Abstract
BACKGROUND
Concussion is a heterogeneous mild traumatic brain injury (mTBI) characterized by a variety of symptoms, clinical presentations, and recovery trajectories. By thematically classifying the most common concussive clinical presentations into concussion subtypes (cognitive, ocular-motor, headache/migraine, vestibular, and anxiety/mood) and associated conditions (cervical strain and sleep disturbance), we derive useful definitions amenable to future targeted treatments.
OBJECTIVE
To use evidence-based methodology to characterize the 5 concussion subtypes and 2 associated conditions and report their prevalence in acute concussion patients as compared to baseline or controls within 3 d of injury.
METHODS
A multidisciplinary expert workgroup was established to define the most common concussion subtypes and their associated conditions and select clinical questions related to prevalence and recovery. A literature search was conducted from January 1, 1990 to November 1, 2017. Two experts abstracted study characteristics and results independently for each article selected for inclusion. A third expert adjudicated disagreements. Separate meta-analyses were conducted to do the following: 1) examine the prevalence of each subtype/associated condition in concussion patients using a proportion, 2) assess subtype/associated conditions in concussion compared to baseline/uninjured controls using a prevalence ratio, and 3) compare the differences in symptom scores between concussion subtypes and uninjured/baseline controls using a standardized mean difference (SMD).
RESULTS
The most prevalent concussion subtypes for pediatric and adult populations were headache/migraine (0.52; 95% CI = 0.37, 0.67) and cognitive (0.40; 95% CI = 0.25, 0.55), respectively. In pediatric patients, the prevalence of the vestibular subtype was also high (0.50; 95% CI = 0.40, 0.60). Adult patients were 4.4, 2.9, and 1.7 times more likely to demonstrate cognitive, vestibular, and anxiety/mood subtypes, respectively, as compared with their controls (P < .05). Children and adults with concussion showed significantly more cognitive symptoms than their respective controls (SMD = 0.66 and 0.24; P < .001). Furthermore, ocular-motor in adult patients (SMD = 0.72; P < .001) and vestibular symptoms in both pediatric and adult patients (SMD = 0.18 and 0.36; P < .05) were significantly worse in concussion patients than in controls.
CONCLUSION
Five concussion subtypes with varying prevalence within 3 d following injury are commonly seen clinically and identifiable upon systematic literature review. Sleep disturbance, a concussion-associated condition, is also common. There was insufficient information available for analysis of cervical strain. A comprehensive acute concussion assessment defines and characterizes the injury and, therefore, should incorporate evaluations of all 5 subtypes and associated conditions.
Keywords: Concussion, subtype, systematic review, meta-analysis, mild traumatic brain injury, head injury, oculomotor, vestibular, traumatic brain injury
ABBREVIATIONS
- CI
confidence interval
- mTBI
mild traumatic brain injury
- NIH
National Institutes of Health
- RCTs
randomized controlled trials
- SIGN
Scottish Intercollegiate Guideline Network
- SD
standard deviation
- SMD
standardized mean difference
- VOR
vestibular ocular reflex
- VMS
visual motion sensitivity
Concussion is a heterogeneous mild traumatic brain injury (mTBI) characterized by a variety of symptoms, clinical presentations, and recovery trajectories. In 2014, the prevalence of key concussion signs and symptoms was described in “Concussion Guidelines Step 1: Systematic Review of Prevalent Indicators,” and concussion was broadly defined.1 In addition to often nonspecific clinical indicators of concussion, there is a wide variability of patient presentations, challenging clinicians and researchers to identify sensitive and specific means of diagnosis. By thematically classifying the most common concussive clinical presentations or profiles into “concussion subtypes and associated conditions,” we derive useful definitions amenable to future targeted treatments.2,3 A collaboration with national multidisciplinary experts aimed to further define and identify evidence supporting 5 predominant concussion subtypes: 1) cognitive, 2) ocular-motor, 3) headache/migraine, 4) vestibular, and 5) anxiety/mood, as well as 2 concussion-associated conditions: 1) sleep disturbance and 2) cervical strain. The primary objective of this effort was to use evidence-based methodology to report the prevalence of subtypes in concussion patients as compared to normal populations, thereby establishing a framework and guidelines for future research. On December 16, 2016, an expert workgroup convened to direct the clinical description of concussion subtypes for the purpose of conducting a systematic review and analysis of the literature with guideline development.
METHODS
Concussion Subtype Workgroup and Invited Observers
To define concussion subtypes, experts were identified from: the 2015 “Targeted Evaluation and Active Management” meeting2; other concussion-focused meetings; review of relevant literature; and via recommendation from various medical and health organizations. Each workgroup candidate was reviewed for potential invitation to participate. In total, 11 nonfederal members ultimately formed the workgroup and they were required to declare financial and intellectual conflicts of interest. In addition, federal representatives, from the U.S. Department of Defense, the FDA/Consumer Product Safety Commission, the Centers for Disease Control and Prevention, the U.S. Department of Veteran Affairs, and the Defense and Veterans Brain Injury Center, as well as organizational representatives from the American Academy of Neurology, American Association of Neurological Surgeons/Congress of Neurological Surgeons, National Athletic Trainer's Association, the American College of Sports Medicine, and the Brain Trauma Foundation participated as observers and reviewers to the process and to the final report. The Concussion Subtypes Workgroup was charged with developing subtype-specific definitions, inclusion and exclusion criteria, acknowledging associated data, identifying primary outcomes, linking data elements, advising systematic review of evidence and analysis, developing recommendations, and positing future directions for research.
Derivation of Definitions and Selection of Clinical Questions
Workgroup members convened to identify and establish thematic descriptions of the most common concussion subtypes based upon expert consensus, and identify clinical questions. The following 4 clinical questions are identified:
What is the prevalence of concussion subtypes and associated conditions compared with controls within the first 3 mo postinjury?
What is the severity of symptoms reported compared to control populations?
What is the temporal recovery trajectory by concussion subtype and associated conditions over the first 3 mo postinjury?
How do subtypes cluster?
Defining Concussion Subtypes and Concussion-Associated Conditions
Subtype-specific criteria, definitions, and prevalence data are presented individually; however, the following features are required for all:
A primary diagnosis of concussion resulting from closed head injury or other transmitted forces to the brain.4
Exclusion criteria include the following: 1) psychiatric or neurological disability preventing accurate self-report, 2) medical condition confounding accurate assessment, and 3) medication, drug, or other substance use that confounds accurate assessment.
Associated data for assessment includes: mechanism of injury and events surrounding injury; history of previous concussion with number, duration, and interval durations between concussions; return to activity details (sport, academics, occupation, service) specifying time to actual return and time to medical clearance for return; and past medical history including surgical history and medication use.
Multiple concussion subtypes may contribute to a patient's clinical presentation of injury following trauma; subtypes are not mutually exclusive.3 For example, a patient with a predominantly vestibular symptomatology may also have headaches.
Concussion subtype predominance may change following injury. For example, a patient may present with predominantly a headache subtype, but later develop signs consistent with the anxiety/mood subtype. And similarly, concussion-associated conditions may vary in presence and predominance.5
Cognitive
The cognitive subtype involves the primary dysfunction of specific cognitive abilities following injury including: attention; impaired reaction time; speed of processing/performance; working memory; new learning; memory storage; memory retrieval; organization of thoughts and behavior.6,7 Patients diagnosed with this subtype have the following: demonstrated deficits in performance testing in the previously specified areas (more than 1 standard deviation (SD) below baseline functioning or 1.5 SD below the normal); reported cognitive symptoms ratings that are significantly greater than baseline levels (>1 SD); and/or significant exacerbation of premorbid cognitive dysfunction.
