Abstract
Introduction
Fever is managed using synthetic drugs such as aspirin, paracetamol among others. Synthetic drugs are associated with many side effects. Herbal medicines form alternative therapy since they possess fewer side effects and are readily available. This study aimed to determine antipyretic potential of DCM extracts of E. globulus and S. didymobotrya in Swiss albino rats.
Materials and methods
The plant leaves samples were obtained from Embu County, Kenya. Dichloromethane solvent was used to extract bioactive constituents from the plant samples. Three grams of DCM leaf extracts of Eucalyptus globulus (Labill) and Senna didymobotrya (Fresenius) samples were obtained and analyzed to determine quantitative phytochemical composition at ICIPE laboratory using GC-MS. Albino rats were used in the antipyretic activity study. Nine groups of five experimental animals were used in each test: Positive control, normal control, negative control and experimental (25, 50, 100, 150, 200 and 250 mg/kg body weight extracts) groups. Pyrexia was induced by injection of turpentine in albino rats intraperitoneally. One hour later, the pyretic animals received the leaf extracts at various dose levels, reference drug (aspirin100 mg/kg body) or the vehicle (DMSO).
Results
Results of antipyretic in vivo bioscreening revealed that E. globulus and S. didymobotrya possess potent antipyretic activity which was comparable to that of the reference drug aspirin. Both extracts exhibited highest antipyretic activity at a dose of 250 mg/kg bw. Results of the GC-MS revealed that these plants possess bio-compounds such as Terpinolene, Alpha-pinene, Borneol, Globulol and Terpineols that are associated with antipyretic activity.
Conclusions
In conclusion, this study revealed that these plants are endowed with bioactive compounds such as terpenoids, and flavonoids that possess antipyretic activity in rats.
Keywords: E.globulus, S.didymobotrya, Pyrexia, Veterinary medicine
Biochemistry; Toxicology; Veterinary medicine; Health sciences; Pharmaceutical science; Pharmacology.
1. Introduction
Fever refers to the body's temperature that is higher than the normal range due to an increase in set-point temperature in the hypothalamus [1]. It is a common sign in medicine that shows body temperature elevation above the standard range of 36.5–37.5 °C [2,3]. Increased prostaglandin E2 (PGE2) biosynthesis in the hypothalamic pre-optic region alters the neuron firing rate, leading to fever induction [4].
Anti-pyretic drugs inhibit the expression of COX-2, leading to the reduction in the PGE2 biosynthesis, which is the major fever mediator [5]. Anti-pyretic drugs have an inhibitory activity on prostaglandin biosynthesis but do influence body temperature if the elevation is caused by an increase in ambient body temperature or body exercise [6]. Other therapeutic agents employed in the treatment of pyrexia include steroids and opioids among others. Fever is managed using antipyretic agents such as diclofenac, aspirin and paracetamol [7].
Other non-conventional interventions employed in fever management include removal of clothes from the patients to expose them to lose heat to the environment. In addition, the patient may undergo massage using a sponge that has been dipped in warm water. This helps in conductive heat loss [8].
Medicinal plants also form an integral part in the management of fever. A wide variety of these medicinal plants are currently used in the management of fever namely Acacia hockii and Kigelia africana [9], Cissus quadrangularis [10], Urtica diocia [11] among others. E. globulus and S. didymobotrya are plants that have numerous medicinal values. E. globulus is used in the treatment of bronchitis, cancer, arthritis, asthma, boils, cold, cough, diabetes, dysentery, dyspepsia, malaria, sore throat, tuberculosis, vaginitis and wounds [12] while S. didymobotrya is used in management of skin infections, malaria, ringworm, jaundice, intestinal worm, bacterial infections, fungal, sickle cell anemia, haemorrhoids and hypertension [13]. E. globulus and S. didymobotrya plants have many medicinal uses. People in Embu County use these plants traditionally in the management of fever. However, no preliminary scientific research has been done to bioscreen these them for antipyretic activity. It is against this background that this study was conceived and designed to explore the antipyretic potential of the DCM leaf extract of E. globulus and S. didymobotrya in rats models.
