Abstract
To reproduce, males have to fertilize the female’s eggs, sometimes in competition with ejaculates of other males. In species where males display alternative reproductive tactics, whereby territorial males secure mating and non-territorial males have to sneak copulations, the latter might be expected to invest relatively more resources towards sperm quality compared with the territorial males. Sperm cells are especially vulnerable to oxidative stress, which reduces male fertility. Therefore, antioxidant resources are expected to modulate sperm quality, and might be allocated differently between reproductive tactics. To test the link between reproductive tactics, redox profile and sperm quality, we experimentally induced changes in the reproductive tactics of 39 captive males Seba’s short-tailed bats Carollia perspicillata. We monitored the blood and ejaculate oxidative balance, and the sperm quality before, 7 days and 21 days after the manipulation of reproductive tactic. Although ejaculates’ oxidative damage was negatively related to sperm velocity, males exhibited similar blood and ejaculates redox profiles and similar sperm quality, regardless of their reproductive tactic. Possibly, these results arise as a consequence of some constraints having been lifted during the experiment. Our results also suggest that, in Seba’s short-tailed bats, the expression of alternative reproductive tactics is not subjected to strong oxidative constraints. Furthermore, our results could reflect an absence of trade-off between pre- and post-copulatory traits in harem males, as they could be selected to invest both in female attraction and sperm quality, as a consequence of their inability to fully monopolize females.
Keywords: oxidative stress, sperm competition, alternative reproductive tactics, Carollia perspicillata
A male’s ability to reproduce depends both on its capacity to acquire mates, determined by pre-copulatory traits (Andersson 1994; Clutton-Brock 2007), and his ability to fertilize the eggs, determined by post-copulatory traits such as sperm quality (Simmons and Fitzpatrick 2012). In the last 3 decades, a large number of sperm competition models assuming costs to both pre- and post-copulatory traits, limited resources, and allocation trade-offs of these resources to both types of traits have been developed to help us understand how males optimize their fitness under sperm competition, that is the circumstance when the sperm of 2 or more males compete for the fertilization of a given set of eggs (Parker 1990; Engqvist and Reinhold 2006; Parker et al. 2013). Specifically, these models predict that that males benefiting from a privileged access to fertile females have a greater fitness return when investing more resources to pre-copulatory traits, that is, to acquire mates, whereas less sexually competitive males will increase their fitness by expending more resources in post-copulatory traits, that is, to improve their fertilization ability. Empirical support for these sperm competition models has been found repeatedly in several taxa (bats: Fasel et al. 2017; birds: Froman et al. 2002; reptiles: Kahrl et al. 2016; beetles: Simmons and Emlen 2006; fishes: Young et al. 2013). Yet, several studies have reported a positive relationship between pre- and post-copulatory selected traits, with more attractive males having higher fertilization abilities (Peters et al. 2004; Malo et al. 2005a; Locatello et al. 2006; Mehlis et al. 2013). These results provide empirical support to the phenotype-linked fertility hypothesis (Sheldon 1994), which states that a male’s attractiveness is an honest signal of its fertility. Recently, a comparative analysis by Lüpold et al. (2014) has reconciled sperm competition models and the phenotype-linked fertility hypothesis by showing that the conditions for a trade-off between pre- versus post-copulatory traits are found when reproductive success is mainly determined by the males’ ability to monopolize females. Variation in female monopolization abilities among males may lead to alternative reproductive tactics (ARTs), which refers to discontinuous phenotypes (e.g., behavioral traits) selected to maximize fitness in alternative and mutually exclusive ways, in the context of intraspecific and intrasexual reproductive competition (Taborsky et al. 2008). Generally, some males will invest resources in order to attract mates and thus monopolize fertile females, whereas other males adopt an alternative sneaker tactic, and consequently inevitably face sperm competition (Oliveira et al. 2008; Engqvist and Taborsky 2016). ARTs can be fixed or plastic throughout an individual’s lifespan. They can have equal fitness pay-offs such as males of the 2 morphs of damselfly Mnais pruinosa costalis (Tsubaki et al. 1997) or unequal fitness pay-offs as in the sassaby antelopes Damaliscus lunatus, where a minority of males defend a territory, whereas the majority forms aggregate of males displaying for females (Emlen and Oring 1977).
Sperm competition represents a major force shaping the evolution of ejaculates characteristics in a competitive environment, for example by selecting for improved sperm quality (Simmons and Fitzpatrick 2012; Fitzpatrick and Lüpold 2014). Sperm membranes are however prone to lipid peroxidation (Agarwal et al. 2014), as they generally contain high concentration of polyunsaturated fatty acids (but see delBarco-Trillo et al. 2015). Furthermore, DNA repair systems are progressively down-regulated during spermatogenesis, leaving genetic oxidative damage potentially affecting the zygote upon fertilization (Lewis and Aitken 2005). When reactive oxygen species production overwhelms the antioxidant machinery, it leads to damage to biological molecules, a condition referred to as oxidative stress (Halliwell and Gutteridge 2007). Specifically, oxidative stress has been shown to have major negative impact on sperm quality (Cocchia et al. 2011; Rojas Mora et al. 2017a) and male fertility (Aitken et al. 2014; Wright et al. 2014). Therefore, oxidative stress could underline the trade-off between pre and post-copulatory traits predicted by the sperm competition models (Parker 1990; Parker et al. 2013). Indeed, oxidative stress has been proposed to be an important constraint shaping the evolution of physiological mechanisms underlying major life-history trade-offs (Monaghan et al. 2009; Metcalfe and Alonso-Alvarez 2010), sometimes in a sex-specific way (Costantini 2018; de Boer et al. 2018).
We used Seba’s short-tailed bats Carollia perspicillata, a species with male ARTs whereby harem males have privileged access to females and face low sperm competition risk, whereas sneaker males always experience sperm competition. In this species, ARTs are flexible, but the probability of transition within a 6-month period from a sneaker tactic to a harem tactic is only about 10% (Fasel et al. 2016). Yet, when given the opportunity, sneakers can rapidly switch tactic to become harem males (pers. observation). ARTs have unequal pay-offs, with harem males, which comprise only 20% of the males in the population, siring 60% of the pups, and a handful of sneakers, which comprise the remaining 80% of the males in our population, siring the other 40% of the pups (Fasel et al. 2016), implying that a vast majority of sneakers never reproduce. Importantly, neither age nor size explain the acquisition of a tactic (Fasel et al. 2016), and testosterone levels do not differ between individuals of different tactics (unpublished data). Harem males exhibit resource defense polygyny, meaning that they defend a small territory on which they have access to females, but do not guard females when the latter are outside of their territory. To defend their territories, harem males are frequently involved in agonistic interactions (Fernandez et al. 2014), and likely have reduced foraging and resting time, leading to higher cellular oxidative stress in the blood, whereas sneaker males show faster and longer-lived sperm than harem males (Fasel et al. 2017). However, this difference in sperm quality between harem and sneaker males disappears after 3 days of sexual abstinence (Wesseling et al. 2016), suggesting that it is linked to physiological constraints rather than differential investment in ejaculates. Since these results were drawn from observational data, we felt a need for an experimental confirmation that, beyond a mere correlation between male status and sperm quality, males do adjust their sperm quality to their reproductive tactics.
