Highlights
-
•
Varied disposition imparts on the tolerance and safety of quinine, and thus constitutes a major limiting consideration for its dosing in uncomplicated malaria. Utilizing a population approach, the effect of body weight and infection status on disposition parameters of quinine were evaluated in Nigerian subjects.
-
•
A reversal of infection-induced changes in volume of distribution and clearance after 48 h of chronic quinine administration was noted.
-
•
It is hypothesized that a downward review of quinine regimen post-48 h of chronic administration, in the event of complete parasitaemia clearance, might be a useful approach in enhancing tolerance and safety.
Keywords: malaria, Nigerians, population pharmacokinetics, quinine, tolerance, toxicity
Abstract
Background
The varied disposition of the antimalarial quinine partly explains its poor tolerance and toxicity in humans.
Objective
Using a population approach, the disposition of quinine in healthy subjects and patients with acute uncomplicated symptomatic malaria from Nigeria was re-examined with a view to providing population-specific attributes.
Methods
Concentration versus time profiles of quinine over 48 hours in healthy individuals, and over 7 days in malaria-infected patients, were stratified to reflect: concentration versus time data during the first 48 hours of quinine administration for healthy subjects and infected patients, concentration versus time data after 48 hours in infected patients, and all concentration versus time data available for healthy subjects and infected patients. Pharmacokinetic parameters were then estimated with a stochastic approximation expectation maximization algorithm.
Results
All datasets were fitted by a 1-compartment model with covariate contributions from body weight and infection status. The absorption rate constant, and volume of distribution and clearance were 1.72 h–1, 86.8 to 157.4 L, and 6.6 to 9.6 L/h, respectively. Infected patients experienced a 38% decrease in volume of distribution and a 31% decrease in clearance in the first 48 hours relative to healthy individuals. The contraction in volume of distribution and clearance diminished significantly after 48 hours of chronic quinine dosing in infected patients.
Conclusions
The study findings suggest that clinical interventions aimed at enhancing the safety and tolerance of quinine might be achieved by a rational decrease in dose size and/or dosing interval, post-48 hours of chronic quinine administration, in malaria-infected patients.
Introduction
Quinine is a naturally occurring alkaloid of the aryl amino alcohol group of drugs. It has been used as an antimalarial for more than 400 years, and it was once a prominent first-line drug for the treatment of uncomplicated malaria.1,2 During the past few decades, multitherapies comprising artemisinin derivatives have become strongly favored ahead of quinine due to their effectiveness, safety, and tolerability.2 Quinine has remained a viable alternative in the face of rising drug resistance and limited access to newer drugs in resource-limited countries, due in part to its affordability.1
Quinine is administered in its salt form through the parenteral or oral route. Following oral administration, more than 70% of the drug is absorbed,3,4 maximum systemic concentration is reached between 1 and 3 hours,3,5 and about 80% of the administered dose is eliminated through the hepatic route.1 The use of quinine for the treatment of malaria is not exempt from caution. It has a narrow therapeutic index6; hence, the risk of adverse reactions increases in the event of significant changes to its disposition.
Previous studies have associated quinine with cinchonism at therapeutic concentrations,1 the development of hyperinsulinemic hypoglycaemia in severe malaria,7 and significant changes in QT interval.8 It is also known that malaria, and its degree of severity, alters the disposition of quinine in humans. Decreased volume of distribution (V) and systemic clearance (CL), as well as increased plasma protein binding of quinine, have been observed in malaria-infected patients.9
Quinine-induced adverse reactions are often dependent on its plasma levels, and population-wide indicators that might advise a re-evaluation of current quinine dose regimens for improved tolerance and safety can be derived from a population approach to the study of its disposition. Although pharmacokinetic parameters of quinine in Nigerians have been estimated and reported in several studies,3,10, 11, 12, 13, 14 a population approach to its disposition is unavailable at this time.
The present study re-examined previously reported data on the disposition of quinine in healthy subjects and malaria-infected Nigerian patients. The analysis was carried out with a view to providing insightful information on the population estimates of pharmacokinetic parameters of the drug as well as the effect of available covariates (age and body weight) on such estimates. The analysis also focused on evaluating the influence of time- and malaria-induced physiological changes on the disposition of quinine in malaria-infected patients.
