Abstract
Background
Delirium is a common severe neuropsychiatric condition secondary to physical illness, which predominantly affects older adults in hospital. Prior to this study, the UK point prevalence of delirium was unknown. We set out to ascertain the point prevalence of delirium across UK hospitals and how this relates to adverse outcomes.
Methods
We conducted a prospective observational study across 45 UK acute care hospitals. Older adults aged 65 years and older were screened and assessed for evidence of delirium on World Delirium Awareness Day (14th March 2018). We included patients admitted within the previous 48 h, excluding critical care admissions.
Results
The point prevalence of Diagnostic and Statistical Manual on Mental Disorders, Fifth Edition (DSM-5) delirium diagnosis was 14.7% (222/1507). Delirium presence was associated with higher Clinical Frailty Scale (CFS): CFS 4–6 (frail) (OR 4.80, CI 2.63–8.74), 7–9 (very frail) (OR 9.33, CI 4.79–18.17), compared to 1–3 (fit). However, higher CFS was associated with reduced delirium recognition (7–9 compared to 1–3; OR 0.16, CI 0.04–0.77). In multivariable analyses, delirium was associated with increased length of stay (+ 3.45 days, CI 1.75–5.07) and increased mortality (OR 2.43, CI 1.44–4.09) at 1 month. Screening for delirium was associated with an increased chance of recognition (OR 5.47, CI 2.67–11.21).
Conclusions
Delirium is prevalent in older adults in UK hospitals but remains under-recognised. Frailty is strongly associated with the development of delirium, but delirium is less likely to be recognised in frail patients. The presence of delirium is associated with increased mortality and length of stay at one month. A national programme to increase screening has the potential to improve recognition.
Keywords: Delirium, Frailty, Older adults, Collaboration
Background
Delirium is a neuropsychiatric syndrome, which disproportionately affects older people in hospital. The Diagnostic and Statistical Manual on Mental Disorders, Fifth Edition (DSM-5) defines delirium as an acute and/or fluctuating change in awareness, arousal, and other cognitive deficits due to physical illness or drugs [1, 2]. Psychomotor subtypes are hyperactive, characterised by motor agitation, perceptual differences, and delusions, and hypoactive, featuring predominantly motor retardation and thought process abnormality; or mixed [3]. It is very common, but prevalence differs across populations: 10–31% for most acute settings outside critical care [4]. Prior to our study, the largest point prevalence study of delirium using DSM criteria reported a prevalence of 19.6% amongst 280 general hospital adult inpatients in a single centre in Ireland [5].
Delirium is consistently associated with increased mortality, accounting for age, co-morbidity, and acute illness [6]. It is also associated with increased length of hospital stay, new institutionalisation, and distress to patients and families [4, 7]. Delirium commonly occurs in people with dementia [8] and is considered to worsen cognitive decline [9]. In people without dementia, an episode of delirium is associated with eightfold increased risk of later-life dementia diagnosis [10]. Few studies have assessed the relationship between delirium and frailty [11–14].
Delirium remains underdiagnosed in up to three quarters of patients [15–18]. Incomplete understanding of delirium and resultant educational needs of healthcare professionals, alongside avoidant behaviours towards a challenging patient group, are likely contributory [19]. National Institute for Health and Care Excellence (NICE) Guidelines recommend that all patients aged 65 or over are screened for delirium upon hospital admission [20]; this can be done using the 4 A’s Test (4AT). The 4AT is a validated screening tool, which can be completed by any healthcare professional in less than 2 min [21]. Diagnosis should be made using DSM-5, recorded in inpatient notes, and communicated to the general practitioner [22].
This study set out to identify the point prevalence of delirium, rates of screening, and rates of recognition of delirium in non-elective admissions of older people within the UK. We aimed to assess patient and hospital factors inclusive of frailty measures that were predictive of delirium, screening, and recognition. Secondary outcomes included one-month mortality and length of stay.
Methods
Study design and setting
We conducted a multi-centre study of delirium screening, recognition, and discharge documentation on Wednesday 14th March 2018: World Delirium Awareness Day. This is an annual international day, which aims to increase awareness of delirium amongst healthcare professionals, patients, carers, and stakeholders [23]. This day was chosen to encourage an increased atmosphere acknowledging the importance of delirium screening and recognition within participating trusts and to encourage quality improvement strategies for the future. This involved acute care trusts within the UK who volunteered to participate. Participation was open to all hospitals within the UK, and the onus was put upon staff within individual sites to volunteer to participant rather than being selected. No financial incentives were provided to trusts to participate. Regional representatives emailed the staff at all sites to encourage participation. Data collected from each site were anonymised and entered into pre-formatted Excel spreadsheets. Spreadsheets were formatted so that data was entered in the same way for all sites. These spreadsheets were collated centrally. Our reporting is in line with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Additional file 3) [24].
Participants
Inclusion criteria were adults aged ≥ 65 years (in line with NICE guidelines), admitted between 08:00 12th March 2018 and 07:59 14th March 2018, and still hospital inpatients at the time of assessment. Exclusion criteria were patients admitted to critical care, imminently approaching the end of life, or in whom it was impractical to assess for logistical reasons (e.g. patient undergoing operation, not at bedside). The minimum data required for inclusion was 4AT score and presence/absence of delirium.
Delirium screening and assessment
Patient assessment as part of the study took place between 08:00 and 20:00 on 14 March 2018. All included patients were screened using the 4AT by the study team; in all patients who scored ≥ 4/12, a further assessment for delirium was conducted. Our approach to further assessment for delirium was operationalised on DSM-5 (Additional file 1). The 4AT was performed by a healthcare professional or student with training and support from the local geriatric medicine site lead. All formal DSM-5 assessments were performed by a healthcare professional; patients with 4AT score ≥ 4/12 identified by students were reviewed by healthcare professionals. Standardised training was provided centrally to all via webcast and video resources. The presence or absence of delirium was classified as definite (meets DSM-5 criteria), possible (meets some DSM-5 criteria but not all), or no delirium. Motor subtype was classified by the Delirium Motor Subtype Scale (DMSS) [19] as hypoactive (reduced alertness), hyperactive (increased alertness or motor agitation), mixed (some features of both hypoactive and hyperactive), or no clear motor subtype.
Additional data collection
Further patient details were recorded from the patient’s hospital notes including age, gender, dementia status (known history—any history documented in the notes/probable—no documented diagnosis but history of progressive cognitive impairment impairing activities of daily living documented/no dementia), and specialty at time of assessment (acute medicine/geriatric medicine/stroke/other medicine/orthopaedic surgery/general surgery/other surgery). Clinical Frailty Scale (CFS) from 1 to 9 was determined prospectively from note review (functional status documentation) and clinical assessment by the student or healthcare professional assessing the patient as part of the study [25]. We recorded if patients had been screened for delirium (using any recognised tool) by the usual care team and if a diagnosis of delirium was recorded in the medical notes by the usual care team prior to 4AT assessment as part of this study. Delirium was considered to have been recognised if a DSM-5 diagnosis was made during the assessment, and this had previously been documented by the usual care team. Each site collected data on local factors: presence of local delirium guidelines, local delirium patient leaflets, delirium screening tools in admission documentation booklets, geriatric medicine team embedded into the admissions unit, or a specialist delirium team. These were defined locally. Length of stay, mortality, and documentation of delirium on discharge documentation were collected up until 13th April 2018.
Statistical methods
Statistical analysis was performed using IBM SPSS Statistics 22 (Chicago, IL, USA). We assessed the differences between patients with and without delirium using chi-squared tests for categorical data and independent t tests for continuous data. Grouping of variables was decided post hoc. Possible delirium was coded as no delirium, and probable dementia was coded as dementia. Frailty status was separated into three categories by CFS (fit, 1–3; frail, 4–6; very frail, 7–9). Delirium subtype was classified as hypoactive or other. Due to small numbers, general and other surgery specialties were grouped for the main analysis. General and other surgery were further grouped with orthopaedic surgery for recognition analysis; stroke was grouped with other medicine when analysing discharge documentation.