To test the interplay between reproductive tactics, sperm quality and redox profile, we experimentally manipulated male reproductive tactics. We aimed at 1) experimentally testing the significance of the correlation between reproductive tactics and sperm quality that was found previously (Fasel et al. 2017); 2) testing for predicted trade-offs between the antioxidant protection of the soma versus the ejaculate depending on the reproductive tactics; 3) investigating the role of antioxidant protection to the ejaculate as a potential mechanism explaining differences in sperm quality. To do so, we experimentally induced males of known reproductive tactics to switch to a different tactic by shuffling males across cages, given that in each cage only 1 male can remain harem male. We monitored the males’ redox profiles in both blood and ejaculate through a set of biomarkers: an endogenous antioxidant (Superoxide dismutase: SOD), an exogenous antioxidant (tocopherol), a marker of cellular oxidative stress (the ratio of oxidized over reduced glutathione) and a marker of oxidative damage to the lipids (malondialdehyde: MDA). To assess sperm quality, we analyzed sperm velocity and the percentage of motile sperm, 2 traits commonly linked to fertilizing abilities (Pusch 1987; Gasparini et al. 2010; Simmons and Fitzpatrick 2012).
As sneaker males face higher level of sperm competition, we predicted that they should privilege the antioxidant protection of their ejaculates, therefore showing lower sperm oxidative damage, and higher sperm quality. Harem males were predicted to favor the antioxidant protection of their soma at the expense of their germline, leading to lower oxidative damage in the blood and potentially higher oxidation levels of their sperm cells. More importantly, we predicted that when forced to switch tactic, males would modify their investment in the antioxidant protection of their soma and their sperm cells to exhibit the physiology of their experimentally assigned reproductive tactics. Therefore, sneakers becoming harem males would exhibit lower oxidative damage in their soma, but produce more oxidized and lower quality sperm than before the switch. Conversely, harem males becoming sneakers would exhibit greater oxidative damage in their soma, but would produce less oxidized and better quality sperm than before the switch.
Material and Methods
Model species and studied population
The study was conducted using bats from 2 captive colonies of Seba’s short-tailed bats (Carollia perspicillata, Yangchiroptera, Phyllostomidae) hosted in a tropical zoo (Papiliorama) located in Switzerland. One population of about 350 individuals fly freely under a 40 m diameter dome (ca. 1, 250 m2 and 12, 500 m3), and a smaller colony of about 200 bats live in another dome (ca. 1, 250m2 and 18, 750 m3), both open to the public. Individuals are fed ad-libitum with a fruit-based mixture. Bats are ringed with a unique combination of 3 plastic rings on their forearm, allowing individual recognition.
Timeline of the experiment
We conducted this experiment using 5 cages at a time, for 1 run. We carried out 4 runs of the experiment from October 2014 until April 2015. Each run lasted about 45 days (± 3 days for hierarchy establishment). Each cage measured 1 m × 2 m × 1 m.
Each cage was constituted with 3 males of known tactics from the colony: 1 harem male with 2 females belonging to his harem, and 2 sneaker males. Individuals were trapped from the colony during their sleep using a hand net. An individual was considered to be a harem male when caught in a harem and reproductive (i.e., with scrotal testes), as harem males do not tolerate other reproductive males on their territory. For each harem male, 2 non-lactating females from the same harem were trapped and transferred to the cages. By having a harem male in a cage with females of his harem, we expected him to maintain his reproductive tactic despite the stress induced by the changes of spatial and social environment. The next day, sneaker males were trapped from bachelor groups in the colony and attributed randomly to the cages. During 3 weeks of acclimation period, the reproductive tactic of the males was determined via daily monitoring of the bats; the 1 male roosting with the females for 2 consecutive days was considered to be the harem male. Once the hierarchy was established, we never observed a change in the social structure.
After the 3 weeks of acclimation, we performed the first blood and ejaculate sampling (see below for details on the procedure). Immediately after sampling, we transferred each male to a different cage, in order to induce changes in some males’ reproductive tactics. Every male was in a novel cage, with individuals they had not yet encountered during the experiment. For each experimental run, we formed 3 types of groups, to maximize the number of reproductive tactics changes. In 1 control cage, we combined 1 harem male with 2 sneaker males and 2 females. In 2 cages, we combined 2 harem males and 1 sneaker male with 2 females. In the remaining 2 cages, we combined 3 sneaker males with 2 females. The different cage composition resulted in 4 categories of reproductive tactic changes: Sneaker males that remained Sneakers (SS), Sneakers that became Harem males (SH), Harem males that became Sneakers (HS) and Harem males that remained Harem males (HH). After the manipulation of reproductive tactics, it took between 3 and 6 days for the social environment to become stable, that is, for 1 male only to stay with the 2 females in 1 spot of the cage. Seven days and 3 weeks after the stabilization of the new hierarchy, we performed the second and third blood and ejaculate samplings, respectively. Establishment of the hierarchy is likely to have induced male-male agonistic interactions (Fernandez et al. 2014). However, no injuries were detected in the males following hierarchy establishment.
A total of 60 males were sampled initially, but several individuals were not included in the analysis. First, 15 males switched their reproductive tactics during the acclimation period compared with the reproductive tactic they exhibited in the colony. These individuals could not be included in the analysis as their first blood and ejaculate sampling would not provide basal measures. Then, males from 4 cages did not exhibit a clear reproductive tactic either during the initial acclimation period (2 cages) or after the manipulation of the reproductive tactics (2 cages); the group remained together without individuals becoming either harem or bachelor males. After a week, all the individuals from these cages (12 males) were released and excluded from the analysis, in order to keep the time spent in the cages comparable, as it might impact the physiology of individuals. Moreover, 1 male injured its wings after the manipulation of reproductive tactics and although he was left in the cage until the end of the experiment, we decided to remove him from the statistical analysis, because challenges to the immune system have been shown to modify the redox balance (Schneeberger et al. 2013). Finally, our sample size for statistical analysis was of 39 males before the manipulation, and 32 males after.