Methods
Study population
The data source for analysis was published concentration versus time values from healthy Nigerian subjects (n = 24)10 and patients with acute symptomatic uncomplicated malaria (n = 6)11 who had been administered quinine. Both studies10,11 utilized validated HPLC-ultraviolet light techniques for quinine quantification with assay limits of quantification of 0.37 µg/mL for the study in healthy subjects10 and 0.03 µg/mL for the study in infected patients.15 The recovery of quinine from plasma matrix was >90%, and assay imprecision was <6% for both studies.
In healthy subjects, 8 mg/kg quinine base was orally administered, and quinine sulphate and blood samples were collected at 0, 1, 2, 4, 6, 8, 12, 24, 36, and 48 hours for quantitation of quinine. The other set of data came from patients with uncomplicated malaria who were also administered 8 mg/kg oral doses of quinine base (as quinine dihydrochloride) every 12 hours on the first day, and every 8 hours for 6 consecutive days. Blood samples were drawn and quantified for quinine in these patients at 0, 0.5, 1, 2, 4, 6, 8, 12, 24, 27, 48, 51, 72, 96, 120, 123, 144, 147, 160, 162, 164, 168, 172, 184, 196, and 208 hours. The demographic characteristics of the study population are presented in Table 1.
Table 1.
Demographic characteristics of the Nigerian study population.*
| Characteristic | Healthy subjects | Malaria-infected patients |
|---|---|---|
| Sample size | 24 | 6 |
| Age, years | 23.95 (2.53) | 13 (4.82) |
| Body weight, kg | 61.63 (7.64) | 34.50 (16.27) |
| Sex | ||
| Male | 18 | 3 |
| Female | 6 | 3 |
Values for sample size and sex are presented as n; whereas values for age and body weight are presented as mean (SD).
Model development and validation
Population pharmacokinetic analysis was performed using Monolix 4.4.0 (http://www.lixoft.com). Pharmacokinetic parameters were estimated by a Bayesian approach that generated maximum a posteriori estimates using the stochastic approximation expectation maximization algorithm. The base population pharmacokinetic parameters model assessed the suitability of different structural models (1 compartment or multicompartment) and error models (constant, additive, or exponential). This model fitted a dataset (dataset 1) containing the concentration-time profiles of quinine for the first 48 hours in healthy subjects who had been administered a single 600-mg oral dose of quinine sulphate (∼8 mg/kg quinine), and the first 48 hours in patients with uncomplicated malaria who had received a total of 5 oral doses of quinine dihydrochloride (∼8 mg/kg quinine) within the said interval. Random effects were initially assumed to be independent with a diagonal variance–covariance matrix, but potential correlations between random effects were also tested.
A log-normal distribution of pharmacokinetic parameters was assumed. The CL and V estimates were standardized using an allometric model: Pi = Ppop x (WTi/WTmedian)β 16 where Ppop, WTi, and WTmedian are the standard value of the parameter estimates, body weight, and population median body weight of the ith individual, respectively. β was then estimated for CL and V. Subsequently, population values of V, absorption rate constant, and CL were estimated with bioavailability fixed at 0.9. A final base model selection was informed by a choice of model with the least values of the Bayesian information criterion and –2 × Log-likelihood, which was estimated by a linearization method. Goodness-of-fit plots and relative standard errors of parameter estimates were also considered.
The contribution of 2 continuous covariates (age and body weight) and 1 categorical covariate (malaria infection status) to variability in population CL and V were tested. The inclusion of these covariates into the model involved an initial forward approach, which only retained covariates that led to a decrease in Bayesian information criterion of the model. Finalization of the covariate model was thereafter carried out by a backward approach. Each covariate was removed 1 at a time using the likelihood ratio test, and only covariates that returned significant likelihood ratio test at P < 0.05 were retained.
The appropriateness of the final model was determined using the goodness-of-fit plots vis-à-vis the observations versus individual or population plots, plots of residuals, and the visual predictive check plots. The population estimates of V and CL were then fixed to further improve the accuracy of the absorption rate constant.
