Abstract
Soils from selected organic and conventional farms in the Philippines were examined for parasite contamination. A total of 600 soil samples from twenty organic and conventional farms were collected and processed through modified sucrose floatation technique. Results revealed that 248 out of 600 (41.33%) soil samples were contaminated with parasite eggs. Parasites recovered were Ascaris spp. (39.0%), Trichuris spp. (26.0%), hookworm/strongylid (22.0%), Toxocara spp. (4.0%), Taenia spp. (3.0%), and some unidentified eggs (6.0%). Contamination rate was found higher in organic (48.30%) than in conventional farms (37.67%) (p = 0.834; not significant); and significantly higher in Southern (64.40%) than in Northern Luzon region (31.40%) (p = 0.015). In addition, contamination rate between soil depths was not statistically significant (p = 0.24), with depth 1 (0-5 cm) at 43% and depth 2 (6-10 cm) at 39.67%. Furthermore, some farming practices were recorded through survey and results revealed that the use of manure as fertilizer (p = 0.017) and wash water (p = 0.014) showed significant positive relationship with parasite contamination in soil. These findings have implications on food safety and could be used to help the agriculture sector and other stakeholders in their efforts to improve food safety policies.
Keywords: Farm soil, STH, Philippines, Agriculture
1. Introduction
Soil-transmitted helminths (STH) infection is common in developing and tropical countries. It is estimated that >1 billion people or one-sixth of the world's population suffer from STH. The most common STH recorded in humans are Ascaris lumbricoides, Trichuris trichiura and hookworms (Necator americanus and Ancylostoma duodenale) (WHO, 2012). In the Philippines, 25% of Filipinos are reported to be infected with these parasites. Of these, children are the most affected (Belizario Jr et al., 2009). STH are transmitted due to poor hygiene practices through accidental ingestion of contaminated food, soil, and fomites. Parasites from soils may infect humans and other animals through accidental ingestion of contaminated soil and eating improperly washed vegetables (Mustafa et al., 2001; Ordoñez et al., 2018). Moreover, there have been increased reports of zoonotic parasites in animal wastes which are commonly used as fertilizers on crops (Andes and Paller, 2018; Ogbolu et al., 2011; Beuchat, 2002).
There are two farming systems practiced in the Philippines - organic and conventional; although diversified integrated farming is also being promoted. Organic farming commonly uses animal manure as fertilizers while conventional farming uses chemical fertilizers. Conventional farming produces higher yields due to higher inputs, however, due to extensive use of pesticides and chemical fertilizers, this type of farming may put the environment at risk. Since people are now more concerned with health and environmental issues, organic farming is on the rise. Furthermore, the Philippine government has placed a huge budget to promote organic agriculture programs through the Department of Agriculture (Maghirang et al., 2013).
In the Philippines, some studies reported the contamination of parasites in soils from selected urban and rural areas in Southern Luzon (Paller and de Chavez, 2014; Horiuchi et al., 2013). However, there are still no reports regarding parasite contamination in soils from agricultural farms and the comparison of parasite contamination between organic and conventional farms. There are also no reports about the association of farming practices with the extent of parasite soil contamination in agricultural lands. Thus, this study generally aimed to determine the extent of contamination of soil with STH eggs and determine the association between some farming practices and contamination rate in soils from selected organic and conventional farms in the Philippines.