Binary logistic regression was performed to assess the effect of covariates upon delirium screening, prevalence, and recognition, and the effect of screening upon recognition. Patients who died within the follow-up period were excluded from the length of stay analysis. Length of stay was visually assessed for normality; Mann-Whitney U test was used to assess the length of stay in those with and without delirium. Effects of the presence of delirium upon length of stay were assessed using robust (bootstrapped) analysis of covariance (ANCOVA), adjusting for the variables above. Bootstrapping was performed due to skewed distribution of length of stay using IBM SPSS Statistics 22, with the number of samples set at 1507, confidence interval set at 95%, and a simple sampling method selected. Bootstrapping was not performed for analysis of other outcomes. Association of delirium with mortality was assessed using binary logistic regression and Cox regression. A secondary analysis was performed to assess the effects of recognition and delirium subtype upon length of stay and mortality. Any missing variables and outcome data were coded as missing data, but these participants were included in all analysis, provided data was available on the presence or absence of delirium.
Ethical approval
All data were collected as part of a multi-centre audit to assess compliance with NICE guidelines and registered through clinical governance departments. Anonymised data were securely transferred to the University of Birmingham. Ethical approval was obtained for a secondary analysis of the anonymised database from the University of Birmingham Science, Technology, Engineering, and Mathematics Ethical Review Committee (ERN_18-1415).
Results
A total of 45 hospitals participated (Additional file 2: Figure S1), and 2385 patients were identified. Reasons for exclusion included the following: pre-admission (77), imminently dying (37), undue distress (45), and logistical reasons (719). Logistical reasons included the patient or notes being unavailable, or unavailability of staff. The final sample included 1507 patients (Fig. 1). The mean age was 80.0 (SD ± 8.3); 54.2% were female, 16.3% had dementia or probable dementia, 43.0% were acute medicine patients, and 68.1% had a CFS score of 4 or greater (Table 1; Additional file 2: Table S1). Mortality to follow-up was 6.7% (97/1507). The rates of missing data were low overall; the rates of missing data are included within Additional file 2: Table S2.
Fig. 1.
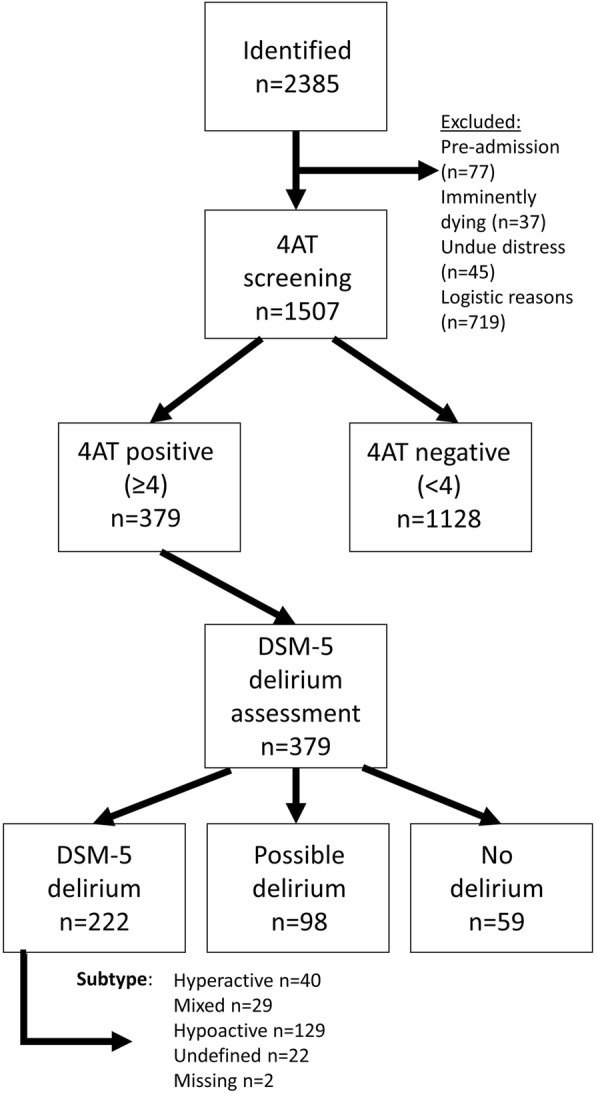
Flow chart demonstrating participation in delirium day study. On 14th March 2018, 45 hospitals participated in World Delirium Day study. Two thousand three hundred and eighty-five individuals met the study criteria of admission between 08:00 on 12th March 2018 and 07:59 on 14th March 2018. Thirty-seven individuals were excluded as they were judged to be imminently dying. Seven hundred and nineteen individuals were excluded for logistical reasons. Seventy-seven were excluded as they had not yet had their initial assessment. Forty-five were excluded because the assessment was deemed to cause undue distress. One thousand five hundred and seven individuals were screened with 4AT. Of these, 366 had a score equal to or greater than four and underwent further assessment of delirium using DSM-5 criteria. Of those who were 4AT positive, 222 were proven to have DSM-5 delirium
Table 1.
Demographics of patients included in this study. Results are shown for the percentage of study participants who met each characteristic. These have been further separated for comparison between participants with and without delirium. Overall, the mean age of participants was 80.0; 54.2% were female, and 16.3% had known or probable dementia; 43.0% were admitted under acute medicine at the time of assessment; 68.0% had a CFS score of 4 or greater
| All | No delirium | Delirium (DSM-5) | p | |
|---|---|---|---|---|
| Age (mean, SD) | 80.0 (8.3) | 79.3 (8.3) | 84.0 (7.4) | < 0.001 |
| Gender | ||||
| Female | 54.2% (798) | 52.9% (663) | 62.0% (218) | 0.013 |
| Dementia | ||||
| Known or probable | 16.3% (244) | 13.0% (166) | 35.5% (78) | < 0.001 |
| Specialty | ||||
| Acute medicine | 43.0% (648) | 42.2% (542) | 47.8% (106) | < 0.001 |
| Geriatric medicine | 17.6% (265) | 16.0% (206) | 26.6% (59) | |
| Other medicine | 20.9% (315) | 22.1% (284) | 14.0% (31) | |
| Stroke | 3.7% (56) | 4.0% (52) | 1.8% (4) | |
| General and other surgery | 8.5% (128) | 9.4% (121) | 3.2% (7) | |
| Orthopaedic surgery | 6.3% (95) | 6.2% (80) | 6.8% (15) | |
| Frailty | ||||
| Fit (CFS 1–3) | 31.9% (468) | 36.3% (453) | 6.9% (15) | < 0.001 |
| Frail (CFS 4–6) | 54.3% (796) | 53.0% (662) | 62.0% (134) | |
| Very frail (CFS 7–9) | 13.7% (201) | 10.7% (134) | 31.0% (67) | |
Delirium prevalence
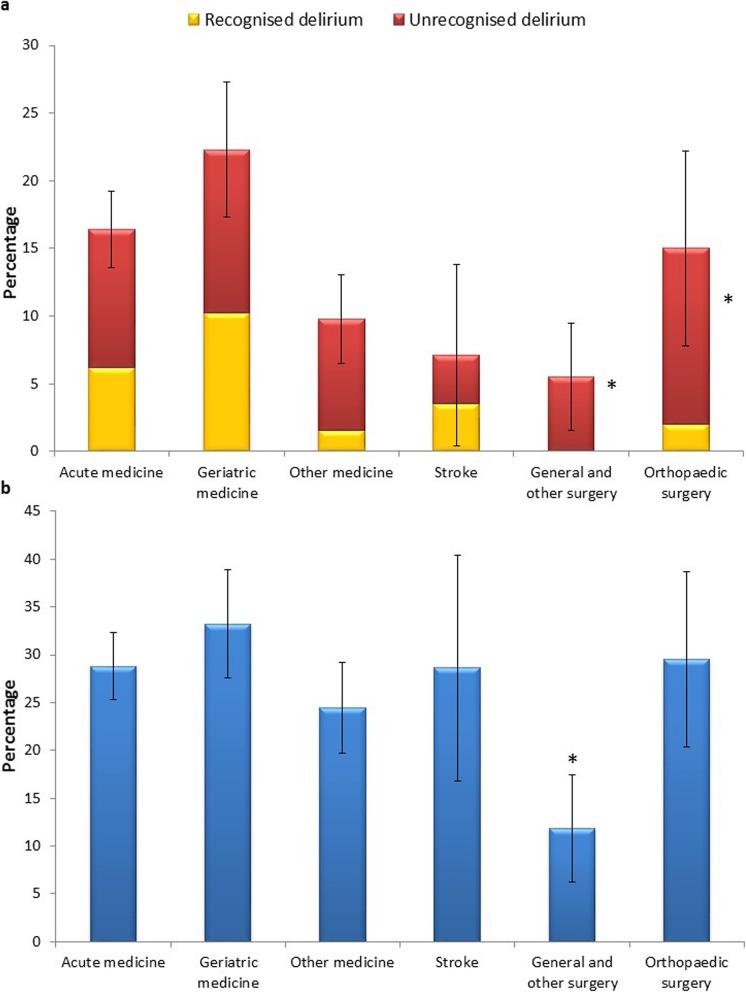
With 4AT assessment, 25.1% (379/1507) scored positive (≥ 4/12). Prevalence of DSM-5 delirium was 14.7% (222/1507); including those with possible delirium as well as DSM-5 delirium, prevalence was 21.2% (320/1507). Considering delirium subtypes, 18.2% (40/220) were hyperactive, 13.2% (29/220) were mixed type, 58.6% (129/220) were hypoactive, and 10.0% (22/220) had no clear subtype (as assessed by local data collectors). The presence of delirium was independently associated with increased age (per year of life: OR 1.04, CI 1.02–1.06; p < 0.001), dementia status (OR 1.95, CI 1.36–2.79; p < 0.001), frailty, frail (OR 4.80, CI 2.63–8.74; p < 0.001), and very frail (OR 9.33, CI 4.79–18.17; p < 0.001). Delirium prevalence was not affected by any hospital factors or gender (Additional file 2: Table S2). Prevalence of delirium differed between specialties (Fig. 2a). However, specialty did not affect the likelihood of delirium after adjusting for age and frailty (Additional file 2: Table S3).