2. Materials and methods
2.1. Plant samples collection, preparation and extraction
After extensive literature review on the medicinal uses of these plants and with bio-conservation aspect considerations fresh leaves of E. globulus (Labill) and S. didymobotrya (Fresenius) were collected from Makunguru village, Nthawa location, Siakago division, Mbeere North Subcounty in Embu County, Kenya. The GPS location for E. globulus (Labill) and S. didymobotrya (Fresenius) specimens were 0o35′12.″S, 37o38′32″E and 0o35′28.″S, 37o38′25″E respectively. The collection of these samples was done based on the ethnobotanical information availed by local herbalists in the area. The samples were cleaned to remove dirt and other contaminants, wrapped in Khaki bags and then transported to Kenyatta University in the Department of Biochemistry, Microbiology and Biotechnology for further processing. The samples were identified and authenticated by Stephen Mwangi, the Chief taxonomist Kenyatta University, Kenya. E. globulus (Labill) was assigned voucher specimen number JKM001, while S. didymobotrya (Fresenius) was assigned JKM002, which were deposited at the Plant Sciences departmental Herbarium of Kenyatta University for future reference.
The leaf samples of the two plants were air-dried at a temperature of 25 °C in the Biochemistry laboratory of Kenyatta University for two weeks. They were then ground using an electric mill into a fine powder and stored at room temperature (25 °C) in appropriately labeled airtight khaki papers until extraction. This study was undertaken in the animal breeding and experimentation laboratory of Kenyatta University, Kenya.
2.2. Extraction
A mass of five hundred grams of the powders of the two plants was each weighed and put into separate labeled conical flasks. A volume of 1500 ml of dichloromethane was then put into each conical flask and corked. The mixture was left to stand for twenty-four hours. Filtration of the extracts was then done in separate well labeled conical flasks using Whatman No.1 filter papers. Five hundred milliliters of dichloromethane was added to each remnant and allowed to stand for twenty-four hours, and then the second filtration was done. This procedure was repeated until the DCM remained clear. DCM is a widely used solvent in the extraction of bioactive compounds in medicinal plants due to its ability to extract more non-polar as well as a significant number of polar compounds. A rotary evaporator was used to concentrate the extract at 40 °C for five hours. The extracts were then put in open 100ml beakers for five days to allow any remaining organic solvent to evaporate until a sticky solid was formed, which was then stored at -4 °C awaiting use in the analysis.
2.3. Gas chromatography-mass spectrometry analysis
Three grams of the DCM leaf extracts of E. globulus (Labill) and S. didymobotrya (Fresenius) were separately obtained and analyzed to determine their quantitative phytochemical composition at ICIPE (International Centre of Insect Physiology and Ecology) laboratory. The protocol followed for analyzing the samples was reviewed by Prof. Baldwyn Torto, the Principal Scientist and Head, Behavioral and Chemical Ecology Department, ICIPE.
A mass of 1.1 mg the DCM leaf extract of E. globulus (Labill) and 1.2 mg S. didymobotrya (Fresenius) were weighed and diluted in respective volumes by partitioning between hexane and methanol. The mixture was then vortexed and centrifuged. The hexane layer was then dried by passing through anhydrous Na2SO4 and analyzed by GC-MS. The peak area was used for quantification. Serial dilutions of authentic standard (1,8-cineole; 99%, Gillingham, Dorset, England) (50 ng/μl, 150 ng/μl, 250 ng/μl, 350 ng/μl and 550 ng/μl) were prepared and analyzed by GC-MS, whose area was used for quantification purposes.
The samples were analyzed on an Agilent Gas Chromatograph 7890A/5975C Mass Spectrometer in full scan mode with the following specifications; gas chromatography Column (HP-5 MS low bleed capillary column (30 m by 0.25 mm i.d., 0.25 μm)) (J&W, Folsom, California, United States of America), flow rate, (Helium) (1.25 ml/min, constant flow mode), injection split mode, temperature of oven (35 °C) for initial five minutes and then elevated by 10 °C per minute to 28 °C Celsius for 10.5 min; run time 70 min.
