SUMMARY
Objective
The endocannabinoid system (ECS) is comprised of cannabinoid receptors 1 and 2 (CB1R and CB2R), endogenous ligands, and regulatory enzymes, and serves to regulate several important physiological functions throughout the brain and body. Recent evidence suggests that the ECS may be a promising target for the treatment of epilepsy, including epilepsy subtypes that arise from mutations in the voltage-gated sodium channel SCN1A. The objective of this study was to explore the effects of modulating CB2R activity on seizure susceptibility.
Methods
We examined susceptibility to induced seizures using a number of paradigms in CB2R knockout mice (Cnr2−/−), and determined the effects of the CB2R agonist, JWH-133, and the CB2R antagonist, SR144528, on seizure susceptibility in wild-type mice. We also examined seizure susceptibility in Cnr2 mutants harboring the human SCN1A R1648H (RH) epilepsy mutation and performed EEG analysis to determine whether the loss of CB2Rs would increase spontaneous seizure frequency in Scn1a R1648H mutant mice.
Results
Both heterozygous (Cnr2+/−) and homozygous (Cnr2−/−) knockout mice exhibited increased susceptibility to PTZ-induced seizures. The CB2R agonist JWH-133 did not significantly alter seizure susceptibility in wild-type mice; however, administration of the CB2R antagonist SR144528 resulted in increased susceptibility to PTZ-induced seizures. In offspring from a cross between the Cnr2 x RH lines, both Cnr2 and RH mutants were susceptible to PTZ-induced seizures; however, seizure susceptibility was not significantly increased in mutants expressing both mutations. No spontaneous seizures were observed in either RH or Cnr2/RH mutants during 336–504 hours of continuous EEG recordings.
Significance
Our results demonstrate that reduced CB2R activity is associated with increased seizure susceptibility. CB2Rs might therefore provide a therapeutic target for the treatment of some forms of epilepsy.
Keywords: Cannabinoid 2 Receptor, SCN1A, seizure susceptibility, CB2R antagonist, CB2R agonist
INTRODUCTION
Over 30% of individuals with epilepsy are refractory to currently available medications, highlighting the need to develop more efficacious treatments.1 The endocannabinoid system (ECS), comprised of cannabinoid receptors, endogenous ligands, and regulatory enzymes, is currently under investigation as a potential therapeutic target for the treatment of several neurological disorders, including epilepsy.2–4
Several studies have demonstrated that drugs targeting the ECS can modulate seizure susceptibility via activity at cannabinoid receptors.5–7 Cannabidiol (CBD), a phytocannabinoid derived from the Cannabis sativa plant, recently received FDA approval for the treatment of Dravet syndrome (DS), a catastrophic, early-onset epilepsy.8; 9 DS is most often caused by mutations in the SCN1A gene, encoding the voltage-gated sodium channel (VGSC) Nav1.1. VGSCs play an important role in regulating neuronal excitability, and SCN1A mutations have been associated with a variety of seizure disorders.10–12 The recent success of the CBD clinical trials in DS suggests a potential role for cannabinoids in other forms of epilepsy.8 However, CBD treatment can be associated with adverse effects, and is not effective in all patients.9; 13 As such, more specific therapies may serve to improve seizure control while reducing off-target side effects.
Although it is well documented that modulation of cannabinoid 1 receptors (CB1Rs) can improve seizure outcomes, activation of CB1Rs can be accompanied by several psychotropic effects, limiting their potential as a therapeutic target.14–18 In contrast, cannabinoid 2 receptors (CB2Rs) have received less attention for their role in the central nervous system (CNS). However, several recent studies have described CB2R expression throughout the brain, including regions associated with seizure generation, such as the hippocampus, cortex, and cerebellum.19–22 Although the cell-specific pattern of CB2R expression in the brain remains controversial, both microglial and neuronal expression have been described.20; 23–25 Furthermore, CB2Rs are highly upregulated in response to brain injury in several mouse models,26; 27 including a rat model of epilepsy in which CB2R expression was upregulated on hippocampal neurons following status epilepticus, suggesting that CB2Rs may play a protective role following injury.28
The few studies that have directly examined the effect of modulating CB2R activity on seizure susceptibility have yielded inconsistent results. Systemic administration of the CB2R agonist, beta caryophyllene, was shown to protect against acutely induced seizures in mice;29,30 however, another study reported that direct brain injection of another CB2R agonist, AM1241, increased seizure severity in rats.31 Additionally, the CB2R agonist HU-308 had no effect on PTZ-induced seizures in young rats.32 Furthermore, the CB2R antagonist, AM630, increased seizure susceptibility in CB1R knockout mice.33 Neither CB1R or CB2R knockout mice exhibit spontaneous seizures, however; CB1R/CB2R double knockout mice exhibit both handling-induced and spontaneous seizures, suggesting that loss of CB2Rs may contribute to increased seizure susceptibility.34 Thus, while there is evidence that modulating CB2R activity can affect seizure susceptibility, this relationship remains unclear, highlighting the impact of differences in study design, including species, seizure model, and dosing.
To elucidate the effect of modulating CB2R activity on seizure susceptibility, we examined susceptibility to induced seizures in a CB2R knockout mouse line (Cnr2−/−) using several seizure induction paradigms. We also administered a CB2R agonist or antagonist to wild-type mice prior to seizure induction in order to determine whether pharmacological manipulation of CB2Rs could similarly alter seizure susceptibility. Furthermore, in order to better understand the potential role of CB2Rs in SCN1A-derived epilepsy, we examined seizure susceptibility in the offspring from a cross between Cnr2 mutant mice and a mouse line expressing the human SCN1A R1648H (RH) mutation. The RH mutation was previously identified in a family with genetic epilepsy with febrile seizures plus (GEFS+).10; 35 Patients with GEFS+ exhibit a wide range of seizure phenotypes, including absence seizures, myoclonic seizures, and both febrile and afebrile seizures.10 Previous work from our laboratory demonstrated that heterozygous Scn1aRH/+ mice are susceptible to induced seizures and exhibit infrequent spontaneous seizures.35
The results of this study demonstrate that reducing CB2R activity increases seizure susceptibility and support a potential role for CB2Rs as a therapeutic target for the development of anti-epileptic drugs.
MATERIALS AND METHODS
Animals
Two CB2R knockout mice (Cnr2−/−) were purchased (Jackson Laboratories, Stock No.005786) and backcrossed to the C57BL/6J background (Jackson Laboratories, Stock No. 000664) for one generation. Resulting heterozygous mice from this cross were then mated to generate the experimental generation.