2. Materials and methods
2.1. Study site and sampling design
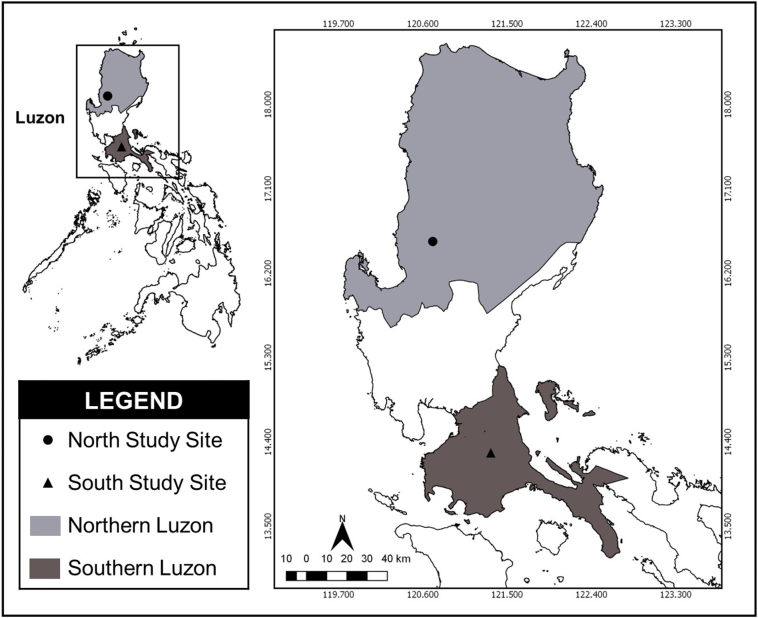
The study sites were selected organic and conventional farms from two provinces in the Northern and Southern Luzon, Philippines that are known produce and supply large quantities of fresh produce within the regions. (Fig. 1). The present study includes a total of 20 farms – 14 from Northern Luzon (7 organic and 7 conventional farms) and six from Southern Luzon (3 organic and 3 conventional farms) – selected from the list provided by the respective Provincial Agricultural Offices (PAO) of the two regions. Farms were chosen based on the following criteria: (a) must be within the range of 300 square meters to 1000 square meters (b) must be practicing organic or conventional farming, (c) must have commercial purposes of farm produce, (d) must include green salad leafy vegetables such as lettuce, and (e) must have consent from the farm owner.
Fig. 1.
Map showing the collection sites in Northern and Southern Luzon Provinces, Philippines.
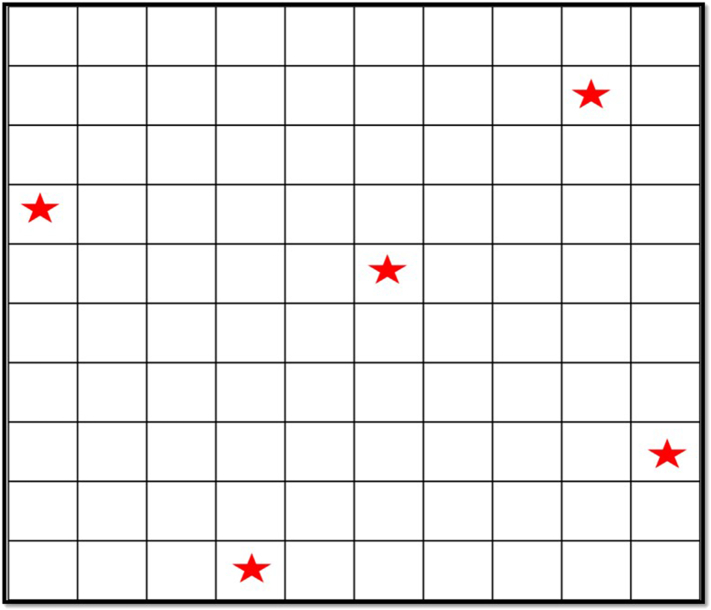
Soil samples were collected from the vegetable plots in the farms using systematic random sampling. The collection period was done during dry season (January to May). The number of samples collected from each farm were based on the size of the vegetable farming area. Soil samples were collected in different five points per 100 sq. meters (Fig. 2). The farm was divided into plots measuring 1 sq. meter and were numbered from 1 to 100. Samples were collected from plots with the number randomly generated by a scientific calculator. Soils were collected from each sampling point at depth 1 (0-5 cm) and depth 2 (6-10 cm). Soils were collected near vegetable plots which were reported as plowed or cultivated every after harvest. Each soil sample was placed in a polyethylene bag, labeled, sealed and brought to the laboratory.
Fig. 2.
Systematic random sampling of 5 points in a 100 square meter farm area.
2.2. Soil collection and processing
Soil samples were processed through modified flotation technique (Horiuchi and Uga, 2016; Horiuchi et al., 2013). Briefly, the soil samples were air-dried for 24–48 h and sifted using a 150-um sieve. Three grams of sieved soil was placed in a 10-ml test tube and washed with 8 ml distilled water by mixing using a vortex mixer (Iwaki, Japan). The suspension was then centrifuged for 10 min at 1800 rpm. The supernatant was decanted and 8 ml of sucrose solution (1.2 specific gravity) was added to the sediment in the tube and mixed thoroughly using a vortex mixer. The tube was centrifuged for 10 min at 1800 rpm. After centrifugation, sucrose solution at 1.3 specific gravity was slowly added up to the brim of the tube using a 10-ml syringe until an upper meniscus was formed. A cover slip was carefully placed on the meniscus to collect the topmost portion of the sucrose suspension. The slides were examined for presence of STH eggs using compound light microscope (Nikon, Tokyo) at 100× and 400× magnification. Egg sizes were measured using an ocular micrometer (Paller and de Chavez, 2014; Matsuo and Nakashio, 2004).
