Abstract
Oncolytic viruses (OVs) are powerful new therapeutic agents in cancer therapy. With the first OV (talimogene laherparepvec [T-vec]) obtaining US Food and Drug Administration approval, interest in OVs has been boosted greatly. Nevertheless, despite extensive research, oncolytic virotherapy has shown limited efficacy against solid tumors. Recent advances in viral retargeting, genetic editing, viral delivery platforms, tracking strategies, OV-based gene therapy, and combination strategies have the potential to broaden the applications of oncolytic virotherapy in oncology. In this review, we present several insights into the limitations and challenges of oncolytic virotherapy, describe the strategies mentioned above, provide a summary of recent preclinical and clinical trials in the field of oncolytic virotherapy, and highlight the need to optimize current strategies to improve clinical outcomes.
Main Text
Over the past few decades, immunotherapy has emerged as an effective therapeutic option against multiple malignancies. Oncolytic viruses (OVs), which can be engineered to replicate selectively in and lyse tumor tissues while sparing the normal non-neoplastic host cells and simultaneously restoring antitumor immunity, offer a novel immunotherapeutic approach for the treatment of tumors.1 Since it was reported that a 42-year-old female patient with myelogenous leukemia underwent tumor remission after an influenza infection, the attempt to use viruses to eliminate tumors has never stopped.2 The antitumor effect of OVs acts in two ways: by directly infecting and lysing tumor cells, or by arousing the immune system to generate an immune attack.3 These two functions of OVs result in two potential directions for therapeutic improvement. One is to improve the tumor targeting of OVs, such as using tumor-specific promoters, viral gene knockout, and even capsid modification, so that the OV can infect tumor tissue more efficiently without damaging normal tissue.4 The other one is to equip the virus with immune system-activating agents such as antibodies, cytokines, and costimulatory molecules to reverse the immunosuppressive tumor microenvironment.5 Compared with traditional administration routes, immune system-activating agents produced by OVs enable the infected tumor cells to be localized and concentrated, reducing the apparent side effects. The oncolytic agent based on the herpes virus, talimogene laherparepvec (T-vec), is an oncolytic herpes virus lacking ribonucleotide reductase and also expressing granulocyte-macrophage colony-stimulating factor (GM-CSF), combining two functions of OVs, and it became the first OV product approved by the US Food and Drug Administration.6 Since then, numerous kinds of viral products, from natural viruses to vectors, have been engineered to promote the safety and efficacy of virotherapy.7, 8, 9
The unique ability of OVs to target malignancies without dependence on specific antigen expression patterns makes them superior to other immunotherapy approaches.10 Moreover, OVs can promote the recruitment of tumor-infiltrating lymphocytes (TILs), reprogram the immunosuppressive tumor microenvironment (TME), and boost systemic antitumor immunity.11 All of these features make them ideal candidates against diverse malignancies.12 Nevertheless, despite extensive research, oncolytic virotherapy has shown limited efficacy against solid tumors because of physical barriers, tumor heterogeneity, and an immunosuppressive TME.10 Therefore, we must acknowledge the limitations and challenges, which include issues with manufacturing OVs, immunological barriers to viral delivery, and limitations to the success of oncolysis. Thanks to the advance of modern genetic engineering technology, some of these challenges and limitations are being addressed in various scientific areas. Today, both preclinical and early-stage clinical trials are intensively investigating the approach to improve oncolytic virotherapy. In this review, we aim to provide an overview of oncolytic virotherapy. We briefly introduce the barriers to oncolytic virotherapy, as well as a summary of recent promising strategies that have been developed to overcome the aforementioned barriers and to enhance the therapeutic potential of OVs.
Current Barriers to Oncolytic Virotherapy
Despite the potential of OVs, there are still many limitations that should be tackled to improve their efficacy in virotherapy. These include factors such as viral tropism, delivery platforms, viral distribution, dosing strategies, antiviral immunity, and oncolysis by the OVs.
In solid tumors, there is a range of hurdles that the OV must circumvent to reach the tumor site. First, physical barriers post a big challenge to delivery because viruses must get past the endothelial layer to reach the target cells.13 In addition, the abnormal lymphatic networks and vascular hyperpermeability inside tumors and the dense extracellular matrix (ECM) of solid tumors result in interstitial hypertension,14 which can impair viral infiltration. Furthermore, OVs can induce a strong innate immune response because of interactions between them and antigen-presenting cells (APCs), together with widespread antiviral immunity, preexisting circulating antibodies, and blood factors such as the coagulation factors FIX, FX, and complement protein C4BP. Subsequently, OVs are more likely to be cleared by the host’s immune system, and it is difficult to make sure whether sufficient numbers reach the tumor site.14,15
Another critical hurdle for OVs is the overwhelming number of individual barriers in the immunosuppressive TME of solid tumors. Tumor cells can escape immune surveillance, proliferate rapidly, and metastasize when coupled with the dysfunction of the TME. Solid tumors can secrete chemokines and cytokines such as interleukin (IL)-10, transforming growth factor β (TGF-β), and arginase-1,16 which suppress the immune cell population and serve to recruit immunosuppressive cells, including T-regulatory cells, myeloid-derived suppressor cells, tumor-associated macrophages (TAMs), tumor-associated fibroblasts, and neutrophils. These can protect tumors from functional antitumor immune responses.17 Consequently, inhibitory signals and immune checkpoint receptors, such as PD-1, CTLA-4, TIM-3, and LAG-3, are upregulated in TILs, contributing to an immunosuppressive tumor environment. Equally importantly, the abnormal organization and structure of the tumor vasculature can impair blood supply.13 The subsequent localized hypoxia and low-pH microenvironment might inhibit tumor cell apoptosis, promote angiogenesis, upregulate tumor growth factors, and make tumor cells more resistant to standard therapeutic methods such as radiotherapy, cytotoxic drugs, and immunotherapy.18, 19, 20 Therefore, once OVs reach the tumor site, it is crucial for them to maintain their functions within the immunosuppressive TME, which plays a key role in the proliferation and survival of cancer cells.
As outlined above, the challenges posed by physical barriers, tumor heterogeneity, antiviral immunity, and an immunosuppressive TME prevent OVs from reaching the target tumor cells specifically, infiltrating into the tumors, and retaining their functions. Several approaches are now being developed to increase the efficiency of tumor targeting, virion delivery, and distribution, as well as techniques arming them with therapeutic transgenes to enhance potency without increasing toxicity and combining with other treatments. Therefore, in this review, we present several further insights into the strategies that are currently being constructed to improve the efficiency of oncolytic virotherapy.
Reinforcing the Selective Replication Strength of OVs
For optimal efficacy and safety, OVs must reach the tumors and destroy cancer cells specifically but should also avoid damaging normal cells. Given each virus with its unique properties, the optimal virus species and suitable design of OVs are crucial for tumor targeting.
Choosing the Optimal OV
A wide range of different viral species have been investigated as prospective cancer therapeutic agents, including oncolytic adenoviruses, type 1 herpes simplex virus (HSV), polioviruses, measles virus (MV), Newcastle disease virus (NDV), reoviruses, vesicular stomatitis virus (VSV), and Zika virus. Here, we discuss some key factors that should be considered when choosing an optimal OV. Given different kinds of tumors of diverse histologic origins, although some viruses exhibit a native tropism for tumors, they cannot readily match given OVs specifically with a certain kind of malignancy. In contrast, most OV studies now show a relatively broad spectrum of antitumor activity, especially for hematological and epithelial malignancies.21 In general, RNA viruses (e.g., reovirus, MV, and coxsackievirus) kill tumor cells faster than do DNA viruses because they replicate in the cytoplasm and do not need to reach the nuclei of the target cells. However, they show fewer tumor-selective properties for the same reason.16 The presence of an envelope is also an important factor when selecting an OV because the oncolytic properties of enveloped viruses are less efficient than those of naked viruses, and enveloped viruses are more likely to be cleared by host immunity. The size of the OV also determines its oncolytic properties. Smaller viruses are easier to infiltrate and are diffuse throughout the tumor, while larger viruses are better able to insert the therapeutic genes. Furthermore, tumor tropism, potential pathogenicity, immunogenicity, druggability, and viral stability are important factors to be considered in virus selection.22 In this regard, many drug engineering approaches have emerged with recent improvements in modern genetic engineering techniques. The pros and cons of these approaches and clinical trials are discussed in the following subsections.
Retargeting OVs
To enhance an OV’s tropism and to reduce its adverse effects, different technologies varying from genetics to chemistry have been used to retarget OVs, and some are being evaluated in clinical trials.15 Retargeting approaches can be divided into three main categories: capsid development, genome engineering, and chemical modifications.
The capsid can be modified to enhance the binding between the virus and the target cell entry receptors. For example, genetically inserting peptides or protein domains into the viral capsid can help to increase transduction efficiencies in some cell lines and facilitate attachment of the OVs to target tumor cell membranes, thereby promoting viral internalization.15,23,24 In these ways, to enhance the OV’s tumor tropism, researchers have recently constructed a kind of random peptide-displaying library to develop vectors that target specific tumor cells.24 However, there is still insufficient knowledge on each OV’s surface binding, internalization, and gene expression properties.
Adaptive strategies comprise other notable directed-evolution approaches. This methodology employs the insertion of a gene coding for single-chain Fv fragments (scFv) that targets receptors only on the tumor cells and increases infection selectivity. For example, an IL-12-armed, human epidermal growth factor receptor 2 (HER2)-retargeted oncolytic HSV showed great efficacy at inhibiting the growth of HER2+ lung cancer in an animal model.25,26 Furthermore, the fiber can be modified to generate a chimeric OV vector, and it is thereby used to circumvent coxsackievirus‐adenovirus receptor eficiency.24 Notably, a novel Ad3/Ad11p chimeric oncolytic Ads (ColoAd1) was designed rationally.27 This capsid exhibits improved tumor cell transduction efficacy.