Fig. 2.
a Prevalence of recognised and unrecognised delirium by specialty. The total of each bar represents the overall prevalence of delirium within each specialty; standard error bars show the 95% confidence intervals of prevalence by specialty. The yellow portion of each bar represents recognised delirium, and the red portion of each bar represents unrecognised delirium. Prevalence differed between specialties; however, after controlling for other confounders (e.g. age), specialty was not predictive of delirium prevalence. There were reduced odds of recognition of delirium in patients admitted to general, other, or orthopaedic surgery as compared to acute medicine. b Screening of delirium by specialty. Each bar represents the total percentage of patients who were screened for delirium by the usual care team prior to assessment as part of this study within each specialty; the standard error bars show the 95% confidence intervals of percentage screened. Reduced odds of screening for delirium were exhibited in patients admitted under general or other surgery as compared to acute medicine
Delirium screening
There was evidence of delirium screening by the usual care team in 27.3% (410/1507). Increasing age (per year of life: OR 1.04, CI 1.02–1.06; p < 0.001) and the presence of a local delirium specialist team (OR 2.03, CI 1.48–2.80; p < 0.001) were associated with an increased chance of screening. Admission under general or other surgery compared to acute medicine resulted in a reduced chance of delirium screening (OR 0.38, CI 0.21–0.70; p = 0.002) (Fig. 2b). Chances of delirium screening by the usual care team were not affected by gender, dementia status, frailty, or other hospital factors (Additional file 2: Table S4).
Delirium recognition
The usual care team recognised DSM-5 delirium in 34.2% of cases (76/222). Increased screening rates were associated with increased recognition rates by the usual care team (OR 5.47, CI 2.67–11.21; p < 0.001). The presence of a delirium team was associated with a decreased chance of recognition (OR 0.33, CI 0.23–0.84; p = 0.020). Delirium was less likely to be recognised in very frail compared to fit patients (OR 0.16, CI 0.04–0.77; p = 0.021) and general, other, and orthopaedic surgery patients compared to acute medicine (OR 0.04, CI 0.01–0.36; p = 0.004) (Fig. 2a). Age, gender, and dementia status did not impact upon delirium recognition. Delirium recognition was not affected by delirium subtype (Additional file 2: Table S5).
Discharge documentation
Discharge documentation was assessed in 69.4% (154/222) with DSM-5 delirium. Delirium was documented on discharge summaries in 28.6% (44/154) of these. Documentation on discharge summaries was not associated with any hospital or patient factors (Additional file 2: Table S6).
Delirium and length of stay
The median length of stay in patients with DSM-5 delirium was 11 days (IQR 5–21), compared to 7 days (IQR 3–14) in those without delirium (p < 0.001). This difference remained in multivariable analysis; delirium presence was associated with a mean (bootstrapped) increased length of stay of + 3.45 days (CI 1.75–5.07) compared to those without (p = 0.001). Possible delirium was associated with increased length of stay when compared to patients without delirium (+ 2.21 days, CI 0.27–4.52; p = 0.038); 4AT-positive status with no evidence of delirium was not associated with increased length of stay (Table 2; Additional file 2: Tables S7-S8). Further post hoc tests are included in Additional file 2: Tables S9-S11. There was no association of delirium recognition or subtype with length of stay (Additional file 2: Tables S12 and S13).
Table 2.
Results of post hoc tests of mean difference following robust (bootstrapped) ANCOVA. The presence of delirium was associated with an increased length of stay of 3.45 days. Considering possible delirium separately, there was an increased length of stay of 2.21 days compared to those without delirium. Results of statistical significance (p<0.005) have been highlighted in bold
| Status (a) | Status (b) | Mean difference (a − b) | Bootstrap | ||||
|---|---|---|---|---|---|---|---|
| Bias | SE | p | 95% confidence interval | ||||
| Lower | Upper | ||||||
| No or possible delirium | Delirium | − 3.45 | 0.017 | 0.84 | 0.001 | − 5.07 | − 1.75 |
| No delirium | 4AT positive, no delirium | − 2.55 | − 0.01 | 1.35 | 0.052 | − 5.21 | 0.04 |
| Possible delirium | − 2.21 | − 0.07 | 1.08 | 0.038 | − 4.52 | − 0.27 | |
| Delirium | − 3.95 | − 0.02 | 0.91 | 0.001 | − 5.78 | − 2.22 | |
| 4AT positive, no delirium | Possible delirium | 0.34 | − 0.06 | 1.65 | 0.820 | − 3.00 | 3.61 |
| Delirium | − 1.41 | − 0.01 | 1.50 | 0.35 | − 4.35 | 1.69 | |
| Possible delirium | Delirium | − 1.74 | 0.05 | 1.31 | 0.183 | − 4.19 | 0.89 |
Delirium and mortality
The presence of DSM-5 delirium was associated with increased mortality at 1 month both before (OR 3.02, CI 1.88–4.87; p < 0.001) and after adjusting for other variables: age, gender, frailty, specialty, and dementia (OR 2.43, CI 1.44–4.09; p = 0.001) (Table 3). Delirium was also associated with increased mortality in time-to-event analysis (HR 1.62, CI 1.00–2.61; p = 0.048) (Table 4). Possible delirium was associated with increased odds of death in univariable but not multivariable analysis (Additional file 2: Table S14). Similarly, 4AT-positive status was associated with increased odds of death in multivariable analysis (OR 2.55, CI 1.53–4.24; p < 0.001) (Additional file 2: Table S15). There was no effect of delirium recognition or subtype upon mortality (Additional file 2: Tables S16 and S17).
Table 3.
Effect of delirium status upon odds of mortality within 30 days. Delirium was associated with an increased odds of death within 30 days both unadjusted and adjusted for other confounders. Of those other confounders measured, only being very frail (CFS 7–9) was associated with increased odds to death to 30 days. Results of statistical significance (p<0.005) have been denoted with *
| Coefficient | SE | Wald | Freedom | p | OR | 95% confidence interval for OR | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Delirium (unadjusted) | 1.10 | 0.24 | 20.62 | 1 | < 0.001* | 3.02 | 1.88 | 4.87 |
| Delirium (adjusted)† | 0.89 | 0.27 | 11.02 | 1 | 0.001* | 2.43 | 1.44 | 4.09 |
| Very frail (adjusted)‡ | 0.95 | 0.38 | 6.20 | 1 | 0.013* | 2.59 | 1.23 | 5.48 |
†Adjusted for age, gender, CFS, dementia status, and specialty
‡Adjusted for delirium status, age, dementia status, and specialty
Table 4.