Ocular-Motor
The ocular-motor subtype involves dysfunction of the visual system (eyesight, eye focusing, eye teaming, and visual perception skills) following injury.8,9 Ocular-motor and visual dysfunction can cause difficulty obtaining, understanding, and processing visual stimuli. Dysfunction can trigger or exacerbate symptoms and impair a patient's ability to integrate and process information. Ocular-motor and visual impairments may be detected by saccades, smooth pursuit, conjugate gaze, convergence, accommodation, and fixation assessments.10 Deficits in the ocular-motor system may mimic cognitive impairment functionally and are frequently found in conjunction with the vestibular symptoms. Patients diagnosed with this subtype have the following: difficulty with visual activities (screen time, reading, near work, driving, etc); asthenopia (eye strain) and eye fatigue; problems with visual focus including changing focus from near to far and back (assessed for as convergence distance, accommodation, and reading issues); photophobia; blurred vision or double vision; frontal headaches or eye pain/pressure behind the eyes; vision-derived nausea; difficulty judging distances; difficulty tolerating complex visual environments; and significant exacerbation of premorbid visual impairment. Hence, these symptoms may contribute to problems concentrating or difficulty in completing written work.
Headache/Migraine
Headache is the most common symptom reported in adults and children following concussion and different types of headaches can occur following head injury.11 Migraine is a headache type characterized by a prodrome and/or aura with associated symptoms including nausea, vomiting, and sensitivity to light, sound, or smell.12 Pre-existing headache types place individuals at greater risk for headache following a concussion12,13 or may be worsened following concussion with increasing frequency or severity. Patients with the headache/migraine subtype of concussion have self-reported history of headaches that differs from their pre-existing history and/or changes in their headache assessments demonstrated on validated headache scales with levels of severities.
Vestibular
The vestibular subtype of concussion is characterized by disruption to the central vestibular system that involves movement and orientation of the body to space and time.14 Symptoms include dizziness, fogginess, lightheadedness, nausea, vertigo, and disequilibrium. This subtype comprises vestibulo-ocular (eg, vestibular ocular reflex [VOR], visual motion sensitivity [VMS]), vestibulo-spinal (eg, imbalance), and gait dysfunction. Peripheral vestibular dysfunction may co-exist with concussion, but it is not common. Dynamic movement, involving integrated head and body movements, may provoke dysfunction and symptoms for these patients.15 Patients with the vestibular subtype have the following: at least 1 symptom of dizziness, fogginess, lightheadedness, nausea, vertigo, or disequilibrium; dysfunction in vestibulo-ocular or vestibulo-spinal tracts affecting gait and/or balance; symptoms and/or dysfunction are provoked with dynamic movement; and they may present with concurrent deficits in neurocognitive testing, feelings of anxiety due to disorientation, and concurrent clinical findings.7
Anxiety/Mood
The anxiety/mood subtype of concussion is characterized by increase in anxiety and mood-related symptoms including the following: nervousness, feeling more emotional, hypervigilance, ruminative thoughts, feelings of being overwhelmed; depressed mood with sadness, feeling more emotional, anger, hostility/irritability, loss of energy, fatigue, and feelings of hopelessness.16 These symptoms are triggered or exacerbated by the concussion directly, or indirectly in relation to other injury-related symptoms. Pre-existing conditions, such as a history of anxiety or migraine, and concurrent stressful events may also predispose or contribute to this subtype. This subtype is often accompanied by sleep disturbance. Physical and social inactivity may trigger or exacerbate the anxiety/mood subtype and physical exertion/exercise often results in improvement.16
Concussion-Associated Conditions
The following concussion-associated conditions represent expert consensus-based thematic descriptions of clinical symptoms and signs commonly seen in conjunction with concussion. Distinct from subtypes, associated conditions do not represent stand-alone diagnostic criteria for concussion following head injury.
Sleep Disturbance
Sleep disturbance refers to the difficulty initiating and/or maintaining quality sleep and may include excessive sleepiness, hyper-somnolence, or insomnia. Sleep disturbance is common in concussion but does not occur in isolation of other postconcussive symptoms, therefore representing a concussion-associated condition. Primarily, sleep disturbance arises from the brain injury itself.17,18 Secondarily, it may occur as sequelae of other concussion-related symptoms and impairments.19,20 Nonrestorative sleep can cause fatigue, tiredness, and/or daytime drowsiness that may be measured by concussion or sleep scales/inventories. Sleep disturbances, regardless if they exist prior to or were caused by a concussion, may adversely affect recovery from concussion.21-25 Patients with this associated condition have new or exacerbated sleep disturbance associated with concussion.
Cervical Strain
The cervical strain concussion-associated condition refers to a head injury resulting in neck pain, neck stiffness, neck or upper extremity weakness, and persistent headache (often occipital/suboccipital in location) in the setting of other concussive symptoms. Injury to structures of the neck leads to somatosensory dysfunction and aberrant signaling/transmission along cervical afferent pathways that travel to the brain.26-29 Signals from these pathways typically are involved in the coordination of cervical and vestibular reflexes and support normal vision and vestibular functioning. Dysfunction in the pathways and their transmissions leads to the afore-mentioned symptoms. Patients may have clinical signs of pain/tenderness in the cervical spine (including midline palpation, as well as paraspinal and suboccipital muscle palpation), weakness with paracervical strength and upper extremity muscle myotome testing, limitation of cervical motion, pain with cervical motion, paresthesia/weakness (radicular symptoms) in upper extremities, pain/paresthesia in occipital region with palpation or head movement. Because cervical strain and concussion share common injury mechanisms, differentiating isolated vs concomitant etiologies, such as whiplash-associated disorder, is important to determine appropriate management and treatment.26-29
Literature Search Strategy and Evidence Review
A systematic review and meta-analysis were conducted following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Meta-Analysis Of Observational Studies in Epidemiology.30-32 A comprehensive literature search for relevant citations was conducted of the following databases: MEDLINE, SCOPUS, COCHRANE controlled trials registers, PsycInfo, and SPORTDiscus databases. Only English language articles that were published between January 1, 1990 and July 1, 2017 were included.
The studies were imported into Covidence software (Veritas Health Innovation Ltd, Melbourne, Victoria, Australia), and duplicate articles were removed. Two assessors independently triaged abstracts following predetermined inclusion/exclusion criteria. Inclusion criteria were studies with 1) patients of any age or sex diagnosed with concussion/mTBI, occurring from any sport, activity, combat, accident or life event, with clinical study evaluations within 3 mo of injury; with or without comparison symptoms or measures of subtypes in patients without concussion or with baseline measures; 2) reported prevalence of symptoms or measures relevant to concussion subtypes and associated conditions (headache/migraine, vestibular, ocular-motor, cognitive, anxiety/mood, sleep disturbance, and cervical strain); 3) study design could include randomized controlled trials (RCTs), cohort studies, case-control studies, pre-post studies, time series, or cross-sectional studies. Exclusion criteria are as follows: 1) studies of patients with moderate or severe traumatic brain injury, or with mixed populations of mild with moderate or severe traumatic brain injury without analyzable data specific to concussion; 2) studies of penetrating head injury; 3) studies in which the concussive event occurred more than 3 mo prior to the clinical study's evaluation; 4) designs including reviews, letter to editor, case report, commentary, or editorial; 5) studies assessing concussion in patients with known complicating underlying neurological disease such as epilepsy or stroke.