Apart from capsid modification, there are two main genome engineering approaches that have been used to construct conditionally replicating OVs. One is to delete the essential viral genes that are required for viral replication in normal cells, such as those in the E1A or E1B regions,28 and HSV-thymidine kinase (HSV-TK). In addition, cancer-related genes can also be deleted to enhance tumor tropism, such as TP53, RAS, RB1, PTEN, and genes coding proteins involved in the WNT signaling pathway,29 because mutations in the tumor-suppressor genes mentioned above result in defects in cell cycle control in tumor cells, thereby making them more sensitive to viral infection.29 Engineered OVs with deletions in certain genes involved in the inhibition of apoptotic cell death can lead to lower viral replication in normal cells compared with tumor cells. For example, ONYX-015, an oncolytic adenovirus with the gene for the viral protein E1B55kd deleted, shows increased tumor-selective replication potency resulting from an inability of the mutant protein to degrade p53.30 Alternatively, tumor-specific promoters of transcription, which are active only in tumor cells, are also targets for regulating tumor selectivity, such as human telomerase reverse transcriptase (hTERT), prostate-specific antigen, α-fetoprotein, carcinoembryonic antigen, or Survivin, which are located upstream of the genes required for viral replication. For instance, hTERT is inactive in most normal cells and can regulate telomerase activity, thus inhibiting the growth of cancer cells, making it a good therapeutic agent to control viral selective transcription.4,31,32 Another approach is the use of simpler and less expensive chemical modification strategies to retargeting OVs, such as the use of polyethylene glycol (PEG), poly-N-(2-hydroxypropyl) methacrylamide, mine-based PEGylation, and thiol-based coupling of transferrin to the fiber knob HI loop.33,34 Similarly, because the pH within tumor sites is lower than that of normal tissue, to this end constructing OV-pH-sensitive polymer complexes is also a viable method to target the TME, resulting in tumor-specific infection.35
However, the strategies outlined above still have limitations in that capsid modifications are not usually heritable. The genetic strategies are limited by the size of the viral genome and may impair viral titers. As well as chemical modification, viral bioactivity might be impaired by excessive polymer shielding. Therefore, a rational design of OVs using combined genetic and chemical modification approaches is needed urgently. Of note, although the strategies discussed above might improve the tumor tropism of OVs, they might also negatively impact other properties of viruses, such as their safety and stability.
Constructing Efficient OV Delivery Platforms
Although the greatest effect of OVs consists of their selective infection and replication in cancer cells, the ability to deliver OV particles efficiently to tumors still constitutes a huge hindrance. The rapid growth of tumors, impaired blood supply,13 abnormal lymphatic networks, vascular hyperpermeability inside tumors, the dense ECM of solid tumors, and the antiviral functions of the host’s immune system14 all reduce the efficacy and delivery of OVs.
OVs Delivered Systemically or via Local Injection
Oncolytic adenoviruses can be delivered systemically or by intratumoral injection, both of which have been applied broadly in studies. Theoretically, a systemic approach is an ideal delivery method because it enables a broad distribution of viruses, which can infect both primary and metastatic tumors, and this relatively noninvasive route can be repeated frequently.36 Nevertheless, viral particles are rapidly sequestered and degraded by the host immune system. Consequent to this, enhancing the homing of OVs to the tumor sites efficiently is another promising strategy for improving their antitumor activity and preventing adverse effects. To avoid these issues, capsid modification has been explored as a means to deliver OVs to tumor sites.15 Apart from engineering the OV itself, making use of other cells and materials is an alternative approach. Ran et al.37 developed a novel carrier system to deliver oncolytic adenoviruses by tumor cell-derived microparticles, which was able to avoid the antiviral effect of host antibodies and induce highly efficient cytolysis of tumor cells. Consistent with this notion, myeloid-derived suppressor cells (MDSCs) have been used to deliver oncolytic VSVs to tumors because they can recruit and specifically migrate to neoplastic lesions by tumor tropism. Importantly, the loading of viruses onto MDSCs enhances their ability to promote tumor regression without affecting their tumor tropism.38 Similarly, chemokine-dependent cellular vehicles, such as cytokine-induced killer cells, neural stem cells,39,40 mesenchymal stem cells,41 or even irradiated tumor cells, have been used for viral delivery. Thus, agents such as nanoparticles, liposomes, PEG, and polymeric particles35 have been employed to transport OVs from the systemic circulation to cancer cells.42,43 Notably, OVs coated with synthesized nanoparticles have longer survival, which can prevent virus clearance by antibodies.14 Another promising strategy is the application of ultrasound44 and magnetic drug-targeting systems.45,46 These are different kinds of strategies for the delivery of OVs because they are noninvasive and have been used for many years in the biomedical imaging field. What is more, magnetic drug-targeted viruses are able to pass through human tissues without attenuation.47
In contrast to systemic delivery, the common route by which OVs have been delivered in preclinical or clinical trials is intratumoral delivery. However, this cannot be employed in multifocal or inaccessible tumors, such as brain tumors or pancreatic cancers. Moreover, functioning cells at the tumor site are needed for viral transfection and immune cell recruitment, and thus it is essential for us to locate the tumor and establish the targets of antineoplastic agents for precise injection. Of note, image-guided delivery might be a promising approach to circumvent these barriers and maximize local virus availability, through directly visualizing the locus in which the OV was administered, and monitor the targeted neoplasm with morphological and molecular analyses.12
Enhancing OV Infiltration and Diffusion
One of the key issues hindering the effectiveness of oncolytic virotherapy is the limited OV distribution throughout tumors, as both the properties of viruses (envelope type and size)16 and the microenvironment of tumors can affect the penetration and diffusion of the OVs. The dense ECM of tumors represents one such hindrance to efficient viral spread because the tumor-associated stroma in the ECM limits their entrance. To address the barriers of the ECM, a promising approach to augment OV infiltration into tumor sites is to construct a novel recombinant virus targeting fibroblast activation protein (FAP), which is expressed on several stromal cells. For instance, de Sostoa et al.48 tried to combine the viral oncolysis of cancer cells and FAP-targeting bispecific T cell engager (FBiTE)-mediated cytotoxicity of FAP-expressing cancer-associated fibroblasts (CAFs) (ICO15K-FBiTE) to enhance the viral spread and T cell-mediated cytotoxicity against the stroma to improve therapeutic activity. Chondroitinase ABC (chase ABC), a natural bacterial enzyme that removes the sugar side chains from proteoglycans found within the ECM,49,50 can be inserted into the HSV genome under an IE4/5 promoter to enhance viral spread. In line with this strategy, collagenase and hyaluronidase can also be inserted to promote the therapeutic OV to spread better.51,52
Another strategy to enhance viral infiltration is to use engineered OVs to express virus fusion proteins, such as fusion-associated small transmembrane (FAST) protein, and fusion proteins of the gibbon ape leukemia virus (GALV), with MV (MV-F) and NDV (NDV-F), which can induce virus-cell fusion and mediate fusion of the infected cells to neighboring uninfected cells, subsequently facilitating cell entry and viral spread.53 For example, Le Boeuf et al.54 engineered a recombinant oncolytic VSV to express the p14 FAST protein of a reovirus (VSV-p14) and demonstrated that VSV-p14 could lead to extensive cell-cell fusion and induce increased dissemination of the viral infection, leading to a 10- to 20-fold increase in virus titers. This subsequently increased OV-induced oncolysis and prolonged survival in animal models of breast cancer. Several other OVs, such as HSV and adenoviruses, have been engineered to express fusion proteins to enhance tumor cell killing and promote the spreading of the OV throughout tumors in vivo.55,56
Maintaining the Balance between Antiviral Immunity and Antitumor Immunity
Regardless of using intravenous delivery or intratumoral injections, another challenging aspect is the presence of innate and adaptive antiviral immune responses evoked by OVs, which can lead to the quick clearance of OVs and limit their antitumor efficacy. Given the significant negative effects of antiviral immunity on OV therapy, multiple strategies have been envisaged to suppress antiviral immunity and the emission of danger signals, including the use of immunomodulators,57,58 genetic manipulation,59,60 histone deacetylase inhibitors, antioxidant sulforaphane,61 cytokines,62 depletion of antibodies,63 and magnetic nanoparticles (MNPs).64 The strategies hinder antiviral immunity, promoting viral replication and enhancing cytotoxicity. However, some investigators advocate that OV-induced antiviral immune responses are beneficial to antitumor immunity because they can overturn tumor-associated immunosuppression, home immune cells to tumors, and lead to virus-induced immunogenic cell death, thereby activating antitumor immunity.65 Thus, another important challenge is to develop strategies to manage antiviral immunity, to enhance antitumor immune activity, and to maintain the balance between them.
Tracking Virotherapy
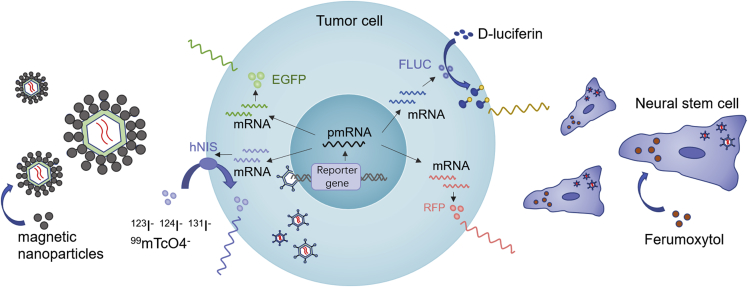
It is becoming increasingly realized that the microenvironment of malignant tumors is complex. Equally complex are the challenges of improving the delivery of OVs, developing strategies to track OV activity, and improving means for in vivo analysis, thereby enabling clinicians to select optimal OV dosing and develop more effective and personalized treatments. Initial localization of viruses has been performed using biopsy specimens, but this procedure is highly invasive for patients, and the data obtained reflect only one time point.66 Accounting for the efficacy of OVs is linked to their effective infection and replication in target cells. Therefore, strategies to track directed virotherapy linked to the viral life cycle are needed. Thus, genes associated with real-time noninvasive imaging of viral replication, such as those encoding luciferase,67 GFP, red fluorescent protein,68 human norepinephrine transporter,69 or the human sodium iodide symporter,70 can be inserted genetically into viral genomes or capsids to track virotherapy. In the case of Ad5luc3 and CF33 (a novel recombinant orthopoxvirus), this has been demonstrated clearly, in which d-luciferin expression can be used for noninvasive quantitation of the amplitude, persistence, and dynamics of viral replication in vivo, which would allow clinicians to track viral activity.67,71 Other investigators have also demonstrated that the intensity of bioluminescence correlates with viral titers.21 Consistent with these possibilities, strategies to load OVs with materials such as iron oxide nanoparticles (ferumoxytol) and MNPs, which allow for magnetic resonance imaging for monitoring OV distribution, are another noninvasive imaging feedback choice (Figure 1).40,64
Figure 1.
Multimodality Tracking Strategies of OVs
Expression of the EGFP reporter gene (EGFP) and red fluorescent protein reporter gene (RFP) in cytoplasm leads to the emission of fluorescent light (green light/red light). Transcription and translation of the firefly luciferase (FLUC) gene lead to cytosolic accumulation of the firefly luciferase enzyme, which subsequently catalyzes a photochemical reaction when d-luciferin is present. The resultant fluorescent light emission can be detected by imaging instruments. The human sodium iodide symporter (hNIS) gene may allow infected tumor cells to concentrate several free radionuclide probes such as 123I, 124I, 131I and 99mTcO4, which could facilitate viral imaging. Ferumoxytol-labeled neural stem cells loaded with OVs allow us to visualize the accumulation of the virus via MRI. In the same manner, magnetic nanoparticle (MNP)-assembled OV particles can be detected by MRI, enabling noninvasive viral monitoring.
