Abstract
Amiodarone is an anti-arrhythmic drug widely used, but its administration can be associated with several adverse side-effects. Among these, amiodarone-induced pulmonary toxicity (APT) occurs in 4–17% of cases and, if not early diagnosed and treated, may evolve towards pulmonary fibrosis and respiratory failure.
A 76 years-old-man went to the hospital for accidental trauma. The patient did not report respiratory symptoms but was suffering from atrial fibrillation treated with amiodarone 200 mg/day from three years (cumulative dose >150 gr). HRCT showed ground-glass opacities and nodules in both lungs. The patient underwent fibreoptic bronchoscopy with BAL. Cytologic examination of BALF sediment put in evidence foamy macrophages. The electronic microscopy revealed into the alveolar macrophages “… the presence of multilamellar intracytoplasmic bodies and lysosomes, loads of lipid material”. LFTs showed a restrictive syndrome and an impairment of DLCO. Amiodarone discontinuation and steroid administration led to the regression of radiological lesions and the recovery of lung function.
Patients taking amiodarone can experience APT. They should perform a basal chest x-ray with LFTs before starting therapy. Monitoring could reveal early the pulmonary toxicity, and patients can respond favourably to the treatment.
Keywords: Amiodarone pulmonary toxicity, Pneumonia, Steroids, Cardiac arrhythmias
1. Introduction
Amiodarone hydrochloride is a very effective drug used to treat and prevent a wide range of cardiac arrhythmias [1,2]. This drug and its metabolite desethylamiodarone have a long half-life (55–60 days) and a lipophilic nature that leads them to accumulate in the tissues at high concentrations and to interfere with the metabolism of phospholipids [3]. Furthermore, adverse side effects can arise or even organ toxicity [[4], [5], [6], [7], [8]]. The most common is related to microdeposits in corneal and thyroid tissue, appearing in 85–100% of patients who receive chronic treatment. The incidence of amiodarone pulmonary toxicity (APT) varies from about 4 to 17% of patients and is related to dosage, age of the patient, and pre-existing lung diseases. These effects reach a plateau with a cumulative-dose >150 gr. Underlying pathologies, general anaesthesia, oxygen administration, invasive or surgical procedures seem to promote the onset of amiodarone-induced pulmonary toxicity [[9], [10], [11], [12]]. APT can cause a variety of clinical presentation, from alveolar/interstitial pneumonitis with a subacute onset to respiratory distress with severe hypoxemia [6,13]. In this context, chest x-rays and high-resolution computed tomography (HRCT) are essential for the diagnosis, revealing changes in pulmonary parenchyma, as ground-glass opacities, or fibrosis areas, or nodules. Also, lung function tests put in evidence a restrictive syndrome with an impaired diffusion of the lung for carbon monoxide (DLCO) [[3], [4], [5], [6], [7], [8]].
In this paper, we describe a patient who received a chronic home dose of amiodarone for more than three years (cumulative dose >150gr) and showed pulmonary toxicity. APT came back with drug discontinuation and steroids.
2. Case presentation
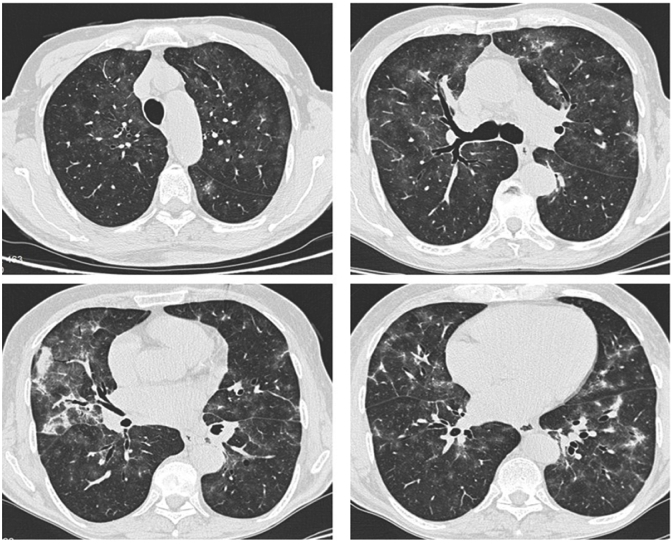
A 76 years old man, never smoker, went to an Emergency Department in July 2016 (three years ago) for an accidental fall. The patient performed chest x-ray that evidenced bilateral pulmonary opacities, but without significant respiratory symptoms. Antibiotics gave poor results, and he underwent HRCT. It revealed multiple confluent ground-glass opacities associated with regions of alveolar filling in both upper lobes, middle one, in the lingula, and the basal segment of the lower area (Fig. 1).
