Summary
Critical to evolutionary fitness, animals regulate social behaviors by integrating signals from both their external environments and internal states. Here we find that population density modulates the courtship behavior of male Drosophila melanogaster in an age-dependent manner. In a competitive mating assay, males reared in a social environment have a marked advantage in courting females when pitted against males reared in isolation. Group housing promotes courtship in mature (7-day) but not immature (2-day) males; this behavioral plasticity requires the Or47b pheromone receptor. Using single-sensillum recordings, we find that group housing increases the response of Or47b olfactory receptor neurons (ORNs) only in mature males. The effect of group housing on olfactory response and behavior can be mimicked by chronically exposing single-housed males to an Or47b ligand. At the molecular level, group housing elevates Ca2+ levels in Or47b ORNs, likely leading to CaMKI-mediated activation of the histone-acetyl transferase CBP. This signaling event in turn enhances the efficacy of juvenile hormone, an age-related regulator of reproductive maturation in flies. Furthermore, the male-specific Fruitless isoform (FruM) is required for the sensory plasticity, suggesting that FruM functions as a downstream genomic coincidence detector in Or47b ORNs—integrating reproductive maturity, signaled by juvenile hormone, and population density, signaled by CBP. In all, we identify a neural substrate and activity-dependent mechanism by which social context can directly influence pheromone sensitivity, thereby modulating social behavior according to animals’ life-history stage.
Keywords: Behavioral flexibility, social context, group housing, juvenile hormone, CBP, CaMKI, Fruitless
Graphical Abstract
eTOC Blurb
Sethi et al. show that molecular pathways signaling population density (CaMKI/CBP) and reproductive maturity (juvenile hormone) are integrated in male Or47b ORNs to modulate pheromone detection, and thereby regulate courtship in Drosophila. Such integration allows coordination of mating behavior with both social context and internal hormonal state.
Introduction
Flexibility of social behavior in changing environments and physiological states is critical to reproductive success and evolutionary fitness. Recent studies advanced our understanding of how individual contextual inputs—such as mating status [1], hormonal state [2], population density [3–5], and prior experience [6]—modulate the display of social behaviors. The influence of environmental cues on social behavior is highly dependent on the physiological state of the animal. However, the cellular and molecular mechanisms that underlie the integration of extrinsic and intrinsic inputs remain poorly understood.
Changes in social environment, such as fluctuations in population density, have a profound impact on mating and aggression behaviors in group-living animals [7]. Recent studies identified key neural circuits and molecules involved in the modulation of aggression by social isolation [3–5,8]. Social context is also known to influence many aspects of male courtship in Drosophila [9–15]. For example, prior exposure to other male flies modulates the courtship display and enhances the duration of copulation [11–14]. These adaptations allow male flies to adjust their courtship intensity in accordance with rival competition and reproductive opportunity.
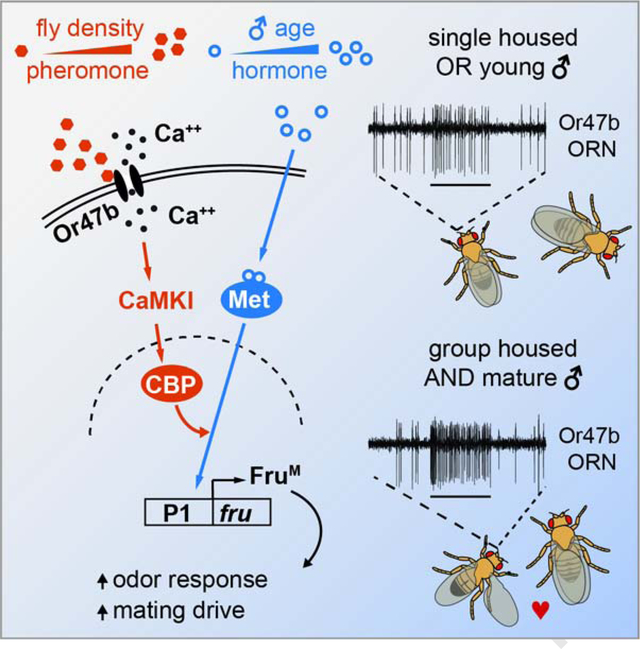
To address how external social cues are integrated with internal state, we focused on the courtship-promoting Or47b ORNs to investigate the effects of age and housing condition on reproductive behavior in male Drosophila. ORNs are often required for the contextual modulation of social behaviors [6,8,16], and several lines of evidence suggest that age-dependent plasticity in Or47b ORNs underlies contextual modulation of courtship behavior [17,18]. Among the ~50 ORN types in the adult olfactory system, Or47b neurons exhibit uncommon anatomical and physiological plasticity [17–19]. Anatomically, the glomerulus innervated by Or47b ORNs increases in size with age [17]. Physiologically, male Or47b ORN responses also increase in an age-dependent manner, leading to higher pheromone sensitivity in older individuals at their fertility peak [18]. This increase in Or47b response is mediated by juvenile hormone, a pleiotropic hormone that also regulates the reproductive maturity in adult Drosophila [18,20]. Notably, such plasticity occurs only in males but not females [18,21], and likely arises from the expression of FruM, a male-specific isoform of the fruitless transcription factor required for many aspects of male courtship behavior [21][22]. Finally, because Or47b ORNs respond to fly pheromones [18,23], their neural activity level may encode population density. Therefore, it is possible that Or47b ORN is a neural substrate which integrates social context and reproductive maturity to regulate pheromone detection and courtship behavior.
In this study, we find that group housing elevates Or47b pheromone responses in mature males, but not in females or young males. Remarkably, the effect of group housing can be mimicked by raising single-housed males in the presence of palmitoleic acid, a pheromone ligand for Or47b ORNs. Mechanistically, group housing increases intracellular Ca2+ levels in Or47b neurons to activate Ca2+/calmodulin-dependent protein kinase I (CaMKI) and CREB binding protein (dCBP), creating a permissive intracellular environment which enhances the efficacy of juvenile hormone. Furthermore, we find that FruM expression levels determine Or47b response and are regulated by juvenile hormone signaling. Our study describes a molecular mechanism by which internal state (age/sexual maturity) and social context (population density) synergistically regulate the expression of the male-specific transcription factor FruM. This mechanism allows male flies to adjust their sensitivity to aphrodisiac pheromones in accordance with their reproductive maturity and social environment.
Results
Group housing enhances pheromone response of Or47b neurons in mature males
We first investigated the effect of housing density on Or47b response. Flies were raised for 7 days either in same-sex groups of 15 (group housing, GH) or in isolation (single housing, SH). We measured odor-evoked spike responses of individual Or47b ORNs to palmitoleic acid, an aphrodisiac pheromone that stimulates male courtship [18]. We found that group housing increased the pheromone sensitivity of Or47b ORNs in males but not females (Figures 1A and 1B; Figure S1). By varying the group size from 5 to 25 in increments of 5, we found that a minimum group size of 10 was required to induce a higher response in males when compared to single-housed males (Figures 1C and 1D; Figure S2A). In contrast, none of the examined housing conditions altered Or47b responses in females (Figures 1C and 1D; Figure S2A). In addition to Or47b ORNs, we examined Or67d and Or88a ORNs that also respond to fly pheromones [24]. Responses of Or67d and Or88a neurons did not increase under similar housing conditions (Figure S3), suggesting that Or47b neurons are unique among pheromone-sensing ORNs in their adaptive responses to housing density.
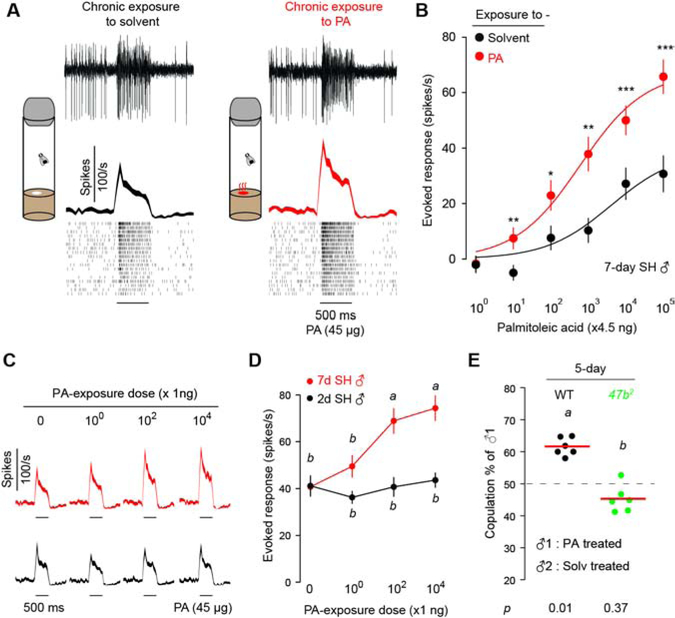
Figure 1. Group housing enhances pheromone responses of Or47b ORNs in mature males.
(A, B) Effect of housing condition on pheromone responses in the Or47b neurons of 7-day old males and females. GH: group-housed, 15 flies per vial. SH: single-housed, flies were raised in isolation. (A) Sample traces (top panel), peri-stimulus time histogram (PSTH, middle panel) and spike rasters (bottom panel) of Or47b neurons in WT males and females. (B) Dosage-response curves comparing male and female flies under GH or SH conditions. Odor stimulation: 4.5 ng to 450 μg palmitoleic acid (PA). n = 17 flies per condition.