Unfortunately, given the differences in genome size, as well as packaging, safety, and efficacy issues, not all oncolytic agents can be modified genetically to express such kinds of genes. Thus, soluble marker peptides—for example, the β chain of human chorionic gonadotropin or human carcinoembryonic antigen, which can be tested from blood or urine samples—can also be used to track the activity of virotherapy.72
Overcoming the Immunosuppressive TME and Enhancing Oncolysis
On arrival at the tumor, the immunosuppressive TME is another main hindrance that prevents OVs from reaching their full therapeutic potential. To improve viral replication and persistence, overcome the effects of the immunosuppressive TME, and thereby extend the oncolysis function of OVs, novel treatment options have been introduced, and are ongoing, by constructing recombinant OVs to deliver therapeutic genes, such as those encoding cytokines, tumor-associated antigens (TAAs), T cell costimulatory molecules, immune checkpoint inhibitors (ICIs), and cell suicide genes.29 In this section, we briefly review the approaches outlined above. In principle, the recombinant transgenes should not compromise the infectivity and replication potency of the OVs. Above all, they should not alter the safety profile of oncolytic virotherapy.17 A summary of these strategies is illustrated in Table 1, and a schematic depiction of OV-based gene therapies is shown in Figure 2.
Table 1.
Main Recombinant Transgenes Tested for Enhancing Antitumor Function of OVs
| Strategy | Detailed Genes |
|---|---|
| Cytokine | GM-CSF, IFN (α, β or γ), IL-2/IL-12/IL-15/IL-18/IL-21/IL-24… |
| Chemokine | CCL5, CCL20, CCL21, CXCL4L1, CXCL10… |
| Tumor-associated antigens | CEA, PSA, hDCT, CLND6 |
| Immune co-stimulatory molecules | CD28, ICOS, OX40, CD30, CD40, and 4-1BB |
| Immune checkpoint inhibitors | PD-1, CTLA4, LAG3, TIM3… |
| Suicide genes | HSV-TK, CD, nitroreductase, cytochrome P450 |
| Tumor suppressor genes | P53, PTEN, P16, Rb, MnSOD |
| Proapoptotic proteins and genes | Apoptin, Lactaptin, TRAIL, SMAC |
| Anti-angiogenesis | VEGI, VEGFR-1-Ig, anti-VEGF single-chain antibody, VEGF promoter-targeting transcriptional repressor (KOX), VEGF promoter-targeted transcriptional repressor zinc finger protein, vasculostatin, canstastin, plasminogen kringle 5, fibroblast growth factor receptor |
GM-CSF, granulocyte‐macrophage colony‐stimulating factor; CEA, carcinoembryonic antigen; PSA, prostate specific antigen; hDCT, human dopachrome tautomerase; CLND6, claudin 6; ICOS, inducible costimulatory molecule; HSV-TK, herpes virus thymidine kinase; CD, cytosine deaminase; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; SMAC, second mitochondria-derived activator of caspases; VEGI, vascular endothelial cell growth inhibitor; VEGFR-1-Ig, vascular endothelial growth factor receptor 1-Ig fusion protein; VEGF, vascular endothelial growth factor.
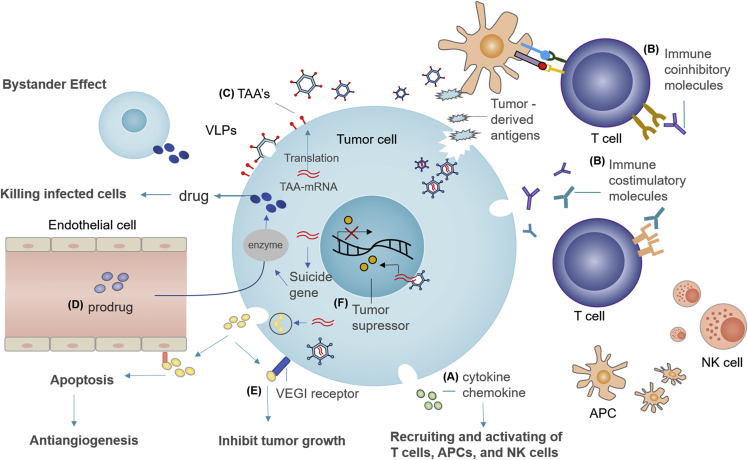
Figure 2.
Schematic Depiction of OV-Based Gene Therapies
(A) Virus-based immunostimulatory cytokine and chemokine expression can recruit and activate T cells, antigen-presenting cells (APCs), and natural killer (NK) cells, and subsequently improve the therapeutic activity of OVs. (B) Combination of OV with agents targeting costimulatory and/or coinhibitory molecules on T cells could lead to a more effective antitumor response. (C) Tumor-associated antigen (TAA)-encoding OVs can give rise to virus-like particle (VLP) presentation, inducing a potent antitumor effect. (D) The enzyme encoded by suicide gene can convert nontoxic prodrugs into toxic products in tumor cells, inducing tumor cell death. In addition, suicide genes also have a unique “bystander” effect. They can diffuse the toxic metabolic products to peripheral uninfected tumor cells through intercellular contact, thereby killing peripheral tumor cells. (E) Encoding OVs with anti-angiogenic transgenes (such as vascular endothelial cell growth inhibitor [VEGI]) can enhance viral permeability and inhibit endothelial cell proliferation. (F) Encoding OVs with tumor-suppressor genes can promote tumor regression and apoptosis.
Cytokines and Chemokines
In the cancer microenvironment, the function of APCs is usually impaired, prompting the need for innovative strategies to facilitate the presentation and recognition of TAAs.73 Because immunostimulatory genes—such as those encoding GM‐CSF, tumor necrosis factor alpha (TNF‐α), and interleukin—play pivotal roles in T cell migration and homing,5 a number of OVs have been engineered to express these transgenes. The goal is to generate more potent OVs to overcome the immunosuppressive TME and enhance oncolysis.36 For example, GM-CSF is a regulatory cytokine that can activate APCs, promote their maturation, increase both CD4+ and CD8+ T cells, as well as polarize TAMs to the more beneficial M1 phenotype.17,74 This should promote potent, long-lasting, specific immunity and prolonged viral replication. Therefore, a great variety of OVs, including oncolytic adenoviruses,75,76 type I HSV, reovirus,77 and poxvirus,17 have been engineered to express GM-CSF, and some have shown compelling outcomes in preclinical tumor models or clinical trials. Importantly, the successful approval of T-vec (a GM-CSF-armed oncolytic HSV) shows that cytokine-secreting OVs have a promising future in effective antitumor therapy and lay a firm foundation for future approvals for other cytokine-encoding OVs. The interferon (IFN) response is important in the immune activities that it improves the adaptive immune and cytotoxic response, and IFN production both enhances antigen presentation and increases antitumor activity.78 Accordingly, several researchers have armed OVs to express type I IFNs (α and β)79,80 or type II IFN (IFN-γ)81 to enhance the immune response and antitumor function. Currently, IFN (-α, -β, or -γ)-encoding oncolytic VSV or oncolytic adenoviruses have demonstrated promise in treating diverse tumor types, including hepatocellular and pancreatic carcinomas, mesotheliomas, myelomas, squamous cell carcinomas of the head and neck, and breast cancers.82, 83, 84 However, excessive IFN production can increase the expression of checkpoint molecules, including PD-1, PDL-1, LAG-3, and TIM-3, leading to the suppression of immunity. However, the increase in these checkpoint molecules provides a suitable environment for combined therapy with ICIs and OVs.22
Similarly, other OV-expressing cytokines (such as IL-2, IL-12, IL-15, IL-18, IL-21, and IL-24)78,85, 86, 87 or chemokines (such as CCL5, CCL20, CCL21, CXCL4L1, and CXCL10)88,89 have been tested in mouse tumor models or clinical trials to stimulate T cell proliferation and differentiation, enhance the proliferation of both cytotoxic T lymphocytes (CTLs) and natural killer cells, stimulate the production of IFN‐γ, induce antitumor inflammation, and improve the therapeutic activity of these OVs.5,90
TAAs
Besides the strategies discussed above, OVs armed with relevant TAA-like tumor vaccines also provide promising therapeutic approaches to retarget solid tumors by inducing a potent and persisting systemic antitumor response.91 The potential of OVs as recombinant cancer vaccine vectors has been explored in diverse animal models. For instance, Hutzler et al.92 developed a novel recombinant oncolytic MV encoding the oncofetal tight junction molecule Claudin 6 that produces virus-like particles (VLPs) expressed on the surface of cells in situ, or as membrane-bound VLPs. This approach has produced promising results in treating highly aggressive tumors and inhibiting tumor metastasis. Furthermore, other novel potentially targetable TAAs include carcinoembryonic antigen (CEA), prostate-specific antigen (PSA), or human dopachrome tautomerase.93, 94, 95 However, because of their heterogeneity, solid tumors represent considerable variability in antigen expression, so it is more difficult to find an ideal target antigen compared with hematological tumors,91 and there are potential risks of TAAs being expressed on healthy tissues.
Immune Costimulatory and Coinhibitory Molecules
The activation status of T cells is regulated by diverse cell receptors through multiple different interactions. Currently, numerous such receptors have been identified, including costimulatory molecules such as CD28 and inducible costimulatory molecule (ICOS), as well as coinhibitory receptors such as PD-1, CTLA-4, VISTA, B7-H3, LAG3, and TIM3. Significant attention has been devoted to modifying OVs to encode T cell costimulatory molecules, to enhance cancer-specific T cell activation, antigen release, and production of IFN, and subsequently to enhance dendritic cell (DC) maturation and antigen presentation of tumor cells, resulting in more potent systemic antitumor immunity.96,97 For instance, poxvirus17 and vaccinia virus98 encoding the costimulatory molecules B7.1, intercellular adhesion molecule 1, and lymphocyte function-associated antigen 3, termed collectively as TRICOM and the enhancer of antigen-presenting DC/pathways, showed activity in animal models and clinical trials.17 Furthermore, NDV that was engineered to express the inducible costimulator ligand was shown to enhance T cell activation and infiltration, enhancing the systemic efficacy of ICIs.99 Furthermore, OVs armed with immune checkpoint molecules that block T cell suppression might also be an effective therapeutic approach,22 as tumor cells usually escape from CTL-mediated lysis through upregulation of inhibitory immune checkpoint proteins,11 such as PDL-1, CTLA-4, and CD40,100 which impede the activity of cytotoxic T cells.
