Abstract
Background:
Cardiogenic shock (CS) following acute myocardial infarction (AMI) portends a poor prognosis. Short-term mechanical circulatory support devices (MCSDs) provide hemodynamic support for patients with CS but predictors of survival and the ability to wean from short-term MCSDs remain largely unknown.
Methods:
All patients > 18 years old treated at our institution with extra-corporeal membrane oxygenation (ECMO) or short-term surgical ventricular assist device (sVAD) for AMI-CS were studied. We collected AMI details with demographic and hemodynamic variables. Primary outcomes were survival to discharge and recovery from MCSD (i.e. survival without heart replacement therapy [HRT] including durable VAD or heart transplant).
Results:
124 patients received ECMO or short-term sVAD following AMI from 2007–2016; 89 received ECMO and 35 short-term VAD. Fifty-five (44.4%) died in the hospital and 69 (55.6%) survived to discharge. Twenty-six (37.7%) required HRT (4 transplant, 22 durable VAD) and 43 (62.3%) were discharged without HRT. Age and cardiac index (CI) at MCSD implantation were predictors of survival to discharge; patients over 60 with CI <1.5L/min/m2 had a low likelihood of survival. The angiographic result after revascularization predicted recovery from MCSD (OR 9.00, 95% confidence interval 2.45–32.99, p=0.001), but 50% of those optimally revascularized still required HRT. CI predicted recovery from MCSD among this group (OR 4.06, 95% confidence interval 1.45–11.55, p=0.009).
Conclusion:
Among AMI-CS patients requiring short-term MCSDs, age and CI predict survival to discharge. Angiographic result and CI predict ventricular recovery but 50% of those optimally revascularized still required HRT.
Introduction:
Cardiogenic shock (CS) remains the leading cause of early mortality following acute myocardial infarction (AMI).1 While recent evidence suggests improved outcomes with this condition,1–2 overall results remain poor, particularly in the most severe cases.2–5 Several randomized trials and observational studies have examined the role of mechanical circulatory support devices (MCSD) for AMI patients.3,6–8 Though studies have demonstrated that some devices provide greater hemodynamic support than others,7–8 the ideal MCSD for AMI remains unclear.
Extra-corporeal membrane oxygenation (ECMO) and short-term surgical ventricular assist devices (sVAD; e.g. CentriMag, Thoratec, Pleasanton, CA) have been used in increasing numbers and are typically reserved for the most severely affected CS patients.2,4,9–10 However, there are risks associated with these devices and despite providing a great deal of circulatory support, mortality remains high. The increased use of durable left ventricular assist devices (LVADs) promises a means of providing long-term survival for those patients without sufficient ventricular recovery to wean from short-term MCSDs.11–14 However, it remains difficult to predict which patients will safely wean from short-term MCSDs and which will not. Furthermore, it is unclear how patients weaned from support will fare after discharge. As such we examined a cohort of patients with severe refractory CS following AMI receiving either ECMO or short-term sVAD at our institution to determine 1) predictors of survival and 2) predictors of the ability to recover from MCSDs altogether.
Methods:
This study was approved by Columbia University’s Institutional Review Board and participants or their surrogate provided written informed consent for inclusion in a CS database. A waiver of consent was granted for those without a surrogate who were too critically–ill to provide informed consent prior to death.
Patient Selection and Data Collection
All patients 18 years or older treated at our institution with either ECMO or short-term sVAD following AMI between 2007 and 2016 were studied retrospectively. Data collected included demographic variables and hemodynamic data whenever available. In addition, AMI details were collected including angiographic result and cardiac biomarkers (e.g. creatine phosphokinase–MB [CK-MB]). Delay to MCSD implantation was defined as device implantation on a calendar day other than the day of AMI presentation.
Outcomes
The primary outcomes were survival to hospital discharge and recovery from MCSD without the need for heart replacement therapy (HRT; either durable LVAD or heart transplant [HT]). Importantly, at our center, there is no age cut-off for durable LVAD therapy, and all patients under 73 are considered potential candidates for HT.
Mechanical Circulatory Support Device Weaning
Our center tests daily whether patients can be weaned from short-term MCSDs. Once vasopressors have been weaned to low levels (i.e. norepinephrine <5mcg/min, vasopressin <2U/hr) we perform a daily “turn-down” of either ECMO or sVAD flows to determine whether the patient is MCSD dependent or not. For both devices, we utilize pulmonary artery (PA) and radial artery catheters to evaluate the change in hemodynamic status as this is done. If the mean arterial pressure falls by more than 15% or below 65mmHg as flows are reduced to 1L/min we consider this a failure to wean from MCSD. Bedside echocardiographic assessment is also used to provide additional information about native cardiac function as flows are reduced, particularly if the patient has already failed one wean attempt. In our institution if a patient fails repeated attempts to wean, they are transitioned to a durable LVAD or undergo HT whenever possible.
Statistical Analysis
Categorical data are presented as percentages and continuous data as means ± standard deviation. Pearson’s chi–squared test was used to compute the significance of the difference between groups for categorical variables. Normality of continuous variables was tested using the Shapiro-Wilk test and the Student’s t–test was used to compare groups for continuous variables. Logistic regression was used to determine significant predictors of the primary outcomes. Variables with a p-value <0.1 in univariable analysis and those felt to be clinically important with respect to the primary outcome (e.g. age and active CPR at MCSD insertion) were included in a multivariable model. Collinear variables were excluded. For time-to-event analyses, Kaplan-Meier estimates of event-free survival were created for groups of interest and the log-rank test was used to compare survivor functions. A p-value <0.05 was considered statistically significant. Data were analyzed using Stata (StataCorp, College Station, TX).