Effect of delirium status upon time to death with follow-up to 30 days. The presence of delirium was associated with a greater risk of an earlier death; no other variables were significant in time to death analysis
| Coefficient | SE | Wald | Freedom | p | HR | 95% confidence interval for OR | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Delirium (unadjusted) | 0.58 | 0.23 | 6.25 | 1 | 0.012 | 1.78 | 1.13 | 2.81 |
| Delirium (adjusted)† | 0.48 | 0.24 | 3.90 | 1 | 0.048 | 1.62 | 1.00 | 2.61 |
†Adjusted for age, gender, CFS, dementia status, and specialty
Discussion
In this multi-centre study of delirium in older acute hospital admissions, delirium was prevalent and associated with significant adverse outcomes. Delirium was more common in individuals with dementia and frailty. Delirium screening and recognition were both low. Importantly, higher screen rates were associated with fivefold higher recognition rates, demonstrating the need for screening in clinical practice. Delirium was associated with increased mortality and length of stay within one month of admission; this association remained after adjusting for age and frailty.
Results in the context of other literature
Prevalence of delirium was lower than a previous single-site point prevalence study (14.7% vs. 19.6%) [5]. However, our study focussed on new admissions only; hospital-wide point prevalence may be higher including cases of incident delirium. Positive 4AT status prevalence was similar to a multihospital study [26]. Increased delirium prevalence was associated with age and dementia, as previously described [27, 28]. Prevalence of different delirium subtypes was similar to results published elsewhere [29].
Screening and recognition rates were similar to previous results [4, 18]. This is the first study to identify differences in delirium screening and recognition rates across specialties. Specialty was not predictive of delirium presence after adjusting for age, frailty, and dementia; specialty alone does not affect chances of delirium. However, individuals admitted under surgery were 3 times less likely to be screened for delirium and 20 times less likely to be recognised as having delirium. This may be related to the training and skill set of responsible healthcare staff.
Documentation of delirium on discharge summaries was poor. This was not affected by any hospital or patient factors. However, this effect is not unique to delirium; a similar rate of poor communication has been described across multiple settings [30]. A multitude of quality improvement projects has suggested techniques to improve discharge communication with varying success [31–33].
Small studies have demonstrated that frailty increases the risk of delirium [34–37] and is associated with greater mortality in delirium [37]. This is the largest study to examine this association. Patients with severe frailty were nine times more likely to have delirium, and delirium and frailty were independent risks of mortality and increased length of stay. The CFS is a widely used and valid method of measuring frailty in clinical practice [25], which can be used with minimal training by non-specialists [38]. We have uniquely demonstrated that frail patients were far less likely to have delirium recognised. This may be due to increased misdiagnosis as chronic cognitive impairment and a perception that features are “expected” for patients with frailty in hospital. By contrast, healthcare professionals may have a different subconscious bias in how they “expect” fit patients to present in hospital. Although we included cases of probable dementia as well as known dementia, it is likely that there were other patients who had undiagnosed dementia and additional patients with mild cognitive impairment; one fifth of patients with delirium have been shown to have undiagnosed dementia [39]. Frailty and cognitive impairment commonly co-exist [40]; therefore, some of the effects of reduced recognition of delirium may relate to pre-existent cognitive impairment.
What is the internal validity of our research?
There are a number of important limitations. Firstly, usual clinical teams were informed of screening and diagnosis results; this may have moderated effects of non-recognition on outcomes. However, only a single study to date has found an association between non-recognition and adverse outcomes [41]. Secondly, non-specialists (healthcare staff other than geriatricians or psychiatrists) carried out the assessments. However, standardised training was provided to assessors, a structured proforma was used for assessment, and positive results were discussed with a local expert. In fact, delirium prevalence was slightly lower than previously published, suggesting if anything this method led to a higher specificity (i.e. fewer false positives). This in itself can be considered a limitation, although we purposefully used a strict interpretation of the DSM-5 criteria. The specificity of the 4AT against DSM-4 has previously been reported at 84.7%, whereas only 58.6% of 4AT-positive patients were considered to have DSM-5 delirium [21]. Thirdly, delirium tends to fluctuate, and delirium may not have been present when reviewed by the usual care team. The nature of delirium ascertainment was such that we may have missed delirium in those who were 4AT negative. Using the published sensitivity of the 4AT as 89.7% [21], we estimate this to be 25 of the 1141 who were 4AT negative. Therefore, results comparing outcomes between delirium and no delirium should be treated with caution. We suggest that given the well-described association of delirium to mortality, this is likely to have tempered our published results.
What are the messages for routine clinical practice?
Specialist delirium teams have developed in hospitals in recent years with an aim to improve delirium management. Delirium teams improved the likelihood of delirium screening but actually reduced the likelihood of recognition. This contradiction could be due to inherent differences in how a delirium team diagnose delirium. Alternative solutions to improve routine delirium screening (e.g. embedded screening tools in admission documentation) were not associated with better screening rates. In the UK, 4AT screening in older trauma patients is financially driven; screening was similar to acute medicine, and yet recognition was reduced [42]. We consider that, although screening can improve recognition, wider training is needed alongside this. Trusts wishing to invest in delirium teams should ensure adequate training is provided to team members. We did not collect data on if formal delirium education or screening training had been targeted towards certain wards within individual hospitals. We were, therefore, unable to assess for intra-hospital factors that may lead to local variations within the same hospital, beyond specialty itself.
Possible delirium was defined as meeting individual but not all reference standard criteria. We consider this synonymous with subsyndromal delirium, defined as the presence of one or more symptoms of delirium, not meeting criteria for or progressing to delirium [43]. Standardised criteria to aid diagnosis do not exist; however, it is increasingly recognised as an important condition in its own right. Associations with increased length of stay [44], institutionalisation [45], and mortality [45] have been demonstrated. We report similar results. The majority of individuals with a positive 4AT score had either possible or definite delirium, and 4AT-positive status by itself predicts adverse outcomes. This highlights the value of the 4AT to identify those at high risk and reiterates that screening should become routine practice. We suggest that in an acute hospital environment, a positive 4AT highlights patients at increased risk of adverse outcome. We recommend pairing of a comprehensive delirium management strategy (such as the TIME Bundle from the Scottish Delirium Association) [46].
Conclusions
Within the UK, delirium is highly prevalent amongst older hospital inpatients across specialties. Delirium is a severe condition associated with increased length of stay and mortality. Older adults with frailty are particularly vulnerable to delirium, and as frailty is associated with adverse outcomes in its own right, these patients exhibit greatest vulnerability. Unfortunately, our results suggest that delirium is less likely to be recognised in the frailest patients. We recommend that national quality improvement strategies should be implemented to increase screening and recognition of delirium, particularly focussing on patients with frailty and those admitted under surgical specialties.
Supplementary information
Additional file 1. Proforma used for data collection including screening and reference standard diagnosis.
Additional file 2. Supplementary figures and tables as referenced within the main text.
Additional file 3. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline checklist.
Acknowledgements
We wish to thank the following individuals who contributed towards data collection but did not contribute to authorship of this paper: Ashish Vasudev (University of Bristol), Asim Ahmad (University of Bristol), Afnan Wahballa (Scarborough General Hospital), Giles Hall (Warwick Hospital), Sammy Carter (Warwick Hospital), Felicity Leishman (Warwick Hospital), William Hunt (Warwick Hospital), Rhianna Davies (Walsall Manor Hospital), Karthik Basker (University of Birmingham), and Jessel Varghese (University Hospitals North Midlands).
