Abstract
Differentiation of embryonic stem cells in vitro is an important tool in dissecting and understanding the mechanisms that govern early embryologic development. In recent years, there has been considerable progress in creating organoids that model gastrulation, neurulation or organogenesis. However, one of the key challenges is reproducibility. Geometrically confining stem cell colonies considerably improves reproducibility and provides quantitative control over differentiation and tissue shape. Here, we review recent advances in controlling the two- or three-dimensional organization of cells and the effect on differentiation phenotypes. Improved methods of geometrical control will allow for an even more detailed understanding of the mechanisms underlying embryologic development and will eventually pave the way for the highly reproducible generation of specific tissue types.
Modeling embryogenesis in vitro
As far back as over 2,300 years ago, Aristotle made the first known written descriptions of animal embryonic development. Looking at a chick inside the egg and dissecting various mammalian embryos, he noticed that their development involves dramatic shape changes of the embryo rather than merely a continuous increase in organism size. The actual signaling pathways and morphogenetic changes were described in great detail in the previous century by manipulating the development of the frog, chick, mouse and many other animal models. Typically, these experiments involved tissue grafting experiments or applying external signals. While extremely valuable, quantitative measurements can be quite challenging in animal models. Moreover, it has become apparent that human development in many aspects follows species-specific developmental programs, often different from other mammals, such as the mouse. Therefore, in recent years, significant efforts have been devoted to designing various in vitro approaches to modeling embryogenesis [1–4].
Embryonic stem cells (ESCs) are an excellent proxy for the early embryo. Protocols of culturing and differentiating ES cells into a variety of tissues have seen appreciable advancements in recent years and have been used to model a number of pathways in early human development. The most common approaches to differentiating stem cells is by applying morphogens—the secreted proteins that activate a signaling program—to either disorganized 2D cell colonies on a dish or to 3D clusters of stem cells suspended in medium or embedded in Matrigel. 3D culture protocols are becoming more and more prominent, especially in creating so-called organoids, i.e. differentiated 3D tissues that mimic certain aspects of adult organs in terms of tissue types and organization [4,5]. Organoids are useful in creating specific tissues or learning about signaling pathways that generate them. However, organoids do not form in a reproducible manner and rarely model the correct size and shapes seen during embryonic development [6]. Growing stem cells on pre-formed scaffolds or combining differentiation protocols with tissue origami [7] could potentially be the next strategy in designing correctly shaped tissues, however coupling differentiation with timely tissue folding would be an extremely challenging task.
Remarkably, stem cells have an innate capacity to self-organize in vitro in a way that mimics morphogenetic changes and cell movements during embryonic development [4]. Based on recent works, it seems that often manipulating the microenvironment of developing tissues, such as by confining cells into micropatterns or by tuning the 3D matrix can be sufficient to recapitulate complex differentiation events in early development in a highly reproducible manner. Confining cells to 2D patterns or within 3D polymeric networks does not simply impose a shape on tissues. Instead, it creates a spatially organized signaling environment, which, combined with the cell capacity to self-organize, can be a powerful tool for mimicking embryonic development in a reproducible and controlled way. Nevertheless, challenges remain, such as correctly reproducing tissue density, mechanical properties of the substrate, developmental timing, and, importantly, achieving symmetry breaking, crucial for the formation of the body axes. Here, we highlight some recent progress in modeling of the early human and mammalian development. We focus on experiments that take advantage of controlled 2D and 3D environments and the self-organization capacity of stem cells to recreate gastrulation, neurulation, and organogenesis.
Modeling development using 2D stem cell micropatterns
Stem cells can differentiate into many different cell types, whose fate is determined depending on the applied morphogen, its concentration, time of application, cell density, and other factors. Considering the complexity of conditions that affect cell fate, there is growing interest to culture stem cells in geometrically defined colonies by seeding them on surfaces with patterned extracellular matrix (ECM) proteins (see Box for technical details) [8]. Micropatterned colonies overcome the important challenge of reproducibility facing organoid research, however they also provide a quantitative platform for microscopy imaging, screening and parallelization. Interestingly, it was recently observed that cell fate can depend on the size of stem cell colonies. When presented with bone morphogenetic protein 2 and activin A, 200-μm-wide circular stem cell colonies consistently produced more endodermal genes while 1,200-μm-wide colonies produced more mesodermal genes [9]. Unconfined colonies under the same conditions would typically differentiate into a mix of mesoderm and endoderm whose ratio is difficult to reproducibly control [9]. It was therefore becoming clear that geometric control not only creates a more quantitative platform for stem cell differentiation, but that it can directly affect the fate choices of cells.