Second-level selection was performed independently by 2 assessors who read the full text of all articles from the first-level selection and applied the same inclusion/exclusion criteria. Discrepancies were resolved by consensus or by a third reviewer at both the first- and second-level selection. Selected studies were classified according to which concussion subtypes/associated conditions they included, with assignment allowed to multiple classifications. In studies with duplicate data (companion publications), the original study or the study reporting more detailed or recent data (with a greater number of patients) was included.
The methodological study quality was assessed by the following means: RCTs were assessed by the COCHRANE Collaboration's tool; cohort and case-control studies were assessed by the Scottish Intercollegiate Guideline Network (SIGN) 50 tool; and pre-post, time-series, and cross-sectional studies were assessed by National Institutes of Health's (NIH’s) Quality Assessment Tool. Study data extraction included information provided on signs and symptoms relevant to the concussion subtypes/associated conditions, concussion assessment modalities, injury mechanisms, and demographic data. Quality assessment was performed by 2 reviewers, with adjudication by a third, until consensus was reached. Data extraction was performed by 3 reviewers and verified by a fourth and fifth.
Meta-Analysis
Separate meta-analyses were conducted to do the following: 1) report the prevalence of each concussion subtype/associated condition in concussion patients using a proportion (binary outcome), 2) compare concussed patients with uninjured/baseline controls using a prevalence ratio (ratio of 2 binary outcomes), and 3) compare differences in concussion assessments between concussed patients and uninjured/baseline controls using a standardized mean difference (SMD; continuous outcome). No studies describing cervical strain met inclusion criteria; therefore, a meta-analysis was not conducted on this associated condition. Consequently, we carried out a meta-analysis for each of the 5 concussion subtypes and sleep disturbance, separately for pediatric and adult patients. If the study included both pediatric and adult patients and only reported the combined results, it was meta-analyzed as part of pediatric or adult patients depending on the larger proportion of these 2 groups in the study. Further, separate meta-analyses were performed for cumulative assessments attributed to each concussion subtype and sleep disturbance. Differing weights were assigned to the included studies, with proportionally larger weight attributed to the studies with larger sample sizes and better precision (ie, smaller standard error).33 A 95% CI was calculated for each effect size measure. Because heterogeneity across the studies was expected in observational studies,34 a random effects model for all meta-analyses was used to quantify the pooled effect size for the included studies.33,34 This was confirmed by heterogeneity and I2 statistics.35 Cohen's criteria were used to assess the magnitude of SMDs (0.2 for small effect, 0.5 for medium effect, and 0.8 for large effect).36
Prevalence at varying time points postinjury, including the acute period of 0 to 3 d described in this work, was meta-analyzed, with the calculations of the pooled estimate using (inverse-variance) Freeman-Tukey double arcsine transformation37,38 and the exact CIs for the effect sizes of individual studies.39 SMDs and their variances were calculated separately for the studies using independent groups and those using pre-post designs, and then were included in a single meta-analysis.33 This was appropriate, as an effect size, such as SMD, provides the same interpretation regardless of the study design.33 A pre-post correlation of 0.6 was used to calculate SMDs for the studies with pre-post designs.40,41 Differences in scores between concussion patients and controls (independent groups) or between baseline and concussion (pre-post designs) were calculated and interpreted to allow for uniform result interpretation. In some measurements/tests, higher scores indicate abnormalities/impairments, whereas it is the opposite for other measurements/scores. In this meta-analysis, numerically positive SMDs represented abnormalities/impairments. Orthopedically injured controls were excluded from the meta-analysis of SMDs because of their marked differences in assessment outcomes compared with other control groups. Forest plots were produced to illustrate the effect of each study as well as the overall effect across the studies.34 In addition, publication bias was examined using Egger's test.42,43 In case publication bias was suspected, the Duval and Tweedie nonparametric trim and fill method was used to provide the meta-analysis results adjusted for publication bias.44 Lastly, we conducted sensitivity analysis to assess the between-study heterogeneity and the impact of an individual study on the overall, pooled effect size, using the leave-one-out approach that recalculated the pooled effect after excluding a study one by one.34,45 All the analyses were conducted using Stata 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, Texas: StataCorp LLC). Specifically, we used the following Stata commands for the meta-analysis: metaprop46 for the prevalence of each concussion subtype, and metan47,48 for the prevalence ratios and SMDs. Further, metabias49 and metatrim50 were used to examine and adjust for publication bias, and metainf51,52 was used for the sensitivity analysis.
Ethics committee approval and patient consent were neither required nor sought for this meta-analysis involving deidentified data.
RESULTS
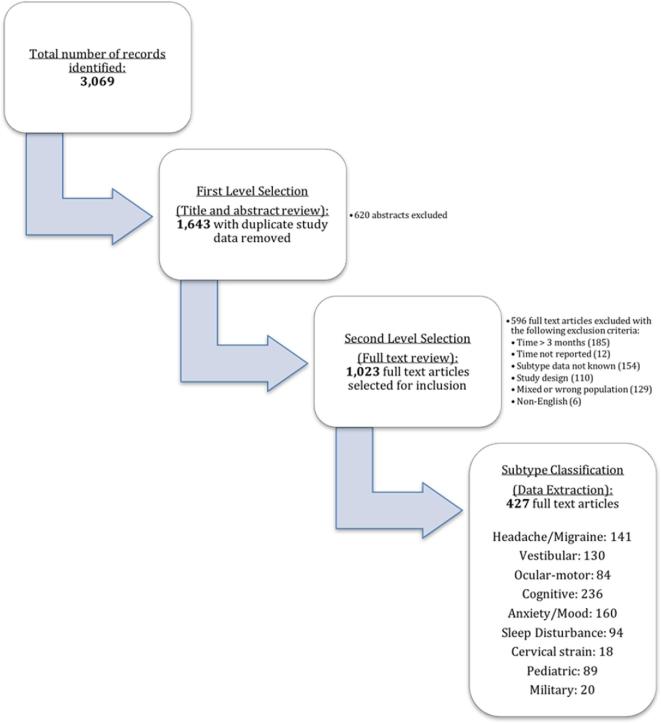
Literature search yielded 3069 records, with full text review of 1643 articles, and 427 articles included for subtype classification and data extraction described in Figure 1. Of these studies, 10 provided data analyzable for concussion prevalence and prevalence ratio (binary outcome), while 15 studies included data applicable for SMD analysis (continuous outcome). Some of the studies included multiple concussion groups. Descriptive characteristics of the included studies are shown in Table 1. The majority of the studies assessed sports-related concussions. The assessment time frame varied by study, ranging from immediately postinjury to 3 d after injury. This systematic review predominantly yielded subtype indicators reported from concussion symptom scales, though some other subjective and objective indicators were also included (Table 2). The results of each subtype's meta-analysis are summarized below, whereas forest plots created from and listing specific symptom/test scores, as well as for the overall effects, are presented in the Supplemental Digital Content (selected forest plots are also presented as the figures in this report). In Tables 3 to 5 and Figure 2 where the summarized results of the subsequent meta-analyses are provided, the number of the studies analyzed (=Study N) indicated the number of data sets, and therefore does not necessarily match the number of studies included in this study mentioned above. That is, some studies provided multiple data sets from, for example, different time points that were still within 3 d postinjury and from different treatment or control groups. A total sample size analyzed (=Sample N) was calculated as the total number of subjects from all data points included in each meta-analysis.
FIGURE 1.
Article workflow.
TABLE 1.