Further, population pharmacokinetic parameters were generated using the developed model with a stratified dataset (dataset 2) that assumed steady-state concentration of quinine after 48 hours and thus computed pharmacokinetic parameters for infected patients from post-48 hour data. In addition, population pharmacokinetic parameter estimates were also derived with a third dataset (dataset 3) that was composed of all CL versus time data from malaria-infected patients (0–208 hours) only. Bootstrapping (2000 nonparametric resampling) of all pharmacokinetic parameter estimates alongside their 95% CIs was determined in all datasets used.
Results
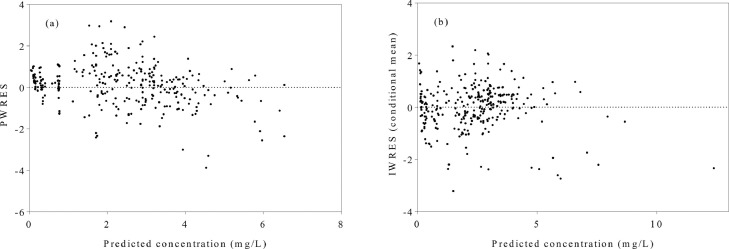
A minimum of 301 quinine CL versus time points from 30 participants, all with corresponding covariate data, was utilized in the final model. A 1-compartment model with first-order absorption and elimination that utilized an exponential error model to describe between-participant variability, adequately described the population. A diagonal variance–covariance matrix explained the random effects, whereas a combined error model was found most suitable. The component covariate model included the effect of body weight and infection status on V as well as the effect of body weight on CL (Table 2). Body weight was observed to reduce the variability of CL from 32.1% to 22.7%, whereas the combined effect of body weight and infection status was found to reduce the variability in V from 48.5% to 32.9%. The appropriateness of the covariance model was validated by the plots of residuals derived for population and individual predictions (Figure 1), whereas individual and population predictions of the final model were also adjudged reliable (Figure 2). Population estimates derived for the assessed parameters are presented in Table 3. CL was estimated by the equation , whereas volume of distribution was derived using the equation [ = 1 for infected individuals and 0 otherwise]. These model estimates were relatively consistent with the 95% CIs of bootstrap means.
Table 2.
Summary of the covariate model construction.
| Model | Covariate Additions | –2LL | BIC | ∆BIC |
|---|---|---|---|---|
| Base model | 0 | 600.28 | 624.08 | – |
| Body weight on V | 1 | 562.41 | 589.62 | –34.46 |
| Body weight on CL | 2 | 545.19 | 575.80 | –48.28 |
| Infection status on V | 3 | 455.13 | 489.14 | –184.94 |
–2LL = –2 × log-likelihood; BIC = Bayesian information criterion, ∆BIC = BIC (model step) – BIC (base model); CL = clearance; V = volume of distribution.
Figure 1.
Plots of residuals against predicted plasma concentrations of quinine in a population of Nigerians comprising 24 healthy subjects and 6 malaria-infected patients. (a) A plot of population-weighted residuals (PWRES) versus predicted plasma concentration of quinine in the final model. (b) A plot of individual-weighted residuals (IWRES) versus predicted plasma concentration of quinine in the final model.
Figure 2.
Plots of observed versus predicted concentration of quinine and a visual predictive plot in Nigerians: 24 healthy subjects and 6 malaria-infected patients. (A) Observed concentration versus individual-predicted concentration for the final model. (B) Observed concentration versus population-predicted concentration of quinine for the final model. (C) A visual predictive check (corrected) for the final model showing observed data points in the first 48 hours of quinine administration after a single oral dose. The continuous green lines represent the 10th, 50th, and the 90th percentiles, whereas the shaded blue and pink regions are the 90% prediction intervals for corresponding percentiles.
Table 3.
