Abstract
Disturbance of the normal gut microbiota has been implicated in the pathogenesis of various diseases, including chronic kidney disease (CKD). A common CKD symptom is chronic constipation. Lubiprostone is a safe and efficacious drug for treating chronic constipation. We herein report 2 patients with IgA nephropathy treated with lubiprostone (24 μg 1×/day). The lubiprostone treatment ameliorated their chronic constipation and, unexpectedly, reduced the urinary protein excretion, urinary liver-type fatty acid binding protein and urine occult blood. These results may indicate that lubiprostone is a useful therapeutic intervention against the progression of IgA nephropathy with chronic constipation.
Keywords: constipation, lubiprostone, chronic kidney disease, urinary protein excretion, gut microbiota
Introduction
Chronic constipation is commonly observed in patients with chronic kidney disease (CKD) and is considered an important component of the medical management for CKD (1). It is also important to regulate the gastrointestinal function and symptoms of CKD patients. The gut microbiota has coevolved with humans for a mutually beneficial coexistence, and it plays an important role in health and disease (2). Quantitative and qualitative alterations in the gut microbiota are noted in CKD patients (2). Chronic constipation worsens the intestinal environment and alters the composition of the gut microbiota. However, the mechanistic link between the gut and kidney diseases is poorly understood.
Lubiprostone is an activator of the type 2 chloride channel that facilitates spontaneous bowel movement (3). It has been shown to increase the weekly average number of spontaneous bowel movements and to enhance the quality of life in patients with chronic constipation (3). Mishima et al. (4) reported that lubiprostone ameliorated the progression of CKD and the accumulation of uremic toxins by improving the gut microbiota and intestinal environment in an adenine-induced CKD model. However, the clinical effects of lubiprostone on the renal function in patients with CKD have not been established.
We herein report that lubiprostone ameliorated the urinary protein excretion and renal dysfunction in two patients with IgA nephropathy.
Case Reports
Case 1
A 32-year-old Japanese woman was referred to us due to proteinuria and chronic constipation. An examination revealed the following: body height 156 cm, weight 54 kg, blood pressure (BP) 122/70 mmHg, and heart rate 76 beats/min. Chest and abdominal X-rays showed no abnormality. Laboratory data demonstrated a normal renal function [serum creatinine 0.66 mg/dL, blood urea nitrogen (BUN) 24.2 mg/dL, estimated glomerular filtration ratio (eGFR) 85.4 mL/min/1.73 m2] but also revealed proteinuria (first voided morning urine) of 66.2 mg/dL, tubular dysfunction [urinary liver-type fatty acid-acid protein (L-FABP)] of 22.6 μg/g.crea (normal range <7.4 μg/g.crea), and positive urine occult blood (+++).
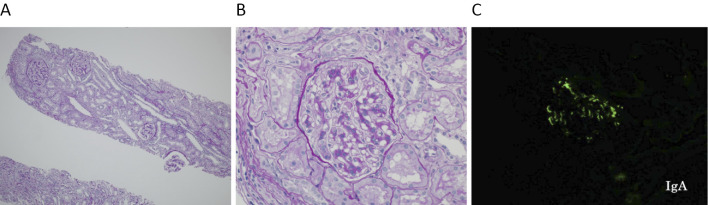
The lipid profiles and proteinemia were within the normal ranges [low-density lipoprotein (LDL)-cholesterol 110 mg/dL, triglyceride (TG) 66 mg/dL, high-density lipoprotein (HDL)-cholesterol 76 mg/dL, total protein 7.1 g/dL, and albumin 4.2 g/dL]. Serologies were negative for antinuclear antibody, anti-basement membrane (GBM) antibody, myeloperoxidase anti-neutrophil cytoplasmic (MPO-ANCA), proteinase 3-ANCA, anti-hepatitis C antibody, and hepatitis B surface antigen. The patient's serum IgA level was high at 488 mg/dL (normal range 110-400 mg/dL). However, the serum complement, IgG, and IgM values were within normal limits. On the third day post-admission, a renal biopsy was performed. The biopsy section contained 16 glomeruli: 8 normal glomeruli and 8 showing slight mesangial hypercellularity and mesangial matrix expansion (H-grade I, Fig. 1A, B). The sample showed IgA (+) and C3 (+) in all glomeruli (Fig. 1C).
Figure 1.
A: PAS staining ×40. A low-power magnification showing the extent of tubulointerstitial injury. B: PAS staining ×400. A glomerulus showing hypercellularity and mesangial expansion. C: Immunofluorescence ×400. IgA was positive in the glomerulus.
