Abstract
Hyperprogressive disease (HPD) is a paradoxical phenomenon involving the acceleration of tumor progression after treatment with immune checkpoint inhibitors (ICIs). A 66-year-old male smoker with advanced lung adenocarcinoma started pembrolizumab for progressive disease following first-line chemotherapy. He developed HPD after two cycles, and a re-biopsy revealed transformation to small-cell carcinoma. He subsequently underwent two lines of chemotherapy for small-cell carcinoma until progression and ultimately died. Transformation to small-cell carcinoma may be a cause of HPD during ICI therapy. The possibility of pathological transformation should be considered in cases of HPD with resistance to ICI therapy.
Keywords: immune checkpoint inhibitors, pembrolizumab, hyperprogressive disease, transformation, small-cell carcinoma
Introduction
Immune checkpoint inhibitors (ICI) that block the programmed death 1 (PD1)/programmed death ligand 1 (PD-L1) pathways are being widely used to successfully treat advanced lung cancers; they are also being increasingly used for other indications (1-3). Immune-related adverse events associated with ICI therapy deserve particular attention (4).
ICI therapy is also known to cause accelerated tumor progression. This paradoxical phenomenon, referred to as hyperprogressive disease (HPD), is defined as a ≥2-fold increase in tumor growth between baseline and the first assessment at 8 weeks, using the response evaluation criteria in solid tumors (RECIST) (5). HPD occurs more commonly than expected and is associated with a poor prognosis (5, 6). Therefore, the early recognition of HPD is important.
Case Report
A 66-year-old man with a 45 pack-year history of smoking was referred to our hospital with suspected advanced lung cancer. Computed tomography (CT) revealed a mass, 35 mm in diameter, in the right apical lung, with enlargement of the mediastinal and right hilar lymph nodes, and multiple metastases in the liver and bones (Fig. 1, 2A). He had a one-month history of back and neck pain and had no remarkable medical history or comorbidities.
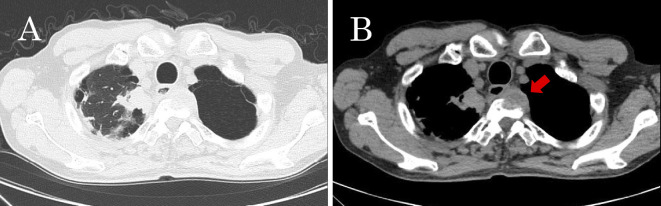
Figure 1.
Computed tomography (CT) of the chest. (A) A mass in the right apical lung, and (B) a metastatic lesion in the body of the third thoracic vertebra (arrow).
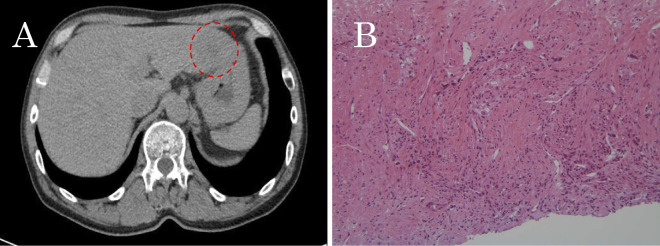
Figure 2.
CT of the abdomen. (A) A low-density lesion in the right lobe of liver (dashed circle). (B) A biopsy specimen showing adenocarcinoma, characterized by glandular formation with necrotic regions (Hematoxylin and Eosin staining).
The levels of carcinoembryonic antigen (CEA), cytokeratin 19 fragment (CYFRA), and pro-gastrin-releasing peptide (pro-GRP) were in the normal range. Since the pulmonary tumor could not be accessed by a bronchoscopic biopsy, an echo-guided needle biopsy was performed from a hepatic lesion, revealing adenocarcinoma (Fig. 2B). The specimen showed indeterminate epidermal growth factor receptor (EGFR) mutations with no anaplastic lymphoma kinase (ALK) translocations. He was diagnosed with clinical stage IVB (cT2aN2M1c) adenocarcinoma of the lung.
