Key Points
In these frail patients, ixazomib 4 mg/wk, the dosage for multiple myeloma, was generally well tolerated with a manageable safety profile.
Ixazomib is active in patients with relapsed/refractory AL amyloidosis with encouraging hematologic (52%) and organ (56%) response rates.
Abstract
This phase 1/2 study assessed the safety, tolerability, and preliminary efficacy of the oral proteasome inhibitor (PI) ixazomib in patients with relapsed/refractory immunoglobulin light chain (AL) amyloidosis. Ixazomib was administered to adult patients with relapsed/refractory AL amyloidosis after 1 or more prior lines of therapy (including bortezomib) on days 1, 8, and 15 of 28-day cycles, for up to 12 cycles. Patients with less than partial response after 3 cycles received oral dexamethasone (40 mg, days 1-4) from cycle 4. A 3+3 dose-escalation phase was followed by 2 expansion cohorts (PI-naive and PI-exposed patients) at the maximum tolerated dose (MTD). Twenty-seven patients were enrolled: 11 during dose escalation (6 at 4.0 mg and 5 at 5.5 mg) and 16 during dose expansion (4.0 mg). Three patients experienced dose-limiting toxicities: 1 at 4.0 mg and 2 at 5.5 mg; the MTD was determined as 4.0 mg. Most common adverse events (AEs) included nausea, skin and subcutaneous tissue disorders (SSTD), diarrhea, and fatigue; grade 3 or higher AEs included dyspnea, fatigue, and SSTD. Overall, the hematologic response rate was 52% in patients treated at the MTD (n = 21). Organ responses were seen in 56% of patients (5 cardiac, 5 renal). Median hematologic progression-free survival was 14.8 months; 1-year progression-free and overall survival rates were 60% and 85%, respectively (median follow-up, 16.9 months). Weekly oral ixazomib appears to be active in patients with relapsed/refractory AL amyloidosis, with a generally manageable safety profile. The study was registered at clinicaltrials.gov as #NCT01318902. A phase 3 study is ongoing (#NCT01659658).
Introduction
Systemic light-chain (AL) amyloidosis is a rare protein misfolding disorder caused by abnormal immunoglobulin light chains produced by a plasma cell clone that is typically small and indolent.1-4 These abnormal light chains form fibrils that are deposited in the organs (kidneys, liver, heart, peripheral and autonomic nervous systems, soft tissues, gastrointestinal system, and lungs), causing toxicity and organ dysfunction.1-5 As the disease is sustained by a plasma cell clone, a number of therapies are effective for the treatment of both multiple myeloma (MM) and AL amyloidosis.3 The current standard treatment of newly diagnosed patients is chemotherapy, often a combination of melphalan and dexamethasone6 or cyclophosphamide, dexamethasone, and bortezomib.5 High-dose melphalan followed by autologous stem-cell transplantation is also highly effective in a subset of patients without multiple organ involvement or severe cardiac dysfunction.4 Although there are no currently approved therapies for patients with relapsed or refractory disease, bortezomib, lenalidomide, and pomalidomide, all of which are approved for the treatment of MM, are effective.5,7-12
Achievement of a hematologic response is required and predictive of an organ response (which can take up to a year),13 as well as prolonged survival.14,15 Response criteria were established in 200516 and updated in 2012 based on the difference between levels of involved and uninvolved free light chains (dFLC)17; these updated criteria were later validated.17-19 The proteasome inhibitor (PI) bortezomib is approved for the treatment of MM and has shown activity in both previously untreated and relapsed/refractory AL amyloidosis (RRAL) patients.9,10,20 Demonstrating the feasibility and activity of PI therapy, single-agent or combination bortezomib treatment results in unprecedented high hematologic response rates and rapid and durable responses,9,20,21 and it has been adopted as a front-line therapy for AL amyloidosis.5,22 However, AL amyloidosis remains a severe condition with a poor prognosis and a median overall survival of approximately 12 months if untreated,23,24 dropping to 6 months when the heart is affected25; new treatment options are therefore needed.2,3,5
There are challenges involved with administering currently available PIs in this patient population. Bortezomib administration is much more challenging in patients with AL than those with MM because of the increased risk for neuropathy (including autonomic neuropathy).26 Cardiotoxicity with carfilzomib often precludes giving a full dose.27,28 Ixazomib is the first oral PI to be tested in the clinic, and encouraging tolerability and efficacy data have been reported in previously untreated and relapsed/refractory MM,29-32 leading to the approval by the US Food and Drug Administration of ixazomib in combination with lenalidomide and dexamethasone for the treatment of patients with relapsed/refractory MM who have received at least 1 prior therapy.33 This phase 1/2 study assessed the safety and preliminary efficacy of weekly oral ixazomib in patients with RRAL.
Patients and methods
Patients
Patients aged 18 years or older with biopsy-proven RRAL after 1 or more prior line of therapy, and requiring further treatment as assessed by the investigator, were enrolled. Other eligibility criteria included measurable major organ (heart/kidney) involvement, Eastern Cooperative Oncology Group performance status 0 to 2, and AL amyloidosis risk stage I/II. Patients who had received prior bortezomib were eligible for the study. Exclusion criteria included treatment with any investigational product within 28 days of the first dose of study drug, failure to fully recover from the effects of prior chemotherapy, New York Heart Association classification III/IV, grade 3 or worse diarrhea, grade 2 or worse or painful peripheral neuropathy (PN), systemic infections, or other malignant disease.
The study was conducted in compliance with the protocol, Good Clinical Practice, and the applicable regulatory requirements (including International Conference on Harmonization guidelines), and in accordance with ethical principles founded in the Declaration of Helsinki. All participants gave written informed consent. All authors had access to the data on request. The study was registered at clinicaltrials.gov as #NCT01318902.
Study design
This open-label, phase 1/2 study evaluated the safety and tolerability of weekly oral ixazomib in patients with RRAL. Patients were enrolled at 9 sites in the United States, Canada, France, Germany, and Italy between May 19, 2011, and December 6, 2012. The study consisted of a dose-escalation phase to determine the maximum tolerated dose (MTD)/recommended phase 2/3 dose (RP2/3D), followed by 2 expansion cohorts (PI-naive and PI-exposed patients) treated at the MTD.
Patients received weekly oral ixazomib on an empty stomach34 on days 1, 8, and 15 of a 28-day cycle for up to 12 cycles; treatment could continue if the patient was deriving clinical benefit. Dose escalation followed a standard 3+3 dose-escalation design, based on dose-limiting toxicities (DLTs) during cycle 1. DLTs were defined as any of the following adverse events considered by the investigator to be possibly related to ixazomib: any grade 4 thrombocytopenia or neutropenia lasting 7 or more days, platelet count below 10 000/mm3, grade 3 thrombocytopenia with clinically significant bleeding, grade 3 febrile neutropenia with fever and/or infection, any grade 3 or worse nonhematologic toxicity apart from grade 3 arthralgia/myalgia or brief (<1 week) grade 3 fatigue, grade 3 or worse nausea and/or emesis despite the use of antiemetic prophylaxis, grade 2 peripheral neuropathy with pain or grade 3 or worse peripheral toxicity, a delay of more than 2 weeks in the initiation of treatment cycle 2 because of a lack of adequate recovery from ixazomib-related toxicities, or other grade 2 or worse ixazomib-related nonhematologic toxicities that, in the opinion of the investigator, required discontinuation of ixazomib therapy.
