Abstract
Idiopathic multicentric Castleman disease (iMCD) is a rare lymphoproliferative disorder, and only a few cases have been reported to be complicated with autoimmune hemolytic anemia (AIHA). A 43-year-old man who presented with multiple swollen lymph nodes was diagnosed with iMCD. He was also diagnosed with AIHA based on laboratory findings, including the results of a bone marrow aspiration study. The patient was treated with tocilizumab; however, the effect was limited, probably due to anti-drug antibodies. Tocilizumab was therefore switched to rituximab, and his anemia was improved. Complication with AIHA should be carefully considered when iMCD patients present with severe anemia.
Keywords: multicentric Castleman disease, autoimmune hemolytic anemia, tocilizumab, anti-drug antibody
Introduction
Castleman disease (CD) is a rare lymphoproliferative disorder that is classified into two types: unicentric CD (UCD) and multicentric CD (MCD) (1). Approximately 50% of MCD cases are associated with human herpesvirus-8 (HHV-8) infection (1). Uncontrolled infection causes hypercytokinemia and polyclonal lymphoproliferation. The other half of MCD cases are HHV-8-negative and are called idiopathic MCD (iMCD). The etiology of iMCD is still unknown; however, excessive production of cytokines, including interleukin-6 (IL-6), is thought to enlarge the lymph nodes and give rise to other symptoms (2,3).
At present, four hypotheses propose that this so-called cytokine storm may be the consequence of uncontrolled infection with some pathogen other than HHV-8 (pathogen hypothesis), autoantibodies or auto-reactive T cells that activate immune reactions (autoimmune hypothesis), germline mutations regulating inflammation (autoinflammatory hypothesis), and somatic mutations in monoclonal lymph node cells that lead to ectopic cytokine secretion (paraneoplastic mechanisms) (3).
The diagnostic criteria of iMCD established by the international working group include the pathologic review of enlarged lymph nodes, typical clinical/laboratory findings, and the exclusion of other diseases that have MCD-like features (4). Common symptoms in patients with iMCD are a fever, night sweats, weight loss, an enlarged liver or spleen, edema, and ascites. Laboratory findings frequently show elevated levels of soluble interleukin 2 receptor (sIL-2R), IL-6, and C-reactive protein (CRP); an elevated erythrocyte sedimentation rate (ESR); hypoalbuminemia; and anemia (3). Patients with iMCD sometimes have, concomitantly, other autoimmune diseases, such as systemic lupus erythematosus and hemophagocytic lymphohistiocytosis (3). Although cases of iMCD accompanied by autoimmune hemolytic anemia (AIHA) have been reported, it remains a very rare complication (5-9).
We herein report a rare case of iMCD complicated with severe AIHA.
Case Report
A 43-year-old man was admitted to our hospital complaining of shortness of breath and general fatigue. He had noticed these symptoms 2 months before admission and had lost 8 kg of body weight in that same period. Starting from the day before he came to our hospital, he had developed a fever of 37°C, and the symptoms had substantially worsened.