Specifically, for the first sampling before the manipulation, there were 23 sneakers and 13 harem males: For the 2nd and 3rd sampling, there were SS: 14; SH: 7; HS: 6; HH: 5. However, due to limitations in blood and ejaculates samples, we did not always manage to have all physiological measures for all males, and sample sizes may vary from 1 specific analysis to another.
Blood collection
For each individual, the blood was collected within 5 min after trapping. Blood samples were drawn from the antebrachial wing vein by puncturing with a sterile needle, and collecting the droplet of blood using a Microvette (CB 300 Hep-Li, Sarstedt). Samples were stored on ice for a maximum of 6 h until transportation to the lab. Back to the lab, blood samples were centrifuged at 2, 000 G for 5 min at 4°C to separate cells from plasma. The plasma was collected and aliquoted for later analyses. Samples were stored at –80°C.
Ejaculate collection
Ejaculates were collected using electro-ejaculation (Fasel et al. 2015). In summary, males were laid dorsally on a warming pad. During the procedure, males were anesthetized using isoflurane. Anesthesia was induced with 5% isoflurane mixed with oxygen for about 5 s, and then was decreased to 1–2% isoflurane. Oxygen was provided at a rate of 0.8 l/min. A probe covered with aqueous lubricant was inserted in the rectum approximately 1 cm deep. Electric stimulations were transmitted using 2 electrodes situated at the distal end of the probe (ICSB, USA). The electrode was linked to an audio amplifier (JVC A-X2) generating 3 series of regular and increasing electric stimulations (maximally 4 mA). Electrical current was continuously monitored with a milliampere meter (Fluke 77 multimeter). After the stimulation, oxygen was provided alone until awareness. The ejaculate collected was transferred into a microcentrifuge tube containing 10 µL of phosphate buffer saline (PBS) to avoid desiccation. According to the estimated volume of ejaculate, PBS was added to obtain a 1:2 dilution. An aliquot of 3 µL was immediately taken for mobility analysis. The remaining of the sample was stored on ice until transportation to the lab, and then kept at −80°C until analysis.
Sperm mobility traits analysis
The 3 µL aliquot was mixed with 15 µL of pre-warmed at 37°C Earle’s balanced salt solution (SpermWash Cryos, Denmark) and gently mixed. Within 10 min of collection, 3 µL of this mix was loaded in a swimming chamber (SC 20-01-04-B, Leja, Nieuw-Vennep, Netherlands), and sperm mobility was recorded in the SC under an Olympus XK41 microscope with dark-field condition, mounted with a Kappa CF 8/5 camera with a 20x magnification objective and a 10x magnification C-mount adaptor. Several 2-s videos (median 8, min 3, max 15) of 25 frames/s with a median number of 15 sperm tracks (min 0, max 224) were then analyzed for each ejaculate using a computer assisted sperm analysis plug-in ImageJ 1.47v (Wilson-Leedy and Ingermann 2007) to obtain estimates of 8 sperm swimming parameters: motility (proportion of motile sperm), curvilinear velocity (VCL, μm/s), velocity average path (VAP), velocity straight line (VSL), linearity (VSL/VAP), wobble (VAP/VCL), progression (average distance of the sperm from its origin on the average path during all frames analyzed) and beat cross frequency (BCF, frequency at which VCL crosses VAP, Hz). Sperm cells swimming with a higher velocity (VCL > 10 μm/s) than non-sperm particles in the sample were considered as motile.
As found previously (Fasel et al. 2015), a principal component analysis (PCA) on all sperm swimming parameters (excluding the percentage of motile sperm, analyzed separately), identified a first principal component (PC) explaining 53.19% of the variance, which was positively loaded with VCL, VAP, VSL but negatively loaded with BCF, and uncorrelated with the wobble, the number of sperm tracked, and linearity. Hence, males with high scores along this first PC produced fast swimming sperm. Results obtained using VCL or PC1 scores were qualitatively similar. Thus, for the sake of comparison with our previous work, and because it might be more intuitive to the readers, we only report analyses with VCL.
Redox markers
Lab analyses were performed by Magali Meniri, blindly with respect to sample identity and the experimental design. All steps were conducted on ice to reduce oxidation. All chemicals were HPLC grade, and chemical solutions were prepared using ultra-pure water H2O (Milli-Q Synthesis; Millipore, Watford, UK).
Blood sample preparation
A homogenate of red blood cells (RBC) was prepared by diluting 10 µL of cell pellet into 10 µL of PBS. The mixture was homogenized with 4 glass beads for 1 min at 30 Hz using a mixer mill, immersed in an ice-cold supersonic bath and sonicated for 5 min, and centrifuged for 5 min at 11, 200 G at 4°C. Of the supernatant 10 µL of RBC homogenate were transferred in an Eppendorf tube for MDA quantification. Another 2 µL were diluted with 748 µL of PBS, vortex-mixed and centrifuged for 15 min at 10, 000 G and 4°C (final dilution RBC 1:750 v:v) for SOD colorimetric assays. Finally, 2 µL were used immediately to quantify the reduced (GSH, ng/mL) and oxidized (glutathione disulfide GSSG, ng/mL) forms of glutathione (see glutathione quantification). We also quantified α, δ, γ-tocopherol using 10 µL of plasma, and MDA in 15 µL of plasma.
Ejaculate sample preparation
The ejaculate was homogenized with 2 glass beads for 1 min at 30 Hz using a mixer mill, then immersed in an ice-cold bath and sonicated for 5 min. Of the supernatant 10 µL of ejaculate were transferred in an Eppendorf tube for MDA quantification. Another 2.5 µL of ejaculate were diluted with 37.5 µL of PBS, vortex-mixed and centrifuged for 15 min at 10, 000 G and 4°C (final dilution ejaculate 1:48 v:v) for SOD colorimetric assays. Finally, 3.5 µL were used immediately to quantify glutathione.
Quantification of redox markers
We assessed MDA (nmol/mL) by its reaction with 2-thiobarbituric acid (TBA) to produce a pink derivate quantifiable by ultra-high performance liquid chromatography with fluorescence detection (UHPLC-FD), using a method adapted from (Losdat et al. 2014). Details about the method can be found in the Supplementary Material. We assessed SOD activity (U/mL) using Cayman’s SOD assay kit (Cayman chemical company, USA), which is based on the detection of superoxide radicals generated by xanthine oxidase and neutralized by SOD. The reduced (GSH, ng/mL) and oxidized (glutathione disulfide GSSG, ng/mL) forms of glutathione were measured by ultra-high performance liquid chromatography tandem mass-spectrometry (UHPLC-MS/MS), according to Bouligand et al. (2006) with some modifications. Further details about the method can be found in the Supplementary Material. We quantified α, δ, γ-tocopherol in the plasma by UHPLC-MS/MS. More details about the method can be found in the Supplementary Material.
