Abstract
A 71-year-old woman with abnormal pulmonary shadows and multiple enlarged thoracic lymph nodes was diagnosed with stage IIB lung adenocarcinoma, pulmonary sarcoidosis, and sarcoidosis-associated lymphadenopathy after biopsies from multiple organ sites. She also had rapidly progressive renal dysfunction, microhematuria, and high myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA) concentrations. A renal biopsy revealed granulomatous tubulointerstitial nephritis and necrotizing glomerulonephritis with crescent formation. She was diagnosed with nephritis caused by both sarcoidosis and ANCA-associated vasculitis. Oral prednisolone was administered to treat her nephritis, resulting in improvement in both her renal dysfunction and her sarcoidosis-associated lymphadenopathy.
Keywords: adenocarcinoma, anti-neutrophil cytoplasmic antibody-associated vasculitis, crescentic glomerulonephritis, lung cancer, sarcoidosis
Introduction
Sarcoidosis is a multisystem granulomatous disease that involves the lungs, eyes, skin, heart and, in rare cases, kidneys. Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), a systemic vasculitis, is characterized by the destruction and inflammation of small vessels in various organs. AAV frequently involves the kidneys, lungs, and skin. These two systemic inflammatory diseases are sometimes associated with cancer (1-5) and can affect the diagnosis and treatment of the latter.
We herein report a case of the simultaneous occurrence of sarcoidosis and AAV in a patient with lung adenocarcinoma.
Case Report
A 71-year-old woman visited our hospital for the evaluation of an abnormal shadow in the left upper lobe of the lung. She was asymptomatic and in good health with no history of smoking or dust inhalation and no appreciable family history. She received celecoxib for spinal canal stenosis and irbesartan and amlodipine for hypertension but had no history of renal disease. She had normal vital signs and normal physical findings.
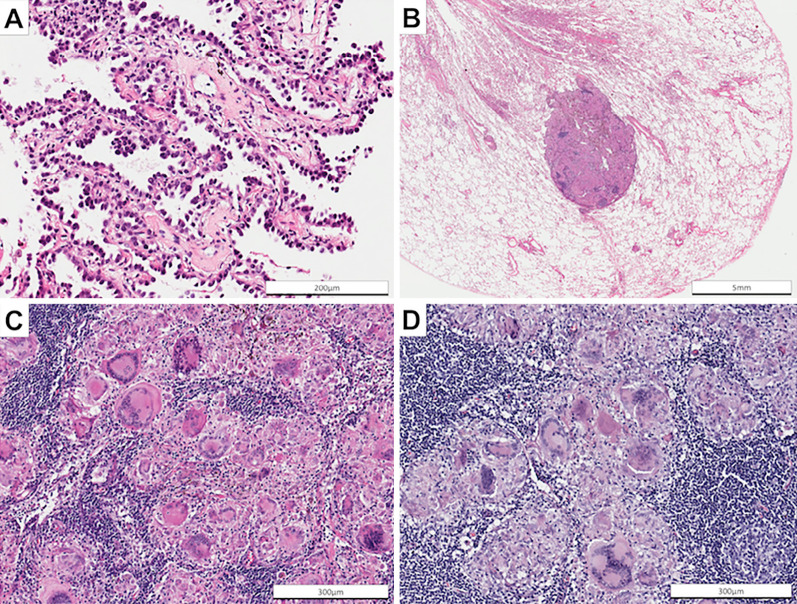
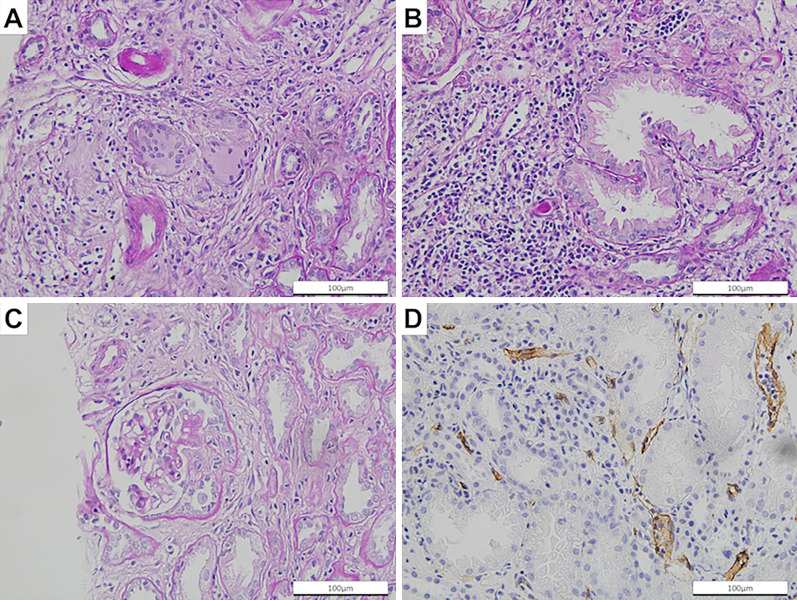