2.3. Survey of farming practices
Survey interviews of the farmer or farm owners were conducted to document the management and practices in the farms. The survey interviews were done to determine the association of Knowledge, Attitudes, and Practices (KAPs) in farming with parasite contamination. The questions included were as follows: use of manure as fertilizer, sources of wash water for animals and crops, hygiene and sanitation practices, and the presence of animals in or near the farm. All the 20 farms participated in the survey. This documentation would provide important information on the potential risk factors of soil contamination in farms. Ethical clearance was obtained from the Institutional Ethics Committee. Informed consent was given and signed by proprietors prior to collection of samples and conduct of survey interview. Questionnaires were pre-tested prior to application in the field.
2.4. Data analysis
Soil contamination rate (%) was calculated as the number of positive samples divided by the total number of soil samples multiplied by 100. Mean density was calculated as the total number of parasites eggs divided by the total number of positive samples. Independent sample t-test was used for the comparison of contamination rate between organic and conventional farms, between samples from the two sampling sites and between the two depths (depth 1 and depth 2) of soil. Point-Biserial correlation was used to determine the association of the contamination rate in soil with the farming practices. Data were analyzed at 95% level of significance.
3. Results
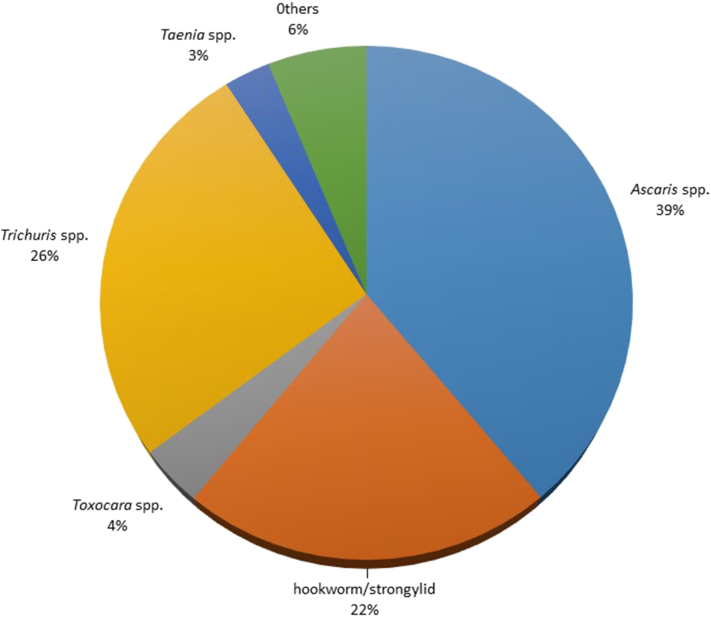
A total of 600 soil samples from selected organic (n = 10) and conventional (n = 10) farms in Northern and Southern Luzon, Philippines were collected and examined for the presence soil-transmitted helminth (STH) eggs. Of the 600 soil samples, 248 were found positive for STH (41.33%). Parasites recovered were Ascaris spp., Trichuris spp., hookworm/strongylid, Toxocara spp., and Taenia spp. with rates of 39.0%, 26.0%, 22.0%, 4.0%, and 3.0%, respectively. Other unidentified eggs had rates of 6.0% (Fig. 3 and Fig. 4).
Fig. 3.
Proportion of STH eggs found in farm soils from selected farms in Northern and Southern Luzon provinces.