In addition, several studies have been modified or are ongoing to target other costimulatory members of the TNF receptor superfamilies, such as OX40, CD30, CD40, and 4-1BB, with evidence of immune activation by these agents in diverse malignancies.97 For example, a modified oncolytic adenovirus Delta-24-RGDOX expressing OX40L has shown high antitumor efficacy because it binds to a unique costimulatory OX40 on T cells, and it is more potent to enhance the in situ expansion of cancer-specific T cells.101
Suicide Genes and Prodrug Conversion
Suicide genes, such as those encoding HSV thymidine kinase (HSV-TK), and cytosine deaminase (CD),102 which are harmless to the cells expressing suicide genes but can induce cell death when activated by an antibody or drug, can also be inserted into OV genomes to enhance viral oncolysis. For example, the thymidine kinase enzyme encoded by the HSV-TK gene is able to convert the antiviral drug ganciclovir into the toxic ganciclovir monophosphate, subsequently interfering with DNA replication and leading to cell apoptosis, and has been tested as a treatment for pancreatic cancer.103 Similarly, CD can convert nontoxic 5-fluorocytosine (5-FC) to the highly toxic anticancer agent 5-fluorouracil, resulting in the killing of infected tumor cells.22,104 Another approach is to engineer OVs to express the fused suicide gene FCU1, which contains CD coupled with the gene for uracil phosphoribosyl transferase, to enhance the sensitivity of tumor cells to 5-FC and increase antitumor efficacy.105 Other suicide genes, such as those encoding nitroreductase and cytochrome P450, have shown compelling results in preclinical tumor models.106,107 Above all, suicide genes have a special “bystander” effect and can kill peripheral tumor cells by enabling the spread of toxic metabolic products.108
Tumor-Suppressor Genes and Proapoptotic Genes
In line with suicide gene strategies, other OVs have been created to express tumor-suppressor or proapoptotic genes, which augment the stimulation of immune responses and promote antitumor effects. Tumor-suppressor genes include P53, PTEN, P16, Rb, and MnSOD.94 Among these, the most common examples are P53 or other family members (P63 or P73). Many types of malignancies have a P53 mutation, and the introduction of these transgenes into the cancer cell genome can enhance OV efficacy and promote tumor apoptosis.108,109 Likewise, Russell et al.110 created an oncolytic HSV coexpressing PTEN to boost immune responses toward tumor cells. This had similar potency in replication and cancer cell killing as its parental control OV. A similar strategy involving recombinant oncolytic adenoviruses encoding apoptosis-inducing proteins, such as apoptin and Lactaptin, or apoptosis-inducing genes, such as TRAIL and SMAC,87 have also been reported to have the potential ability to promote tumor cell apoptosis in diverse malignancies.32,111
Anti-angiogenesis
Considering that angiogenesis plays a crucial role in tumor growth and invasion, another antitumor strategy is the engineering of OVs that encode anti-angiogenic transgenes or the direct targeting of viruses to the tumor-associated vascular endothelial cells and partially enhancing their permeability.21 To this end, a range of viruses have been armed with the vascular endothelial cell growth inhibitor, vascular endothelial growth factor (VEGF) receptor 1-Ig fusion protein, anti-VEGF single-chain antibody, VEGF promoter-targeting transcriptional repressor (KOX), or VEGF promoter-targeted transcriptional repressor zinc finger protein, aiming to induce apoptosis in proliferating endothelial cells and inhibition of neovascularization.112, 113, 114, 115, 116 Similarly, the endostatin- or angiostatin-encoding genes have been engineered to be expressed by oncolytic adenoviruses, oncolytic vaccinia viruses, adeno-associated viruses, and oncolytic HSV, showing promising efficacy in diverse tumor types, directly disrupting the delivery of nutrients and oxygen to the tumor, tumor clearance, and animal survival. Furthermore, Vasculostatin, Canstatin, Plasminogen kringle 5, and fibroblast growth factor receptor are among potential anti-angiogenesis transgene candidate targets for a similar strategy to enhance OV function.117, 118, 119, 120
Combination Therapies
Although various OVs and numerous modified strategies have been constructed to enhance efficiency and overcome the barriers in solid tumors, the clinical results of OV-like monotherapy remain to be improved. With recent advances in tumor immunology, the combination of OVS and immunotherapy has become an appealing choice. However, there are multiple treatment options for combination therapies, including ICIs,97,121 adoptive cell therapy,22,122 radiotherapy,123 and chemotherapy.124 To ensure the best possible responses, it is essential for us to have a thorough understanding of the rationale of each combination therapy. Here, we briefly review two potent therapies for combination with OVs.
The first and most common strategy to increase the potency of oncolytic virotherapies is to combine them with ICIs. Because tumor cells usually escape from CTL-mediated lysis through upregulation of inhibitory immune checkpoints,11 combining these two therapies has been shown to relieve the tumor’s immunosuppressive environment. Simultaneously, infection with OVs stimulates antitumor immune responses that in turn augment the efficacy of ICIs.121 For this reason, there have been multiple preclinical and clinical trials that combined oncolytic virotherapy and ICIs. For example, a phase Ib trial (ClinicalTrials.gov: NCT02263508) combining T-vec with an anti-PD-1 antibody (pembrolizumab) in 21 patients with surgically unresectable and previously untreated stage IIB and IV melanomas reported an increased overall objective response rate up to 62% without any dose-limiting toxicities.125,126 Consistent with this, an anti-CTLA-4 antibody (ipilimumab) was used with T-vec in a phase II trial (ClinicalTrials.gov: NCT01740297), and 198 patients with stages IIIB to IV melanomas were randomly allocated combination therapy or ipilimumab alone. The objective response rate to T-vec plus ipilimumab (39%) was significantly higher than with ipilimumab alone (18%; p = 0.002).127 Furthermore, the combination of ICIs with various OVs, such as vaccinia virus, adenovirus, reovirus, coxsackieviruses, maraba virus, and VSV, is under evaluation in different phase I or phase II clinical trials.121
Another strategy to improve the potency of oncolytic virotherapy is in combination with adoptive cell therapy because OVs can kill cancer cells specifically, and they have the potential to convert the TME from an immunosuppressive to an immunostimulatory environment that is permissive to T cell entry and activation.122 Another interesting aspect of this type of combination therapy is that OV-induced necrosis of tumor cells leads to the release of TAAs, thus promoting the priming and activation of neoantigen-specific T cells.122 OVs can also be modified genetically to deliver the therapeutic transgenes outlined above locally in the TME, which makes OVs appealing partners for adoptive cell therapy. A preclinical study of this combination strategy, using GD2-chimeric antigen receptor (CAR) T cells and a recombinant oncolytic adenovirus (armed with CCL5 and IL-15) in a xenogeneic mouse model of neuroblastoma, reported significantly increased overall survival of mice compared with either of the monotherapies.89 Similarly, the combination of OAd-TNF-α-IL-2 (a recombinant oncolytic adenovirus expressing TNF-α and IL-2) with meso-CAR T cells in an animal model of pancreatic ductal adenocarcinoma induced significantly better tumor regression and increased the antitumor efficacy of CAR T cells.128 In different preclinical trials, OVs armed with ICIs,127 cytokines,128 or bispecific T cell engagers129 enhanced the antitumor functions of CAR T cells and promoted CAR T cell homing, accumulation, and activation in the tumor, addressing tumor escape caused by the loss of antigen or heterogeneity of antigen expression and enhancing animal survival.130
Thus, by combining oncolytic virotherapy with other immunotherapies, researchers are now about to open a new chapter in oncotherapy. Many preclinical studies have confirmed such combinations to enhance therapeutic responses in animal models. However, note that further studies focusing on the optimal timing of the delivery of immune checkpoint inhibition, adoptive cells, or other therapeutic agents are needed urgently.
Clinical Trials
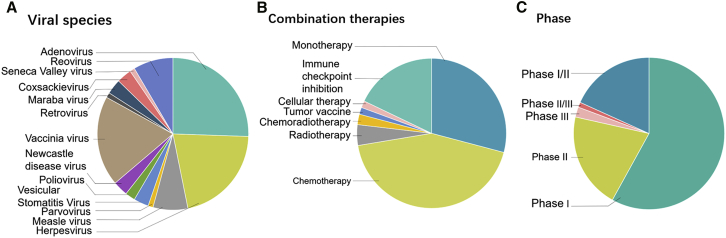
A number of clinical trials and translational studies involving OV have been registered at ClinicalTrials.gov. As of July 16, 2019, there are 118 clinical trials testing OVs. To date, 13 kinds of viruses have been investigated, and most have focused on oncolytic adenoviruses, HSV, and the vaccinia virus (Figure 3A). Oncolytic virotherapy has functioned well across different cancers, routes of administration, and monotherapy as well as in combination therapies. Apart from the traditional therapeutic approaches—for instance, chemoradiotherapy—the recent more efficient combination therapies mentioned above are also being tested in patients with different cancers (Figure 3B). Among these, immune checkpoint inhibition and cellular therapies are increasingly being utilized in clinical trials with or without OVs, indicating enormous potential for combination therapies, especially in patients with refractory tumors. A selection of ongoing clinical trials is summarized in Table 2.
Figure 3.
Overview of OV Clinical Trials
The dataset is from ClinicalTrials.gov, accessed on July 16, 2019. The 118 clinical trials are categorized on the basis of viral species (A), combination therapies (B), and clinical trial phase (C).
Table 2.