Heterozygous Scn1aRH/+ mice harboring the human SCN1A R1648H mutation were generated as previously described and maintained on a C57BL/6J background.35 To generate Cnr2−/− and Scn1aRH/+ double-mutants, Scn1aRH/+ mice were first bred to Cnr2−/− mutants. Heterozygous Cnr2+/− offspring that also expressed the RH mutation were then crossed to Cnr2+/− heterozygous mutants to generate six possible genotypes: Cnr2+/+/Scn1a+/+ (WT), Cnr2+/+/ Scn1aRH/+ (RH), Cnr2+/−/Scn1a+/+/ (Cnr2+/−), Cnr2+/−/Scn1aRH/+ (Cnr2+/−/RH), Cnr2−/−/Scn1a+/+ (Cnr2−/−), and Cnr2−/−/Scn1aRH/+ (Cnr2−/−/RH). Mice were housed in groups of 3–5 on a 12-hour light/dark cycle with standard laboratory rodent chow (Lab Diet, #5001) and water available ad libitum. Animal housing facilities were maintained at 69–72 °F and 30–70% humidity. All experiments were performed in accordance with the Emory University Institutional Animal Care and Use Committee guidelines. Furthermore, the principles outlined in the ARRIVE guidelines and the Basel declaration were implemented in the planning of all experiments.36 Unless otherwise stated, adult 8–12 week old mutant animals and wild-type (WT) littermates (20–25 g) were used for all experiments. For all experiments, animals were randomly distributed to groups, and experimenters were blinded to treatment group during data analysis.
Genotyping
Genotyping was performed prior to all experiments. To screen for Cnr2−/− mutants, tail DNA was amplified with the following primers: Mutant Forward- GGGGATCGATCCGTCCTGTAAGTCT, WT Forward- GGAGTTCAACCCCATGAAGGAGTAC, and Reverse- GACTAGAGCTTTGTAGGTAGGCGGG. Amplification was performed for 35 cycles of: 94 °C for 30 s, 60 °C for 60 s, and 72 °C for 30 s. Wild-type animals yielded a 385 bp PCR product, heterozygous animals (Cnr2+/−) yielded 385 bp and 550 bp PCR products, and homozygous animals (Cnr2−/−) generated a single 550 bp PCR band.
To screen for the Scn1aRH/+ mutation, tail DNA was amplified using the following primers: Forward- TTGATGACTTCTTCACTGATTGAT, Reverse- AGAGGCTCTGCACTTTCTTC. Amplification was performed for 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s. The PCR product was digested with EcoRI to distinguish between wild-type (591 bp) and mutant (461 bp).
Pentylenetetrazole (PTZ)-Induced Seizures
Seizures were induced using the chemiconvulsant pentylenetetrazole (PTZ) as previously described.37 Mice were administered PTZ subcutaneously (100 mg/kg, Sigma-Aldrich) and placed in a plexiglass chamber. The latency to the first myoclonic jerk (MJ) and generalized tonic-clonic seizure (GTCS) were recorded over a 30-minute observation period. n= 8–12/group.
6 Hz-Induced Seizures
Seizures induced by the 6 Hz paradigm were conducted as previously described.38; 39 Thirty minutes prior to seizure induction, the topical anesthetic proparacaine hydrochloride ophthalmic solution (Patterson Veterinary) was applied to each eye. Mice were subjected to corneal electrostimulation (6 Hz, 2 ms pulse width, 3 s duration) at 18 mA using an ECT unit (Ugo Basile; Comerio, Italy). Behavioral seizures were scored using a modified Racine Scale: 0- no seizure, 1- staring/immobile > 3 s, 2- forelimb clonus, and 3- rearing and falling. n= 16–30/group.
Flurothyl-Induced Seizures
Thresholds to flurothyl induced seizures were determined as previously described.40 Briefly, mice were placed in a plexiglass chamber (13.5 × 8 × 6 inches), and exposed to flurothyl (Bis(2,2,2-trifluoroethyl) ether, Sigma Aldrich) at a rate of 20 μL/minute. Latencies to the first MJ and GTCS were recorded. n= 8–12/group.
Kainic Acid (KA)-Induced Seizures
Mice were administered kainic acid (intraperitoneal, i.p. 25 mg/kg, Sigma-Aldrich) and behavioral seizures were recorded over a 2-hour period. Seizures were scored on a modified Racine Scale: 0 – no behavior, 1 – freezing/staring, 2- head nodding, 3 – tail clonus, 4 – forelimb clonus, 5 – rearing and falling, and 6 – death. n= 8/group.
Hyperthermia-Induced Seizures (a model of febrile seizure susceptibility)
Susceptibility to hyperthermia-induced seizures was determined as previously described.38; 41 Temperature was monitored using a rectal temperature probe connected to a heating lamp and temperature controller (TCAT 2DF, Physitemp, Clifton, NJ, USA). Mice aged P14 or P21 were placed in a clear cylinder, and the core body temperature of each mouse was increased by 0.5 oC every two minutes until either a GTCS occurred or 42.5 oC was reached. The temperature at which each mouse exhibited a GTCS was recorded. Behavioral seizures were scored on a modified Racine Scale: 1 – staring, 2 – head nodding, 3 – unilateral forelimb clonus, 4 – bilateral forelimb clonus, 5 – GTCS. n= 8/group.
Drug Treatment
For pharmacological studies, the CB2R antagonist, SR144528 (3 mg/kg, Tocris), or vehicle (0.9% saline with 5% tween 80) was administered by i.p. injection 30 minutes prior to seizure induction. The CB2R agonist, JWH-133 (3 mg/kg, Tocris), or vehicle (0.9% saline with 5% tween 80) was administered by i.p. injection 30 minutes prior to seizure induction.
EEG Surgery and Analysis
Electroencephalogram (EEG) electrodes were surgically implanted in adult RH and Cnr2−/−/RH mice as previously described.42; 43 All mice were administered meloxicam immediately prior to the surgical procedure (5 mg/kg, Patterson Veterinary). Anesthesia was maintained with approximately 1.5% isoflurane (Patterson Veterinary) throughout the surgical procedure. Four bipolar stainless-steel screws (Vintage Machine Supplies) were implanted at the following coordinates relative to Bregma: anterior-poster (AP) + 0.5 mm and medial-lateral (ML) −2.2 mm; AP + 2.0 mm and ML + 1.2 mm; AP − 3.5 mm and ML − 2.2 mm; AP − 1.5 mm and ML + 1.2 mm. Mice were allowed to recover from surgery for one week prior to beginning EEG recordings. Two to three continuous weeks of electrographic recordings were obtained for each mouse. EEG signals were analyzed with Stellate Harmonie EEG software using a high pass filter of 5 Hz and a low pass filter of 35 Hz. Seizure activity was defined as synchronous discharges of increased amplitude at least twice the background and ≥ 3 seconds in duration. n= 4/group.