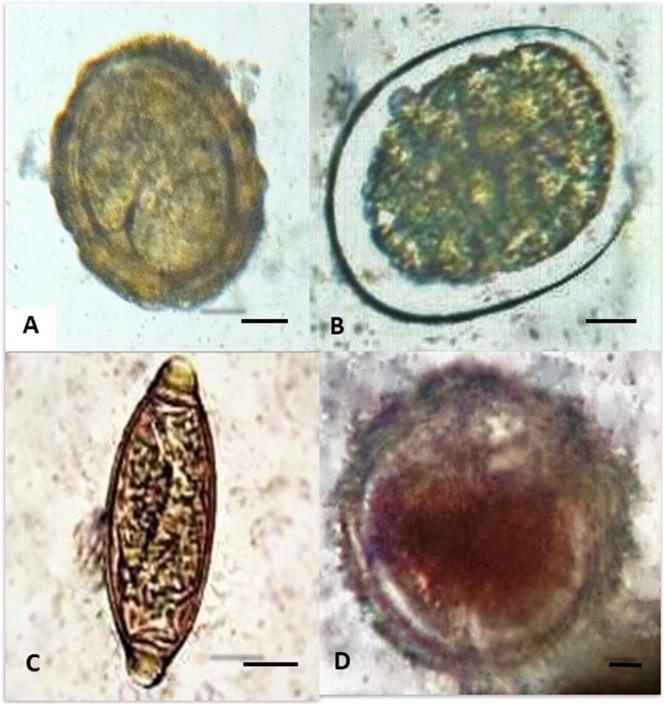
Fig. 4.
STH eggs found in soil farms from Northern and Southern Luzon Provinces: A) Ascaris sp.; B) Hookworm; C) Trichuris sp.; D) Toxocara sp. (Scale bar: 10 μm).
3.1. STH contamination rate of farm soils
Table 1 shows the STH contamination rate in soils collected from Northern and Southern Luzon. Southern Luzon showed higher STH contamination (64.40%) than Northern Luzon region (31.40%). Statistical analysis revealed significant difference between the two selected areas (p = 0.015). Table 1 also summarizes the parasite contamination between organic and conventional farms. Organic farms showed higher STH contamination rate (48.30%) than conventional farms (37.67%). However, statistical analysis showed no significant difference between the two types of farms (p = 0.834).
Table 1.
Contamination rate and mean density of STH recovered from organic and conventional farms in Northern and Southern Luzon Provinces.
| Northern Luzon |
Southern Luzon |
Over-all contamination rate (%) | |||
|---|---|---|---|---|---|
| Contamination Rate (%) | Mean density (epg) | Contamination Rate (%) | Mean density (epg) | ||
| Organic farms | |||||
| Ascaris sp. | 18.00 | 1 | 25.56 | 1 | |
| Hookworm/strongylid | 6.67 | 1 | 17.78 | 1 | |
| Trichiuris sp. | 6.00 | 1 | 20.00 | 1 | |
| Toxocara sp. | 2.00 | 1 | 2.22 | 1 | |
| Taenia sp. | 0.67 | 1 | 2.22 | 1 | |
| Total | 35.24 | 1 | 67.78 | 1 | 48.30 |
| Conventional farms | |||||
| Ascaris sp. | 10.00 | 1 | 24.44 | 1 | |
| Hookworm/strongylid | 6.00 | 1 | 13.33 | 1 | |
| Trichiuris sp. | 5.33 | 1 | 21.11 | 1 | |
| Toxocara sp. | 3.33 | 1 | – | 1 | |
| Taenia sp. | 0.67 | 1 | 2.22 | 1 | |
| Total | 31.43 | 1 | 64.44 | 1 | 37.67 |
| Over-all contamination rate (%) | 31.40 | 64.40 | |||
3.2. Comparison of contamination rate between depth 1 and depth 2
The extent of contamination in various soil depths was also examined in this study. Results showed that 43.0% of the soil samples from depth 1 (0–5 cm) were positive with STH eggs while 39.67% were found positive in depth 2 (6–10 cm) (Fig. 5). However, statistical analysis showed that there was no significant difference in the contamination rate between the two depths (p = 0.968). The mean density of eggs per gram of soil in both depths was found to be 1 egg/g soil.
Fig. 5.