A Selection of Ongoing Recruiting OV Clinical Trials
| Viral Species | Virus Name | Condition | Route of Administration | Co-therapy | Phase | ClinicalTrials. gov Identifier |
|---|---|---|---|---|---|---|
| Adenovirus | DNX-2401 | CNS cancer | intra-arterial | therapeutic conventional surgery | I | NCT0389656 |
| CNS cancer | i.t. | chemoradiotherapy | I | NCT03178032 | ||
| NSC-CRAd-Survivin-pk7 | CNS cancer | i.t. | surgery; chemoradiotherapy | I | NCT03072134 | |
| Ad5-yCD/mutTKSR39rep-ADP | NSCLC | i.t. | chemoradiotherapy; valacyclovir | I | NCT03029871 | |
| Ad5-yCD/mutTKSR39rep-hIL12 | pancreatic cancer | i.t. | 5-FC; chemotherapy | I | NCT03281382 | |
| prostate cancer | i.t. | radiation | I | NCT02555397 | ||
| DNX-2440 | CNS cancer | i.t. | – | I | NCT03714334 | |
| LOAd703 | pancreatic cancer | i.t. | gemcitabine; nab-paclitaxel | I/IIa | NCT02705196 | |
| pancreatic, ovarian, biliary, colorectal cancer | i.t. | chemotherapy | I/II | NCT03225989 | ||
| ADV/HSV-tk | NSCLC | i.t. | valacyclovir; pembrolizumab; SBRT | II | NCT03004183 | |
| ONCOS-102 | melanoma | i.t. | cyclophosphamide; pembrolizumab | I | NCT03003676 | |
| prostate cancer | i.t. | DCVac/Pca; cyclophosphamide | I/II | NCT03514836 | ||
| NG-350A | metastatic cancer; epithelial tumor | i.t./i.v. | – | I | NCT03852511 | |
| Enadenotucirev | rectal cancer | i.t. | capecitabine; radiotherapy | I | NCT03916510 | |
| ovarian cancer | i.t./i.p. | – | I | NCT02028117 | ||
| VCN-01 | head and neck cancer | i.t. | durvalumab | I | NCT03799744 | |
| Telomelysin (OBP-301) | esophageal cancer | i.t. | radiation | I | NCT03213054 | |
| Herpes virus | OH2 | solid tumor; gastrointestinal cancer | i.t. | HX 008 | I | NCT03866525 |
| rQNestin34.5v.2 | CNS cancer | i.t. | cyclophosphamide | I | NCT03152318 | |
| T-VEC | melanoma | i.t. | CD1c (BDCA-1); myeloid DCs | I | NCT03747744 | |
| peritoneal surface malignancies | i.p. | – | I | NCT03663712 | ||
| breast cancer | i.t. | paclitaxel | I/II | NCT02779855 | ||
| M032 | CNS cancer | i.t. | – | I | NCT02062827 | |
| G207 | CNS cancer | i.t. | radiotherapy | I | NCT02457845 | |
| Vaccinia virus | Pexa-Vec (JX-594) | colorectal cancer | i.v. | durvalumab; tremelimumab | I/II | NCT03206073 |
| HCC | i.t. | nivolumab | I/IIa | NCT03071094 | ||
| HCC | i.t. | sorafenib | III | NCT02562755 | ||
| metastatic tumor; advanced tumor | i.t. | ipilimumab | I | NCT02977156 | ||
| solid tumors; soft-tissue sarcoma; breast cancer | i.v. | cyclophosphamide | I/II | NCT02630368 | ||
| renal cell carcinoma | i.v. | REGN2810 | I | NCT03294083 | ||
| GL-ONC1 | ovarian cancer | i.p. | chemotherapy; bevacizumab | I/II | NCT02759588 | |
| TG6002 | CNS cancer | i.v. | 5-FC | I/II | NCT03294486 | |
| ASP9801 | solid tumors | i.t. | –- | I | NCT03954067 | |
| Poliovirus/rhinovirus | PVSRIPO | CNS cancer | i.t. | – | II | NCT02986178 |
| CNS cancer | i.t. | – | Ib | NCT03043391 | ||
| triple-negative breast cancer | i.t. | surgery | I | NCT03564782 | ||
| melanoma | i.t. | – | I | NCT03712358 | ||
| Measles virus | MV-NIS | multiple myeloma | i.t. | cyclophosphamide | II | NCT02192775 |
| ovarian, fallopian, peritoneal cancer | i.p. | paclitaxel; pegylated liposomal doxorubicin hydrochlorid | II | NCT02364713 | ||
| ovarian cancer | i.p. | mesenchymal stem cell transplantation | I/II | NCT02068794 | ||
| CNS cancer | i.t. | –- | I | NCT02700230 | ||
| VSV | VSV-IFNβ-NIS | solid tumor; HCC; NSCLC | i.v. | pembrolizumab | I | NCT03647163 |
| endometrial cancer | i.v. | – | I | NCT03120624 | ||
| Reovirus | plasma cell myeloma | i.v. | carfilzomib; dexamethasone; nivolumab | I | NCT03605719 | |
| multiple myeloma | i.v. | lenalidomide; pomalidomide | I | NCT03015922 | ||
| Coxsackie virus | CVA21 | NSCLC | i.v. | pembrolizumab | I | NCT02824965 |
VSV, vesicular stomatitis virus; HCC, hepatocellular carcinoma; CNS, central nervous system; NSCLC, non-small cell lung cancer; i.t., intratumoral; i.v., intravenous; i.p., intraperitoneal; 5-FC, 5-fluorocytosine; SBRT, stereotactic body radiation therapy.
Conclusions
Oncolytic virotherapy is a promising immunotherapy for malignancies. With the development of modern genetic engineering techniques, increasing numbers of researchers are discovering strategies to optimize the construction of OVs, to reduce their clinical toxicity, to construct efficient OV delivery platforms, and to increase the efficacy of OVs, with the aim of achieving the greatest therapeutic benefit. In this review, we have described the multiple strategies that have been constructed for improving the efficacy of oncolytic virotherapy. Our comprehensive understanding of the challenges posed by physical barriers, antiviral immunity, and an immunosuppressive TME preventing OV delivery, infiltration, and oncolysis functions will be important for the rational design of recombinant OVs. Of note, genetically manipulating viruses might also lead to unexpected toxic reactions as well. It is essential for us to consider all factors in the safety analysis of OVs, either natural or engineered. Furthermore, because solid cancers usually have high mutational burdens, it is hard for a single agent to achieve promising long-term remission. Therefore, the combination of oncolytic virotherapy with other possible immune-based therapies is eagerly awaited.97 Future studies in oncolytic virotherapy can focus on systemic delivery approaches and OV-based gene therapies. The strategies outlined above highlight the vast expanse of preclinical and clinical research that is yet to be explored. To harness OVs in developing innovative strategies and overcoming current challenges, contributions from the fields of molecular biology, structural biology, immunology, genomics, and bioinformatics are required. Increased understanding of the challenges and limitations in cancer therapy and continued study of advances in recombinant OVs should lay solid foundations for future clinical success.
Author Contributions
All listed authors contributed to the writing of this review. All authors read and provided critical revision of the manuscript and approved the final version.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by a grant from the Science and Technology Department of Sichuan Province, P.R. China (2017SZ0015).
References
- 1.Muik A., Stubbert L.J., Jahedi R.Z., Geiβ Y., Kimpel J., Dold C., Tober R., Volk A., Klein S., Dietrich U. Re-engineering vesicular stomatitis virus to abrogate neurotoxicity, circumvent humoral immunity, and enhance oncolytic potency. Cancer Res. 2014;74:3567–3578. doi: 10.1158/0008-5472.CAN-13-3306. [DOI] [PubMed] [Google Scholar]
- 2.Dock G. The influence of complicating diseases upon leukaemia. Am. J. Med. Sci. 1904;127:563–592. [Google Scholar]
- 3.Melcher A., Parato K., Rooney C.M., Bell J.C. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol. Ther. 2011;19:1008–1016. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Erp E.A., Kaliberova L.N., Kaliberov S.A., Curiel D.T. Retargeted oncolytic adenovirus displaying a single variable domain of camelid heavy-chain-only antibody in a fiber protein. Mol. Ther. Oncolytics. 2015;2:15001. doi: 10.1038/mto.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goradel N.H., Mohajel N., Malekshahi Z.V., Jahangiri S., Najafi M., Farhood B., Mortezaee K., Negahdari B., Arashkia A. Oncolytic adenovirus: a tool for cancer therapy in combination with other therapeutic approaches. J. Cell. Physiol. 2019;234:8636–8646. doi: 10.1002/jcp.27850. [DOI] [PubMed] [Google Scholar]
- 6.Ledford H. Cancer-fighting viruses win approval. Nature. 2015;526:622–623. doi: 10.1038/526622a. [DOI] [PubMed] [Google Scholar]
- 7.Heo J., Reid T., Ruo L., Breitbach C.J., Rose S., Bloomston M., Cho M., Lim H.Y., Chung H.C., Kim C.W. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andtbacka R.H., Curti B.D., Hallmeyer S., Feng Z., Paustian C., Bifulco C., Fox B., Grose M., Shafren D. Phase II calm extension study: coxsackievirus A21 delivered intratumorally to patients with advanced melanoma induces immune-cell infiltration in the tumor microenvironment. J. Immunother. Cancer. 2015;3(S2):P343. [Google Scholar]
- 9.Desjardins A., Gromeier M., Herndon J.E., 2nd, Beaubier N., Bolognesi D.P., Friedman A.H., Friedman H.S., McSherry F., Muscat A.M., Nair S. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 2018;379:150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulley J.L., Madan R.A., Pachynski R., Mulders P., Sheikh N.A., Trager J., Drake C.G. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J. Natl. Cancer Inst. 2017;109:djw261. doi: 10.1093/jnci/djw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell S.J., Barber G.N. Oncolytic viruses as antigen-agnostic cancer vaccines. Cancer Cell. 2018;33:599–605. doi: 10.1016/j.ccell.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raja J., Ludwig J.M., Gettinger S.N., Schalper K.A., Kim H.S. Oncolytic virus immunotherapy: future prospects for oncology. J. Immunother. Cancer. 2018;6:140. doi: 10.1186/s40425-018-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuczynski E.A., Vermeulen P.B., Pezzella F., Kerbel R.S., Reynolds A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019;16:469–493. doi: 10.1038/s41571-019-0181-9. [DOI] [PubMed] [Google Scholar]
- 14.Jain R.K., Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagedorn C., Kreppel F. Capsid engineering of adenovirus vectors: overcoming early vector-host interactions for therapy. Hum. Gene Ther. 2017;28:820–832. doi: 10.1089/hum.2017.139. [DOI] [PubMed] [Google Scholar]
- 16.Guedan S., Alemany R. CAR-T cells and oncolytic viruses: joining forces to overcome the solid tumor challenge. Front. Immunol. 2018;9:2460. doi: 10.3389/fimmu.2018.02460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharp D.W., Lattime E.C. Recombinant poxvirus and the tumor microenvironment: oncolysis, immune regulation and immunization. Biomedicines. 2016;4:19. doi: 10.3390/biomedicines4030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nayyar G., Chu Y., Cairo M.S. Overcoming resistance to natural killer cell based immunotherapies for solid tumors. Front. Oncol. 2019;9:51. doi: 10.3389/fonc.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh E., Hong J., Kwon O.J., Yun C.O.J.S.R. A hypoxia- and telomerase-responsive oncolytic adenovirus expressing secretable trimeric TRAIL triggers tumour-specific apoptosis and promotes viral dispersion in TRAIL-resistant glioblastoma. Sci. Rep. 2018;8:1420. doi: 10.1038/s41598-018-19300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9(Suppl 5):10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- 21.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bommareddy P.K., Shettigar M., Kaufman H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018;18:498–513. doi: 10.1038/s41577-018-0014-6. [DOI] [PubMed] [Google Scholar]