Results:
In total 124 patients received either ECMO or short-term sVAD following AMI complicated by CS between 2007 and 2016. Of these, 42 (33.9%) presented initially to our institution while 82 (66.1%) presented initially to another institution and were transferred to our center for management of CS. During this study period, 710 patients underwent primary percutaneous coronary intervention (PCI) after presenting to our center with ST elevation myocardial infarction. Of the patients in our cohort, 89 (71.8%) received ECMO as the first device and 35 (28.2%) sVAD as the first device; all sVADs used during this period were Centrimag VADs.
Prior to MCSD implantation 61 (49.2%) patients had an IABP, 26 (21.0%) had a percutaneous LVAD, and 10 (8.1%) had received both sequentially. Seventy-nine (64.8%) had suffered a cardiac arrest prior to MCSD implantation, 96 (91.4%) were mechanically ventilated, and 25 (20.5%) had active CPR during MCSD implantation. The mean lactate was 5.41±5.00mmol/L. Additional patient demographics are displayed in Table 1.
Table 1:
Patient Demographics
| Variable | All | Died | Alive | P value |
|---|---|---|---|---|
| Age (years) | 59.1 ± 10.2 | 60.7 ± 11.7 | 58.0 ± 10.2 | 0.19 |
| Gender, n (% male) | 93 (75.0) | 41 (74.5) | 52 (75.4) | 0.92 |
| Diabetes Mellitus, n (%) | 52 (42.6) | 25 (45.5) | 27 (40.3) | 0.57 |
| Hypertension, n (%) | 70 (57.3) | 33 (60.0) | 37 (55.2) | 0.60 |
| Dyslipidemia, n (%) | 56 (45.9) | 20 (36.4) | 36 (53.7) | 0.06 |
| Cardiac Arrest, n (%) | 79 (64.8) | 35 (66.0) | 69 (63.8) | 0.80 |
| Active CPR, n (%) | 20 (16.1) | 12 (21.8) | 8 (11.6) | 0.12 |
| Creatinine (mg/dL) | 1.60 ± 0.86 | 1.65 ± 0.94 | 1.55 ± 0.78 | 0.56 |
| Lactate (mmol/L) | 5.41 ± 5.00 | 7.19 ± 6.14 | 4.40 ± 3.95 | 0.04 |
| pH | 7.32 ± 0.16 | 7.30 ± 0.20 | 7.33 ± 0.12 | 0.40 |
| Aspartate aminotransferase (U/L) | 449.34 ± 899.51 | 655.18 ± 1264.49 | 274.83 ± 899.51 | 0.052 |
| Alanine aminotransferase (U/L) | 228.32 ± 514.17 | 315.56 ± 715.42 | 154.35 ± 221.55 | 0.15 |
| Total Bilirubin (mg/dL) | 1.13 ± 1.10 | 1.38 ± 1.38 | 0.91 ± 0.74 | 0.051 |
| Intubated, n (%) | 96 (91.4) | 46 (95.8) | 50 (87.7) | 0.14 |
| Systolic BP (mmHg) | 100.6 ± 20.2 | 96.9 ± 19.6 | 103.4 ± 20.4 | 0.14 |
| Diastolic BP (mmHg) | 58.6 ± 13.4 | 60.0 ± 15.2 | 57.6 ± 12.0 | 0.40 |
| Mean Arterial Pressure (mmHg) | 72.3 ± 13.4 | 72.1 ± 14.7 | 72.5 ± 12.5 | 0.88 |
| Number Inotropes/Vasopressors | 2.3 ± 1.1 | 2.5 ± 1.1 | 2.1 ± 1.0 | 0.04 |
| Cardiac Output (L/min) | 3.62 ± 1.20 | 3.20 ± 1.05 | 3.95 ± 1.22 | 0.007 |
| Cardiac Index (L/min/m2) | 1.83 ± 0.55 | 1.63 ± 0.53 | 1.98 ± 0.51 | 0.007 |
| Cardiac Power Output (W) | 0.59 ± 0.25 | 0.52 ± 0.20 | 0.65 ± 0.27 | 0.02 |
| Cardiac Power Index (W/m2)) | 0.30 ± 0.11 | 0.26 ± 0.10 | 0.32 ± 0.11 | 0.02 |
CPR, cardiopulmonary resuscitation; BP, blood pressure; W, watts
Fifty-five (44.4%) patients died in the hospital, 2 of whom had received HRT (1 OHT and 1 durable LVAD). Thirty-six (67.9%) died while still on a short-term MCSD while 17 (32.1%) died after being removed from circulatory support. Of these deaths, 18 (34.0%) were due to anoxic brain injury, 31 (58.5%) were due to refractory CS or asystole, 2 (3.8%) were due to overwhelming infection, and 2 (3.8%) were due to other causes (e.g. severe hemorrhage). Sixty-nine (55.6%) patients survived to discharge; twenty-six (37.7%) required HRT (4 OHT, 22 durable LVAD), and 43 (62.3%) were weaned from the MCSD and discharged without HRT (Figure 1).
Figure 1.