Slight interstitial fibrosis and tubular atrophy were also observed. The patient had chronic constipation (frequency of stool: 1×/5 days), and lubiprostone (24 μg 1×/day) was therefore administered. No other medications were administered in this case. Table 1 shows the changes in the patient's renal parameters. After 1 month, the frequency of stools was improved to 1×/day and continued beyond 12 months. Her body weight, blood pressure, and serum levels of creatinine showed no change for 12 months. Interestingly, the patient's urinary protein excretion and L-FABP showed a gradual decline for 12 months. The urine occult blood and numbers of red blood cells were also gradually attenuated through and after 12 months.
Table 1.
Changes in the Clinical Parameters in Case 1.
| Before | After 1 month | After 3 months | After 6 months | After 12 months | |
|---|---|---|---|---|---|
| Frequency of stools | 1×/5 days | 1×/day | 1×/day | 1×/day | 1×/day |
| Body weight, kg | 54.0 | 54.0 | 53.8 | 53.8 | 53.6 |
| Blood pressure, mm Hg | 122/70 | 122/72 | 120/72 | 122/74 | 120/72 |
| Creatinine, mg/dL | 0.66 | 0.65 | 0.66 | 0.65 | 0.65 |
| Blood urea nitrogen, mg/dL | 24.2 | 22.8 | 23.2 | 22.8 | 23.2 |
| Urinary protein excretion, mg/g.crea | 110.8 | 92.2 | 66.8 | 44.8 | 25.8 |
| Urinary liver-type fatty acid binding protein, µg/g.crea | 22.6 | 22.0 | 18.8 | 17.6 | 14.8 |
| Urine occult blood | (+++) | (++) | (++) | (+) | (±) |
| Urine red blood cell/high-power field | Many | Many | 10 to 25 | 1 to 5 | 1 to 5 |
Case 2
A 44-year-old Japanese woman was referred to us due to renal dysfunction, severe proteinuria, and chronic constipation. Three years earlier, proteinuria (morning voided urine 120 mg/dL) had been detected at another clinic, but renal dysfunction had not been observed (serum creatinine 0.82 mg/dL). No treatment had been administered for three years. Our examination revealed the following: body height 160 cm, weight 56 kg, BP 142/82 mmHg, and heart rate 70 beats/min. Chest and abdominal X-ray showed no abnormalities. Laboratory data revealed renal dysfunction (serum creatinine 1.88 mg/dL, BUN 23.8 mg/dL, eGFR 24.2 mL/min/1.73 m2), proteinuria (first voided morning urine) of 280.8 mg/dL, tubular dysfunction (L-FABP 88.2 μg/g.crea), normal lipid profiles (LDL-C 124 mg/dL, TG 128 mg/dL, HDL-C 70 mg/dL), and normal proteinemia (total protein 7.2 g/dL, albumin 4.0 g/dL).
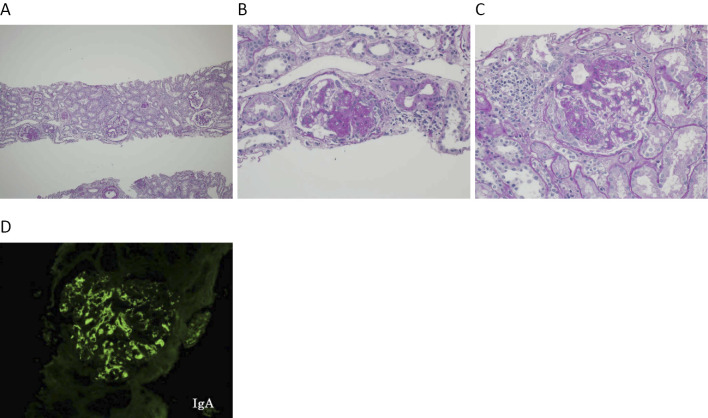
Her serologies were negative for antinuclear antibody, anti-GBM antibody, MPO-ANCA, proteinase 3-ANCA, anti-hepatitis C antibody, and hepatitis B surface antigen. The patient's serum IgA level was high (498 mg/dL), but serum complements, IgG, and IgM were within normal limits. On the third day post-admission, a renal biopsy was performed. The biopsy section contained 20 glomeruli: 5 glomeruli with global sclerosis, 4 with segmental sclerosis, 1 with crescent formation, and 10 with mesangial hypercellularity and matrix expansion (H-grade II, Fig. 2A-C). The sample showed IgA (+) and C3 (+) in all glomeruli (Fig. 2D). Interstitial fibrosis and tubular atrophy were also observed.
Figure 2.
A: PAS staining ×40. A low-power magnification showing the extent of tubulointerstitial injury. B: PAS staining ×400. A glomerulus showing hypercellularity and mesangial expansion. C: PAS staining ×400. A glomerulus showing crescent formation. D: Immunofluorescence ×400. IgA was strongly positive in the glomerulus.