Patients with no major hematologic response (less than partial response [PR] after 3 cycles) received oral dexamethasone (40 mg, days 1-4) in addition to ixazomib from cycle 4. If no response was detected after cycle 6, the patient was discontinued from treatment; if a response was achieved, ixazomib plus dexamethasone was continued. Prohibited medications included strong CYP1A2 and CYP3A inhibitors, strong CYP3A inducers, angiotensin converting enzyme inhibitors, St. John’s wort, Ginkgo biloba, and warfarin.
Study objectives
The primary objectives were to determine the safety, tolerability, and MTD and RP2/3D of weekly oral ixazomib in patients with relapsed/refractory AL amyloidosis. Secondary objectives included analysis of plasma and blood pharmacokinetics (PK) of ixazomib, assessment of overall hematologic and organ response rates, determination of time to and duration of hematologic and organ responses, and assessment of hematologic progression-free survival (PFS) and overall survival. Quality of life was included as an exploratory objective.
Assessments
Adverse events were graded using NCI-CTCAE version 4.03. Blood samples were collected for ixazomib PK analysis at multiple points pre- and postdosing during cycle 1: before dosing (within 1 hour) on days 1 and 15 of cycle 1, and day 1 of cycle 2, as well as at 30 minutes and 1, 2, 4, 6, 24, and 168 hours after ixazomib administration on days 1 and 15 of cycle 1. Plasma and whole-blood concentrations of ixazomib were measured using a validated liquid chromatography/tandem mass spectrometry assay35 after dosing on days 1 and 15. Hematologic response assessment was based on the difference between involved and uninvolved free light chain according to standardized criteria17; the baseline was set at screening, and levels were evaluated during the rest period of each cycle, at the end-of-treatment visit, and every 6 weeks thereafter by central laboratory. Amyloid-related organ assessments were performed according to standardized criteria16,17,36 after cycles 3, 6, 9, and 12; at the end-of-treatment visit; and every 6 months thereafter until disease progression. These assessments included, but were not limited to, NT-proBNP, B-type natriuretic peptide, troponin, echocardiography, physical examination (heart), 24-hour urine proteinuria, serum creatinine, estimated glomerular filtration rate, serum albumin (kidney), alkaline phosphatase, alanine aminotransferase, ultrasound/computed tomography scan/magnetic resonance imaging (liver), patient-reported symptoms, physical examination, and electromyography (peripheral nervous system). Renal responses were also assessed retrospectively, using updated response criteria.36 Hematologic disease PFS was defined as the time from first ixazomib dose to hematologic disease progression (a 50% increase in serum M-protein to >0.5 g/dL from baseline, or a 50% increase in urine M-protein to >200 mg/24 hours with a visible peak present; free light chain increase of 50% from baseline to >10 mg/dL [100 mg/L]; or death). Quality of life was analyzed using the European Organization for Research and Treatment of Cancer QLQ-C30 instrument (functional subscale) for patients in the dose-expansion cohort only. Global scores were calculated on the basis of patients’ level of functioning in 5 categories: physical, emotional, cognitive, social, and role up to a maximum score of 100. A change in score of 10 or more points is considered clinically meaningful for this instrument.37
Statistical analyses
Six populations were defined in the statistical analysis plan: the safety population included all patients who received at least 1 dose of ixazomib; the DLT-evaluable population included all patients who received all cycle 1 doses of ixazomib or who experienced a DLT in cycle 1 of the dose-escalation phase; the hematologic response-evaluable population included patients who received at least 1 cycle of ixazomib at the RP2/3D of 4.0 mg, had measurable disease at baseline, and had at least 1 postbaseline hematologic response assessment; the organ response-evaluable population comprised patients who received at least 1 cycle of ixazomib at the RP2/3D, had kidney or heart involvement at baseline, and had at least 1 postbaseline organ response assessment; and the PK analysis population included patients who received at least 1 dose of ixazomib and had sufficient data to permit the calculation of ixazomib plasma PK parameters. Plasma and blood PK parameters were calculated for individual patients on day 1 and day 15, using noncompartmental methods (WinNonlin v6.2). Calculated parameters included maximum observed plasma concentration, time of maximum observed plasma concentration, and area under the ixazomib plasma concentration vs time curve from 0 to 168 hours postdose, which were summarized using descriptive statistics. PFS and overall survival were estimated using Kaplan-Meier methodology.
Results
Patients
A total of 27 patients were enrolled and received at least 1 dose of ixazomib; these patients comprised the safety population. The first 11 patients were enrolled to the dose-escalation cohort: 6 received ixazomib 4.0 mg and 5 received ixazomib 5.5 mg. A further 16 patients were enrolled to the dose-expansion cohort.
Demographics and baseline disease characteristics of the dose-escalation and dose-expansion cohorts are shown in Table 1. Major organ involvement, defined by standard criteria, included the kidney (but not the heart) in 10 (37%) patients, the heart (but not the kidney) in 11 (41%) patients, and both the heart and kidney in 6 (22%) patients. The median time from diagnosis to first dose of ixazomib was 51 months (range, 10-148 months). The median number of prior therapies received was 3 (range, 1-8), and 18 (67%) patients had undergone prior autologous stem-cell transplantation. The most common prior therapies were regimens containing melphalan (96%), dexamethasone (74%), bortezomib (70%), immunomodulatory drugs (52%), or cyclophosphamide (44%). In total, 6 (22%) patients had received 2 or more prior bortezomib-containing regimens, and 5 (19%) patients were considered refractory to bortezomib.
Table 1.