Fig. 1.
Baseline CT scans showing bilateral ground-glass opacities and pulmonary consolidation.
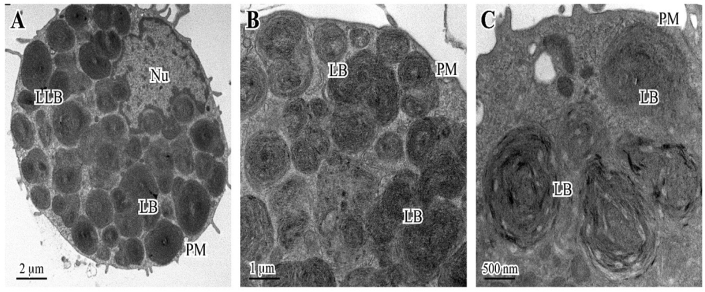
From medical history emerged that the patient was taking amiodarone 200 mg once daily from more than three years for atrial fibrillation. He underwent fiberoptic bronchoscopy, and bronchoalveolar lavage (BAL) in the middle lobe got a recovery of 50%. The cytologic exam on the sediment did not show atypical cells. Cell count/ml was equal to 200,000/mm3, with 51% of macrophages, 24% of lymphocytes, 5% of neutrophils and 20% of eosinophils. BALF specimens did not reveal any bacterial growth. Electron microscopy analysis showed alveolar macrophages with multilamellar intracytoplasmic bodies and lysosomes loads of lipid material; sometimes, this material also precipitates in the form of crystals” (Fig. 2).
Fig. 2.
Transmission electron micrographs of bronchoalveolar lavage fluid of the case report. Ultrastructural analysis showed “… presence of cells in large part consisting of alveolar macrophages with multilamellar intracytoplasmic bodies and lysosomes loads of lipid material that, sometimes, also precipitates in the form of crystals”.
(A) Alveolar macrophage with many lysosomes and lamellated inclusions. (B–C) Details of macrophage cytoplasm containing large lysosomal bodies, no uniform in size. Legend: Nu, nucleus; PM, plasma membrane; LB, lysosomal bodies. [TEM, uranyl acetate/lead citrate; Morgagni 268D Electron Microscopy - FEI Company, Hillsboro].
Lung function tests showed a restrictive syndrome (Table 1, A); also, the DLCO was impaired (61%).
Table 1.
Lung function tests before (A) and after three months of amiodarone withdrawal and steroid therapy (B). The assessment showed a restrictive basal syndrome and a reduced diffusing capacity for CO (A); data returned to normal after treatment (B).
| A | B | |
|---|---|---|
| FVC (%) | 3.14 (75%) | 4.02 (96%) |
| FEV1 (%) | 2.40 (76%) | 3.09 (98%) |
| FEV1/FVC.100 | 74.1 | 73.9 |
| RV (%) | 2.15 (77%) | 2.68 (96%) |
| TLC (%) | 5.81 (78%) | 7.01 (94%) |
| VR/TLC .100 | 31.10 | 32.32 |
| DLCO/VA | 0.67 (61%) | 1.11 (101%) |
FVC (forced vital capacity), FEV1 (forced expiratory volume in the 1st second), RV (residual volume), TLC (total lung capacity) are expressed as litres and % of theoretical (in brackets); FEV1/FVC.100 and VR/TLC 0.100: are expressed as a percentage; DLCO/VA (diffusing capacity of the lung/alveolar ventilation) is shown as mmol/min/kPa/L ad % of theoretical (in brackets).