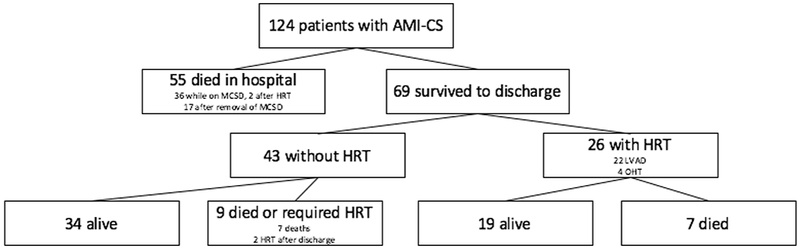
Overview of patient outcomes. AMI; acute myocardial infarction; CS, cardiogenic shock; MCSD, mechanical circulatory support device; HRT, heart replacement therapy; LVAD, left ventricular assist device; OHT, orthotopic heart transplant.
Hemodynamics
Seventy-four (59.6%) patients had an invasive hemodynamic assessment prior to MCSD insertion. Amongst those with invasive hemodynamics, there was evidence of severe hemodynamic compromise. The mean arterial pressure was 72.3±13.4mmHg, mean cardiac index (CI) 1.83±0.55L/min/m2 and mean cardiac power index (CPI) 0.30±0.11W despite the majority already having either IABP or percutaneous LVAD. Patients were also receiving an average of 2.3±1.1 inotropic or vasopressor infusions at MCSD insertion.
AMI Characteristics
Ninety-eight (79.0%) patients had suffered an ST elevation myocardial infarction and 26 (21.0%) suffered a non-ST elevation myocardial infarction; the left anterior descending coronary artery was the most common culprit vessel. Patients had, on average, 2.2±0.8 epicardial coronary vessels diseased (>50% stenosis). All patients had coronary angiography; ninety-eight (79.0%) underwent PCI, 9 (7.3%) underwent coronary artery bypass grafting, and in 17 (13.7%) revascularization attempts were unsuccessful. Thrombolysis In Myocardial Infarction (TIMI) 3 flow was achieved in 65.9% of patients. The mean CK-MB peak was 357.7±362.0ng/ml and mean left ventricular ejection fraction (LVEF) at MCSD implantation was 21.3±12.1% as measured by echocardiography or ventriculography in 120 (96.8%) of the patients.
Survival to Discharge
In univariable analysis, serum lactate, the number of vasoactive medications at MCSD insertion, dyslipidemia, aspartate aminotransferase, total bilirubin, and CI at MCSD implantation met our pre-specified criteria for inclusion in our multivariable model. In addition to these, age and on-going CPR during MCSD insertion were included in the model while total bilirubin was excluded due to collinearity; only age and CI remained independent predictors of survival to discharge (Table 3).
Table 3:
Predictors of In-hospital Death
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age* | 1.12 | 0.90 – 1.70 | 0.19 | 3.90 | 1.19 – 12.78 | 0.03 |
| Gender (male) | 0.96 | 0.42 – 2.17 | 0.92 | |||
| Diabetes Mellitus | 1.23 | 0.60 – 2.54 | 0.57 | |||
| Hypertension | 1.21 | 0.59 – 2.51 | 0.60 | |||
| Dyslipidemia | 0.49 | 0.24 – 1.02 | 0.06 | 0.99 | 0.08 – 11.91 | 0.99 |
| Cardiac Arrest | 1.10 | 0.52 – 2.34 | 0.80 | |||
| Active CPR | 2.13 | 0.80 – 5.65 | 0.13 | 2.00 | 0.07 – 56.35 | 0.68 |
| Creatinine (mg/dL) | 1.16 | 0.71 – 1.88 | 0.56 | |||
| Lactate (mmol/L) | 1.12 | 1.00 – 1.25 | 0.049 | 1.28 | 0.91 – 1.79 | 0.16 |
| Aspartate aminotransferase‖ | 1.06 | 1.00 – 1.12 | 0.045 | 1.08 | 0.93 – 1.25 | 0.29 |
| Alanine aminotransferase‖ | 1.05 | 0.97 – 1.14 | 0.23 | |||
| Total Bilirubin | 1.62 | 0.94 – 2.81 | 0.08 | |||
| pH | 0.29 | 0.02 – 4.92 | 0.39 | |||
| Mechanically Ventilated | 3.22 | 0.64 – 16.30 | 0.16 | |||
| Systolic BP | 0.98 | 0.96 – 1.01 | 0.14 | |||
| Diastolic BP | 1.01 | 0.98 – 1.05 | 0.39 | |||
| Number Inotropes/Vasopressors | 1.49 | 1.01 – 2.21 | 0.045 | 2.66 | 0.80 – 8.83 | 0.11 |
| Cardiac Index† | 0.77 | 0.63 – 0.94 | 0.01 | 0.37 | 0.16 – 0.87 | 0.02 |
| Pulmonary Artery Pulsatility Index | 1.08 | 0.65 – 1.79 | 0.76 | |||
| LVEF at Implant‡ | 0.88 | 0.75 – 1.04 | 0.13 | |||
OR, odds ratio; CI, confidence interval; CPR, cardiopulmonary resuscitation; BP, blood pressure; LVEF, left ventricular ejection fraction.