- 23.Hesse A., Kosmides D., Kontermann R.E., Nettelbeck D.M. Tropism modification of adenovirus vectors by peptide ligand insertion into various positions of the adenovirus serotype 41 short-fiber knob domain. J. Virol. 2007;81:2688–2699. doi: 10.1128/JVI.02722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto Y., Nagasato M., Yoshida T., Aoki K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci. 2017;108:831–837. doi: 10.1111/cas.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menotti L., Cerretani A., Hengel H., Campadelli-Fiume G. Construction of a fully retargeted herpes simplex virus 1 recombinant capable of entering cells solely via human epidermal growth factor receptor 2. J. Virol. 2008;82:10153–10161. doi: 10.1128/JVI.01133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Totsch S.K., Schlappi C., Kang K.D., Ishizuka A.S., Lynn G.M., Fox B., Beierle E.A., Whitley R.J., Markert J.M., Gillespie G.Y. Oncolytic herpes simplex virus immunotherapy for brain tumors: current pitfalls and emerging strategies to overcome therapeutic resistance. Oncogene. 2019;38:6159–6171. doi: 10.1038/s41388-019-0870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhn I., Harden P., Bauzon M., Chartier C., Nye J., Thorne S., Reid T., Ni S., Lieber A., Fisher K. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS ONE. 2008;3:e2409. doi: 10.1371/journal.pone.0002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauthoff H., Pipiya T., Heitner S., Chen S., Bleck B., Reibman J., Chang W., Norman R.G., Rom W.N., Hay J.G. Impact of E1a modifications on tumor-selective adenoviral replication and toxicity. Mol. Ther. 2004;10:749–757. doi: 10.1016/j.ymthe.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Twumasi-Boateng K., Pettigrew J.L., Kwok Y.Y.E., Bell J.C., Nelson B.H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer. 2018;18:419–432. doi: 10.1038/s41568-018-0009-4. [DOI] [PubMed] [Google Scholar]
- 30.Hamid O., Hoffner B., Gasal E., Hong J., Carvajal R.D. Oncolytic immunotherapy: unlocking the potential of viruses to help target cancer. Cancer Immunol. Immunother. 2017;66:1249–1264. doi: 10.1007/s00262-017-2025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulasov I.V., Zhu Z.B., Tyler M.A., Han Y., Rivera A.A., Khramtsov A., Curiel D.T., Lesniak M.S. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum. Gene Ther. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 32.Cui C.X., Li Y.Q., Sun Y.J., Zhu Y.L., Fang J.B., Bai B., Li W.J., Li S.Z., Ma Y.Z., Li X. Antitumor effect of a dual cancer-specific oncolytic adenovirus on prostate cancer PC-3 cells. Urol. Oncol. 2019;37:352.e1–352.e18. doi: 10.1016/j.urolonc.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Kreppel F., Gackowski J., Schmidt E., Kochanek S. Combined genetic and chemical capsid modifications enable flexible and efficient de- and retargeting of adenovirus vectors. Mol. Ther. 2005;12:107–117. doi: 10.1016/j.ymthe.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Fisher K.D., Stallwood Y., Green N.K., Ulbrich K., Mautner V., Seymour L.W. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 35.Choi J.W., Lee Y.S., Yun C.O., Kim S.W. Polymeric oncolytic adenovirus for cancer gene therapy. J. Control. Release. 2015;219:181–191. doi: 10.1016/j.jconrel.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reale A., Vitiello A., Conciatori V., Parolin C., Calistri A., Palù G. Perspectives on immunotherapy via oncolytic viruses. Infect. Agent. Cancer. 2019;14:5. doi: 10.1186/s13027-018-0218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ran L., Tan X., Li Y., Zhang H., Ma R., Ji T., Dong W., Tong T., Liu Y., Chen D. Delivery of oncolytic adenovirus into the nucleus of tumorigenic cells by tumor microparticles for virotherapy. Biomaterials. 2016;89:56–66. doi: 10.1016/j.biomaterials.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Eisenstein S., Coakley B.A., Briley-Saebo K., Ma G., Chen H.M., Meseck M., Ward S., Divino C., Woo S., Chen S.H., Pan P.Y. Myeloid-derived suppressor cells as a vehicle for tumor-specific oncolytic viral therapy. Cancer Res. 2013;73:5003–5015. doi: 10.1158/0008-5472.CAN-12-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mooney R., Majid A.A., Batalla-Covello J., Machado D., Liu X., Gonzaga J., Tirughana R., Hammad M., Lesniak M.S., Curiel D.T., Aboody K.S. Enhanced delivery of oncolytic adenovirus by neural stem cells for treatment of metastatic ovarian cancer. Mol. Ther. Oncolytics. 2018;12:79–92. doi: 10.1016/j.omto.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morshed R.A., Gutova M., Juliano J., Barish M.E., Hawkins-Daarud A., Oganesyan D., Vazgen K., Yang T., Annala A., Ahmed A.U. Analysis of glioblastoma tumor coverage by oncolytic virus-loaded neural stem cells using MRI-based tracking and histological reconstruction. Cancer Gene Ther. 2015;22:55–61. doi: 10.1038/cgt.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duebgen M., Martinez-Quintanilla J., Tamura K., Hingtgen S., Redjal N., Wakimoto H., Shah K. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. J. Natl. Cancer Inst. 2014;106:dju090. doi: 10.1093/jnci/dju090. [DOI] [PubMed] [Google Scholar]
- 42.Doronin K., Shashkova E.V., May S.M., Hofherr S.E., Barry M.A. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum. Gene Ther. 2009;20:975–988. doi: 10.1089/hum.2009.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green N.K., Herbert C.W., Hale S.J., Hale A.B., Mautner V., Harkins R., Hermiston T., Ulbrich K., Fisher K.D., Seymour L.W. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11:1256–1263. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
- 44.Greco A., Di Benedetto A., Howard C.M., Kelly S., Nande R., Dementieva Y., Miranda M., Brunetti A., Salvatore M., Claudio L. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Mol. Ther. 2010;18:295–306. doi: 10.1038/mt.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tresilwised N., Pithayanukul P., Holm P.S., Schillinger U., Plank C., Mykhaylyk O. Effects of nanoparticle coatings on the activity of oncolytic adenovirus-magnetic nanoparticle complexes. Biomaterials. 2012;33:256–269. doi: 10.1016/j.biomaterials.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 46.Choi J.W., Park J.W., Na Y., Jung S.J., Hwang J.K., Choi D., Lee K.G., Yun C.O. Using a magnetic field to redirect an oncolytic adenovirus complexed with iron oxide augments gene therapy efficacy. Biomaterials. 2015;65:163–174. doi: 10.1016/j.biomaterials.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Hill C., Carlisle R. Achieving systemic delivery of oncolytic viruses. Expert Opin. Drug Deliv. 2019;16:607–620. doi: 10.1080/17425247.2019.1617269. [DOI] [PubMed] [Google Scholar]
- 48.de Sostoa J., Fajardo C.A., Moreno R., Ramos M.D., Farrera-Sal M., Alemany R. Targeting the tumor stroma with an oncolytic adenovirus secreting a fibroblast activation protein-targeted bispecific T-cell engager. J. Immunother. Cancer. 2019;7:19. doi: 10.1186/s40425-019-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahramanian A., Kuroda T., Wakimoto H. Construction of oncolytic herpes simplex virus with therapeutic genes of interest. Methods Mol. Biol. 2019;1937:177–188. doi: 10.1007/978-1-4939-9065-8_10. [DOI] [PubMed] [Google Scholar]
- 50.Dmitrieva N., Yu L., Viapiano M., Cripe T.P., Chiocca E.A., Glorioso J.C., Kaur B. Chondroitinase ABC I-mediated enhancement of oncolytic virus spread and antitumor efficacy. Clin. Cancer Res. 2011;17:1362–1372. doi: 10.1158/1078-0432.CCR-10-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKee T.D., Grandi P., Mok W., Alexandrakis G., Insin N., Zimmer J.P., Bawendi M.G., Boucher Y., Breakefield X.O., Jain R.K. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 52.Guedan S., Rojas J.J., Gros A., Mercade E., Cascallo M., Alemany R. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol. Ther. 2010;18:1275–1283. doi: 10.1038/mt.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krabbe T., Altomonte J. Fusogenic viruses in oncolytic immunotherapy. Cancers (Basel) 2018;10:E216. doi: 10.3390/cancers10070216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Boeuf F., Gebremeskel S., McMullen N., He H., Greenshields A.L., Hoskin D.W., Bell J.C., Johnston B., Pan C., Duncan R. Reovirus FAST protein enhances vesicular stomatitis virus oncolytic virotherapy in primary and metastatic tumor models. Mol. Ther. Oncolytics. 2017;6:80–89. doi: 10.1016/j.omto.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simpson G.R., Horvath A., Annels N.E., Pencavel T., Metcalf S., Seth R., Peschard P., Price T., Coffin R.S., Mostafid H. Combination of a fusogenic glycoprotein, pro-drug activation and oncolytic HSV as an intravesical therapy for superficial bladder cancer. Br. J. Cancer. 2012;106:496–507. doi: 10.1038/bjc.2011.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guedan S., Grases D., Rojas J.J., Gros A., Vilardell F., Vile R., Mercade E., Cascallo M., Alemany R. GALV expression enhances the therapeutic efficacy of an oncolytic adenovirus by inducing cell fusion and enhancing virus distribution. Gene Ther. 2012;19:1048–1057. doi: 10.1038/gt.2011.184. [DOI] [PubMed] [Google Scholar]
- 57.Lun X.Q., Jang J.H., Tang N., Deng H., Head R., Bell J.C., Stojdl D.F., Nutt C.L., Senger D.L., Forsyth P.A., McCart J.A. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin. Cancer Res. 2009;15:2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 58.Yang D.G., Chung Y.C., Lai Y.-K., Lai C.W., Liu H.J., Hu Y.C. Avian influenza virus hemagglutinin display on baculovirus envelope: cytoplasmic domain affects virus properties and vaccine potential. Mol. Ther. 2007;15:989–996. doi: 10.1038/mt.sj.6300131. [DOI] [PubMed] [Google Scholar]
