Abstract
Background
Due to concerns about hypertriglyceridemia, liver enzyme abnormalities, and leukopenia during isotretinoin therapy for acne, patients are often followed closely with routine laboratory monitoring, although the value of this practice has been questioned.
Methods
We conducted a cohort study of patients receiving isotretinoin for acne between January 1, 2008 and June 30, 2017 using the OptumInsights Electronic Health Record Database to evaluate the frequency of laboratory abnormalities. Poisson regression was used to evaluate for changes to the frequency of routine laboratory monitoring over time.
Results
Among 1,863 patients treated with isotretinoin, grade 3 or greater triglyceride and liver function testing abnormalities were noted in fewer than 1% and 0.5% of patients screened, respectively. No grade 3 or greater cholesterol or complete blood count abnormalities were observed. There were no meaningful changes in the frequency of laboratory monitoring over time.
Conclusions and Relevance
While laboratory abnormalities are rare and often do not influence management, frequent laboratory monitoring remains a common practice. There are opportunities to improve the quality of care among patients being treated with isotretinoin for acne by reducing the frequency of lipid and liver function monitoring and by eliminating complete blood count monitoring.
Capsule Summary
• Little is known about whether lab monitoring practices for patients being treated for acne with isotretinoin are changing over time.
• Although laboratory abnormalities are rare, frequent monitoring remains a common practice and there are opportunities to improve quality and cost of care by reducing the frequency of this monitoring.
Introduction
Isotretinoin is a highly effective treatment for acne which can reliably lead to remission of disease activity following treatment; however, its use has been associated with several important adverse events, most notably teratogenicity.1 In addition, due to concerns about hypertriglyceridemia, potential elevation of liver enzymes, leukopenia, and thrombocytopenia during therapy, patients are frequently monitored as often as monthly for lipid, liver enzyme, and complete blood count abnormalities.1 However, several reports over the past two decades have questioned the clinical utility of frequent (e.g. monthly) lab monitoring and have suggested reduced monitoring practices.1–7 For example, the 2003 Global Alliance to Improve Outcomes in Acne guidelines suggest limiting testing to labs at baseline and then after 1 to 2 months of therapy, and recommend no further testing if these initial lab results are normal and there are no other risk factors present.8 A 2006 study by Zane and colleagues suggested that complete blood count monitoring may be of low value.6 More recently Hansen and colleagues have suggested that, in the absence of known risk factors, complete blood count monitoring should be eliminated, monitoring of lipid panel and liver function tests should be performed at baseline and after the peak dose is obtained, and the should be no further monitoring if these results are normal.7
While lab monitoring practices have been a subject of several studies over the past two decades, there remains uncertainty regarding the type and frequency of lab monitoring being ordered for patients on isotretinoin in routine clinical practice.5–7 In addition, little is known about whether monitoring practices are changing over time in response to findings from these studies that question frequent lab monitoring practices for isotretinoin. The purpose of this study was to evaluate the frequency of monitoring practices and laboratory abnormalities among a large cohort of patients and to examine whether isotretinoin monitoring practices are changing over time.
Methods
Study design and data source
We performed a cohort study using a 10% random sample of the Optumlnsights Electronic Health Record database that included data from January 1, 2007 to June 30, 2017; this was the dataset available to the investigators. The full dataset includes de-identified data abstracted from the electronic medical records of over 150,000 providers, 2,000 hospitals, and 7,000 clinics and is based on integrating data from the electronic medical record and other health information technology platforms used in these practices.9–12 With de-identified patient-level data for 81 million individuals and their associated healthcare encounters, it is the largest electronic health record source in the United States.10 The data captured in the database include diagnoses, prescriptions written, and laboratory data as well as patient demographic information such as age and gender. This study was granted exempt status by the University of Pennsylvania Institutional Review Board.
Study population
Inclusion criteria were: (1) patients with at least one International Classification of Diseases (ICD) 9 or 10 code for acne (706.1, L70.0, L70.1, L70.8, L70.9); (2) at least one prescription for isotretinoin on or after the acne diagnosis date, with the first prescription meeting all inclusion criteria being defined as the index date; (3) at least six months of continuous enrollment prior to the index date with no prescriptions for isotretinoin; (4) at least one year of continuous enrollment after the index date. The final two criteria were chosen to increase the likelihood of capturing a complete course of isotretinoin therapy. Due to few patients meeting study inclusion criteria in 2007, we limited our analyses to 2008 onwards. Previous studies have validated the accuracy of ICD codes to identify patients with acne.13 Individual consecutive prescriptions for isotretinoin were identified to define courses of therapy. To allow for minor delays in prescribing due to logistical and other factors, prescriptions separated by fewer than 15 days were considered part of the same course of therapy. For each patient, only the first course of isotretinoin was evaluated.