Box: Methods of creating 2D micropatterned ESC colonies.
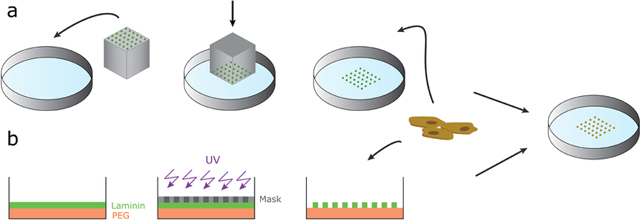
There are several ways of controlling the geometry of cell colonies. One common approach comprises casting a mold using an elastomeric material such as poly(dimethylsiloxane) (PDMS). The elastomer is coated with an ECM protein, such as laminin, which is then mechanically transferred like a stamp onto the dish [32] (see Box figure, a). Other approaches involve directly manipulating the surface by etching the surface with UV. In the depicted example, (see Box figure, b), the laminin is deposited on the PDMS-coated surface, covered with a photomask in the shape of desired patterns, then the laminin covering the remaining surface is etched away with UV [15].
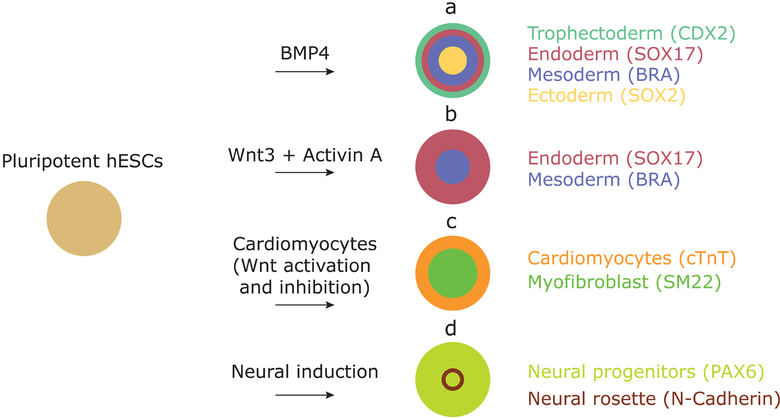
The mechanism by which geometric confinement determines cell fate was revealed in recent works where micropatterned colonies of human ESCs (hESCs) were presented with the bone morphogenetic protein 4 (BMP4). Within 48h, cells acquired fates of all three germ layers and trophectoderm that were radially organized forming, from edges inwards: trophectoderm, endoderm, mesoderm, and ectoderm (Figure 1) [10]. Similar to the aforementioned study [9], here small colonies mostly acquired the outermost—trophectoderm—fate, with larger colonies progressively acquiring inner fates [10]. Therefore, it seems that size per se is not the key factor in differentiation; rather, cell fate depends on the distance from the edge. Combined experimental and theoretical efforts demonstrated that this edge sensing is a result of (A), secretion of the BMP4 inhibitor noggin whose concentration is maximal at the colony center and (B), the localization of BMP4 receptors at the basolateral membrane of epithelial hECSs, which restricts BMP4 signaling to the outer perimeter of micropatterns [11]. As a result, the secreted inhibitor interacts with BMP4 in a reaction-diffusion mechanism, establishing a BMP4 concentration gradient from edges inward and causing the concentric organization of germ layers. The central role of the BMP4-noggin pair was later confirmed in similar experiments, where it was suggested that a combination of the reaction-diffusion and the duration of induction are important for gastrulation-like patterning [12]. Employing an analogous approach using mouse ESCs (mESCs) and combinations of BMP, Wnt, activin and FGF, it was demonstrated that cells in the radially organized germ layers indeed recapitulate regionally specific cell types seen in mouse embryos at gastrulation [13].
Figure 1:
Examples of differentiation of hESCs on micropatterns. Upon stimulation with differentiation signals, different tissues can be generated in a reproducible manner in order to study (a) germ layer and (b) primitive streak formation in gastrulation models, (c) beating heart chambers, and (d) neural rosette formation.
Interestingly, hESCs confined to very small colonies (<10 cells per colony) showed an unexpected behavior when presented with a low dose of BMP4 for 42h. Based on the expression of CDX2, a trophectoderm marker, and of SOX2, a pluripotency marker, colonies either fully differentiated into trophectoderm cells or remained pluripotent [14]. The authors suggest that there may be a mechanism that enforces a common fate and acts at a particular distance, which may be seen as a form of community effect among cells [14].