Statistical analysis
To test the prediction that males redox profile and sperm quality would match their reproductive tactic, we ran a first set of models. Separate linear mixed-effects models were run for each sampling event, that is, before the manipulation, 7 days after the manipulation and 21 days after the manipulation. For the redox profile of the blood, the response variables of the univariate analyses were either MDARBC, MDAplasma, the GSSG/GSH ratio (log-transformed), SOD activity RBC or α, δ, γ-tocopherolplasma. For the redox profile of the ejaculates the response variable of the univariate analyses was either MDAejaculates, or SOD activityejaculates. Regarding the sperm quality traits, the response variables were either sperm velocity or the percentage of motile sperm (logit-transformed). For traits measured before manipulating male tactics, the fixed factor was the initial reproductive tactic (Sneaker or Harem). For traits measured 7 days or 21 days after the manipulation, the fixed factor was the type of change in reproductive tactic (SS, SH, HS, or HH). For each of these models, we included the cage number at the time of sampling (i.e., cage number before or after the manipulation of reproductive tactic for a specific run) as a random factor.
To test the prediction that sperm quality was correlated with the individual redox markers we built a second set of univariate models where the percentage of motile sperm (logit-transformed) or sperm velocity were included as response variables, and ejaculate redox markers (MDA, SOD, or GSH/GSSG ratio) were used as explanatory variables. For this set of models, individual identity was used as a random factor to account for the repeated measures taken on the same male.
Data were analyzed using R, version 3.3.3. The “nlme” package was used for the linear mixed-effects models, and we used the “lsmeans” package to run post-hoc tests based on least-square means with Tukey correction for test multiplicity. The significance level was set at 0.05. As we did not have measures for all redox markers and sperm quality for each male, we could not apply linear mixed-effect models with repeated measures and individual ID as a random factor. For similar reasons, we could not use data reduction methods such as PCAs to reduce the number of models. However, we applied the false discovery rate procedure to account for the multiplicity of tests (Benjamini and Hochberg 1995) and reduce the likelihood of type-I errors.
Results
Before the manipulation of reproductive tactics, neither the levels of redox markers, nor the sperm mobility traits differed between the 2 initial reproductive tactics (Tables 1 and 2).
Table 1.
Models investigating whether blood and sperm redox markers as well as sperm quality traits differed between initial male reproductive tactics (harem or sneaker) before the manipulation, or among tactic change categories (SS, SH, HS, and HH) 7 days and 21 days after the manipulation
| Before manipulation |
7 days after |
21 days after |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Response variable | N | F | P | N | F | P | N | F | P |
| Redox markers blood | |||||||||
| MDA RBC | 33 | 0.101,15 | 0.76 | 28 | 1.313,10 | 0.32 | 25 | 1.113,7 | 0.40 |
| MDA plasma (sqrt) | 34 | 0.091,16 | 0.76 | 26 | 0.253,10 | 0.85 | 26 | 1.803,8 | 0.22 |
| Ratio GSSG/GSH blood (log) | 28 | 0.091,10 | 0.77 | 23 | 0.043,7 | 0.98 | 28 | 0.153,12 | 0.93 |
| SOD blood | 24 | 0.121,11 | 0.74 | 25 | 2.133,7 | 0.18 | 27 | 0.793,11 | 0.52 |
| α-tocopherol | 21 | 0.161,10 | 0.69 | 15 | 0.593,5 | 0.64 | – | – | – |
| δ-tocopherol | 20 | 1.051,9 | 0.33 | 15 | 1.993,5 | 0.23 | – | – | – |
| γ-tocopherol | 19 | 3.271,9 | 0.10 | 14 | 1.813,4 | 0.28 | – | – | – |
| Redox markers ejaculate | |||||||||
| MDA ejaculate (sqrt) | 24 | 0.781,10 | 0.39 | 21 | 0.523,5 | 0.68 | 22 | 0.333,8 | 0.80 |
| SOD ejaculate | 26 | 0.391,9 | 0.54 | 25 | 0.993,4 | 0.43 | 25 | 0.503,8 | 0.69 |
| Sperm quality traits | |||||||||
| Sperm velocity | 36 | 0.631,16 | 0.44 | 31 | 0.253,10 | 0.86 | 30 | 1.233,8 | 0.36 |
| % motile sperm | 36 | 0.061,20 | 0.81 | 31 | 2.843,13 | 0.08 | 30 | 0.713,13 | 0.56 |
All linear mixed-effects models included the cage as a random factor.
Table 2.