Data Analysis
All data was analyzed using GraphPad Prism version 6 or 7 (GraphPad Software). Latencies to PTZ and flurothyl-induced seizures in Cnr2 mutant mice were analyzed using 1-way ANOVA followed by Tukey post-hoc comparison. Analysis of the percent of mice reaching a GTCS following PTZ administration was performed using the Mantel-Cox log-rank test. A 2-way rANOVA followed by a Bonferroni post-hoc comparison was used to analyze the time course of KA-induced seizure severity in Cnr2 mutant mice. Behavioral seizures in the 6 Hz paradigm were analyzed with a Kruskall-Wallis test followed by Dunn’s multiple comparisons. A 2-way ANOVA with Tukey’s post-hoc comparison was used to compare the effect of agonist or antagonist treatment and genotype on seizure susceptibility, as well as differences between seizure susceptibility in Cnr2/RH double mutants. Differences between groups were considered to be significant if P < 0.05.
RESULTS
Cnr2 mutant mice exhibit increased susceptibility to induced seizures
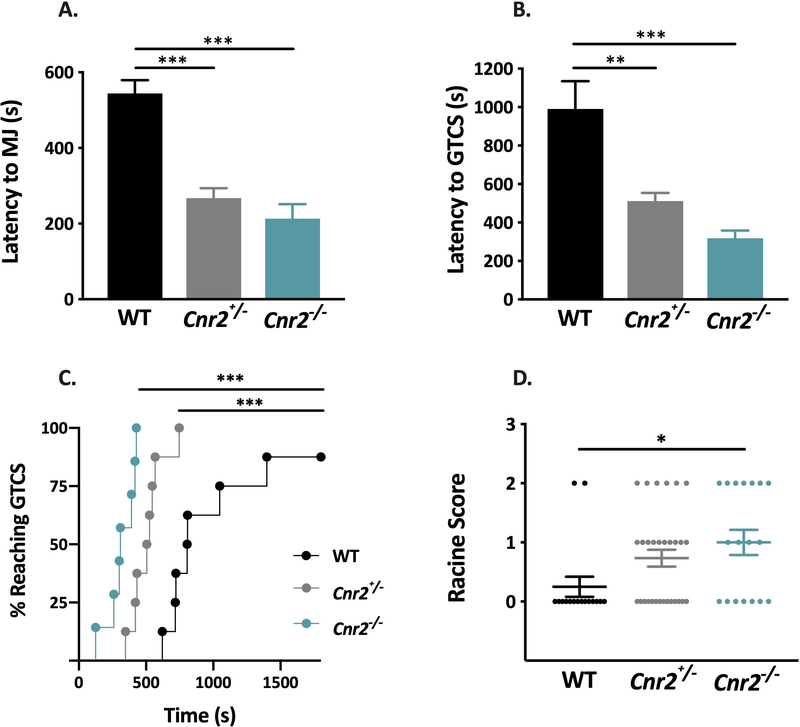
We first compared seizure susceptibility between male Cnr2 mutants and WT littermates using the PTZ seizure induction paradigm. Cnr2+/− and Cnr2−/− mutants exhibited significantly lower latencies to the first MJ (P < 0.001, Fig 1A) and GTCS (Cnr2+/−, P < 0.01; Cnr2−/−, P < 0.001, Fig 1B) compared to WT littermates. The percent of mice reaching GTCS over time in Cnr2+/− and Cnr2−/− mutants was also significantly different from WT littermates (P < 0.001, Fig 1C). Seizure latencies did not significantly differ between Cnr2+/− and Cnr2−/− mutants (Fig 1A–C).
Figure 1. Cnr2 mutant mice exhibit increased susceptibility to induced seizures.
A. Cnr2+/− and Cnr2−/− mice exhibit significantly reduced latencies to the first MJ (A) and GTCS (B) following PTZ administration compared to WT littermates. 1-way ANOVA followed by Tukey’s post-hoc comparison, n= 8/group. C. Percent of Cnr2+/− and Cnr2−/− mutants reaching GTCS, and latency to GTCS, are significantly different from WT littermates. Mantel-Cox log-rank test. D. Cnr2−/− mice exhibit higher average Racine scores when compared to WT littermates following an 18 mA electrical stimulus. Each symbol represents one mouse. Kruskall-Wallis Test with Dunn’s multiple comparisons. WT, n=16; Cnr2+/−, n=30; Cnr2−/−, n=18. For A-D, *P < 0.05, **P < 0.01, ***P < 0.001. All error bars represent mean +/− SEM.
We then examined seizure susceptibility using the 6 Hz seizure induction paradigm. Male Cnr2−/− mice exhibited a higher average Racine score compared to WT littermates following an 18 mA stimulus, indicating a more severe seizure response (P < 0.05, Fig 1D). Heterozygous mutants did not differ significantly from WT littermates or homozygous mutants.
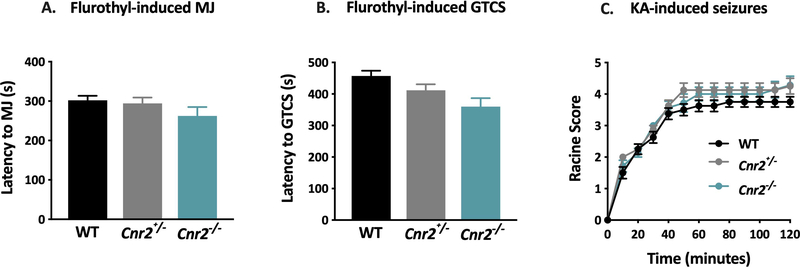
Susceptibility to seizures induced by flurothyl (Fig 2A–B) and KA (Fig 2C) were comparable between Cnr2 mutant mice and WT littermates. To determine whether Cnr2 mutant mice were susceptible to febrile seizures, we investigated the effect of hyperthermia on seizure generation in mutants and WT littermates at P14 and P21. Neither WT nor mutant mice exhibited hyperthermia-induced seizures at either age.
Figure 2. Cnr2 mutant mice are not susceptible to flurothyl- or kainic acid-induced seizures.
Latency to the first MJ (A) and GTCS (B) was comparable between genotypes following flurothyl exposure. 1- way ANOVA with Tukey’s post-hoc comparison, n=8/group. C. The response of Cnr2 mutant mice to KA was similar to WT littermates. 2-way rANOVA with Bonferroni post-hoc comparison, n= 8/group. All error bars represent mean +/− SEM.