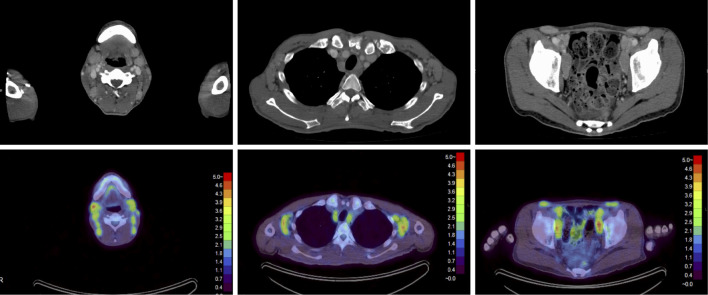
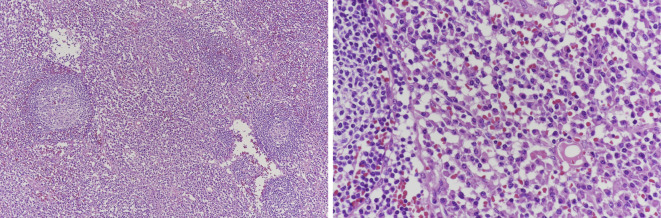
On admission, his body temperature was 39°C, his blood pressure 100/52 mmHg, and his heart rate 100 bpm. A physical examination revealed jaundice; anemic conjunctiva; hepatosplenomegaly; systolic murmur; and swollen lymph nodes in the bilateral cervix, axilla, and inguen. Laboratory findings were as shown in Table 1. Serum protein immunoelectrophoresis showed polyclonal hypergammaglobulinemia with a free light chain κ/λ ratio of 1.57. Bence Jones protein was not detected in the urine analysis. Computed tomography (CT) showed the enlargement of the lymph nodes at multiple sites (bilateral cervix, axilla, inguen, mediastinum, and abdominal and retroperitoneal cavities) and splenomegaly. Fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) revealed an abnormal uptake in the lymph nodes in the cervix [maximum standardized uptake value (SUVmax): right 4.65, left 3.80], axilla (SUVmax: right 4.51, left 4.51), inguen (SUVmax: right 4.50, left 4.30), mediastinum (SUVmax: right 3.96, left 4.06), and external iliac (SUVmax: right 4.94, left 5.03) (Fig. 1). A biopsy of a right posterior cervical lymph node revealed hyperplastic lymphoid follicles and plasma cell infiltration in the interfollicular areas, which was consistent with the plasma cell type of CD (Fig. 2).
Table 1.
Laboratory Data of the Present Case.
| Hematology | Immunology | |||||
| WBC | 7,484 | /µL | CRP | 9.04 | mg/dL | |
| Neutro | 57.8 | % | IL-6 | 67.5 | pg/mL | |
| Lymph | 31.5 | % | IgG | 8,338 | mg/dL | |
| Eosino | 1.4 | % | IgA | 649 | mg/dL | |
| Baso | 0.9 | % | IgM | 121 | mg/dL | |
| RBC | 220×104 | /µL | sIL-2R | 2,134 | U/mL | |
| MCV | 90.2 | fL | Antineuclear antibody | (-) | ||
| MCHC | 31.3 | % | Rherumatoid factor | (-) | ||
| Hb | 2.2 | g/mL | Cold agglutinin | (-) | ||
| Ret | 88.1 | ‰ | Direct Coombs’ test | (+) | ||
| Plt | 32.5×104 | /µL | Indirect Coombs’ test | (+) | ||
| Biochemistry | Infection | |||||
| Total bilirubin | 2.25 | mg/dL | EBV-IgM | (-) | ||
| Direct bilirubin | 1.16 | mg/dL | CMV-IgM | (+) | ||
| AST | 12 | U/L | HHV-8 DNA PCR | (-) | ||
| ALT | 6 | U/L | HIV Ab | (-) | ||
| Na | 132 | mEq/L | T-SPOT | (-) | ||
| K | 3.3 | mEq/L | 1,3-β-D-glucan | 12 | pg/mL | |
| Cl | 103 | mEq/L | Urine | |||
| Ca | 7 | mg/dL | pH | 7.5 | ||
| Fe | 34 | µg/L | Gravity | 1.022 | ||
| TIBC | 132 | µg/L | Urobilinogen | >8.0 | mg/dL | |
| Ferritin | 263.9 | ng/mL | Bilirubin | (-) | ||
| Haptoglobin | 166 | mg/dL | Protein | (+) | ||
| LDH | 138 | U/L | Blood | (±) | ||
| BUN | 15 | mg/dL | ||||
| Cre | 0.89 | mg/dL | ||||
| Total protein | 12.4 | g/dL | ||||
| Albumin | 2.1 | g/dL | ||||
| Folic acid | 1.8 | ng/mL | ||||
| Vit. B12 | 310 | pg/mL | ||||
WBC: white blood cell, Neutro: neutrocyte, Lymph: lymphocyte, Eosino: eosinocyte, Baso: basocyte, RBC: red blood cell, MCV: mean corpuscular volume, MCHC: mean corpuscular hemoglobin concentration, Hb: hemoglobin, Ret: reticulocyte, Plt: platelet, AST: aspartate aminotransferase, ALT: alanine aminotransferase, TIBC: total iron-binding capacity, LDH: lactate dehydrogenase, BUN: blood urea nitrogen, Cre: creatinine, CRP: C-reactive protein, EBV: Epstein Barr virus, CMV: cytomegalo virus, HHV-8 DNA PCR: human herpes virus-8, DNA polymerase chain reaction
Figure 1.