(C, D) Effect of housing density on Or47b response. WT flies were raised for 7 days either in isolation or in same-sex groups of 5 to 25 flies. n = 16 flies for males, n = 12 flies for females.
(E, F) Effect of age and housing condition on Or47b response. WT males were raised in groups of 15 or in isolation from eclosion to different ages. n = 20 flies for each condition.
(G, H) Courtship competition assay with one virgin female and two naïve males. The copulation percentages of group-housed males (♂1) are shown with the age and genotypes of the males indicated above. Chi-square test was used to determine if copulation percentage was different from chance. p-values are indicated below. Bars indicate mean copulation percentage. Dots represent results from individual experiments. n = 5 experiments per condition, 12–26 matches per experiment. Dashed line indicates chance level.
(I) Effect of 2-day group housing on Or47b pheromone response in male flies of different age. Filled bars indicate group housing. n=15 flies per condition.
Significant differences are denoted by ***, p<0.001 (B) or different letters (p<0.05) (D, F, H), determined by two-way ANOVA followed by Tukey’s post-hoc test. For (I) *, p<0.012, **, p<0.002 as determined by two-tailed unpaired t-test with Bonferroni correction. 45 μg PA was used as the odor stimulus unless specified otherwise. Data are presented as mean ±SEM. Line width in PSTH indicates SEM.
See also Figures S1–S3 and Table S1.
We next investigated the effect of group housing on males of different ages. Adult males were raised in groups of 15 or in isolation from eclosion up to 10 days of age. In males aged 5–10 days but not younger, we observed that group-housing increased their Or47b responses when compared to single-housed controls (Figures 1E and 1F). This result is consistent with our previous study indicating that Or47b responses are higher in males at the age of 7 days compared to those at 2 days [18]. In Drosophila melanogaster males, the peak of both courtship activity and fertility is reached around 7 days post-eclosion [25,26], an age we therefore refer to as mature.
Does group housing-induced sensitization of Or47b neurons translate into a higher mating drive? Using an established behavioral assay that measures the differential courtship motivation between two males (Figure 1G; [18]), we found that group-housed males had a higher copulation rate than single-housed males at the age of 7 days but not 2 days (Figure 1H), consistent with the observation that group housing-induced Or47b sensitization occurred in 7-day but not 2-day old males (Figure 1F; Figure S2B). These results suggest that the effect of group housing on courtship in males is mediated by changes in Or47b pheromone responses. Indeed, we found that group housing did not affect the copulation rate of Or47b mutant males at either age (Figure 1H).
This age-dependent disparity in group-housing effect can in principle arise from differences in the duration of social experience or in the age of flies when group housing takes place. To distinguish between these two possibilities, we first investigated the impact of different group-housing durations. Flies that were group housed for 7 days had higher Or47b responses when compared to flies group housed for only one day, but not for two or more days (Figure S2C). This result demonstrates that group housing for 2 days is sufficient to enhance Or47b response.
Next, to determine the importance of age when social encounter commences, we group housed males of different ages for a fixed duration of 2 days. We found that two days of group housing was sufficient to enhance Or47b responses in 4-day, 6-day and 8-day old males but not in 2-day old males (Figure 1I). This observation argues for a minimum age at which group housing can sensitize Or47b ORNs. Collectively, these results show that male Or47b ORNs can integrate age and population density information to adapt their pheromone responses. Specifically, group housing enhances Or47b pheromone responses in mature males, allowing for an age-dependent modulation of courtship behavior by prior exposure to other flies.
Group-housing effect can be mimicked by chronic exposure to an Or47b ligand
What is the input signal that leads to the adaptive Or47b responses to group housing? Given that Or47b is a pheromone receptor, group-housed flies are likely exposed to higher levels of Or47b ligands such as palmitoleic acid (PA) than single-housed flies. We therefore hypothesized that the group-housing effect is mediated by chronic exposure to Or47b ligands, which trigger activity-dependent plasticity in Or47b ORNs. In support of our hypothesis, single-housed males raised in vials perfumed with palmitoleic acid had higher Or47b responses compared to control males (Figures 2A and 2B). Furthermore, the effect of PA exposure is age-dependent and observed in 7-day but not 2-day old males (Figures 2C and 2D). This result suggests that in mature males, PA-evoked activity initiates a positive feedback mechanism which enhances Or47b pheromone sensitivity.
Figure 2. Chronic exposure to an Or47b ligand is sufficient to enhance pheromone response and mating drive in mature males.
(A, B) Effect of chronic exposure to palmitoleic acid (PA) on pheromone responses in Or47b neurons. (A) Newly eclosed male flies were single housed in vials containing filter paper blotted with solvent (left) or 1 μg PA (right) for 7 days. Sample traces (top panel), peri-stimulus time histogram (PSTH, middle panel) and spike rasters (bottom panel) of Or47b ORNs in males housed with solvent or PA. (B) Dosage-response curves comparing Or47b ORN responses in males housed with solvent or 1 μg PA. Odor stimulation: 4.5 ng to 450 μg palmitoleic acid (PA). n = 13 flies per condition.
(C, D) Effect of chronic exposure to 0–10 μg PA on Or47b ORN responses in 7-day and 2-day single-housed males. Flies were exposed to varying amounts of PA from eclosion until the time of experiment. n=18 flies for each condition.
(E) Courtship competition assay between two males: ♂1 - exposed to 1 μg PA for 5 days, ♂2 - exposed to solvent alone. Both males in the assay were either WT or Or47b mutants. The copulation percentages of males exposed to PA (♂1) are shown. Bars indicate mean copulation percentage. Dots represent results from individual experiments. n=6 experiments per condition, 12~20 matches per experiment. Comparison between WT and Or47b mutant males was made using two-tailed unpaired t-test and significant difference is indicated as different letters (p<0.05). Chi-square test was used to determine if copulation percentage was different from chance and p-values are indicated below.
Significant differences for recording experiments are denoted by *, p<0.05; **, p<0.01; ***, p<0.001 as determined by unpaired two-tailed t-test (B) or different letters (p<0.05) as determined by two-way ANOVA followed by Tukey’s post-hoc test (D). 45 μg PA was used as the odor stimulus unless specified otherwise. Data are presented as mean ±SEM. Line width in PSTH indicates SEM.
See also Figure S4.
Next, we determined if chronic exposure to PA is sufficient to enhance courtship behavior in single-housed males. In courtship competition assays using wildtype males, we found that flies exposed to PA had a higher copulation rate than control males (Figure 2E). In contrast, chronic PA exposure did not alter the copulation rate of Or47b mutants (Figure 2E), suggesting that Or47b sensitization is required for the courtship enhancement. As with group housing, we found that the effect of PA exposure was sexually dimorphic; raising single-housed females in PA-perfumed vials did not result in enhanced Or47b responses (Figure S4A). Notably, female and male flies carry similar amounts of PA and other Or47b ligands [18,23], suggesting that the dimorphic Or47b plasticity is likely mediated by the genetic differences between the two sexes instead of differing PA levels in the environment. In summary, these results indicate that heightened levels of Or47b ligands in group-housing conditions are sufficient to sensitize Or47b ORNs in mature males.
CaMKI and dCBP are both required for the group-housing effect
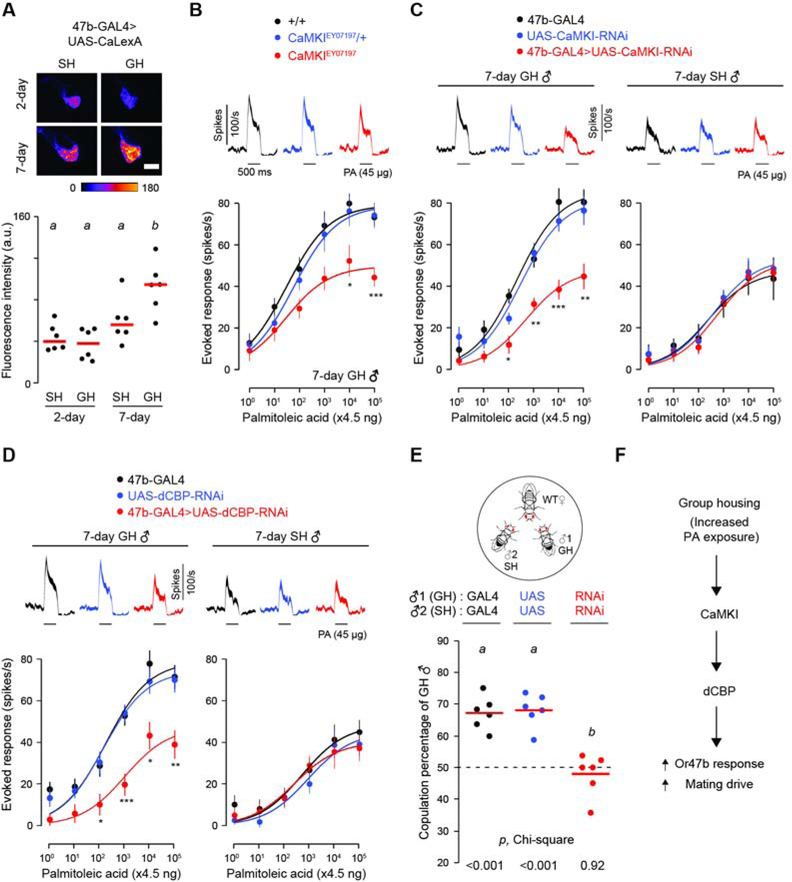
We next determined the molecular mechanisms which underlie the sensitization of Or47b ORNs. Given that calcium signaling is typically required for activity-dependent neuronal plasticity [27], we first asked whether group-housed males have higher baseline calcium levels in their Or47b neurons. Using CaLexA, a transcriptional reporter of intracellular Ca2+ [28], we observed that group housing enhanced Or47b neuronal calcium levels in 7-day but not 2-day males (Figure 3A). To determine the subsequent signaling events following rising intracellular Ca2+, we first focused on Ca2+/calmodulin-dependent protein kinases (CaMKs), which have known functions in mediating activity-dependent gene expression [27,29]. In particular, CaM kinase signaling has been implicated in regulating gene expression downstream of the Or47b receptor [30]. We found that a loss-of-function mutation in CaMKI reduced Or47b ORN responses in 7-day, group-housed males (Figure 3B). Similarly, RNAi-mediated knockdown of CaMKI in Or47b neurons lowered the pheromone response of group-housed males to that of single-housed males (Figure 3C). Moreover, CaMKI knockdown precluded the effect of chronic PA exposure (Figure S4B). In comparison, CaMKII knockdown did not have any effect on Or47b response in 7-day group-housed males (Figure S4C). These data collectively suggest that group housing elevates Or47b pheromone response by means of activity-dependent CaMKI signaling.