Mean ± standard error for each response variables investigated, for the initial male reproductive tactics (harem or sneaker) before the manipulation, or among tactic change categories (SS, SH, HS, and HH) 7 days and 21 days after the manipulation on raw data
| Tactic changes | Redox markers blood |
Redox markers ejaculate |
Sperm quality traits |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDARBC | MDAplasma(1) | Ratio GSSG/ GSHblood(2) | SODblood | α-tocopherolplasma | δ-tocopherolplasma | γ-tocopherolplasma | MDAejaculate(1) | SODejaculate | Sperm velocity | % motile sperm (3) | |
| Before manipulation | |||||||||||
| Sneaker | 0.79 ± 0.11 | 0.28 ± 0.05 | 14.19 ± 2.36 | 7597.87 ± 445.35 | 45.89 ± 5.02 | 9.51 ± 1.26 | 1.12 ± 0.28 | 1.67 ± 0.28 | 250.05 ± 29.80 | 62.15 ± 3.48 | 0.27 ± 0.05 |
| Harem | 0.71 ± 0.24 | 0.20 ± 0.07 | 10.33 ± 1.68 | 7319.76 ± 602.52 | 49.22 ± 7.73 | 8.12 ± 0.55 | 0.57 ± 0.17 | 1.31 ± 0.35 | 277.81 ± 40.09 | 66.90 ± 4.99 | 0.31 ± 0.07 |
| 7 days after | |||||||||||
| SS | 0.64 ± 0.22 | 0.26 ± 0.06 | 12.21 ± 4.02 | 7104.39 ± 376.55 | 55.10 ± 9.92 | 14.02 ± 1.12 | 2.15 ± 0.35 | 1.50 ± 0.25 | 288.60 ± 48.72 | 65.44 ± 4.16 | 0.13 ± 0.03 |
| SH | 1.2 ± 0.25 | 0.42 ± 0.25 | 8.49 ± 2.02 | 6292.46 ± 945.23 | 63.35 ± 15.45 | 10.53 ± 2.45 | 1.77 ± 0.81 | 1.42 ± 0.64 | 240.53 ± 66.88 | 68.69 ± 4.34 | 0.33 ± 0.10 |
| HS | 0.99 ± 0.39 | 0.31 ± 0.10 | 7.91 ± 3.37 | 7366.51 ± 769.24 | 54.69 ± 6.86 | 8.07 ± 2.36 | 1.38 ± 0.72 | 1.81 ± 0.66 | 193.48 ± 48.02 | 67.26 ± 7.35 | 0.26 ± 0.12 |
| HH | 0.50 ± 0.08 | 0.27 ± 0.04 | 12.65 ± 6.24 | 6665.89 ± 705.36 | 87.20 ± NA | 13.5 ± NA | 4.67 ± NA | 1.94 ± 1.78 | 181.54 ± 56.87 | 56.01 ± 9.21 | 0.14 ± 0.13 |
| 21 days after | |||||||||||
| SS | 0.67 ± 0.12 | 0.21 ± 0.06 | 8.23 ± 1.34 | 7003.23 ± 510.10 | – | – | – | 1.71 ± 0.25 | 233.43 ± 57.44 | 59.43 ± 4.50 | 0.25 ± 0.07 |
| SH | 0.73 ± 0.19 | 0.07 ± 0.02 | 6.12 ± 1.29 | 8581.10 ± 339.10 | – | – | – | 1.62 ± 0.54 | 320.93 ± 95.85 | 71.94 ± 4.01 | 0.26 ± 0.08 |
| HS | 0.75 ± 0.29 | 0.19 ± 0.04 | 11.14 ± 1.91 | 8195.52 ± 1379.07 | – | – | – | 1.69 ± 0.44 | 220.75 ± 53.92 | 65.73 ± 8.54 | 0.21 ± 0.15 |
| HH | 1.12 ± 0.13 | 0.23 ± 0.07 | 7.12 ± 2.10 | 6863.31 ± 1101.64 | – | – | – | 2.03 ±NA | 260.60 ± 50.43 | 53.62 ± 10.93 | 0.17 ± 0.10 |
Mean and standard were computed or raw data. The transformations used for statistical analyses were: 1): square-root transformed; 2): log transformed; 3) logit transformed.
Seven days after the experimental manipulation of the reproductive tactics, none of the redox markers investigated, nor the mobility traits covaried with the changes in reproductive tactics (Tables 1 and 2). After 21 days, none of the blood and sperm redox markers differed across changes in reproductive tactics (Tables 1 and 2). Similarly, there were no differences in sperm mobility traits among reproductive tactics (Tables 1 and 2).
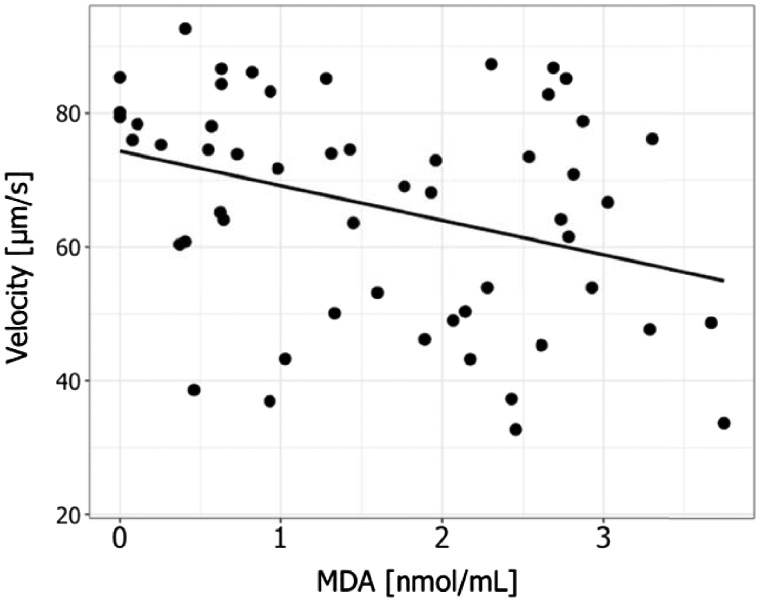
Finally, we investigated the link between sperm mobility traits and markers of ejaculate redox balance. Sperm velocity was negatively correlated with the level of MDA in the ejaculate (F1, 22=7.17; P = 0.01; Figure 1). The percentage of motile sperm was not correlated with ejaculate MDA level. Sperm mobility traits did not correlate with SOD activity in the ejaculate, nor with the GSSG/GSH ratio (Table 3).
Figure 1:
Correlation between sperm velocity and levels of MDA in the ejaculate. The black line is the regression line of the model.
Table 3.
Sperm mobility traits and redox markers
| Explanatory variable | Sperm velocity |
Percentage of motile sperm (logit) |
||||
|---|---|---|---|---|---|---|
| N | F | P | N | F | P | |
| MDA ejaculate | 54 | 7.171,22 | 0.01 | 63 | 0.011,29 | 0.90 |
| Ratio GSSG/GSH ejaculate (log) | 31 | 0.291,9 | 0.60 | 33 | 2.091,10 | 0.18 |
| SOD ejaculate | 60 | 0.051,26 | 0.83 | 71 | 0.321,35 | 0.57 |
Linear mixed-effects models, with individual identity as a random effect. P-values highlighted in bold remained significant (P < 0.05) after correction using the false discovery rate procedure.
Discussion
Our manipulation of the male reproductive tactics led to 4 categories of males, with males that remained in their initial tactic, and males that switched to the opposite tactic. Our results suggest that males did not adjust their sperm quality to their tactic. Numerous studies in various taxa have found that in species with ARTs, sneaker males exhibit higher sperm quality than males with a favored access to females in both plastic (birds: Froman et al. 2002; Rowe et al. 2010; insects: Kelly 2008; fishes: Haugland et al. 2009; squid: Hirohashi et al. 2016), and fixed ARTs (insects: Simmons and Emlen 2006; fishes: Fu et al. 2001; Makiguchi et al. 2016; Smith and Ryan 2010; Vladić and Järvi 2001; Young et al. 2013). In mammals however, results are more contrasted. Some studies have found the predicted trade-off between pre-copulatory expenditure and ejaculate quality (plastic ARTs: Fitzpatrick et al. 2012; Stockley and Purvis 1993), but others did not find a relationship between pre-copulatory expenditure and ejaculate quality according to the reproductive tactic (plastic ARTs: Kruczek and Styrna 2009; Lemaître et al. 2012; Schradin et al. 2012), or even found a positive relationship (plastic ARTs: Malo et al. 2005b; Preston et al. 2003).