Population parameter estimates for quinine in healthy subjects (n = 24) and malaria-infected patients (n = 6) in Nigeria
| Parameter | Quinine administration in the first 48 h for healthy subjects and infected patients (Dataset 1)* |
Quinine administration after 48 h in infected patients (Dataset 2, steady-state assumed)† |
||
|---|---|---|---|---|
| Model estimate Bootstrap mean (95% CI) | RSE (%) | Model estimate Bootstrap mean (95% CI) | RSE (%) | |
| F | 0.9 (fixed) | – | 0.9 (fixed) | – |
| ka (h–1) | 1.716 1.684 (1.371–2.0428) |
20 | 1.716 (fixed) | – |
| V (L) | 117.793 129.624 (112.362–146.534) |
7 | 130.703 157.415 (93.918–241.983) |
22 |
| βV, infection status | 0.993 | 27 | 0.993 | – |
| βV, body weight | 2.198 | 15 | 2.198 | – |
| CL (L/h) | 9.139 9.019 (8.101–9.879) |
5 | 10.485 8.893 (5.878–12.944) |
15 |
| βCL, body weight | 0.722 | 19 | 0.722 | |
| Between-subject variability | ||||
| ωKa | 0.833 | 21 | – | – |
| ωV | 0.329 | 15 | 0.568 | 29 |
| ωCL | 0.227 | 15 | 0.375 | 28 |
βV, body weight = estimated fixed effect of body weight on volume of distribution; βV, infection status = estimated fixed effect of malaria infection on volume of distribution; CL = clearance; F = bioavailability, ka = absorption rate constant; V = volume of distribution.
Dataset 1 captured the concentration versus time following 8 mg/kg doses of quinine in healthy subjects (single oral dose) and malaria-infected (5 oral doses) patients in the first 48 hours.
Dataset 2 captured the concentration versus time data in malaria-infected patients, after 48 hours (48–208 hours), following chronic dosing (15 additional oral doses) of 8 mg/kg quinine at intervals of 8 hours.
The population estimate for V increased from about 87 L to 157 L, and that of CL increased from 6.6 L/h to 8.9 L/h after 48 hours of quinine therapy in malaria-infected patients (Table 4).
Table 4.
A comparison of pharmacokinetic parameters from model estimates in healthy subjects (n = 24) and malaria-infected patients (n = 6) in Nigeria.*
| Model parameter | Quinine administration during the first 48 h |
Quinine administration after 48 h [steady-state assumed] |
Quinine administration for 0 to 208 h† |
|||
|---|---|---|---|---|---|---|
| Mean bootstrap estimates | 95% CI | Mean bootstrap estimates | 95% CI | Mean bootstrap estimates | 95% Confidence interval | |
| V (L) in healthy subjects | 140.435 | 129.921–151.360 | – | – | – | – |
| V (L) in patients with malaria | 86.833 | 34.725–153.373 | 157.415 | 93.918–241.983 | 103.464 | 48.209–169.731 |
| CL (L/h) in healthy subjects | 9.587 | 8.867–10.363 | – | – | – | – |
| CL (L/h) in patients with malaria | 6.640 | 4.496–8.894 | 8.893 | 5.878–12.944 | 8.912 | 5.589–12.271 |
V = volume of distribution; CL = clearance.
Healthy subjects were administered single oral doses of 8 mg/kg quinine, whereas malaria-infected patients were administered 5 oral doses of 8 mg/kg quinine during the first 48 hours, and 15 additional oral doses of quinine after 48 hours.
Plasma levels of quinine were documented between 0 and 48 hours in healthy subjects, and between 0 and 208 hours in malaria-infected patients.
Discussion
This study utilized a population pharmacokinetic parameters approach to investigate the disposition of quinine in Nigerians who were healthy subjects or patients infected with a malaria parasite. An adequate fit of the study data was achieved with a 1-compartment model and a fixed value for the fraction of drug absorbed. Infection-induced changes in the volume of distribution and clearance were noted to be consistent with previous reports in other cohorts.6,17 These disposition parameters in infected patients steadily reversed to levels comparable with those in healthy individuals after 48 hours of chronic quinine administration.
Considering that data from 2 separate studies were merged for analysis, it was necessary to minimize data- or study-related sources of bias in model estimates. To this end, a fixed value for bioavailability was deemed necessary—first, because of the different salt forms of quinine administered (although quite comparable absolute bioavailability has been reported18), and second, because information on feeding habits (which might have some effect19) before and during quinine administration was unreported for both groups in the study. The present study model utilized a 90% value for the fixed value for bioavailability based on a previous report that showed the near complete bioavailability of quinine after administration.5 Reliable population parameter estimates of CL and V, relative to the 95% CI of the bootstrap mean, were subsequently derived.