Chest computed tomography (CT) showed a crescent-shaped shadow with a maximum diameter of 49 mm in the left upper lobe of the lung and a 7-mm diameter nodule in the left lower lobe (Fig. 1A and B). Positron emission tomography (PET)-CT showed the increased accumulation of fluorodeoxyglucose (FDG) in the hilar and mediastinal lymph nodes bilaterally, as well as in the crescent-shaped lesion in the left upper lobe (Fig. 1C and D). No extra-thoracic lesions were detected. She had anemia, hypercalcemia, renal dysfunction, proteinuria, microhematuria, and slightly increased serum concentrations of carcinoembryonic antigen and sialyl Lewis X-i. (Table). A further investigation revealed high serum levels of myeloperoxidase-ANCA (MPO-ANCA) and soluble interloikin-2 receptor (sIL-2R). A transbronchial biopsy of the crescent-shaped lesion in the left upper lobe revealed adenocarcinoma (Fig. 2A). An endobronchial ultrasound-guided transbronchial needle biopsy of the mediastinal lymph nodes was not performed because it was unavailable in our hospital at that time. Thoracoscopic biopsies of both the left hilar lymph nodes and the small nodule in the left lower lobe of the lung showed noncaseating granuloma without cancer cells (Fig. 2B-D). A renal biopsy revealed granulomatous interstitial nephritis (GIN) and necrotizing glomerulonephritis with 40% formation of crescents (Fig. 3). She was therefore diagnosed with stage IIB lung adenocarcinoma, pulmonary sarcoidosis, sarcoidosis-associated lymphadenopathies, and nephritis caused by both sarcoidosis and AAV.
Figure 1.
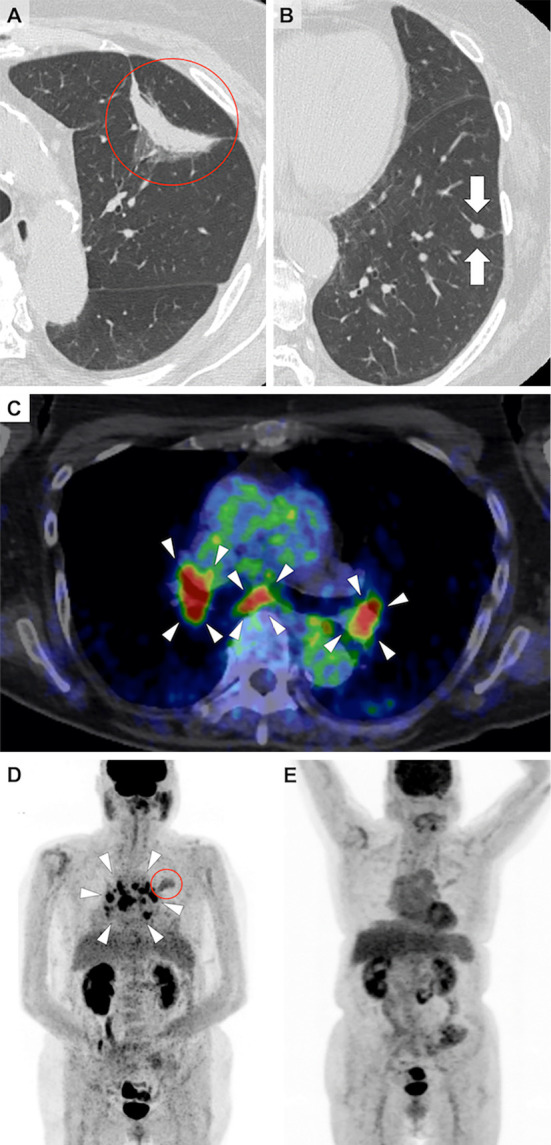
Chest computed tomography (CT) showing a crescent-shaped shadow in the left upper lobe (A, circle) and a small nodule in the left lower lobe of the lung (B, arrows). Positron emission tomography-CT image demonstrating accumulation of fluorodeoxyglucose (FDG) in the crescent-shaped shadow in the left upper lobe (circle) and bilateral enlarged hilar and mediastinal lymph nodes (arrowheads) (C, D). After steroid therapy, FDG no longer accumulated in the hilar and mediastinal lymph nodes (E).