by 5 year increment
by 50 U/L increment
by 0.2L/min/m2 increment
by 5 % increment
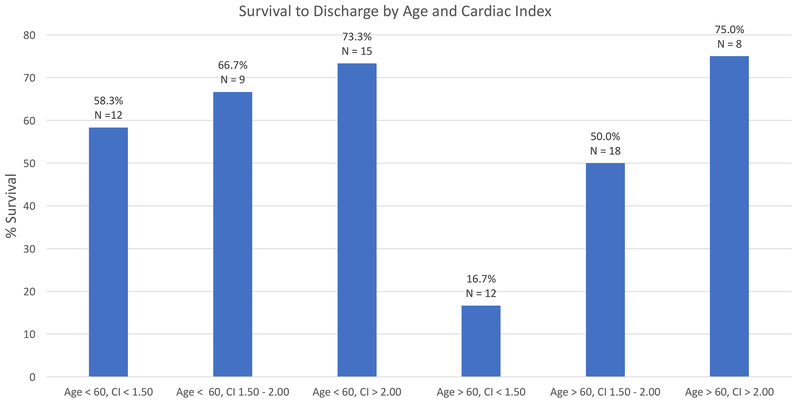
Overall survival of patients with invasive hemodynamic measurements prior to MCSD insertion stratified by CI tertile are displayed in Figure 2. When further stratified into groups guided by median of age, there were significant differences in the likelihood of survival to discharge (p=0.049, Figure 3). Specifically, patients <60 years survived to discharge at rates of 58.3% (N = 12), 66.7% (N = 9), and 73.3% (N = 15) when stratified by CI tertile (<1.50, 1.50–2.00, and >2.00, respectively). Patients >60 years survived to discharge at rates of 16.7% (N = 12), 50.0% (N = 18), and 75.0% (N = 8) when stratified into similar CI categories. Importantly, only 13 (10.5%) patients were above our institutional age criterion for HT consideration and none were excluded from durable LVAD consideration based on age.
Figure 2.
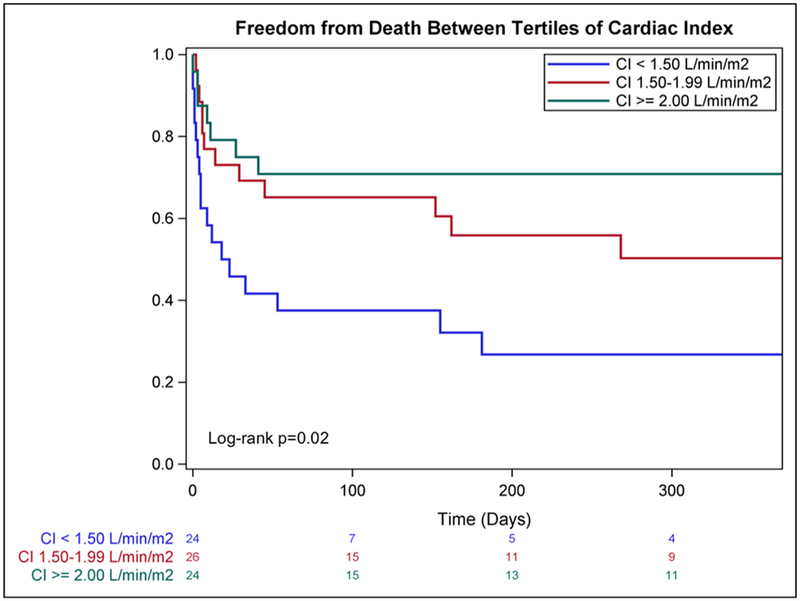
Kaplan-Meier survival estimates with stratification by cardiac index at the time of MCSD insertion. MCSD, Mechanical Circulatory Support Device; CI, cardiac Index.
Figure 3.
Survival to hospital discharge with stratification by age and cardiac index at the time of MCSD insertion. MCSD, Mechanical Circulatory Support Device; CI, cardiac index.
Recovery from MCSD
Characteristics of the AMI were examined as potential predictors of the ability to wean from MCSD and leave the hospital without HRT. Those treated with ECMO and short-term sVAD as the initial support device had similar rates of recovery from MCSD. In univariable analysis, predictors of the ability to wean from MCSD included achievement of TIMI 3 flow in the infarct vessel, fewer epicardial coronary arteries diseased, lack of residual coronary artery disease after revascularization, MCSD implantation on the day of presentation, as well as systolic blood pressure, CI, central venous pressure, and LVEF all at MCSD initiation (Table 4). Patient with TIMI 0-2 flow following attempted revascularization were unlikely (10%) to recover from MCSD, whereas 50% with TIMI 3 flow did (Figure 4A).