Outcomes
Laboratory values for triglycerides, total cholesterol, aspartate aminotransferase (AST), alanine aminotransferase (ALT), white blood cell count (WBC), and platelet count were identified for each patient. Labs were categorized as baseline labs or by month of therapy for those performed while the patient was receiving isotretinoin. To account for delays in initiating therapy related to iPLEDGE requirements, baseline labs were defined as those performed up to 60 days prior to the first prescription. Labs performed up to 15 days after the end date of the course of therapy were included and attributed to the final month of treatment. Laboratory abnormalities were defined based on the Cancer Institute Common Terminology Criteria for Adverse Events v5.0 grading system (see Table 4 for details).6,7,14 The frequency of abnormalities that were Grade 3 or higher was evaluated since this cutoff corresponds to values that are often felt to be clinically meaningful (e.g. triglycerides>500mg/dl) and is consistent with thresholds used in prior studies.7 In an effort to include patients being treated with typical courses of therapy, we restricted our analysis of changes in the frequency of lab monitoring over time to the first six months of therapy among those who were prescribed isotretinoin for at least 3 months.
Table 4.
Number of patients with a lab abnormality by month of therapy
| Baseline | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 | |
|---|---|---|---|---|---|---|---|
| Triglycerides, mg/dL | |||||||
| Grade 1 (150–300), n (%) | 161 (13.3) | 146 (19.8) | 190 (22.2) | 234 (29.5) | 195 (29.3) | 144 (32.7) | 92 (39.0) |
| Grade 2 (300–500), n (%) | 17 (1.4) | 18 (2.4) | 21 (2.5) | 30 (3.8) | 38 (5.6) | 16 (3.7) | 13 (5.3) |
| Grade 3 (500–1000), n (%) | 1 (0.1) | 1 (0.1) | 4 (0.5) | 6 (0.7) | 0 (0) | 2 (0.4) | 0 (0) |
| Grade 4 (1000), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total Cholesterol, mg/dL | |||||||
| Grade 1 (ULN-300), n (%) | 117 (12.1) | 101 (19.2) | 123 (20.3) | 115 (20.7) | 113 (24.5) | 74 (25.4) | 45 (26.1) |
| Grade 2 (300–400), n (%) | 1 (0.1) | 2 (0.4) | 2 (0.3) | 3 (0.5) | 6 (1.3) | 3 (1) | 2 (1.1) |
| Grade 3 (400–500), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Grade 4 (>500), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| AST, IU/L | |||||||
| Grade 1 (ULN-3x ULN), n (%) | 46 (3.8) | 29 (4.0) | 45 (5.5) | 49 (6.6) | 38 (5.9) | 25 (5.9) | 16 (6.7) |
| Grade 2 (3x ULN-5x ULN), n (%) | 2 (0.2) | 1 (0.1) | 2 (0.2) | 1 (0.1) | 0 (0) | 1 (0.2) | 1 (0.4) |
| Grade 3 (5x ULN-20x ULN), n (%) | 2 (0.2) | 1 (0.1) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) |
| Grade 4 (>20x ULN), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ALT, IU/L | |||||||
| Grade 1 (ULN-3x ULN), n (%) | 38 (3.1) | 26 (3.7) | 43 (5.4) | 34 (4.6) | 24 (3.8) | 13 (3.1) | 7 (2.9) |
| Grade 2 (3x ULN-5x ULN), n (%) | 1 (0.1) | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0.8) |
| Grade 3 (5x ULN-20x ULN), n (%) | 1 (0.1) | 0 (0) | 1 (0.1) | 2 (0.3) | 4 (0.6) | 0 (0) | 0 (0) |
| Grade 4 (>20x ULN), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| WBC, ×10^3/mL | |||||||
| Grade 1 (3-LLN), n (%) | 41 (4.1) | 33 (6.7) | 38 (7.1) | 31 (6.6) | 31 (7.3) | 20 (7.2) | 15 (10.1) |
| Grade 2 (2–3), n (%) | 1 (0.1) | 3 (0.6) | 2 (0.4) | 2 (0.4) | 2 (0.5) | 1 (0.4) | 1 (0.7) |
| Grade 3 (1–2), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Grade 4 (<1), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Platelets, ×10^3/mL | |||||||
| Grade 1 (75-LLN), n (%) | 19 (1.9) | 11 (2.2) | 12 (2.3) | 7 (1.5) | 5 (1.2) | 8 (2.9) | 2 (1.4) |
| Grade 2 (50–75), n (%) | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Grade 3 (25–50), n (%) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Grade 4 (<25), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
AST: aspartate aminotransferase; ALT: alanine aminotransferase; WBC: white blood cell Details on the absolute number of patients screened each month can be found in Table 2.