Patient demographics and baseline disease characteristics
| Dose-escalation cohort (n = 11) | Dose-expansion cohort (n = 16) | RP2/3D cohort (N = 22) | Total (N = 27) | |
|---|---|---|---|---|
| Median age, y (range) | 66.0 (54-77) | 64.5 (57-78) | 64.5 (54-78) | 65.0 (54-78) |
| Male, n (%) | 5 (45) | 9 (56) | 13 (59) | 14 (52) |
| White, n (%) | 10 (91) | 13 (81) | 18 (82) | 23 (85) |
| NYHA classification, n (%)* | ||||
| Class I | 4 (36) | 3 (19) | 6 (27) | 7 (26) |
| Class II | 4 (36) | 9 (56) | 10 (45) | 13 (48) |
| Mayo cardiac biomarker stage, n (%) | ||||
| Stage I | 5 (45) | 8 (50) | 11 (50) | 13 (48) |
| Stage II | 5 (45) | 8 (50) | 10 (45) | 13 (48) |
| Stage III† | 1 (9) | 0 | 1 (5) | 1 (4) |
| Site of AL organ involvement at study entry, n (%) | ||||
| Kidney and heart | 4 (36) | 2 (13) | 5 (23) | 6 (22) |
| Heart (not kidney) | 3 (27) | 8 (50) | 9 (41) | 11 (41) |
| Kidney (not heart) | 4 (36) | 6 (38) | 8 (36) | 10 (37) |
| Median number of organ systems involved, n (range) | 2.0 (1-6) | 1.5 (1-4) | 2.0 (1-6) | 2.0 (1-6) |
| Median dFLC at screening, mg/L (range) | 104 (40-502) | 126 (46-1064) | 121 (40-1064) | 121 (40-1064) |
| Median NTproBNP at screening, pg/mL (range) | 1490.5 (105.1-4033) | 471 (51-5691) | 669.5 (51-5691) | 897.4 (51-5691) |
| Median 24-h urine protein, g/24 h (range) | 4.8 (0.1-22) | 0.5 (0.004-16.5) | 0.6 (0.004-22) | 0.5 (0.004-22) |
| Median time from diagnosis, months (range) | 33.0 (11-86) | 72.5 (10-148) | 37.5 (10-148) | 51.0 (10-148) |
| Median number of prior therapies, n (range) | 3.0 (1-7) | 3.0 (1-8) | 3.0 (1-8) | 3.0 (1-8) |
| Prior therapy type, n (%)‡ | ||||
| Melphalan-containing regimen | 10 (91) | 16 (100) | 21 (95) | 26 (96) |
| Dexamethasone-containing regimen | 9 (82) | 11 (69) | 16 (73) | 20 (74) |
| Bortezomib-containing regimen§ | 8 (73) | 11 (69) | 16 (73) | 19 (70) |
| Autologous stem-cell transplant | 7 (64) | 11 (69) | 16 (73) | 18 (67) |
| Immunomodulatory drug-containing regimen | 5 (45) | 9 (56) | 11 (50) | 14 (52) |
| Cyclophosphamide-containing regimen | 5 (45) | 7 (44) | 10 (45) | 12 (44) |
NYHA, New York Heart Association.
Class I and II only included, n = 7 were Class 0.
One patient with cardiac biomarker stage III was enrolled as a result of a laboratory imprecision.
Eleven patients refractory to any line of therapy.
Five patients refractory to bortezomib.
DLTs and MTD
One patient in the 4.0-mg dose cohort experienced a DLT of grade 3 thrombocytopenia in cycle 1; the ixazomib dose was reduced to 3.0 mg in cycle 2, and the patient’s platelet count returned to baseline levels. Two patients in the 5.5-mg dose cohort experienced DLTs. The first patient experienced grade 3 diarrhea on day 9, cycle 1; ixazomib was reduced to 4.0 mg on day 15, and the diarrhea resolved. The second patient had grade 2 renal failure and grade 2 respiratory failure (dyspnea) after the first ixazomib dose and was hospitalized, and the next dose was held; these events were considered related to ixazomib. The patient then experienced cardiac arrest (grade 4), possibly related to metoprolol, ixazomib, or a combination of the 2, and the drug was permanently discontinued. The MTD, and RP2/3D, of weekly oral ixazomib was determined as 4.0 mg.
Safety
In total, 26 (96%) patients experienced adverse events (AEs; 22 [81%] drug-related); 22 (81%) patients experienced grade 3 or worse AEs (11 [41%] drug-related), and 17 (63%) reported serious AEs (4 [15%] drug-related; Table 2). The most common any-grade AEs included nausea (n = 15 [56%]), diarrhea and fatigue (n = 14 [52%] each), and constipation and decreased appetite (n = 8 [30%] each; Table 3). The most frequent grade 3 AEs included dyspnea, fatigue, and skin and subcutaneous tissue disorders (MedDRA system organ class; each n = 4 [15%]; Table 3). Grade 4 AEs occurred in 4 patients: 1 cardiac arrest (drug-related), 1 hypokalemia (drug-related), 1 hyperkalemia, and 2 hyponatremia (drug-related in 1 case). Most common serious AEs were atrial fibrillation, congestive cardiac failure, and pleural effusion (2 [7%] each). There was only 1 case of herpes zoster infection; 81% of patients received antiviral drugs during the study (administered prophylactically in 74% of patients, including the patient with herpes zoster infection; other indications included shingles and herpes).
Table 2.
Summary of adverse events
| Dose-escalation cohort, n = 11 (%) | Dose-expansion cohort, n = 16 (%) | RP2/3D cohort, N = 22 (%) | Total, N = 27 (%) | |
|---|---|---|---|---|
| Any AE | 11 (100) | 15 (94) | 21 (95) | 26 (96) |
| Drug-related AE | 9 (82) | 13 (81) | 18 (82) | 22 (81) |
| Grade ≥3 AE | 9 (82) | 13 (81) | 18 (82) | 22 (81) |
| Drug-related grade ≥3 AE | 6 (55) | 5 (31) | 8 (36) | 11 (41) |
| Serious AE | 8 (73) | 9 (56) | 12 (55) | 17 (63) |
| Drug-related serious AE | 3 (27) | 1 (6) | 2 (9) | 4 (15) |
| AEs resulting in drug discontinuation | 3 (27) | 2 (13) | 3 (14) | 5 (19) |
| On-study deaths | 0 | 1 (6) | 1 (5) | 1 (4) |
Table 3.