The question then arises as to why males in our experiment did not adjust their sperm quality according to their reproductive tactics. Indeed, it was previously found in our model species that sneakers have higher sperm quality compared with harem males (Fasel et al. 2017). However, it was also shown that following 3 days of sexual abstinence, males of all reproductive tactics exhibited similar sperm quality (Wesseling et al. 2016). Therefore, these results suggest that the difference in sperm quality did not arise from differential investment in sperm quality, but rather from physiological constraints imposed by the higher copulation rate of harem males (Wesseling et al. 2016). Therefore, in our experiment, the conditions in the cages such as ad libitum feeding (Tuomi et al. 1983; Catoni et al. 2008), or low copulation rate (Wesseling et al. 2016) may have lifted a number of constraints, allowing all males to exhibit sperm of similar quality.
In this study, we also aimed at investigating whether allocation of resources in the antioxidant protection of the soma versus the germline may underlie differences in sperm quality, and may differ between harem and sneaker males. Although we found a negative correlation between the level of oxidative damage in the sperm and sperm velocity, we found no differences in somatic or ejaculate redox profile between individuals exhibiting different ARTs. Thus, and contrary to recent findings in other species (Rojas Mora et al. 2017b; Tomášek et al. 2017), oxidative stress does not seem to constraint the expression of reproductive tactics in Seba’s short-tailed bats, and thus does not lead to different redox profile according to male tactics (harem vs. sneaker). A comparative study conducted on tropical bats found that frugivorous bats showed both lower levels of oxidative damage and higher antioxidant level compared with species with other diets (Schneeberger et al. 2014). Therefore, Seba’s short-tailed bats may be able to acquire enough antioxidant from their diet to avoid oxidative stress, at least in our experimental cages. Furthermore, the protection of sperm membrane against lipid peroxidation could be offered by other means than antioxidants. Indeed, it was shown recently that PUFAs are reduced along epididymal maturation in C. perspicillata (Fasel et al. 2018). A membrane with lower concentration of PUFAs should thus better resist ROS (delBarco-Trillo et al. 2015).
Overall, our results suggest that a resource allocation trade-off between the soma and the ejaculate might not occur in Seba’s short-tailed bats. Lüpold et al. (2014) recently proposed that the level of female monopolization might mediate the relationship between pre- and post-copulatory traits. In a comparative study, they showed that if males are able to fully monopolize the females, therefore reducing the risk of sperm competition, they invest relatively more towards pre-copulatory traits. However, if the monopolization is incomplete, males invest in both types of traits, as sperm competition risk is distributed more evenly among males, regardless of their reproductive tactic. Similar results were found in a comparative study conducted by Stockley and Purvis (1993), who showed that in mammalian continuous breeders, a trade-off is found between pre- and post-copulatory selected traits, with subordinate males exhibiting relatively bigger testes compared with dominant males. However, in seasonal breeders, dominant and subordinate males exhibit testes of similar size, as dominant males might be overwhelmed by the number of simultaneously fertile females, and thus unable to monopolize females efficiently Harem males are also forced to leave their territory to forage, leaving their females unattended. Therefore, females are free to explore other territories, but also to mate with sneaker males away from the harem males’ territory. Moreover, females are not faithful to a single harem male during their life, but have been found to move often between harems (Fleming 1988). Although the harem-holding tactic has the highest pay-off, 40% of the pups are sired by sneakers (Fasel et al. 2016). Moreover, these estimates come from our captive population where reproduction is not synchronized among females. It is likely that in the wild, where reproduction occurs during 2 annual peaks, harem males may suffer from sperm depletion (Preston et al. 2001; Wesseling et al. 2016), and thus loose out more fertilization opportunities to sneakers. Overall, the mating system, the females’ behavior and the high proportion of pups sired by sneakers suggest that in the Seba’s short-tailed bats, females’ monopolization might be incomplete. Therefore, it is likely that contrary to our initial predictions, harem males might invest heavily in both pre- and post- copulatory traits, to both attract females and secure fertilizations.
Half of our initial males had to be removed from the analysis, as some did not form a hierarchy in the cages. These males represents yet another example of how stress induced by detention can impact an individual’s behavior, which can limit and even prevent the study of some behaviors (Tauson 1998; McCobb et al. 2005; Uetake et al. 2013; Schneeberger et al. 2014). For other males, the reproductive tactics that they exhibited in the colony changed during the acclimation period. It highlights the plasticity of these ARTs, and the fact that males may not be able to retain their territorial status when the environmental conditions change, that is, when put in captivity with a different social surrounding. It also shows that some males can readily take over the harem position, as soon as they are given the opportunity.
To conclude, we did not find differences in sperm quality according to reproductive tactics. We propose that a number of energetic constraints may have been lifted in the experimental cages, possibly allowing all males to exhibit sperm of similar quality regardless of their reproductive tactic. Furthermore, our results suggest that, in Seba’s short-tailed bats, the expression of ARTs is not subjected to strong oxidative constraints, possibly due to their antioxidant rich diet. Overall, our results suggest that harem males do not trade-off their investment in pre- versus post-copulatory traits, as a possible consequence of harem males’ inability to fully monopolize females.
Ethical statement
This study was supported by grants from the Swiss National Science Foundation n° PP00P3_139011 and n° PP00P3_165840 to FH, and by a grant from the Swiss National Science Foundation n° P2BEP3_168709 to NJF.