Geriatric Medicine Research Collaborative:
Manuscript preparation: Carly Welch (University of Birmingham), Lauren McCluskey (University of Birmingham), Daisy Wilson (University of Birmingham), and George E Chapman (University of Birmingham)
Steering and data analysis group: Carly Welch (University of Birmingham), Lauren McCluskey (University of Birmingham), Daisy Wilson (University of Birmingham), and Thomas A Jackson (University of Birmingham) (overall guarantor and senior author)
Advisory group: Jonathan Treml (University Hospitals Birmingham NHS Foundation Trust), Daniel Davis (University College London), Emma Cunningham (Queen’s University Belfast), Claire Copeland (NHS Forth Valley), Terrence Quinn (University of Glasgow), Thomas Pinkney (University of Birmingham), and Rahul Mahida (University of Birmingham)
Data analysis advisor: Peter Nightingale (University Hospitals Birmingham NHS Foundation Trust)
Regional leads: Sarah Richardson (North East), Oliver Todd (Yorkshire), Ruth Willott (East Midlands), Kelli Torsney (East of England), Mary Ni Lochlainn (London), Kumudhini Giridharan (Kent, Surrey, and Sussex), Natalie Cox (Wessex), Jane Masoli (Peninsula), Lindsay Ronan (Peninsula), Victoria Gaunt (Severn), Benjamin Jelley (Welsh Geriatricians’ research Network, Wales), Joanne Taylor (North West), and Roisin Healy (Northern Ireland)
Other collaborators: Emily Rose (University Hospital Ayr), Megan Parkinson (University Hospital Ayr), Ajay Macharouthu (University Hospital Ayr), Eilidh McKenzie (NHS Forth Valley), Roisin McCormack (NHS Forth Valley), Jasmine Hart (Perth Royal Infirmary), Alison McCulloch (Perth Royal Infirmary), Neil Henderson (Perth Royal Infirmary), Louise Beveridge (Perth Royal Infirmary), Emma Elliott (University of Glasgow), Bogna Drozdowska (University of Glasgow), Martin Taylor-Rowan (University of Glasgow), Natasha Christodoulides (Oxford University Hospitals), James Allen (Oxford University Hospitals), Harriet Brown (Oxford University Hospitals), Jennifer Champion (Oxford University Hospitals), Riana Patel (Stoke Mandeville Hospital), Ghazal Hodhody (Stoke Mandeville Hospital), Kara Mayor (Stoke Mandeville Hospital), Christopher James Miller (University Hospitals of Leicester NHS Trust), Mark Studley (University Hospitals of Leicester NHS Trust), Vishnu Prasad (University Hospitals of Leicester NHS Trust), Emma Mumtaz (University Hospitals of Leicester NHS Trust), Sam Cohen (University Hospitals of Leicester NHS Trust), Sherif Abdelbadiee (University Hospitals of Leicester NHS Trust), Anna Lewis (King’s Mill Hospital), Bushra Khizar (King’s Mill Hospital), Hannah Pendleton (King’s Mill Hospital), Teresa Harkin (King’s Mill Hospital), Steve Rutter (King’s Mill Hospital), Ayoub Behbahani (Southport Hospital), Abolfazl Behbahani (Liverpool University), Ani Tencheva (South Tyneside Hospital), Rachel King (South Tyneside Hospital), Laura Jones (South Tyneside Hospital), Alex Hornsby (South Tyneside Hospital), Robbie Horton (South Tyneside Hospital), Kate Foster (South Tyneside Hospital), Kirsty Moore (University Hospital of North Durham), Vincent McCormack (University Hospital of North Durham), Matthew Kearney (University Hospital of North Durham), Emma Fisken (Newcastle Hospitals), Rory Durcan (Newcastle Hospitals), Elizabeth Deacon (Newcastle Hospitals), Jane Noble (Newcastle Hospitals), Arunkumar Annamalai (North Tees and Hartlepool NHS Foundation Trust), Roxana Taranu (North Tees and Hartlepool NHS Foundation Trust), Michael Sen (North Tees and Hartlepool NHS Foundation Trust), Pryankaran Mithrakumar (North Tees and Hartlepool NHS Foundation Trust), Laura Briggs (North Tees and Hartlepool NHS Foundation Trust), Jamal Bhatti (North Tees and Hartlepool NHS Foundation Trust), Shiv Bhakta (Cambridge University Hospitals), Amaka Achara (Hinchingbrooke Hospital), Elizabeth Ellis (Hinchingbrooke Hospital), Sejlo Koshedo (Hinchingbrooke Hospital), Ayesha Aamir (Hinchingbrooke Hospital), Edward Wu (Hinchingbrooke Hospital), Abdullah B Khalid (Hinchingbrooke Hospital), Parrthiepan Visvaratnam (Hinchingbrooke Hospital), Ijeoma Obi (Peterborough City Hospital), Nader Nashed (Peterborough City Hospital), Chioma Iwu (Peterborough City Hospital), Sneha Gurung (Peterborough City Hospital), Shonit Nagumantry (Peterborough City Hospital), Olugbenro Akintade (Peterborough City Hospital), Valerie Page (West Hertfordshire Hospitals NHS Trust), Kwasi Debrah (West Hertfordshire Hospitals NHS Trust), Katie Ball (West Hertfordshire Hospitals NHS Trust), Jabed Ahmed (West Hertfordshire Hospitals NHS Trust), Zhao Xiao Bei (West Hertfordshire Hospitals NHS Trust), Sarah B McClelland (Weston General Hospital), Michael Haley (Weston General Hospital), Norman Pang (Weston General Hospital), Andre Le Poideven (Weston General Hospital), Emily Moore (Weston General Hospital), Freya Cooper (Weston General Hospital), Natalie Grundmann (Weston General Hospital), Elizabeth Lonsdale-Eccles (Yeovil District Hospital), Janine Valentine (Yeovil District Hospital), Emma Stratton (University Hospitals Bristol NHS Trust), Emily Bowen (University Hospitals Bristol NHS Trust), Miriam Thake (University Hospitals Bristol NHS Trust), Dorothy Kuek (University of Bristol), Wioletta Pyc (University of Bristol), Deborah Scott (University Hospitals Bristol NHS Trust), Frances Rickard (University Hospitals Bristol NHS Trust), Natalie Gaskell (University Hospitals Bristol NHS Trust), Helen McDonald (Gloucester Royal Hospital), Victoria Gaunt (Gloucester Royal Hospital), Sam Mills (Gloucester Royal Hospital), Stuart Winearls (Gloucester Royal Hospital), Paapa A-Odame (University of Bristol), Ciaran Barlow (University of Bristol), Isabelle Nicholls (University of Bristol), Emma Norman (University of Bristol), Kim Kirrane (Derriford Hospital), Peter Jackson (Derriford Hospital), Christian Chourot (Derriford Hospital), Laura Jayne Beeley (Royal Cornwall Hospital Trust), Aaron Kay (Royal Cornwall Hospital Trust), Victoria Clayton (Royal Cornwall Hospital Trust), John Marshall (Royal Cornwall Hospital Trust), Hannah Morgan (Royal Cornwall Hospital Trust), George Naish (Royal Cornwall Hospital Trust), Clare Hunt (Maidstone and Tunbridge Wells NHS Trust), Rajeev Mishra (Maidstone and Tunbridge Wells NHS Trust), Saurav Bhattacharya (Maidstone and Tunbridge Wells NHS Trust), Nisha Rai (East Kent Hospitals), Ahmad Alareed (East Kent Hospitals), Clementine Anderson (East Kent Hospitals), Ganapathy Bhat (East Kent Hospitals), Sandra Darko (East Kent Hospitals), Pedro Vila De Mucha (East Kent Hospitals), David Saliu (East Kent Hospitals), Karen Beharry (East Kent Hospitals), Laurence Caines (East Kent Hospitals), Sanojan Bremakumar (East Kent Hospitals), Daniel Furmedge (Guy’s & St Thomas’ NHS Foundation Trust), Celine Bultynck (Guy’s & St Thomas’ NHS Foundation Trust), Esther Hindley (Guy’s & St Thomas’ NHS Foundation Trust), Elaine Seymour (Guy’s & St Thomas’ NHS Foundation Trust), Darmiga Thayabaran (Guy’s & St Thomas’ NHS Foundation Trust), Cal Doherty (Guy’s & St Thomas’ NHS Foundation Trust), John Frewen (King’s College Hospital), Oluwatosin O Abiola (King’s College