Figure 3. CaMKI and dCBP are required for the effect of group housing on Or47b pheromone response and courtship behavior.
(A) Effect of housing condition on intracellular Ca2+ concentration of Or47b neurons in 7-day and 2-day old males. Pseudocolor images show GFP fluorescence intensity of Or47b axon terminals in the antennal lobe. Bars indicate mean GFP intensity. Dots represent GFP intensity in antennal lobes of individual flies. n = 6 flies per condition. Scale bar = 15 μm.
(B) Effect of CaMKI mutation on pheromone response in Or47b ORNs of 7-day old GH males. n=14 flies per genotype.
(C and D) Effect of CaMKI (C) and dCBP (D) knockdown in Or47b ORNs on pheromone responses in GH and SH flies. n=14–15 flies per condition, 7-day old males.
(E) Effect of dCBP knockdown in Or47b neurons on copulation percentage in the courtship-competition assay. ♂1 - GH, ♂2 - SH. Both males were 7-day old and of the same genotype, as indicated. Dots represent results from individual experiments. n=6 experiments per condition, 13~22 matches per experiment. Different letters denote significant differences between genotypes (p<0.05) as determined by one-way ANOVA followed by Tukey’s post-hoc test. Chi-square test was used to determine if copulation percentages of GH males were different from chance and p-values are indicated below. GAL4: Or47b-GAL4, UAS: UAS-dCBP-RNAi, RNAi: Or47b-GAL4>UAS-dCBP-RNAi.
(F) Model for the molecular pathway mediating the effect of group housing on Or47b ORN pheromone responses and courtship behavior.
GH: raised in a group of 15 per vial. SH: raised in isolation. Significant differences between conditions in (A) were determined by two-way ANOVA followed by Tukey’s post-hoc test and are indicated by different letters (p<0.05). Significant differences for recording experiments are denoted by *, p<0.05; **, p<0.01; ***, p<0.001 as determined by one-way (B) or two-way (C, D) ANOVA followed by Tukey’s post-hoc test. Odor stimulation: 4.5 ng to 450 μg palmitoleic acid (PA). Data are presented as mean ±SEM. Line width in PSTH indicates SEM.
See also Figure S4.
To identify the signaling molecules downstream of CaMKI, we examined Drosophila CREB binding protein (dCBP, also known as nejire). CBP is a lysine acetyltransferase that functions as a transcriptional co-activator in a variety of physiological processes [31]. In addition, CBPs can directly acetylate histones and participate in chromatin remodeling, thereby regulating gene expression [32]. The vertebrate homolog of Drosophila CaMKI is known to phosphorylate and activate CBP, which in turn initiates transcription in response to neuronal activity [33]. Furthermore, dCBP is also involved in regulating gene expression downstream of the Or47b receptor [30]. We found that RNAi-mediated knockdown of dCBP in the Or47b neurons of group-housed males reduced their responses to a level similar to that of single-housed males (Figure 3D). Moreover in courtship competition assays, dCBP knockdown eliminated the difference in the copulation rate between group- and single-housed males (Figure 3E). Together, these data support a model in which group housing enhances courtship behavior by activating a CaMKI-dCBP signaling pathway in male Or47b ORNs (Figure 3F).
Juvenile hormone signaling is required for the effect of group housing on Or47b ORNs
Why is the group-housing effect observed only in mature males but not in younger, 2-day old males? Given that Or47b ORNs adapt their responses to changes in housing density in an age-dependent manner (Figure 1), we hypothesize that the signaling events initiated by the two conditions interact to modulate Or47b responses. Age-dependent sensitization in Or47b ORNs is mediated by juvenile hormone [18], a pleiotropic hormone that also regulates reproductive maturity in adult flies [20]. For adult males, juvenile hormone levels are low at eclosion, increase with age, and return to baseline at 10 days [34]. To determine if juvenile hormone signaling is required for activity-dependent Or47b plasticity, we knocked down the juvenile hormone receptor, Methoprene-tolerant (Met) [35], in Or47b ORNs. This manipulation abolished the effect of chronic PA exposure (Figure 4A), suggesting that juvenile hormone signaling is also required for the effect of group-housing. If a low level of juvenile hormone in immature males renders them insensitive to group housing, then treating 2-day old males with methoprene, a juvenile hormone analog (JHA), should augment their Or47b ORN responses by group housing. In support of this hypothesis, treating 2-day old males with 2.5 μg of JHA increased their Or47b responses following chronic PA exposure (Figure 4B). These results suggest that the two molecular pathways—one signaling population density and the other fly age—are both required for the activity-dependent Or47b neuronal plasticity.
Figure 4. Group housing enhances the effect of juvenile hormone signaling on Or47b ORN pheromone response.
(A) Juvenile hormone binds to its receptor, Methoprene-tolerant (Met), in Or47b ORNs (left). Red cross indicates RNAi-mediated knockdown of Met. (Right) Effect of Met-knockdown on pheromone responses in Or47b ORNs of males chronically exposed to solvent or palmitoleic acid (PA). SH male flies were exposed to 10 μg PA or solvent for 7 days. n = 13 flies per condition. Odor stimulation: 4.5 ng to 450 μg PA.
(B) Effect of chronic exposure to 0–10 μg PA on Or47b ORN pheromone responses in 7-day and 2-day SH males. Males were treated either with solvent or 2.5 μg of a juvenile hormone analog (JHA, Methoprene). n=18 flies per condition.
(C–E) Or47b ORN pheromone responses in 2-day old male flies treated with 0–25 μg of JHA. Effect of housing condition (C), chronic exposure to 10 μg PA (D) and dCBP knockdown (E) on JHA-dependent enhancement in Or47b ORN pheromone response. n=17–18 flies per condition.
(F) Effect of varying dosage of JHA treatment (0–25 μg) and PA exposure (0–10 μg) on Or47b ORN pheromone responses in 2-day SH males. Heatmap indicates normalized mean evoked response for a given rearing condition. Response for each rearing condition was normalized to that of males with no JHA treatment or PA exposure (bottom left).
(G) Model for interaction between group housing and juvenile hormone signaling pathways.
GH: raised in groups of 15 per vial. SH: raised in isolation. Significant differences are denoted by *, p<0.05; **, p<0.01; ***, p<0.001 (A), or different letters (p<0.05) (B–E) as determined by two-way ANOVA followed by Tukey’s post-hoc test. 45 μg PA was used as the odor stimulus unless specified otherwise. Data are presented as mean ±SEM. Line width in PSTH indicates SEM.
Group housing enhances the efficacy of juvenile hormone signaling
What then is the nature of interaction between these two signaling pathways? Do they operate in synergy? Can strong activation of one pathway compensate for the low signaling level of the other? To address these questions, we first investigated the efficacy of hormonal signaling in 2-day old males by systematically manipulating JHA dosage and housing conditions. We found that JHA treatment was more effective in enhancing Or47b ORN responses in group-housed males, when compared to their single-housed counterparts (Figure 4C). These results indicate that the two signaling pathways functionally interact in a synergistic manner. Surprisingly, a higher dosage (25 μg) of JHA was able to enhance Or47b responses even in single-housed males (Figure 4C), suggesting that a high level of hormonal signaling can compensate for low levels of neuronal activity. In addition, chronically exposing single-housed males to PA enhanced the efficacy of JHA in the sensitization of Or47b ORNs (Figure 4D). Given that dCBP is required for the group-housing effect (Figures 3D and 3E), its absence should reduce the efficacy of juvenile hormone signaling. As expected, RNAi-mediated knockdown of dCBP in Or47b ORNs reduced the effect of JHA treatment in group-housed males (Figure 4E). These results suggest that in Or47b ORNs, the crosstalk between dCBP and juvenile hormone signaling is central to the integration of housing density and fly age.
To further determine the relative contribution of neuronal activity and juvenile hormone signaling, we systematically varied the strength of these two inputs in single-housed young flies (Figure 4F). We raised 2-day old males in vials containing varying amounts of PA (0–10 μg) and JHA (0–25 μg). In the absence of JHA treatment, increasing PA levels did not alter Or47b response (Figure 4F), indicating that neuronal activity, and by extension dCBP activation, is not sufficient to enhance Or47b response. In contrast, a high-dose of JHA treatment was sufficient to sensitize Or47b pheromone response in the absence of exogenous PA exposure (Figure 4F). Notably, treatment with 2.5 μg JHA enhanced odor responses of flies raised in PA-perfumed vials (0.1–10 μg), while either treatment alone did not affect Or47b responses (Figure 4F). This result further argues for a synergistic interaction between juvenile hormone signaling and neuronal activity, while the compensation of low Or47b activity by 25μg JHA (Figure 4D) highlights an asymmetry between these two pathways.