Descriptive Characteristics of Included Studies
| Outcome variable | Population | Study, Year | Total N | Age (Mean ± SD in yr) for concussion patients | Control | Mechanism | Concussion assessment time frame |
|---|---|---|---|---|---|---|---|
| Binary | Pediatric | Collins (Revolution Helmet), 200653 | 62 | 16.3 ± 1.1 | N/A | Sports | Within 72 h |
| Collins (Standard Helmet), 200653 | 74 | 15.9 ± 1.3 | N/A | Sports | Within 72 h | ||
| Grubenhoff, 201154 | 348 | 10.8 ± 3.3, 11.9 ± 3.1 | Orthopedic | Mixed | 1 d | ||
| Nance, 201655 | 85 | 13.9 (SD not reported) | Orthopedic | Mixed | < 3 d | ||
| Schatz, 200656 | 138 | 16.5 ± 2.3 | Uninjured | Sports | Within 72 h | ||
| Sroufe, 201057 | 28 | 13.5 (range: 10-17) | Orthopedic | Mixed | Within 24 h | ||
| Adult | Guskiewicz, 200158 | 36 | 19.5 ± 1.3 | N/A | Sports | Time of injury, 1 d, and 3 d | |
| Kontos, 201259 | 75 | 15.7 ± 1.3, 19.7 ± 1.3 | Baseline | Sports | 2 d | ||
| McCrea, 200560 | 150 | 20.0 ± 1.4 | Uninjured | Sports | Time of injury, immediately postinjury, postgame/practice, 2 to 3 h, 1 d, 2 d, and 3 d | ||
| Ponsford, 201161 | 223 | 35.0 ± 13.1 | Trauma | Mixed | 2 d | ||
| Stone, 201562 | 114 | 36.1 ± 13.0 | Uninjured | Mixed | ≤ 3 d | ||
| Continuous | Pediatric | Chin, 201663 | 330 | 17.5 ± 2.0 | Uninjured/Baseline | Sports | 24 h |
| Collins (Revolution Helmet), 200653 | 62 | 16.3 ± 1.1 | Baseline | Sports | Within 72 h | ||
| Collins (Standard Helmet), 200653 | 74 | 15.9 ± 1.3 | Baseline | Sports | Within 72 h | ||
| Covassin (AM J Sports), 201364 | 598 | 17.3 ± 2.3, 17.0 ± 2.2, 17.8 ± 2.4, 19.0 ± 2.2 | Baseline | Sports | 3 d | ||
| Covassin (Brain Inj), 201365 | 165 | 16.7 ± 2.4 | Baseline | Sports | 3 d | ||
| Hammeke, 201366 | 24 | 16.5 ± 0.5 | Uninjured | Sports | 13 h | ||
| Iverson, 200667 | 30 | 16.1 ± 2.1 | Baseline | Sports | 1 to 2 d | ||
| Schatz, 200656 | 138 | 16.5 ± 2.3 | Uninjured | Sports | Within 72 h | ||
| Sufrinko, 201568 | 265 | 17.4 ± 2.3 | Baseline | Mixed | 2 d | ||
| Adult | Crevitts (Intoxicated Patients), 200069 | 33 | Not reported* | Uninjured | Mixed | Within 1 d | |
| Crevitts (No Intoxication Patients), 200069 | 52 | 26.9 ± 11.8 | Uninjured | Mixed | Within 1 d | ||
| De Monte (No PTA), 200670 | 91 | 22.7 ± 6.8 | Uninjured | Mixed | Within 1 d | ||
| De Monte (PTA), 200670 | 85 | 26.5 ± 10.1 | Uninjured | Mixed | Within 1 d | ||
| Echlin, 201271 | 45 | Not reported** | Baseline | Sports | 72 h | ||
| Guskiewicz, 200158 | 72 | 19.5 ± 1.3 | Uninjured/Baseline | Sports | 1 d and 3 d | ||
| Kontos, 201072 | 96 | 19.3 ± 2.1 | Baseline | Sports | 2 d | ||
| McCrea, 200560 | 150 | 20.0 ± 1.4 | Uninjured/Baseline | Sports | Immediately postinjury, 2 to 3 h, 1 d, 2 d, and 3 d | ||
| Sheedy, 200973 | 100 | 33.6 ± 12.7 | Baseline | Mixed | 3 d |
*Age-matched controls whose ages were 27.6 ± 10.5 yr.
**Canadian Interuniversity Sports ice hockey players.
TABLE 2.
Concussion Symptom Scales, and Other Subjective and Objective Indicators Used for Meta-Analysis of Prevalence using Prevalence Ratio and Standardized Mean Difference (SMD)
| Concussion subtype or associated condition | Classification | Measurements used for prevalence ration | Measurements used for SMD |
|---|---|---|---|
| Concussion subtype | Cognitive | Concentration, remembering, retrograde amnesia, anterograde amnesia, posttraumatic amnesia, cognitive problems, feeling slow | ImPACT - verbal memory, ImPACT—visual motor speed, ImPACT - reaction time, ImPACT—impulse control, verbal learning test—immediate memory, verbal learning test—delayed recall, verbal learning test—recognition, trail making test A, trail making test B, Stroop word, Stroop color, Stroop word-colors test, controlled oral word association test, symbol digit, symbol digit recall, digit symbol substitution test, learning trial, Wechsler digit span test forward, Wechsler digit span test backward, letter-number sequencing, total sentences, concentration, remembering, Sternberg task—percent accuracy, Sternberg task—reaction time (ms) |
| Ocular-motor | Visual problems, blurred vision, visual changes, sensitivity to light, double vision | Antisaccade (errors), antisaccade (latency), remembered saccade (errors), remembered saccade (latency), visual problems, visual acuity, sensitivity to light, King-Devick (K-D) test | |
| Headache-migraine | Headache, sensitivity to light, sensitivity to light or sound, sensitivity to noise, neck pain, vomiting, nausea, nausea/vomiting, nausea, nausea/vomiting, abnormal coordination | Headache, sensitivity to light, sensitivity to noise, vomiting, nausea | |
| Vestibular | Dizziness, balance problem, tinnitus, fogginess, disequilibrium, confusion/disorientation, disorientation, vomiting, nausea, nausea/vomiting, abnormal coordination | BESS, mBESS, dizziness, balance problem, fogginess, vomiting, nausea | |
| Anxiety-mood | Depression, irritability, emotional problem, nervousness, confusion/disorientation, confusion, sadness, slow down, photophobia, personality changes, numbness, tingling, numbness/tingling | Anxiety, depression, irritability, emotional problem, nervousness, sadness, slow down, stress, numbness | |
| Associated condition | Sleep disturbance | Drowsiness, sleeping more than usual, sleeping less than usual, trouble falling asleep, sleepiness | Drowsiness, sleep symptoms |
TABLE 3.
