Abstract
BACKGROUND
Admitting patients to an intensive care or medium care unit (ICU/MCU) after adult supratentorial tumor craniotomy remains common practice even though some studies have suggested lower level care is sufficient for selected patients. We have introduced a “no ICU, unless” policy for tumor craniotomy patients.
OBJECTIVE
To provide a quieter postoperative environment for patients, reduce the burden on the ICU department, and to evaluate whether costs can be reduced.
METHODS
A cohort study was performed comparing patients that underwent tumor craniotomy for supratentorial tumors during 1 yr after introduction (n = 109) of the new policy with the year before (n = 107). Rate of complications was evaluated, as was the length of stay and patient satisfaction using qualitative evaluation. Finally, costs were evaluated comparing the situation before and after implementation of the new protocol.
RESULTS
A reduction in ICU/MCU admittance from 64% to 24% of patients was found resulting in 13.3% cost reduction (€1950 per case), without increasing the length of stay at the ward. The length of stay in the hospital was similar. Complications were significantly reduced after implementing the new policy (0.98 vs 0.53 per patient, P = .003). Patients that were interviewed after the new policy reported feeling safe and at ease at the ward.
CONCLUSION
Changing our policy from “ICU, unless” to “no ICU, unless” reduced complication rates and length of stay in the hospital while keeping patients satisfied. Hospital costs related to the admission have been significantly reduced by the new policy.
Keywords: Brain tumor, Neurosurgery, Healthcare costs, Postoperative care, Quality improvement
ABBREVIATIONS
- CD
Clavien-Dindo
- CI
confidence interval
- ICU
intensive care unit
- IOM
intraoperative monitoring
- MCU
medium care unit
Traditionally, craniotomy patients are admitted to an (neurosurgical) intensive care unit (ICU) or other high- or medium-care (MCU) facilities for the first 12 to 24 h and transferred to the neurosurgical ward on the day after.1,2 This regimen is recommended in order to be able to early detect severe postoperative complications (like hemorrhage, epilepsy, edema, etc), which are thought to occur mostly on the first postoperative day.3-6 The need for postoperative intensive care admittance has been challenged, and even day-care tumor craniotomy has been advocated.1,2,7-12 Most postoperative complications do not need urgent intervention (like transient neurological deficits, infection, or CSF circulation problems), but immediate intervention is needed in case of postoperative hemorrhage or epileptic seizures.
In the Radboud University Medical Center each year more than 500 craniotomies are performed. When reviewing our own database of all supratentorial elective craniotomies between 2015 and 2016 (over 600 cases, including other cases than tumor) for severe complications, only one case of postop general epilepsy (that was treated medically) and one postoperative hemorrhage that needed urgent surgery were retrieved. The latter occurred on day 2 postoperatively, while the patient was already at the neurosurgical ward. This low rate of serious postop complications is consistent with the work of others.2,8
This has prompted us to question the need for routine postoperative ICU/MCU admittance after craniotomy. Moreover, the last couple of years the burden on ICU units has increased, and it has become a bottleneck in the planning of neurosurgical cases. Occasionally, cases had to be postponed because of lack of ICU beds. Also, yearly evaluations of patients’ satisfaction showed that the first day after surgery at the ICU was regarded as especially stressful because of the regular checks and lack of sleep. Finally, this can result in significant savings.2,8,12,13 In our hospital, 1-d admittance to ICU or MCU costs €2154 and €1421, respectively, whereas a day at the neurosurgical ward costs €395. This has to be balanced against potential increases in complication and length of stay.
Therefore, this study is aimed at comparing 2 postoperative regimens for patients admitted for elective tumor craniotomy; standard postop ICU/MCU admittance vs only when indicated. We want to study changes in length of stay and complication rates as well as a possible cost reduction. Secondary, we have qualitatively evaluated patient satisfaction after implementation of the new regimen in order to detect issues concerning patient satisfaction.
METHODS
Identification of Target Population
With the neurosurgical, the anesthesiological, and the ICU staff, cases were defined that could be included in the new postoperative regimen. It was decided not to include infratentorial, emergency, pediatric, or vascular cases, because these were expected to have higher risks. For this study, all patients treated electively for supratentorial tumors, aged >18 that underwent a resection or (open) biopsy through a craniotomy were included. Purely calvarian bone tumors, pituitary tumors, and endoscopic procedures were excluded for this analysis in order to present a homogeneous group.