Any-grade and grade 3 AEs occurring in more than 10% of patients overall
| Any-grade AE | Dose-escalation cohort, n = 11 (%) | Dose-expansion cohort, n = 16 (%) | RP2/3D cohort, N = 22 (%) | Total, N = 27 (%) |
|---|---|---|---|---|
| All-cause | ||||
| Nausea | 8 (73) | 7 (44) | 11 (50) | 15 (56) |
| Skin and subcutaneous tissue disorders* | 6 (55) | 9 (56) | 13 (59) | 15 (56) |
| Diarrhea | 6 (55) | 8 (50) | 11 (50) | 14 (52) |
| Fatigue | 5 (45) | 9 (56) | 11 (50) | 14 (52) |
| Constipation | 4 (36) | 4 (25) | 5 (23) | 8 (30) |
| Decreased appetite | 4 (36) | 4 (25) | 6 (27) | 8 (30) |
| Hypotension | 2 (18) | 5 (31) | 6 (27) | 7 (26) |
| Peripheral edema | 3 (27) | 4 (25) | 7 (32) | 7 (26) |
| Pyrexia | 2 (18) | 5 (31) | 6 (27) | 7 (26) |
| Pain in extremity | 4 (36) | 3 (19) | 7 (32) | 7 (26) |
| Asthenia | 1 (1) | 5 (31) | 5 (23) | 6 (22) |
| Dyspnea | 3 (27) | 3 (19) | 4 (18) | 6 (22) |
| Thrombocytopenia | 2 (18) | 4 (25) | 6 (27) | 6 (22) |
| Upper respiratory tract infection | 2 (18) | 4 (25) | 6 (27) | 6 (22) |
| Contusion | 1 (1) | 4 (25) | 5 (23) | 5 (19) |
| Headache | 1 (1) | 4 (25) | 5 (23) | 5 (19) |
| Insomnia | 2 (18) | 3 (19) | 4 (18) | 5 (19) |
| Vomiting | 3 (27) | 2 (13) | 3 (14) | 5 (19) |
| Abdominal pain | 3 (27) | 1 (6) | 2 (9) | 4 (15) |
| Anemia | 2 (18) | 2 (13) | 4 (18) | 4 (15) |
| Back pain | 0 | 4 (25) | 4 (18) | 4 (15) |
| Cough | 1 (1) | 3 (19) | 4 (18) | 4 (15) |
| Dizziness | 1 (1) | 3 (19) | 4 (18) | 4 (15) |
| Dysgeusia | 1 (1) | 3 (19) | 4 (18) | 4 (15) |
| Muscular weakness | 0 | 4 (25) | 4 (18) | 4 (15) |
| Peripheral neuropathies not elsewhere classified† | 2 (18) | 2 (13) | 4 (18) | 4 (15) |
| Arthralgia | 0 | 3 (19) | 3 (14) | 3 (11) |
| Cardiac failure | 1 (1) | 2 (13) | 3 (14) | 3 (11) |
| Congestive cardiac failure | 1 (1) | 2 (13) | 3 (14) | 3 (11) |
| Dehydration | 2 (18) | 1 (6) | 2 (9) | 3 (11) |
| Dry skin | 1 (1) | 2 (13) | 3 (14) | 3 (11) |
| Fall | 0 | 3 (19) | 3 (14) | 3 (11) |
| Fluid retention | 1 (1) | 2 (13) | 2 (9) | 3 (11) |
| Hyponatremia | 1 (1) | 2 (13) | 3 (14) | 3 (11) |
| Increased blood creatinine | 1 (1) | 2 (13) | 3 (14) | 3 (11) |
| Nasal congestion | 1 (1) | 2 (13) | 3 (14) | 3 (11) |
| Drug-related | ||||
| Nausea | 5 (45) | 5 (31) | 7 (32) | 10 (37) |
| Diarrhea | 4 (36) | 5 (31) | 7 (32) | 9 (33) |
| Fatigue | 3 (27) | 4 (25) | 6 (27) | 7 (26) |
| Skin and subcutaneous tissue disorders* | 3 (27) | 4 (25) | 6 (27) | 7 (26) |
| Thrombocytopenia | 2 (18) | 4 (25) | 6 (27) | 6 (22) |
| Pyrexia | 1 (9) | 3 (19) | 4 (18) | 4 (15) |
| Peripheral neuropathies not elsewhere classified† | 2 (18) | 2 (13) | 4 (18) | 4 (15) |
| Vomiting | 2 (18) | 2 (13) | 3 (14) | 4 (15) |
| Decreased appetite | 2 (18) | 2 (13) | 3 (14) | 4 (15) |
| Abdominal pain | 3 (27) | 1 (6) | 2 (9) | 4 (15) |
| Asthenia | 1 (9) | 2 (13) | 2 (9) | 3 (11) |
| Headache | 1 (9) | 2 (13) | 3 (14) | 3 (11) |
| Anemia | 2 (18) | 1 (6) | 3 (14) | 3 (11) |
| Constipation | 2 (18) | 1 (6) | 2 (9) | 3 (11) |
| Grade 3 AE | Dose escalation, n = 11 (%) | Dose expansion, n = 16 (%) | Total at 4.0 mg, N = 22 (%) | Total, N = 27 (%) |
|---|---|---|---|---|
| All-cause | ||||
| Dyspnea | 1 (9) | 1 (6) | 2 (9) | 4 (15) |
| Fatigue | 1 (9) | 3 (19) | 3 (14) | 4 (15) |
| Skin and subcutaneous tissue disorders* | 2 (18) | 2 (6) | 3 (14) | 4 (15) |
| Cardiac failure | 1 (9) | 2 (13) | 3 (14) | 3 (11) |
| Diarrhea | 1 (9) | 2 (13) | 2 (9) | 3 (11) |
| Thrombocytopenia | 2 (18) | 1 (6) | 3 (14) | 3 (11) |
| Drug-related | ||||
| Diarrhea | 1 (9) | 2 (13) | 2 (9) | 3 (11) |
| Skin and subcutaneous tissue disorders* | 2 (18) | 1 (6) | 2 (9) | 3 (11) |
| Thrombocytopenia | 2 (18) | 1 (6) | 3 (14) | 3 (11) |
MedDRA system organ class term including maculopapular rash, papular rash, pruritus, dry skin, cutaneous amyloidosis, and skin hyperpigmentation.
Higher-level term including PN and peripheral sensory neuropathy.
Four patients (15%) reported grade 1/2 PN not elsewhere classified (higher-level term); of these, 2 had baseline PN. There were no cases of grade 3 or higher PN. In total, 15 (56%) patients had skin and subcutaneous tissue disorders, including maculo-papular rash (n = 5 [19%]; 3 at grade 3), macular rash (n = 1 [4%]), and pruritus (n = 4 [15%]; grade 3 in 1 patient). Among a total of 9 patients experiencing rash events, 6 received ixazomib alone, and in 3 patients, the rash occurred with ixazomib plus dexamethasone.
Patients received a median of 4 cycles of ixazomib overall (range, 1-31): 4 cycles (range, 1-12) in the dose-escalation cohort and 5 cycles (range, 1-31) in the dose-expansion cohort. PI-naive patients (n = 6) received a median of 12 cycles (range, 1-31) compared with 3.5 cycles (range, 1-24) in PI-exposed patients (n = 16). In total, 11 (41%) patients received 6 or more cycles of ixazomib, and 8 (30%) were deemed to be deriving clinical benefit and continued receiving treatment beyond 12 cycles. At data cutoff (July 20, 2015), 3 patients continued receiving therapy, 5 had completed the maximum 12 cycles of treatment, and 19 had discontinued therapy. Reasons for discontinuation included disease progression (n = 8), symptomatic deterioration, patient withdrawal (each n = 4), unsatisfactory response (less than PR; n = 2), and AEs (n = 1). There was 1 on-study death in the dose-expansion cohort, resulting from amyloidosis-related heart failure, which was not considered to be related to ixazomib.