The CB2R specific antagonist, SR144528, increases seizure susceptibility in WT mice
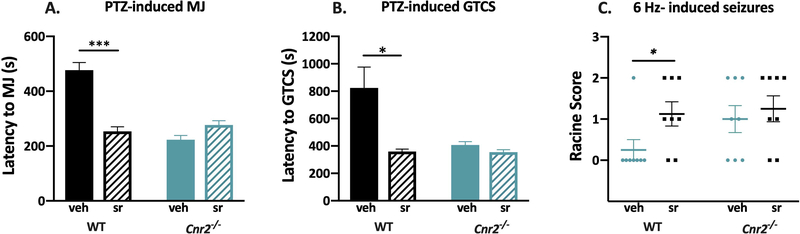
To determine whether the results observed with the Cnr2 mutants could be recapitulated with pharmacological methods, we administered the CB2R antagonist, SR144528, to male Cnr2−/− and WT littermates 30 minutes prior to PTZ administration. Latencies to the first MJ and GTCS were significantly lower in WT mice treated with SR144528 compared to vehicle-treated mice (MJ, P < 0.001; GTCS, P < 0.05, Fig 3A–B). SR144528 treatment had no effect on latency to the MJ or GTCS in Cnr2−/− mice, indicating that the effects of SR144528 are CB2R-specific (Fig 3A–B). Vehicle-treated Cnr2−/− mutants exhibited decreased latencies to both the MJ and GTCS when compared to vehicle-treated WT littermates (P < 0.001, Fig 3A–B), consistent with the data shown in Fig 1A–B.
Figure 3. The CB2R specific antagonist, SR144528, increases seizure susceptibility.
A. SR144528 significantly reduced latencies to the first MJ following PTZ administration in WT mice compared to vehicle-treated controls. B. WT mice treated with SR144528 exhibit significantly reduced latencies to the first GTCS following PTZ administration compared to vehicle-treated WT mice. SR144528 treatment did not affect latencies to the first MJ or GTCS in Cnr2−/− mutants. 2-way ANOVA with Tukey’s post-hoc comparison, n=8/group. C. WT mice treated with SR144528 exhibit significantly higher average Racine scores when compared to vehicle-treated WT mice. SR144528 treatment did not affect susceptibility to 6 Hz-induced seizures in Cnr2−/− mutants. Each symbol represents one mouse. 2-way ANOVA with Tukey’s post hoc comparison, n= 8/group. *P < 0.05, ***P < 0.001. Veh, vehicle; sr, SR144528. All error bars represent mean +/− SEM.
We also tested whether SR144528 could impact seizure severity using the 6 Hz seizure induction paradigm. Consistent with the results observed following PTZ administration, WT mice administered SR144528 exhibited significantly higher Racine scores compared to vehicle-treated WT mice (P < 0.05, Fig 3C). SR144528 had no effect on susceptibility to 6 Hz-induced seizures in Cnr2−/− mice (Fig 3C).
The CB2R specific agonist, JWH-133, does not increase resistance to PTZ-induced seizures
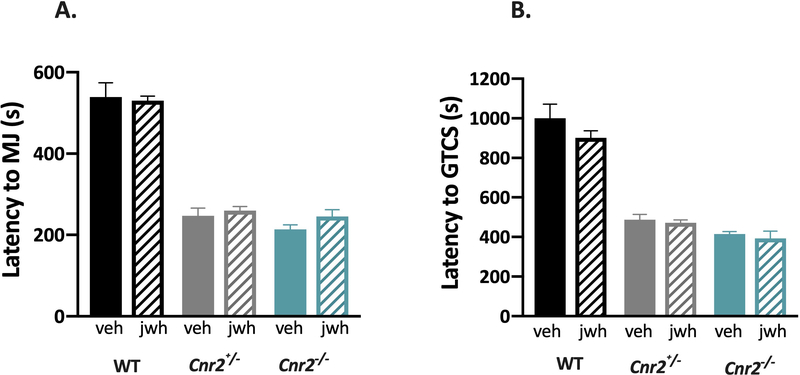
To determine whether increasing CB2R activity could be seizure protective, we administered the CB2R selective agonist, JWH-133, to WT, Cnr2+/−, and Cnr2−/− mice 30 minutes prior to inducing seizures with PTZ. As expected, vehicle-treated Cnr2−/− and Cnr2+/− mutants show reduced latencies to both the MJ and GTCS when compared to vehicle-treated WT littermates; however, JWH-133 did not have any effect on seizure susceptibility regardless of genotype (Fig 4).
Figure 4. The CB2R specific agonist, JWH-133, does not alter susceptibility to PTZ-induced seizures.
The CB2R agonist JWH-133 did not significantly alter latencies to the first MJ (A) or GTCS (B) in WT, Cnr2+/− or Cnr2−/− mutant mice. 2-way ANOVA with Tukey’s post-hoc comparison, n=8/group. Veh, vehicle; jwh, JWH-133. All error bars represent mean +/− SEM.