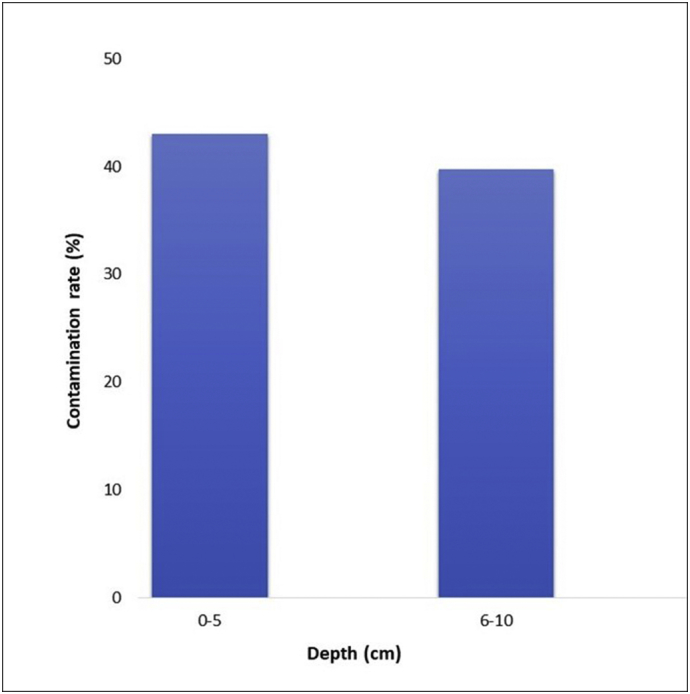
STH contamination rate in two different depths of soils from organic and conventional farms in Northern and Southern Luzon, Philippines.
3.3. Association between farming practices and STH contamination
Association between farming practices and parasite contamination in soil was also analyzed. Table 2 shows the summary of farming practices in selected farms in Northern and Southern Luzon. Farm practices such as type of fertilizer used (p = 0.017) and water sources (p = 0.014) showed significant relationship with parasite contamination in soil. Other factors such as use of gloves, presence of toilet inside the farm, washing of hands, deworming and presence of farm animals were also considered but showed no significant relationship with the parasite contamination rates.
Table 2.
Farming practices in organic and conventional farms in Northern Luzon and Southern Luzon.
| Farming practices | Northern Luzon (n = 14) |
Southern Luzon (n- = 6) |
P value | ||
|---|---|---|---|---|---|
| Organic | Conventional | Organic | Conventional | ||
| Fertilizers used | |||||
| Animal manure | 7 | 5 | 2 | 1 | 0.017⁎ |
| Synthetic | 0 | 2 | 1 | 2 | |
| Human excreta | 0 | 0 | 0 | 0 | |
| Water source | |||||
| Wash water (tap) | 6 | 6 | 3 | 2 | 0.014⁎ |
| Mountain spring | 1 | 1 | 0 | 1 | |
| Hygiene | |||||
| Toilet facilities | |||||
| Present | 3 | 2 | 2 | 2 | 0.456 |
| Absent | 4 | 5 | 1 | 1 | |
| Using gloves | |||||
| Yes | 2 | 3 | 2 | 1 | 0.636 |
| No | 5 | 4 | 1 | 2 | |
| Washing of hands before and after treating the soil | |||||
| Yes | 5 | 2 | 1 | 1 | 0.472 |
| No | 2 | 5 | 2 | 2 | |
| Deworming of farmers and farm animals | |||||
| Yes | 2 | 1 | 1 | 1 | 0.485 |
| No | 5 | 6 | 2 | 2 | |
| Farm animals in or near the farm | |||||
| Present | 6 | 2 | 3 | 2 | 0.102 |
| Absent | 1 | 5 | 0 | 1 | |
Significant at p ≤ 0.05.
4. Discussion
In the present study, Ascaris spp., Trichuris spp., Toxocara spp., hookworm/strongylid, and Taeniid eggs were observed in the soils from the selected farms. Interestingly, the study of Ordoñez et al. (2018) reported the presence of the same parasites in vegetables harvested from the same study sites. Through molecular analysis, parasites were identified as Ancylostoma ceylanicum, Ascaris suum, Toxocara canis, T. cati. and Trichiuris trichiura. A. ceylanicum, and T. canis originate from dogs, T. cati from cats, A. suum from pigs, and T. trichiura from humans.
Parasite contamination in soils with STH eggs is not only distinct to this study but it has been reported worldwide (Sunil et al., 2014, and Klapec and Borecka, 2012). The presence of STH eggs in the environment is an indication of human and animal activities that involve unhygienic practices such as open defecation. STH exhibit high fecundity and requires soil for development. For instance, Ascaris lumbricoides and A. suum lay about 200,000 eggs daily, while Trichuris trichiura and Toxocara canis lay approximately 10,000 eggs. High fecundity is one of parasites' strategy to increase their chance for transmission to next hosts. Moreover, most STH eggs are thick-shelled and resistant to different unfavorable conditions such as dehydration and exposure to chemicals. These characteristics of parasite eggs allow them to withstand adverse environmental conditions so they can survive longer in soil environments.