Applying Wnt3A, an effector that acts downstream of BMP4, to hESC micropattern colonies, it was shown that Wnt is sufficient to induce the induction of mesoderm and endoderm genes at the periphery of hESC colonies, without the expression of extraembryonic markers (Figure 1) [15]. Complementing Wnt3A with activin gave rise to cells expressing markers of the human organizer, also in a radially symmetric fashion. Differentiation of micropatterned hESC colonies thus helped delineate the role of early morphogens in patterning of the primitive streak, and it also helped efficiently produce specialized human cell types that would have otherwise been challenging in a standard dish culture [15].
Geometric confinement was also successful in modeling other early developmental events, further downstream of gastrulation. Applying a protocol that would produce disorganized cardiomyocytes on disorganized colonies, human induced pluripotent stem cells disks of 400–600 μm in diameter produced beating cardiac microchambers at the center of each colony (Figure 1) [16]. Although the precise mechanism remains unclear, it is also edge-sensitive based on the restriction of the pluripotency marker OCT4 to the outer perimeter one day following stimulation [16]. Recently, this technique has been extended to generate radially symmetric neural rosettes [17] and neural ectodermal organization [18] by applying neural differentiation protocols on confined hESCs.
Finally, geometric confinement of stem cells is not only useful to induce embryonic patterning, but the precise colony organization is extremely helpful in quantification. In a recent example, tail bud explants from the mouse pre-somitic mesoderm were cultured on small micropatterns to study the mechanism of genetic oscillations in developing somites [17].
These studies mark only the beginnings of applying geometric control to more conventional stem cell dish culture. Most importantly, they shed light on developmental events in a more quantitative fashion. The techniques described above could potentially be combined with other innovative bioengineering methods to model more complex tissues and dynamic events. For instance, the surface outside of patterns, which initially does not permit the growth of cells, could be coated with a compound that, when activated with click chemistry, promotes cell migration from patterns. An interesting application of this method has been proposed where human neural progenitors were cultured on sub-millimeter micropatterns and allowed to form a neural rosette (Figure 1) before activating the remaining surface and allowing nascent neurons to emerge from geometrically confined colonies [18].
Modeling gastrulation and neurulation in 3D
While 2D micropatterns have shown to be very successful in modeling early developmental events, it is still appealing to create 3D models of the embryo. One important benefit is a more realistic representation of the structure of the embryo and its mechanical environment. For instance, the pre-gastrulation epiblast surrounds the amniotic cavity, which could play an important role in signaling and tissue patterning during gastrulation. Another advantage is that 2D cultures suffer from edge effects at the colony border, while 3D cultures are isotropically symmetric. Much effort has thus been devoted to modeling embryogenesis by using organoids, typically by embedding dissociated stem cells into soft and porous gels, such as Matrigel. However, reproducibility and quantitative control still present tremendous challenges in modeling organogenesis.
The inspiration to growing stem cells in Matrigel comes from studies where Madin-Darby canine kidney (MDCK) cells embedded in Matrigel formed spherical epithelial cysts [19]. Analogously, hESCs grown in Matrigel form 3D epithelial colonies with a cavity in the center [20], reminiscent of the pre-gastrulation epiblast [21,22]. The spontaneous formation of the lumen is due to the innate apicobasal polarity of human pluripotent cells, where each stem cell inherits a highly organized and asymmetrically placed apicosome at division [23]. This apicosome helps to rapidly establish cell polarization and, when multiple stem cells form an aggregate, apicosomes of different cells are trafficked such that the entire aggregate forms a common lumen [23].
Matrigel also helps promote spontaneous differentiation of hESC cysts into squamous tissue expressing many of the extraembryonic markers, which the authors suggest to be amnion [24]. These structures form when hESCs are cultured on very soft substrates in a Matrigel-rich environment. The observed amniogenesis is BMP dependent, however its mechanism is still unknown [24]. Occasionally, this culture method gives rise to asymmetric differentiation where only one side of the cyst turns into squamous tissue whereas the other remains OCT4+, therefore resembling the post-implantation epiblast disk with the amnion [25].
