Abstract
Stauffer's syndrome is a paraneoplastic phenomenon associated with renal cell carcinoma (RCC) characterized by cholestatic hepatitis. We explore the effects of perioperative immunotherapy in a case of Stauffer's syndrome. A 70-year-old female with a locally advanced clear cell RCC (ccRCC) developed severe hyperbilirubinemia. The patient's cholestasis progressed despite initial systemic immunotherapy, but improved after cytoreductive nephrectomy. The patient continued immunotherapy post-operatively and regained normalized hepatic function. To our knowledge, this is the first case reporting use of systemic immunotherapy with surgery in Stauffer's syndrome, and we provide clinical insight into a treatment regimen which may be employed in future cases.
Keywords: Renal cell carcinoma, Stauffer's syndrome, Immunotherapy
Introduction
Paraneoplastic hepatopathy associated with RCC was first described in 1961.1 The syndrome has been designated as Stauffer's syndrome and entails cholestatic liver dysfunction in the absence of underlying hepatobiliary disease. It is estimated that hepatic dysfunction, in the absence of liver metastases, occurs in 10–15% of RCC cases.
Stauffer's syndrome classically presents with elevated total bilirubin (TB), direct bilirubin (DB), and alkaline phosphatase (ALP). It is associated with an unfavorable prognosis. It is theorized that the hepatic dysfunction is immunogenic in etiology and due to overexpression of interleukin-6 from the tumor.2
Treatment entails removing the source of the paraneoplastic syndrome via nephrectomy. Some reports suggest resolution of the hepatopathy upon removal of the tumor,3 and there is a consensus that for localized RCC, radical nephrectomy represents the optimal treatment. However, for patients with mRCC management is uncertain, and to our knowledge there are no reports in the literature outlining a case of mRCC and employment of systemic immunotherapy. Herin, we present a case of mRCC complicated by severe cholestatic hepatitis treated with immunotherapy and surgery.
Case presentation
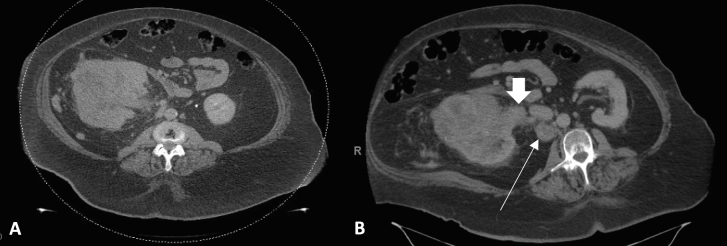
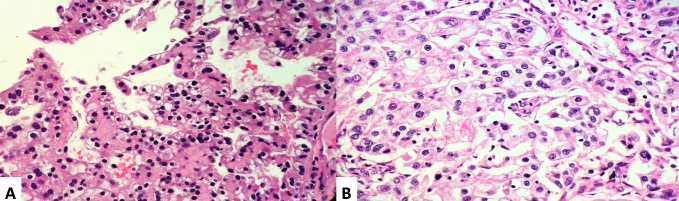
A 70-year-old female developed hematuria and underwent a computed tomography (CT) scan of the abdomen/pelvis. Imaging revealed a normal sized liver, no intrahepatic or extrahepatic bile duct dilation, gallbladder with no calcified stones or abnormal wall thickness, and an unremarkable pancreas without ductal dilation. There was a large mass replacing much of the right kidney which measured 11.7 x 10.8 × 11.9cm; there was invasion into the right renal vein with a R.E.N.A.L. Nephrometry score of 12xh (Fig. 1a). Additionally, there was an enlarged retrocaval lymph node measuring 3.9 × 3.6cm (Fig. 1b), a right adrenal nodule, and several basilar lung nodules which were consistent with metastatic disease. An ultrasound-guided core biopsy revealed ccRCC WHO/ISUP grade 2 in a background of extensive tumor necrosis, fibrosis, and inflammation (Fig. 2a).
Fig. 1.
CT abdomen and pelvis with contrast demonstrating (A) an 11.7 x 10.8 x 11.9 right renal mass replacing the majority parenchyma with R.E.N.A.L. Nephrometry score of 12xh; (B) enlarged metastatic retrocaval lymph node measuring 3.9 × 3.6 cm (indicated by thin arrow) and right renal vein tumor thrombus (indicated by the wide arrow).
Fig. 2.