Contrast-enhanced computed tomography (CT) showing the enlargement of the lymph nodes at multiple sites, including the bilateral cervix, axilla, inguen, mediastinum, abdominal cavity, and retroperitoneal cavity (upper panel). Fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG-PET) findings revealed that FDG had accumulated at these sites as well (lower panel).
Figure 2.
The histopathological findings of a right posterior cervical lymph node revealed the parafollicular plasma cell proliferation that was consistent with plasma cell-type Castleman disease. Right panel: original magnification ×100. Left panel: original magnification ×400.
Laboratory findings included severe normocytic anemia, spherocytosis, increased reticulocyte count, positive direct and indirect Coombs test results, total/indirect hyperbilirubinemia, and increased urine urobilinogen. In addition, the results of bone marrow aspiration indicated increased erythropoiesis. Based on the above findings, a diagnosis of iMCD and AIHA was made.
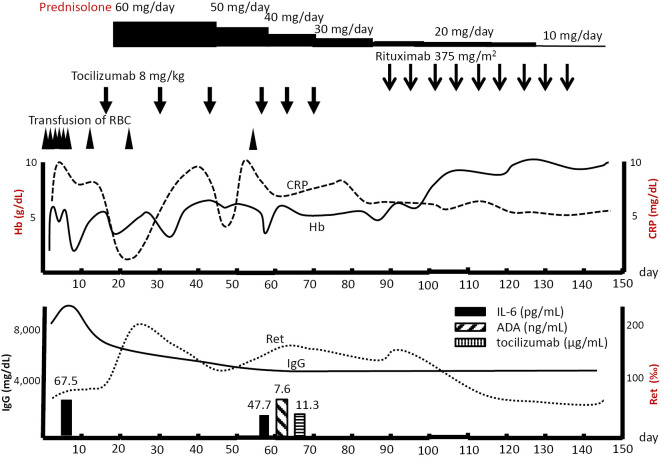
Initially, the patient was treated with tocilizumab at a dose of 8 mg/kg biweekly. A few days after the first tocilizumab administration, the serum CRP levels and pyrexia improved, but the anemia showed no improvement. Six days after the first tocilizumab administration, oral prednisolone (60 mg/day) was added to the treatment. However, after the second administration of tocilizumab (30 days after his admission), the patient's laboratory results worsened: the CRP levels increased and Hb levels decreased. We shortened the interval of tocilizumab to 8 mg/kg weekly, but the patient responded poorly. A blood test revealed that anti-drug antibody (ADA) against tocilizumab had been produced (7.60 ng/mL; reference value upper limit was 3.91 ng/mL), and the serum tocilizumab levels were low (11.3 μg/mL) considering the drug dose (serum levels of tocilizumab, IL-6 and ADA against tocilizumab were measured via an enzyme-linked immunosorbent assay with the cooperation of Chugai Pharmaceutical). The serum IL-6 level was 47.7 pg/mL, which should have been much higher if tocilizumab had blocked the IL-6 receptors sufficiently and worked effectively. The relatively low serum IL-6 levels, low serum tocilizumab levels, and detected ADA suggested a neutralizing effect of ADA on tocilizumab (10).