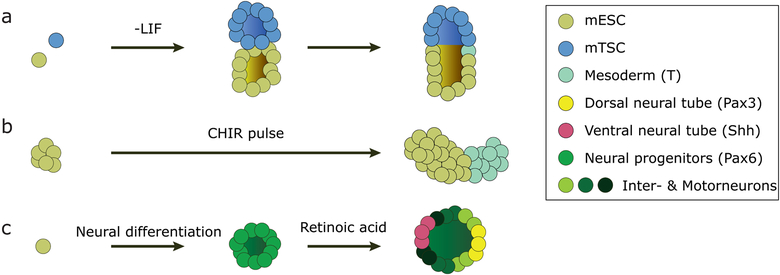
Most efforts in modeling early embryogenesis in 3D have been done using mouse cells. For example, synthetic structures remarkably similar to the peri-implantation mouse embryo were obtained by mixing aggregates of mESCs and mouse trophectoderm stem cells (mTSCs) and embedding them in Matrigel. The two cell clusters merge and spontaneously create a common lumen, in a process dependent on nodal signaling (Figure 2) [26]. Within five days in culture, a fraction of mESC-only and mixed mESC-mTSC colonies expressed the mesoderm marker Brachyury, although more prominently in the co-culture and, importantly, at the interface of mESC and mTSC regions, just like in the real mouse embryo at gastrulation. In another study, polarized expression of Brachyury was achieved by applying a pulse of the Wnt agonist CHIR99201 to disorganized mESC clusters (Figure 2) [27]. It was further shown that these so-called gastruloids have the capacity to asymmetrically express markers consistent with the anterior-posterior and the dorso-ventral axis of the gastrulating mouse embryo, for which Wnt and nodal signaling were important [28]. Interestingly, these structures showed signs of axis formation despite the lack of amniotic cavity or epithelial character and the absence of extraembryonic tissues at the onset of the experiment. Curiously, a different study showed that mESCs can differentiate into concentrically organized germ layers in 3D by first withdrawing LIF from mESCs cultured in a very soft (90-Pa stiffness) fibrin gel and then transferring the formed clusters 2.5 days later to a soft (1-kPa stiffness) 2D substrate coated with collagen-I [29]. The positioning of the individual germ layers was highly variable and crucially dependent on the stiffness of the substrate and the presence of collagen-I, suggesting an important role of mechanics in this process. The correct positioning of all three germ layers only took place in 2.5% of the cases [29].
Figure 2:
Examples of differentiating 3D mESC colonies. (a) Differentiation by removal of pluripotency factors and combination of mESCs and mTSCs leads to cell co-cultures. They combine to structures with a single lumen that express gastrulation markers on one side, replicating mouse gastrulation. (b) A pulse of the Wnt agonist CHIR causes collections of pluripotent stem cells to differentiate asymmetrically, with one part expressing the primitive streak marker Brachyury (T). (c) Neural induction generates neuroepithelial cysts with a central lumen, subsequent stimulation with retinoic acid leads to dorsal-ventral patterning mimicking patterning of the neural tube.
These studies together demonstrate that the presence of extraembryonic tissues is not key in inducing gastrulation and point to important signaling pathways involved in this process, at least in vitro. An important next step will be to investigate the precise molecular mechanisms and minimal requirements to break axis symmetry in the epithelial epiblast tissue of the early embryo. Driven by this question, we have recently proposed a model of axial symmetry breaking in 3D hESC cysts induced by a low dose of BMP4. Our model implicates WNT and its inhibitor DKK1 to be important molecular players in this process [31].
Another example of a symmetry-breaking event studied in mESC cysts is the asymmetric patterning of the mouse neural tube. Dissociated mESCs were embedded in Matrigel or, in the subsequent improvement, into a polyethylene glycol (PEG)-based hydrogel supplemented with various ECM proteins. After neural induction, mESCs self-organized into 3D neurepithelial cysts and, after a timely addition of retinoic acid, they asymmetrically expressed markers consistent with the dorso-ventral patterning of the neural tube (Figure 2) [30,31].
Synthetic embryology: outlook
Geometric confinement of stem cell colonies in both 2D and 3D gives tremendous control in modeling embryonic tissues and thus they are extremely valuable as quantitative tools for early development. We expect that both micropatterning and 3D modeling will become increasingly popular in the near future to gain a deeper understanding of molecular and signaling mechanisms underlying human embryonic patterning. We envision micropatterns as an excellent tool in creating and studying regional specification downstream of gastrulation, such as the formation of the neural tube and neural patterning, somitogenesis, gut formation, and other embryonic processes. With further improvements, it would be of tremendous interest to use surface architecture methods to study the development of different tissues juxtaposed to one another, as they would be in the embryo, such as the formation of somites along the neural tube, paving the way toward advanced synthetic embryology.