Prevalence of Concussion Subtypes and Sleep Disturbance in Concussion Patients
| Concussion subtype/ | Study N | Proportion | |
|---|---|---|---|
| associated condition | Population | (Sample N) | (95% CI) |
| Cognitive | Pediatric | 10 (654) | 0.32 (0.21, 0.43)a |
| Adult | 16 (1233) | 0.40 (0.25, 0.55) | |
| Ocular-motor | Pediatric | 8 (600) | 0.34 (0.27, 0.41) |
| Adult | 6 (438) | 0.34 (0.18, 0.53) | |
| Headache/migraine | Pediatric | 15 (1320) | 0.52 (0.37, 0.67) |
| Adult | 16 (1107) | 0.38 (0.26, 0.52) | |
| Vestibular | Pediatric | 21 (1705) | 0.50 (0.40, 0.60)b |
| Adult | 26 (1853) | 0.25 (0.18, 0.33)c | |
| Anxiety/mood | Pediatric | 15 (989) | 0.30 (0.21, 0.39) |
| Adult | 15 (975) | 0.23 (0.15, 0.33) | |
| Sleep disturbance | Pediatric | 4 (156) | 0.33 (0.19, 0.49) |
| Adult | 7 (600) | 0.34 (0.18, 0.51) |
CI = confidence interval.
aPublication bias suspected by Egger's test (P = .001); adjusted proportion (95% CI) = 0.33 (0.20, 0.46).
bPublication bias suspected by Egger's test (P = .013); adjusted proportion (95% CI) = 0.49 (0.39, 0.59).
cPublication bias suspected by Egger's test (P = .028); adjusted proportion (95% CI) = 0.29 (0.22, 0.37).
TABLE 5.
Standardized Mean Differences in Symptom/Test Scores for Concussion Subtypes and Sleep Disturbance in Concussion Patients vs Controls
| Concussion subtype/ | Study N | Standardized mean | |
|---|---|---|---|
| associated condition | Population | (Sample N) | difference (95% CI) |
| Cognitive | Pediatric | 56 (25 566) | 0.66 (0.57, 0.75)* |
| Adult | 61 (6310) | 0.24 (0.16, 0.32)* | |
| Ocular-motor | Pediatric | 2 (660) | 0.04 (−0.07, 0.14)a |
| Adult | 8 (336) | 0.72 (0.36, 1.09)* | |
| Headache/migraine | Pediatric | 5 (1650) | −0.01 (−0.08, 0.07) |
| Adult | N/A | – | |
| Vestibular | Pediatric | 10 (2998) | 0.18 (0.04, 0.32)* |
| Adult | 12 (1946) | 0.36 (0.18, 0.55)* | |
| Anxiety/mood | Pediatric | 6 (1980) | −0.05 (−0.14, 0.04) |
| Adult | N/A | – | |
| Sleep disturbance | Pediatric | 2 (860) | 0.44 (−0.72, 1.61) |
| Adult | N/A | – |
*Significantly different from standardized mean difference = 0.
CI = Confidence interval. N/A = No applicable data.
Standardized mean difference was calculated by the mean difference between the concussion and control groups over an appropriate within-groups standard deviation. Positive standardized mean difference represents abnormalities/impairments.
Controls include uninjured subjects and baseline controls (excluding orthopedic controls).
aPublication bias suspected by Egger's test (P < .001); adjusted SMD (95% CI) = 0.54 (0.14, 0.93).
FIGURE 2.
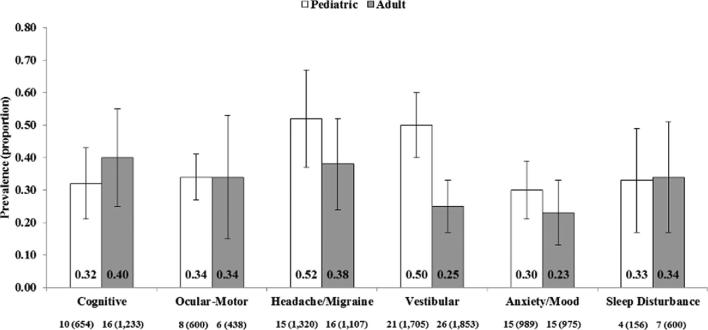
Prevalence of concussion subtypes and sleep disturbance in concussion patients. Bars are 95% CI. Values below each subtype/associated condition are study N (sample N).
Prevalence of Concussion Subtypes and Sleep Disturbance in Concussion Patients
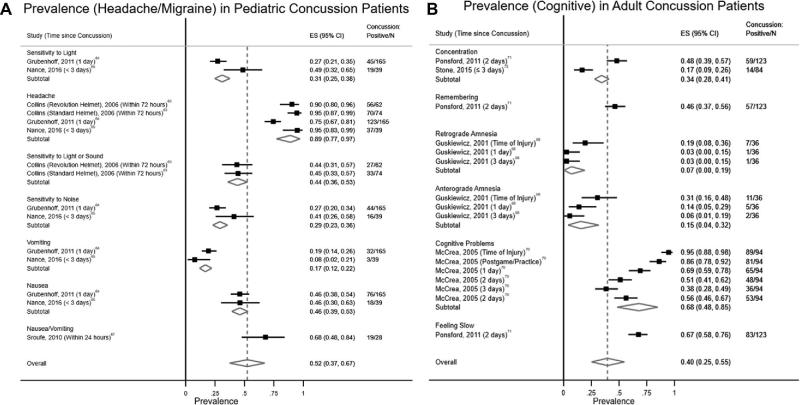
The results of the analysis on the prevalence of concussion subtypes and sleep disturbance in concussion patients are summarized in Table 3 and Figure 2. The headache/migraine subtype was the most prevalent in children (0.52; 95% CI = 0.37, 0.67; Figure 3A), whereas the cognitive subtype (0.40; 95% CI = 0.25, 0.55; Figure 3B) was most prevalent in the adult population. In children, the prevalence of the vestibular subtype was also high (0.50; 95% CI = 0.40, 0.60). Prevalence of the ocular-motor subtype and sleep disturbance was similar between the pediatric and adult populations (0.33-0.34).
FIGURE 3.
A, Forest plot for prevalence (headache/migraine) in pediatric concussion patients. B, Forest plot for prevalence (cognitive) in adult concussion patients.
Prevalence Ratios of Concussion Subtypes and Sleep Disturbance in Concussion Patients vs Controls
Table 4 summarizes the prevalence ratios of concussion subtypes and sleep disturbance in concussion patients as compared with uninjured/baseline controls. There were no applicable studies with appropriate control groups to supply data for ocular-motor, headache/migraine, vestibular, and anxiety/mood subtypes in children, as well as sleep disturbance in the pediatric and adult populations. Adult patients were 4.4, 2.9, and 1.7 times more likely to show cognitive, vestibular, and anxiety/mood subtypes of concussion, respectively, as compared with their controls (P < .05).
TABLE 4.
Prevalence Ratios of Concussion Subtypes and Sleep Disturbance in Concussion Patients vs Controls
| Concussion subtype/ | Study N | Prevalence ratio | |
|---|---|---|---|
| associated condition | Population | (Sample N) | (95% CI) |
| Cognitive | Pediatric | 1 (138) | 1.83 (0.17, 19.75) |
| Adult | 7 (1014) | 4.40 (2.80, 6.91)* | |
| Ocular-motor | Pediatric | N/A | – |
| Adult | 1 (114) | 11.31 (0.70, 183.33) | |
| Headache/migraine | Pediatric | N/A | – |
| Adult | 2 (228) | 1.48 (0.96, 2.27) | |
| Vestibular | Pediatric | N/A | – |
| Adult | 9 (1206) | 2.88 (1.91, 4.33)* | |
| Anxiety/mood | Pediatric | N/A | – |
| Adult | 2 (264) | 1.70 (1.31, 2.20)* | |
| Sleep disturbance | Pediatric | N/A | – |
| Adult | N/A | – |
*Significantly different from prevalence ratio = 1.
CI = Confidence interval. N/A = No applicable data.
Prevalence ratio was calculated by the prevalence in injured subjects over the prevalence in uninjured or baseline controls (excluding orthopedic controls).