New Postoperative Regimen
Differences between ICU, MCU, and neurosurgical ward prior to the new regimen are shown in Table 1. In short, MCU and ICU only differ in the availability of ventilatory support. Before introduction of the new regimen, patients were routinely admitted to MCU or ICU postoperatively, unless the surgeon and anesthesiologist decided otherwise (short procedure, young healthy patient), resulting in a ICU or MCU admittance of 64%. After the new regimen, patients were routinely admitted to the standard neurosurgical ward, unless the surgeon (based on high expected blood loss or expected duration of surgery >6 h) or anesthesiologist (based on cardiopulmonary comorbidity and functional status) decided otherwise.
TABLE 1.
Comparison of Monitoring Capacities on ICU, MCU, and Regular Care Neurosurgical Ward prior to New Regimen
| ICU | MCU | Ward | |
|---|---|---|---|
| Daytime nurse:patient ratio | 0.83 | 0.5 | 0.25 |
| Nighttime nurse:patient ratio | 0.57 | 0.4 | 0.1 |
| Interval of Neuro checks (min) | 15-30 | 30-60 | 120 |
| Continuous pulse and saturation measurement | + | + | − |
| Non-invasive RR registration | + | + | + |
| Central intravenous line (monitoring) | + | + | − |
| Arterial line monitoring | + | + | − |
| Continuous EEG Registration | + | + | − |
| IV pressor/inotropic medication | + | + | − |
| High dose IV pressor/inotropic medication | + | − | − |
| Advanced (thermodilution) hemodynamic monitoring | + | − | − |
| Intracranial pressure monitoring | + | − | − |
Within the new regimen the interval of neuro checks at the ward changed from 120 to 60 min and continuous monitoring of pulse and saturation was added. Frequency of RR checks was increased from every 4 h to once each hour.
All standard operating procedures (SOP) for tumor craniotomy were adopted to the new policy. Nurses of the recovery department were instructed by the anesthesiologists and nurses of the neurosurgical ward by the neurosurgeons. Extra monitors for continuous postoperative monitoring of saturation and pulse were acquired for the neurosurgical ward and nurses were trained to use them. The following SOP was introduced:
Patients who do need to be admitted to the ICU/MCU postoperatively are identified by the neurosurgeon.
Based on comorbidities and functional status, the anesthesiologist can order ICU/MCU admittance, even if the neurosurgeon does not expect this to be necessary.
Postoperatively, patients stay at the recovery ward for at least 1 h, or until standard discharge criteria are met (see Text, Supplemental Digital Content 1).14,15
During stay at the recovery ward in addition to routine monitoring, pupillary function, level of consciousness (EMV), and global motor function are checked every 15 min.
Each patient is checked by an attending neurosurgeon or trainee within 1 h postop.
When transferred to the ward monitoring of heart rate and saturation is continued until 6 h postop, and EMV and pupils are checked every hour.
Alarms are set at sO2 < 96%, heart rate <50 or >90 per minute, systolic blood pressure <90 or >160 mmHg.
Analyses
The data of a prospective cohort of patients after the implementation of the policy were compared to the data of a series of patients that were operated the year before the implementation in order to estimate (1) complication rates and length of stay before and after the change of regimen, (2) a cost difference, and (3) patient satisfaction when applying the new postop regimen.
Data were collected from the April 1st 2016 until March 31th 2017 for the preintervention cohort (cohort A) and from April 1st 2017 until March 31th 2018 for the intervention cohort (cohort B). Length of stay and complications were taken from hospital registries. All hospital costs per patient were collected from the hospital billing department, adhering to a third party payer perspective. Complications were graded according Clavien-Dindo.16
Patients were interviewed from the postintervention cohort for a qualitative assessment of patient satisfaction. Semistructured interviews were taken by phone from patients and or caregivers. Subjects that were addressed were feeling of safety, experience of privacy and rest or unrest, and family experiences. We tried to reach all patients that were included in the new policy during the first 3 mo and contacted additional patients after the first 6 mo until data saturation was reached (no new topics emerged).