In the Philippines, recent studies have also been conducted on soil contamination with STH eggs. Horiuchi et al. (2013) found out that >70.0% of the soil samples from rural villages in Southern Luzon were positive for STH eggs. Furthermore, according to Paller and de Chavez (2014), 31% of the soil samples in urban and rural areas in Southern Luzon were positive for STH eggs. Their results showed that Toxocara spp. was the most prevalent (77.0%), followed by Ascaris spp. (11.0%), hookworms/strongylids (7.0%) and Trichuris spp. (5.0%).
Meanwhile, in the present study, STH soil contamination in selected farms was found to be higher in Southern than in Northern Luzon region. The difference could be due to locations and varying altitude. Northern Luzon selected farms were 4130 ft above sea level, while the farms in Southern Luzon range from 118 to 962 ft above sea level. In the study of Appleton and Gouws (1996), it was reported that as the altitude increases, the number of parasites decreases. Parasites are said to be highly affected by surface temperatures and altitude (Hotez, 2008). Helminths such as Trichuris and Ascaris are predominant in low altitude places. During the sampling period, Southern Luzon and Northern Luzon had average temperatures of 27.75 °C and 20.25 °C, respectively. Temperature is a contributing factor in the development of parasites in soil; a mean temperature of 31.54 ± 2.76 °C allows development of the STH (Paller and de Chavez (2014). Furthermore, temperature affects the viability of eggs. Parasite eggs can be viable for ten to twelve months in tropical climates (Sunil et al., 2014). The ability of these parasites to withstand the temperature in the soil might be a factor in having more chances of the parasites to survive and contaminate the environment.
However, the difference in contamination rate between the two provinces could be highly attributed to the differences in farming practices. In the selected sites in Northern Luzon, both organic and conventional farms are strictly being monitored by organized cooperatives. Regardless of the type of farming, there is a set of common guidelines for good farming practices. According to these guidelines, farms should: (World Health Organization, 2012) be located far from residential areas, (Belizario Jr et al., 2009) have an area of at least 500m2, (Mustafa et al., 2001) be barred with fences, and (Ordoñez et al., 2018) not allow animals to roam inside the farms (Food and Agriculture Organization, 1998). On the other hand, selected farms in Southern Luzon are randomly located in the province and vary in farming practices with no strict guidelines.
This study also compared organic versus conventional farms. Organic farms showed higher STH contamination rate than conventional farms. Although not significant, the difference could be due also to different farming practices between the two types of farms. Organic farms are involved with extensive use of manure as fertilizer which could contribute to the contamination of soil with STH eggs. On the other hand, the selected conventional farms do not exclusively use synthetic fertilizer but also apply animal manure which could explain the contamination of soils. Some animals were also observed roaming in some farms. According to the interview, both conventional and organic farms in Southern Luzon and Northern Luzon use animal manure as fertilizers. However, no disclosure was made whether these fertilizers were treated to kill potential pathogens. The presence of parasites in untreated manure may increase contamination rate in soils with parasite eggs. According to Rai et al., 1994, Rai et al., 1995 presence of farm animals and the use of their dung for fertilizer can be a possible source of STH eggs. As observed by Klapec and Borecka (2012), the abundance of farm animals is directly proportional to the contamination rate of soils with STH eggs.
In terms of soil depth, this study revealed presence of STH eggs up to 10 cm depth. According to Stojcevic et al. (2010), a depth of 0-4 cm gives the optimum environment for STH eggs while Beaver (1980) added that T. trichiura eggs survive more in shallow parts of the soil by 20–30%. Furthermore, Hotez et al. (2003) reported that the survivability of eggs is higher at increased soil depths. It was also reported that STH, such as Ascaris could still survive in soil as deep as 10 cm (Beaver, 1980). Earthworms may also act to transport parasite eggs allowing surface soils to be carried deeper into the soils (Mizgajska, 1997). Also, at greater depth (6-10 cm), STH eggs are protected from direct sunlight; thus, surviving in the environment for longer periods (Paller and De Chavez, 2014).