Acknowledgements
This work was supported by a National Institutes of Health/Department of Health and Human Services grant (#5RO1Hd080699) to A.B. M.S. is a Junior Fellow of the Simons Foundation.
Footnotes
Conflict of interest statement
Nothing declared.
References
- 1.Davies J: Using synthetic biology to explore principles of development. Development 2017, 144:1146–1158. [DOI] [PubMed] [Google Scholar]
- 2.Laurent J, Blin G, Chatelain F, Vanneaux V, Fuchs A, Larghero J, Thery M: Convergence of microengineering and cellular self-organization towards functional tissue manufacturing. Nature Biomedical Engineering 2017, 1:939–956. [DOI] [PubMed] [Google Scholar]
- 3.Simunovic M, Brivanlou AH: Embryoids, organoids and gastruloids: new approaches to understanding embryogenesis. Development 2017, 144:976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasai Y, Eiraku M, Suga H: In vitro organogenesis in three dimensions: self-organising stem cells. Development 2012, 139:4111–4121. [DOI] [PubMed] [Google Scholar]
- 5.Clevers H: Modeling Development and Disease with Organoids. Cell 2016, 165:1586–1597. [DOI] [PubMed] [Google Scholar]
- 6.Huch M, Knoblich JA, Lutolf MP, Martinez-Arias A: The hope and the hype of organoid research. Development 2017, 144:938–941. [DOI] [PubMed] [Google Scholar]
- 7.Hughes AJ, Miyazaki H, Coyle MC, Zhang J, Laurie MT, Chu D, Vavrusova Z, Schneider RA, Klein OD, Gartner ZJ: Engineered Tissue Folding by Mechanical Compaction of the Mesenchyme. Dev Cell 2018, 44:165–178 e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peerani R, Bauwens C, Kumacheva E, Zandstra PW: Patterning mouse and human embryonic stem cells using micro-contact printing In Stem Cells in Regenerative Medicine. Edited by: Springer; 2009:21–33. [DOI] [PubMed] [Google Scholar]
- 9.Lee LH, Peerani R, Ungrin M, Joshi C, Kumacheva E, Zandstra P: Micropatterning of human embryonic stem cells dissects the mesoderm and endoderm lineages. Stem Cell Res 2009, 2:155–162. [DOI] [PubMed] [Google Scholar]
- 10.Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH: A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods 2014, 11:847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etoc F, Metzger J, Ruzo A, Kirst C, Yoney A, Ozair MZ, Brivanlou AH, Siggia ED: A Balance between Secreted Inhibitors and Edge Sensing Controls Gastruloid Self-Organization. Developmental Cell 2016, 39:302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Dissection of the mechanisms leading to the gastrulation phenotype of hESCs on micropatterns.
- 12.Tewary M, Ostblom J, Prochazka L, Zulueta-Coarasa T, Shakiba N, Fernandez-Gonzalez R, Zandstra PW: A stepwise model of reaction-diffusion and positional information governs self-organized human peri-gastrulation-like patterning. Development 2017, 144:4298–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgani SM, Metzger JJ, Nichols J, Siggia ED, Hadjantonakis AK: Micropattern differentiation of mouse pluripotent stem cells recapitulates embryo regionalized cell fate patterning. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemashkalo A, Ruzo A, Heemskerk I, Warmflash A: Morphogen and community effects determine cell fates in response to BMP4 signaling in human embryonic stem cells. Development 2017, 144:3042–3053. [DOI] [PubMed] [Google Scholar]
- 15.Martyn I, Kanno T, Ruzo A, Siggia E, Brivanlou A: Self-organization of a functional human organizer by combined WNT and NODAL signalling. Nature, in press [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Generation of a human organizer on micropatterns and functional verification by grafting into chick embryos.
- 16.Ma Z, Wang J, Loskill P, Huebsch N, Koo S, Svedlund FL, Marks NC, Hua EW, Grigoropoulos CP, Conklin BR, et al. : Self-organizing human cardiac microchambers mediated by geometric confinement. Nat Commun 2015, 6:7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight GT, Sha J, Ashton RS: Micropatterned, clickable culture substrates enable in situ spatiotemporal control of human PSC-derived neural tissue morphology. Chem Commun (Camb) 2015, 51:5238–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]; Derivation of organized, polarized neuroepithela on micropatterns.