Deletion of CB2Rs does not worsen seizure phenotypes in mice expressing the SCN1A R1648H mutation
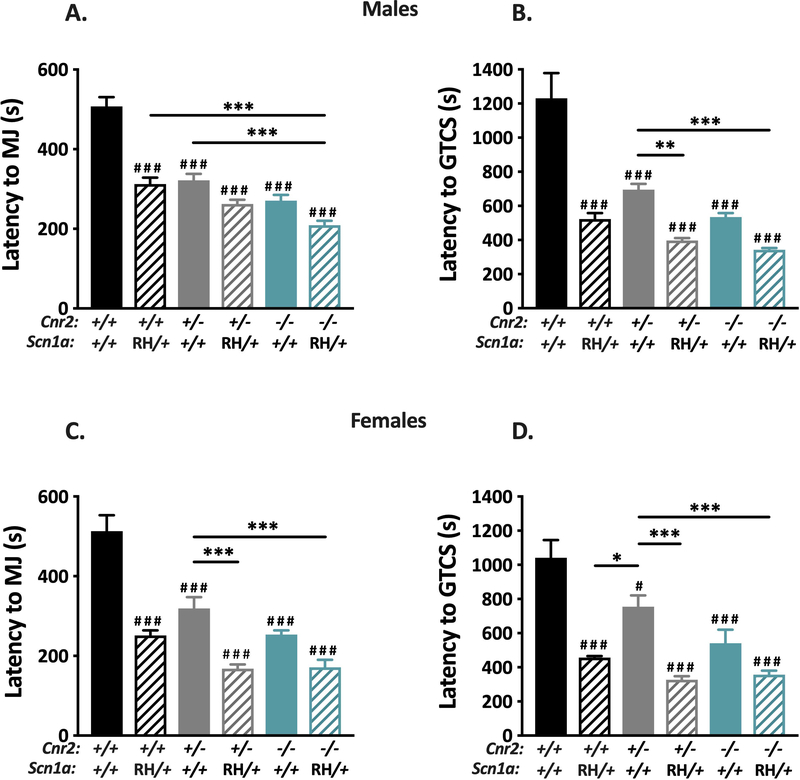
We next examined whether decreasing CB2R expression would influence seizure susceptibility in a mouse model of SCN1A derived epilepsy. To do so, we crossed Cnr2 mutant mice to a mouse line harboring the human R1648H SCN1A mutation, and subsequently evaluated susceptibility to PTZ-induced seizures in male and female offspring of each genotype. Male and female mutants of all genotypes exhibited significantly lower average latencies to the first MJ and GTCS when compared to WT littermates (Fig 5). Further, male Cnr2−/−/RH mutant mice exhibited significantly reduced latencies to the MJ compared to RH mutant mice (P < 0.001, Fig 5A), although average latency to the first GTCS was not significantly different (Fig 5B). Female Cnr2−/−/RH mice did not show significantly altered latencies to either the MJ or GTCS when compared to female RH mutants (Fig 5C–D). These results indicate that although reduced Cnr2 expression and the SCN1A R1648H mutation both result in increased seizure susceptibility, the absence of Cnr2 does not exacerbate seizure susceptibility in Scn1a mutant mice.
Figure 5. Deletion of CB2Rs does not worsen PTZ-induced seizures in Scn1aRH/+ mutant mice.
All groups of mutant male (A-B) and female (C-D) mice exhibited reduced average latencies to both the MJ and GTCS compared to WT (###P < 0.001, #P < 0.05). A. Male Cnr2−/−/RH mutants (teal stripe) show decreased latency to the MJ compared to both Cnr2+/− (gray) and RH (black stripe) (***P < 0.001). B. Both Cnr2+/−/RH (gray stripe) and Cnr2−/−/RH male mice (teal stripe) exhibit decreased latency to GTCS when compared to Cnr2+/− mice (gray) (**P < 0.01, ***P < 0.001). C. Female Cnr2+/−/RH (gray stripe) and Cnr2−/−/RH (teal stripe) mice exhibit decreased latencies to the MJ when compared to Cnr2+/− (gray) mutant mice (***P < 0.001). D. Female Cnr2+/−/RH (gray stripe) and Cnr2−/−/RH (teal stripe) mice exhibit decreased latencies to the GTCS when compared to Cnr2+/− (gray) mutant mice (***P < 0.001). RH female mice (black stripe) exhibit decreased latency GTCS when compared to Cnr2+/− (gray) mutant mice (*P < 0.05). 2-way ANOVA with Tukey’s post-hoc test, n=8–12/group. Symbols above individual bars denote statistical significance from WT. All error bars represent mean +/− SEM.
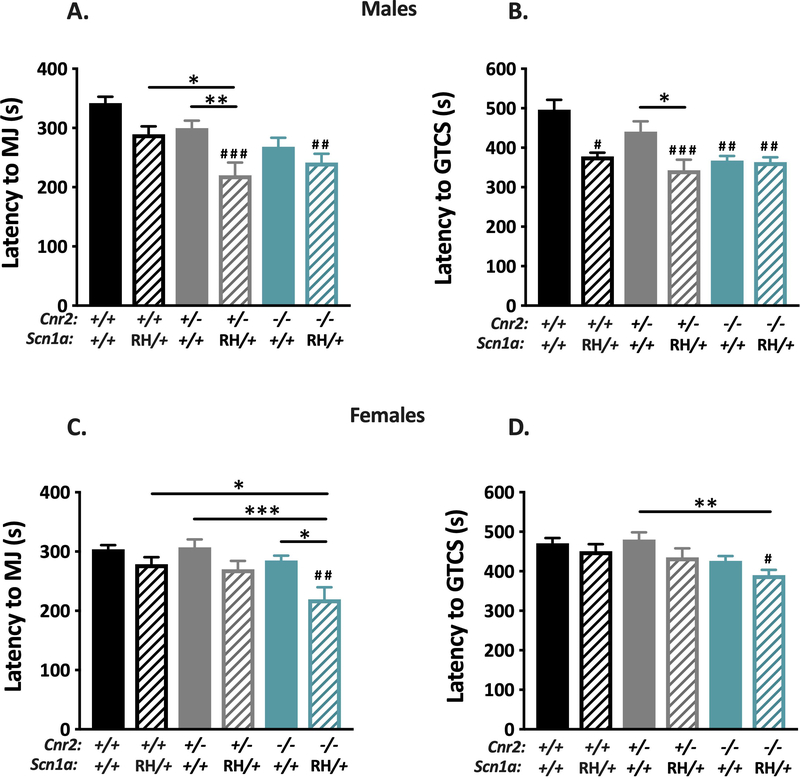
To determine whether the results observed with PTZ were paradigm specific, we also examined the effect of co-expression of both mutations on susceptibility to flurothyl-induced seizures. Male Cnr2+/−/RH mutants showed significantly reduced latencies to the MJ when compared to RH mutants (P < 0.05, Fig 6A), but did not show significantly different latencies to the GTCS (Fig 6B). Cnr2−/−/RH male mice did not show any significant differences in seizure latencies when compared to RH-only mutants (Fig 6A–B). Female Cnr2−/−/RH mutants showed significantly reduced latencies to the MJ when compared to RH mutants (P < 0.05, Fig 6C), but did not show significant differences in latency to the GTCS (Fig 6D). Similar to the observations with PTZ, these results indicate that the absence of Cnr2 does not consistently alter susceptibility to flurothyl-induced seizures in SCN1A mutant mice.
Figure 6. Deletion of CB2Rs does not worsen flurothyl-induced seizures in Scn1aRH/+ mutant mice.