Table.
Laboratory Findings on Admission.
| WBC | 4,550 | /μL | CRP | 0.20 | mg/dL |
| Neutrophil | 74.0 | % | C3 | 97 | mg/dL |
| Lymphocyte | 17.6 | % | C4 | 38 | mg/dL |
| Monocyte | 5.9 | % | CH50 | >60 | U/mL |
| Basophil | 0.7 | % | Anti-nuclear antibody | <1:40 | |
| Eosinophil | 1.8 | % | MPO-ANCA (<3.5 U/mL) | 267 | U/mL |
| Hemoglobin | 9.9 | g/dL | PR3-ANCA (<1.0 U/mL) | <1.0 | U/mL |
| PLT | 26.3×104/ | μL | Anti-GBM antibody | <2.0 | U/mL |
| Albumin | 3.5 | g/dL | CEA | 6.8 | ng/mL |
| AST | 12 | IU/L | SLX | 46 | U/mL |
| ALT | 4 | IU/L | sIL-2R | 2,197 | U/mL |
| LDH | 167 | IU/L | ACE (8.3-21.4 U/L) | 14.0 | U/L |
| BUN | 33.4 | mg/dL | Urinary protein | 3+ | |
| Creatinine | 1.49 | mg/dL | Urinary occult blood | 3+ | |
| eGFR | 27 | mL/min/1.73m2 | Urinary sugar | - | |
| Na | 136 | mEq/L | Urinary β2-microglobulin | 40,697 | μg/L |
| K | 2.5 | mEq/L | Urinary α1-microglobulin | 73.0 | mg/L |
| Cl | 99 | mEq/L | Urinary Ca | 9.5 | mg/dL |
| P | 2.7 | mg/dL | |||
| Ca | 12.0 | mg/dL |
WBC: white blood cells, PLT: platelet, Alb: albumin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, BUN: blood urea nitrogen, eGFR: estimate glomerular filtration rate, CRP: C-reactive protein, antibody MPO-ANCA: myeloperoxidase anti-neutrophil cytoplasmic antibody, PR3-ANCA: proteinase3 antineutrophil cytoplasmic antibody, GBM: glomerular basement membrane, CEA: carcinoembryonic antigen, SLX: sialyl Lewis X-i antigen, sIL-2R: soluble Interloikin-2 receptor, ACE: angiotensin-converting enzyme
Figure 2.
Representative photomicrograph of a biopsy of the crescent-shaped shadow in the left upper lobe revealing invasive adenocarcinoma with lepidic growth [A, Hematoxylin and Eosin (H&E) staining, ×400]. Representative photomicrograph of a biopsy of the nodule in the left lower lobe of the lung showing noncaseating granuloma (B, H&E staining, ×10, C: ×400). Representative photomicrograph of a biopsy of the hilar lymph nodes also showing noncaseating granuloma (D: H&E staining, ×200).
Figure 3.
Representative photomicrograph of a renal biopsy revealing noncaseating epithelioid cell granuloma (A), tubulitis (B), cellular crescent formation (C), and peritubular capillaritis (D) (A, B, and C, Periodic acid-Schiff stain, ×400; and D, CD34 stain, ×400).
There were no ophthalmologic, dermatologic, or cardiologic findings (on echocardiogram or cardiac magnetic resonance imaging) associated with sarcoidosis. The severity of sarcoidosis was grade III because of the need for corticosteroid treatment and involvement of two organs (lung and kidney) according to the definition of Japanese Society of Sarcoidosis and other Granulomatous Disorders (6). Her AAV was renal-limited disease, and her Birmingham vasculitis activity score was 12 because of hematuria, proteinuria, a serum creatinine level 1.49 mg/dL (1.41-2.82 mg/dL), and a more than 30% increase in her serum creatinine level (maximum total score of 63) (7).