Statistical analysis
The monitoring frequency of each lab was calculated as the number of patients who had one or more of the tests of interest performed each month divided by the number of patients on therapy each month. Univariate Poisson regression models were used to assess changes in the frequency of lab monitoring over time whereby number of labs ordered over time was considered count data. Goodness of fit testing confirmed that the model fit the distribution of the data. Costs of monitoring were estimated based on the Medicare clinical laboratory fee schedule.15 Statistical analyses were performed in Stata 15 (StataCorp, College Station, Texas).
Results
Cohort
There were 1,863 patients included in the analysis and 49.0% were women. The median age was 18.2 years (IQR 16.3–24.5). The median course duration for isotretinoin treatment was 148 days (IQR 65–183) (Table 1). Regional distribution of patients across the U.S. were: 11.5% Northwest, 61.9% Midwest, 13.6% South, and 9.7% West.
Table 1.
Patient characteristics (n=1,863).
| Age, median (IQR) | 18.2 (16.3–24.5) |
| Female, n (%) | 912 (49.0) |
| Race, n (%) | |
| White | 1620 (87.0) |
| African American | 46 (2.5) |
| Asian | 38 (2.0) |
| Other/Unknown | 159 (8.5) |
| Ethnicity, n (%) | |
| Hispanic | 58 (3.1) |
| Non-Hispanic | 1669 (89.6) |
| Unknown | 136 (7.3) |
| Region, n (%) | |
| Northeast | 214 (11.5) |
| Midwest | 1153 (61.9) |
| South | 254 (13.6) |
| West | 181 (9.7) |
| Other/Unknown | 61 (3.3) |
| Treatment duration, days, median (IQR) | 148 (65–183) |
| Number of prescriptions, median (IQR) | 5 (2–6) |
Monitoring frequency and changes over time
In general, absolute numbers of patients on therapy and, consequently, absolute numbers of patients being monitored decreased with each successive month. Baseline triglyceride and total cholesterol levels were evaluated in 65.3% and 52.5% of patients, respectively. The range of patients who received triglyceride monitoring each month while on therapy was between 39.6% and 61.4% of patients. Baseline AST and ALT levels were evaluated in 64.6% and 64.8% of patients, respectively. The range of patients who received AST and ALT monitoring each month while on therapy was between 37.6% and 58.5% of patients. Baseline WBC and platelet count levels were evaluated in 53.9% and 53.5% of patients, respectively. The range of patients who received complete blood count monitoring each month while on therapy was between 26.8% and 37.4% of patients (Table 2).
Table 2.