Thus far, our findings show that: (1) a juvenile hormone receptor is required for the effect of chronic PA exposure (Figure 4A), (2) group housing alone is not sufficient to enhance Or47b response in immature males (Figure 4F), and (3) dCBP is required for group housing to enhance the efficacy of juvenile hormone (Figure 4E). Based on these results, we propose a model whereby dCBP activation by group housing plays a permissive role in Or47b ORNs, giving rise to an intracellular environment that facilitates juvenile hormone signaling (Figure 4G). As such, when juvenile hormone signaling is abolished or at a low level, as in the case of Met knockdown or in 2-day old males, dCBP activation alone is not sufficient to regulate Or47b pheromone responses.
FruM expression levels determine Or47b response
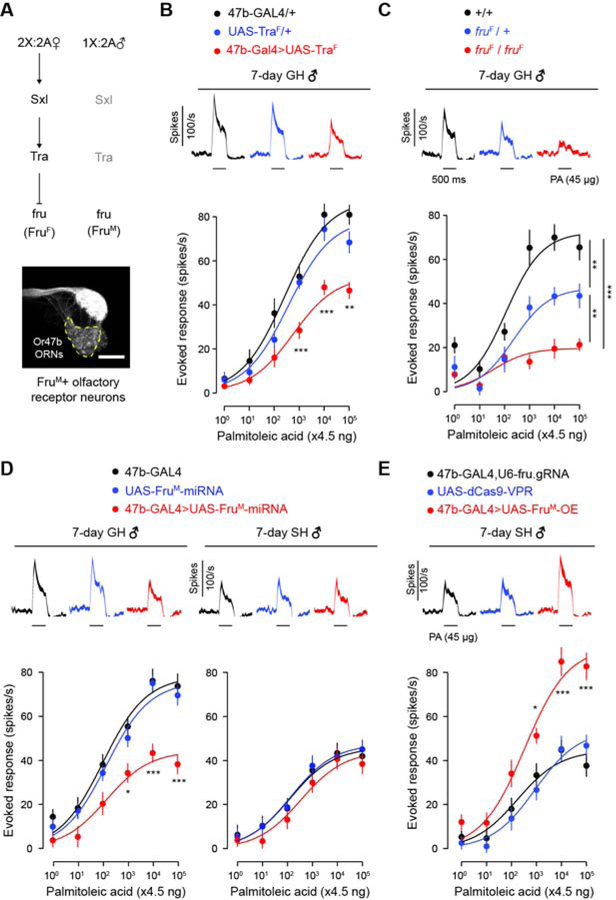
A striking feature of the adaptive Or47b pheromone response is its sexual dimorphism: housing density and age only affect the Or47b ORNs of males but not females ([18]; Figures 1C and 1D). What then could be the molecular basis of this sex difference? Given that FruM is expressed in Or47b ORNs ([21], Figure 5A), it likely plays a role in regulating the male-specific adaptive responses. To test this hypothesis, we first feminized Or47b neurons by ectopically expressing the female splicing factor Transformer (TraF), and found that this manipulation suppressed odor response in group-housed mature males (Figure 5B). In addition, we observed a dosage effect of FruM expression on Or47b responses; the evoked spike frequency was the lowest in FruM homozygous mutants (fruF/fruF), followed by heterozygous mutants (fruF/+) and then wild type controls (all 7-day old, group-housed males, Figure 5C). This haploinsufficiency raises the possibility that FruM expression level in Or47b ORNs is a limiting factor in regulating the pheromone sensitivity in a cell autonomous manner.
Figure 5. FruM expression levels determine Or47b ORN response.
(A) Illustration of the signaling cascade leading to the sexually dimorphic expression of Fruitless. A representative confocal image shows GFP expression in the axon terminals of Or47b neurons (dashed line) in the antennal lobe of a male fly carrying the following transgenes: fruP1.GAL4, UAS-GFP, ey-FLP, Tub-FRT-GAL80-FRT. Scale bar = 25 μm.
(B) Effect of TraF ectopic expression in Or47b ORNs on pheromone responses in 7-day GH males. n=14 flies per condition.
(C) Effect of FruM mutation on pheromone responses in Or47b ORNs of 7-day GH males. fruF is a mutant allele that does not produce functional FruM protein. n=13 flies per condition.
(D) Effect of FruM knockdown in Or47b ORNs on pheromone responses in GH and SH flies. n=14 flies per condition, 7-day old males.
(E) Effect of FruM overexpression (OE) in Or47b ORNs on pheromone responses in 7-day SH males. n=14 flies per condition. FruM-OE = U6-fru.gRNA, UAS-dCas9-VPR.
GH: raised in groups of 15 per vial. SH: raised in isolation. Significant differences are denoted by *, p<0.05; **, p<0.01; ***, p<0.001 as determined by one-way ANOVA followed by Tukey’s post-hoc test (B–E). Odor stimulation: 4.5 ng to 450 μg palmitoleic acid (PA). Data are presented as mean ±SEM. Line width in PSTH indicates SEM.
See also Figure S5.
The observation that heterozygous mutant males have an intermediate phenotype led us to hypothesize that group housing upregulates FruM expression, which in turn gives rise to the elevated pheromone responses in Or47b neurons. This hypothesis is also supported by the observation that FruM expression in Or47b ORNs is reduced in Or47b receptor mutant flies, suggesting that neuronal activity is required for sustaining FruM expression in adult Or47b neurons [30]. To test this hypothesis, we devised a genetic approach to manipulate FruM expression levels in Or47b ORNs. FruM knockdown reduced Or47b responses in group-housed but not single-housed males (Figure 5D). If FruM is a limiting factor that regulates Or47b response plasticity, overexpression of FruM in single-housed males should then enhance their pheromone responses. To overexpress FruM, we used Or47b-GAL4 to drive the expression of dCas9-VPR, a genetically engineered molecule capable of recruiting transcriptional activation machinery. Two single-guide RNAs (sgRNAs) were ubiquitously expressed to target dCas9-VPR upstream of the P1 promotor of the fruitless gene, allowing for overexpression of FruM only in Or47b ORNs ([36], Figure S5A). Indeed, dCas9-mediated overexpression of FruM was sufficient to enhance Or47b response in single-housed males regardless of their age (Figure 5E; Figure S5B). As an alternative approach, we generated a knock-in UAS transgenic line (Figure S5C) to overexpress FruM in Or47b ORNs from its endogenous locus, and found that this manipulation yielded a similar outcome (Figure S5D). Together, these results indicate that pheromone sensitivity in adult Or47b ORNs is determined by FruM expression levels. Thus, FruM appears to have a post-developmental function in fine-tuning neuronal physiology.
dCBP and juvenile hormone signaling require FruM to elevate pheromone responses
Having determined the relationship between FruM expression level and Or47b pheromone sensitivity, we next asked whether FruM operates downstream of dCBP (group housing/neuronal activity) and juvenile hormone signaling (age). This hypothesis is supported by multiple lines of evidence. Firstly, juvenile hormone signaling can enhance FruM expression in adult male neurons [37]. In addition, the effect of group housing on Or47b response requires CaMKI-dCBP (Figure 3), a signaling pathway that also modulates FruM expression in Or47b neurons [30]. Furthermore, dCBP can directly bind upstream of the fru-P1 promoter in adult males ([38], modEncode).
To test whether FruM expression is required for the effect of JHA treatment, we feminized Or47b ORNs by ectopically expressing TraF, and found that this manipulation abolished JHA-induced sensitization (2-day old, group-housed males, Figure 6A), Conversely, overexpression of FruM abolished the reduction in Or47b pheromone response caused by Met knockdown (7-day old, group-housed males, Figure 6B) Likewise, the response reduction caused by dCBP knockdown was also abolished by FruM overexpression (7-day old, group-housed males, Figure S6A). Thus, FruM operates downstream of juvenile hormone and dCBP signaling.
Figure 6. FruM expression is downstream of juvenile hormone signaling in Or47b neurons.
(A) Effect of ectopic expression of TraF on JHA-dependent enhancement in Or47b ORN pheromone response. n=18 flies per condition, 7-day SH males. Odor stimulation: 45 μg palmitoleic acid (PA).
(B) Pheromone responses in 7-day GH males with the following manipulations in Or47b ORNs: GAL4 control (black), FruM overexpression (blue), Met knockdown (red), and simultaneous FruM overexpression and Met knockdown (green). n=14 flies per condition. Odor stimulation: 4.5 ng to 450 μg PA.
(C) Expression of fruP1 transcript in antennae of 7-day GH males following RNAi-mediated knockdown of Met in Or47b neurons, n=10 samples per condition. For each sample, RNA was extracted from antennal tissues of 50 males. Quantitative RT-PCR was used to determine fruP1 transcript expression levels. Data normalized to mean fruP1 expression in Or47b-GAL4 controls. The fruP1 transcript encodes the FruM protein in males. Dots represent individual samples. Bars represent mean of 10 samples.
(D) Model showing that the FruM locus acts as a genomic coincidence detector to integrate social context with the hormonal state of a fly. Group housing activates the CaMKI/CBP pathway, which in turn enhances the effect of juvenile hormone signaling in Or47b neurons. Graded levels of FruM expression fine-tune neuronal properties, with significant impacts on mating behavior.