Bias in parameter estimates arising from a fewer number of patients with malarial infection (n = 6) for which plasma quinine data were available, relative to the number uninfected subjects (n = 24), was assumed minimal because blood sampling in each of these groups was quite extensive. In addition, visual predictive checks (Figures 1 and 2) showed no apparent trend, affirming the reliability of model parameter estimates generated for both healthy subjects and malaria-infected patients.
A fit of the plasma concentration data in the first 48 hours of single oral doses of quinine in healthy subjects and 8-hourly doses in infected patients showed that CL and V decreased by about 31% and 81%, respectively, in infected patients. These changes in pharmacokinetic parameters are known20 and have been explained as resulting from the cumulative effects of the increased partitioning of quinine into erythrocytes relative to parasitaemia,21 and the increased levels of α-1-acid glycoprotein available for binding quinine in infected patients.6,22 Although a contraction of CL and V in infected patients might suggest longer systemic residence of quinine and perhaps greater exposure, the concentration-dependent association of quinine with α-1-acid glycoprotein8 portends a substantial decrease in levels of unbound drug available for therapeutic action following chronic administration.
In malaria, chronic dosing of quinine, a drug with a narrow therapeutic index,6 would appear justifiable at the onset of treatment. This claim takes into account that infection-induced changes in the body, which reverse as parasites are being cleared, might be crucial to the levels of free drug available for therapeutic action. The stratification of data in the present study was, thus conducted to assess the likely effects of changing body physiology on quinine disposition during the period of therapy. To achieve this, stratified plasma data reflecting events after >90% of steady-state level had been reached after 48 hours of chronic quinine administration were studied.
A fit of plasma data after 48 hours in malaria-infected patients showed an expansion in CL and V, suggesting the steady reversal of infection-induced changes in the course of chronic quinine dosing. Clinical data acquired in malaria-infected patients whose data were utilized in the present study11 did show that malaria parasites were indeed completely cleared within 24 hours of quinine therapy. Thus, the expansion of CL and V is believed to be a reflection of rapid changes in the plasma levels of α-1-acid glycoprotein levels, and/or erythrocyte levels/intraerythrocytic concentration of quinine23,24 24 hours after malaria parasites could no longer be detected. Although expanding values of CL and V have previously been reported in convalescing patients after a few weeks,11,21,25, 26, 27 the present study is the first to note these changes during chronic drug administration in malaria-infected patients.
A comparison of quinine disposition between infected patients and healthy subjects was also expected to provide insight into how infection-induced changes in pharmacokinetic parameters in the course of malaria treatment might be leveraged upon to improve tolerance and minimize susceptibility to adverse reactions. Plasma quinine levels above a threshold of 20 µg/mL are generally believed to result in toxicity. Loss of hearing and some other adverse reactions are known to be concentration-dependent and may be seen at lesser plasma concentrations.18,28,29 For example, despite the highest Cmax value of 5.2 µg/mL observed in the healthy Nigerian subjects in this study, cinchonism10 and spontaneous menstruation (unreported) were observed in some subjects. It is thus likely that serious adverse reactions can occur in some individuals in the population during quinine administration either for the treatment of malaria or as a prophylactic.
We hypothesize that a decreased dose regimen of quinine in the event of parasitaemia CL after 48 hours might prove beneficial for chronic dosing in instances of poor tolerance. In other words, such patients may experience fewer adverse effects with reduced dose and/or duration of treatment. It will also be critical that dosage adjustments be combined with the monitoring of the clinical end points that will be needed to validate such regimen reviews. The assertions of the present study are limited to cases of mild severity, which the data reflect because there were no simulations for the effect of disease severity nor prolonged parasitaemia in the course of chronic drug administration.
Conclusions
This study provided and described some population estimates of quinine disposition parameters in healthy and malaria-infected Nigerians. The stratification of data demonstrated that quinine disposition may not be uniform throughout the period of its chronic administration in malaria-infected patients. Newer studies in this population that assess the effectiveness of rational decreases in dose size and frequency of quinine dosing, especially in malaria-infected patients, may provide approaches to optimizing tolerance and efficacy while minimizing adverse reactions.