The patient refused drugs other than those for constipation. Therefore, lubiprostone (24 μg 1×/day) was administered. Before the treatment, the frequency of stools was 1×/5 days, but it increased to 1×/2 days after 1 month, and to 1×/day at 3, 6 and 12 months. Her body weight and blood pressure showed no apparent change for 12 months. The serum levels of creatinine, proteinuria, urinary L-FABP, and urine occult blood and red blood cells gradually decreased after treatment (Table 2).
Table 2.
Changes in the Clinical Parameters in Case 2.
| Before | After 1 month | After 3 months | After 6 months | After 12 months | |
|---|---|---|---|---|---|
| Frequency of stools | 1×/5 days | 1×/2 days | 1×/day | 1×/day | 1×/day |
| Body weight, kg | 56.0 | 55.8 | 55.8 | 55.8 | 55.7 |
| Blood pressure, mm Hg | 142/82 | 142/80 | 140/78 | 140/80 | 140/80 |
| Creatinine, mg/dL | 1.88 | 1.86 | 1.72 | 1.66 | 1.54 |
| Blood urea nitrogen, mg/dL | 23.8 | 23.2 | 23.0 | 23.2 | 23.0 |
| Urinary protein excretion, mg/g.crea | 312.8 | 278.8 | 228.6 | 188.8 | 112.6 |
| Urinary liver-type fatty acid binding protein, µg/g.crea | 88.2 | 80.6 | 66.2 | 62.8 | 34.4 |
| Urine occult blood | (++++) | (++++) | (+++) | (+++) | (++) |
| Urine red blood cell/high-power field | Many | Many | 10 to 25 | 10 to 25 | 1 to 5 |
Discussion
In our two IgA nephropathy patients with chronic constipation, lubiprostone reduced proteinuria, the tubular injury marker L-FABP, and urine occult blood. In addition, although Case 2 showed renal impairment prior to the lubiprostone treatment, her serum creatinine level also slightly decreased over 12 months. Both patients received no other medications, such as renin-angiotensin-aldosterone system (RAAS) inhibitors, dilazep, or tonsillectomy and steroid pulse therapy, due to their refusal of those medications. Furthermore, their BP and body weight did not show obvious changes for 12 months after initiation of lubiprostone. Thus, the present report suggests that lubiprostone itself plays a renoprotective role in patients with IgA nephropathy through the improvement of constipation.
There are an increasing number of studies suggesting that the gut microbiota plays a pathophysiological role in the development of kidney diseases, and this “gut-kidney axis” has been recognized as a promising target for novel treatment strategies in kidney diseases (5,6). Furthermore, recent data demonstrate that impaired gastrointestinal mucosal immune responses and altered gut microbiota may also be associated with the pathogenesis of IgA nephropathy (7-9). Thus, improving the milieu of gut microbiota is expected to be a potential therapeutic approach in patients with IgA nephropathy. Thus far, however, no effective therapies backed by clinical evidence have been shown capable of achieving that aim.
Lubiprostone is a selective type 2 chloride-channel activator that increases interstitial fluid secretion. Patients administered lubiprostone have been shown to have a greater mean number of spontaneous complete bowel movements than placebo (10). Li et al. (11) conducted a meta-analysis of 1,468 patients in a lubiprostone group and 841 in a placebo group and concluded that lubiprostone is a safe and efficacious drug for the treatment of chronic constipation with limited adverse effects. Mishima et al. (4) reported that lubiprostone exerted a renoprotective effect on the progression of CKD with a reduction in the plasma concentration of uremic toxins and improvement in the gut microbiota population, suggesting a potential therapeutic approach to CKD based on the improvement of the intestinal environment. They also reported that lubiprostone improved renal fibrosis and inflammation on histological evaluations. Unfortunately, we did not measure the uremic toxins, including indoxyl sulfuric acid levels, or the microbiota population in the present two patients. Measuring these parameters may help clarify the mechanisms underlying the kidney protection afforded by lubiprostone treatment.
Dysbiosis is a state of unfavorable gut microbiota and is related to various diseases (12-14). Several examinations using fecal and intestinal samples and animal models of renal insufficiency have demonstrated that CKD is likely associated with unfavorable changes in the microbiota and thereby dysbiosis (2,15,16). It is thus expected that an improvement of the gut microbiota and intestinal environment will become a viable therapeutic option for CKD treatment. Although clinical studies have assessed the impact of the microbiota on uremic toxins as a surrogate marker in CKD (17), whether or not improving the gut microbiota in CKD will yield renal and cardiovascular benefits remains unclear at present.