The lung lesions were attributed to APT considering the characteristics of the pathology (history, clinical and radiological and functional presentation, electron microscopy results on BALF sediment).
Since the cytotoxic drug-induced damage appeared to progress despite the lack of related symptoms, an empirical course of steroids seemed appropriate even in light of the data in the literature (8–9,13). In our case, the HRCT and LFTs data appeared severely compromised and to avoid progression to fibrosis, we adopt a treatment based on the discontinuation of the drug associated with steroids administration. The aim was to favour the reabsorption of the inflammatory lesions and quickly avoid his possible evolution. So, the patient stopped amiodarone and started prednisone intake for eight months (12,5 mg once a day) monitoring laboratory parameters.
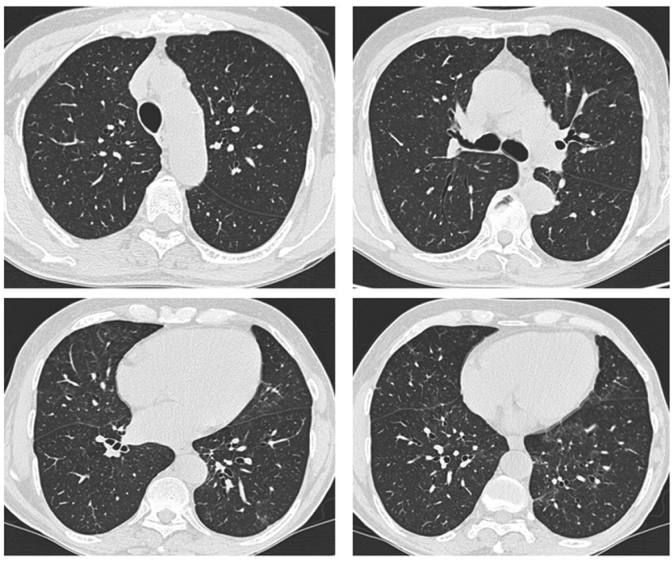
Quickly, CT scans showed complete resolution, and LFTs with the DLCO returned to the standard value (Fig. 3; Table 1, B). HRCT check confirmed results two years after steroids discontinuation.
Fig. 3.
CT control of the case-report showing resolution of opacities and pulmonary consolidation after steroid therapy for three months.
The patient signed informed consent for the publication of his clinical details and radiological images.
3. Discussion and conclusions
We have described a case suffering from amiodarone-induced pulmonary toxicity (APT) with an excellent clinical response to drug discontinuation and steroids administration.
Amiodarone is a drug widely used in the treatment of cardiac arrhythmias, but sometimes it can cause side effects, interesting all the organs [1,2,8]. Pulmonary toxicity is less frequent than thyroid and eye involvement but is potentially the most dangerous event for the patient leading to respiratory failure or even death [[3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]]. Lung toxicities may occur both in acute and in subacute/chronic forms. Among the first events, we can expect bronchospasm, bronchial asthma exacerbation, interstitial pneumonia, ARDS, diffuse alveolar haemorrhage; among the second events, we can expect lipoid pneumonia, chronic eosinophilic pneumonia, nodules or pulmonary masses. Also, amiodarone toxicity may induce pleural effusions or thickening [6,8].
The clinical presentation can range from the absence of respiratory symptoms to acute onset or chronic evolution. Radiological manifestations of pulmonary toxicity, without a strong clinical suspicion, are frequently not recognised. Risk factors for pulmonary toxicity include a high cumulative dose, duration of therapy exceeding two months (but sometimes even after a few days), existing lung diseases, increased patient age, surgery and pulmonary angiography [[8], [9], [10], [11], [12]].