After 6 doses of tocilizumab, we switched the therapy to rituximab (10 mg/kg biweekly). The patient's CRP levels and anemia subsequently improved (Fig. 3). He was discharged from our hospital on the 99th day and continued rituximab until the 8th dose on an outpatient basis. After the 8th dose of rituximab, his anemia, swelling of lymph nodes, and general fatigue had improved, although mild fever was still shown, and the CRP level was high.
Figure 3.
The clinical course of our patient with AIHA associated with MCD. Day 0 is the day when the patient was admitted to our hospital. AIHA: autoimmune hemolytic anemia, MCD: multicentric castleman disease, RBC: Red blood cells, CRP: C-reactive protein, Hb: hemoglobin, ADA: anti-drug antibody, Ret: reticulocytes
Discussion
The treatment guideline established by the international working group and published in 2018 present treatment options for iMCD patients classified as severe or nonsevere based on their performance status and the presence of organ failure (11). Our case was categorized as nonsevere, as although our patient's Hb was 2.2 g/dL, which is lower than 8 g/dL, a diagnostic criterion of severe organ failure, his performance status was not poor, and renal or pulmonary failure and anasarca/ascites were not detected. First-line therapy for nonsevere patients is siltuximab or tocilizumab with or without corticosteroids. Roughly 50% of patients with iMCD will not respond to IL-6 therapy, and second-line therapy for inadequate responders is rituximab and steroids with or without immunomodulatory agents (11). In our case, we administered tocilizumab biweekly plus prednisolone 60 mg/day (1 mg/kg/day) as first-line therapy, which is consistent with the treatment guideline.
Anemia is a common symptom of iMCD, and iMCD is occasionally accompanied by autoimmune diseases (3). In most cases, however, anemia is due to chronic inflammation, and complication with AIHA is quite rare. To our knowledge, only five cases of MCD accompanied by AIHA have been reported to date (5-9) (Table 2). In all of those cases, the patients were successfully treated with prednisolone plus an immunosuppressive agent (i.e., rituximab, chemotherapy, or tocilizumab). All but one case showed a substantial time difference between the occurrence of MCD and hemolytic anemia (5). The patients were in remission for years, and none of the symptoms of iMCD other than AIHA were identified. This suggests that AIHA can occur irrespective of the activity of MCD itself. In our patient, anemia was normocytic with increased reticulocytes and bilirubin levels. In contrast, LDH and haptoglobin levels were within the normal range, which is inconsistent with hemolytic anemia. Although detailed mechanisms have not been revealed, LDH is often decreased in iMCD patients (12).
Table 2.
Reports Describing Cases of AIHA Accompanied by IMCD.
| No. | Reference | Age & Sex | Duration between diagnosis of MCD and AIHA | Treatment | Hb at worst (g/dL) |
WBC (/μL) |
CRP (mg/dL) |
Hb after treatment (g/dL) |
Direct Coomb’s test | LDH (IU/L) |
Haptoglobin (mg/dL) |
Ret (‰) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 49F | 9 years later | predonisolone, azathioprine, cyclophosphamid, vincristine, danazol, plasmapheresis | 4.7 | 6,300 | 12.4 | 11.2 | positive | 606 | 9.6 | 118 |
| 2 | 5 | 72M | at the same time | CHOP | 5 | 4,900 | not mentioned | 9 | positive | 312 | not mentioned | not mentioned |
| 3 | 7 | 53M | 3 years later | R-CHOP | 2.2 | 9,420 | 0.95 | 9.9 | positive | not mentioned | not mentioned | 113 |
| 4 | 6 | 23M | several times since the age of 7 until 2 years before the diagnosis of MCD | rituximab | normal | not mentioned | not mentioned | not mentioned | not mentioned | not mentioned | not mentioned | not mentioned |
| 5 | 8 | 58F | 13 years later | predonisolone, tocilizmab | 4.7 | 8,300 | 10.1 | 10.5 | positive | not mentioned | not mentioned | 188 |
CHOP: cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisolone, R-CHOP: rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisolone
The pathogenesis of AIHA has not been fully elucidated. However, IL-6 may have an important role (13). AIHA patients reportedly have increased serum levels of IL-6 as well as IL-4, IL-10, IL-13, IL-17, and IL-21, whereas IFN-γ levels are reduced (11). Furthermore, the presence of T helper 2 (Th2) cells, regulatory T cells, and T helper 17 (Th17) cells, which can react with the Rh peptide fraction, has been reported, suggesting that these cytokine/cells are closely associated with the activity of the disease (14). Th2 cells secrete cytokines, including IL-6, as well as IL-4, IL-10, IL-13, and TGF-β, thus stimulating B-cells to produce antibodies. In addition, IL-6 induces Th17 differentiation, amplifying the proinflammatory and autoimmune responses (15,16). Taken together, the reported evidence indicates that increased IL-6 levels in iMCD can lead to autoimmunity, which is consistent with our case, where the patient showed elevated IL-6 levels.