2.4. The experimental animals
Male Swiss albino rats were utilized in this study. The rats were aged between 2 and 3 months with an average weight of 150 g. The animals were obtained and bred at Kenyatta University animal breeding and research facility. They were kept in approved polyethylene cages at room temperature (25 ± 2 °C) with 40–60 % humidity and 12h dark hours and 12h light cycle. They were provided with standard diet and water ad libitum [13]. The authors sought authorization to use animal experimentation from The National Commission For Science, Technology and Innovation (NACOSTI/P/16/6765/14525). This study received approval. The animals were cared for and handled according to the ethical guidelines and procedures for handling animals for Kenyatta University. The animals were handled and cared for according to the ethical guidelines and procedures for handling animals stipulated in the American Institute of Laboratory Animal Resources [14]. After experiments, the animals were sacrificed using anesthesia, incinerated and disposed appropriately [15].
2.5. Experimental design
This study adopted a completely randomized controlled study design from which an experimental design was drawn. Experimental rats were split into nine groups of five animals each (n = 5) as summarized in Table 1. The body temperatures of rats in all the groups were taken one hour before and after fever induction and at hourly intervals following administration of treatments for four hours [16]. Approximately 3cm of a well-lubricated digital thermometer (thermistor probe®) was inserted into the anal region of the rats to measure the rectal temperature [17,18].
Table 1.
Antipyretic activity evaluation of DCM leaves extracts of E. globulus and S. didymobotrya on turpentine-induced pyrexia in rats.
| Groups | Treatment dose |
|---|---|
| Group1 | 3% DMSO |
| Group II | Turpentine + Normal saline |
| Group 111 | Turpentine + 100 mg/kg bw asprin |
| Group 1V | Turpentine + 25 mg/kg bw extract |
| Group V | Turpentine + 50 mg/kg bw extract |
| Group V1 | Turpentine + 100 mg/kg bw extract |
| Group V11 | Turpentine + 150 mg/kg bw extract |
| Group VIII | Turpentine + 200 mg/kg bw extract |
| Group IX | Turpentine + 250 mg/kg bw extract |
The thermistor probe® was first quantified against a mercury thermometer. The baseline/initial mean rectal temperature was calculated by measuring the rectal temperature of rats at fifteen minutes intervals for 1 h before the induction of fever.
The rectal temperatures of rats were measured and recorded at hourly intervals for 4 h after the administration of different treatments. The rats whose rectal temperatures rose by one degree Celsius one hour after intraperitoneal injection of turpentine (20 mg/kg body weight) were termed pyretic and were used for the studies. The difference in rectal temperatures prior to and after treatments was obtained and the % inhibition in the rectal temperature computed according to the formula as described by [19].
where,
B - Rectal temperature at one hour following turpentine injection
Cn - Rectal temperature after treatments.
2.6. Data management and statistical analysis
Data on pyrexia was obtained, recorded into a spread-sheet. Descriptive statistics was then done and the data expressed as mean ± SEM. Inferential statistics were done using one-way ANOVA followed by Tukey's post hoc test for pairwise separation and comparison of means. Unpaired student t-test was used to compare the antipyretic effects of the two plant extracts. The confidence level was set at 99.5% (p ≤ 0.005). Statistical analysis was done using Minitab statistical software (version 17). Data was presented in form of tables and graphs.
3. Results
3.1. Quantitative phytochemical analysis of antipyretic compounds of E. globulus and S. didymobotrya
Results of the GC-MS revealed that these plants possess bio-compounds such as Terpinolene, Alpha-pinene, Borneol, Globulol and Terpineols (Table 2).
Table 2.