Table 4:
Predictors of Recovery from MCSD
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 1.00 | 0.98 – 1.02 | 0.98 | |||
| Gender | 0.95 | 0.41 – 2.23 | 0.91 | |||
| Acute Coronary Syndrome type (STEMI) | 0.53 | 0.22 – 1.30 | 0.17 | |||
| PCI | 0.51 | 0.13 – 2.01 | 0.33 | |||
| Culprit Vessel | ||||||
| Left Main | ||||||
| Left Anterior Descending | 1.08 | 0.31 – 3.76 | 0.91 | 0.58 | 0.03 – 10.04 | 0.71 |
| Circumflex | 0.63 | 0.09 – 4.32 | 0.63 | 1.16 | 0.03 – 44.05 | 0.94 |
| Right Coronary Artery | 7.5 | 1.48 – 37.9 | 0.02 | 6.00 | 0.15 – 241.28 | 0.34 |
| Number Vessels Diseased | 0.55 | 0.34 – 0.89 | 0.015 | 0.36 | 0.12 – 1.08 | 0.07 |
| TIMI 3 Flow in Culprit | 9.00 | 2.45 – 33.00 | 0.001 | |||
| Residual CAD | 0.16 | 0.049 – 0.51 | 0.002 | |||
| Delay to MCSD | 0.46 | 0.22 – 0.99 | 0.046 | 0.46 | 0.09 – 2.31 | 0.34 |
| Systolic BP | 1.02 | 1.00 – 1.05 | 0.045 | |||
| Diastolic BP | 0.97 | 0.94 – 1.01 | 0.14 | |||
| Cardiac Index at implant* | 5.47 | 1.91 – 15.69 | 0.002 | 1.38 | 1.01 – 1.88 | 0.04 |
| Systolic PA Pressure | 0.99 | 0.94 – 1.03 | 0.55 | |||
| Diastolic PA Pressure | 0.94 | 0.85 – 1.03 | 0.18 | |||
| PCWP | 0.98 | 0.91 – 1.04 | 0.46 | |||
| Central Venous Pressure | 0.88 | 0.78 – 1.00 | 0.04 | |||
| CK-MB | 1.00 | 1.00 – 1.00 | 0.20 | |||
| LVEF at Implant‖ | 1.04 | 1.01 – 1.07 | 0.013 | 1.03 | 0.69 – 1.53 | 0.90 |
| ECMO as first device | 0.86 | 0.38 – 1.94 | 0.72 | |||
| Pulmonary Artery Pulsatility Index | 1.22 | 0.73 – 2.04 | 0.45 | |||
MCSD, mechanical Circulatory Support Device; OR, odds ratio; CI, confidence interval; STEMI, ST elevation myocardial infarction; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction; CAD, coronary artery disease; BP, blood pressure; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; CK – MB, creatine phosphokinase – MB; LVEF, left ventricular ejection fraction; ECMO, extra-corporeal membrane oxygenation
by 0.2L/min/m2 increment
by 5% increment
Figure 4.
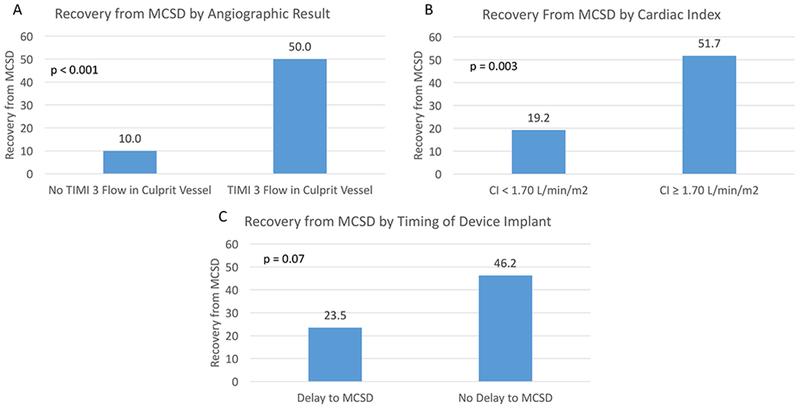
Recovery from Short-Term Mechanical Circulatory Support Device Following Acute Myocardial Infarction. A) The differences in probability of recovery based on achievement of TIMI 3 flow in culprit vessel. Among those achieving TIMI 3 flow, the probabilities of recovery by B) cardiac index at device implantation and C) by timing of implantation. MCSD, Mechanical Circulatory Support Device; TIMI, Thrombolysis in Myocardial Infarction; CI, cardiac index
Among the subset of patients achieving TIMI 3 flow, CI at device implant (as binary variable guided by median value) was predictive of recovery from MCSD (Figure 4B). In addition, there was a 22.7% absolute difference in the probability of successful wean when comparing those who had MCSD implantation on the day of presentation compared to those who had a delay to implantation. However, this difference was not statistically significant (46.2% vs. 23.5%, respectively, p=0.07; Figure 4C).
In a multivariable model analyzing only those who achieved TIMI 3, only CI remained a significant predictor of recovery (OR 1.38 per 0.2L/min/m2 increment, 95% confidence interval 1.01–1.88, p=0.04; Table 4). The number of vessels diseased was inversely related to the likelihood of recovery but this was not statistically significant in our model (OR 0.36; 95% confidence interval 0.12–1.08, p=0.07).
Long-term survival
Median follow-up after discharge was 365 days (IQR: 111–942 days). Following discharge, 2 (4.7%) patients who had been weaned from MCSD required HRT (durable LVADs at 857 and 1309 days after discharge) and 7 died. Among those requiring in-hospital HRT, 7 died in follow-up. None of the patients with durable LVAD at discharge underwent explant for significant recovery on device support. The survival estimates did not differ significantly between those discharged with or without HRT (83.8% vs 85.7% 1-year survival, respectively; Figure 5).
Figure 5.
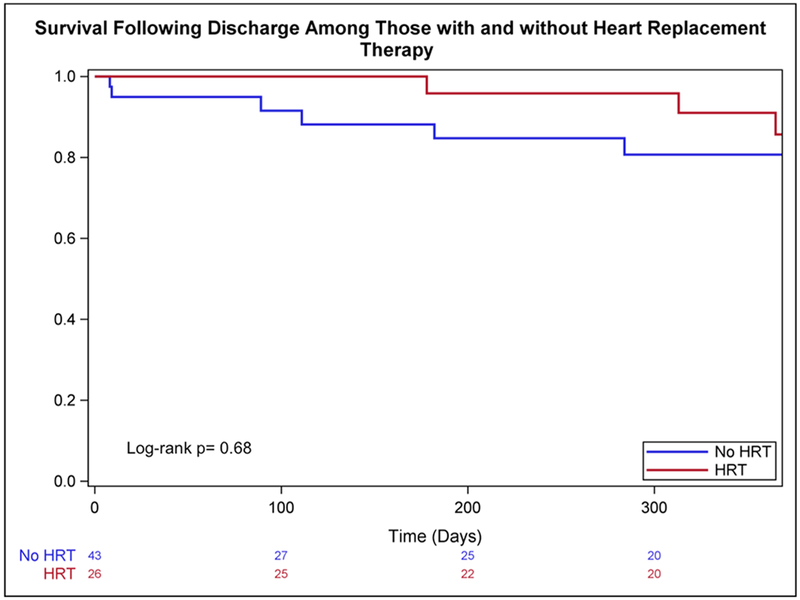
Kaplan-Meier estimates of post-discharge survival among patients with and without HRT. HRT, heart replacement therapy.