- 59.Ahn D.G., Sharif T., Chisholm K., Pinto D.M., Gujar S.A., Lee P.W. Ras transformation results in cleavage of reticulon protein Nogo-B that is associated with impairment of IFN response. Cell Cycle. 2015;14:2301–2310. doi: 10.1080/15384101.2015.1044187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noser J.A., Mael A.A., Sakuma R., Ohmine S., Marcato P., Lee P.W., Ikeda Y. The RAS/Raf1/MEK/ERK signaling pathway facilitates VSV-mediated oncolysis: implication for the defective interferon response in cancer cells. Mol. Ther. 2007;15:1531–1536. doi: 10.1038/sj.mt.6300193. [DOI] [PubMed] [Google Scholar]
- 61.Olagnier D., Lababidi R.R., Hadj S.B., Sze A., Liu Y., Naidu S.D., Ferrari M., Jiang Y., Chiang C., Beljanski V. Activation of Nrf2 signaling augments vesicular stomatitis virus oncolysis via autophagy-driven suppression of antiviral immunity. Mol. Ther. 2017;25:1900–1916. doi: 10.1016/j.ymthe.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han J., Chen X., Chu J., Xu B., Meisen W.H., Chen L., Zhang L., Zhang J., He X., Wang Q.E. TGFβ treatment enhances glioblastoma virotherapy by inhibiting the innate immune response. Cancer Res. 2015;75:5273–5282. doi: 10.1158/0008-5472.CAN-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alvarez-Breckenridge C.A., Yu J., Caligiuri M.A., Chiocca E.A. Uncovering a novel mechanism whereby NK cells interfere with glioblastoma virotherapy. OncoImmunology. 2013;2:e23658. doi: 10.4161/onci.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Almstätter I., Mykhaylyk O., Settles M., Altomonte J., Aichler M., Walch A., Rummeny E.J., Ebert O., Plank C., Braren R. Characterization of magnetic viral complexes for targeted delivery in oncology. Theranostics. 2015;5:667–685. doi: 10.7150/thno.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gujar S., Pol J.G., Kim Y., Lee P.W., Kroemer G. Antitumor benefits of antiviral immunity: an underappreciated aspect of oncolytic virotherapies. Trends Immunol. 2018;39:209–221. doi: 10.1016/j.it.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto M., Curiel D.T. Current issues and future directions of oncolytic adenoviruses. Mol. Ther. 2010;18:243–250. doi: 10.1038/mt.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Leary M.P., Warner S.G., Kim S.I., Chaurasiya S., Lu J., Choi A.H., Park A.K., Woo Y., Fong Y., Chen N.G. A novel oncolytic chimeric orthopoxvirus encoding luciferase enables real-time view of colorectal cancer cell infection. Mol. Ther. Oncolytics. 2018;9:13–21. doi: 10.1016/j.omto.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le L.P., Le H.N., Dmitriev I.P., Davydova J.G., Gavrikova T., Yamamoto S., Curiel D.T., Yamamoto M. Dynamic monitoring of oncolytic adenovirus in vivo by genetic capsid labeling. J. Natl. Cancer Inst. 2006;98:203–214. doi: 10.1093/jnci/djj022. [DOI] [PubMed] [Google Scholar]
- 69.Chen N., Zhang Q., Yu Y.A., Stritzker J., Brader P., Schirbel A., Samnick S., Serganova I., Blasberg R., Fong Y., Szalay A.A. A novel recombinant vaccinia virus expressing the human norepinephrine transporter retains oncolytic potential and facilitates deep-tissue imaging. Mol. Med. 2009;15:144–151. doi: 10.2119/molmed.2009.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haddad D., Chen C.H., Carlin S., Silberhumer G., Chen N.G., Zhang Q., Longo V., Carpenter S.G., Mittra A., Carson J. Imaging characteristics, tissue distribution, and spread of a novel oncolytic vaccinia virus carrying the human sodium iodide symporter. PLoS ONE. 2012;7:e41647. doi: 10.1371/journal.pone.0041647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guse K., Dias J.D., Bauerschmitz G.J., Hakkarainen T., Aavik E., Ranki T., Pisto T., Särkioja M., Desmond R.A., Kanerva A., Hemminki A. Luciferase imaging for evaluation of oncolytic adenovirus replication in vivo. Gene Ther. 2007;14:902–911. doi: 10.1038/sj.gt.3302949. [DOI] [PubMed] [Google Scholar]
- 72.Peng K.W., Facteau S., Wegman T., O’Kane D., Russell S.J.J.N.M. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat. Med. 2002;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 73.Brown M.C., Holl E.K., Boczkowski D., Dobrikova E., Mosaheb M., Chandramohan V., Bigner D.D., Gromeier M., Nair S.K. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci. Transl. Med. 2017;9:eaan4220. doi: 10.1126/scitranslmed.aan4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nebiker C.A., Han J., Eppenberger-Castori S., Iezzi G., Hirt C., Amicarella F., Cremonesi E., Huber X., Padovan E., Angrisani B. GM-CSF production by tumor cells is associated with improved survival in colorectal cancer. Clin. Cancer Res. 2014;20:3094–3106. doi: 10.1158/1078-0432.CCR-13-2774. [DOI] [PubMed] [Google Scholar]
- 75.Kuryk L., Møller A.W., Jaderberg M. Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pembrolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. OncoImmunology. 2018;8:e1532763. doi: 10.1080/2162402X.2018.1532763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Packiam V.T., Lamm D.L., Barocas D.A., Trainer A., Fand B., Davis R.L., 3rd, Clark W., Kroeger M., Dumbadze I., Chamie K. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: interim results. Urol. Oncol. 2018;36:440–447. doi: 10.1016/j.urolonc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Kemp V., van den Wollenberg D.J.M., Camps M.G.M., van Hall T., Kinderman P., Pronk-van Montfoort N., Hoeben R.C. Arming oncolytic reovirus with GM-CSF gene to enhance immunity. Cancer Gene Ther. 2019;26:268–281. doi: 10.1038/s41417-018-0063-9. [DOI] [PubMed] [Google Scholar]
- 78.Pearl T.M., Markert J.M., Cassady K.A., Ghonime M.G. Oncolytic virus-based cytokine expression to improve immune activity in brain and solid tumors. Mol. Ther. Oncolytics. 2019;13:14–21. doi: 10.1016/j.omto.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel M.R., Jacobson B.A., Ji Y., Drees J., Tang S., Xiong K., Wang H., Prigge J.E., Dash A.S., Kratzke A.K. Vesicular stomatitis virus expressing interferon-β is oncolytic and promotes antitumor immune responses in a syngeneic murine model of non-small cell lung cancer. Oncotarget. 2015;6:33165–33177. doi: 10.18632/oncotarget.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.LaRocca C.J., Han J., Gavrikova T., Armstrong L., Oliveira A.R., Shanley R., Vickers S.M., Yamamoto M., Davydova J. Oncolytic adenovirus expressing interferon alpha in a syngeneic Syrian hamster model for the treatment of pancreatic cancer. Surgery. 2015;157:888–898. doi: 10.1016/j.surg.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bourgeois-Daigneault M.-C., Roy D.G., Falls T., Twumasi-Boateng K., St-Germain L.E., Marguerie M., Garcia V., Selman M., Jennings V.A., Pettigrew J. Oncolytic vesicular stomatitis virus expressing interferon-γ has enhanced therapeutic activity. Mol. Ther. Oncolytics. 2016;3:16001. doi: 10.1038/mto.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Armstrong L., Arrington A., Han J., Gavrikova T., Brown E., Yamamoto M., Vickers S.M., Davydova J. Generation of a novel, cyclooxygenase-2-targeted, interferon-expressing, conditionally replicative adenovirus for pancreatic cancer therapy. Am. J. Surg. 2012;204:741–750. doi: 10.1016/j.amjsurg.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shinozaki K., Ebert O., Suriawinata A., Thung S.N., Woo S.L. Prophylactic alpha interferon treatment increases the therapeutic index of oncolytic vesicular stomatitis virus virotherapy for advanced hepatocellular carcinoma in immune-competent rats. J. Virol. 2005;79:13705–13713. doi: 10.1128/JVI.79.21.13705-13713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naik S., Nace R., Barber G.N., Russell S.J. Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-β. Cancer Gene Ther. 2012;19:443–450. doi: 10.1038/cgt.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosewell Shaw A., Porter C.E., Watanabe N., Tanoue K., Sikora A., Gottschalk S., Brenner M.K., Suzuki M. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T cells against metastatic head and neck cancer. Mol. Ther. 2017;25:2440–2451. doi: 10.1016/j.ymthe.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ashshi A.M., El-Shemi A.G., Dmitriev I.P., Kashentseva E.A., Curiel D.T. Combinatorial strategies based on CRAd-IL24 and CRAd-ING4 virotherapy with anti-angiogenesis treatment for ovarian cancer. J. Ovarian Res. 2016;9:38. doi: 10.1186/s13048-016-0248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu Y., He J., Geng J., An Y., Ye X., Yan S., Yu Q., Yin J., Zhang Z., Li D. Recombinant Newcastle disease virus expressing human TRAIL as a potential candidate for hepatoma therapy. Eur. J. Pharmacol. 2017;802:85–92. doi: 10.1016/j.ejphar.2017.02.042. [DOI] [PubMed] [Google Scholar]
- 88.Dinsart C., Pervolaraki K., Stroh-Dege A., Lavie M., Ronsse I., Rommelaere J., Van Damme J., Van Raemdonck K., Struyf S. Recombinant parvoviruses armed to deliver CXCL4L1 and CXCL10 are impaired in their antiangiogenic and antitumoral effects in a Kaposi sarcoma tumor model due to the chemokines’ interference with the virus cycle. Hum. Gene Ther. 2017;28:295–306. doi: 10.1089/hum.2016.108. [DOI] [PubMed] [Google Scholar]
- 89.Nishio N., Dotti G. Oncolytic virus expressing RANTES and IL-15 enhances function of CAR-modified T cells in solid tumors. OncoImmunology. 2015;4:e988098. doi: 10.4161/21505594.2014.988098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pol J.G., Lévesque S., Workenhe S.T., Gujar S., Le Boeuf F., Clements D.R., Fahrner J.E., Fend L., Bell J.C., Mossman K.L. Trial watch: oncolytic viro-immunotherapy of hematologic and solid tumors. Oncoimmunology. 2018;7:e1503032. doi: 10.1080/2162402X.2018.1503032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez M., Moon E.K. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front. Immunol. 2019;10:128. doi: 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hutzler S., Erbar S., Jabulowsky R.A., Hanauer J.R.H., Schnotz J.H., Beissert T., Bodmer B.S., Eberle R., Boller K., Klamp T. Antigen-specific oncolytic MV-based tumor vaccines through presentation of selected tumor-associated antigens on infected cells or virus-like particles. Sci. Rep. 2017;7:16892. doi: 10.1038/s41598-017-16928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bridle B.W., Boudreau J.E., Lichty B.D., Brunellière J., Stephenson K., Koshy S., Bramson J.L., Wan Y. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus. Mol. Ther. 2009;17:1814–1821. doi: 10.1038/mt.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang R., Zhang X., Ma B., Xiao B., Huang F., Huang P., Ying C., Liu T., Wang Y. Enhanced antitumor effect of combining TRAIL and MnSOD mediated by CEA-controlled oncolytic adenovirus in lung cancer. Cancer Gene Ther. 2016;23:168–177. doi: 10.1038/cgt.2016.11. [DOI] [PubMed] [Google Scholar]