Statistical Analyses
Differences in length of stay and complications within 30 d were analyzed univariately and by a general linear model to correct for the following: age, ASA score, procedure (intra-axial tumor, extra-axial tumors, skull base tumors, open biopsy, or open cyst fenestration) and use of intraoperative monitoring (IOM). We chose to correct for these confounders because higher age and ASA score increases complication risk, the use of IOM can result in more short time neurological deficits, and possibly specific procedures result in more or less complications.
Costs were analyzed by a generalized linear model with a log link relating the conditional mean to confounders age, ASA score, procedure type, and IOM using a gamma distribution specifying the relationship between the variance and the mean. Statistical significance was assumed when P < .05.
Hospital billing (pricing) did not significantly differ between the 2 cohorts.
Statistical analyses were done using SPSS statistics (IBM SPSS Statistics for Windows, Version 22.0, IBM Corp, Armonk, New York).
Because this study does not imply any burden for the patients and all data were retrieved from existing databases and registries, no ethical approval had to be sought according to Dutch law.
RESULTS
Cohort A consists of 107 patients (63 intra-axial tumors and 41 extra-axial or skull base), cohort B consists of 109 patients (69 intra-axial, 39 extra-axial, or skull base). In cohort A 64% (before introduction of the new policy, sometimes surgeons chose to admit their patients to the neurosurgical ward after a few hours at the postanesthesia care unit, instead of ICU/MCU), and in cohort B, 24% of patients were admitted to ICU or MCU postoperatively. Age, sex, ASA score, and procedure type were not statistically significantly different between cohorts. IOM was used more in cohort A (Table 2).
TABLE 2.
Baseline Patient Characteristics
| Cohort A | Cohort B | |
|---|---|---|
| N = | 107 | 109 |
| Male | 50(47) | 57(52) |
| Mean Age | 55.5 | 54.6 |
| Admitted to ICU/MCU | 68(64) | 25(24) |
| Physical status | ||
| ASA 1 | 13(12) | 7(6) |
| ASA 2 | 73(68) | 71(65) |
| ASA 3 | 21(20) | 30(28) |
| ASA 4 | 0(0) | 1(1) |
| Procedure type | ||
| Intra-axial | 58(54) | 63(58) |
| Extra-axial | 30(28) | 30(23) |
| Open biopsy | 5(5) | 6(6) |
| Skull base | 11(10) | 9(8) |
| Open Cyst fenestration | 3(3) | 1(1) |
| IOM used | 18(17) | 8(7) |
Numbers are expressed as absolute values (percentage). ASA: American Society of Anaesthesiologists physical status classification, ICU: intensive care unit, IOM: intra-operative monitoring, MCU: medium care unit.
Length of Stay
Length of stay at ICU and MCU unit was statistically shorter in cohort B as compared to cohort A (P < .05). Length of stay at the neurosurgical ward (including 1 d prior to surgery and the time at the OR) did not show a statistical difference. Mean total length of stay was 1 d shorter in cohort B, but this was not statistically significant (Table 3). Length of stay per tumor type is shown in the Table, Supplemental Digital Content 2.
TABLE 3.
Length of Stay at the Ward, MCU, and ICU as prior to New Regimen (Cohort A) and after New Regimen (Cohort B)
| Cohort A | Cohort B | |
|---|---|---|
| Ward | 5.8 (5.14-6.67) | 5.4 (4.26-6.55) |
| MCU | 0.53 (0.39-0.67) | 0.11 (0.05-0.17)* |
| ICU | 0.47 (0.29-0.66) | 0.23 (0.11-0.58)* |
| Total stay | 6.8 (5.93-7.76) | 5.75 (4.55-6.94) |
Numbers represent means (95% CI).
*Significant difference (P < .05).