Pharmacokinetics
Ixazomib was rapidly absorbed after oral administration, with a median Tmax in plasma and whole blood of approximately 1 hour on days 1 and 15 (Table 4). At the 4.0-mg dose, day 15 geometric mean plasma Cmax was 40.7 ng/mL and AUC0-168 was 990.2 hr ⋅ ng/mL. Higher concentrations were observed in whole blood throughout the dosing interval. After Cmax, the disposition profiles of ixazomib in plasma and whole blood were different, with plasma concentrations declining more rapidly during the first 24 hours postdose than those in whole blood. After administration of ixazomib 4.0 mg, the geometric mean whole blood-to-plasma ratio for Cmax was 2.38 on day 1 and 2.89 on day 15. The corresponding ratios for AUC0-168 were 13 and 10, respectively. Half-life was not estimated in this study as a result of limited PK sampling; population PK analysis will estimate this in a future publication.
Table 4.
Pharmacokinetic parameters of ixazomib in plasma and whole blood
| Parameter (units) | 4.0 mg | 5.5 mg | |||
|---|---|---|---|---|---|
| Plasma | Whole blood | Plasma | Whole blood | ||
| Day 1 | |||||
| N | 15* | 16 | — | — | |
| Tmax (hr) | 1.0 (0.5-2.0) | 1.0 (0.5-6.0) | — | — | |
| Cmax (ng/mL) | 41.6 (80) | 98.9 (40) | — | — | |
| AUC0-168 (hr*ng/mL) | 577 (142) | 7340 (41) | — | — | |
| DN Cmax (ng/mL/mg) | 10.4 (80) | 24.7 (40) | — | — | |
| DN AUC0-168 (hr*ng/mL/mg) | 144 (142) | 1840 (41) | — | — | |
| Cmax blood-to-plasma ratio† | — | 2.38 (56) | — | — | |
| AUC0-168 blood-to-plasma ratio‡ | — | 12.7 (64) | — | — | |
| Day 15 | |||||
| N | 18§ | 17ǁ | 2 | 2 | |
| Tmax (hr) | 1.0 (0.5-6.1) | 1.0 (0.5-6.0) | 0.5, 1.0 | 0.5, 1.0 | |
| Cmax (ng/mL) | 40.7 (66) | 125 (17) | 61.4, 123 | 176, 203 | |
| AUC0-168 (hr*ng/mL) | 990 (42) | 9780 (20) | 1240, 2210 | 13 700, 15 200 | |
| DN Cmax (ng/mL/mg) | 10.2 (66) | 31.3 (17) | 11.2, 22.4 | 32.0, 36.9 | |
| DN AUC0-168 (hr*ng/mL/mg) | 248 (42) | 2440 (20) | 225, 402 | 2490, 2760 | |
| Cmax blood-to-plasma ratio | — | 2.89 (65) | — | 1.65, 2.87 | |
| AUC0-168 blood-to-plasma ratio | — | 9.86 (50) | — | 6.2, 12.3 | |
| Accumulation ratio for AUC0-168 | 2.09 (18) | 1.45 (9) | — | — | |
Parameters are presented as geometric mean (% CV), except for Tmax, which is presented as median (range). Individual values are reported if n < 3.
AUC0-168, area under the ixazomib plasma concentration versus time curve from 0 to 168 h post-dose; Cmax, maximum observed concentration; DN, dose-normalized; Tmax, time to Cmax.
n = 14 for AUC0-168 and DN AUC0-168.
n = 15.
n = 14.
n = 15 for AUC0-168, DN AUC0-168, and n = 10 for the accumulation ratio.
ǁn = 14 for AUC0-168, DN AUC0-168, and AUC0-168 blood-to-plasma ratio and n = 11 for the accumulation ratio.
Efficacy
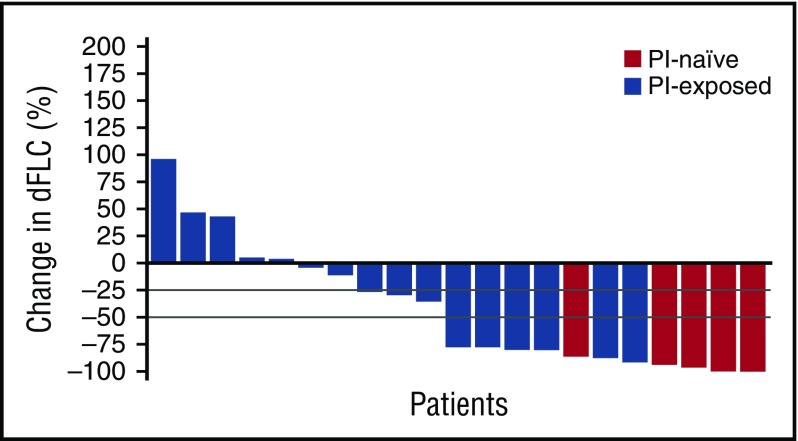
Best hematologic and organ responses to treatment are shown in Table 5. The overall hematologic response rate (ORR) was 52% in hematologic response-evaluable patients (who received at least 1 cycle of ixazomib at the RP2/3D of 4.0 mg, including those who received dexamethasone; who had measurable disease at baseline and at least 1 post-baseline hematologic response assessment). As shown in Figure 1, a reduction in dFLC of 50% or more was demonstrated in 11/21 (52%) hematologic response-evaluable patients who received ixazomib 4.0 mg. Ten patients overall had not yet achieved PR or better after 3 cycles of ixazomib, and thus dexamethasone was added; 4 patients subsequently achieved a response (1 PR, 2 VGPR, 1 CR). Of the 5 PI-naive patients, all achieved a response; of the 16 PI-exposed patients, 38% achieved a response. Of the 12 patients who were bortezomib-exposed but not refractory, 3 patients achieved a VGPR, 1 achieved a PR, and 8 achieved stable disease. Of the 4 PI-exposed, bortezomib-refractory patients, 1 achieved a VGPR, 1 achieved a PR, 1 achieved stable disease, and 1 had progressive disease. The times to first and best hematologic response were 1.8 and 2.7 months, respectively. After 1, 2, 3, and 6 cycles of therapy, respectively, 24%, 33%, 33%, and 52% of patients had a response (PR or better). Eight patients had a deepening of their response during the course of therapy: 4 with ixazomib alone (1 PR to CR, 2 PR to VGPR, 1 no change [NC] to VGPR) and 4 with the addition of dexamethasone (1 NC to CR, 2 NC to VGPR, 1 NC to PR). The median duration of hematologic response was 24.9 months, both overall (n = 11) and in patients who received ixazomib 4.0 mg (n = 11). The median duration of hematologic response was also 24.9 months in PI-naive patients (n = 5) compared with 12.9 months in PI-exposed patients (n = 6).
Table 5.