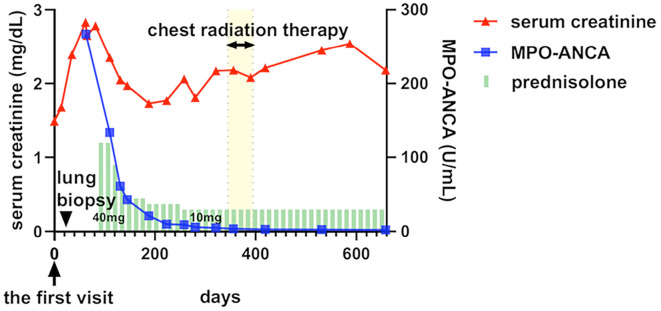
Her renal function had deteriorated by 8 weeks after the initial visit [serum creatinine 2.83 mg/dL and estimated glomerular filtration rate (eGFR) 13 mL/min/1.73 m2]. Oral prednisolone at 40 mg/day was administered to treat her nephritis, and her renal function improved (serum creatinine 1.73 mg/dL and eGFR 23 mL/min/1.73 m2 16 weeks after initiating steroid treatment). After six months of oral prednisolone therapy, the level of MPO-ANCA became undetectable. During the same period, the mediastinal and hilar lymphadenopathy were also reduced (Fig. 1E). In parallel with the steroid therapy, radical radiation therapy was administered to the lung cancer (total of 70 Gy in 35 fractions). A radical operation was avoided because of the risk of postoperative complications due to steroid therapy and the patient's preference. The prednisolone dose was gradually decreased to 10 mg/day over 6 months; there was no subsequent exacerbation of her renal dysfunction, lymphadenopathy, or the pulmonary nodule caused by sarcoidosis (Fig. 4).
Figure 4.
Clinical course of the patient. After oral prednisolone therapy, myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA) became undetectable, and the renal function was partially improved. Serum creatinine, MPO-ANCA, chest radiation therapy, and prednisolone therapy are shown in red lines, blue lines, yellow bars, and green bars, respectively.
Discussion
We herein report a patient with simultaneous sarcoidosis, AAV, and lung adenocarcinoma. The pulmonary nodule and thoracic lymphadenopathy caused by sarcoidosis, which radiologically mimicked lung cancer metastases, were correctly diagnosed by a thoracoscopic biopsy. The nephritis was considered to have been caused by both sarcoidosis and AAV and improved to a degree with oral prednisolone. Because sarcoidosis and AAV occasionally occur in association with cancer and influence the diagnosis and treatment of that cancer, their accurate diagnosis and appropriate management are crucial.
Vasculitis is known to be associated with malignant tumors; about 5% of cases of systemic vasculitis are associated with cancer (8). Among cases of cancer-associated vasculitis, hematologic cancers are the most common in all cancers (63.1-77.5%), and lung cancer is the most common in solid tumors (5-7.7%) (9,10). The most common types of vasculitis associated with malignant tumors are leukocytoclastic vasculitis (45%) and polyarteritis nodosa (36.7%), with AAV being the third-most common (11.7%) (10). It has been reported that 3.3-17.1% of AAV cases occur in association with cancer (4,11,12). Leukemia and bladder cancer are the most common malignancies associated with AAV [standardized incidence rate (SIR), 4.9-5.7 and 3.8-4.8, respectively] (5,13). Lung cancer is also known to be associated with AAV (SIR of 1.67) (5). It is considered that the dysfunctional immune system associated with vasculitis may increase the risk of cancer. In addition, immunosuppressive agents administered for vasculitis, such as cyclophosphamide, may also increase the risk of cancer (11). Vasculitis sometimes occurs after a cancer has developed; it is then considered a paraneoplastic syndrome (14-17). Although the precise mechanisms are unknown, cancer cells may have cross-reactive antigens to vascular endothelium; alternatively, cancer cells may invade vessel walls and damage the vascular endothelium, triggering an immunologic response against blood vessels (15).