Supplementary Material
References
- Agarwal A, Virk G, Ong C, Du Plessis SS, 2014. Effect of oxidative stress on male reproduction. World J Mens Health 32:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. et al. , 2014. Oxidative stress and male reproductive health. Asian J Androl 16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson MB, 1994. Sexual Selection. Princeton, USA: Princeton University Press. [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc S B Methodol 57:289–300. [Google Scholar]
- Bouligand J, Deroussent A, Paci A, Morizet J, Vassal G, 2006. Liquid chromatography-tandem mass spectrometry assay of reduced and oxidized glutathione and main precursors in mice liver. J. Chromatogr B Analyt Technol Biomed Life Sci 832:67–74. [DOI] [PubMed] [Google Scholar]
- Catoni C, Peters A, Schaefer MH, 2008. Life history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Animal Behav 76:1107–1119. [Google Scholar]
- Clutton-Brock T, 2007. Sexual selection in males and females. Science 318:1882–1885. [DOI] [PubMed] [Google Scholar]
- Cocchia N, Pasolini MP, Mancini R, Petrazzuolo O, Cristofaro I. et al. , 2011. Effect of sod (superoxide dismutase) protein supplementation in semen extenders on motility, viability, acrosome status and ERK (extracellular signal-regulated kinase) protein phosphorylation of chilled stallion spermatozoa. Theriogenology 75:1201–1210. [DOI] [PubMed] [Google Scholar]
- Costantini D, 2018. Meta-analysis reveals that reproductive strategies are associated with sexual differences in oxidative balance across vertebrates. Curr Zool 64:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer RA, Costantini D, Casasole G, AbdElgawad H, Asard H. et al. , 2018. Sex-specific effects of inbreeding and early life conditions on the adult oxidative balance. Curr Zool 64:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- delBarco-Trillo J, Mateo R, Roldan ERS, 2015. Differences in the fatty-acid composition of rodent spermatozoa are associated to levels of sperm competition. Biology Open 4:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen ST, Oring LW, 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223. [DOI] [PubMed] [Google Scholar]
- Engqvist L, Reinhold K, 2006. Theoretical influence of female mating status and remating propensity on male sperm allocation patterns. J Evol Biol 19:1448–1458. [DOI] [PubMed] [Google Scholar]
- Engqvist L, Taborsky M, 2016. The evolution of genetic and conditional alternative reproductive tactics. Proc Biol Sci 283:20152945.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasel N, McMillian K, Jakop U, Méné-Saffrané L, Engel K. et al. , 2018. Modification of sperm fatty acid composition during epididymal maturation in bats. Reproduction 157:77–85. [DOI] [PubMed] [Google Scholar]
- Fasel N, Saladin V, Richner H, 2016. Alternative reproductive tactics and reproductive success in male Carollia perspicillata (Seba’s short-tailed bat). J Evol Biol 29:2242–2255. [DOI] [PubMed] [Google Scholar]
- Fasel NJ, Helfenstein F, Buff S, Richner H, 2015. Electroejaculation and semen buffer evaluation in the microbat Carollia perspicillata. Theriogenology 83:904–910. [DOI] [PubMed] [Google Scholar]
- Fasel NJ, Wesseling C, Fernandez AA, Vallat A, Glauser G. et al. , 2017. Alternative reproductive tactics, sperm mobility and oxidative stress in Carollia perspicillata (Seba’s short-tailed bat.). Behav Ecol Sociobiol 71:11. [Google Scholar]
- Fernandez AA, Fasel N, Knörnschild M, Richner H, 2014. When bats are boxing: aggressive behaviour and communication in male Seba’s short-tailed fruit bat. Animal Behav 98:149–156. [Google Scholar]
- Fitzpatrick JL, Almbro M, Gonzalez-Voyer A, Kolm N, Simmons LW, 2012. Male contest competition and the coevolution of weaponry and testes in Pinnipeds. Evolution 66:3595–3604. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JL, Lüpold S, 2014. Sexual selection and the evolution of sperm quality. Mol Hum Reprod 20:1180–1189. [DOI] [PubMed] [Google Scholar]
- Fleming TH, 1988. The Short-Tailed Fruit Bat: A Study in Plant-Animal Interactions. Chicago, USA: University of Chicago Press. [Google Scholar]
- Froman DP, Pizzari T, Feltmann AJ, Castillo-Juarez H, Birkhead TR, 2002. Sperm mobility: mechanisms of fertilizing efficiency, genetic variation and phenotypic relationship with male status in the domestic fowl Gallus gallus domesticus. Proc Biol Sci 269:607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Neff BD, Gross MR, 2001. Tactic-specific success in sperm competition. Proc Biol Sci 268:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini C, Simmons LW, Beveridge M, Evans JP, 2010. Sperm swimming velocity predicts competitive fertilization success in the green swordtail Xiphophorus helleri. PLoS ONE 5: e12146.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J, 2007. Free Radicals in Biology and Medicine. 4th edn. Oxford, NY: Oxford University Press. [Google Scholar]
- Haugland T, Rudolfsen G, Figenschou L, Folstad I, 2009. Sperm velocity and its relation to social status in Arctic charr Salvelinus alpinus. Animal Reproduction Sci 115:231–237. [DOI] [PubMed] [Google Scholar]
- Hirohashi N, Tamura-Nakano M, Nakaya F, Iida T, Iwata Y, 2016. Sneaker male squid produce long-lived spermatozoa by modulating their energy metabolism. J Biol Chem 291:19324–19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahrl AF, Cox CL, Cox RM, 2016. Correlated evolution between targets of pre- and postcopulatory sexual selection across squamate reptiles. Ecol Evol 6:6452–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CD, 2008. Sperm investment in relation to weapon size in a male trimorphic insect? Behav Ecol 19:1018–1024. [Google Scholar]
- Kruczek M, Styrna J, 2009. Semen quantity and quality correlate with bank vole males’ social status. Behav Processes 82:279–285. [DOI] [PubMed] [Google Scholar]
- Lemaître J-F, Ramm SA, Hurst JL, Stockley P, 2012. Sperm competition roles and ejaculate investment in a promiscuous mammal. J Evol Biol 25:1216–1225. [DOI] [PubMed] [Google Scholar]
- Lewis SEM, Aitken RJ, 2005. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res 322:33–41. [DOI] [PubMed] [Google Scholar]
- Locatello L, Rasotto MB, Evans JP, Pilastro A, 2006. Colourful male guppies produce faster and more viable sperm. J Evol Biol 19:1595–1602. [DOI] [PubMed] [Google Scholar]
- Losdat S, Helfenstein F, Blount JD, Richner H, 2014. Resistance to oxidative stress shows low heritability and high common environmental variance in a wild bird. J Evol Biol 27:1990–2000. [DOI] [PubMed] [Google Scholar]
- Lüpold S, Tomkins JL, Simmons LW, Fitzpatrick JL, 2014. Female monopolization mediates the relationship between pre- and postcopulatory sexual traits. Nat Commun 5:3184.. [DOI] [PubMed] [Google Scholar]