Best hematologic and organ response to treatment
| Hematologic response-evaluable patients | PI-naive cohort, n = 5 (%) | PI-exposed cohort, n = 16 (%) | RP2/3D cohort, N = 21 (%) |
|---|---|---|---|
| ORR | 5 (100) | 6 (38) | 11 (52) |
| CR | 2 (40) | 0 | 2 (10)* |
| VGPR | 3 (60) | 4 (25) | 7 (33)† |
| PR | 0 | 2 (13) | 2 (10)* |
| No change | 0 | 9 (56) | 9 (43) |
| Progressive disease | 0 | 1 (6) | 1 (5) |
| Organ response-evaluable patients | PI-naïve cohort, n = 5 (%) | PI-exposed cohort, n = 13 (%) | RP2/3D cohort, N = 18 (%) |
|---|---|---|---|
| Response | 5 (100) | 5 (38) | 10 (56) |
| Cardiac response‡ | 2/3 (67)§ | 3/8 (38) | 5/11 (45) |
| Renal response¶ | 3/3 (100) | 2/8 (25) | 5/11 (45) |
| No change | 0 | 8 (62) | 8 (44) |
| Progression | 0 | 0 | 0 |
ORR indicates overall response rate; CR, complete response; PR, partial response; VGPR, very good partial response.
One patient received dexamethasone.
Two patients received dexamethasone.
According to NT-proBNP levels in 11 response-evaluable patients with cardiac involvement at baseline.
One additional patient had a cardiac response by echo.
Assessed retrospectively by updated renal response criteria36 in 11 response-evaluable patients with renal involvement at baseline.
Figure 1.
Waterfall plot of percentage reduction in dFLC for all hematologic response-evaluable patients (n = 21).
Eighteen patients were included in the organ response-evaluable population. Post hoc analysis based on cardiac and renal response criteria17,36 indicated that 10/18 (56%) patients had an organ response: 5/5 (100%) PI-naive patients and 5/13 (38%) PI-exposed patients. Of the 11 patients with renal/cardiac involvement at baseline, 5 (45%) had cardiac responses and 5 (45%) had renal responses. Seven (35%) hematologic response-evaluable patients (the population in whom NT-proBNP levels were assessable) achieved a cardiac response (>30% and >300 ng/L reduction in NT-proBNP).
At data cutoff, median hematologic PFS was 13.6 months overall (n = 27; median follow-up, 27.2 months) and 14.8 months in patients who received ixazomib 4.0 mg (n = 22; median follow-up, 36.3 months; Figure 2A). The 1-year hematologic PFS rate was 50% in the overall population (n = 27) and 60% in patients who received ixazomib 4.0 mg (n = 22). PI-naive patients had a median hematologic PFS of 25.8 months compared with 10.7 months in PI-exposed patients; the 1-year hematologic PFS rate was 83% in PI-naive patients compared with 41% in PI-exposed patients. The 1-year overall survival rate was 80% in the overall population (n = 27) and 85% in patients who received ixazomib 4.0 mg (n = 22; Figure 2B); 1-year overall survival was 83% in PI-naive and 86% in PI-exposed patients.
Figure 2.
Survival in patients who received ixazomib 4.0 mg (n = 22). (A) PFS; (B) overall survival.
Quality of life
Quality of life (QOL) was analyzed using the European Organization for Research and Treatment of Cancer QLQ-C30 instrument (functional subscale) for patients in the dose-expansion cohort only (n = 16). For the 14 patients with available data, the median score for global QOL was 66.7 (25-100) at baseline and 62.5 (42-100) at end of treatment. For the symptom subscales of the EORTC QLQ-C30 instrument, overall, small mean reductions in dyspnea and small mean increases and/or no change in fatigue, diarrhea, nausea and vomiting, appetite loss, and constipation occurred at most assessments during treatment. No consistent pattern was observed for pain and insomnia. Therefore, patients receiving ixazomib maintain their QOL without negatively affecting patient-reported symptoms. Patients who were classed as responders (CR+VGPR+PR; n = 6) had higher scores than nonresponders (n = 7), both at baseline (79.2 vs 50.0) and at the end of treatment (83.3 vs 47.2).
Discussion
In this phase 1/2 study, the first to assess the oral PI ixazomib in patients with RRAL, good tolerability, and promising efficacy, with an ORR of 52%, were observed. The MTD of weekly ixazomib in patients with RRAL was determined as 4.0 mg; this dose is now being used in a phase 3 study of ixazomib in patients with RRAL (NCT01659658) and is the same dose used in phase 3 MM trials (NCT01850524, NCT02181413, and NCT02312258).31
Weekly ixazomib dosing appeared feasible and generally well tolerated in this patient population. The most common drug-related AEs were nausea, diarrhea, fatigue, skin and subcutaneous tissue disorders, and thrombocytopenia; drug-related grade 3 or worse AEs included thrombocytopenia, diarrhea, and skin and subcutaneous tissue disorders. This is similar to the safety profile reported in a phase 3 study of ixazomib in patients with relapsed/refractory MM, where grade 3 or worse AEs included 18% neutropenia, 12% thrombocytopenia, 10% anemia, 6% diarrhea, and 5% rash.31 Consistent with other ixazomib studies,29,31,32,38 PN was mild and infrequent, and there were no cases of grade 3 or worse PN in this study; ixazomib may be more appropriate than bortezomib for patients with RRAL who may have neuropathy. The limited gastrointestinal toxicity in patients with RRAL is notable, considering the frequent gastrointestinal amyloid involvement in AL amyloidosis. Cardiac AEs were mostly related to disease, rather than treatment. There was 1 on-study death resulting from amyloidosis-related heart failure, which should be put in the context of a phase 1/2 study of bortezomib in a similar patient population with RRAL (50% of patients had cardiac involvement compared with 63% of patients in the present study), where there were 11 on-study deaths in a cohort of 70 patients.10
Ixazomib PK parameters in RRAL appear similar to ixazomib PK data in other disease populations.29,32,38,39 Ixazomib was rapidly absorbed after oral administration, with a geometric mean Tmax of approximately 1 hour when administered at the RP2/3D of 4.0 mg. In vitro, ixazomib is partitioned into red blood cells in an extensive and concentration-dependent manner40; in this study, higher concentrations of ixazomib were present in whole blood than in plasma after once-weekly dosing at 4.0 or 5.5 mg. Collectively, these data support preferential distribution of ixazomib into red blood cells, which are known to contain high concentrations of the 20S proteasome.41,42
This was one of the first studies to prospectively use the updated consensus criteria for assessment of hematologic and cardiac responses.17 These criteria recommend the measurement of dFLC reduction for assessment of hematologic responses and NT-proBNP for assessment of cardiac responses; a reduction in dFLC has been previously shown to be associated with improved survival.43,44 Renal response criteria, based on a 30% or more decrease in proteinuria or below 0.5 g/24 h without renal progression, were defined after the start of this study and applied retrospectively.36 Preliminary data indicated hematologic (ORR, 52% in the RP2/3D cohort) as well as early organ responses (56% in the RP2/3D cohort) in this heavily pretreated population (median, 3 prior lines of therapy [range, 1-8]). Importantly, responses appeared durable, with 60% of patients treated at the RP2/3D remaining in response at 1 year. Patients who achieved a hematologic response of VGPR or better had improved PFS compared with those who did not (median, 17.0 vs 10.7 months; P = .1968), in line with previous findings.17
Responses were achieved by patients regardless of prior PI exposure; however, it should be emphasized that the sample size of the PI-naive population is small. Response rate and hematologic PFS rate in PI-naive patients (n = 5) were 100% (40% CR) and 83% at 1 year, respectively. In patients who received prior bortezomib (n = 16), the ORR was 38% (0% CR), and the 1-year PFS rate was 41%. Of the 4 patients who were considered refractory to bortezomib, 2 achieved a response (1 VGPR, 1 PR). Administration of dexamethasone to patients who did not achieve PR or better after 3 cycles of ixazomib was able to salvage a response in 4 of 10 patients. Although it is a smaller study, the preliminary efficacy data reported here compare favorably with those from a large phase 1/2 study of bortezomib in patients with RRAL, all of whom were PI-naive; bortezomib treatment resulted in an ORR of 69%, with 38% CR; renal and cardiac responses were seen in 45% of patients each.10
This study also evaluated QOL in patients with RRAL. Patients who responded to treatment (PR or better) had higher scores than nonresponders, both at baseline (79.2 vs 50.0) and at the end of treatment (83.3 vs 47.2). This difference in score of 10 or more points is considered clinically meaningful, denoting that responders had a better QOL than nonresponders throughout the treatment period.37 In addition, QOL was maintained in both groups throughout with ixazomib treatment. The ongoing phase 3 study of ixazomib in RRAL (NCT01659658) will define the effect of ixazomib treatment on QOL in a larger patient population.