A double-blind placebo-controlled randomized clinical trial to evaluate the effectiveness and safety of lubiprostone in CKD patients at the pre-dialysis stage is underway (UMIN000023850). In this context, our present finding that modulation of the enteral environment may improve the renal function may be crucial. Further experimental and clinical studies are needed in order to evaluate the ability of this agent to exert medicinal adjustment of the enteral environment in order to suppress the progression of CKD.
The relationships among intestinal bacteria and the brain and cardiovascular system have also recently attracted attention. Amaral et al. (18) reported that the sensation of pain due to inflammatory stimulation in germ-free mice was duller than that in conventional mice, and they proposed that the existence of the enterobacterial flora is indispensable for normal pain perception. Tang et al. (19) reported that intestinal bacteria allow arteriosclerosis to progress through a metabolism product called trimethylamaine-N-oxide. Thus, the enterobacterial flora strongly affect the maintenance of multiple organs' homeostasis, suggesting that abnormality of the enterobacterial flora (dysbiosis) plays pathological roles in various organs and disorders, including CKD. Health management efforts may soon target the enterobacterial flora.
In summary, we reported for the first time that lubiprostone may provide renoprotection in IgA nephropathy patients with constipation. Further studies are needed in order to confirm the effect of lubiprostone in various types of CKD with constipation as well as to further elucidate the action mechanism of lubiprostone.
Author's disclosure of potential Conflicts of Interest (COI).
Koichi Node: Honoraria, MSD, Astellas, Amgen, AstraZeneca, Eli Lilly Japan, Otsuka, Daiichi-Sankyo, Takeda, Boehringer Ingelheim Japan, Bayer, Pfizer, Ono and Mitsubishi Tanabe.
Acknowledgement
We thank Professor Yoshihiko Ueda, Department of Pathology, Koshigaya Hospital, Dokkyo University School of Medicine, Saitama, Japan, for the technical help with the renal histology preparation and examination.
References
- 1. Quimby JM. Update on medical management of clinical manifestation of chronic kidney disease. Vet Clin North Am Small Anim Pract 46: 1163-1181, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Ramezani A, Raj DS. The gut microbiomes, kidney disease, and targeted interventions. J Am Soc Nephrol 25: 657-670, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fukudo S, Hongo M, Kaneko H, Takano M, Ueno R. Lubiprostone increases spontaneous bowel movement frequency and quality of life in patients with chronic idiopathic constipation. Clin Gastroenterol Hepatol 13: 294-301, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Mishima E, Fukuda S, Shima H, et al. . Alteration of the intestinal environment by lubiprostone is associated with amelioration of adenine-induced CKD. J Am Soc Nephrol 26: 1787-1794, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25: 657-670, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evenepoel P, Poesen R, Meijers B. The gut-kidney axis. Pediatr Nephrol 32: 2005-2014, 2017. [DOI] [PubMed] [Google Scholar]
- 7. De Angelis M, Montemurno E, Piccolo M, et al. . Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS One 9: e99006, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coppo R. The gut-renal connection in IgA nephropathy. Semin Nephrol 38: 504-512, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe H, Goto S, Mori H, et al. . Comprehensive microbiome analysis of tonsillar crypts in IgA nephropathy. Nephrol Dial Transplant 32: 2072-2079, 2017. [DOI] [PubMed] [Google Scholar]
- 10. Thayalasekeran S, Ali H, Tsai HH. Novel therapies for constipation. World J Gastroenterol 19: 8247-8251, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li F, Fu T, Tong WD, et al. . Lubiprostone is effective in the treatment of chronic idiopathic constipation and irritable bowel syndrome. Mayo Clin Proc 91: 456-468, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Antza C, Stabouli S, Kotsis V. Gut microbiota in Kidney disease and hypertension. Pharmacol Res 130: 198-203, 2018. [DOI] [PubMed] [Google Scholar]
- 13. Kitai T, Kirsop J, Tang WH. Exploring the microbiome in heart failure. Curr Heart Fail Rep 13: 103-109, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S. Microbiome, probiotics and neurodegenerative diseases:deciphering the gut brain axis. Cell Mol Life Sci 74: 3769-3787, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farhadi A, Bana A, Fields J, Keshavarzian A. Intestinal barrier. A interface between health and disease. J Gastroenterol Hepathol 18: 479-497, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Vaziri ND, Wong J, Pahl M, et al. . Chronic kidney disease alters intestinal microbial flora. Kidney Int 83: 308-315, 2013. [DOI] [PubMed] [Google Scholar]
- 17. Mishima E, Fukuda S, Mukawa C, et al. . Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney int 92: 634-645, 2017. [DOI] [PubMed] [Google Scholar]
- 18. Amaral FA, Sachs D, Costa VV, et al. . Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci U S A 105: 2193-2197, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang WH, Wang Z, Levison BS, et al. . Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368: 1575-1584, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]