 88–92. [DOI] [PubMed] [Google Scholar]
- Melzack R, 1999. From the gate to the neuromatrix. Pain Suppl 6, S121–126. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ, 2005. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage 28, 175–184. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ, 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey H, 1994. Part III pain terms, a current list with definitions and notes on usage. Classification of chronic pain-Descriptions of chronic pain syndromes and definitions of pain terms, 207–214. [Google Scholar]
- Mischkowski D, Palacios-Barrios EE, Banker L, Dildine TC, Atlas LY, 2019. Pain or nociception? Subjective experience mediates the effects of acute noxious heat on autonomic responses-corrected and republished. Pain 160, 1469–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayedi M, 2014. All roads lead to the insula. Pain 155, 1920–1921. [DOI] [PubMed] [Google Scholar]
- Moayedi M, Davis KD, 2013. Theories of pain: from specificity to gate control. J Neurophysiol 109, 5–12. [DOI] [PubMed] [Google Scholar]
- Moltner A, Holzl R, Strian F, 1990. Heart rate changes as an autonomic component of the pain response. Pain 43, 81–89. [DOI] [PubMed] [Google Scholar]
- Montavont A, Mauguiere F, Mazzola L, Garcia-Larrea L, Catenoix H, Ryvlin P, Isnard J, 2015. On the origin of painful somatosensory seizures. Neurology 84, 594–601. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD, 2011. A multisensory investigation of the functional significance of the “pain matrix”. Neuroimage 54, 2237–2249. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD, 2018. The search for pain biomarkers in the human brain. Brain 141, 3290–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Davis T, Poldrack RA, 2014. The impact of study design on pattern estimation for single-trial multivariate pattern analysis. Neuroimage 103, 130–138. [DOI] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R, 2008. Brain correlates of autonomic modulation : Combining heart rate variability with fMRI. Neuroimage 42, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel MM, May ES, Tiemann L, Postorino M, Dinh ST, Ploner M, 2017. Autonomic responses to tonic pain are more closely related to stimulus intensity than to pain intensity. Pain 158, 2129–2136. [DOI] [PubMed] [Google Scholar]
- Norman GJ, 2016. Comment: Emotional and Autonomic Arousal Constructs in Psychophysiological Research: Where Do We Go From Here? Emotion Review 8, 79–80. [Google Scholar]
- Ohman A, Soares JJ, 1994. “Unconscious anxiety”: phobic responses to masked stimuli. J Abnorm Psychol 103, 231–240. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC, 1992. Cardiovascular effects of human insular cortex stimulation. Neurology 42, 1727–1732. [DOI] [PubMed] [Google Scholar]
- Opthof T, 2000. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res 45, 173–176. [PubMed] [Google Scholar]
- Paine P, Kishor J, Worthen SF, Gregory LJ, Aziz Q, 2009. Exploring relationships for visceral and somatic pain with autonomic control and personality. Pain 144, 236–244. [DOI] [PubMed] [Google Scholar]
- Palma J-A, Benarroch EE, 2014. Neural control of the heart: recent concepts and clinical correlations. Neurology 83, 261–271. [DOI] [PubMed] [Google Scholar]
- Parr T, Friston KJ, 2018. Attention or salience? Curr Opin Psychol 29, 1–5. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E, 1937. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389–443. [Google Scholar]
- Perl ER, 2007. Ideas about pain, a historical view. Nat Rev Neurosci 8, 71–80. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M, 2010. A prefrontal nonopioid mechanism in placebo analgesia. Pain 150, 59–65. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L, 2000. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 30, 263–288. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Martin EM, Faber D, 2012. Origins of arousal: Roles for medullary reticular neurons. Trends in Neurosciences 35, 468–476. [DOI] [PubMed] [Google Scholar]
- Piché M, Arsenault M, Rainville P, 2010. Dissection of perceptual, motor and autonomic components of brain activity evoked by noxious stimulation. Pain 149, 453–462. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Paton JF, 2006. A decerebrate, artificially-perfused in situ preparation of rat: utility for the study of autonomic and nociceptive processing. J Neurosci Methods 155, 260–271. [DOI] [PubMed] [Google Scholar]