H + E stained sections showing: (A) biopsy specimen with clear cell renal cell carcinoma, WHO/ISUP grade 2; (B) resection specimen shows clear cell renal cell carcinoma, WHO/ISUP grade 4 with larger, eosinophilic nucleoli, pleomorphic nuclei, and numerous mitotic figures.
Soon thereafter, the patient developed scleral icterus, pruritis, and light-colored stools. Lab work was notable for a TB of 5.6mg/dL, ALP 397 U/L, aspartate aminotransferase (AST) 263 U/L, and alanine aminotransferase (ALT) 296 U/L. Magnetic resonance angiography identified tumor thrombus extending into the right renal vein up to its confluence with the inferior vena cava (IVC), without IVC involvement.
The patient's TB and DB concentrations continuously rose and a decision was made to offer a litmus test of emergent systemic therapy. Due to the theoretical risk of hepatotoxicity with employing tyrosine-kinase inhibitors (TKI) in the setting of severe hyperbilirubinemia, the patient was treated with one cycle of the programmed death-1 (PD-1) inhibitor nivolumab followed two weeks later by nivolumab with the cytotoxic T-lymphocyte associated protein-4 (CTLA-4) inhibitor ipilimumab. The hyperbilirubinemia worsened to a TB of 19.7. Ultimately, the decision was made to pursue cytoreductive nephrectomy (CN). The patient underwent open radical nephrectomy, adrenalectomy, tumor thrombectomy, resection of radiographically pathologic nodal tissue, and liver biopsy. Final surgical pathology was consistent with ccRCC WHO/ISUP grade 4, 10cm with areas of necrosis, fibrosis and inflammation, hilar fat with fibrosis and chronic inflammation; and negative renal vein margin (Fig. 2b). The retrocaval lymph node and tumor thrombus excisions revealed extensive fibrosis and focal granulomatous-like inflammation with no viable tumor seen. Intra-operative liver biopsy identified cholestasis with no tumor infiltration.
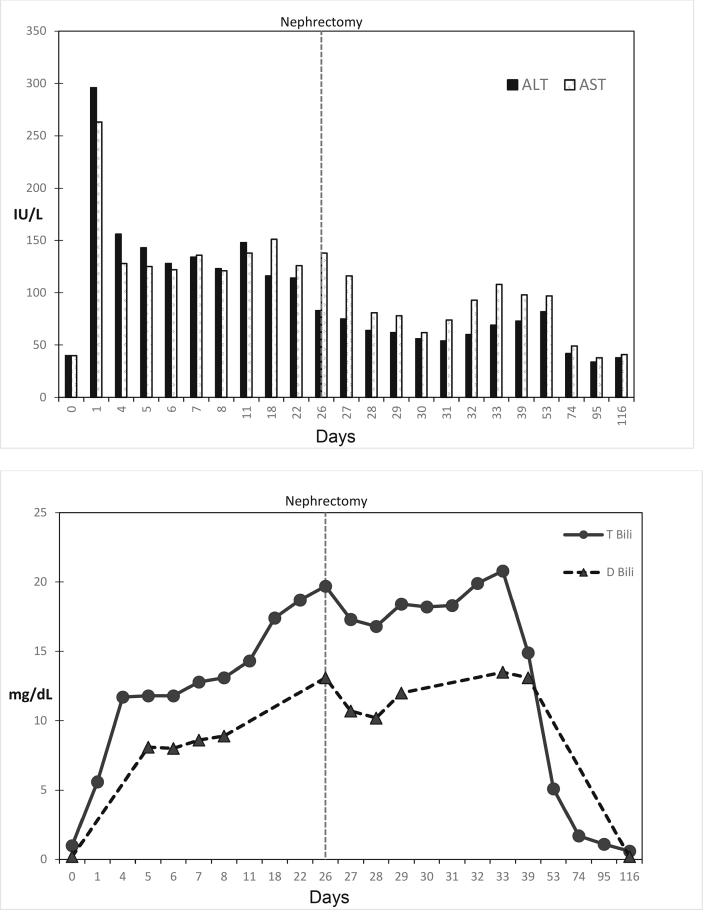
Two weeks post-operatively, there was a steady downtrend in the TB with normalization of liver function tests within 10 weeks (Fig. 3a and b). The patient completed three more cycles of nivolumab/ipilimumab therapy. CT restaging revealed no clear measurable disease consistent with a complete response. The patient has since been undergoing disease surveillance.
Fig. 3.