Significant differences are denoted by *, p<0.05 or different letters (p<0.05), determined by two-way (A) or one-way (B, C) ANOVA followed by Tukey’s post-hoc test. Data are presented as mean ±SEM (A, B). Line width in PSTH indicates SEM.
See also Figure S6.
Furthermore, if juvenile hormone signaling influences the pheromone response of Or47b ORNs through FruM expression, manipulating the former should affect the latter. We therefore measured mRNA levels of FruM in fly antennae with RT-qPCR. Indeed, knocking down Met expression in Or47b neurons reduced FruM expression (Figure 6C). Our findings support a model in which the two signaling pathways—one communicating housing condition and the other signaling hormonal state—act in concert to regulate the expression of FruM in Or47b ORNs. Thus, the FruM locus may function as a genomic coincidence detector that integrates social context with reproductive maturity to adapt Or47b pheromone responses in a sexually dimorphic manner (Figure 6D).
Discussion
Modulation of physiology and behavior by population density is a conserved feature across animal species [39,40]. Here we find that group housing promotes male courtship in Drosophila. From an evolutionary standpoint, it is beneficial for male flies to elevate their mating drive in an environment of high population density, likely to gain a competitive edge over an increased number of rivals. Conversely, a low population density may signal an environment of scarce reproductive opportunity in which flies may lower their pheromone sensitivity to prioritize other survival behaviors. In addition to its ethological significance, our study also identifies a signaling cue for population density—the levels of a fly pheromone—which by itself recapitulates the effect of group housing on Or47b ORN sensitization.
In Drosophila, neural circuits underlying male courtship behavior are orchestrated by FruM. Prior efforts have largely been focused on understanding its developmental role in organizing sex-typical behavior [22,41]. For example, FruM promotes male fate by inhibiting cell death, altering dendritic arborization and instructing synaptic connectivity [41–43]. Although FruM is expressed in the adult brain [21], its function in mature neurons is largely unknown. In this study, we find that FruM directly regulates the responses to aphrodisiac pheromones in adult ORNs. Our results suggest that FruM expression level is regulated by social context, thereby allowing neuronal properties to be fine-tuned in mature neurons. These findings are in agreement with the observation that FruM regulates the expression of several genes that are known to control neuronal physiology [44]. Taken together, our results highlight a novel function for FruM in adulthood, which directly impacts social behavior beyond its previously established roles during development.
Our study uncovers an interaction between dCBP and juvenile hormone signaling that underlies the integrative effect of age and social context on courtship behavior. The critical role of dCBP in the adaptive Or47b response suggests an epigenetic mechanism that allows for modulation of pheromone detection by social context. Such neuronal plasticity induced by high population density may enable animals to adapt to environmental changes at a rate faster than that afforded by genetic changes under selection pressure [45]. How might dCBP regulate juvenile hormone signaling? One possibility is that dCBP activation leads to chromatin modification by means of histone acetylation, thereby enhancing the accessibility of enhancers upstream of the fruitless P1 promoter to juvenile hormone receptors. dCBP may also function as a transcriptional co-activator that forms a complex with juvenile hormone receptors to enhance the expression of FruM in Or47b ORNs. A similar interaction between CBP and juvenile hormone signaling is likely at play in the red flour beetle, where knockdown of CBP reduces expression of several juvenile hormone-responsive genes [46]. In mammals, CBP can enhance the transcriptional activity of sex hormone receptors [47,48]. Thus, activity-dependent modulation by CBP may represent a conserved mechanism for generating hormone-mediated polyphenism, a phenomenon wherein distinct phenotypes are produced by the same genotype [49]. In the context of hormone signaling, our study suggests a novel means by which social experience impacts reproductive behaviors by modulating the interaction between a hormone receptor and its target genes [50].
Sex-typical behaviors in mammals are controlled by sex hormones that organize the developing brain and control the activation of the underlying neural circuits in adults, while genetic sex plays a minor role in modulating mammalian reproductive behaviors [51]. In contrast, courtship display in insects like Drosophila was long considered to be determined entirely by genetic sex [51]. However, this view has evolved in light of a growing body of evidence implicating the activational effect of juvenile hormone on insect reproductive behaviors [52]. The molecular pathway described here illustrates how juvenile hormone activates the Or47b olfactory circuit exclusively in males by promoting the expression of FruM. On the basis of these findings, we propose that genetic sex plays a dominant role in sculpting sex-typical behavior, whereas reproductive hormones modulate the vigor of the behavioral display in Drosophila.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
Materials (fly lines, plasmids) generated in this study are available upon request and will be deposited with commonly used stock centers. Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jing W. Wang (jw800@ucsd.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Vinegar flies, Drosophila melanogaster, were raised on standard fly food (containing yeast, cornmeal, agar and molasses) at 25°C in a 12:12 light-dark cycle in cylindrical glass vials (24 mm diameter, 94 mm height). Glass vials were filled with 8 to 10 mL of fly food and plugged with cotton balls. Flies were collected within 12 hours of eclosion, separated by sex and raised either in isolation (1 per vial) or in groups of 15 (unless otherwise indicated). All flies were flipped every 24 hours to avoid the potential effect of odor build up on single housed flies. Wild-type males were in the Berlin background. The following transgenes were used in this study - or47b2 (BDSC_51306) [53], Or47b-GAL4 (RRID:BDSC_9984, BDSC_9983) [54], 13XLexAop2-6XGFP (RRID:BDSC_52265) [55], 10XUAS-mLexA-VP16-NFATdC (this study), UAS-CaMKI-RNAi (TRiP.JF02268 - BDSC_26726) [56], UAS-dCBP-RNAi (RRID:BDSC_32576) [57], UAS-Met-RNAi (VDRC_100638), ey-FLP (RRID:BDSC_5580), Tb-(FRT-GAL80-FRT)-STOP (RRID:BDSC_38880) [58], UAS-TraF (RRID:BDSC_4590) [59], fruF (RRID:BDSC_66873) [60], UAS-fru-miRNA [61], U6-fru-sgRNA (RRID:BDSC_80225) [36], UAS-dCas9-VPR (RRID:BDSC_66561) [36], CaMKI[EY07197] (RRID:BDSC_16799) [62], UAS-CaMKII-RNAi (RRID:BDSC_29401) [56], fru[GAL4.P1] (RRID:BDSC_66696)[21], fru[UAS.P1] (this study). Detailed genotypes and rearing conditions of flies for each experiment are listed in Table S1.
METHOD DETAILS
Generation of transgenic reagents
To generate 10XUAS-mLexA-VP16-NFATΔC fly, mLexA-VP16-NFATΔC was cloned from the original CaLexA plasmid [28] and inserted into the pJRC81 plasmid. The original XbaI site in the pJFRC81 plasmid [63] was converted to a SpeI site using site-directed mutagenesis. mLexA-VP16-NFATΔC was cloned with 5’ Syn21-NotI and 3’ SpeI overhangs. The NFATΔC domain is composed of 1–588 amino acids of the human NFATc1 protein. The construct was transformed using phiC31 integrase-mediated recombination into the attP2 landing site by Bestgene Inc. (Chino Hills, CA).
To generate the knock-in fru[UAS.p1] line, CRISPR-mediated mutagenesis was performed by WellGenetics Inc. (Taipei City, Taiwan) based on a previously published strategy with some modifications [64]. In brief, gRNA sequence CACATAAACGCAGTACATGG[TGG] was cloned into U6 promoter plasmid(s). Cassette RFP-20×UAS containing two loxP sites, 3XP3-RFP, 20×UAS, hsp70 promoter, intervening sequences (IVS, introns) and two homology arms were cloned into pUC57-Kan as donor template for repair. fru/CG14307-targeting gRNAs and hs-Cas9 were supplied in DNA plasmids, together with donor plasmid for microinjection into embryos of control strain w1118. F1 flies carrying selection marker of 3×P3-RFP were further validated by genomic PCR and sequencing. CRISPR generates a 73 bp deletion in fru/CG14307 and is replaced by cassette RFP-20×UAS.
Single-sensillum recording
A step-by-step protocol for single-sensillum recordinghas been described previously [65]. In brief, a sharp aluminosilicate glass electrode containing artificial hemolymph solution [66] was inserted in the at4 sensillum to record odor-evoked changes in local field potential and spike responses. Odor cartridges were prepared by placing filter paper disks in truncated 200 μL pipette tips (53508–810, VWR, Radnor, PA). Ethanol (E7023, Sigma-Aldrich) was used as a solvent for all odorants and 4.5 μL of individual odorants of different concentrations was added to the filter paper disk and the cartridge was placed in a vacuum desiccator (VX-06514–30, Cole-Parmer, Vernon Hills, IL USA) for 60 minutes to evaporate the solvent prior to experiments. Odor cartridges were positioned with filter disks placed at a distance of 6–7 mm and pointed directly at the antenna. Odor stimulus was delivered via a 500-ms pulse of air (500 mL/min) directed through the odor cartridge towards the antenna in the presence of humidified air flow at 2 L/min from a different direction. The following odorants were used in this study: trans-palmitoleic acid (Cayman Chemical, 9001798, CAS 10030-73-6), methyl palmitate (Sigma-Aldrich, P5177, CAS 112-39-0), and 11-cis-Vaccenyl Acetate (Cayman Chemical, 10010101, CAS 6186-98-7).