- Ploner M, Lee MC, Wiech K, Bingel U, Tracey I, 2010. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci U S A 107, 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, 2011. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron 72, 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Fustos J, Critchley HD, 2012. On the generalised embodiment of pain: how interoceptive sensitivity modulates cutaneous pain perception. Pain 153, 1680–1686. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC, 1997. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277, 968–971. [DOI] [PubMed] [Google Scholar]
- Reyes del Paso GA, Garrido S, Pulgar Á, Duschek S, 2011. Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. Journal of Psychosomatic Research 70, 125–134. [DOI] [PubMed] [Google Scholar]
- Reyes Del Paso GA, Montoro C, de Guevara Muñóz Ladrón C., Duschek S, Jennings JR, 2014. The effect of baroreceptor stimulation on pain perception depends on the elicitation of the reflex cardiovascular response: Evidence of the interplay between the two branches of the baroreceptor system. Biological Psychology 101, 82–90. [DOI] [PubMed] [Google Scholar]
- Rissman J, Greely HT, Wagner AD, 2010. Detecting individual memories through the neural decoding of memory states and past experience. Proceedings of the National Academy of Sciences 107, 9849–9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ME, Staud R, Price DD, 2013. Pain measurement and brain activity: will neuroimages replace pain ratings? J Pain 14, 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Iannetti GD, Liang M, Wood JN, 2016. The “Pain Matrix” in Pain-Free Individuals. JAMA Neurol 73, 755–756. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Kragel PA, Feldman L, Wager TD, Bianciardi M, 2018. Deconstructing arousal into wakeful, autonomic and affective varieties. Neuroscience Letters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, 1981. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science 214, 685–687. [DOI] [PubMed] [Google Scholar]
- Schestatsky P, Valls-Sole J, Costa J, Leon L, Veciana M, Chaves ML, 2007. Skin autonomic reactivity to thermoalgesic stimuli. Clinical Autonomic Research 17, 349–355. [DOI] [PubMed] [Google Scholar]
- Schneider M, Hathway P, Leuchs L, Samann MC, Spoormaker VI, 2016. Spontaneous pupil dilations during the resting state are associated with activation of the salience network. Neuroimage 139, 189–201. [DOI] [PubMed] [Google Scholar]
- Schulz E, Zherdin A, Tiemann L, Plant C, Ploner M, 2012. Decoding an individual’s sensitivity to pain from the multivariate analysis of EEG data. Cereb Cortex 22, 1118–1123. [DOI] [PubMed] [Google Scholar]
- Sclocco R, Beissner F, Desbordes G, Polimeni JR, Wald LL, Kettner NW, Kim J, Garcia RG, Renvall V, Bianchi AM, Cerutti S, Napadow V, Barbieri R, 2016a. Neuroimaging brainstem circuitry supporting cardiovagal response to pain: a combined heart rate variability/ultrahigh-field (7 T) functional magnetic resonance imaging study. Philos Trans A Math Phys Eng Sci 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclocco R, Beissner F, Desbordes G, Polimeni JR, Wald LL, Kettner NW, Kim J, Garcia RG, Renvall V, Bianchi AM, Cerutti S, Napadow V, Barbieri R, Hospital MG, Beissner F, Desbordes G, Hospital MG, Polimeni JR, Hospital MG, 2016b. Neuroimaging brainstem circuitry supporting cardiovagal response to pain: A combined heart rate variability/ultrahigh-field (7 T) functional magnetic resonance imaging study. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I, 2015. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci 18, 499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharvit G, Vuilleumier P, Delplanque S, Corradi-Dell’Acqua C, 2015. Cross-modal and modality-specific expectancy effects between pain and disgust. Scientific reports 5, 17487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RF, Lang PJ, 1976. Psychophysical judgment: electro-cortical and heart rate correlates of accuracy and uncertainty. Biol Psychol 4, 51–64. [DOI] [PubMed] [Google Scholar]
- Smith DB, Strawbridge PJ, 1969. The heart rate response to a brief auditory and visual stimulus. Psychophysiology 6, 317–329. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL, 1991. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V, 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 105, 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC, 2009. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci 29, 2684–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD, 1989. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 491, 156–162. [DOI] [PubMed] [Google Scholar]