Complications
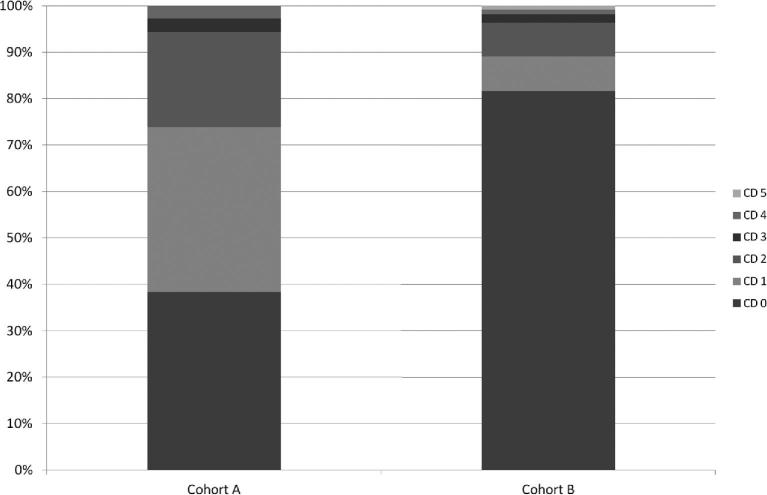
Incidence of complications within 30 d after surgery (total complication count per patient per admittance) was 0.98 for cohort A and 0.53 for cohort B, a statistically significant reduction (P < .003). Distribution of complications according to Clavien-Dindo (CD) classification16 is shown in the Figure. Details of the minor complications are shown in the Table, Supplemental Digital Content 3. Although incidence of serious complications (CD > 2) was low in both cohorts (5.6% and 3.7% of cases, respectively), overall, in cohort B, less patients had complications (P < .0001). Less severe complications (CD 1 or 2) were found more often in cohort A (Figure).
Figure.
Distribution of complications within 30 d after surgery, graded according to CD scale. If a patient experienced multiple complications, only the highest CD grade is counted.
In cohort B ICU/MCU admission was planned because of expected blood loss or long duration of surgery in 8 cases, because of expected surgical risks in 8 cases and because of comorbidity in 9 cases. No unplanned ICU/MCU admissions occurred in the second cohort immediately postoperatively. Two patients of cohort B had to be admitted to the MCU, even though initially they went to the ward postoperatively. In one patient, a perforated diverticulitis was the cause, occurring 4 d postsurgery. The other patient was transferred to the MCU for heart-rate monitoring after bradycardia occurred during vomiting on the evening postsurgery. Other CD > 2 complications were wound infections treated surgically (twice, cohort A), venous infarction, secondary hydrocephalus that needed surgery and respiratory distress syndrome (all once) in cohort A, and an epidural hematoma and CSF leakage that needed surgical treatment (both once) in cohort B. The only reported death within 30 d was a patient that died of a heart attack 6 d after surgery (cohort B). Patients admitted to ICU/MCU in cohort B did not show higher complication rates.
Costs
Mean total costs (including ICU/MCU or ward stay, surgery, laboratory costs, imaging, and consultations) per admittance was €13,607 for cohort A and €11,654 for cohort B (P < .0001). For statistical analysis correction for the confounders age, ASA score, surgery type, and IOM use was done in a generalized linear model approach showing 13.3% lower overall costs in cohort B (factor 1.133, 95% c.i. 1.035-1.240 or on average €1950 per case). Onetime costs included training of nurses (€2624) and 2 new monitors (€19,905) for the ward.
Qualitative Analyses
Three months after implementation, 20 patients had been admitted to the ward postsurgery. Of these, 5 patients could not be questioned because of health issues; 4 could not be reached. Another 7 patients were interviewed 6 to 9 mo after implementation of the new regimen, but data saturation was already reached after interview 12 (details in appendix). In general, patients were very content with the care they had received during their stay at the neurosurgical ward. They reported feeling safe en experienced enough rest during their postoperative care, although some noise from neighboring patients was reported. Frequent checkups were not troublesome; it was reported that being watched closely added to the feeling of safety. The fact that family could be present during their stay (also at night) was appreciated. Family felt well-informed and involved in decision making.
DISCUSSION
Summary
Our study showed that a selection of patients after tumor craniotomy does not need routine admission to the ICU or MCU ward postoperatively. Complication rates were similar, although the rate of less severe complications was statistically significantly lower. The length of stay at the ward did not increase. Furthermore, patients felt safe and at ease at the ward.
Interpretation
Even though still 24% of cases were admitted to MCU or ICU, savings were higher than anticipated. This was, in part, due to the fact that decrease in ICU/MCU days did not increase the stay at the ward. We postulate this is the result of earlier mobilization on the ward than on the ICU/MCU units, resulting in earlier discharge. The incidence of major complications did not change between the cohorts, but incidence was low, and this study is underpowered to draw significant conclusions. Minor complications were significantly reduced after introduction of the new regimen. The more frequent use of IOM in the first cohort can in part explain this because these patients tend to experience relatively more transient neurological deficits. Possibly a reduction was achieved because of the shorter ICU/MCU stay; intravenous infusions are less frequent, catheters are discontinued sooner, and patients mobilize quicker at the ward. On the other hand, more intensive monitoring at the ICU/MCU units can lead to more detection of minor complications like hypertension and electrolyte problems. Future studies are needed to verify these results.