Hospital), Simon Tetlow (King’s College Hospital), Guy Tinson (King’s College Hospital), Olivia Morrow (King’s College Hospital), Isabel Greaves (King’s College Hospital), Rachael Bygate (King’s College Hospital), Aayenah Yunus (King’s College Hospital), Catherine Bryant (King’s College Hospital), Howell Jones (University College London), Helen Bowden (University College London), Rose Laud (University College London Hospitals), Keziah Austin (University College London Hospitals), Farrah Bahsoon (University College London Hospitals), Martin Glasser (Royal Free London NHS Foundation Trust), Khai Lee Cheah (Royal Free London NHS Foundation Trust), James Speed (University College London), Lucy Porter (University College London), James Dove (Camden & Islington NHS Foundation Trust), Katrin Hoffman (University College London), Olivia Evans (Lewisham & Greenwich NHS Trust), Taran Nandra (Lewisham & Greenwich NHS Trust), Leeying Giet (Lewisham & Greenwich NHS Trust), Simon Stapley (Scarborough General Hospital), Imola Bargaoanu (Scarborough General Hospital), Ismail Kadir (Scarborough General Hospital), Adam McClean (Scarborough General Hospital), Pranav Mishra (Scarborough General Hospital), Katie Houldershaw (Scarborough General Hospital), Ana Andrusca (Scarborough General Hospital), Emmy Abu (Scarborough General Hospital), Adam Swietoslawski (Scarborough General Hospital), Bilquis Ahmed (Scarborough General Hospital), Matthew Ansell (Scarborough General Hospital), Saad Abdullah (Scarborough General Hospital), Shoaib Iqbal (Scarborough General Hospital), James Wilcockson (Scarborough General Hospital), Angela Kabia (Scarborough General Hospital), Karthika Velusamy (Scarborough General Hospital), Nihaad Syed (Scarborough General Hospital), Charlotte Chuter (Scarborough General Hospital), Hamza Ahmed (Scarborough General Hospital), Sarah Ahmad (Scarborough General Hospital), Gladys Ofoche (Scarborough General Hospital), Jacqueline Ibanichuka (Scarborough General Hospital), Alice Wheeler (Bradford Royal Infirmary), Angharad Chilton (Bradford Royal Infirmary), Zainab Hussain (Bradford Royal Infirmary), Felicia Tan (Bradford Royal Infirmary), Sinead Quinn (Warwick Hospital), Paul Croft (Warwick Hospital), Amy Walker (Warwick Hospital), Charlotte Bell (Warwick Hospital), Claire Wilkes (Warwick Hospital), Eliza Griffiths (Warwick Hospital), James Reid (Warwick Hospital), Ahmed Abras (Walsall Manor Hospital), Muhammad Adam (Walsall Manor Hospital), Awolkhier Mohammedseid-Nurhussien (Walsall Manor Hospital), Sohail Shakeel (Walsall Manor Hospital), Zarah Amin (Walsall Manor Hospital), Georgia R Layton (Walsall Manor Hospital), Nathan Ingamells (University of Birmingham), Jemima Taylor (University of Birmingham), Luke Wynne (University of Birmingham), Wan Idoracaera Calisa Ikhwan (Walsall Manor Hospital), Hanna Waraich (Walsall Manor Hospital), Olivia Cooper (Walsall Manor Hospital), Philip Thomas (Sandwell and West Birmingham NHS Trust), Emily Williamson (Sandwell and West Birmingham NHS Trust), Huma Naqvi (Sandwell and West Birmingham NHS Trust), Helena Lee (Sandwell and West Birmingham NHS Trust), Elizabeth Holmes (University Hospitals North Midlands), Megan Offer (University Hospitals North Midlands), Alex McQuillan (University Hospitals North Midlands), Emma Jay (University Hospitals North Midlands), Hannah Currie (Wye Valley NHS Trust), Sureena Janagal (Wye Valley NHS Trust), Gary Kumbun (Wye Valley NHS Trust), Rodric Jenkin (Wye Valley NHS Trust), Holly Jacques (University Hospitals Birmingham (Good Hope Hospital)), James Gaywood (University Hospitals Birmingham (Good Hope Hospital)), Laura Babb (University Hospitals Birmingham (Good Hope Hospital)), Moe Su Su San (University Hospitals Birmingham (Good Hope Hospital)), Sasha Porter-Bent (University Hospitals Birmingham (Good Hope Hospital)), Daisy Wilson (University Hospitals Birmingham (Birmingham Heartlands Hospital)), Tarunya Vedutla (University Hospitals Birmingham (Birmingham Heartlands Hospital)), Asiodu Nneamaka (University Hospitals Birmingham (Solihull Hospital)), Anum Cheema (University Hospitals Birmingham (Solihull Hospital)), Hannah Moorey (Sandwell and West Birmingham NHS Trust), Asma Khan (Sandwell and West Birmingham NHS Trust), Zeinab Majid (Sandwell and West Birmingham NHS Trust), Puja Jatti (Sandwell and West Birmingham NHS Trust), Abhishek Gupta (University Hospitals Birmingham NHS Trust), Tammy Lee (University Hospitals Birmingham NHS Trust), Helen Chamberlain (University Hospitals Birmingham NHS Trust), Clare Hughes (University Hospitals Birmingham NHS Trust), Alexis Giles (University Hospitals Birmingham NHS Trust), Tamsin Critchlow (University Hospitals Birmingham NHS Trust), Bethan Morgan (University of Birmingham), Alice Moseley (University of Birmingham), Grace Fennelly (University of Birmingham), Sophie Pettler (University of Birmingham), Edward Bilton (University of Birmingham), Emma Astaire (University of Birmingham), William McKeown (Belfast Health and Social Care Trust), Katherine Williamson (Belfast Health and Social Care Trust), Caroline Rice (Belfast Health and Social Care Trust), Sharan Ramakrishna (Anuerin Bevan University Health Board), Zahid Subhan (Anuerin Bevan University Health Board), Nedaa Haddad (Anuerin Bevan University Health Board), and Anjli Krishan (Scarborough General Hospital)
Authors’ contributions
CW, LM, DW, and TAJ designed the protocol for this study, collected the data, and analysed and interpreted the results. CW, LM, DW, and GEC prepared the first manuscript draft. JT, DD, EC, CC, and TQ made substantial contributions to the design of the work, data collection, and manuscript revisions. TP and RM contributed to the interpretation of the data and substantive revisions. PN substantially contributed to and supported the data analysis. SR, OT, RW, KT, MN, KG, NC, JM, LR, VG, BJ, JT, and RH substantially contributed to the conception, design, and acquisition of the data. All other collaborators substantially contributed to the acquisition, analysis, and interpretation of the data. All authors read and approved the final manuscript.
Funding
No specific funding was obtained for this study. The Geriatric Medicine Research Collaborative has received funding from the British Geriatrics Society to cover running costs of the collaborative.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted at each individual site as part of a national audit of delirium screening; confirmation was obtained from the Birmingham Research Ethics Committee that review by a research ethics committee was not required, as per HRA guidance. The audit was registered at each site as per local governance policies. Anonymous data was collected centrally, and the University of Birmingham ethical review committee provided approval for secondary data analysis of the anonymised database (ERN_18-1415).
Consent for publication
Not applicable—no personal information has been included within this publication, and all data used in the analysis was fully anonymised.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Geriatric Medicine Research Collaborative, Email: gemresearchuk@gmail.com.