Quantitative phytochemical analysis of antipyretic compounds.
| Compound | Molecular formula | E. globulus (μg/mg) | S. didymobotrya (μg/mg) |
|---|---|---|---|
| Globulol | (C15H26O) | 7.2 | - |
| Terpinolene | (C10H16) | 2.9 | 2.3 |
| Alpha-pinene | (C10H16) | 17.9 | 2.7 |
| Borneol | (C10H18O) | 2.8 | - |
| Terpineols | (C10H18O) | 2.3 | - |
3.2. Antipyretic activity of dichloromethane leaf extract of E. globulus (Labill) in Swiss albino rats
The DCM leaf extract of E. globulus (Labill) generally exhibited in vivo antipyretic activities in rats, which was evidenced by a reduction in rectal temperature against turpentine-induced fever (Table 3). After one hour of treatment, the groups of Swiss albino rats that received aspirin (100 mg/kg body weight) and the extract dose levels of 25, 50, 100, 150, 200 and 250 mg/kg body weight lowered the rectal temperature to 98.07%, 98.96%, 98.53%, 98.49%, 98.13%, 97.57% and 97.71% respectively (Table 3). The E. globulus extract at the dose level of 200 mg/kg body weight caused the highest antipyretic activity, which reduced pyrexia by 2.43 % in the first hour. This change was higher than that caused by the reference drug, aspirin, which reduced pyrexia by 1.93%. However, the effect of aspirin was comparable to that of extracts dose levels of 50, 100, 150, 200 and 250 mg/kg body weight (p > 0.005).
Table 3.
Antipyretic effects of dichloromethane leaf extract of E. globulus on turpentine-induced pyrexia in rat.
| Group | Treatment | Percentage change in rectal temperatures (ºC) |
||||
|---|---|---|---|---|---|---|
| 0hr | 1hr | 2hr | 3hr | 4hr | ||
| Normal control | DMSO | 100.00 ± 0.00 | 99.89 ± 0.13a (0.11) | 100.16 ± 0.22a (-0.16) | 99.78 ± 0.13a (0.22) | 99.89 ± 0.18a (0.11) |
| Negative Control | Turpentine + DMSO | 100.00 ± 0.00 | 100.42 ± 0.06a (-0.42) | 100.16 ± 0.10a (-0.57) | 100.68 ± 0.18b (-0.68) | 100.52 ± 0.12a (-0.52) |
| Positive Control | Turpentine + Aspirin | 100.00 ± 0.00 | 98.07 ± 0.07cd (1.93) | 97.55 ± 0.15bc (2.45) | 96.82 ± 0.12cde (3.18) | 95.31 ± 0.16d (4.69) |
| DCM: leaf Extract | Turpentine + 25 mg/kg bw | 100.00 ± 0.00 | 98.96 ± 0.08b (1.04) | 98.18 ± 0.23b (1.82) | 97.46 ± 0.25c (2.54) | 97.04 ± 0.26b (2.96) |
| Turpentine + 50 mg/kg bw | 100.00 ± 0.00 | 98.53 ± 0.06bc (1.47) | 97.91 ± 0.17b (2.09) | 97.28 ± 0.07cd (2.72) | 96.28 ± 0.13bc (3.72) | |
| Turpentine + 100 mg/kg bw | 100.00 ± 0.00 | 98.49 ± 0.10bc (1.51) | 97.44 ± 0.05bcd (2.56) | 96.60 ± 0.01de (3.40) | 95.98 ± 0.10cd (4.02) | |
| Turpentine + 150 mg/kg bw | 100.00 ± 0.00 | 98.13 ± 0.15cd (1.87) | 96.94 ± 0.05cd (3.06) | 96.37 ± 0.09e (3.63) | 95.53 ± 0.07cd (4.47) | |
| Turpentine + 200 mg/kg bw | 100.00 ± 0.00 | 97.57 ± 0.10d (2.43) | 96.80 ± 0.06cd (3.20) | 96.13 ± 0.08e (3.87) | 95.20 ± 0.07d (4.80) | |
| Turpentine + 250 mg/kg bw | 100.00 ± 0.00 | 97.71 ± 0.05d (2.29) | 96.73 ± 0.07d (3.27) | 96.41 ± 0.05e (3.59) | 95.17 ± 0.10d (4.83) | |
Descriptive statistics are expressed as mean ± SEM for 5 rats per group. Values with a similar superscript letter are statistically insignificant (p > 0.005) along the same column by one-way ANOVA followed by Tukey's post hoc test. Aspirin = 100 mg/kg body weight; DMSO = 10%; Turpentine = 20%; bw = body weight. The figures in brackets represent mean % inhibition.