Results revealed that the type of fertilizer used (manure or synthetic) and water sources (tap wash water or mountain spring) have showed significant contribution to STH contamination in farm soils. Agricultural practices such as the use of raw manure as fertilizer contribute to soil contamination with STH parasites (Andes and Paller, 2018). Piggeries whose runoffs may also contribute to contamination of STH eggs in farm soils were observed to be in proximity to some of the selected farms. Moreover, pig manure was observed to be an additional component in the fertilizers in some selected farms; although some farmers practice vermicomposting. None of the farms was reported to use human excreta as fertilizer. The study of Miller et al. (2015), stated that the use of pig bedding for compost and the location of pigpens near farms may serve as additional sources of human infection and may also contribute to soil contamination. Aside from fertilizers, wash water showed significant relationship with the presence of parasites. During the sampling period, it was observed that farmers handle farm animals and collect animal manure without gloves or any protective suits. In addition, some farmers usually wash their hands in water tanks which are also used to water their crops. This unhygienic practice could increase the contamination of farm soils including crops and animals in the area. The study of Erdogrul and Sener (2004) in Turkey also found vegetables, fruits, soil, and water used in the farms to be contaminated with parasites.
Agriculture is one of the major sources of livelihood in the Philippines. However, the findings of the present study revealing the occurrence of STH in soil farms pose threat to public health. In fact, this study reinforced the recent findings of Ordoñez et al. (2018) regarding the parasite contamination of vegetables collected from the same sites. Most of the helminths found in this study were zoonotic parasites that are potentially capable of infecting humans. Some of the current farm practices by both conventional and organic farms, such as use of untreated manure and contaminated wash water, greatly pose threats for further parasite transmission to humans, animals and the environment. Consumers of farm produce may also be at risk of infection.
Agricultural systems, both conventional and organic farming, embrace the idea of sustainable approach for agricultural production. It aims to maintain and increase the soil fertility to ensure a healthy environment. However, based on the findings of the current study, there is a need to review some of the guidelines on farming systems to ensure that not only the integrity of the environment but also the health of the farmers and consumers are being addressed.
For further studies, it is recommended to extend the study using other environmental samples such as water and fertilizers to further identify other sources of contamination. Other water-borne parasites may also be evaluated. In addition, participatory rural appraisals should be encouraged to enable local people to enhance and analyze their knowledge on good farming practices. Finally, the information gathered in this study can be used to create awareness and action plans to improve farming practices to ensure food safety.
Acknowledgements
The authors would like to extend their gratitude to the National Research Council of the Philippines-Department of Science and Technology (NRCP-DOST) for the financial support to complete the study and the Department of Agriculture – Bureau of Agricultural Research (DA-BAR) for the thesis grant awarded to Ms. Shiela Babia-Abion.
References
- Andes A.L., Paller V.G.V. Effect of various composting methods on the concentration and viability of Ascaris suum eggs in organic fertilisers. Pertanika J Trop Agric Sci. 2018;41(2):687–698. [Google Scholar]
- Appleton C.G., Gouws E. The distribution of common intestinal nematodes along an altitudinal transect in KwaZulu-Natal, South Africa. Tropical. Medicine. Parasitology. 1996;90:181–188. doi: 10.1080/00034983.1996.11813042. [DOI] [PubMed] [Google Scholar]
- Beaver P.C. Recent knowledge in control of soil-transmitted helminthes. Scientific Group on Intestinal Protozoan and Helminthic. 1980:1–7. Infections; 4.3. [Google Scholar]
- Belizario V.Y., Jr., De Leon W.U., Lumampao Y.F., Anastacio M.B.M., Tai C.M. Sentinel surveillance of soil- transmitted helminthiasis in selected local government units in the Philippines. Asia Pac. J. Public Health. 2009;21(1):26–42. doi: 10.1177/1010539508327245. [DOI] [PubMed] [Google Scholar]
- Beuchat L. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 2002;4:413–423. doi: 10.1016/s1286-4579(02)01555-1. [DOI] [PubMed] [Google Scholar]
- Erdogrul O., Sener H. Elsevier; 2004. The Contamination of Various Fruit and Vegetable with Enterobius vermicularis, Ascaris Eggs, Entamoeba histolyca Cysts and Giardia Cysts; pp. 559–562. [Google Scholar]
- Food and Agriculture Organization (FAO). 1998. Farm structures in tropical climates Retrieved on July 2016 Retrieved from http://www.fao.org/docrep/s1250e/S1250E00.htm#Contents.