A. Male Cnr2−/−/RH (teal stripe) and Cnr2+/−/RH (gray stripe) mutants exhibit decreased latency to the MJ when compared to WT (black) (##P < 0.01, ###P < 0.001). Cnr2+/−/RH mutants (gray stripe) also show decreased latency to the MJ when compared to RH (black stripe) (*P < 0.05) and Cnr2+/− (gray) (**P < 0.001). B. When compared to WT, RH (black stripe) (#P < 0.05), Cnr2+/−/RH (gray stripe) (###P < 0.001), Cnr2−/− (teal) (##P < 0.01 ), and Cnr2−/−/RH (teal stripe) male mutants (##P <0.01) exhibit significantly decreased latencies to GTCS. Cnr2+/−/RH mutants (gray stripe) exhibit significantly decreased latency to the GTCS compared to Cnr2+/− mice (gray) (*P < 0.05). C. Cnr2−/− /RH female mice (teal stripe) show significantly decreased latency to the MJ when compared to WT (black) (##P < 0.01), RH (black stripe) (*P < 0.05), Cnr2+/− (gray) (***P < 0.001), and Cnr2−/−(teal) (*P < 0.05) mice. D. Cnr2−/− /RH female mice (teal stripe) exhibit decreased latency to the GTCS when compared to WT (#P < 0.05) and Cnr2+/− mutant mice (gray) (**P < 0.01). 2-way ANOVA with Tukey’s post hoc comparison, n=8–12/group. Symbols above individual bars indicate significance from WT. Error bars represent mean +/− SEM
Deletion of CB2Rs does not increase spontaneous seizure frequency in mice expressing the SCN1A R1648H mutation
We previously demonstrated that heterozygous RH mutant mice exhibit infrequent spontaneous seizures.35 In order to test whether the lack of CB2Rs could increase spontaneous seizure frequency, length, or severity in RH mice, we performed continuous EEG recordings for 336–504 hours in RH and Cnr2−/−/RH mutant mice. Spontaneous seizures were not detected in any mouse during the recording period (n= 4/genotype).
DISCUSSION
We show that the loss of CB2Rs (Cnr2−/− mutants) results in increased susceptibility to PTZ- and 6 Hz-induced seizures, and administration of the CB2R antagonist, SR144528, in WT mice increases seizure susceptibility, suggesting that increasing CB2R expression or activity may confer seizure protection. Interestingly, while both heterozygous (Cnr2+/−) and homozygous (Cnr2−/−) mutants exhibited increased susceptibility to PTZ-induced seizures, there was no significant difference between Cnr2+/− and Cnr2−/− mutants, indicating that partial reduction of CB2R activity is sufficient to increase seizure susceptibility. While Cnr2+/− mutants presumably have 50% loss of CB2R protein compared to WT littermates, the lack of reliable CB2R antibodies44; 45 makes it difficult to quantify differences in protein levels between genotypes.
Studies which have previously examined the effects of CB2R compounds on seizure phenotypes have yielded inconsistent results.29–33 Although some studies reported that the CB2R agonist beta caryophyllene can be seizure protective,29; 30 other studies observed either no effect of a CB2R agonist (HU308),32 or greater seizure severity following administration of the CB2R agonist AM1241.31 We found no effect of the CB2R agonist, JWH-133, on PTZ-induced seizures (Fig 4). These inconsistencies may be due to differences in study design, including seizure induction paradigm, species, and the specific agonist or antagonist tested.29–33 We selected JWH-133 and SR144528 for our studies because they are highly potent and CB2R-specific, exhibiting few off-target effects in vitro.46–48 Furthermore, at doses similar to those used in our study, JWH-133 has shown therapeutic effects in the brain including attenuation of stress-induced neuroinflammation49 and reduction of post-surgical cognitive impairment and associated neuroinflammation.50In addition, administration of SR144528 at doses comparable to that in the current study was associated with detrimental effects in the brain, including increased infarct size and delayed motor recovery following stroke.51; 52 Thus, both drugs have been shown to be effective at doses similar to those used in our study. The inclusion of Cnr2−/− mice in our study also served as a control for drug specificity, as drugs acting at CB2Rs would not be expected to have any effect on mice lacking functional receptors.
It is possible that we did not observe any effects of JWH-133 on seizure susceptibility because endogenous endocannabinoids were already eliciting maximal receptor occupancy. It is well-accepted that elevated neuronal activity, including seizures, can cause transient release of endocannabinoids.23; 53; 54 This increase in endocannabinoids could potentially act on and occupy CB2Rs yielding a “ceiling effect,” thereby limiting the potential for JWH-133 to elicit an additional response at CB2Rs. A similar effect has been described for CB1Rs, in which treatment with a CB1R agonist prior to pilocarpine-induced seizures had no effect on seizure severity, yet CB1R knockout mice and CB1R-specific antagonists exacerbated the effects of pilocarpine.14 This raises the possibility that antagonism of CB2Rs may have a greater effect on seizure susceptibility than agonism of CB2Rs.
While the results of this study indicate a role for CB2Rs in modulating seizure susceptibility, it is unclear whether these effects might be mediated via neuronal or glial mechanisms. CB2R expression is observed on both neurons and glial cells,20; 23–25 and recent studies have shown that CB2Rs play a role in regulating neuronal excitability.20; 23; 55 For example, application of JWH-133 reduces the excitability of cortical pyramidal neurons in brain slices from WT mice.55 More recently, Stempel et. al observed that pyramidal neurons in the CA2 and CA3 regions of the hippocampus become hyperpolarized in response to a train of action potentials, and that this hyperpolarization is dependent on CB2Rs, but not CB1Rs.23 Thus, it appears that CB2Rs can facilitate inhibition of excitatory cells, thereby reducing excitability of the hippocampus in response to excessive neuronal activity. An increase in CB2R expression on hippocampal neurons has been observed following status epilepticus in a rat model of epilepsy.28 This upregulation could be involved in a neuroprotective mechanism which reduces excitability following excessive neuronal firing, raising the possibility that targeting CB2Rs may be therapeutic in epilepsy.
Several studies indicate that CB2R-mediated effects in the brain might also be due to their effects on microglial function. A significant increase in expression of CB2Rs have been observed on activated microglia compared to microglia in the resting state,56 and stimulation of CB2Rs has been shown to suppress microglia activation and subsequently reduce microglia-mediated release of pro-inflammatory cytokines and phagocytosis of cells.57 Given the potential roles for CB2R on neurons and microglia, future experiments should be designed to evaluate the distinct roles of neuronal and non-neuronal CB2Rs on modulating seizure susceptibility.
Although both Cnr2−/− and RH mutant mice were susceptible to PTZ-induced seizures, there was no additive effect of the mutations on seizure susceptibility. We previously reported a low frequency of spontaneous seizures in RH mice35; however, we did not observe spontaneous seizures in either RH or Cnr2−/−/RH mice in the current study. While preclinical studies examining the role of the ECS in SCN1A derived epilepsy are limited, a previous study showed that cannabidiol (CBD) is protective against hyperthermia-induced seizures as well as spontaneous seizures and sociability deficits in heterozygous Scn1a knockout mice, which serve as a model of Dravet syndrome.58 Despite the fact that CBD has shown success in a mouse model of DS and patients with SCN1A-derived epilepsy, accumulating evidence suggests that its anti-epileptic effects are not likely through CB1R- or CB2R- mediated mechanisms.58; 59 This is consistent with our observation that the loss of CB2R did not worsen seizure phenotypes in RH mutant mice.