Standardized Mean Difference in Concussion Patients vs Controls
SMDs were calculated for all concussion subtypes and sleep disturbance in pediatric patients, whereas the analysis for adult patients was performed on all but headache/migraine and anxiety/mood subtypes, along with sleep disturbance, due to the lack of applicable data (Table 5). Concussed patients in both pediatric and adult populations showed significantly more cognitive symptoms than did their respective controls (SMD = 0.66 and 0.24; P < .001). Further, concussed patients showed significantly worse scores (ie, more errors and longer latency time, leading to higher SMDs) for the ocular-motor subtype in pediatric patients (SMD = 0.72; 95% CI = 0.36, 1.09; P < .001) and for the vestibular subtype in both pediatric and adult patients (SMD = 0.18 and 0.36; 95% CI = 0.04, 0.32 and 0.18, 0.55; P = .012 and < 0.001) than their controls. Scores on sleep disturbance were not significantly different between pediatric concussion and controls (SMD = 0.44; 95% CI = −0.72, 1.61; P = .456).
Publication Bias
Egger's test revealed that publication bias was suspected for the meta-analysis of the proportions of the cognitive subtype in pediatric patients and the vestibular subtype in both pediatric and adult patients (P = .001, .013, and .028, respectively). Meanwhile, the meta-analysis results adjusted for publication bias using the Duval and Tweedie nonparametric trim and fill method were not substantially different from the original results (Table 3). There was no publication bias suspected for the meta-analysis of prevalence ratios of concussion subtypes and sleep disturbance in concussion patients vs controls (P > .05). Publication bias was possible for the meta-analysis of the SMD for the ocular-motor subtype in the adult population (P < .001). The adjusted SMD by the Duval and Tweedie nonparametric trim and fill method was 0.54 (95% CI = 0.14, 0.93) which remained significantly different from zero.
Sensitivity Analysis
The results of the sensitivity analysis indicated that none of the point estimates and CIs of the pooled prevalence for each concussion subtype and sleep disturbance changed substantially with the exclusion of any individual study. This was the case for the pooled prevalence ratio and SMDs. Specifically, the maximum differences in the point estimates are as follows: 0.07 for the prevalence (sleep disturbance in both pediatric and adult patients), 0.40 for the prevalence ratios (headache/migraine subtype in adult patients), and 0.59 for the SMDs (sleep disturbance in pediatric patients). The maximum difference in the point estimate of the SMD was 0.10 (ocular-motor subtype in adult patients), after excluding the SMD analysis of sleep disturbance in pediatric patients that only included 2 studies.
DISCUSSION
This study was the first meta-analytic review to identify evidence of concussion subtypes among children and adults from the extant literature. The most common acute (within 3 d) concussion subtype in adults and children was headache/migraine and this is consistent with previous reports of headache being the most common postconcussive symptom.74,75 The vestibular subtype, populated in this analysis from symptom reports and the Balance Error Scoring System test results, was as common as headache/migraine in children, possibly representing the vulnerability of their developing spatial skills.76 Recent literature has similarly identified vestibulo-oculomotor impairment following concussion.9,77 While previous studies have supported anxiety and mood disturbances following concussion, this study highlights this symptom cluster within an initial clinical encounter in up to a third of adults and children.16 Notably, some individual symptoms, such as “headache” and “dizziness,” are more common than their culminative and overarching subtype due to weighting of studies for sample size and precision. However, this could indicate that there may be the opportunity for refined classifications within a subtype, for example migraine headache vs nonmigraine headache.
The results of this large, heterogeneous, and generalizable sample of adults and children support trends in the clinical consensus of subtype-oriented concussion diagnosis.3,7 This study identified evidence for concussion subtypes within the acute time frame of 3 d following injury, capturing patients that may have shortened courses of symptomatology and impairments following injury and indicating that subtype assessments should begin within the first clinical encounter.
A limited number of studies were included for analysis because many potentially applicable studies failed to report individual outcomes and had wide variability in acute concussion assessment times. Additionally, lack of literature reporting robust objective assessment in this acute time period limited subtype specific meta-analysis to largely symptom reports. None of the studies ultimately included for analysis within the reported time frame informed the cervical strain associated condition, and we could not comment on its prevalence as an associated condition. The number of studies informing the ocular-motor subtype and sleep disturbance were also limited, with these topics representing newer areas of investigation. For the purposes of this research, and relevant to clinical care, the accurate and timely report of postconcussive symptoms is critical to the reliability and stability of their assessments and these descriptions. This study examined the prevalence of concussion subtypes within the acute time-frame to identify the need for targeted diagnostic and management approaches in the acute setting, acknowledging that postconcussive symptoms may evolve over time and persist longer than the 3-mo time frame of the literature search. Further research is needed to understand the subtype-specific recovery trajectories. Concussion subtypes are not mutually exclusive; however, clustering could not be reported in this meta-analysis as comprehensive individual patient data were not available, though have been reported elsewhere.3 This study examined 5 subtypes and 2 associated conditions that the multidisciplinary expert workgroup deemed prominent upon their insights to the applicable body of scientific literature and in their experience in the clinical care of concussed patients. Expert consensus-driven subtype description may lend to potential bias in definition and less commonly assessed subtypes such as auditory disturbance, autonomic dysfunction, and endocrine dysfunction were not included in this study. The majority of studies included for meta-analysis studies sports-concussion and were North American-centric representing a limitation to the generalizability of our findings. Finally, 2 studies, Echlin et al 201271 and Covassin et al 201358, represented data outliers in our analyses in that concussed patients were reported to have less impairments than their control groups on several measures, and this is represented in the Appendix. Taken as part of pooled studies, these results have minimal effect. However, the SMD calculations for some pediatric indicators are impacted when a single study (Covassin et al64) was represented, though these results were ultimately not statistically significant.
This review highlights the need for clinical evaluation for concussion subtypes and their associated conditions immediately following injury. Because a large proportion of pediatric patients exhibit the vestibular subtype, care should be taken to assess for this subtype and prescribe early rehabilitation strategies to facilitate timely recovery. Clinicians should assess for anxiety, mood, and sleep disturbances even in the acute setting following injury and provide prognostic counseling as well as advise appropriate sleep management.78 Challenges to implementing a clinical classification of concussion include developing a well-defined approach to methodology, effective involvement of stakeholders, and financial constraints. Future research on concussion subtypes will better direct meaningful outcomes by utilizing consistent definitions and criteria such as those established by this study. Meaningful anticipated outcomes include targeted approaches in rehabilitation including specific strategies for physical and cognitive activity.
This research team will report the recovery trajectories and patterns of subtype predominance from the acute period through 3 mo following injury in Concussion Guidelines Step 3. Several studies are under way utilizing large data sets that may further influence subtype classification systems.79-81
CONCLUSION
This research establishes that 5 concussion subtypes and associated sleep disturbance are common and vary in prevalence within 3 d following injury. A comprehensive acute concussion assessment should include evaluation for subtypes and associated conditions.
Disclosures
This material is based in part upon work supported by (1) the US Army Contracting Command, Aberdeen Proving Ground, Natick Contracting Division, through a contract awarded to Stanford University (W911 QY-14-C-0086). Any opinions, findings, and conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the US Army Contracting Command, Aberdeen Proving Ground, Natick Contracting Division, or Stanford University. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Supplemental Digital Content. Figures. Forest plots for specific symptom/test scores and overall effects. The Supplemental Digital Content includes 27 forest plots to show the specific symptom/test scores, along with the overall effects, for the meta-analysis on each concussion subtype and associated condition.