Geriatric Medicine Research Collaborative:
Carly Welch, Lauren McCluskey, Daisy Wilson, George E. Chapman, Thomas A. Jackson, Jonathan Treml, Daniel Davis, Emma Cunningham, Claire Copeland, Terrence Quinn, Thomas Pinkney, Rahul Mahida, Peter Nightingale, Sarah Richardson, Oliver Todd, Ruth Willott, Kelli Torsney, Mary Ni Lochlainn, Kumudhini Giridharan, Natalie Cox, Jane Masoli, Lindsay Ronan, Victoria Gaunt, Benjamin Jelley, Joanne Taylor, Roisin Healy, Emily Rose, Megan Parkinson, Ajay Macharouthu, Eilidh McKenzie, Roisin McCormack, Jasmine Hart, Alison McCulloch, Neil Henderson, Louise Beveridge, Emma Elliott, Bogna Drozdowska, Martin Taylor-Rowan, Natasha Christodoulides, James Allen, Harriet Brown, Jennifer Champion, Riana Patel, Ghazal Hodhody, Kara Mayor, Christopher James Miller, Mark Studley, Vishnu Prasad, Emma Mumtaz, Sam Cohen, Sherif Abdelbadiee, Anna Lewis, Bushra Khizar, Hannah Pendleton, Teresa Harkin, Steve Rutter, Ayoub Behbahani, Abolfazl Behbahani, Ani Tencheva, Rachel King, Laura Jones, Alex Hornsby, Robbie Horton, Kate Foster, Kirsty Moore, Vincent McCormack, Matthew Kearney, Emma Fisken, Rory Durcan, Elizabeth Deacon, Jane Noble, Arunkumar Annamalai, Roxana Taranu, Michael Sen, Pryankaran Mithrakumar, Laura Briggs, Jamal Bhatti, Shiv Bhakta, Amaka Achara, Elizabeth Ellis, Sejlo Koshedo, Ayesha Aamir, Edward Wu, Abdullah B. Khalid, Parrthiepan Visvaratnam, Ijeoma Obi, Nader Nashed, Chioma Iwu, Sneha Gurung, Shonit Nagumantry, Olugbenro Akintade, Valerie Page, Kwasi Debrah, Katie Ball, Jabed Ahmed, Zhao Xiao Bei, Sarah B. McClelland, Michael Haley, Norman Pang, Andre Le Poideven, Emily Moore, Freya Cooper, Natalie Grundmann, Elizabeth Lonsdale-Eccles, Janine Valentine, Emma Stratton, Emily Bowen, Miriam Thake, Dorothy Kuek, Wioletta Pyc, Deborah Scott, Frances Rickard, Natalie Gaskell, Helen McDonald, Victoria Gaunt, Sam Mills, Stuart Winearls, Paapa A-Odame, Ciaran Barlow, Isabelle Nicholls, Emma Norman, Kim Kirrane, Peter Jackson, Christian Chourot, Laura Jayne Beeley, Aaron Kay, Victoria Clayton, John Marshall, Hannah Morgan, George Naish, Clare Hunt, Rajeev Mishra, Saurav Bhattacharya, Nisha Rai, Ahmad Alareed, Clementine Anderson, Ganapathy Bhat, Sandra Darko, Pedro Vila De Mucha, David Saliu, Karen Beharry, Laurence Caines, Sanojan Bremakumar, Daniel Furmedge, Celine Bultynck, Esther Hindley, Elaine Seymour, Darmiga Thayabaran, Cal Doherty, John Frewen, Oluwatosin O. Abiola, Simon Tetlow, Guy Tinson, Olivia Morrow, Isabel Greaves, Rachael Bygate, Aayenah Yunus, Catherine Bryant, Howell Jones, Helen Bowden, Rose Laud, Keziah Austin, Farrah Bahsoon, Martin Glasser, Khai Lee Cheah, James Speed, Lucy Porter, James Dove, Katrin Hoffman, Olivia Evans, Taran Nandra, Leeying Giet, Simon Stapley, Imola Bargaoanu, Ismail Kadir, Adam McClean, Pranav Mishra, Katie Houldershaw, Ana Andrusca, Emmy Abu, Adam Swietoslawski, Bilquis Ahmed, Matthew Ansell, Saad Abdullah, Shoaib Iqbal, James Wilcockson, Angela Kabia, Karthika Velusamy, Nihaad Syed, Charlotte Chuter, Hamza Ahmed, Sarah Ahmad, Gladys Ofoche, Jacqueline Ibanichuka, Alice Wheeler, Angharad Chilton, Zainab Hussain, Felicia Tan, Sinead Quinn, Paul Croft, Amy Walker, Charlotte Bell, Claire Wilkes, Eliza Griffiths, James Reid, Ahmed Abras, Muhammad Adam, Awolkhier Mohammedseid-Nurhussien, Sohail Shakeel, Zarah Amin, Georgia R. Layton, Nathan Ingamells, Jemima Taylor, Luke Wynne, Wan Idoracaera Calisa Ikhwan, Hanna Waraich, Olivia Cooper, Philip Thomas, Emily Williamson, Huma Naqvi, Helena Lee, Elizabeth Holmes, Megan Offer, Alex McQuillan, Emma Jay, Hannah Currie, Sureena Janagal, Gary Kumbun, Rodric Jenkin, Holly Jacques, James Gaywood, Laura Babb, Moe Su Su San, Sasha Porter-Bent, Daisy Wilson, Tarunya Vedutla, Asiodu Nneamaka, Anum Cheema, Hannah Moorey, Asma Khan, Zeinab Majid, Puja Jatti, Abhishek Gupta, Tammy Lee, Helen Chamberlain, Clare Hughes, Alexis Giles, Tamsin Critchlow, Bethan Morgan, Alice Moseley, Grace Fennelly, Sophie Pettler, Edward Bilton, Emma Astaire, William McKeown, Katherine Williamson, Caroline Rice, Sharan Ramakrishna, Zahid Subhan, Nedaa Haddad, and Anjli Krishan
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12916-019-1458-7.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) 5 2013. [Google Scholar]
- 2.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grover S, Sharma A, Aggarwal M, Mattoo SK, Chakrabarti S, Malhotra S, et al. Comparison of symptoms of delirium across various motoric subtypes. Psychiatry Clin Neurosci. 2014;68(4):283–291. doi: 10.1111/pcn.12131. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350–364. doi: 10.1093/ageing/afl005. [DOI] [PubMed] [Google Scholar]
- 5.Ryan Daniel James, O'Regan Niamh Annmarie, Caoimh Ronán Ó, Clare Josie, O'Connor Marie, Leonard Maeve, McFarland John, Tighe Sheila, O'Sullivan Kathleen, Trzepacz Paula T, Meagher David, Timmons Suzanne. Delirium in an adult acute hospital population: predictors, prevalence and detection. BMJ Open. 2013;3(1):e001772. doi: 10.1136/bmjopen-2012-001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pendlebury S, Lovett N, Smith S, Dutta N, Bendon C, Lloyd-Lavery A, et al. Observational, longitudinal study of delirium in consecutive unselected acute medical admissions: age-specific rates and associated factors, mortality and re-admission. BMJ Open. 2015;5(11):e007808. doi: 10.1136/bmjopen-2015-007808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partridge JS, Martin FC, Harari D, Dhesi JK. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry. 2013;28(8):804–812. doi: 10.1002/gps.3900. [DOI] [PubMed] [Google Scholar]
- 8.Jackson TA, MacLullich AMJ, Gladman JRF, Lord JM, Sheehan B. Diagnostic test accuracy of informant-based tools to diagnose dementia in older hospital patients with delirium: a prospective cohort study. Age Ageing. 2016;45(4):505–511. doi: 10.1093/ageing/afw065. [DOI] [PubMed] [Google Scholar]
- 9.Davis DH, Muniz-Terrera G, Keage HA, Stephan BC, Fleming J, Ince PG, et al. Association of delirium with cognitive decline in late life: a neuropathologic study of 3 population-based cohort studies. JAMA Psychiatry. 2017;74(3):244–251. doi: 10.1001/jamapsychiatry.2016.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135(Pt 9):2809–2816. doi: 10.1093/brain/aws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung P, Pereira MA, Hiebert B, Song X, Rockwood K, Tangri N, et al. The impact of frailty on postoperative delirium in cardiac surgery patients. J Thorac Cardiovasc Surg. 2015;149(3):869–75.e1–2. doi: 10.1016/j.jtcvs.2014.10.118. [DOI] [PubMed] [Google Scholar]
- 12.Assmann P, Kievit P, van der Wulp K, Verkroost M, Noyez L, Bor H, et al. Frailty is associated with delirium and mortality after transcatheter aortic valve implantation. Open Heart. 2016;3(2):e000478. doi: 10.1136/openhrt-2016-000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chew J, Lim WS, Chong MS, Ding YY, Tay L. Impact of frailty and residual subsyndromal delirium on 1-year functional recovery: a prospective cohort study. Geriatr Gerontol Int. 2017;17(12):2472–2478. doi: 10.1111/ggi.13108. [DOI] [PubMed] [Google Scholar]