Discussion:
Our data demonstrate the following:
Despite a high severity of illness following AMI including high rates of cardiac arrest, significant hemodynamic compromise, and markedly elevated lactate, 55.6% of patients treated with ECMO or short-term VAD survived to discharge either with or without HRT.
Both age and CI at device insertion were predictors of survival to discharge and those older than 60 with lower CI had an exceedingly low likelihood of survival.
The most important predictor of recovery from MCSD without durable LVAD or transplant was restoration of TIMI 3 flow in the infarct vessel.
Amongst those with an optimal angiographic result, only 50% of patients had ventricular recovery sufficient for wean from MCSD altogether. Beyond angiographic result, CI was predictive of ventricular recovery.
While several studies have examined predictors of survival in patients with AMI and CS,15–18 little is known about the predictors of ability to recover from MCSD. Ours is the first dataset to try to help answer an important clinical question: will a patient be able to recover sufficiently to be weaned from circulatory support or will they require durable HRT? This information is valuable in two respects. First, premature transition to durable HRT instead of weaning a recoverable patient from support may expose the patient to unnecessary risk of durable LVAD or HT and the associated adverse events. Second, unnecessary delay on a short-term device awaiting an unlikely recovery may expose the patient to higher risk of complication prior to transition to durable HRT.
Our patient population is notable for the severity of illness and importantly, is similar to that of other studies of severe refractory CS following AMI.3,5–8,15–18 Two-thirds had suffered a cardiac arrest prior to MCSD insertion and almost all were mechanically ventilated. For those with an invasive hemodynamic assessment prior to device insertion, there was evidence of severe compromise; the mean CI and CPI were comparable or even worse than those in the SHOCK registry.19 Furthermore, there was evidence of end-organ dysfunction and high serum lactate.
With respect to survival, age and CI were found to be powerful predictors of survival to discharge. Most strikingly, when stratified by age and CI, all categories experienced rates of survival to discharge between 50 and 75% with one exception: patients over 60 with CI <1.50L/min/m2. This group experienced a much lower rate of survival than all other groups.
Historically, the short-term mortality of patients with CS following AMI has ranged between 40 and 60%.1–6 As such, there has been an increase in MCSD use in hope of altering this sobering statistic.2 However, as MCSDs become more commonly utilized with AMI-CS, it is important to select patients carefully to avoid exposing those unlikely to survive. To do so may only prolong a dying process which may be particularly difficult for patients’ families. While our data do not support a strict age cut-off for MCSD implantation, it emphasizes the need to be highly selective when treating those with low likelihood of survival.
We observed that despite the presence of severe refractory CS requiring either ECMO or a short-term sVAD, about two-thirds of survivors had sufficient ventricular recovery to be weaned from the support device. The most important determinant of ventricular recovery was the angiographic result: those with TIMI 0-2 flow had only a 10% chance of successful wean from MCSD. However, even those with an optimal angiographic result still only had a 50% chance of successful wean from MCSD. CI was a powerful predictor of recovery among those with TIMI 3 flow; less than 20% of those with CI below 1.70L/min/m2 had recovery from MCSD. These data are consistent with other reports highlighting the importance of achieving TIMI 3 flow.16,18 Indeed, patients without TIMI 3 flow in the culprit vessel or lower CI despite optimal revascularization should be evaluated early for durable HRT to minimize time on short-term MCSD and the associated complications.
Interestingly, we noted a trend towards higher likelihood of recovery for patients undergoing MCSD implantation without a delay following AMI. This observation was consistent with other reports highlighting improved outcomes among patients undergoing device insertion earlier rather than later.20–21 These data are hypothesis generating, and other investigators have already begun to test this hypothesis in prospective study.22 While this finding in our study was not statistically significant (p=0.07), the contrast in probability of recovery between those with and without delay to MCSD implantation was striking with a 23% percent absolute difference.
While our weaning protocol for short-term MCSDs is institution-specific, we observed that those with and without HRT at discharge experienced good long-term outcomes despite high illness severity at presentation. This suggests that our protocol for assessing a patient’s ability to safely wean from MCSD effectively identified the optimal treatment for each patient. Specifically, only two patients required LVAD implantation after discharge, both occurring more than 2 years after AMI. We also have an institution-specific protocol for identifying durable LVAD recipients as possible candidates for device explant due to heart recovery; importantly none of the patients with an LVAD at discharge underwent device explant for recovery.
These data are informative as the incidence of MCSDs used to treat CS patients is rapidly rising.2 In order to minimize the risk of complication from short-term MCSDs, the time on these devices should be minimized whenever possible. If the likelihood of recovery is low, transition to durable HRT, either OHT or LVAD, should be expedited. Furthermore, for patients receiving short-term MCSDs at hospitals without durable LVAD programs, early transfer should be pursued if the likelihood of recovery is low. Alternatively, if the chance of recovery is high, then it may be best to support longer on a short-term device in the hope that the patient will be eventually weaned from support entirely.