- Sutherland MR, Mather M, 2018. Arousal (but not valence) amplifies the impact of salience. Cogn Emot 32, 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH, 1991. Multiple representations of pain in human cerebral cortex. Science 251, 1355–1358. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD, 2009. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30, 2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkelsen AJ, Molgaard H, Hansen J, Andersen OK, Jensen TS, 2005. Acute pain increases heart rate: differential mechanisms during rest and mental stress. Auton Neurosci 121, 101–109. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD, 2012. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience and biobehavioral reviews 36, 747–756. [DOI] [PubMed] [Google Scholar]
- Torta DM, Legrain V, Mouraux A, Valentini E, 2017. Attention to pain! A neurocognitive perspective on attentional modulation of pain in neuroimaging studies. Cortex 89, 120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treister R, Kliger M, Zuckerman G, Aryeh IG, Eisenberg E, 2012a. Differentiating between heat pain intensities: The combined effect of multiple autonomic parameters. Pain 153, 1807–1814. [DOI] [PubMed] [Google Scholar]
- Treister R, Kliger M, Zuckerman G, Goor Aryeh I, Eisenberg E, 2012b. Differentiating between heat pain intensities: the combined effect of multiple autonomic parameters. Pain 153, 1807–1814. [DOI] [PubMed] [Google Scholar]
- Turpin G, Siddle DA, 1979. Effects of stimulus intensity on electrodermal activity. Psychophysiology 16, 582–591. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, 2015. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16, 55–61. [DOI] [PubMed] [Google Scholar]
- Uno T, Grings WW, 1965. Autonomic components of orienting behavior. Psychophysiology 1, 311–321. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Legrain V, Vogt J, Crombez G, 2010. Keeping pain in mind: a motivational account of attention to pain. Neurosci Biobehav Rev 34, 204–213. [DOI] [PubMed] [Google Scholar]
- Victor RG, Leimbach WN, Seals DR, Wallin BG, Mark AL, 1987. Effects of the Cold Pressor Test on Muscle Sympathetic Nerve Activity in Humans. Hypertension 9, 429–436. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E, 2013. An fMRI-based neurologic signature of physical pain. N Engl J Med 368, 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey HY, Catana C, Hooker JM, Dougherty DD, Knudsen GM, Wang DJ, Chonde DB, Rosen BR, Gollub RL, Kong J, 2014. Simultaneous fMRI-PET of the opioidergic pain system in human brain. Neuroimage 102 Pt 2, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G, 2003. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron 40, 655–664. [DOI] [PubMed] [Google Scholar]
- Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I, 2010. Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci 30, 16324–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Tracey I, 2013. Pain, decisions, and actions: a motivational perspective. Front Neurosci 7, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Chang LJ, Lindquist MA, Wager TD, 2017a. Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci 20, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, Andrews-Hanna JR, Wager TD, 2014. Separate neural representations for physical pain and social rejection. Nat Commun 5, 5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Schmidt L, Krishnan A, Jepma M, Roy M, Lindquist MA, Atlas LY, Wager TD, 2017b. Quantifying cerebral contributions to pain beyond nociception. Nat Commun 8, 14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Touroutoglou A, Quigley KS, Feldman Barret L, Dickerson BC, 2017. Salience Network Connectivity Modulates Skin Conductance Responses in Predicting Arousal Experience. Journal of cognitive neuroscience 29, 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Hillstrom AP, 1994. Stimulus-driven attentional capture: evidence from equiluminant visual objects. J Exp Psychol Hum Percept Perform 20, 95–107. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD, 2011. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CP, Kung SS, Su YF, Lin WC, Howng SL, Kwan AL, 2005. Stereotactic bilateral anterior cingulotomy for intractable pain. J Clin Neurosci 12, 886–890. [DOI] [PubMed] [Google Scholar]
- Young CB, Raz G, Everaerd D, Beckmann CF, Tendolkar I, Hendler T, Fernández G, Hermans EJ, 2017. Dynamic Shifts in Large-Scale Brain Network Balance As a Function of Arousal. The Journal of Neuroscience 37, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunhammer M, Bingel U, Wager TD, 2016. Issues in pain prediction-more gain than pain. Trends in neurosciences 39, 639–640. [DOI] [PubMed] [Google Scholar]