Individual spikes were sorted based on spike amplitude using Clampfit 10.7 (Molecular Devices). Sorted spikes were manually inspected to ensure accuracy. Evoked response was calculated as (2 × number of spikes during the 500 ms of odor stimulation) - (number of spikes during the period of 1000 ms before odor stimulation). Peri-stimulus time histograms (PSTHs) were calculated by averaging spike numbers in 50 ms time bins.
Courtship competition assay
Courtship experiments were conducted in a cylindrical mating chamber (2 cm in diameter and 1 cm in height) with a wire mesh at the bottom placed over a petri dish containing standard fly food. The courtship chamber has been previously described in detail [18]. All experiments were conducted under 660-nm dim red light at 25°C and 50 % relative humidity. Two naive males were placed in a courtship chamber with one 2-day old virgin Canton-S female. To facilitate identification of the males, one of the two males was dusted with a fluorescent dye (UVXPBR, LDP LLC, Carlstadt, NJ) 48 hours prior to experiment. Dye application was alternated between group- and single-housed males on a trial-by-trial basis to minimize possible dye-induced behavioral bias. The identity of the male which copulated first with the female and the latency to copulation were manually recorded during a 2-hour observation period. Courtship chambers in which neither of the males copulated with the female during the 2-hour period were excluded from further analysis. Each experiment consisted of 20–29 parallel courtship matches. Mating advantage was indicated by the percentage of matches in which males of a given condition copulated with the female. Five to six independent trials were conducted for each experiment to allow statistical analysis of mating advantage among the two males.
Chronic odor exposure
A piece of filter paper (approximately 8×8 mm) was placed on the surface of fly food in standard fly vials. One microliter of a given odorant at the specified concentration was applied to the filter paper. Ethanol (E7023, Sigma-Aldrich) was used as a solvent to dilute the odorants. Flies were flipped into fresh vials containing the odorants every 24 hours.
Pharmacological manipulations of juvenile hormone
Methoprene (33375, Sigma-Aldrich), 20 μL at given concentrations (between 0.00125% to 0.125% v/v), was applied to the surface of fly food. Ethanol was used as a solvent. The fly vials were placed in the fume hood for 2 hrs to evaporate the solvent. Flies were flipped into fresh vials containing methoprene or the solvent every 24 hours.
Histology
Fly brains were dissected in cold PBS, fixed first for 3 min in 4% (w/v) paraformaldehyde, followed by 3 min in 4% (w/v) paraformaldehyde containing 0.25% Triton-X-100. Fixation was facilitated by microwaving the sample on ice. Next, samples were placed in blocking solution (2% Triton X-100, 0.02% sodium azide and 10% normal goat serum in PBS) and degassed in a vacuum chamber for 15 minutes. This step was repeated for 6 times to remove air in the trachea. In CaLexA experiments, native GFP was imaged for quantification. Samples were mounted in Focusclear (Cedarlane Labs, Canada). Parallel experiments were conducted with samples processed and imaged on the same day. Samples were imaged with a 20×/0.75 objective using a Zeiss LSM 510 confocal microscope to collect Z-stacks at 2-μm intervals. Laser power and detector gain were held constant to allow for comparison of GFP expression between samples. Maximum intensity Z-projection images were processed using ImageJ (NIH) and background-subtracted images were used to quantify GFP expression.
Quantitative RT-PCR
Male flies were collected within 12 hr of eclosion and aged 7 days in group housing condition of 15 flies per vial. Anesthetized flies were dipped in liquid nitrogen and antennae were brushed off using a needle into a 1.5 ml centrifuge tube filled with liquid nitrogen. A single sample contained antennae collected from 50 flies and stored at −80°C. Each condition had 10 samples. mRNA was extracted from each sample using the QIAGEN RNeasy Mini and QIAGEN QIAshredder kits. A hand-held pestle drill was used during extraction. RNA yield was determined using a ThermoFisher Scientific NanoDrop spectrophotometer and cDNA was prepared using Invitrogen SuperScript VILO™ MasterMix. RT-qPCR was performed on the Bio-Rad CFX machine using iQ™ SYBR® Green Supermix from Bio-Rad and the following primer sets: fruP1 (forward primer - GCCACGCCCACTCGCATTAC, reverse primer - TGGTCAGTGTTGTACCTAG); and rp49 (forward primer - AGGGTATCGACAACAGAGTG, reverse primer - CACCAGGAACTTCTTGAATC). rp49 served as a reference gene to which FruM was normalized. RT-qPCR was performed with each primer pair in three technical replicates for each cDNA sample. CT values were recorded with Bio-Rad CFX Manager software to determine relative transcript levels.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical results (p-value and n) are indicated in figure legends corresponding to each experiment. In cases where a dosage curve for odor concentration was performed, statistical tests comparing the experimental groups were performed for each concentration and the p-value is indicated on the figure for conciseness. All statistical analyses were performed in Igor Pro (V.6, Wavemetrics). To determine the minimum sample size for SSR recording experiments, we used power analysis based on pilot data (GPower 3.1, [67]). For experiments with one factor (f=0.68, α=0.05, power=0.95): 13 samples each are required (3 conditions); 14 samples each are required (4 conditions). For experiments with two factors, n=13 per condition is required to determine interaction between the two factors for 12 (4×3) conditions (f=0.3, α=0.05, power=0.80, df=6); n=16 per condition is required to determine interaction between the two factors for 8 (4×2) conditions (f=0.26, α=0.05, power=0.80, df=3).
To fit dosage response curves to the experimental data, we used Hill equation fitting (Igor Pro V.6, Wavemetrics). Using free fitting parameters on the pooled data showed in Figure S1, we determined that group housed and single housed Or47b neuron dosage-response curves have the same rate but differ in their Vmax. Based on this initial characterization, we fit all dosage-response curves using mean responses at each odor concentration with the same parameters (base=0, rate=0.5). The dosage-response curves were made for illustration purpose only and were not used for any statistical analysis.
DATA AND CODE AVAILABILITY
Raw data for single-sensillum recording experiments are available upon request to the Lead Contact.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| trans-Palmitoleic acid | Cayman Chemical, MI | Cat#9001798; CAS:10030-73-6 |
| Methyl Palmitate | Sigma-Aldrich, MO | Cat#P5177; CAS: 112-39-0 |
| 11-cis Vaccenyl Acetate | Cayman Chemical, MI | Cat#10010101; CAS: 6186-98-7 |
| Methoprene | Sigma-Aldrich, MO | Cat#33375; CAS: 40596-69-8 |
| Ethanol | Sigma-Aldrich, MO | Cat#E7023; CAS: 64-17-5 |
| Focusclear | CelExplorer Labs Co., Taiwan | Cat#FC-101 |
| Critical Commercial Assays | ||
| RNeasy Mini Kit | QIAGEN, Germany | Cat#74104 |
| QIAshredder Kit | QIAGEN, Germany | Cat#79654 |
| SuperScript VILO Master Mix | Thermo Fisher Scientific, MA | Cat#11755050 |
| iQ SYBR Green Supermix | Bio-Rad Laboratories, CA | Cat#1708880 |
| Experimental Models: Organisms/Strains | ||
| D. melanogaster: or47b2 | [53]; Bloomington Drosophila Stock Center | BDSC: 51306 |
| D. melanogaster: Or47b-GAL4 | [54]; Bloomington Drosophila Stock Center | BDSC: 9984 |
| D. melanogaster: Or47b-GAL4 | [54]; Bloomington Drosophila Stock Center | BDSC: 9983 |
| D. melanogaster: LexAop2-6XGFP | [55]; Bloomington Drosophila Stock Center | BDSC: 52265 |
| D. melanogaster: UAS-CaMKI-RNAi | [56]; Bloomington Drosophila Stock Center | BDSC: 26726 |
| D. melanogaster: UAS-dCBP-RNAi | [57]; Bloomington Drosophila Stock Center | BDSC: 32576 |
| D. melanogaster: ey-FLP | Bloomington Drosophila Stock Center | BDSC: 5580 |
| D. melanogaster: Tub-(FRT-GAL80-FRT)-STOP | [58]; Bloomington Drosophila Stock Center | BDSC: 38880 |
| D. melanogaster: UAS-traF | [59]; Bloomington Drosophila Stock Center | BDSC: 4590 |
| D. melanogaster: fruF | [60]; Bloomington Drosophila Stock Center | BDSC: 66873 |
| D. melanogaster: U6-fru-sgRNA | [36]; Bloomington Drosophila Stock Center | BDSC: 80225 |
| D. melanogaster: CaMKI[EY07197] | [62]; Bloomington Drosophila Stock Center | BDSC: 16799 |
| D. melanogaster: UAS-dCas9-VPR | [36]; Bloomington Drosophila Stock Center | BDSC: 66561 |
| D. melanogaster: UAS-CaMKII-RNAi | [56]; Bloomington Drosophila Stock Center | BDSC: 29401 |
| D. melanogaster: fru[GAL4.P1] | [21]; Bloomington Drosophila Stock Center | BDSC: 66696 |
| D. melanogaster: UAS-Met-RNAi | Vienna Drosophila Stock Center | VDRC: 100638 |
| D. melanogaster: UAS-fru-miRNA | [61] | N/A |
| D. melanogaster: fru[UAS.P1] | This study | N/A |
| D. melanogaster: 10XUAS-mLexA-VP16-NFATΔC | This study | N/A |
| Oligonucleotides | ||
| fruP1 forward primer: GCCACGCCCACTCGCATTAC | This study | N/A |
| fruP1 reverse primer: TGGTCAGTGTTGTACCTAG | This study | N/A |
| rp49 forward primer: AGGGTATCGACAACAGAGTG | This study | N/A |
| rp49 reverse primer: CACCAGGAACTTCTTGAATC | This study | N/A |
| fruP1 gRNA: CACATAAACGCAGTACATGG TGG | This study | N/A |
| Recombinant DNA | ||
| Plasmid: pJRC81 | [63] | Addgene, Cat#104615 |
| Plasmid: 10XUAS-mLexA-VP16-NFATΔC | This study | N/A |
| Software and Algorithms | ||
| MATLAB | MathWorks | RRID:SCR_001622; https://www.mathworks.com/products/matlab.html |
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| pCLAMP 10.7 | Molecular Devices | RRID: SCR_011323; http://www.moleculardevices.com/products/software/pclamp.html |
| Igor Pro V.6 | Wavemetrics | RRID:SCR_000325; https://www.wavemetrics.com/products/igorpro/ |
| GPower 3.1 | [67]; Universität Düsseldorf | http://www.gpower.hhu.de |
| CFX Manager | Bio-Rad Laboratories, CA | Cat# 1845000 |
| Other | ||
| Aluminosilicate glass electrodes | Sutter Instrument Co., CA | AF100-64-10 |
| Vacuum desiccator | Cole-Parmer, IL | VX-06514-30 |
| Fluorescent dust | LDP LLC, NJ | UVXPBR |
Highlights.