Percentage of Patients on Isotretinoin with Labs Checked by Month
| Baseline | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 | |
|---|---|---|---|---|---|---|---|
| Number of patients on therapy | 1863 | 1863 | 1448 | 1295 | 1130 | 895 | 498 |
| Triglycerides, n (%) | 1216 (65.3) | 737 (39.6) | 855 (59.0) | 795 (61.4) | 667 (59.0) | 440 (49.2) | 237 (47.6) |
| Total Cholesterol, n (%) | 978 (52.5) | 533 (28.6) | 615 (42.5) | 562 (43.4) | 468 (41.4) | 298 (33.3) | 176 (35.3) |
| AST, n (%) | 1203 (64.6) | 723 (38.8) | 815 (56.3) | 757 (58.5) | 641 (56.7) | 422 (47.2) | 238 (47.8) |
| ALT, n (%) | 1208 (64.8) | 700 (37.6) | 794 (54.8) | 732 (56.5) | 626 (55.4) | 411 (45.9) | 237 (47.6) |
| WBC, n (%) | 1005 (53.9) | 504 (27.1) | 531 (36.7) | 472 (36.4) | 423 (37.4) | 276 (30.8) | 148 (29.7) |
| Platelets, n (%) | 997 (53.5) | 499 (26.8) | 528 (36.5) | 470 (36.3) | 422 (37.3) | 274 (30.6) | 147 (29.5) |
AST: aspartate aminotransferase; ALT: alanine aminotransferase; WBC: white blood cell
Between 2008 and 2016, the frequency of triglyceride monitoring decreased 16.4% from 0.67 to 0.56 labs per month (incidence rate ratio (IRR) per year 0.98; 95% CI 0.96 – 0.99). The frequency of cholesterol monitoring decreased 12.5% from 0.48 to 0.42 labs per month (IRR per year 0.97; 95% CI 0.95 – 1.00). The frequency of AST monitoring decreased 20.9% from 0.67 to 0.53 labs per month (IRR 0.98 per year; 95% CI 0.96 – 0.99). The frequency of ALT monitoring decreased 25.0% from 0.68 to 0.51 labs per month (IRR 0.99 per year; 95% CI 0.97 – 1.00). The frequency of WBC monitoring decreased 12.2% from 0.41 to 0.36 labs per month (IRR per year 1.00; 95% CI 0.97–1.02) The frequency of platelet count monitoring decreased 12.2% from 0.41 to 0.36 labs per month (IRR 0.99 per year; 95% CI 0.97 – 1.02). Statistically significant decreases in laboratory monitoring frequency over the study duration were noted for triglycerides (IRR 0.82 for the study period; 95% CI 0.72 – 0.93), total cholesterol (IRR 0.80 for the study period; 95% CI 0.66 – 0.98) and AST (IRR 0.84 for the study period; 95% CI 0.73 – 0.95) only (Table 3).
Table 3.
Labs Per Month of Therapy
| Year | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | IRR per year (95% CI) | IRR for study period (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Triglycerides | 0.67 | 0.72 | 0.67 | 0.68 | 0.64 | 0.69 | 0.63 | 0.61 | 0.56 | 0.98 (0.96–0.99) | 0.82 (0.72–0.93) | |
| Cholesterol | 0.48 | 0.46 | 0.50 | 0.49 | 0.47 | 0.47 | 0.46 | 0.41 | 0.42 | 0.97 (0.95–1.00) | 0.80 (0.66–0.98) | |
| AST | 0.67 | 0.64 | 0.65 | 0.61 | 0.60 | 0.62 | 0.60 | 0.58 | 0.53 | 0.98 (0.96–0.99) | 0.84 (0.73–0.95) | |
| ALT | 0.68 | 0.62 | 0.57 | 0.59 | 0.56 | 0.62 | 0.59 | 0.59 | 0.51 | 0.99 (0.97–1.00) | 0.90 (0.78–1.04) | |
| WBC | 0.41 | 0.45 | 0.35 | 0.39 | 0.39 | 0.43 | 0.41 | 0.40 | 0.36 | 1.00 (0.97–1.02) | 0.97 (0.77–1.22) | |
| Platelets | 0.41 | 0.45 | 0.35 | 0.39 | 0.39 | 0.42 | 0.41 | 0.40 | 0.36 | 0.99 (0.97–1.02) | 0.96 (0.76–1.20) |