Serial liver function tests. x-axis denotes days into therapy, Surgical removal of the tumor occurred on day 26 as indicated by the vertical dotted line; (A) y concentrations of ALT, and AST as indicated in the bar graphs (IU/L); (B) concentration of total and direct bilirubin (mg/dL).
Discussion
Stauffer's syndrome is a paraneoplastic of RCC that presents with hepatic cholestasis. The accepted approach to management, at least in the setting of localized disease, remains removing the primary tumor with nephrectomy.3 However, the treatment of mRCC has undergone dramatic changes in recent years. Whereas neoadjuvant CN has been the standard of care in mRCC, in the face of recent evidence from the CARMENA and SURTIME trials, we know with relative certainty that some patients with intermediate-and poor-risk mRCC may be harmed by upfront nephrectomy. Treatment guidelines recommend starting with a systemic therapy litmus test.4,5 For patients with Stauffer's syndrome, the treatment paradigm becomes more complex. Multiple systemic therapies are approved for first-line treatment of mRCC, however, nearly all the regimens target angiogenesis via TKIs. TKIs harbor their own risk of hepatotoxicity, and their use is relatively contraindicated in the setting of hyperbilirubinemia. Immune checkpoint blockade (ICB) represents a newer, feasible treatment option available in the current treatment landscape of mRCC.
This case demonstrates that ICB may be safely employed in patients with RCC associated Stauffer's syndrome. Our patient experienced a complete response after perioperative nivolumab and ipilimumab, and surgical resection of the primary tumor and largest ipsilateral lymph node. Final surgical pathology revealed no residual tumor in the resected lymph node or tumor thrombus which is presumably the effect of the ICB therapy, and the primary tumor harbored extensive necrosis and fibrosis associated with chronic inflammation. Although the cholestatic hepatitis did not improve until surgical nephrectomy, neoadjuvant ICB served as a safe and effective conduit to CN. Given the significant pathologic, clinical, and radiologic response, it is tempting to wonder if the patient's outcome may have been similar with systemic treatment alone. Since she was considered surgically resectable in the setting of worsening hyperbilirubinemia, it was ultimately determined that CN was the best chance to attempt to reverse the paraneoplastic process. However, in our opinion, in a patient scenario with mRCC and either more extensive metastatic disease, or with an inoperable patient, combination ICB represents a safe and reasonable approach, with surgery reserved for later if the risk/benefit assessment changes.
Conclusion
This report highlights a unique case of paraneoplastic Stauffer's syndrome in mRCC. Although clinicians may be enticed to forgo systemic treatments in Stauffer's syndrome due to the toxicities of anti-angiogenesis, the precarious nature of ICB, and worsening hepatic function, we provide an outline for a potentially successful treatment regimen with durable tumor response and safe outcome.
Declaration of competing interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgments
We would like to thank Eli Barshan, MD, for his contributions to the report. We would also like to thank Susan Roethke, NP, for her contributions and skillful editing of this report.
Abbreviations
- RCC
renal cell carcinoma
- ccRCC
clear cell RCC
- TB
total bilirubin
- DB
direct bilirubin
- ALP
alkaline phosphatase
- mRCC
metastatic RCC
- CT
computed tomography
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- IVC
inferior vena cava
- TKI
tyrosine-kinase inhibitors
- PD-1
programmed death 1
- CTLA-4
cytotoxic T-lymphocyte associated protein 4
- CN
cytoreductive nephrectomy
- ICB
immune checkpoint blockade
References
- 1.MH S: Nephrogenic hepatomegaly. Gastroenterology 2961, 40:694.
- 2.Blay J.Y., Rossi J.F., Wijdenes J. Role of interleukin-6 in the paraneoplastic inflammatory syndrome associated with renal-cell carcinoma. Int J Cancer. 1997;72(3):424–430. doi: 10.1002/(sici)1097-0215(19970729)72:3<424::aid-ijc9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Morla D., Alazemi S., Lichtstein D. Stauffer's syndrome variant with cholestatic jaundice: a case report. J Gen Intern Med. 2006;21(7):C11–C13. doi: 10.1111/j.1525-1497.2006.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mejean A., Ravaud A., Thezenas S. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379(5):417–427. doi: 10.1056/NEJMoa1803675. [DOI] [PubMed] [Google Scholar]
- 5.Bex A., Mulders P., Jewett M. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial. JAMA Oncol. 2019;5(2):164–170. doi: 10.1001/jamaoncol.2018.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]