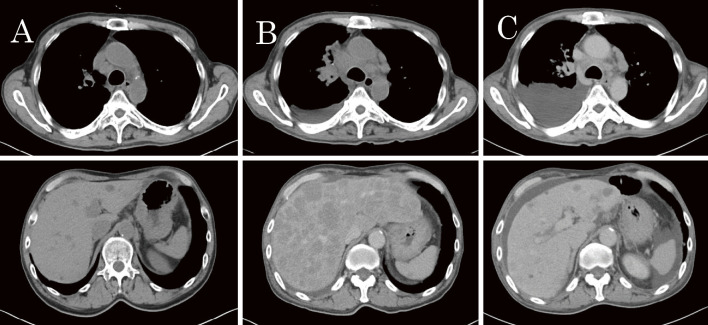
Combination first-line chemotherapy with carboplatin (CBDCA), pemetrexed (PEM), and bevacizumab (BEV) was initiated in December 2016. After four courses of therapy and two courses of maintenance therapy with PEM and BEV, the thoracic lesions progressed. Owing to a high tumor proportion score (TPS) of programmed death-ligand 1 (PD-L1) (90-100%), pembrolizumab was initiated as second-line therapy in May 2017. At that time, his general condition corresponded to Eastern Cooperative Oncology Group performance status (ECOG-PS) 1. Approximately five weeks after starting pembrolizumab (after the administration of two cycles), he visited our hospital complaining of fatigue and loss of appetite. During that period, his ECOG-PS score had also rapidly deteriorated to 3. CT was performed, revealing enlargement of the mediastinal and right hilar lymph nodes, right-sided pleural effusion, and numerous new hepatic lesions (Fig. 3B). This progression was in accordance with the definition of HPD.
Figure 3.
A series of CT images presented in the order of the clinical course. (A) Progressive disease observed during treatment with the first regimen. (B) Enlarged mediastinal lymph nodes and numerous hepatic lesions, which emerged after five weeks of pembrolizumab treatment. (C) Moderate shrinkage of thoracic lesions and considerable improvement of hepatic lesions after one month of chemotherapy for small-cell carcinoma.
A cytological examination for pleural effusion was performed, and the cell block of the fluid revealed small-cell carcinoma with positive immunohistochemical staining for CD56 and cytokeratin (AE1/AE3) and negative staining for chromogranin A and synaptophysin (Fig. 4). Serum neuron-specific enolase (NSE) was found to be extremely high (364.6 ng/mL), while pro-GRP remained within the normal range. We suspected that the adenocarcinoma had transformed to small-cell carcinoma. Third-line therapy was initiated in June 2017 with a combination of CBDCA and etoposide (VP16). After the therapy, his ECOG-PS score temporarily recovered to 1. CT performed four weeks after starting the therapy showed moderate shrinkage of thoracic lesions and considerable improvement of hepatic metastases; however, right pleural effusion had increased, ascites had developed due to hypoalbuminemia and hydration (Fig. 3C), and NSE levels had decreased to 82.5 ng/mL. Following three courses of CBDCA and VP16 until disease progression, he received three courses of amrubicin as fourth-line therapy. His general condition gradually worsened until his death in November 2017.
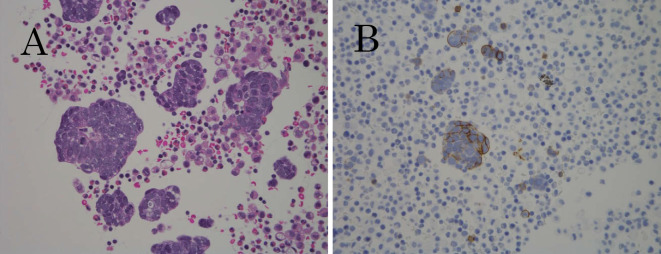
Figure 4.
Cell block from pleural effusion in the right thorax. (A) Small-cell carcinoma, characterized by tumor cells with high nucleo-cytoplasmic ratios (Hematoxylin and Eosin staining), and (B) tumor cell positivity for CD56 on immunohistochemical staining.
Discussion
This report describes our experience with the transformation of metastatic lung adenocarcinoma to small-cell carcinoma in a patient undergoing therapy with pembrolizumab. After a short period of ICI therapy, he presented with features of HPD; a re-biopsy revealed histopathologic transformation to small-cell carcinoma. The findings on a pathological examination and imaging during the initial diagnosis were concordant with those of metastatic adenocarcinoma of the lung. Furthermore, the clinical response after chemotherapy with the combination of CBDCA, PEM, and BEV was also compatible with that of lung adenocarcinomas. After the development of HPD, the pathological findings from the cell block obtained from the pleural fluid were indicative of small-cell carcinoma, both morphologically and immunohistologically. In addition, the good response to chemotherapy with CBDCA and VP16 seemed to be typical of small-cell carcinoma.