The previous findings also suggest that monoclonal antibodies targeting IL-6 (IL-6 mAbs) are effective for the treatment of AIHA. Kunitomi et al. described a patient with AIHA that was successfully treated with tocilizumab (17). Yuzuriha et al. successfully treated AIHA accompanied by MCD with tocilizumab (8). These reports show that Il-6 mAbs are an attractive therapeutic option for iMCD, AIHA, and overlap cases.
ADA production might be the reason why tocilizumab was not sufficiently effective in our case. When the efficacy of tocilizumab was diminished, we detected the presence of ADA in the patient's serum, along with serum tocilizumab levels that were lower than the effective blood concentration. In addition, the fact that IL-6 levels did not rise as much as they should have if tocilizumab had properly inhibited the IL-6 receptors suggests that tocilizumab's effect was not at its maximum.
Tocilizumab's immunogenicity and its impact on drug effectiveness have been evaluated in several studies. Burmester et al. assessed the data of 8,974 rheumatoid arthritis patients from previous clinical trials who were treated with intravenous or subcutaneous tocilizumab for up to five years (18). ADA production was found in only 1.2-1.5% of all patients. This study showed that ADA does not affect tocilizumab's pharmacokinetics nor the emergence of adverse effects; in fact, none of the patients who developed ADA experienced a loss of efficacy. However, another study has shown statistically significant correlations between serum tocilizumab levels higher than 10 μg/ml and ESR, CRP, and the 28-joint disease activity score in 126 rheumatoid arthritis patients after 6 months of treatment (19). These findings strongly suggest that further research is needed to clarify the relationship between ADA and tocilizumab therapy.
In our case, anemia was improved (hemoglobin: 6.6 g/dL at the 126th hospital day), but the disease activity was not completely controlled, as the CRP levels remained high and a mild fever persisted. According to the guideline, switching the therapeutic regimen to chemotherapy or immunosuppressive agents should be considered when the symptoms are not fully ameliorated. However, tocilizumab remains a viable option to consider, as there are some reports that the production of ADA is not persistent (20).
In conclusion, we encountered a rare case of iMCD accompanied by AIHA in which the effect of tocilizumab might have been reduced, at least in part, because of ADA production. In addition, we want to highlight that measuring the serum levels of IL-6, tocilizumab, and ADA may be useful for evaluating the ADA production in iMCD patients and that the possibility of AIHA complications should be carefully considered in cases of iMCD presenting with severe anemia.
The authors state that they have no Conflict of Interest (COI).
Finalcial Support
We would like to thank Chugai Pharmaceutical for the measurement and interpretation of the serum levels of tocilizumab, IL-6, and ADA against tocilizumab.