Declaration of Competing Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
Acknowledgments
All authors contributed to the data analysis and the preparation of the manuscript.
References
- 1.Achan J., Talisuna A.O., Erhart A., Yeka A., Tibenderana J.K., Baliraine F.N., Rosenthal P.J., D'Alessandro U. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:144. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation . Third Edition. 2015. Guidelines for the treatment of malaria. [Google Scholar]
- 3.Salako L.A., Sowunmi A. Disposition of quinine in plasma, red blood cells and saliva after oral and intravenous administration to healthy adult Africans. Eur J Clin Pharmacol. 1992;42(2):171–174. doi: 10.1007/BF00278479. [DOI] [PubMed] [Google Scholar]
- 4.Fuder H., Herzog R., Vaupel W., Wetzelsberger N., Lucker P.W. Study on the absolute bioavailability of quinine and theophylline from tablets after single dose oral administration as compared to intravenous infusion in healthy male non-smoking volunteers. Methods Find Exp Clin Pharmacol. 1994;16(9):651–660. [PubMed] [Google Scholar]
- 5.Paintaud G., Alvan G., Ericsson O. The reproducibility of quinine bioavailability. Br J Clin Pharmacol. 1993;35(3):305–307. doi: 10.1111/j.1365-2125.1993.tb05698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White N.J. The treatment of malaria. N Engl J Med. 1996;335(11):800–806. doi: 10.1056/NEJM199609123351107. [DOI] [PubMed] [Google Scholar]
- 7.Okitolonda W., Delacollette C., Malengreau M., Henquin J.C. High incidence of hypoglycaemia in African patients treated with intravenous quinine for severe malaria. Br Med J (Clin Res Ed) 1987;295(6600):716–718. doi: 10.1136/bmj.295.6600.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihaly G.W., Ching M.S., Klejn M.B., Paull J., Smallwood R.A. Differences in the binding of quinine and quinidine to plasma proteins. Br J Clin Pharmacol. 1987;24(6):769–774. doi: 10.1111/j.1365-2125.1987.tb03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishna S., White N.J. Pharmacokinetics of quinine, chloroquine and amodiaquine. Clinical implications. Clin Pharmacokinet. 1996;30(4):263–299. doi: 10.2165/00003088-199630040-00002. [DOI] [PubMed] [Google Scholar]
- 10.Igbinoba S.I., Onyeji C.O., Akanmu M.A., Soyinka J.O., Pullela S.S., Cook J.M., Nathaniel T.I. Effect of dehusked Garcinia kola seeds on the overall pharmacokinetics of quinine in healthy Nigerian volunteers. J Clin Pharmacol. 2015;55(3):348–354. doi: 10.1002/jcph.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babalola C.P., Bolaji O.O., Ogunbona F.A., Sowunmi A., Walker O. Pharmacokinetics of quinine in African patients with acute falciparum malaria. Pharm World Sci. 1998;20(3):118–122. doi: 10.1023/a:1008699022244. [DOI] [PubMed] [Google Scholar]
- 12.Falade O.B., Falusi A.G., Olaniyi A.A., Ezeasor C., Kwasi D.A., Babalola C.P. Significant Pharmacokinetic Interactions Between Quinine and Ampicillin-Cloxacillin Combination. Drugs R D. 2016;16(2):193–203. doi: 10.1007/s40268-016-0128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babalola C.P., Bolaji O., Ogunbona F.A., Sowunmi A., Walker O. Dose linearity of quinine in healthy human subjects. Eur J Pharm Biopharm. 1997;44(2):143–147. [Google Scholar]
- 14.Soyinka J.O., Onyeji C.O., Omoruyi S.I., Owolabi A.R., Sarma P.V., Cook J.M. Pharmacokinetic interactions between ritonavir and quinine in healthy volunteers following concurrent administration. Br J Clin Pharmacol. 2010;69(3):262–270. doi: 10.1111/j.1365-2125.2009.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babalola C.P., Bolaji O.O., Dixon P.A., Ogunbona F.A. Column liquid chromatographic analysis of quinine in human plasma, saliva and urine. J Chromatogr. 1993;616(1):151–154. doi: 10.1016/0378-4347(93)80482-j. [DOI] [PubMed] [Google Scholar]