CB2R-targeted treatment may also be indicated for other forms of epilepsy which are not SCN1A-derived. CB2Rs play a well-documented role in reducing neuroinflammation in mouse models of neurological disease. Specifically, treatment with CB2R agonists have been shown to reduce levels of reactive gliosis and pro-inflammatory cytokines following neuronal injury in models of Parkinson’s disease, Alzheimer’s disease, and traumatic brain injury.60–62 This reduction in neuroinflammation has been associated with improved outcomes in these models.62–64 Several forms of drug-resistant epilepsy, such as mesial temporal lobe epilepsy (MTLE), are characterized by high levels of neuroinflammation.65; 66 Given the reduction in inflammation that is observed following CB2R activation, treatments targeting CB2R might prove to be particularly efficacious in epilepsy disorders like MTLE.
The results of this study indicate that the loss of CB2R activity increases seizure susceptibility in mice. Although modulation of CB2R activity did not alter seizure phenotypes in a mouse model of SCN1A-derived epilepsy, treatments that target CB2Rs may still be indicated for other forms of epilepsy, particularly those which are characterized by extensive neuroinflammation.
KEY POINTS.
Genetic deletion of the cannabinoid 2 receptor (CB2R) increases seizure susceptibility in mice.
The CB2R antagonist, SR144528, increases seizure susceptibility in wild-type mice.
Deletion of CB2Rs in a mouse model of human SCN1A-derived epilepsy does not further increase seizure susceptibility.
Spontaneous seizure frequency was not increased in mice expressing both Cnr2 and Scn1a mutations.
CB2Rs may provide a therapeutic target for the treatment of some forms of epilepsy.
ACKNOWLEDGMENTS
The research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R21NS098776 (AE). The content of this publication is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.French JA. Refractory epilepsy: clinical overview. Epilepsia 2007;48 Suppl 1:3–7. [DOI] [PubMed] [Google Scholar]
- 2.Kaur R, Ambwani SR, Singh S. Endocannabinoid System: A Multi-Facet Therapeutic Target. Curr Clin Pharmacol 2016;11:110–117. [DOI] [PubMed] [Google Scholar]
- 3.Brodie MJ, Ben-Menachem E. Cannabinoids for epilepsy: What do we know and where do we go? Epilepsia 2018;59:291–296. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell BK, Gloss D, Devinsky O. Cannabinoids in treatment-resistant epilepsy: A review. Epilepsy Behav 2017;70:341–348. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg EC, Patra PH, Whalley BJ. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav 2017;70:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg EC, Tsien RW, Whalley BJ, et al. Cannabinoids and Epilepsy. Neurotherapeutics 2015;12:747–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaston TE, Friedman D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav 2017;70:313–318. [DOI] [PubMed] [Google Scholar]
- 8.Devinsky O, Cross JH, Laux L, et al. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N Engl J Med 2017;376:2011–2020. [DOI] [PubMed] [Google Scholar]
- 9.Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol 2016;15:270–278. [DOI] [PubMed] [Google Scholar]
- 10.Escayg A, MacDonald BT, Meisler MH, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet 2000;24:343–345. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara T Clinical spectrum of mutations in SCN1A gene: severe myoclonic epilepsy in infancy and related epilepsies. Epilepsy Res 2006;70 Suppl 1:S223–230. [DOI] [PubMed] [Google Scholar]
- 12.Mulley JC, Scheffer IE, Petrou S, et al. SCN1A mutations and epilepsy. Hum Mutat 2005;25:535–542. [DOI] [PubMed] [Google Scholar]
- 13.Iffland K, Grotenhermen F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res 2017;2:139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kow RL, Jiang K, Naydenov AV, et al. Modulation of pilocarpine-induced seizures by cannabinoid receptor 1. PLoS One 2014;9:e95922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair RE, Deshpande LS, Sombati S, et al. Activation of the cannabinoid type-1 receptor mediates the anticonvulsant properties of cannabinoids in the hippocampal neuronal culture models of acquired epilepsy and status epilepticus. J Pharmacol Exp Ther 2006;317:1072–1078. [DOI] [PubMed] [Google Scholar]
- 16.Luszczki JJ, Czuczwar P, Cioczek-Czuczwar A, et al. Arachidonyl-2’-chloroethylamide, a highly selective cannabinoid CB1 receptor agonist, enhances the anticonvulsant action of valproate in the mouse maximal electroshock-induced seizure model. Eur J Pharmacol 2006;547:65–74. [DOI] [PubMed] [Google Scholar]
- 17.Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc Lond B Biol Sci 2012;367:3353–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Amsterdam J, Brunt T, van den Brink W. The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J Psychopharmacol 2015;29:254–263. [DOI] [PubMed] [Google Scholar]
- 19.Chen DJ, Gao M, Gao FF, et al. Brain cannabinoid receptor 2: expression, function and modulation. Acta Pharmacol Sin 2017;38:312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Kim J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience 2015;311:253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onaivi ES, Ishiguro H, Gong JP, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci 2006;1074:514–536. [DOI] [PubMed] [Google Scholar]
- 22.Liu QR, Pan CH, Hishimoto A, et al. Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav 2009;8:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stempel AV, Stumpf A, Zhang HY, et al. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 2016;90:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmole AC, Lundt R, Gennequin B, et al. Expression Analysis of CB2-GFP BAC Transgenic Mice. PLoS One 2015;10:e0138986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez A, Aparicio N, Pazos MR, et al. Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. J Neuroinflammation 2018;15:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Concannon RM, Okine BN, Finn DP, et al. Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson’s disease. Exp Neurol 2016;283:204–212. [DOI] [PubMed] [Google Scholar]
- 27.Yu SJ, Reiner D, Shen H, et al. Time-Dependent Protection of CB2 Receptor Agonist in Stroke. PLoS One 2015;10:e0132487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q, Wang H. The spatiotemporal expression changes of CB2R in the hippocampus of rats following pilocarpine-induced status epilepticus. Epilepsy Res 2018;148:8–16. [DOI] [PubMed] [Google Scholar]
- 29.de Oliveira CC, de Oliveira CV, Grigoletto J, et al. Anticonvulsant activity of beta-caryophyllene against pentylenetetrazol-induced seizures. Epilepsy Behav 2016;56:26–31. [DOI] [PubMed] [Google Scholar]