Group housing enhances courtship motivation in mature but not immature males.
Group housing elevates the pheromone response of Or47b ORNs only in mature males.
CaMKI/CBP pathway synergizes with juvenile hormone in sensitizing Or47b ORNs.
FruM levels fine-tune pheromone sensitivity according to both fly density and age.
Acknowledgments
We thank members of the Wang, Su and Volkan labs for advice on experiments and comments on the manuscript; Renny Ng for editing; Dr. Jonathan Zirin and Dr. Norbert Perrimon for sharing the fruitless gRNA fly line; Dr. Yishi Jin for sharing the confocal microscope facility; Dr. Benjamin de Bivort for sharing the fly clipart images. This study is supported by NIH grants (R01NS109401 to P.C.V and J.W.W; R01DC016466 to J.W.W. and C.-Y.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Yapici N, Kim Y-J, Ribeiro C, and Dickson BJ (2008). A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451, 33–37. Available at: http://www.nature.com/articles/nature06483. [DOI] [PubMed] [Google Scholar]
- 2.Dey S, Chamero P, Pru JK, Chien M-S, Ibarra-Soria X, Spencer KR, Logan DW, Matsunami H, Peluso JJ, and Stowers L (2015). Cyclic Regulation of Sensory Perception by a Female Hormone Alters Behavior. Cell 161, 1334–1344. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0092867415005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Dankert H, Perona P, and Anderson DJ (2008). A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc. Natl. Acad. Sci 105, 5657–5663. Available at: http://www.pnas.org/cgi/doi/10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zelikowsky M, Hui M, Karigo T, Choe A, Yang B, Blanco MR, Beadle K, Gradinaru V, Deverman BE, and Anderson DJ (2018). The Neuropeptide Tac2 Controls a Distributed Brain State Induced by Chronic Social Isolation Stress. Cell 173, 1265–1279.e19. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0092867418303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang T, Yang CF, Chizari MD, Maheswaranathan N, Burke KJ, Borius M, Inoue S, Chiang MC, Bender KJ, Ganguli S, et al. (2017). Social Control of Hypothalamus-Mediated Male Aggression. Neuron 95, 955–970.e4. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0896627317305986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keleman K, Vrontou E, Krüttner S, Yu JY, Kurtovic-Kozaric A, and Dickson BJ (2012). Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature 489, 145–149. Available at: http://www.nature.com/articles/nature11345. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira RF (2009). Social behavior in context: Hormonal modulation of behavioral plasticity and social competence. Integr. Comp. Biol 49, 423–440. Available at: https://academic.oup.com/icb/article-lookup/doi/10.1093/icb/icp055. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, Liang X, Gong J, Yang Z, Zhang Y-H, Zhang J-X, and Rao Y (2011). Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat. Neurosci 14, 896–902. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21685916. [DOI] [PubMed] [Google Scholar]
- 9.Hosken DJ, Bretman A, Goodwin SF, and Ruth Archer C (2019). Genes and Environments in Drosophila Sex In Genes and Behaviour (Chichester, UK: John Wiley & Sons, Ltd; ), pp. 111–129. Available at: http://doi.wiley.com/10.1002/9781119313663.ch6. [Google Scholar]
- 10.Li X, Ishimoto H, and Kamikouchi A (2018). Auditory experience controls the maturation of song discrimination and sexual response in Drosophila. Elife 7, 1–19. Available at: https://elifesciences.org/articles/34348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marie-Orleach L, Bailey NW, and Ritchie MG (2019). Social effects on fruit fly courtship song. Ecol. Evol 9, 410–416. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/ece3.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McRobert SP, and Tompkins L (1988). Two Consequences of Homosexual Courtship Performed by Drosophila melanogaster and Drosophila affinis Males. Evolution (N. Y). 42, 1093 Available at: https://www.jstor.org/stable/2408925?origin=crossref. [DOI] [PubMed] [Google Scholar]
- 13.Dankert H, Wang L, Hoopfer ED, Anderson DJ, and Perona P (2009). Automated monitoring and analysis of social behavior in Drosophila. Nat. Methods 6, 297–303. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24363022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bretman A, Fricke C, and Chapman T (2009). Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc. R. Soc. B Biol. Sci 276, 1705–1711. Available at: http://www.royalsocietypublishing.org/doi/10.1098/rspb.2008.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim WJ, Jan LY, and Jan YN (2012). Contribution of visual and circadian neural circuits to memory for prolonged mating induced by rivals. Nat. Neurosci 15, 876–883. Available at: http://www.nature.com/articles/nn.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bentzur A, Shmueli A, Omesi L, Ryvkin J, Knapp J-M, Parnas M, Davis FP, and Shohat-Ophir G (2018). Odorant binding protein 69a connects social interaction to modulation of social responsiveness in Drosophila. PLOS Genet. 14, e1007328 Available at: https://dx.plos.org/10.1371/journal.pgen.1007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayser MS, Yue Z, and Sehgal A (2014). A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science 344, 269–74. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24744368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin H-H, Cao D-S, Sethi S, Zeng Z, Chin JSR, Chakraborty TS, Shepherd AK, Nguyen CA, Yew JY, Su C-Y, et al. (2016). Hormonal Modulation of Pheromone Detection Enhances Male Courtship Success. Neuron 90, 1272–1285. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0896627316301647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto D, and Kohatsu S (2017). What does the fruitless gene tell us about nature vs. nurture in the sex life of Drosophila? Fly (Austin). 11, 139–147. Available at: 10.1080/19336934.2016.1263778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flatt T, Tu M-P, and Tatar M (2005). Hormonal pleiotropy and the juvenile hormone regulation ofDrosophila development and life history. BioEssays 27, 999–1010. Available at: http://doi.wiley.com/10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- 21.Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, and Dickson BJ (2005). Neural Circuitry that Governs Drosophila Male Courtship Behavior. Cell 121, 795–807. Available at: https://linkinghub.elsevier.com/retrieve/pii/S009286740500406X. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto D, and Koganezawa M (2013). Genes and circuits of courtship behaviour in Drosophila males. Nat. Rev. Neurosci 14, 681–92. Available at: 10.1038/nrn3567. [DOI] [PubMed] [Google Scholar]
- 23.Dweck HKM, Ebrahim SAM, Thoma M, Mohamed AAM, Keesey IW, Trona F, Lavista-Llanos S, Svatoš A, Sachse S, Knaden M, et al. (2015). Pheromones mediating copulation and attraction in Drosophila. Proc. Natl. Acad. Sci 112, E2829–E2835. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25964351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Goes van Naters W, and Carlson JR (2007). Receptors and Neurons for Fly Odors in Drosophila. Curr. Biol 17, 606–612. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0960982207010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kvelland I (1965). Some observations on the mating activity and fertility of Drosophila melanogaster males. Hereditas 53, 281–306. Available at: http://doi.wiley.com/10.1111/j.1601-5223.1965.tb01997.x. [DOI] [PubMed] [Google Scholar]
- 26.Long CE, Markow TA, and Yaeger P (1980). Relative male age, fertility, and competitive mating success inDrosophila melanogaster. Behav. Genet 10, 163–170. Available at: http://link.springer.com/10.1007/BF01066266. [DOI] [PubMed] [Google Scholar]
- 27.Zucker RS (1999). Calcium- and activity-dependent synaptic plasticity. Curr. Opin. Neurobiol 9, 305–313. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0959438899800452. [DOI] [PubMed] [Google Scholar]
- 28.Masuyama K, Zhang Y, Rao Y, and Wang JW (2012). Mapping Neural Circuits with Activity-Dependent Nuclear Import of a Transcription Factor. J. Neurogenet 26, 89–102. Available at: http://www.tandfonline.com/doi/full/10.3109/01677063.2011.642910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu YV, Bell HW, Glauser DA, VanHooser SD, Goodman MB, and Sengupta P (2014). CaMKI-Dependent regulation of sensory gene expression mediates experience-dependent plasticity in the operating range of a thermosensory neuron. Neuron 84, 919–926. Available at: 10.1016/j.neuron.2014.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hueston CE, Olsen D, Li Q, Okuwa S, Peng B, Wu J, and Volkan PC (2016). Chromatin Modulatory Proteins and Olfactory Receptor Signaling in the Refinement and Maintenance of Fruitless Expression in Olfactory Receptor Neurons. PLOS Biol. 14, e1002443 Available at: http://dx.plos.org/10.1371/journal.pbio.1002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman RH, and Smolik S (2000). CBP/p300 in cell growth, transformation, and development. Genes Dev. 14, 1553–77. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10887150. [PubMed] [Google Scholar]
- 32.Turner BM, and La Thangue NB (1991). Histone acetylation and control of gene expression. J. Cell Sci 99 (Pt 1), 13–20. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1757496. [DOI] [PubMed] [Google Scholar]