In the 2nd hour, the E. globulus leaf extract reduced the elevated rectal temperature in a dose-dependent fashion. At dose levels of 25, 50, 100, 150, 200 and 250 mg/kg body weight, the extract lowered the raised rectal temperature to 98.18%, 97.91%, 97.44%, 96.94%, 96.80% and 96.73% respectively (Table 3). The antipyretic activities of the DCM leaf extract dose levels of 25, 50, 100, 150 and 200 mg/kg body weight were statistically similar and comparable to that of aspirin (p > 0.005; Table 3).
In the 3rd hour post-treatment, the leaf extract dose levels of 25, 50, 100, 150, 200 and 250 mg/kg body weight lowered the elevated rectal temperature in rats to 97.46%, 97.28%, 96.60%, 97.37%, 96.31% and 96.41% respectively (Table 3). Similarly, at this hour the extract showed a dose-independent antipyretic potential. The rats that received the E. globulus extract at dose levels of 25, 50, 100, 150, 200 and 250 mg/kg body weight exhibited antipyretic activities that were significantly different (p < 0.005; Table 3). However, the antipyretic activity of aspirin was statistically similar compared to that of the extract at all tested dose levels (p > 0.005; Table 3).
In the 4th hour, the E. globulus leaf extract reduced raised rectal temperature in a dose-dependent manner. The extract of E. globulus (Labill) dose levels of 25, 50, 100, 150, 200 and 250 mg/kg body weight reduced pyrexia to 97.04%, 96.28%, 95.98%, 95.53%, 95.20% and 95.17%, respectively (Table 3). At this hour, the group that received leaf extract of E. globulus (Labill) at a dose level of 250 mg/kg body weight recorded the highest antipyretic effects, which was higher than that of aspirin (Table 3). The antipyretic effects of the extract at the dose levels of 100, 150, 200 and 250 mg/kg body weight were not significantly different from each other and were comparable to that of the reference drug, aspirin (p > 0.005; Table 3).
The DCM leaf extract of S. didymobotrya (Fresenius) in vivo equally showed antipyretic potential in rats. This was exhibited by a decrease in previously raised rectal on turpentine-induced fever in rats (Table 4). After the first hour of treatment, the groups of rats that received the reference drug aspirin at 100 mg/kg body weight and DCM leaf extract of S. didymobotrya (Fresenius) at dose levels of 25, 50, 100, 150, 200 and 250 mg/kg body weight lowered the raised rectal temperature to 98.75%, 99.48%, 98.97%, 98.86%, 99.00%, 98.70% and 98.69% respectively (Table 4). At this hour, 250 mg/kg body weight dose level recorded the highest antipyretic effect with a 1.31% fever reduction compared to aspirin, which recorded a reduction of 2.24%. The antipyretic effect of S. didymobotrya (Fresenius) extract dose levels exhibited no significant differences and were comparable to the effect of the aspirin at this hour (p > 0.005; Table 4). The effect of the extract at this hour was dose- independent.
Table 4.