Sarcoidosis is known to increase the risk of cancer (18). Although reports are conflicting (19-23), several have suggested an association between sarcoidosis and lung cancer (24-28). The immunologic abnormalities in sarcoidosis may result in attenuation of immune reactions for tumors or oncogenic viruses. Alternatively, chronic inflammation associated with sarcoidosis may lead to development of cancer (2,20,29).
It is also important to consider “cancer-associated sarcoid reactions” if sarcoidosis simultaneously occurs with cancer. Sarcoid reactions are defined as noncaseating granulomas in patients who do not meet the criteria for having systemic sarcoidosis. Cancer-associated sarcoid reactions are postulated to be caused by induced T-cell-mediated host responses to antigenic tumor factors. Sarcoid reactions occur in 4.4% of patients with cancer, most commonly in lymph nodes draining the tumor (29). The current patient had involvement of multiple organs, including the lymph nodes, lung, and kidney, and was therefore diagnosed with sarcoidosis rather than cancer-associated sarcoid reactions. In patients with cancer, sarcoid reactions cannot be distinguished from tumor lesions by diagnostic imaging techniques, including FDG-PET (30). In the present patient, the high sIL-2R concentration was suggestive of sarcoidosis, and this diagnosis was confirmed by an examination of biopsies from both the pulmonary lesion and lymph nodes. In addition, the subsequent steroid therapy for her nephritis collaterally diminished the lymphadenopathy, which is consistent with the diagnosis of sarcoidosis.
Both sarcoidosis and AAV are considered to be associated with autoimmune mechanisms against intrinsic or extrinsic antigens (31,32). In the present case, there may have been some overlapping immune responses for tumor antigens and/or antigens provoked by tumors.
We were unable to determine the relative contributions of AAV and sarcoidosis to our patient's nephritis. There were a variety of pathological findings. The crescent formation in her glomeruli and pauci-immune deposition may have been caused by AAV. GIN is a common form of renal sarcoidosis (33). Although GIN sometimes develops in AAV, our patient had severe sarcoidosis with involvement of multiple organs other than the kidneys. It is therefore possible that sarcoidosis contributed to the development of her nephritis.
Sarcoidosis and vasculitis were recently reported to occur after immune checkpoint inhibitor therapy (34,35). Immune-related diseases, including sarcoidosis and vasculitis, may become increasingly recognized as immune-related adverse events associated with cancer therapy, in addition to being risk factors for cancer and paraneoplastic syndromes.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Dr Trish Reynolds, MBBS, FRACP for editing a draft of this manuscript.
References
- 1.Brinker H. Sarcoidosis and malignancy. Chest 108: 1472-1474, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Boffetta P, Rabkin CS, Gridley G. A cohort study of cancer among sarcoidosis patients. Int J Cancer 124: 2697-2700, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi N, Nakamura R, Kurishima K, Sato Y, Satoh H. Sarcoidosis and lung cancer. Acta Medica (Hradec Kralove) 53: 115-118, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Pankhurst T, Savage COS, Gordon C, Harper L. Malignancy is increased in ANCA-associated vasculitis. Rheumatology 43: 1532-1535, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Shang W, Ning Y, Xu X, et al. Incidence of cancer in ANCA-associated vasculitis: a meta-analysis of observational studies. PLoS One 10: e0126016, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clinical practice guideline on sarcoidosis Japan Soc Sarcoidosis other Granulomatous Disord 2019 http://www.jssog.com/www/top/kenkai20118.html 2019424(in Japanese)
- 7.Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham vasculitis activity score (version 3). Ann Rheum Dis 68: 1827-1832, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Guerrero J, Gutiérrez-Ureña S, Vidaller A, Reyes E, Iglesias A, Alarcón-Segovia D. Vasculitis as a paraneoplastic syndrome. Report of 11 cases and review of the literature. J Rheumatol 17: 1458-1462, 1990. [PubMed] [Google Scholar]