- Horiuchi, S., Uga, S. 2016. Modified flotation method, an effective technique for recovering helminth eggs in soil. Parasitol Int. 2016 Oct;65(5 Pt B):576–579. doi: 10.1016/j.parint.2016.04.010. Epub 2016 May 1. [DOI] [PubMed]
- Horiuchi S., Paller V.G., Uga S. Soil contamination by parasite eggs in rural village in the Philippines. Trop. Biomed. 2013;30:495–503. [PubMed] [Google Scholar]
- Hotez P.J. In: Hookworm and Poverty. Annals of the New York Academy of Sciences. New York Academy of Sciences, editor. vol. 1136. 2008. pp. 38–39. [DOI] [PubMed] [Google Scholar]
- Hotez P., De Silva N., Brooker S., Bethony J. 2003. Soil Transmitted Helminth Infections: The Nature, Causes and Burden of the Condition. (Disease Control Priorities Project working paper no. 3. pp 81) [Google Scholar]
- Klapec, T, Borecka, A., 2012. Contamination of vegetables, fruits and soil with geohelmints eggs on organic farms in Poland. Ann Agric Environ Med. 2012;19(3):421–5. [PubMed]
- Maghirang R.G., De la Cruz R., Villareal R.L. How sustainable is organic agriculture in the Philippines? National. Academic. Science and Technology. Philippines. 2013;33(2):289–321. [Google Scholar]
- Matsuo J, Nakashio S. 2004. Prevalence of fecal contamination is sandpits in public parks in Sapporo City, Japan. Veterinary Parasitology; 2005, (10) p.115. [DOI] [PubMed]
- Miller L.A., Colby K., Manning S.E., Sears S., Montgomery S., Mathison B. Ascariasis in humans and pigs on small- scale farms, Maine, USA, 2010–2013. Maine Center for Disease Control and Prevention, Maine Department of Health and Human Services, Augusta, Maine. USA. 2015;21(2) doi: 10.3201/eid2102.140048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizgajska H. The role of some environmental factorsin the contamination of soil with Toxocara spp. and other geohelminth eggs. Parasitol. Int. 1997;46(1):67–72. [Google Scholar]
- Mustafa U., Adnan S., Gonul A., Hatice O., Suleyman A. Environmental pollution with soil transmitted helminths in Sanliurfa, Turkey. Oswaldo Cruz, Rio de Janeiro. 2001;96(7):903–909. doi: 10.1590/s0074-02762001000700004. [DOI] [PubMed] [Google Scholar]
- Ogbolu D.O., Alli O.A., Amoo A.O., Olaosun I.I. vol. I. High-level parasitic contamination of soil sampled in Ibadan metropolis. Department of Medical Microbiology and Parasitology, University College Hospital; OZAVBIE, G.W., OLUSOGA-OGBOLU, F.F: 2011. I. [PubMed] [Google Scholar]
- Ordoñez K.N., Lim Y.A.L., Goh X.T., Paller V.G.V. 2018. Parasite contamination of vegetables from selected organic and conventional farms in the Philippines. Pertanika J Trop Agric Sc. 2018;41(4):1–16. [Google Scholar]
- Paller V.G.V., de Chavez E.R.C. Toxocara (Nematoda: Ascaridida) and other soil-transmitted helminth eggs contaminating soils in selected urban and rural areas in the Philippines. Sci. World J. 2014;2014:2014. doi: 10.1155/2014/386232. article ID 386232, 6 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S.K., Kubo T., Nakanishi M. Status of soil transmitted helminthic infection in Nepal. Kansenshogaku Zassi. 1994;68:625–630. doi: 10.11150/kansenshogakuzasshi1970.68.625. [DOI] [PubMed] [Google Scholar]
- Rai S.K., Bajracharya K., Budhathoki S. Status of intestinal parasitoses at TU teaching hospital. Journal Institute Medical (Nepal) 1995;17:134–142. [Google Scholar]
- Stojcevic D., Susic V., Lucinger S. Contamination of soil and sand with parasite elements as a risk factor for human health in public parks and playgrounds in Pula. Croatia. Vet. Archive. 2010;80(6):733–742. [Google Scholar]
- Sunil B., Thomas D.R., Latha C., Shameem H. Assessment of parasitic contamination of raw vegetables in Mannuthy, Kerala state, India. VeterinaryWorld. 2014;7(4):253–256. [Google Scholar]
- World Health Organization (WHO). 2012. Soil transmitted helminths. Retrieved July 3, 2012, from http://who.int/intestinal_worms/en/49-52.