Antipyretic effects of dichloromethane leaf extracts of S. didymobotrya on turpentine-induced pyrexia in rats.
| Group | Treatment | Percentage change in rectal temperatures (ºC) |
||||
|---|---|---|---|---|---|---|
| 0h | 1h | 2h | 3h | 4h | ||
| Normal control | DMSO | 100.00 ± 0.00 | 99.73 ± 0.12b (0.27) | 99.95 ± 0.21b (0.05) | 100.11 ± 0.20a (-0.11) | 100.00 ± 0.19b (-0.00) |
| Negative Control | Turpentine + DMSO | 100.00 ± 0.00 | 100.52 ± 0.08a (-0.52) | 100.67 ± 0.06a (-0.67) | 100.67 ± 0.06a (-0.67) | 100.72 ± 0.05a (-0.72) |
| Positive Control | Turpentine + Aspirin | 100.00 ± 0.00 | 98.75 ± 0.15d (1.25) | 97.45 ± 0.09d (2.55) | 96.67 ± 0.05e (3.33) | 95.89 ± 0.13f (4.11) |
| DCM: leaf Extract | Turpentine + 25 mg/kg bw | 100.00 ± 0.00 | 99.48 ± 0.08bc (0.52) | 98.79 ± 0.16c (1.21) | 98.27 ± 0.23b (1.73) | 97.64 ± 0.16c (2.36) |
| Turpentine + 50 mg/kg bw | 100.00 ± 0.00 | 98.97 ± 0.08cd (1.03) | 97.83 ± 0.14d (2.17) | 97.47 ± 0.04c (2.53) | 97.01 ± 0.05cd (2.99) | |
| Turpentine + 100 mg/kg bw | 100.00 ± 0.00 | 98.86 ± 0.13cd (1.14) | 97.56 ± 0.06d (2.44) | 97.40 ± 0.01cd (2.60) | 96.36 ± 0.01def (3.64) | |
| Turpentine + 150 mg/kg bw | 100.00 ± 0.00 | 99.00 ± 0.15cd (1.00) | 98.05 ± 0.07d (1.95) | 97.36 ± 0.01cde (2.64) | 96.67 ± 0.10de (3.33) | |
| Turpentine + 200 mg/kg bw | 100.00 ± 0.00 | 98.70 ± 0.12d (1.30) | 97.60 ± 0.09d (2.40) | 96.77 ± 0.06de (3.23) | 96.25 ± 0.07ef (3.75) | |
| Turpentine + 250 mg/kg bw | 100.00 ± 0.00 | 98.69 ± 0.09d (1.31) | 97.76 ± 0.11d (2.24) | 96.92 ± 0.14cde (3.08) | 96.04 ± 0.08ef (3.97) | |
Descriptive statistics are expressed as mean ± SEM for 5 rats per group. Values with a similar superscript letter are statistically insignificant (p > 0.005) along the same column by one-way ANOVA followed by Tukey's post hoc test. Aspirin = 100 mg/kg body weight; DMSO = 10%; Turpentine = 20%; bw = body weight. The figures in brackets represent mean % inhibition.
In the second hour, the DCM leaf extract of S. didymobotrya (Fresenius) at the dose levels of 25, 50, 100, 150, 200 and 250 mg/kg body weight, lowered the elevated rectal temperatures to 98.79%, 97.83%, 97.56%, 98.05%, 97.60% and 97.76% respectively (Table 4). The reference drug reduced the raised rectal temperature to 97.45% (Table 4). The 100 mg/kg body weight dose level recorded the highest antipyretic activity with a 2.44% reduction (Table 3). There was no significant difference in the anti-pyretic activities of the S. didymobotrya extract dose levels of 50, 100, 150, 200 and 250 mg/kg body weight. Their effects were comparable with that of aspirin (p > 0.005; Table 4).
In the third hour, the S. didymobotrya (Fresenius) leaf extract dose levels of 25, 50, 100, 150, 200 and 250 mg/kg body weight lowered the elevated rectal temperature to 98.27%, 97.47%, 97.40%, 97.36%, 96.77% and 96.92% respectively (Table 4). At this hour, the extract showed a dose-independent antipyretic potential. At 200 mg/kg body weight dose level, the extract revealed the highest antipyretic activity with a fever reduction of 3.23% (Table 4). The antipyretic activities of the S. didymobotrya (Fresenius) at dose levels of 100, 200 and 250 mg/kg body weight revealed no significant difference (p > 0.005; Table 4). Further, the effect of aspirin was comparable to that of the extract dose levels of 150, 200 and 250 mg/kg body weight (p > 0.005; Table 4).