Limitations
Our study has several limitations. It is a single–center study and subject to inherent limitations of practice pattern and bias. Our institutional protocol for weaning short-term MCSDs is based on hemodynamic assessment during device flow reduction but has not been validated. Thus it is possible that patients we deemed unable to wean from MCSD might have been safely weaned with a longer period of time on short-term support. However, the complication rates associated with short-term MCSDs are considerable so we attempt to minimize this risk by moving towards durable HRT if there has not been significant recovery within 2 weeks.
We were also limited by missing data. Specifically, not all patients had pre-implant hemodynamics, highlighting the heterogeneity of patient presentation. While we routinely use PA catheters to manage patients with suspected CS, other referring institutions may not always insert one prior to MCSD implantation and transfer to our institution. Additionally, a subset of patients was too unstable to undergo placement of a PA catheter prior to MCSD insertion. We opted not to impute critical data points like pre-implant hemodynamics in order to understand the true significance of this data, limiting our sample size for some analyses. Lastly, we lacked granularity with respect to the exact time of MCSD implantation compared to onset of AMI. Because of these limitations in our dataset, it is important to recognize that our power to detect important predictors of outcomes for this population is limited.
Conclusions:
Among patients with AMI and severe refractory CS requiring ECMO or short-term sVAD, age and CI are predictors of survival to discharge. In particular, older adults with severe hemodynamic compromise had an exceedingly low likelihood of survival to discharge despite use of powerful MCSDs. Restoration of TIMI 3 flow was a powerful predictor of ventricular recovery from MCSD, but 50% of those with an optimal angiographic result still required HRT for survival. Among those with TIMI 3 flow, CI at device insertion predicted the need for long-term HRT. The number of coronary arteries diseased and timing of device insertion may also be important in determining the likelihood of ventricular recovery. Larger studies are needed to validate these findings and also identify additional predictors of outcomes that might have been missed in this analysis. Such information is crucial to optimizing outcomes for patients with AMI and CS so that so that those unlikely to recover can be transitioned quickly to durable HRT and those with a good chance of recovery can be targeted for wean from short-term MCSD.
Table 2:
Patient Profiles
| Variable | All | Died or HRT | Recovery | P value |
|---|---|---|---|---|
| STEMI, n (%) | 98 (79.0) | 67 (82.7) | 31 (72.1) | 0.17 |
| Culprit Vessel, n (%) | 0.004 | |||
| Left Main | 14 (11.4) | 10 (12.5) | 4 (9.3) | |
| Left Anterior Descending | 83 (67.5) | 58 (72.5) | 25 (58.1) | |
| Circumflex | 10 (8.1) | 8 (10.0) | 2 (4.7) | |
| Right coronary artery | 16 (13.0) | 4 (5.0) | 12 (27.9) | |
| PCI, n (%) | 98 (91.5) | 60 (93.8) | 38 (88.4) | 0.33 |
| Number of Diseased Vessels | 2.2 ± 0.8 | 2.3 ± 0.7 | 1.9 ± 0.9 | 0.01 |
| TIMI 3 in culprit, n (%) | 58 (65.9) | 29 (51.9) | 29 (90.6) | < 0.001 |
| Residual CAD, n (%) | 32 (54.2) | 26 (70.3) | 6 (27.3) | 0.001 |
| Systolic BP (mmHg) | 100.6 ± 20.2 | 97.6 ± 19.7 | 107.0 ± 20.2 | 0.04 |
| Diastolic BP (mmHg) | 58.6 ± 13.4 | 60.1 ± 13.8 | 55.5 ± 12.4 | 0.13 |
| Mean Arterial Pressure (mmHg) | 72.3 ± 13.4 | 72.2 13.7 | 72.6 ± 12.8 | 0.89 |
| Cardiac Output (L/min) | 3.62 ± 1.20 | 3.26 ± 1.01 | 4.32 ± 1.25 | 0.0002 |
| Pulmonary Artery Pulsatility Index | 1.63 ± 1.20 | 1.53 ± 1.20 | 1.82 ± 1.21 | 0.45 |
| Cardiac Index (L/min/m2) | 1.83 ± 0.55 | 1.67 ± 0.49 | 2.13 ± 0.53 | 0.0004 |
| Cardiac Power Output (W) | 0.59 ± 0.25 | 0.53 ± 0.19 | 0.71 ± 0.30 | 0.003 |
| Cardiac Power Index (W/m2) | 0.30 ± 0.11 | 0.27 ± 0.10 | 0.35 ± 0.13 | 0.007 |
| Central Venous Pressure (mmHg) | 13.8 ± 5.4 | 15.0 ± 5.9 | 11.8 ± 3.8 | 0.04 |
| Systolic PA Pressure (mmHg) | 39.8 ± 13.2 | 40.5 ± 13.3 | 38.1 ± 13.3 | 0.56 |
| Diastolic PA Pressure (mmHg) | 22.2 ± 7.1 | 23.0 ± 6.9 | 20.1 ± 7,3 | 0.18 |
| Mean PA Pressure (mmHg) | 28.2 ± 9.0 | 29.0 ± 8.6 | 26.2 ± 10.0 | 0.32 |
| PCWP (mmHg) | 26.8 ± 13.0 | 28.2 ± 12.9 | 24.2 ± 13.6 | 0.47 |
| CK - MB peak (ng/ml) | 357.7 ± 362.0 | 393.9 ± 408.1 | 275.8 ± 362.0 | 0.19 |
| CK peak (U/L) | 6284.1 ± 7992.6 | 6800.6 ± 8238.3 | 5350.9 ± 7569.8 | 0.42 |
| LVEF at Implant (%) | 21.3 ± 12.1 | 19.2 ± 11.1 | 25.1 ± 13.1 | 0.01 |
| Delay to MCSD, n (%) | 61 (49.6) | 45 (56.3) | 16 (37.2) | 0.04 |
| ECMO as first device, n (%) | 89 (72.8) | 59 (72.8) | 30 (69.8) | 0.72 |
HRT, heart replacement therapy; STEMI, ST elevation myocardial infarction; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction; CAD, coronary artery disease; BP, blood pressure; W, watts; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; CK – MB, creatine phosphokinase – MB; CK, creatine phosphokinase; LVEF, left ventricular ejection fraction; MCSD, mechanical circulatory support device; ECMO, extra-corporeal membrane oxygenation.