Lung function tests typically show a restrictive syndrome. A reduction in DLCO more than 15% is a reliable indicator for amiodarone-induced pulmonary toxicity, with high sensitivity and specificity [4,5,8]. Lung biopsy, alveolar cytogram, foamy macrophages in BAL can help in the formulation of the diagnosis, but this is an exclusion diagnosis. Today, no tests are available for establishing the onset of APT and the diagnosis. To make a clinical diagnosis of APT requires the exclusion of other diagnostic possibilities (especially congestive heart failure) together with a reasonable constellation of symptoms or findings.
Pulmonary imaging is essential. The high-resolution CT scan reveals ground-glass opacities, localised or diffuse parenchymal infiltrates mono or bilateral, parenchymal nodules, pneumonia, pleural thickening and effusions.
Once made diagnosis of APT, the clinicians have a limited number of therapeutic options.
Firstly, it is necessary to stop drug intake and replace it with another antiarrhythmic agent or arrange other possibilities, in agreement with the cardiologist. If the toxicity manifestations are limited, usually the suspension alone of the drug leads to clinical and radiological improvement. But if the signs of toxicity are of a relevant entity or if chest x-ray changes do not regress, systemic corticosteroids are recommended for APT although controlled trials are lacking. Prednisolone is usually used at a dose of 0.5/1mg per Kg of weight and for a long time, up to a year. The goal is the regression of complication and complete recovery. In severe APT, the therapy can range from steroids at a high dose to mechanical ventilation. To underline that after the initial attack phase, corticosteroids should be continued for a long time, checking the evolution of clinical data, blood gases, LFTs and chest x-ray [4,5,[8], [9], [10], [11], [12], [13]]. Pulmonary toxicity may initially progress despite drug stopping or even recur upon steroid withdrawal, probably for the long half-life of amiodarone. The clinical answer depends on the individual sensitivity to treatment and the prolonged persistence of the drug at high concentration in the lung tissue, even over 90 days [14].
In this report, we focused our attention to a patient suffering from APT. He had taken amiodarone 200 mg once a day for more years for atrial fibrillation (cumulative dose > 150 gr). At admission in hospital for an accidental trauma, he did not have respiratory symptoms. But HRCT showed widespread ground-glass opacities and pulmonary nodules, while LFTs revealed a restrictive syndrome and a reduced DLCO, more than 15%. Cytology and electronic microscopy performed on BALF revealed foamy macrophages, lipid-laden. The drug discontinuation and steroids administration for a long time, even if at low doses to avoid the side effects of cortisone, shortly led to the disappearance of symptoms and the improvement of radiological and functional findings. Controls after two years did not reveal any relapse.
In conclusion, diagnosis of APT is not easy because of multiform clinical aspects of this complication. Indeed, we must consider it among the differential diagnoses in patients who use of amiodarone, and with progressive or acute respiratory symptoms or who are presenting radiological interstitial lesions, also in the absence of symptoms. There is no diagnostic test pathognomonic for APT, but this is a diagnosis of exclusion made on clinical, laboratory and radiographic findings. All patients taking amiodarone should perform a chest x-ray, and LFTs before starting therapy, and then patients should be followed carefully and monitored appropriately. With a prompt diagnosis, patients can respond favourably to drug discontinuation and steroids administration. Of course, this statement requires controlled studies based on a large number of cases.
Author's contribution
(a designed study, b-collected data, c-wrote the paper, d - ultrastructural analysis)
Terzo Fabriziob,c, Ricci Albertoa,c, D'Ascanio Michelab,c, Raffa Salvatorec,d, Mariotta Salvatorea,b,c
Declarations
-
•
The informed consent for publication, signed by the patient, is available from the corresponding author;
-
•
The data used for the current study are available from the corresponding author;
-
•
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare no conflict of interest.
Contributor Information
Fabrizio Terzo, Email: fabrizio.terzo@uniroma1.it.
Alberto Ricci, Email: alberto.ricci@uniroma1.it.
Michela D'Ascanio, Email: dascaniomichela87@gmail.com.
Salvatore Raffa, Email: salvatore.raffa@uniroma1.it.
Salvatore Mariotta, Email: salvatore.mariotta@uniroma1.it.