- 18.Xue X, Sun Y, Resto-Irizarry AM, Yuan Y, Yong KM, Zheng Y, Weng S, Shao Y, Chai Y, Studer L, Fu J: Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nat Mater 2018. May 21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubaud A, Regev I, Mahadevan L, Pourquie O: Excitable Dynamics and Yap-Dependent Mechanical Cues Drive the Segmentation Clock. Cell 2017, 171:668–682 e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elia N, Lippincott-Schwartz J: Culturing MDCK cells in three dimensions for analyzing intracellular dynamics. Curr Protoc Cell Biol 2009, Chapter 4:Unit 4 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taniguchi K, Shao Y, Townshend RF, Tsai YH, DeLong CJ, Lopez SA, Gayen S, Freddo AM, Chue DJ, Thomas DJ, et al. : Lumen Formation Is an Intrinsic Property of Isolated Human Pluripotent Stem Cells. Stem Cell Rep 2015, 5:954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahbazi MN, Jedrusik A, Vuoristo S, Recher G, Hupalowska A, Bolton V, Fogarty NM, Campbell A, Devito LG, Ilic D, et al. : Self-organization of the human embryo in the absence of maternal tissues. Nat Cell Biol 2016, 18:700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deglincerti A, Croft GF, Pietila LN, Zernicka-Goetz M, Siggia ED, Brivanlou AH: Self-organization of the in vitro attached human embryo. Nature 2016, 533:251–254. [DOI] [PubMed] [Google Scholar]
- 24.Taniguchi K, Shao Y, Townshend RF, Cortez CL, Harris CE, Meshinchi S, Kalantry S, Fu J, O’Shea KS, Gumucio DL: An apicosome initiates self-organizing morphogenesis of human pluripotent stem cells. J Cell Biol 2017, 10.1083/jcb.201704085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao Y, Taniguchi K, Gurdziel K, Townshend RF, Xue X, Yong KM, Sang J, Spence JR, Gumucio DL, Fu J: Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat Mater 2016, 10.1038/nmat4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao Y, Taniguchi K, Townshend RF, Miki T, Gumucio DL, Fu J: A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat Commun 2017, 8:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison SE, Sozen B, Christodoulou N, Kyprianou C, Zernicka-Goetz M: Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 2017, 356. [DOI] [PubMed] [Google Scholar]; Co-cultures of mouse ES and TS cells can spontaneously form structures with a common lumen that exhibit the expression of a primitive streak marker at the interface of the two tissues like in the mouse embryo.
- 28.van den Brink SC, Baillie-Johnson P, Balayo T, Hadjantonakis AK, Nowotschin S, Turner DA, Martinez Arias A: Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development 2014, 141:4231–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner DA, Girgin M, Alonso-Crisostomo L, Trivedi V, Baillie-Johnson P, Glodowski CR, Hayward PC, Collignon J, Gustavsen C, Serup P, et al. : Anteroposterior polarity and elongation in the absence of extraembryonic tissues and spatially localised signalling in Gastruloids, mammalian embryonic organoids. Development 2017:dev.150391. [DOI] [PMC free article] [PubMed] [Google Scholar]; Embryoid bodies of mouse ES cells upon stimulation with Wnt agonist display asymetric expression of anterior-posterior markers
- 30.Poh YC, Chen J, Hong Y, Yi H, Zhang S, Chen J, Wu DC, Wang L, Jia Q, Singh R, et al. : Generation of organized germ layers from a single mouse embryonic stem cell. Nat Commun 2014, 5:4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simunovic M, Metzger JJ, Etoc F, Yoney A, Ruzo A, Martyn I, Croft G, Brivanlou AH, Siggia ED: Molecular mechanism of symmetry breaking in a 3D model of a human epiblast. bioRxiv 2018. January 1:330704. [DOI] [PubMed] [Google Scholar]
- 32.Ranga A, Girgin M, Meinhardt A, Eberle D, Caiazzo M, Tanaka EM, Lutolf MP: Neural tube morphogenesis in synthetic 3D microenvironments. Proc Natl Acad Sci U S A 2016, 113:E6831–E6839. [DOI] [PMC free article] [PubMed] [Google Scholar]; Method of a generating synthetic mouse neural tube-like cysts from mouse ES cells that break dorsal-ventral symmetry upon uniform stimulation with morphogens.
- 33.Meinhardt A, Eberle D, Tazaki A, Ranga A, Niesche M, Wilsch-Brauninger M, Stec A, Schackert G, Lutolf M, Tanaka EM: 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Rep 2014, 3:987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM: Patterning proteins and cells using soft lithography. Biomaterials 1999, 20:2363–2376. [DOI] [PubMed] [Google Scholar]