Therapy with EGFR-tyrosine kinase inhibitors (TKIs) occasionally transforms lung adenocarcinomas to small-cell carcinomas. The transformation to small-cell carcinoma is one of the known mechanisms of acquired resistance to EGFR-TKIs, accounting for 3-14% of resistant cases (7, 8). Although the mechanism has not been identified, one of the possible explanations is that as the number of tumor cells sensitive to treatment decline, small-cell carcinoma present in the initial tumor becomes dominant by selective pressure (9). Recent studies revealed that transformation to small-cell carcinoma is associated with inactivation of Rb1 and p53, which may derive small-cell carcinoma clones from adenocarcinoma at a very early stage, owing to divergent evolutionary processes (10). Transformation to small-cell carcinoma has already been reported in patients treated with ICI for adenocarcinomas of the lung (11, 12). The mechanisms are believed to be similar to those of patients treated with EGFR-TKIs. These cases usually receive treatment with several courses of ICI, which may lead to translocation. In our patient, rapid tumor progression and spread of hepatic metastases were observed after only two courses of ICI, despite a high TPS of PD-L1 in the initial adenocarcinoma. We suspected that small-cell carcinoma had already been dominant before ICI therapy in the tumor components insensitive to prior chemotherapy. We should consider the possibility that the original tumor was a combined small-cell carcinoma with adenocarcinoma and that a needle biopsy at the initial diagnosis failed to detect this.
Interestingly, HPD during ICI therapy is known to be a relatively common phenomenon, with reported incidence rates of 8-14% in non-small cell lung cancer (NSCLC) (6, 13). HPD has been reported to be associated with certain independent factors, including older age (>65 years of age) (5) and a higher number of metastatic lesions at baseline (>2) (6). The tumor status of PD-L1 expression has not been reported to be associated with the occurrence of HPD (5, 6). Although the mechanisms underlying the development of HPD after PD-1/PD-L1 blockage have not been fully elucidated, some immunological explanations are proposed, including the expansion of PD-1+ regulatory T cells, exhaustion of compensatory T cells, modulation of pro-tumorigenic immune cell subsets, activation of aberrant inflammation, and activation of oncogenic signaling (14). Our patient also developed HPD after two courses of pembrolizumab. After prior chemotherapy eliminated the chemo-sensitive tumor cells, residual insensitive tumor, including small-cell carcinoma, might have rapidly progressed because of these mechanisms, which worked to accelerate tumor growth.
In conclusion, this report describes the development of HPD in a case of adenocarcinoma of the lung with transformation to small-cell carcinoma during pembrolizumab therapy. This demonstrates that one of the causes of HPD during ICI can be transformation to small-cell carcinoma. Further investigations will be needed to elucidate the mechanisms underlying the development of HPD and tumor transformation. The possibility of pathological transformation should be considered in all cases of HPD with resistance to ICI therapy.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. New Engl J Med 373: 1627-1639, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377: 1919-1929, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379: 2040-2051, 2018. [DOI] [PubMed] [Google Scholar]
- 4. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158-168, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23: 1920-1928, 2017. [DOI] [PubMed] [Google Scholar]
- 6. Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol 4: 1543-1552, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 19: 2240-2247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3: 75ra26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang SY, Zhao J, Wang MZ, et al. Small-cell lung cancer transformation in patients with pulmonary adenocarcinoma: a case report and review of the literature. Medicine 95: e2752, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JK, Lee J, Kim S, et al. Clonal history and genetic predictors of transformation into small-cell Carcinomas from lung adenocarcinomas. J Clin Oncol 35: 3065-3074, 2017. [DOI] [PubMed] [Google Scholar]
- 11. Imakita T, Fujita K, Kanai O, et al. Small cell lung cancer transformation during immunotherapy with nivolumab: a case report. Respir Med Case Rep 21: 52-55, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abdallah N, Nagasaka M, Abdulfatah E. Non-small cell to small cell lung cancer on PD-1 inhibitors: two cases on potential histologic transformation. Lung Cancer (Auckl) 9: 85-90, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kato S, Goodman A, Walavalkar V, et al. Hyper-progressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 23: 4242-4250, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Champiat S, Ferrara R, Massard C, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol 15: 748-762, 2018. [DOI] [PubMed] [Google Scholar]