In the fourth hour, the leaf extract also reduced the raised rectal temperature in pyretic rats in a dose-independent response. The DCM leaf extract of S. didymobotrya, at dose levels of 25, 50, 100, 150, 200 and 250 mg/kg body weight, lowered rectal temperatures to 97.64%, 97.01%, 96.36%, 96.67%, 96.25% and 96.04% respectively (Table 4). There was no significant variation in the antipyretic activities among the extract at the dose levels of 100, 150, 200 and 250 mg/kg body (p > 0.005; Table 4). Similarly, the effect of the reference drug aspirin was not significantly different from the effect of the extract dose levels of 100, 200 and 250 mg/kg body weight (p > 0.005; Table 4).
3.3. Comparison of in vivo antipyretic activities of DCM extracts of E. globulus and S. didymobotrya
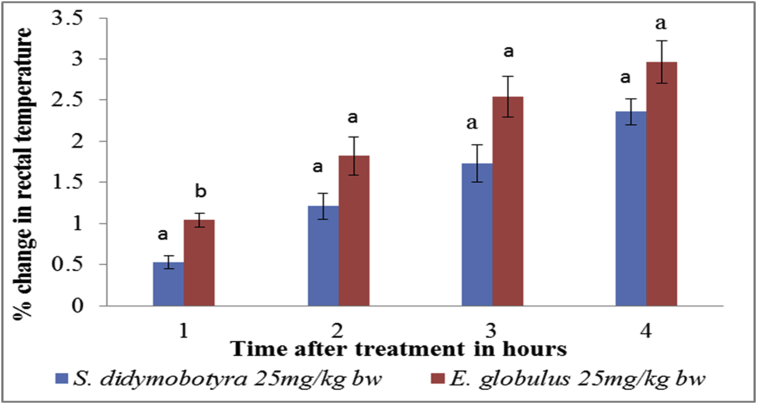
In comparison, the antipyretic activity of E. globulus extract was significantly higher compared to S. didymobotrya dose of 25 mg/kg body weight in the first hour (p < 0.005; Figure 1). However, the antipyretic effects of the DCM leaf extracts of E. globulus (Labill) and S. didymobotrya (Fresenius) in rats, at the dose of 25 mg/kg body weight, were not significantly different in the 2nd, 3rd and 4th hours (p > 0.005; Figure 1).
Figure 1.
Comparison between antipyretic effects of DCM leaf extracts of E. globulus (Labill) and S. didymobotyra (Fresenius) at the dose level of 25 mg/kg body weight on turpentine-induced pyrexia in rats. Means with the same letter are statistically insignificant in each hour of treatment (p > 0.005); bw = bodyweight.
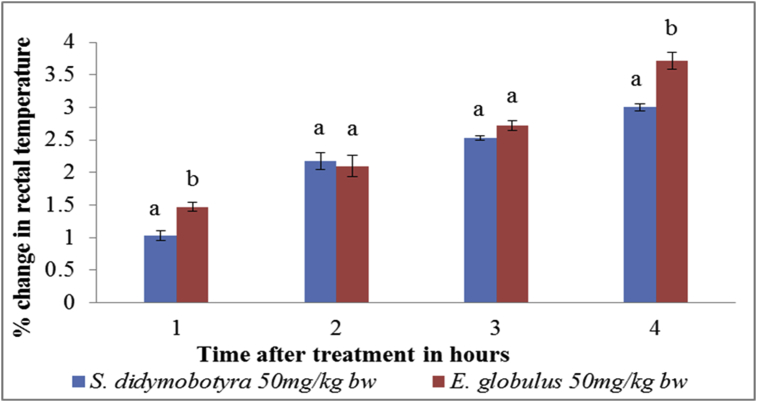
At the dose level of 50 mg/kg body weight, the antipyretic effect of the E. globulus (Labill) was significantly higher compared to that of S. didymobotrya in the first and fourth hours (p < 0.005; Figure 2). In contrast, the antipyretic activities of the leaf extracts of S. didymobotrya (Fresenius) and E. globulus (Labill) exhibited no significant difference in the second and third hours (p > 0.005; Figure 2).