Our qualitative analysis showed patients were content, and no major issues were reported. We think clear SOP’s and proper instruction and involvement of nursing staff are paramount to achieve this.
Our finding that less intensive postoperative monitoring after craniotomy is safe is in line with previous publications.2,7-9,11,12,17 Others have suggested comparable regimens could reduce costs. 2,8,12,13 Previous reported selection criteria for reduced postoperative monitoring were quite strict (eg age <65, small tumors, no comorbidity13), whereas we chose to allow all supratentorial tumor cases to be admitted to the general neurosurgical ward, except when the surgeon or anesthesiologist judged otherwise based on expected duration of surgery, expected blood loss, comorbidities, and functional status. Even though selection might be more subjective this way, more patients can be included in the new regimen, which we have shown is safe; therefore, we do not see a need for more stringent criteria for ICU/MCU admission postcraniotomy. We do not routinely perform postoperative imaging to rule out complications, and in the population we have studied, there is no need to do so. When extending this kind of regimen to higher-risk patients, postoperative imaging can be a way to exclude immediate postoperative complications.
Limitations
This is the first study to perform an analysis of costs and complications comparing postoperative care regimens in craniotomy for brain tumors comparing a prospective cohort with a retrospective cohort. Strengths of our study are that we compared 2 otherwise comparable and homogeneous cohorts as for costs and complications, we have corrected for possible confounders and we have included patient satisfaction. Also we have reported the changes we performed in operating procedures and instructions for nursing staff. A limitation of our study is the fact that we did not include a control group for qualitative analysis (patients were not interviewed prior to our change in regimen). Also the small nature of the cohorts makes that the study is underpowered for detecting changes in major complications, because of their low incidence. Data for cohort A were collected retrospectively and data for cohort B prospectively, possibly resulting in bias like better registration and higher incidence of complications in cohort B; something we have not seen in the data.
CONCLUSION
In conclusion postoperative care on the regular neurosurgical ward instead of ICU/MCU after craniotomy for selected patients is safe and significantly reduces hospital costs. Patients reported being satisfied and feeling safe and at ease within the new policy. Proper preparation and instruction of nursing staff is mandatory.
Disclosures
This study was cofunded by Dutch Healthcare Insurance Company VGZ. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
We would like to thank Mrs F. M. Cuijpers-Vollenberg for all her efforts to obtain data from our hospital and billing administration.
Supplemental Digital Content 1. Text. Recovery discharge criteria. For references, see reference list in main manuscript.
Supplemental Digital Content 2. Table. Total length of stay per type of operation. Numbers of patients (n) and mean length of stay per type of operation. Within the low numbers of skull base and cyst fenestration means are affected by incidental problematic cases with long stay (eg, one case of cyst fenestration in a craniopharyngioma was complicated by long-lasting hypothermia).
Supplemental Digital Content 3. Table. Minor complications per cohort (Clavien-Dindo [CD] grade 1 and 2). Minor complications (CD 1 and 2; not requiring surgical intervention) in Cohort A and Cohort B. CSF: cerebral spinal fluid.
COMMENTS
This is an interesting study on the effect of the change of strategy considering immediate postoperative care of a portion of craniotomy patients. The reduction of ICU admissions did not result in unwanted effects; rather, a reduction of complications, length of stay, and hospital costs could be observed, while the patients even felt better. The selection of suitable patients for the new ‘no ICU, unless’ strategy has to be done very carefully. It has to account for specific hospital and department structures. A follow-up study prospectively monitoring the effects of the new strategy is highly advised. It would also be interesting to analyze if there are any positive effects of intra- or immediate postoperative imaging for complication avoidance, or whether some delayed postoperative imaging, eg, 4 to 6 h after surgery, are recommended.