References
- 1. Fajgenbaum DC. Novel insights and therapeutic approaches in idiopathic multicentric Castleman disease. Blood 132: 2323-2330, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koga T, Sumiyoshi R, Kawakami A, Yoshizaki K. A benefit and the prospects of IL-6 inhibitors in idiopathic multicentric Castleman's disease. Mod Rheumatol 29: 302-305, 2019. [DOI] [PubMed] [Google Scholar]
- 3. Liu AY, Nabel CS, Finkelman BS, et al. Idiopathic multicentric Castleman's disease: a systematic literature review. Lancet Haematol 3: E163-E175, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Fajgenbaum DC, Uldrick TS, Bagg A, et al. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood 129: 1646-1657, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liberato NL, Bollati P, Chiofalo F, Filipponi M, Poli M. Autoimmune hemolytic anemia in multicentric Castleman's disease. Haematologica 81: 40-43, 1996. [PubMed] [Google Scholar]
- 6. Ocio EM, Sanchez-Guijo FM, Diez-Campelo M, et al. Efficacy of rituximab in an aggressive form of multicentric Castleman disease associated with immune phenomena. Am J Hematol 78: 302-305, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Tajima K, Yamamoto H, Suzuki I, et al. Autoimmune hemolytic anemia with warm-reactive immunoglobulin M antibody in multicentric Castleman disease. Ann Hematol 92: 849-851, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Yuzuriha A, Saitoh T, Koiso H, et al. Successful treatment of autoimmune hemolytic anemia associated with multicentric Castleman disease by anti-interleukin-6 receptor antibody (tocilizumab) therapy. Acta Haematol 126: 147-150, 2011. [DOI] [PubMed] [Google Scholar]
- 9. Hisatake J, Ishiyama T, Akimoto Y, et al. Autoimmune hemolytic anemia associated with multicentric Castleman's disease with a 28-year history. Rinsho Ketsueki (Jpn J Clin Hematol) 35: 768-773, 1994. [PubMed] [Google Scholar]
- 10. Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112: 3959-3964, 2008. [DOI] [PubMed] [Google Scholar]
- 11. van Rhee F, Voorhees P, Dispenzieri A, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood 132: 2115-2124, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshizaki K. A reference guide for management of Castleman disease. Rinsho Ketsueki (Jpn J Clin Hematol) 58: 97-107, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Barcellini W. New insights in the pathogenesis of autoimmune hemolytic anemia. Transfus Med Hemother 42: 287-293, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamesaki T. Recent progress of diagnosis and treatment for immune-mediated hematological diseases. Topics: III. Diagnosis and treatment; 2. Autoimmune hemolytic anemia. Nihon Naika Gakkai Zasshi (J Jpn Soc Intern Med) 103: 1599-1608, 2014. [DOI] [PubMed] [Google Scholar]
- 15. Xu L, Zhang T, Liu Z, Li Q, Xu Z, Ren T. Critical role of Th17 cells in development of autoimmune hemolytic anemia. Exp Hematol 40: 994-1004, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Kimura A, Kishimoto T. IL-6: Regulator of Treg/Th17 balance. Eur J Immunol 40: 1830-1835, 2010. [DOI] [PubMed] [Google Scholar]
- 17. Kunitomi A, Konaka Y, Yagita M, Nishimoto N, Kishimoto T, Takatsuki K. Humanized anti-interleukin 6 receptor antibody induced long-term remission in a patient with life-threatening refractory autoimmune hemolytic anemia. Int J Hematol 80: 246-249, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Burmester GR, Choy E, Kivitz A, et al. Low immunogenicity of tocilizumab in patients with rheumatoid arthritis. Ann Rheum Dis 76: 1078-1085, 2017. [DOI] [PubMed] [Google Scholar]
- 19. Benucci M, Meacci F, Grossi V, et al. Correlations between immunogenicity, drug levels, and disease activity in an Italian cohort of rheumatoid arthritis patients treated with tocilizumab. Biologics 10: 53-58, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sigaux J, Hamze M, Daien C, et al. Immunogenicity of tocilizumab in patients with rheumatoid arthritis. Joint Bone Spine 84: 39-45, 2017. [DOI] [PubMed] [Google Scholar]