Acknowledgements:
Dr. Garan is supported by National Institutes of Health Grant No. KL2TR001874 and has received honoraria from Abiomed (Danvers, MA). Dr. Clerkin is supported by National Institutes of Health Grant No. T32 HL007854. Dr. Naka has received consulting fees from St. Jude Medical (St. Paul, MN).
Disclosures:
Dr. Garan is supported by National Institutes of Health Grant No. KL2TR001874 and has received honoraria from Abiomed (Danvers, MA).
Dr. Naka has received consulting fees from St. Jude Medical/Abbott Vascular (St. Paul, MN).
References:
- 1.Goldberg RJ, Makam RC, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade-Long Trends (2001-2011) in the Incidence and Hospital Death Rates Associated with the In-Hospital Development of Cardiogenic Shock after Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes. 2016;9:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–15. [DOI] [PubMed] [Google Scholar]
- 3.Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69:278–287. [DOI] [PubMed] [Google Scholar]
- 4.Takayama H, Truby L, Koekort M, et al. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant. 2013;32:106–11. [DOI] [PubMed] [Google Scholar]
- 5.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625–34. [DOI] [PubMed] [Google Scholar]
- 6.Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–96. [DOI] [PubMed] [Google Scholar]
- 7.Burkhoff D, Cohen H, Brunckhorst C, et al. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152:469.e1–8. [DOI] [PubMed] [Google Scholar]
- 8.Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584–8. [DOI] [PubMed] [Google Scholar]
- 9.Truby L, Naka Y, Kalesan B, et al. Important role of mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock. Eur J Cardiothorac Surg. 2015;48:322–8. [DOI] [PubMed] [Google Scholar]
- 10.Takayama H, Soni L, Kalesan B, et al. Bridge-to-decision therapy with a continuous-flow external ventricular assist device in refractory cardiogenic shock of various causes. Circ Heart Fail. 2014;7:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–504. [DOI] [PubMed] [Google Scholar]
- 12.Acharya D, Loyaga-Rendon RY, Pamboukian SV, et al. Ventricular assist device in acute myocardial infarction. J Am Coll Cardiol. 2016;67(16):1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang NC, Topkara VK, Leacche M, John R, Byrne JG, Naka Y. Left ventricular assist device implantation after acute anterior wall myocardial infarction and cardiogenic shock: a two-center study. J Thorac Cardiovasc Surg. 2005;130:693–8. [DOI] [PubMed] [Google Scholar]
- 14.Chou J, Bermudez C, Kormos R, Teuteberg J. Permanent Continuous Flow Left Ventricular Assist Devices Use After Acute Stabilization for Cardiogenic Shock in Acute Myocardial Infarction. ASAIO J. 2017;63:e13–e17. [DOI] [PubMed] [Google Scholar]
- 15.Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016;42:370–8. [DOI] [PubMed] [Google Scholar]
- 16.Pöss J, Köster J, Fuernau G, et al. Risk Stratification for Patients in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69:1913–1920. [DOI] [PubMed] [Google Scholar]
- 17.Lee WC, Fang CY, Chen HC, et al. Associations with 30-day survival following extracorporeal membrane oxygenation in patients with acute ST segment elevation myocardial infarction and profound cardiogenic shock. Heart Lung. 2016;45:532–537. [DOI] [PubMed] [Google Scholar]
- 18.Chung SY, Tong MS, Sheu JJ, et al. Short-term and long-term prognostic outcomes of patients with ST-segment elevation myocardial infarction complicated by profound cardiogenic shock undergoing early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention. Int J Cardiol. 2016;223:412–417. [DOI] [PubMed] [Google Scholar]
- 19.Fincke R, Hochman JS, Lowe AM, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004;44:340–8. [DOI] [PubMed] [Google Scholar]
- 20.Basir MB, Schreiber TL, Grines CL, et al. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am J Cardiol. 2017;119:845–851. [DOI] [PubMed] [Google Scholar]
- 21.O’Neill WW, Schreiber T, Wohns DH, et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol. 2014;27:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Neill W, Basir M, Dixon S, Patel K, Schreiber T, Almany S. Feasibility of Early Mechanical Support During Mechanical Reperfusion of Acute Myocardial Infarct Cardiogenic Shock. JACC Cardiovasc Interv. 2017;10:624–625. [DOI] [PubMed] [Google Scholar]