AST: aspartate aminotransferase; ALT: alanine aminotransferase; WBC: white blood cell count
Number of labs per month for each patient was calculated as the number of labs performed while receiving treatment with isotretinoin divided by the total number of months of therapy received
Frequency of laboratory abnormalities
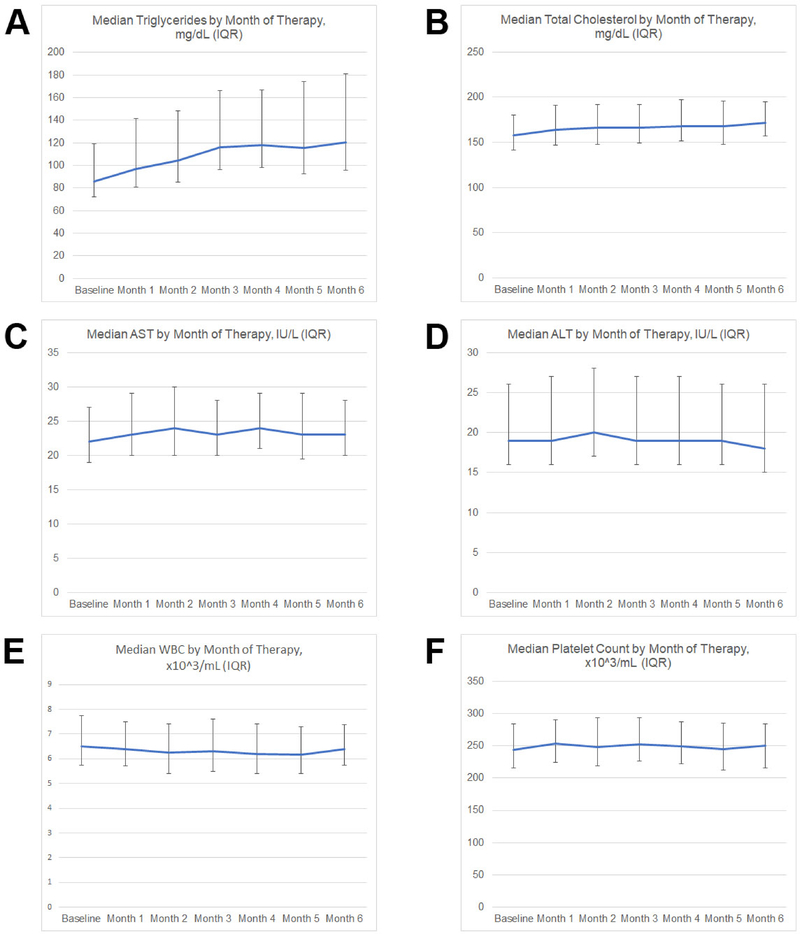
Grade 3 triglyceride abnormalities were noted in fewer than 1% of patients screened (Table 4). Among the 12 patients who developed grade 3 triglyceride abnormalities while on therapy, seven (58.3%) had elevated baseline triglycerides, and six (50%) had their triglycerides repeated, of whom four (66.7%) had improved triglycerides on repeat testing. Nine (76.9%) of the 12 patients with grade 3 triglyceride abnormalities continued their course of isotretinoin. Most lipid changes occurred early in the course of therapy before stabilizing (Figure 1). No grade 3 or higher cholesterol abnormalities were noted. Grade 3 AST and ALT abnormalities were noted in fewer than 0.5% of patients screened and were not more common on therapy than at baseline. Among the five patients who developed grade 3 AST or ALT abnormalities, four (80%) had improved values when these were rechecked and three patients (60%) continued their course of isotretinoin. No significant WBC or platelet abnormalities were noted, except for one patient who had a likely spurious platelet count of 32 (all other values were above 300 for this patient).
Figure 1. Laboratory value trends during isotretinoin therapy.
Median values and interquartile ranges (error bars) are presented for triglycerides (A), total cholesterol (B), aspartate aminotransferase (AST) (C), alanine aminotransferase (ALT) (D), white blood cell count (WBC) (E), and platelet count (F).
Cost of monitoring
Based on the Medicare clinical laboratory fee schedule reimbursement for lipid panel ($15), hepatic function panel ($9), and complete blood count ($9), the average patient in our study population undergoing a 6-month course of isotretinoin would be expected to incur approximately $134 in laboratory charges.15 In contrast, if monitoring was reduced to baseline lipid panel and liver function panel testing, repeated at approximately two months after achieving peak dose, as suggested by Hansen and colleagues, laboratory costs would decrease by approximately $87 per patient.7 Eliminating routine complete blood count testing alone would be expected to reduce patient costs by approximately $30 per course of therapy. Extrapolating these savings to the nearly 200,000 patients registered in iPLEDGE annually, reducing the frequency of monitoring as above would be expected to decrease patient care costs for isotretinoin monitoring by approximately $17.4 million annually. Based on the frequency of abnormalities detected, the cost of identifying one Grade 3 triglyceride or hepatic enzyme abnormality would be estimated at approximately $6,000 and $7,750, respectively.