- Makiguchi Y, Torao M, Kojima T, Pitcher TE, 2016. Reproductive investment patterns and comparison of sperm quality in the presence and absence of ovarian fluid in alternative reproductive tactics of masu salmon Oncorhynchus masou. Theriogenology 86:2189–2193.e2. [DOI] [PubMed] [Google Scholar]
- Malo AF, Garde JJ, Soler AJ, García AJ, Gomendio M. et al. , 2005a. Male fertility in natural populations of red deer is determined by sperm velocity and the proportion of normal spermatozoa. Biol Reprod 72:822–829. [DOI] [PubMed] [Google Scholar]
- Malo AF, Roldan ERS, Garde J, Soler AJ, Gomendio M, 2005b. Antlers honestly advertise sperm production and quality. Proc Biol Sci 272:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCobb EC, Patronek GJ, Marder A, Dinnage JD, Stone MS, 2005. Assessment of stress levels among cats in four animal shelters. J Am Vet Med Assoc 226:548–555. [DOI] [PubMed] [Google Scholar]
- Mehlis M, Hilke LK, Bakker TCM, 2013. Attractive males have faster sperm in three-spined sticklebacks Gasterosteus aculeatus. Curr Zool 59:761–768. [Google Scholar]
- Metcalfe NB, Alonso-Alvarez C, 2010. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death: oxidative stress as a life-history constraint. Funct Ecol 24:984–996. [Google Scholar]
- Monaghan P, Metcalfe NB, Torres R, 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12:75–92. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Taborsky M, Brockmann HJ, 2008. Alternative Reproductive Tactics: An Integrative Approach. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Parker GA, 1990. Sperm competition games: sneaks and extra-pair copulations. Proc Biol Sci 242:127–133. [Google Scholar]
- Parker GA, Lessells CM, Simmons LW, 2013. Sperm competition games: a general model for precopulatory male-male competition. Evolution 67:95–109. [DOI] [PubMed] [Google Scholar]
- Peters A, Denk AG, Delhey K, Kempenaers B, 2004. Carotenoid-based bill colour as an indicator of immunocompetence and sperm performance in male mallards. J Evol Biol 17:1111–1120. [DOI] [PubMed] [Google Scholar]
- Preston BT, Stevenson IR, Pemberton JM, Coltman DW, Wilson K, 2003. Overt and covert competition in a promiscuous mammal: the importance of weaponry and testes size to male reproductive success. Proc Biol Sci 270:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston BT, Stevenson IR, Pemberton JM, Wilson K, 2001. Dominant rams lose out by sperm depletion. Nature 409:681–682. [DOI] [PubMed] [Google Scholar]
- Pusch HH, 1987. The importance of sperm motility for the fertilization of human oocytes in vivo and in vitro. Andrologia 19:514–527. [DOI] [PubMed] [Google Scholar]
- Rojas Mora A, Firth A, Blareau S, Vallat A, Helfenstein F, 2017a. Oxidative stress affects sperm performance and ejaculate redox status in subordinate house sparrows. J Exp Biol 220:2577–2588. [DOI] [PubMed] [Google Scholar]
- Rojas Mora A, Meniri M, Gning O, Glauser G, Vallat A. et al. , 2017b. Antioxidant allocation modulates sperm quality across changing social environments. PLoS ONE 12:e0176385.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M, Swaddle JP, Pruett-Jones S, Webster MS, 2010. Plumage coloration, ejaculate quality and reproductive phenotype in the red-backed fairy-wren. Animal Behav 79:1239–1246. [Google Scholar]
- Schneeberger K, Czirják GÁ, Voigt CC, 2013. Inflammatory challenge increases measures of oxidative stress in a free-ranging, long-lived mammal. J Exp Biol 216:4514–4519. [DOI] [PubMed] [Google Scholar]
- Schneeberger K, Czirják GÁ, Voigt CC, 2014. Frugivory is associated with low measures of plasma oxidative stress and high antioxidant concentration in free-ranging bats. Naturwissenschaften 101:285–290. [DOI] [PubMed] [Google Scholar]
- Schradin C, Eder S, Müller K, 2012. Differential investment into testes and sperm production in alternative male reproductive tactics of the African striped mouse Rhabdomys pumilio. Horm Behav 61:686–695. [DOI] [PubMed] [Google Scholar]
- Sheldon BC, 1994. Male phenotype, fertility, and the pursuit of extra-pair copulations by female birds. Proc Biol Sci 257:25–30. [Google Scholar]
- Simmons LW, Emlen DJ, 2006. Evolutionary trade-off between weapons and testes. PNAS 103:16346–16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons LW, Fitzpatrick JL, 2012. Sperm wars and the evolution of male fertility. Reproduction 144:519–534. [DOI] [PubMed] [Google Scholar]
- Smith CC, Ryan MJ, 2010. Evolution of sperm quality but not quantity in the internally fertilized fish Xiphophorus nigrensis. J Evol Biol 23:1759–1771. [DOI] [PubMed] [Google Scholar]
- Stockley P, Purvis A, 1993. Sperm competition in mammals: a comparative study of male roles and relative investment in sperm production. Funct Ecol 7:560–570. [Google Scholar]
- Taborsky M, Oliveira RF, Brockmann HJ, 2008. The evolution of alternative reproductive tactics: concepts and questions In: Oliveira RF, Taborsky M, Brockmann HJ, editors. Alternative Reproductive Tactics. Cambridge: Cambridge University Press, 1–22. [Google Scholar]
- Tauson R, 1998. Health and production in improved cage designs. Poult Sci 77:1820–1827. [DOI] [PubMed] [Google Scholar]
- Tomášek O, Albrechtová J, Němcová M, Opatová P, Albrecht T, 2017. Trade-off between carotenoid-based sexual ornamentation and sperm resistance to oxidative challenge. Proc R Soc B 284:20162444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubaki Y, Rowan EH, Siva-Jothy MT, 1997. Differences in adult and reproductive lifespan in the two male forms of Mnais pruinosa costalis Selys. Res Popul Ecol 39:149–155. [Google Scholar]
- Tuomi J, Hakala T, Haukioja E, 1983. Alternative concepts of reproductive effort, costs of reproduction, and selection in life-history evolution. Am Zool 23:25–34. [Google Scholar]
- Uetake K, Goto A, Koyama R, Kikuchi R, Tanaka T, 2013. Effects of single caging and cage size on behavior and stress level of domestic neutered cats housed in an animal shelter. Anim Sci J 84:272–274. [DOI] [PubMed] [Google Scholar]
- Vladić TV, Järvi T, 2001. Sperm quality in the alternative reproductive tactics of Atlantic salmon: the importance of the loaded raffle mechanism. Proc Biol Sci 268:2375–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling C, Fasel N, Richner H, Helfenstein F, 2016. Modification of sperm quality after sexual abstinence in Seba’s short-tailed bat, Carollia perspicillata. J Exp Biol 219:1363–1368. [DOI] [PubMed] [Google Scholar]
- Wilson-Leedy JG, Ingermann RL, 2007. Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 67:661–672. [DOI] [PubMed] [Google Scholar]
- Wright C, Milne S, Leeson H, 2014. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online 28:684–703. [DOI] [PubMed] [Google Scholar]
- Young B, Conti DV, Dean MD, 2013. Sneaker “jack” males outcompete dominant “hooknose” males under sperm competition in Chinook salmon Oncorhynchus tshawytscha. Ecol Evol 3:4987–4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.