- 95.Yang Y.F., Xue S.Y., Lu Z.Z., Xiao F.J., Yin Y., Zhang Q.W., Wu C.T., Wang H., Wang L.S. Antitumor effects of oncolytic adenovirus armed with PSA-IZ-CD40L fusion gene against prostate cancer. Gene Ther. 2014;21:723–731. doi: 10.1038/gt.2014.46. [DOI] [PubMed] [Google Scholar]
- 96.Zitvogel L., Galluzzi L., Kepp O., Smyth M.J., Kroemer G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015;15:405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 97.Zamarin D., Wolchok J.D. Potentiation of immunomodulatory antibody therapy with oncolytic viruses for treatment of cancer. Mol. Ther. Oncolytics. 2014;1:14004. doi: 10.1038/mto.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DiPaola R.S., Plante M., Kaufman H., Petrylak D.P., Israeli R., Lattime E., Manson K., Schuetz T. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J. Transl. Med. 2006;4:1. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zamarin D., Holmgaard R.B., Ricca J., Plitt T., Palese P., Sharma P., Merghoub T., Wolchok J.D., Allison J.P. Intratumoral modulation of the inducible co-stimulator ICOS by recombinant oncolytic virus promotes systemic anti-tumour immunity. Nat. Commun. 2017;8:14340. doi: 10.1038/ncomms14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Passaro C., Alayo Q., De Laura I., McNulty J., Grauwet K., Ito H., Bhaskaran V., Mineo M., Lawler S.E., Shah K. Arming an oncolytic herpes simplex virus type 1 with a single-chain fragment variable antibody against PD-1 for experimental glioblastoma therapy. Clin. Cancer Res. 2019;25:290–299. doi: 10.1158/1078-0432.CCR-18-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang H., Rivera-Molina Y., Gomez-Manzano C., Clise-Dwyer K., Bover L., Vence L.M., Yuan Y., Lang F.F., Toniatti C., Hossain M.B., Fueyo J. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Cancer Res. 2017;77:3894–3907. doi: 10.1158/0008-5472.CAN-17-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee E.S., Jin S.Y., Kang B.K., Jung Y.T. Construction of replication-competent oncolytic retroviral vectors expressing R peptide-truncated 10A1 envelope glycoprotein. J. Virol. Methods. 2019;268:32–36. doi: 10.1016/j.jviromet.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 103.Rouanet M., Lebrin M., Gross F., Bournet B., Cordelier P., Buscail L. Gene therapy for pancreatic cancer: specificity, issues and hopes. Int. J. Mol. Sci. 2017;18:E1231. doi: 10.3390/ijms18061231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guffey M.B., Parker J.N., Luckett W.S., Jr., Gillespie G.Y., Meleth S., Whitley R.J., Markert J.M. Engineered herpes simplex virus expressing bacterial cytosine deaminase for experimental therapy of brain tumors. Cancer Gene Ther. 2007;14:45–56. doi: 10.1038/sj.cgt.7700978. [DOI] [PubMed] [Google Scholar]
- 105.Foloppe J., Kintz J., Futin N., Findeli A., Cordier P., Schlesinger Y., Hoffmann C., Tosch C., Balloul J.M., Erbs P. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008;15:1361–1371. doi: 10.1038/gt.2008.82. [DOI] [PubMed] [Google Scholar]
- 106.Green N.K., Youngs D.J., Neoptolemos J.P., Friedlos F., Knox R.J., Springer C.J., Anlezark G.M., Michael N.P., Melton R.G., Ford M.J. Sensitization of colorectal and pancreatic cancer cell lines to the prodrug 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954) by retroviral transduction and expression of the E. coli nitroreductase gene. Cancer Gener Ther. 1997;4:229–238. [PubMed] [Google Scholar]
- 107.Löhr M., Müller P., Karle P., Stange J., Mitzner S., Jesnowski R., Nizze H., Nebe B., Liebe S., Salmons B. Targeted chemotherapy by intratumour injection of encapsulated cells engineered to produce CYP2B1, an ifosfamide activating cytochrome P450. Gener Ther. 1998;5:1070–1078. doi: 10.1038/sj.gt.3300671. [DOI] [PubMed] [Google Scholar]
- 108.Yang X., Huang B., Deng L., Hu Z. Progress in gene therapy using oncolytic vaccinia virus as vectors. J. Cancer Res. Clin. Oncol. 2018;144:2433–2440. doi: 10.1007/s00432-018-2762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bressy C., Hastie E., Grdzelishvili V.Z. Combining oncolytic virotherapy with p53 tumor suppressor gene therapy. Mol. Ther. Oncolytics. 2017;5:20–40. doi: 10.1016/j.omto.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Russell L., Swanner J., Jaime-Ramirez A.C., Wang Y., Sprague A., Banasavadi-Siddegowda Y., Yoo J.Y., Sizemore G.M., Kladney R., Zhang J. PTEN expression by an oncolytic herpesvirus directs T-cell mediated tumor clearance. Nat. Commun. 2018;9:5006. doi: 10.1038/s41467-018-07344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koval O., Kochneva G., Tkachenko A., Troitskaya O., Sivolobova G., Grazhdantseva A., Nushtaeva A., Kuligina E., Richter V. Recombinant vaccinia viruses coding transgenes of apoptosis-inducing proteins enhance apoptosis but not immunogenicity of infected tumor cells. BioMed Res. Int. 2017;2017:3620510. doi: 10.1155/2017/3620510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiao T., Fan J.K., Huang H.L., Gu J.F., Li L.Y., Liu X.Y. VEGI-armed oncolytic adenovirus inhibits tumor neovascularization and directly induces mitochondria-mediated cancer cell apoptosis. Cell Res. 2010;20:367–378. doi: 10.1038/cr.2009.126. [DOI] [PubMed] [Google Scholar]
- 113.Guse K., Sloniecka M., Diaconu I., Ottolino-Perry K., Tang N., Ng C., Le Boeuf F., Bell J.C., McCart J.A., Ristimäki A. Antiangiogenic arming of an oncolytic vaccinia virus enhances antitumor efficacy in renal cell cancer models. J. Virol. 2010;84:856–866. doi: 10.1128/JVI.00692-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kang Y.A., Shin H.C., Yoo J.Y., Kim J.H., Kim J.S., Yun C.O. Novel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirus. Mol. Ther. 2008;16:1033–1040. doi: 10.1038/mt.2008.63. [DOI] [PubMed] [Google Scholar]
- 115.Adelfinger M., Bessler S., Frentzen A., Cecil A., Langbein-Laugwitz J., Gentschev I., Szalay A.A. Preclinical testing oncolytic vaccinia virus strain GLV-5b451 expressing an anti-VEGF single-chain antibody for canine cancer therapy. Viruses. 2015;7:4075–4092. doi: 10.3390/v7072811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Choi J.W., Jung S.J., Kasala D., Hwang J.K., Hu J., Bae Y.H., Yun C.O. pH-sensitive oncolytic adenovirus hybrid targeting acidic tumor microenvironment and angiogenesis. J. Control. Release. 2015;205:134–143. doi: 10.1016/j.jconrel.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fan J.K., Xiao T., Gu J.F., Wei N., He L.F., Ding M., Liu X.Y. Increased suppression of oncolytic adenovirus carrying mutant k5 on colorectal tumor. Biochem. Biophys. Res. Commun. 2008;374:198–203. doi: 10.1016/j.bbrc.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 118.Liu T.C., Zhang T., Fukuhara H., Kuroda T., Todo T., Canron X., Bikfalvi A., Martuza R.L., Kurtz A., Rabkin S.D. Dominant-negative fibroblast growth factor receptor expression enhances antitumoral potency of oncolytic herpes simplex virus in neural tumors. Clin. Cancer Res. 2006;12:6791–6799. doi: 10.1158/1078-0432.CCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 119.Hardcastle J., Kurozumi K., Dmitrieva N., Sayers M.P., Ahmad S., Waterman P., Weissleder R., Chiocca E.A., Kaur B. Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Mol. Ther. 2010;18:285–294. doi: 10.1038/mt.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He X.-P., Su C.-Q., Wang X.-H., Pan X., Tu Z.-X., Gong Y.-F., Gao J., Liao Z., Jin J., Wu H.-Y. E1B-55kD-deleted oncolytic adenovirus armed with canstatin gene yields an enhanced anti-tumor efficacy on pancreatic cancer. Cancer Lett. 2009;285:89–98. doi: 10.1016/j.canlet.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 121.Sivanandam V., LaRocca C.J., Chen N.G., Fong Y., Warner S.G. Oncolytic viruses and immune checkpoint inhibition: the best of both worlds. Mol. Ther. Oncolytics. 2019;13:93–106. doi: 10.1016/j.omto.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ajina A., Maher J. Prospects for combined use of oncolytic viruses and CAR T-cells. J. Immunother. Cancer. 2017;5:90. doi: 10.1186/s40425-017-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Cathail S.M., Pokrovska T.D., Maughan T.S., Fisher K.D., Seymour L.W., Hawkins M.A. Combining oncolytic adenovirus with radiation—a paradigm for the future of radiosensitization. Front. Oncol. 2017;7:153. doi: 10.3389/fonc.2017.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roulstone V., Pedersen M., Kyula J., Mansfield D., Khan A.A., McEntee G., Wilkinson M., Karapanagiotou E., Coffey M., Marais R. BRAF- and MEK-targeted small molecule inhibitors exert enhanced antimelanoma effects in combination with oncolytic reovirus through ER stress. Mol. Ther. 2015;23:931–942. doi: 10.1038/mt.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Puzanov I., Milhem M.M., Minor D., Hamid O., Li A., Chen L., Chastain M., Gorski K.S., Anderson A., Chou J. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J. Clin. Oncol. 2016;34:2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E. Oncolytic virotherapy promotes intratumoral t cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanoue K., Rosewell Shaw A., Watanabe N., Porter C., Rana B., Gottschalk S., Brenner M., Suzuki M. Armed oncolytic adenovirus-expressing PD-L1 mini-body enhances antitumor effects of chimeric antigen receptor t cells in solid tumors. Cancer Res. 2017;77:2040–2051. doi: 10.1158/0008-5472.CAN-16-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Watanabe K., Luo Y., Da T., Guedan S., Ruella M., Scholler J., Keith B., Young R.M., Engels B., Sorsa S. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight. 2018;3:99573. doi: 10.1172/jci.insight.99573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fajardo C.A., Guedan S., Rojas L.A., Moreno R., Arias-Badia M., de Sostoa J., June C.H., Alemany R. Oncolytic adenoviral delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Cancer Res. 2017;77:2052–2063. doi: 10.1158/0008-5472.CAN-16-1708. [DOI] [PubMed] [Google Scholar]
- 130.Guedan S., Ruella M., June C.H. Emerging cellular therapies for cancer. Annu. Rev. Immunol. 2019;37:145–171. doi: 10.1146/annurev-immunol-042718-041407. [DOI] [PMC free article] [PubMed] [Google Scholar]