- 14.Guerini F, Frisoni GB, Morghen S, Speciale S, Bellelli G, Trabucchi M. Clinical instability as a predictor of negative outcomes among elderly patients admitted to a rehabilitation ward. J Am Med Dir Assoc. 2010;11(6):443–448. doi: 10.1016/j.jamda.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Ritter SRF, Cardoso AF, Lins MMP, Zoccoli TLV, Freitas MPD, Camargos EF. Underdiagnosis of delirium in the elderly in acute care hospital settings: lessons not learned. Psychogeriatrics. 2018;18(4):268–275. doi: 10.1111/psyg.12324. [DOI] [PubMed] [Google Scholar]
- 16.Welch C, Jackson TA. Can delirium research activity impact on routine delirium recognition? A prospective cohort study. BMJ Open. 2018;8(10):e023386. doi: 10.1136/bmjopen-2018-023386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kales HC, Kamholz BA, Visnic SG, Blow FC. Recorded delirium in a national sample of elderly inpatients: potential implications for recognition. J Geriatr Psychiatry Neurol. 2003;16(1):32–38. doi: 10.1177/0891988702250535. [DOI] [PubMed] [Google Scholar]
- 18.Collins N, Blanchard MR, Tookman A, Sampson EL. Detection of delirium in the acute hospital. Age Ageing. 2010;39(1):131–135. doi: 10.1093/ageing/afp201. [DOI] [PubMed] [Google Scholar]
- 19.Eeles EM, England R, Armstrong A, Pinsker D, Pandy S, Teodorczuk A. Understanding our patients better will lead to better recognition of delirium: an opinion piece. Australas J Ageing. 2018;37(4):241–242. doi: 10.1111/ajag.12585. [DOI] [PubMed] [Google Scholar]
- 20.National Insitute for Health and Clinical Excellence (NICE) Delirium: prevention, diagnosis and management - clinical guideline [CG103] 2010. [PubMed] [Google Scholar]
- 21.Bellelli G, Morandi A, Davis DH, Mazzola P, Turco R, Gentile S, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43(4):496–502. doi: 10.1093/ageing/afu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Insitute for Health and Clinical Excellence (NICE) Cartographer delirium in adults - quality statement 5: communication of diagnosis to GPs. 2014. [Google Scholar]
- 23.Leah V. World Delirium Awareness Day provides us all with the opportunity to highlight that delirium is a medical emergency, but you can make a difference. 2018. [Google Scholar]
- 24.Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ (Clinical research ed) 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellelli G, Morandi A, Di Santo SG, Mazzone A, Cherubini A, Mossello E, et al. “Delirium Day”: a nationwide point prevalence study of delirium in older hospitalized patients using an easy standardized diagnostic tool. BMC Med. 2016;14:106. doi: 10.1186/s12916-016-0649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326–333. doi: 10.1093/ageing/afu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eeles EM, Hubbard RE, White SV, O’Mahony MS, Savva GM, Bayer AJ. Hospital use, institutionalisation and mortality associated with delirium. Age Ageing. 2010;39(4):470–475. doi: 10.1093/ageing/afq052. [DOI] [PubMed] [Google Scholar]
- 29.Stagno D, Gibson C, Breitbart W. The delirium subtypes: a review of prevalence, phenomenology, pathophysiology, and treatment response. Palliat Support Care. 2004;2(2):171–179. doi: 10.1017/S1478951504040234. [DOI] [PubMed] [Google Scholar]
- 30.Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841. doi: 10.1001/jama.297.8.831. [DOI] [PubMed] [Google Scholar]
- 31.Cresswell Aynsley, Hart Matthew, Suchanek Ondrej, Young Tania, Leaver Laurence, Hibbs Stephen. Mind the gap: Improving discharge communication between secondary and primary care. BMJ Quality Improvement Reports. 2015;4(1):u207936.w3197. doi: 10.1136/bmjquality.u207936.w3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buurman BM, Verhaegh KJ, Smeulers M, Vermeulen H, Geerlings SE, Smorenburg S, et al. Improving handoff communication from hospital to home: the development, implementation and evaluation of a personalized patient discharge letter. Int J Qual Health Care. 2016;28(3):384–390. doi: 10.1093/intqhc/mzw046. [DOI] [PubMed] [Google Scholar]
- 33.Hesselink G, Zegers M, Vernooij-Dassen M, Barach P, Kalkman C, Flink M, et al. Improving patient discharge and reducing hospital readmissions by using intervention mapping. BMC Health Serv Res. 2014;14(1):389. doi: 10.1186/1472-6963-14-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verloo H, Goulet C, Morin D, von Gunten A. Association between frailty and delirium in older adult patients discharged from hospital. Clin Interv Aging. 2016;11:55–63. doi: 10.2147/CIA.S100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pol RA, van Leeuwen BL, Visser L, Izaks GJ, van den Dungen JJ, Tielliu IF, et al. Standardised frailty indicator as predictor for postoperative delirium after vascular surgery: a prospective cohort study. Eur J Vasc Endovasc Surg. 2011;42(6):824–830. doi: 10.1016/j.ejvs.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Leung JM, Tsai TL, Sands LP. Brief report: preoperative frailty in older surgical patients is associated with early postoperative delirium. Anesth Analg. 2011;112(5):1199–1201. doi: 10.1213/ANE.0b013e31820c7c06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eeles EM, White SV, O’Mahony SM, Bayer AJ, Hubbard RE. The impact of frailty and delirium on mortality in older inpatients. Age Ageing. 2012;41(3):412–416. doi: 10.1093/ageing/afs021. [DOI] [PubMed] [Google Scholar]
- 38.Parmar KL, Law J, Carter B, Hewitt J, Boyle JM, Casey P, et al. Frailty in older patients undergoing emergency laparotomy: results from the UK Observational Emergency Laparotomy and Frailty (ELF) Study. Ann Surg. 2019. 10.1097/SLA.0000000000003402. Epub ahead of print. [DOI] [PubMed]
- 39.Jackson TA, MacLullich AMJ, Gladman JRF, Lord JM, Sheehan B. Undiagnosed long-term cognitive impairment in acutely hospitalised older medical patients with delirium: a prospective cohort study. Age Ageing. 2016;45(4):493–499. doi: 10.1093/ageing/afw064. [DOI] [PubMed] [Google Scholar]
- 40.Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019;18(2):177–184. doi: 10.1016/S1474-4422(18)30371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakuma R, du Fort GG, Arsenault L, Perrault A, Platt RW, Monette J, et al. Delirium in older emergency department patients discharged home: effect on survival. J Am Geriatr Soc. 2003;51(4):443–450. doi: 10.1046/j.1532-5415.2003.51151.x. [DOI] [PubMed] [Google Scholar]
- 42.Royal College of Physicians . National Hip Fracture Database (NHFD) annual report 2017. 2017. [Google Scholar]
- 43.Cole MG, Ciampi A, Belzile E, Dubuc-Sarrasin M. Subsyndromal delirium in older people: a systematic review of frequency, risk factors, course and outcomes. Int J Geriatr Psychiatry. 2013;28(8):771–780. doi: 10.1002/gps.3891. [DOI] [PubMed] [Google Scholar]
- 44.Ouimet S, Riker R, Bergeron N, Cossette M, Kavanagh B, Skrobik Y. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med. 2007;33(6):1007–1013. doi: 10.1007/s00134-007-0618-y. [DOI] [PubMed] [Google Scholar]
- 45.Marcantonio E, Ta T, Duthie E, Resnick NM. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 46.Bauernfreund Y, Butler M, Ragavan S, Sampson EL. TIME to think about delirium: improving detection and management on the acute medical unit. BMJ Open Qual. 2018;7(3):e000200. doi: 10.1136/bmjoq-2017-000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Proforma used for data collection including screening and reference standard diagnosis.
Additional file 2. Supplementary figures and tables as referenced within the main text.
Additional file 3. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline checklist.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.