Discussion
In this cohort study of patients across the United States, clinically significant abnormalities for patients receiving isotretinoin for acne were rare and often did not result in changes in management. In particular, no significant WBC or platelet count abnormalities were noted. These findings are consistent with prior studies and suggest that extensive laboratory monitoring in this population may be of low value.7,5,6 In addition, changes to lipid levels observed in this study typically occurred during the first 2–3 months of therapy before stabilizing, which is consistent with findings in prior studies.7
Despite the growing evidence base supporting reduced laboratory monitoring for patients being treated with isotretinoin for acne, we observed only modest decreases in the frequency of lipid and liver function laboratory monitoring between 2008 and 2016. Complete blood count monitoring frequency was unchanged over time at an average of 0.36 complete blood counts per month (or one complete blood count per 2.8 months) in 2016. Given the pain and psychologic distress experienced by young adults undergoing phlebotomy, concerns about frequent laboratory monitoring may deter eligible patients from receiving isotretinoin, which could result in underutilization of this treatment.16 In addition, the expense of and inconvenience associated with frequent testing could result in patients opting for other treatments. This potential underutilization of isotretinoin could result in worse outcomes for patients with moderate-to-severe acne. Finally, since patients who eventually receive isotretinoin may be treated with prolonged courses of oral antibiotics, underutilization of isotretinoin could result in higher use of oral antibiotics, increasing the risk of antibiotic associated complications.17,18
Consistent with prior research, the frequent laboratory monitoring observed in this study is associated with a significant cost burden to the patient and health system.7 As more patients become enrolled in high-deductible insurance plans, these laboratory charges will have increasing impact on patients’ out-of-pocket costs making it ever more important for clinicians to consider the clinical and financial effects of their practices.19 Additionally, frequent laboratory monitoring increases indirect costs to patients from missed work or school.
Using relatively conservative estimates based on the Medicare clinical laboratory fee schedule, the cost of identifying one Grade 3 triglyceride or liver enzyme abnormality was over $6,000. Since many patients who had an abnormal test continued therapy in our study, the costs to identify one clinically relevant abnormality may be even higher, particularly for liver enzyme testing. These findings raise the question of whether any laboratory testing is cost-effective in this patient population, although further research is needed to identify whether more optimized screening practices can improve the cost-effectiveness of monitoring by limiting screening to patients who are at highest risk.2
Given the costs of frequent laboratory monitoring for patients being treated with isotretinoin and the lack of significant changes in practice over time despite accumulating evidence that frequent monitoring may be of low value, it will be important to identify strategies to reduce this practice gap. One approach may be to provide more specific guideline recommendations regarding laboratory monitoring. While the 2003 Global Alliance to Improve Outcomes in Acne Group recommend limiting laboratory monitoring, the 2016 American Academy of Dermatology guidelines do not include particularly specific recommendations for the frequency of laboratory monitoring.1,8 While laboratory monitoring should be individualized to the needs of the specific patient, including consideration of baseline risk factors, adopting more specific guideline recommendations may encourage clinicians to feel comfortable reducing the frequency of monitoring for the typical patient. In addition, there may be opportunities to educate clinicians through specialty society publications and newsletters. As we continue to develop a stronger evidence-base regarding optimal laboratory monitoring patterns for patients on isotretinoin, it will be important to identify the best strategies to ensure this evidence results in appropriate changes to practice patterns.
Limitations
Given that the study was conducted using automated data from an electronic medical records database, we are unable to evaluate the clinical notes to understand the exact clinical decision when clinicians encountered abnormal laboratory values. However, since many patients received subsequent prescriptions for isotretinoin after the laboratory abnormalities were detected, it is likely that clinicians felt comfortable continuing therapy despite the laboratory abnormalities. In addition, we were unable to assess the exact dosages prescribed and to evaluate for associations between different dosing regimens and the frequency and degree of laboratory abnormalities. Nevertheless, given the geographic diversity and large sample size of our study population, our findings are likely generalizable to the typical use of isotretinoin in the community. Since patients may not have been fasting for their laboratory tests, some lipid abnormalities noted in this study may have been due to lack of fasting and the rate of true abnormalities may be lower than what was observed. The Medicare clinical laboratory fee schedule may underestimate the true costs of laboratory testing in this population and actual costs of testing may be higher for many patients with commercial insurance coverage.20
Conclusions
Among acne patients undergoing treatment with isotretinoin, laboratory abnormalities are rare and often do not influence management. However, frequent laboratory monitoring remains a common practice and the rate of laboratory monitoring has not decreased substantially over time. There are opportunities to improve the quality and cost of care by reducing the frequency of lipid and liver function monitoring and by eliminating the practice of complete blood count monitoring in this patient population, which should be highlighted in future clinical guidelines.
Acknowledgments
Funding sources: Funded in part through NIAMS 1P30AR069589-01. Dr. Barbieri is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number T32-AR-007465 and receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Junko Takeshita is supported by NIAMS K23-AR068433.