In conclusion, in this phase 1/2 study of 27 patients, weekly oral ixazomib, with dexamethasone added if necessary, appeared feasible and reasonably well tolerated in patients with RRAL and was associated with encouraging preliminary evidence of clinical activity. A phase 3 study of weekly ixazomib plus dexamethasone vs physician’s choice in patients with RRAL is ongoing (NCT01659658). On the basis of these encouraging data, a US Food and Drug Administration Breakthrough Therapy Designation was granted in December 2014.
Acknowledgments
The authors acknowledge Helen Johns of FireKite, an Ashfield company, part of UDG Healthcare plc, for writing support during the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc., and complied with Good Publication Practice 3 ethical guidelines.
G.M. and G.P. are supported by grants from Associazione Italiana per la Ricerca sul Cancro–Special Program Molecular Clinical Oncology 5 per mille n. 9965, from Cassa di Risparmio delle Provincie Lombarde (CARIPLO) “Structure-function relation of amyloid: understanding the molecular bases of protein misfolding diseases to design new treatments n. 2013-0964,” and from CARIPLO “Molecular mechanisms of Ig toxicity in age-related plasma cell dyscrasias n. 2015-0591.” This study was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.Y., N.G., and A.-M.H. performed statistical analysis; G.P., D.B., H.Y., N.G., and G.M. wrote the manuscript; and all authors designed the research and collected data, analyzed and interpreted data, and reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: V.S. received research support from Celgene; Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited; Onyx; and Prothena Biosciences, Inc. G.P. received honoraria and travel grants from Prothena Biosciences, Inc.; travel grants from Celgene; and advisory board for Janssen-Cilag. V.K. received honoraria from Celgene, Lundbeck, and Amgen. J.A.Z. received research support from Celgene and Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited; consultancy for Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, and BMS, Janssen, Amgen, Prothena Biosciences, Inc., and Seattle Genetics. A.D.C. received honoraria and membership on the board of directors or advisory committee for Celgene, BMS, and Onyx. A.D. received research funding from Celgene; Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited; and Janssen Research & Development. A.J. received research funding from Celgene; honoraria from Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited; Janssen; and Celgene. S.O.S. received research funding from Celgene and Janssen and honoraria from Janssen, Prothena Biosciences, Inc., GlaxoSmithKline, and Celgene. D.B., H.Y., N.G., and A.-M.H. are employed by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. R.L.C. had a consultancy and membership on board of directors or advisory committee for Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, and received research funding from Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. G.M. had a consultancy from Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited; Pfizer; Janssen; and Prothena Biosciences, Inc.
The current affiliation for A.D.C. is Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA.
The current affiliation for A.-M.H. is Sanofi Oncology, Cambridge, MA.
David C. Seldin died on 27 June 2015.
Correspondence: Giampaolo Merlini, Amyloidosis Research and Treatment Center, Fondazione IRCCS Policlinico San Matteo, University of Pavia, Italy; e-mail: gmerlini@unipv.it.