REFERENCES
- 1. Carney N, Ghajar J, Jagoda A et al.. Concussion guidelines step 1. Neurosurgery. 2014;75(suppl 1):S3-S15. [DOI] [PubMed] [Google Scholar]
- 2. Collins MW, Kontos AP, Okonkwo DO et al.. Statements of agreement from the targeted evaluation and active management (TEAM) approaches to treating concussion meeting held in Pittsburgh, October 15-16, 2015. Neurosurgery. 2016;79(6):912-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maruta J, Lumba-Brown A, Ghajar J. Concussion subtype identification with the Rivermead Post-concussion Symptoms Questionnaire. Front Neurol. 2018;9. (doi: 10.3389/fneur.2018.01034). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCrory P, Meeuwisse W, Dvorak J et al.. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. [DOI] [PubMed] [Google Scholar]
- 5. Eisenberg MA, Meehan WP 3rd, Mannix R. Duration and course of post-concussive symptoms. Pediatrics. 2014;133(6):999-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howell DR, Kriz P, Mannix RC, Kirchberg T, Master CL, Meehan WP III. Concussion symptom profiles among child, adolescent, and young adult athletes. Clin J Sport Med. published online:June 21, 2018. (doi: 10.1097/JSM.0000000000000629). [DOI] [PubMed] [Google Scholar]
- 7. Collins MW, Kontos AP, Reynolds E, Murawski CD, Fu FH. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235-246. [DOI] [PubMed] [Google Scholar]
- 8. Maruta J, Jaw E, Modera P, Rajashekar U, Spielman LA, Ghajar J. Frequency responses to visual tracking stimuli may be affected by concussion. Mil Med. 2017;182(S1):120-123. [DOI] [PubMed] [Google Scholar]
- 9. Sussman ES, Ho AL, Pendharkar AV, Ghajar J. Clinical evaluation of concussion: the evolving role of oculomotor assessments. Neurosurg Focus. 2016;40(4):E7. [DOI] [PubMed] [Google Scholar]
- 10. Kapoor N, Ciuffreda KJ. Vision disturbances following traumatic brain injury. Curr Treat Options Neurol. 2002;4(4):271-280. [DOI] [PubMed] [Google Scholar]
- 11. Seifert T. The relationship of migraine and other headache disorders to concussion. Handb Clin Neurol. 2018;158:119-126. [DOI] [PubMed] [Google Scholar]
- 12. Rizzoli P, Mullally WJ. Headache. Am J Med. 2018;131(1):17-24. [DOI] [PubMed] [Google Scholar]
- 13. Sufrinko A, McAllister-Deitrick J, Elbin RJ, Collins MW, Kontos AP. Family history of migraine associated with posttraumatic migraine symptoms following sport-related concussion. J Head Trauma Rehabil. 2018;33(1):7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mucha A, Fedor S, DeMarco D. Vestibular dysfunction and concussion. Handb Clin Neurol. 2018;158:135-144. [DOI] [PubMed] [Google Scholar]
- 15. Mucha A, Collins MW, Elbin RJ et al.. A brief vestibular/ocular motor screening (VOMS) assessment to evaluate concussions. Am J Sports Med. 2014;42(10):2479-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandel N, Reynolds E, Cohen PE, Gillie BL, Kontos AP. Anxiety and mood clinical profile following sport-related concussion: from risk factors to treatment. Sport Exerc Perform Psychol. 2017;6(3):304-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sandsmark DK, Elliott JE, Lim MM. Sleep-wake disturbances after traumatic brain injury: synthesis of human and animal studies. Sleep. 2017;40(5):zsx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomasy HE, Opp MR. Hypocretin mediates sleep and wake disturbances in a mouse model of traumatic brain injury. J Neurotrauma. 2019;36(5):802-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sateia MJ. International classification of sleep disorders-third edition. Chest. 2014;146(5):1387-1394. [DOI] [PubMed] [Google Scholar]
- 20. DoD Clinical Recommendation | June 2014: Management of Sleep Disturbances Following Concussion/Mild Traumatic Brain Injury: Guidance for Primary Care Management in Deployed and Non-Deployed Settings. 2014. https://pueblo.gpo.gov/DVBIC/pdf/DV-1839.pdf. Accessed May 7, 2019. [Google Scholar]
- 21. Clinchot DM, Bogner J, Mysiw WJ, Fugate L, Corrigan J. Defining sleep disturbance after brain injury. Am J Phys Med Rehabil. 1998;77(4):291-295. [DOI] [PubMed] [Google Scholar]
- 22. Holcomb EM, Schwartz DJ, McCarthy M, Thomas B, Barnett SD, Nakase-Richardson R. Incidence, characterization, and predictors of sleep apnea in consecutive brain injury rehabilitation admissions. J Head Trauma Rehabil. 2016;31(2):82-100. [DOI] [PubMed] [Google Scholar]
- 23. St-Onge MP, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes. 2014;38(3):411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wickwire EM, Williams SG, Roth T et al.. Sleep, sleep disorders, and mild traumatic brain injury. What we know and what we need to know: findings from a national working group. Neurotherapeutics. 2016;13(2):403-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim MM, Baumann CR. Sleep-wake disorders in patients with traumatic brain injury. UpToDate 2018. https://www.uptodate.com/contents/sleep-wake-disorders-in-patients-with-traumatic-brain-injury. Accessed May 7, 2019. [Google Scholar]
- 26. Cheever K, Kawata K, Tierney R, Galgon A. Cervical Injury assessments for concussion evaluation: a review. J Athl Train. 2016;51(12):1037-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaremski JL, Herman DC, Clugston JR, Hurley RW, Ahn AH. Occipital neuralgia as a sequela of sports concussion. Curr Sports Med Rep. 2015;14(1):16-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marshall CM, Vernon H, Leddy JJ, Baldwin BA. The role of the cervical spine in post-concussion syndrome. Phys Sportsmed. 2015;43(3):274-284. [DOI] [PubMed] [Google Scholar]
- 29. Craton N, Leslie O. Time to re-think the Zurich guidelines? Clin J Sport Med. 2014;24(2):93-95. [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. [DOI] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stroup DF, Berlin JA, Morton SC et al.. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 33. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, West Sussex, UK: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 34. Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta-Analysis in Medical Research. Chichester, West Sussex, UK: John Wiley & Sons, Ltd; 2000. [Google Scholar]
- 35. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 37. Miller JJ. The inverse of the Freeman–Tukey double arcsine transformation. Am Stat. 1978;32(4):138-138. [Google Scholar]
- 38. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21(4):607-611. [Google Scholar]
- 39. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857-872. [DOI] [PubMed] [Google Scholar]
- 40. Saltychev M, Barlund E, Paltamaa J, Katajapuu N, Laimi K. Progressive resistance training in Parkinson's disease: a systematic review and meta-analysis. BMJ Open. 2016;6(1):e008756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fu R, Vandermeer BW, Shamliyan TA et al.. Handling continuous outcomes in quantitative synthesis. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 42. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443-3457. [DOI] [PubMed] [Google Scholar]
- 44. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89-98. [Google Scholar]