- 16.Anderson B.J., Holford N.H. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 17.Supanaranond W., Davis T.M., Pukrittayakamee S., Silamut K., Karbwang J., Molunto P., Chanond L., White N.J. Disposition of oral quinine in acute falciparum malaria. Eur J Clin Pharmacol. 1991;40(1):49–52. doi: 10.1007/BF00315138. [DOI] [PubMed] [Google Scholar]
- 18.Sowunmi A., Salako L.A., Ogunbona F.A. Bioavailability of sulphate and dihydrochloride salts of quinine. Afr J Med Med Sci. 1994;23(3):275–278. [PubMed] [Google Scholar]
- 19.Saggers V.H., Hariratnajothi N., McLean A.E. The effect of diet and phenobarbitone on quinine metabolism in the rat and in man. Biochem Pharmacol. 1970;19(2):499–503. doi: 10.1016/0006-2952(70)90206-6. [DOI] [PubMed] [Google Scholar]
- 20.White N.J., Looareesuwan S., Warrell D.A., Warrell M.J., Bunnag D., Harinasuta T. Quinine pharmacokinetics and toxicity in cerebral and uncomplicated Falciparum malaria. Am J Med. 1982;73(4):564–572. doi: 10.1016/0002-9343(82)90337-0. [DOI] [PubMed] [Google Scholar]
- 21.White N.J., Looareesuwan S., Silamut K. Red cell quinine concentrations in falciparum malaria. Am J Trop Med Hyg. 1983;32(3):456–460. doi: 10.4269/ajtmh.1983.32.456. [DOI] [PubMed] [Google Scholar]
- 22.Mansor S.M., Molyneux M.E., Taylor T.E., Ward S.A., Wirima J.J., Edwards G. Effect of Plasmodium falciparum malaria infection on the plasma concentration of alpha 1-acid glycoprotein and the binding of quinine in Malawian children. Br J Clin Pharmacol. 1991;32(3):317–321. doi: 10.1111/j.1365-2125.1991.tb03905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotepui M., Piwkham D., PhunPhuech B., Phiwklam N., Chupeerach C., Duangmano S. Effects of malaria parasite density on blood cell parameters. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verdier M.C., Bentue-Ferrer D., Tribut O. pour le groupe Suivi Therapeutique Pharmacologique de la Societe Francaise de Pharmacologie et de T: [Therapeutic drug monitoring of quinine] Therapie. 2011;66(6):507–516. doi: 10.2515/therapie/2011071. [DOI] [PubMed] [Google Scholar]
- 25.White N.J. Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokinet. 1985;10(3):187–215. doi: 10.2165/00003088-198510030-00001. [DOI] [PubMed] [Google Scholar]
- 26.Looareesuwan S., Charoenpan P., Ho M., White N.J., Karbwang J., Bunnag D., Harinasuta T. Fatal Plasmodium falciparum malaria after an inadequate response to quinine treatment. J Infect Dis. 1990;161(3):577–580. doi: 10.1093/infdis/161.3.577. [DOI] [PubMed] [Google Scholar]
- 27.Newton P.N., Ward S., Angus B.J., Chierakul W., Dondorp A., Ruangveerayuth R., Silamut K., Teerapong P., Suputtamongkol Y., Looareesuwan S. Early treatment failure in severe malaria resulting from abnormally low plasma quinine concentrations. Trans R Soc Trop Med Hyg. 2006;100(2):184–186. doi: 10.1016/j.trstmh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen-Abbring F.W., Perenboom R.M., van der Hulst R.J. Quinine-induced hearing loss. ORL J Otorhinolaryngol Relat Spec. 1990;52(1):65–68. doi: 10.1159/000276106. [DOI] [PubMed] [Google Scholar]
- 29.Roche R.J., Silamut K., Pukrittayakamee S., Looareesuwan S., Molunto P., Boonamrung S., White N.J. Quinine induces reversible high-tone hearing loss. Br J Clin Pharmacol. 1990;29(6):780–782. doi: 10.1111/j.1365-2125.1990.tb03704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]