References
- 1.Comenzo RL. Managing systemic light-chain amyloidosis. J Natl Compr Canc Netw. 2007;5(2):179-187. [DOI] [PubMed] [Google Scholar]
- 2.Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol. 2011;29(14):1924-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merlini G, Wechalekar AD, Palladini G. Systemic light chain amyloidosis: an update for treating physicians. Blood. 2013;121(26):5124-5130. [DOI] [PubMed] [Google Scholar]
- 4.Merlini G, Comenzo RL, Seldin DC, Wechalekar A, Gertz MA. Immunoglobulin light chain amyloidosis. Expert Rev Hematol. 2014;7(1):143-156. [DOI] [PubMed] [Google Scholar]
- 5.Palladini G, Merlini G. What is new in diagnosis and management of light chain amyloidosis? Blood. 2016;128(2):159-168. [DOI] [PubMed] [Google Scholar]
- 6.Palladini G, Milani P, Foli A, et al. . Oral melphalan and dexamethasone grants extended survival with minimal toxicity in AL amyloidosis: long-term results of a risk-adapted approach. Haematologica. 2014;99(4):743-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dispenzieri A, Lacy MQ, Zeldenrust SR, et al. . The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood. 2007;109(2):465-470. [DOI] [PubMed] [Google Scholar]
- 8.Palladini G, Milani P, Foli A, et al. . A phase 2 trial of pomalidomide and dexamethasone rescue treatment in patients with AL amyloidosis. Blood. 2017;129(15):2120-2123. [DOI] [PubMed] [Google Scholar]
- 9.Reece DE, Hegenbart U, Sanchorawala V, et al. . Long-term follow-up from a phase 1/2 study of single-agent bortezomib in relapsed systemic AL amyloidosis. Blood. 2014;124(16):2498-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reece DE, Hegenbart U, Sanchorawala V, et al. . Efficacy and safety of once-weekly and twice-weekly bortezomib in patients with relapsed systemic AL amyloidosis: results of a phase 1/2 study. Blood. 2011;118(4):865-873. [DOI] [PubMed] [Google Scholar]
- 11.Sanchorawala V, Shelton AC, Lo S, Varga C, Sloan JM, Seldin DC. Pomalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 1 and 2 trial. Blood. 2016;128(8):1059-1062. [DOI] [PubMed] [Google Scholar]
- 12.Sanchorawala V, Wright DG, Rosenzweig M, et al. . Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood. 2007;109(2):492-496. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman GP, Dispenzieri A, Gertz MA, et al. . Kinetics of organ response and survival following normalization of the serum free light chain ratio in AL amyloidosis. Am J Hematol. 2015;90(3):181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gertz MA, Lacy MQ, Dispenzieri A, et al. . Effect of hematologic response on outcome of patients undergoing transplantation for primary amyloidosis: importance of achieving a complete response. Haematologica. 2007;92(10):1415-1418. [DOI] [PubMed] [Google Scholar]
- 15.Palladini G, Russo P, Nuvolone M, et al. . Treatment with oral melphalan plus dexamethasone produces long-term remissions in AL amyloidosis. Blood. 2007;110(2):787-788. [DOI] [PubMed] [Google Scholar]
- 16.Gertz MA, Comenzo R, Falk RH, et al. . Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79(4):319-328. [DOI] [PubMed] [Google Scholar]
- 17.Palladini G, Dispenzieri A, Gertz MA, et al. . New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541-4549. [DOI] [PubMed] [Google Scholar]
- 18.D’Souza A, Huang J, Hari P. New light chain amyloid response criteria help risk stratification of patients by day 100 after autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(4):768-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girnius S, Seldin DC, Cibeira MT, Sanchorawala V. New hematologic response criteria predict survival in patients with immunoglobulin light chain amyloidosis treated with high-dose melphalan and autologous stem-cell transplantation. J Clin Oncol. 2013;31(21):2749-2750. [DOI] [PubMed] [Google Scholar]
- 20.Palladini G, Sachchithanantham S, Milani P, et al. . A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126(5):612-615. [DOI] [PubMed] [Google Scholar]
- 21.Kastritis E, Roussou M, Gavriatopoulou M, et al. . Long-term outcomes of primary systemic light chain (AL) amyloidosis in patients treated upfront with bortezomib or lenalidomide and the importance of risk adapted strategies. Am J Hematol. 2015;90(4):E60-E65. [DOI] [PubMed] [Google Scholar]
- 22.Mahmood S, Palladini G, Sanchorawala V, Wechalekar A. Update on treatment of light chain amyloidosis. Haematologica. 2014;99(2):209-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen AD, Comenzo RL. Systemic light-chain amyloidosis: advances in diagnosis, prognosis, and therapy. Hematology Am Soc Hematol Educ Program. 2010;2010:287-294 [DOI] [PubMed] [Google Scholar]
- 24.Merlini G, Palladini G. Light chain amyloidosis: the heart of the problem. Haematologica. 2013;98(10):1492-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32(1):45-59. [PubMed] [Google Scholar]
- 26.Mikhael JR, Schuster SR, Jimenez-Zepeda VH, et al. . Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;119(19):4391-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atrash S, Tullos A, Panozzo S, et al. . Cardiac complications in relapsed and refractory multiple myeloma patients treated with carfilzomib. Blood Cancer J. 2015;5:e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen A, Liedtke M, Scott E, et al. . Safety and efficacy of carfilzomib (CFZ) in previously-treated systemic light-chain (AL) amyloidosis. Clin Lymphoma Myeloma Leuk. 2015;15(Suppl 3):e58-e59. [Google Scholar]
- 29.Kumar SK, Bensinger WI, Zimmerman TM, et al. . Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma. Blood. 2014;124(7):1047-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar SK, Berdeja JG, Niesvizky R, et al. . Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15(13):1503-1512. [DOI] [PubMed] [Google Scholar]
- 31.Moreau P, Masszi T, Grzasko N, et al. ; TOURMALINE-MM1 Study Group. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621-1634. [DOI] [PubMed] [Google Scholar]
- 32.Richardson PG, Baz R, Wang M, et al. . Phase 1 study of twice-weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood. 2014;124(7):1038-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ninlaro (ixazomib) capsules, for oral use [package insert]. Osaka, Japan: Takeda Pharmaceuticals Limited; 2015.
- 34.Gupta N, Hanley MJ, Venkatakrishnan K, et al. . The effect of a high-fat meal on the pharmacokinetics of ixazomib, an oral proteasome inhibitor, in patients with advanced solid tumors or lymphoma. J Clin Pharmacol. 2016;56(10):1288-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta N, Hanley MJ, Venkatakrishnan K, et al. . Pharmacokinetics of ixazomib, an oral proteasome inhibitor, in solid tumour patients with moderate or severe hepatic impairment. Br J Clin Pharmacol. 2016;82(3):728-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palladini G, Hegenbart U, Milani P, et al. . A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124(15):2325-2332. [DOI] [PubMed] [Google Scholar]
- 37.Snyder CF, Blackford AL, Sussman J, et al. . Identifying changes in scores on the EORTC-QLQ-C30 representing a change in patients’ supportive care needs. Qual Life Res. 2015;24(5):1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta N, Goh YT, Min CK, et al. . Pharmacokinetics and safety of ixazomib plus lenalidomide-dexamethasone in Asian patients with relapsed/refractory myeloma: a phase 1 study. J Hematol Oncol. 2015;8:103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta N, Diderichsen PM, Hanley MJ, et al. . Population pharmacokinetic analysis of ixazomib, an oral proteasome inhibitor, including data from the phase III TOURMALINE-MM1 study to inform labelling [published online ahead of print 13 March 2017]. Clin Pharmacokinet. doi:10.1007/s40262-017-0526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kupperman E, Lee EC, Cao Y, et al. . Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70(5):1970-1980. [DOI] [PubMed] [Google Scholar]
- 41.Lightcap ES, McCormack TA, Pien CS, Chau V, Adams J, Elliott PJ. Proteasome inhibition measurements: clinical application. Clin Chem. 2000;46(5):673-683. [PubMed] [Google Scholar]
- 42.Neelam S, Kakhniashvili DG, Wilkens S, Levene SD, Goodman SR. Functional 20S proteasomes in mature human red blood cells. Exp Biol Med (Maywood). 2011;236(5):580-591. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S, Dispenzieri A, Katzmann JA, et al. . Serum immunoglobulin free light-chain measurement in primary amyloidosis: prognostic value and correlations with clinical features. Blood. 2010;116(24):5126-5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S, Dispenzieri A, Lacy MQ, et al. . Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989-995. [DOI] [PMC free article] [PubMed] [Google Scholar]