- 45. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bradburn MJ, Deeks JJ, Altman DG. metan—-a command for meta-analysis in Stata. In: Palmer TM, Sterne JAC, eds. Meta-Analysis in Stata: An Updated Collection From the Stata Journal. College Station, TX: Stata Press; 2016:3-28. [Google Scholar]
- 48. Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, Sterne JAC. metan: fixed- and random-effects meta-analysis. In: Palmer TM, Sterne JAC, eds. Meta-Analysis in Stata: An Updated Collection From the Stata Journal. College Station, TX: Stata Press; 2016:3-28. [Google Scholar]
- 49. Harbord RM, Harris RJ, Sterne JAC. Updated tests for small-study effects in meta-analyses. Stata J. 2009;9(2):197-210. [Google Scholar]
- 50. Steichen TJ. Nonparametric trim and fill analysis of publication bias in meta-analysis. Stata Tech Bull. 2000;57(STB-57):8-14. [Google Scholar]
- 51. Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Techn Bull. 1999;47(STB-47):15-17. [Google Scholar]
- 52. Tobias A. Update of metainf. Stata Tech Bull. 2001;10(56):sbe26.1. [Google Scholar]
- 53. Collins M, Lovell MR, Iverson GL, Ide T, Maroon J. Examining concussion rates and return to play in high school football players wearing newer helmet technology: a three-year prospective cohort study. Neurosurgery. 2006;58(2):275-286; discussion 275-286. [DOI] [PubMed] [Google Scholar]
- 54. Grubenhoff JA, Kirkwood MW, Deakyne S, Wathen J. Detailed concussion symptom analysis in a paediatric ED population. Brain Inj. 2011;25(10):943-949. [DOI] [PubMed] [Google Scholar]
- 55. Nance ML, Callahan JM, Tharakan SJ et al.. Utility of neurocognitive testing of mild traumatic brain injury in children treated and released from the emergency department. Brain Inj. 2016;30(2):184-190. [DOI] [PubMed] [Google Scholar]
- 56. Schatz P, Pardini JE, Lovell MR, Collins MW, Podell K. Sensitivity and specificity of the ImPACT test battery for concussion in athletes. Arch Clin Neuropsychol. 2006;21(1):91-99. [DOI] [PubMed] [Google Scholar]
- 57. Sroufe NS, Fuller DS, West BT, Singal BM, Warschausky SA, Maio RF. Postconcussive symptoms and neurocognitive function after mild traumatic brain injury in children. Pediatrics. 2010;125(6):e1331-e1339. [DOI] [PubMed] [Google Scholar]
- 58. Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263-273. [PMC free article] [PubMed] [Google Scholar]
- 59. Kontos AP, Covassin T, Elbin RJ, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756. [DOI] [PubMed] [Google Scholar]
- 60. McCrea M, Barr WB, Guskiewicz K et al.. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11(1):58-69. [DOI] [PubMed] [Google Scholar]
- 61. Ponsford J, Cameron P, Fitzgerald M, Grant M, Mikocka-Walus A. Long-term outcomes after uncomplicated mild traumatic brain injury: a comparison with trauma controls. J Neurotrauma. 2011;28(6):937-946. [DOI] [PubMed] [Google Scholar]
- 62. Stone ME Jr., Safadjou S, Farber B et al.. Utility of the Military Acute Concussion Evaluation as a screening tool for mild traumatic brain injury in a civilian trauma population. J Trauma Acute Care Surg. 2015;79(1):147-151. [DOI] [PubMed] [Google Scholar]
- 63. Chin EY, Nelson LD, Barr WB, McCrory P, McCrea MA. Reliability and validity of the Sport Concussion Assessment Tool-3 (SCAT3) in high school and collegiate athletes. Am J Sports Med. 2016;44(9):2276-2285. [DOI] [PubMed] [Google Scholar]
- 64. Covassin T, Moran R, Wilhelm K. Concussion symptoms and neurocognitive performance of high school and college athletes who incur multiple concussions. Am J Sports Med. 2013;41(12):2885-2889. [DOI] [PubMed] [Google Scholar]
- 65. Covassin T, Crutcher B, Wallace J. Does a 20 minute cognitive task increase concussion symptoms in concussed athletes? Brain Inj. 2013;27(13-14):1589-1594. [DOI] [PubMed] [Google Scholar]
- 66. Hammeke TA, McCrea M, Coats SM et al.. Acute and subacute changes in neural activation during the recovery from sport-related concussion. J Int Neuropsychol Soc. 2013;19(8):863-872. [DOI] [PubMed] [Google Scholar]
- 67. Iverson GL, Brooks BL, Collins MW, Lovell MR. Tracking neuropsychological recovery following concussion in sport. Brain Inj. 2006;20(3):245-252. [DOI] [PubMed] [Google Scholar]
- 68. Sufrinko A, Pearce K, Elbin RJ et al.. The effect of preinjury sleep difficulties on neurocognitive impairment and symptoms after sport-related concussion. Am J Sports Med. 2015;43(4):830-838. [DOI] [PubMed] [Google Scholar]
- 69. Crevits L, Hanse MC, Tummers P, Van Maele G. Antisaccades and remembered saccades in mild traumatic brain injury. J Neurol. 2000;247(3):179-182. [DOI] [PubMed] [Google Scholar]
- 70. De Monte VE, Geffen GM, Massavelli BM. The effects of post-traumatic amnesia on information processing following mild traumatic brain injury. Brain Inj. 2006;20(13-14):1345-1354. [DOI] [PubMed] [Google Scholar]
- 71. Echlin PS, Skopelja EN, Worsley R et al.. A prospective study of physician-observed concussion during a varsity university ice hockey season: incidence and neuropsychological changes. Part 2 of 4. Neurosurg Focus. 2012;33(6):E2: 1-11. [DOI] [PubMed] [Google Scholar]
- 72. Kontos AP, Elbin RJ 3rd, Covassin T, Larson E. Exploring differences in computerized neurocognitive concussion testing between African American and White athletes. Arch Clin Neuropsychol. 2010;25(8):734-744. [DOI] [PubMed] [Google Scholar]
- 73. Sheedy J, Harvey E, Faux S, Geffen G, Shores EA. Emergency department assessment of mild traumatic brain injury and the prediction of postconcussive symptoms. J Head Trauma Rehabil. 2009;24(5):333-343. [DOI] [PubMed] [Google Scholar]
- 74. Starkey NJ, Jones K, Case R, Theadom A, Barker-Collo S, Feigin V. Post-concussive symptoms after a mild traumatic brain injury during childhood and adolescence. Brain Inj. 2018;32(5):617-626. [DOI] [PubMed] [Google Scholar]
- 75. Begasse de Dhaem O, Barr WB, Balcer LJ, Galetta SL, Minen MT. Post-traumatic headache: the use of the sport concussion assessment tool (SCAT-3) as a predictor of post-concussion recovery. J Headache Pain. 2017;18(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wiener-Vacher SR, Hamilton DA, Wiener SI. Vestibular activity and cognitive development in children: perspectives. Front Integr Neurosci. 2013;20(3):245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kontos AP, Deitrick JM, Collins MW, Mucha A. Review of vestibular and oculomotor screening and concussion rehabilitation. J Athl Train. 2017;52(3):256-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lumba-Brown A, Yeates K, Sarmiento K et al.. Centers for disease control and prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;172(11):e182853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Maas AI, Menon DK, Steyerberg EW et al.. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery. 2015;76(1):67-80. [DOI] [PubMed] [Google Scholar]
- 80. Cnossen MC, Winkler EA, Yue JK et al.. Development of a prediction model for post-concussive symptoms following mild traumatic brain injury: a TRACK-TBI pilot study. J Neurotrauma. 2017;34(16):2396-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pac-12. Student-Athlete Health & Well-Being Initiative: Prior Grant Awardees 2019. https://pac-12.com/conference/sahwbgp/prior-grant-awardees. Accessed May 24, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.