List of abbreviations
- APT
amiodarone-induced pulmonary toxicity
- LFTs
lung function tests
- HRCT
high resolution computed tomography
- DLCO
diffusing lung capacity for carbon monoxide
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
References
- 1.Marcus F.I., Fontaine G.H., Frank R., Grosgogeat Y. Clinical pharmacology and therapeutic applications of the antiarrhythmic agent, amiodarone. Am. Heart J. 1981;101:480–493. doi: 10.1016/0002-8703(81)90140-x. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda N., Nademanee K., Kannan R., Singh B.N. Electrophysiologic effects of amiodarone: experimental and clinical observation relative to serum and tissue drug concentrations. Am. Heart J. 1984;108:890–898. doi: 10.1016/0002-8703(84)90451-4. [DOI] [PubMed] [Google Scholar]
- 3.Brien F., Jimmo S., Brennan F.J., Ford S.E., Armstrong P.W. Distribution of amiodarone and its metabolite, desethylamiodarone, in human tissues. Can. J. Physiol. Pharmacol. 1987;65:360–364. doi: 10.1139/y87-062. [DOI] [PubMed] [Google Scholar]
- 4.Van Erven L., Schalij M.J. Amiodarone: an effective antiarrhythmic drug with unusual side effects. Heart. 2010;96:1593–1600. doi: 10.1136/hrt.2008.152652. [DOI] [PubMed] [Google Scholar]
- 5.Pitcher W.D. Amiodarone pulmonary toxicity. Am. J. Med. Sci. 1992;303:206–212. doi: 10.1097/00000441-199203000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Camus P., Fanton A., Bonniaud P., Camus C., Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71:301–326. doi: 10.1159/000079633. [DOI] [PubMed] [Google Scholar]
- 7.Schwaiblmair M., Berghaus T., Haeckel T., Wagner T., von Scheidt W. Amiodarone-induced pulmonary toxicity: an under-recognized and severe adverse effect? Clin. Res. Cardiol. 2010;99:693–700. doi: 10.1007/s00392-010-0181-3. [DOI] [PubMed] [Google Scholar]
- 8.Papiris S.A., Triantafillidou C., Kolilekas L., Markoulaki D., Manali E.D. Amiodarone: review of pulmonary effects and toxicity. Drug Saf. 2010;33:539–558. doi: 10.2165/11532320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Lee W., Ryu D.R., Han S.S., Ryu S.W., Cho B.R., Kwon H., Kim B.R. Very early onset of amiodarone-induced pulmonary toxicity. Korean Circ. J. 2013;43:699–701. doi: 10.4070/kcj.2013.43.10.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handschin A.E., Lardinois D., Schneiter D., Bloch K., Weder W. Acute amiodarone-induced pulmonary toxicity following lung resection. Respiration. 2003;70:310–312. doi: 10.1159/000072016. [DOI] [PubMed] [Google Scholar]
- 11.Baumann H., Fichtenkamm P., Schneider T., Biscoping J., Henrich M. Rapid onset of amiodarone-induced pulmonary toxicity after lung lobe resection - a case report and review of recent literature. Ann. Med. Surg. (Lond) 2017;21:53–57. doi: 10.1016/j.amsu.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teerakanok J., Tantrachoti P., Chariyawong P., Nugent K. Acute Amiodarone pulmonary toxicity after surgical procedures. Am. J. Med. Sci. 2016;352:646–651. doi: 10.1016/j.amjms.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Garg J., Agrawal N., Marballi A., Agrawal S., Rawat N., Sule S., Lehrman S.G. Amiodarone induced pulmonary toxicity: an unusual response to steroids. Am. J. Case Rep. 2012;13:62–65. doi: 10.12659/AJCR.882757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esinger W., Schleiffer T., Leinberger H., Hertrich F., Köhler G., Gröger C. Raute-Kreinsen U Steroid-refractory amiodarone-induced pulmonary fibrosis. Clinical features and morphology after an amiodarone-free interval of 3 months. Dtsch. Med. Wochenschr. 1988;113:1638–1641. doi: 10.1055/s-2008-1067863. [DOI] [PubMed] [Google Scholar]