- 33.Impey S, Fong AL, Wang Y, Cardinaux JR, Fass DM, Obrietan K, Wayman GA, Storm DR, Soderling TR, and Goodman RH (2002). Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron 34, 235–44. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11970865. [DOI] [PubMed] [Google Scholar]
- 34.Lee SS, Ding Y, Karapetians N, Rivera-Perez C, Noriega FG, and Adams ME (2017). Hormonal Signaling Cascade during an Early-Adult Critical Period Required for Courtship Memory Retention in Drosophila. Curr. Biol 27, 2798–2809.e3. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0960982217310266. [DOI] [PubMed] [Google Scholar]
- 35.Jindra M, Bellés X, and Shinoda T (2015). Molecular basis of juvenile hormone signaling. Curr. Opin. insect Sci 11, 39–46. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28285758. [DOI] [PubMed] [Google Scholar]
- 36.Ewen-Campen B, Yang-Zhou D, Fernandes VR, González DP, Liu L-P, Tao R, Ren X, Sun J, Hu Y, Zirin J, et al. (2017). Optimized strategy for in vivo Cas9-activation in Drosophila. Proc. Natl. Acad. Sci 114, 9409–9414. Available at: http://www.pnas.org/lookup/doi/10.1073/pnas.1707635114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu B, Ma L, Zhang E, Du J, Liu S, Price J, Li S, and Zhao Z (2018). Sexual dimorphism of sleep regulated by juvenile hormone signaling in Drosophila. PLOS Genet. 14, e1007318 Available at: https://dx.plos.org/10.1371/journal.pgen.1007318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nègre N, Brown CD, Ma L, Bristow CA, Miller SW, Wagner U, Kheradpour P, Eaton ML, Loriaux P, Sealfon R, et al. (2011). A cis-regulatory map of the Drosophila genome. Nature 471, 527–531. Available at: http://www.nature.com/articles/nature09990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Applebaum SW, and Heifetz Y (1999). Density-dependent physiological phase in insects. Annu. Rev. Entomol 44, 317–341. Available at: http://www.annualreviews.org/doi/10.1146/annurev.ento.44.1.317. [DOI] [PubMed] [Google Scholar]
- 40.Brennan PLR, Gereg I, Goodman M, Feng D, and Prum RO (2017). Evidence of phenotypic plasticity of penis morphology and delayed reproductive maturation in response to male competition in waterfowl. Auk 134, 882–893. Available at: https://academic.oup.com/auk/article/134/4/882-893/5149125. [Google Scholar]
- 41.Ito H, Sato K, Koganezawa M, Ote M, Matsumoto K, Hama C, and Yamamoto D (2012). Fruitless Recruits Two Antagonistic Chromatin Factors to Establish Single-Neuron Sexual Dimorphism. Cell 149, 1327–1338. Available at: 10.1016/j.cell.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 42.Kimura K-I, Ote M, Tazawa T, and Yamamoto D (2005). Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 438, 229–233. Available at: http://www.nature.com/articles/nature04229. [DOI] [PubMed] [Google Scholar]
- 43.Kohl J, Ostrovsky AD, Frechter S, and Jefferis GSXE (2013). A Bidirectional Circuit Switch Reroutes Pheromone Signals in Male and Female Brains. Cell 155, 1610–1623. Available at: 10.1016/j.cell.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalton JE, Fear JM, Knott S, Baker BS, McIntyre LM, and Arbeitman MN (2013). Male-specific Fruitless isoforms have different regulatory roles conferred by distinct zinc finger DNA binding domains. BMC Genomics 14, 659 Available at: http://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-14-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cardoso SD, Teles MC, and Oliveira RF (2015). Neurogenomic mechanisms of social plasticity. J. Exp. Biol 218, 140–149. Available at: http://jeb.biologists.org/cgi/doi/10.1242/jeb.106997. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Roy A, and Palli SR (2018). CREB-binding protein plays key roles in juvenile hormone action in the red flour beetle, Tribolium Castaneum. Sci. Rep 8, 1426 Available at: http://www.nature.com/articles/s41598-018-19667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frønsdal K, Engedal N, Slagsvold T, and Saatcioglu F (1998). CREB Binding Protein Is a Coactivator for the Androgen Receptor and Mediates Cross-talk with AP-1. J. Biol. Chem 273, 31853–31859. Available at: http://www.jbc.org/lookup/doi/10.1074/jbc.273.48.31853. [DOI] [PubMed] [Google Scholar]
- 48.Smith CL, Oñate SA, Tsai MJ, and O’Malley BW (1996). CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc. Natl. Acad. Sci. U. S. A 93, 8884–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8799122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartfelder K, and Emlen DJ (2012). Endocrine Control of Insect Polyphenism In Insect Endocrinology (Elsevier; ), pp. 464–522. Available at: https://linkinghub.elsevier.com/retrieve/pii/B9780123847492100111. [Google Scholar]
- 50.Ball GF, and Balthazart J (2008). Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Philos. Trans. R. Soc. B Biol. Sci 363, 1699–1710. Available at: https://royalsocietypublishing.org/doi/10.1098/rstb.2007.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang CF, and Shah NM (2014). Representing Sex in the Brain, One Module at a Time. Neuron 82, 261–278. Available at: 10.1016/j.neuron.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elekonich MM, and Robinson GE (2000). Organizational and activational effects of hormones on insect behavior. J. Insect Physiol 46, 1509–1515. Available at: https://pdfs.semanticscholar.org/presentation/9c9c/34ad8643fd37de299e3fcc5f5c46065ca4c6.pdf?_ga=2.2663600.1267028239.1528779483-960716145.1527041992. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Han X, Mehren J, Hiroi M, Billeter J-C, Miyamoto T, Amrein H, Levine JD, and Anderson DJ (2011). Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat. Neurosci 14, 757–762. Available at: http://www.nature.com/articles/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fishilevich E, and Vosshall LB (2005). Genetic and Functional Subdivision of the Drosophila Antennal Lobe. Curr. Biol 15, 1548–1553. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0960982205008936. [DOI] [PubMed] [Google Scholar]
- 55.Shearin HK, Macdonald IS, Spector LP, and Stowers RS (2014). Hexameric GFP and mCherry Reporters for the Drosophila GAL4, Q, and LexA Transcription Systems. Genetics 196, 951–960. Available at: http://www.genetics.org/lookup/doi/10.1534/genetics.113.161141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perkins LA, Holderbaum L, Tao R, Hu Y, Sopko R, McCall K, Yang-Zhou D, Flockhart I, Binari R, Shim H-S, et al. (2015). The Transgenic RNAi Project at Harvard Medical School: Resources and Validation. Genetics 201, 843–852. Available at: http://www.genetics.org/lookup/doi/10.1534/genetics.115.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smolik S, and Jones K (2007). Drosophila dCBP Is Involved in Establishing the DNA Replication Checkpoint. Mol. Cell. Biol 27, 135–146. Available at: http://mcb.asm.org/cgi/doi/10.1128/MCB.01283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gordon MD, and Scott K (2009). Motor Control in a Drosophila Taste Circuit. Neuron 61, 373–384. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0896627309000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferveur J, Stortkuhl K, Stocker R, and Greenspan R (1995). Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science (80-.). 267, 902–905. Available at: http://www.sciencemag.org/cgi/doi/10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- 60.Demir E, and Dickson BJ (2005). fruitless Splicing Specifies Male Courtship Behavior in Drosophila. Cell 121, 785–794. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0092867405004071. [DOI] [PubMed] [Google Scholar]
- 61.Meissner GW, Luo SD, Dias BG, Texada MJ, and Baker BS (2016). Sex-specific regulation of Lgr3 in Drosophila neurons. Proc. Natl. Acad. Sci. U. S. A 113, E1256–65. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26884206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, et al. (2004). The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167, 761–81. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15238527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pfeiffer BD, Truman JW, and Rubin GM (2012). Using translational enhancers to increase transgene expression in Drosophila. Proc. Natl. Acad. Sci 109, 6626–6631. Available at: http://www.pnas.org/cgi/doi/10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kondo S, and Ueda R (2013). Highly Improved Gene Targeting by Germline-Specific Cas9 Expression in Drosophila. Genetics 195, 715–721. Available at: http://www.genetics.org/lookup/doi/10.1534/genetics.113.156737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ng R, Lin H-H, Wang JW, and Su C-Y (2017). Electrophysiological Recording from Drosophila Trichoid Sensilla in Response to Odorants of Low Volatility. J. Vis. Exp Available at: https://www.jove.com/video/56147/electrophysiological-recording-from-drosophila-trichoid-sensilla. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang JW, Wong AM, Flores J, Vosshall LB, and Axel R (2003). Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, 271–82. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12553914. [DOI] [PubMed] [Google Scholar]
- 67.Faul F, Erdfelder E, Buchner A, and Lang A-G (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160. Available at: http://www.springerlink.com/index/10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data for single-sensillum recording experiments are available upon request to the Lead Contact.