- 9.Kurzrock R, Cohen PR. Vasculitis and cancer. Clin Dermatol 11: 175-187, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Fain O, Hamidou M, Cacoub P, et al. Vasculitides associated with malignancies: Analysis of sixty patients. Arthritis Care Res 57: 1473-1480, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Kermani TA, Warrington KJ, Amin S. Malignancy risk in vasculitis. Ther Adv Musculoskelet Dis 3: 55-63, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hommel C, Rihova Z, Mokaddem F, Libotte B. pANCA-vasculitis associated with rectal adenocarcinoma. Acta Clin Belg 69: 463-466, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Mahr A, Heijl C, Le Guenno G, Faurschou M. ANCA-associated vasculitis and malignancy: current evidence for cause and consequence relationships. Best Pract Res Clin Rheumatol 27: 45-56, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Navarro JF, Quereda C, Rivera M, Navarro FJ, Ortunfo J. Anti-neutrophil cytoplasmic antibody-associated paraneoplastic vasculitis. Postgr Med J 70: 373-375, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Díez-Porres L, Ríos-Blanco JJ, Robles-Marhuenda A, Gutiérrez-Molina M, Gil-Aguado A, Vázquez-Rodríguez JJ. ANCA-associated vasculitis as paraneoplastic syndrome with colon cancer: a case report. Lupus 14: 632-634, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Rivera M, González C, Gonzalo A, Quereda C, Fogué L, Ortuño J. Vasculitis associated with non-hodgkin's lymphoma. Nephron 65: 167-168, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Hamidou MA, El Kouri D, Audrain M, Grolleau J-Y. Systemic antineutrophil cytoplasmic antibody vasculitis associated with lymphoid neoplasia. Ann Rheum Dis 60: 293-295, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonifazi M, Bravi F, Gasparini S, et al. Sarcoidosis and cancer risk: systematic review and meta-analysis of observational studies. Chest 147: 778-791, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Seersholm N, Vestbo J, Viskum K. Risk of malignant neoplasms in patients with pulmonary sarcoidosis. Thorax 52: 892-894, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer 29: 247-251, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rømer FK, Hommelgaard P, Schou G. Sarcoidosis and cancer revisited: a long-term follow-up study of 555 Danish sarcoidosis patients. Eur Respir J 12: 906-912, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Askling J, Grunewald J, Eklund A, Hillerdal G, Ekbom A. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med 160: 1668-1672, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi M, Odaka M, Hosoda Y, Iwai K, Tachibana T. Excess death of lung cancer among sarcoidosis patients. Sarcoidosis 8: 51-55, 1991. [PubMed] [Google Scholar]
- 24.Kumar S, Baghdadi S, Cale A.R.J. Concurrence of sarcoidosis and lung cancer: a diagnostic dilemma. Thorax 61: 1100, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeill M, Zanders TB, Morris MJ. A 49-year-old man with concurrent diagnoses of lung cancer, sarcoidosis, and multiple regions of adenopathy on positron emission tomography. Chest 135: 546-549, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Tokuyasu H, Izumi H, Mukai N, et al. Small cell lung cancer complicated by pulmonary sarcoidosis. Intern Med 49: 1997-2001, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Kachalia AG, Ochieng P, Kachalia K, Rahman H. Rare coexistence of sarcoidosis and lung adenocarcinoma. Respir Med Case Reports 12: 4-6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iijima Y, Sugiyama Y, Sawahata M, Nakayama M, Bando M. Clinical features of pulmonary sarcoidosis complicated by lung cancer. Intern Med 56: 1957-1960, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanimoto Y, Kataoka M. Sarcoidosis and malignancy. Jpn J Chest Dis 72: 838-845, 2013. [Google Scholar]
- 30.Koo HJ, Kim MY, Shin SY, et al. Evaluation of mediastinal lymph nodes in sarcoidosis, sarcoid reaction, and malignant lymph nodes using CT and FDG-PET/CT. Medicine (Baltimore) 94: e1095, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet 383: 1155-1167, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Chen M, Kallenberg CGM. ANCA-associated vasculitides-advances in pathogenesis and treatment. Nat Rev Rheumatol 6: 653-664, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Khedher A, Mahfoudhi M, Barbouche S, et al. Interstitial and glomerular renal involvement in sarcoidosis. Eur J Intern Med 24: e186, 2013. [Google Scholar]
- 34.Tfayli A, Fakhri G, Akel R, Salem Z, Tawil A. Pulmonary sarcoidosis activation following neoadjuvant pembrolizumab plus chemotherapy combination therapy in a patient with non-small cell lung cancer: a case report. Case Rep Oncol 10: 1070-1075, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol 37: 2579-2584, 2018. [DOI] [PubMed] [Google Scholar]