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication
Abbreviations
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- WBC
white blood cell count
Footnotes
Conflict of Interest Disclosure: Junko Takeshita receives a research grant from Pfizer Inc (to the Trustees of the University of Pennsylvania) for work that is unrelated to this study and has received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly and Novartis. The authors have no other conflicts of interest to disclose.
Institutional Review Board Approval: This study was approved the Institutional Review Board of the University of Pennsylvania.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33. [DOI] [PubMed] [Google Scholar]
- 2.Opel D, Kramer ON, Chevalier M, Bigby M, Albrecht J. Not every patient needs a triglyceride check, but all can get pancreatitis: a systematic review and clinical characterization of isotretinoin-associated pancreatitis. Br J Dermatol. 2017;177(4):960–966. [DOI] [PubMed] [Google Scholar]
- 3.Barth JH, Macdonald-Hull SP, Mark J, Jones RG, Cunliffe WJ. Isotretinoin therapy for acne vulgaris: a re-evaluation of the need for measurements of plasma lipids and liver function tests. Br J Dermatol. 1993;129(6):704–707. [DOI] [PubMed] [Google Scholar]
- 4.Altman RS, Altman LJ, Altman JS. A proposed set of new guidelines for routine blood tests during isotretinoin therapy for acne vulgaris. Dermatol Basel Switz. 2002;204(3):232–235. [DOI] [PubMed] [Google Scholar]
- 5.Lee YH, Scharnitz TP, Muscat J, Chen A, Gupta-Elera G, Kirby JS. Laboratory Monitoring During Isotretinoin Therapy for Acne: A Systematic Review and Meta-analysis. JAMA Dermatol. 2016;152(1):35–44. doi: 10.1001/jamadermatol.2015.3091 [DOI] [PubMed] [Google Scholar]
- 6.Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142(8):1016–1022. [DOI] [PubMed] [Google Scholar]
- 7.Hansen TJ, Lucking S, Miller JJ, Kirby JS, Thiboutot DM, Zaenglein AL. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75(2):323–328. [DOI] [PubMed] [Google Scholar]
- 8.Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(1 Suppl):S1–37. [DOI] [PubMed] [Google Scholar]
- 9.Optum, Inc. Clinical/EHR Data. https://www.optum.com/solutions/government/federal/data-analytics-federal/clinical-data.html. Accessed December 12, 2018.
- 10.Nunes AP, Yang J, Radican L, et al. Assessing occurrence of hypoglycemia and its severity from electronic health records of patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2016;121:192–203. [DOI] [PubMed] [Google Scholar]
- 11.Walker AM, Zhou X, Ananthakrishnan AN, et al. Computer-assisted expert case definition in electronic health records. Int J Med Inf. 2016;86:62–70. [DOI] [PubMed] [Google Scholar]
- 12.Mannino DM, Yu T-C, Zhou H, Higuchi K. Effects of GOLD-Adherent Prescribing on COPD Symptom Burden, Exacerbations, and Health Care Utilization in a Real-World Setting. Chronic Obstr Pulm Dis Miami Fla. 2015;2(3):223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ejaz A, Malaiyandi V, Kim WB, Rogalska T, Alhusayen R. Validating the diagnostic code for acne in a tertiary care dermatology centre. Eur JDermatol EJD. 2015;25(5):469–471. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Common Terminology Criteria for Adverse Events v5.0 (CTCAE). 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf. Accessed December 12, 2018.
- 15.Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/index.html. Published January 10, 2018. Accessed January 17, 2019.
- 16.Birnie KA, Noel M, Chambers CT, Uman LS, Parker JA. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2018;10:CD005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74(2):273–279. [DOI] [PubMed] [Google Scholar]
- 18.Barbieri JS, Spaccarelli N, Margolis DJ, James WD. Approaches to limit systemic antibiotic use in acne: Systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. October 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.America’s Health Insurance Plans. Health Savings Accounts and High Deductible Health Plans Grow as Valuable Financial Planning Tools. https://www.ahip.org/wp-content/uploads/2018/04/HSA_Report_4.12.18.pdf. Accessed January 12, 2019.
- 20.Transparency in Pricing for the Hospital of the University of Pennsylvania - Penn Medicine. https://www.pennmedicine.org/for-patients-and-visitors/patient-information/insurance-and-billing/financial-transparency/financial-transparency-for-the-hospital-of-the-university-of-pennsylvania. Accessed January 12, 2019.