Christopher Nimsky
Marburg, Germany
The authors present an interesting study summarizing their experience before and after a policy change for craniotomy for supratentorial tumors from ‘ICU, unless’ to ‘no ICU, unless’. They present 109 patients from the year after the policy change and 107 patients from the year before the policy change. With the new policy, they report reduced complication rates, reduced length of stay in the hospital, reduced hospital costs, and unchanged patient satisfaction. This is an important study as we practice in an era of hospital bed shortages and cost considerations. The authors are to be commended on this study because the ability to reconsider the needs of our neurosurgical patients in a manner that reduces costs while preserving safety and patient satisfaction will enable neurosurgeons to provide timely care for our growing population with neurosurgical needs.
Manish K. Aghi
San Francisco, California
REFERENCES
- 1. Ziai WC, Varelas PN, Zeger SL, Mirski MA, Ulatowski JA. Neurologic intensive care resource use after brain tumor surgery: an analysis of indications and alternative strategies. Crit Care Med. 2003;31(12):2782-2787. [DOI] [PubMed] [Google Scholar]
- 2. de Almeida CC, Boone MD, Laviv Y, Kasper BS, Chen CC, Kasper EM. The utility of routine intensive care admission for patients undergoing intracranial neurosurgical procedures: a systematic review. Neurocrit Care. 2018;28(1):35-42. [DOI] [PubMed] [Google Scholar]
- 3. Kelly DF. Neurosurgical postoperative care. Neurosurg Clin N Am 1994;5(4):789-810. [PubMed] [Google Scholar]
- 4. Taylor WA, Thomas NW, Wellings JA, Bell BA. Timing of postoperative intracranial hematoma development and implications for the best use of neurosurgical intensive care. J Neurosurg. 1995;82(1):48-50. [DOI] [PubMed] [Google Scholar]
- 5. Lonjaret L, Guyonnet M, Berard E et al.. Postoperative complications after craniotomy for brain tumor surgery. Anaesth Crit Care Pain Med. 2017;36(4):213-218. [DOI] [PubMed] [Google Scholar]
- 6. Henker C, Schmelter C, Piek J. Complications and monitoring standards after elective craniotomy in Germany. Anaesthesist. 2017;66(6):412-421. [DOI] [PubMed] [Google Scholar]
- 7. Au K, Bharadwaj S, Venkatraghavan L, Bernstein M. Outpatient brain tumor craniotomy under general anesthesia. JNS. 2016;125(5):1130-1135. [DOI] [PubMed] [Google Scholar]
- 8. Beauregard CL, Friedman WA.. Routine use of postoperative ICU care for elective craniotomy: a cost-benefit analysis. Surg Neurol. 2003;60(6):483-489; dicussion 489. [DOI] [PubMed] [Google Scholar]
- 9. Bui JQ, Mendis RL, van Gelder JM, Sheridan MM, Wright KM, Jaeger M. Is postoperative intensive care unit admission a prerequisite for elective craniotomy?? JNS. 2011;115(6):1236-1241. [DOI] [PubMed] [Google Scholar]
- 10. Hanak BW, Walcott BP, Nahed BV et al.. Postoperative intensive care unit requirements after elective craniotomy. World Neurosurg. 2014;81(1):165-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mirza FA, Wang C, Pittman T. Can patients safely be admitted to a ward after craniotomy for resection of intra-axial brain tumors? Br J Neurosurg. 2017;32(2):1-5. [DOI] [PubMed] [Google Scholar]
- 12. Florman JE, Cushing D, Keller LA, Rughani AI. A protocol for postoperative admission of elective craniotomy patients to a non-ICU or step-down setting. J Neurosurg. 2017;127(6):1392-1397. [DOI] [PubMed] [Google Scholar]
- 13. Osorio JA, Safaee MM, Viner J et al.. Cost-effectiveness development for the postoperative care of craniotomy patients: a safe transitions pathway in neurological surgery. Neurosurg Focus. 2018;44(5):E19. [DOI] [PubMed] [Google Scholar]
- 14. Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7(1):89-91. [DOI] [PubMed] [Google Scholar]
- 15. Gardner-Thorpe J, Love N, Wrightson J, Walsh S, Keeling N. The value of Modified Early Warning Score (MEWS) in surgical in-patients: a prospective observational study. annals. 2006;88(6):571-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dindo D, Demartines N, Clavien PA. Classification of surgical complications. Ann Surg. 2004;240(2):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bernstein M. Outpatient craniotomy for brain tumor: a pilot feasibility study in 46 patients. Can j neurol sci. 2001;28(2):120-124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.