- 30.Tchekalarova J, da Conceicao Machado K, Gomes AL Junior, et al. Pharmacological characterization of the cannabinoid receptor 2 agonist, beta-caryophyllene on seizure models in mice. Seizure 2018;57:22–26. [DOI] [PubMed] [Google Scholar]
- 31.de Carvalho CR, Hoeller AA, Franco PL, et al. The cannabinoid CB2 receptor-specific agonist AM1241 increases pentylenetetrazole-induced seizure severity in Wistar rats. Epilepsy Res 2016;127:160–167. [DOI] [PubMed] [Google Scholar]
- 32.Huizenga MN, Wicker E, Beck VC, et al. Anticonvulsant effect of cannabinoid receptor agonists in models of seizures in developing rats. Epilepsia 2017;58:1593–1602. [DOI] [PubMed] [Google Scholar]
- 33.Sugaya Y, Yamazaki M, Uchigashima M, et al. Crucial Roles of the Endocannabinoid 2-Arachidonoylglycerol in the Suppression of Epileptic Seizures. Cell Rep 2016;16:1405–1415. [DOI] [PubMed] [Google Scholar]
- 34.Rowley S, Sun X, Lima IV, et al. Cannabinoid receptor 1/2 double-knockout mice develop epilepsy. Epilepsia 2017;58:e162–e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin MS, Dutt K, Papale LA, et al. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem 2010;285:9823–9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kilkenny C, Browne W, Cuthill IC, et al. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 2010;160:1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loscher W, Honack D, Fassbender CP, et al. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy Res 1991;8:171–189. [DOI] [PubMed] [Google Scholar]
- 38.Wong JC, Dutton SB, Collins SD, et al. Huperzine A Provides Robust and Sustained Protection against Induced Seizures in Scn1a Mutant Mice. Front Pharmacol 2016;7:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong JC, Thelin JT, Escayg A. Donepezil increases resistance to induced seizures in a mouse model of Dravet syndrome. Ann Clin Transl Neurol 2019;6:1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutton SBB, Dutt K, Papale LA, et al. Early-life febrile seizures worsen adult phenotypes in Scn1a mutants. Exp Neurol 2017;293:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oakley JC, Kalume F, Yu FH, et al. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci U S A 2009;106:3994–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong JC, Makinson CD, Lamar T, et al. Selective targeting of Scn8a prevents seizure development in a mouse model of mesial temporal lobe epilepsy. Sci Rep 2018;8:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamar T, Vanoye CG, Calhoun J, et al. SCN3A deficiency associated with increased seizure susceptibility. Neurobiol Dis 2017;102:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baek JH, Darlington CL, Smith PF, et al. Antibody testing for brain immunohistochemistry: brain immunolabeling for the cannabinoid CB(2) receptor. J Neurosci Methods 2013;216:87–95. [DOI] [PubMed] [Google Scholar]
- 45.Zhang HY, Shen H, Jordan CJ, et al. CB2 receptor antibody signal specificity: correlations with the use of partial CB2-knockout mice and anti-rat CB2 receptor antibodies. Acta Pharmacol Sin 2019;40:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soethoudt M, Grether U, Fingerle J, et al. Cannabinoid CB(2) receptor ligand profiling reveals biased signalling and off-target activity. Nature Communications 2017;8:13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rinaldi-Carmona M, Barth F, Millan J, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther 1998;284:644–650. [PubMed] [Google Scholar]
- 48.Huffman JW. CB2 receptor ligands. Mini Rev Med Chem 2005;5:641–649. [DOI] [PubMed] [Google Scholar]
- 49.Zoppi S, Madrigal JL, Caso JR, et al. Regulatory role of the cannabinoid CB2 receptor in stress-induced neuroinflammation in mice. Br J Pharmacol 2014;171:2814–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun L, Dong R, Xu X, et al. Activation of cannabinoid receptor type 2 attenuates surgery-induced cognitive impairment in mice through anti-inflammatory activity. J Neuroinflammation 2017;14:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bravo-Ferrer I, Cuartero MI, Zarruk JG, et al. Cannabinoid Type-2 Receptor Drives Neurogenesis and Improves Functional Outcome After Stroke. Stroke 2017;48:204–212. [DOI] [PubMed] [Google Scholar]
- 52.Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke 2012;43:211–219. [DOI] [PubMed] [Google Scholar]
- 53.Marsicano G, Goodenough S, Monory K, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003;302:84–88. [DOI] [PubMed] [Google Scholar]
- 54.Marinelli S, Pacioni S, Cannich A, et al. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci 2009;12:1488–1490. [DOI] [PubMed] [Google Scholar]
- 55.den Boon F, Chemeau P, Schaafsma-Zhao Q, et al. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellulartype-2 cannabinoid receptors. PNAS 2012;109:3534–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maresz K, Carrier EJ, Ponomarev ED, et al. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem 2005;95:437–445. [DOI] [PubMed] [Google Scholar]
- 57.Ehrhart J, Obregon D, Mori T, et al. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation 2005;2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaplan JS, Stella N, Catterall WA, et al. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A 2017;114:11229–11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014;55:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomez-Galvez Y, Palomo-Garo C, Fernandez-Ruiz J, et al. Potential of the cannabinoid CB(2) receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 2016;64:200–208. [DOI] [PubMed] [Google Scholar]
- 61.Amenta PS, Jallo JI, Tuma RF, et al. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J Neurosci Res 2012;90:2293–2305. [DOI] [PubMed] [Google Scholar]
- 62.Aso E, Juves S, Maldonado R, et al. CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AbetaPP/PS1 mice. J Alzheimers Dis 2013;35:847–858. [DOI] [PubMed] [Google Scholar]
- 63.Elliott MB, Tuma RF, Amenta PS, et al. Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury. J Neurotrauma 2011;28:973–981. [DOI] [PubMed] [Google Scholar]
- 64.Malfitano AM, Basu S, Maresz K, et al. What we know and do not know about the cannabinoid receptor 2 (CB2). Semin Immunol 2014;26:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gales JM, Prayson RA. Chronic inflammation in refractory hippocampal sclerosis-related temporal lobe epilepsy. Ann Diagn Pathol 2017;30:12–16. [DOI] [PubMed] [Google Scholar]
- 66.Tezer FI, Firat A, Tuzun E, et al. Immunopathology in drug resistant mesial temporal lobe epilepsy with different types of hippocampal sclerosis. Int J Neurosci 2018;128:421–428. [DOI] [PubMed] [Google Scholar]