Figure 2.
Comparison between antipyretic effects of DCM leaf extracts of E. globulus (Labill) and S. didymobotyra (Fresenius) at the dose level of 50 mg/kg body weight on turpentine-induced pyrexia in rats. Means with the same letter are statistically insignificant in each hour of treatment (p > 0.005); bw = bodyweight.
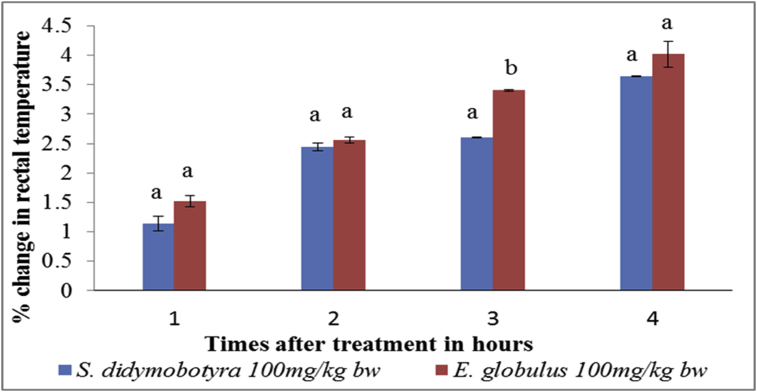
At the extract dose level of 100 mg/kg body weight, the antipyretic effects of the DCM leaf extracts of S. didymobotrya (Fresenius) and E. globulus (Labill) exhibited no significant differences in the first, second and fourth hours (p > 0.005; Figure 3). However, the antipyretic effect of E. globulus was significantly higher compared to that of the S. didymobotrya extract in the third hour (p < 0.005; Figure 3).
Figure 3.
Comparison between antipyretic effects of DCM leaf extracts of E. globulus (Labill) and S. didymobotyra (Fresenius) at the dose level of 100 mg/kg body weight on turpentine-induced pyrexia in rats. Means with the same letter are statistically insignificant in each hour of treatment (p > 0.005); bw = bodyweight.
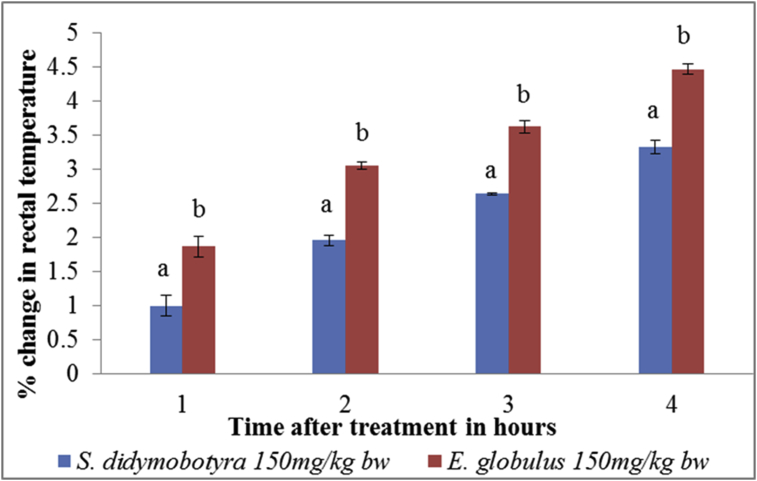
At the dose level of 150 mg/kg body weight, the antipyretic activity of the DCM leaf extract of E. globulus (Labill) was significantly higher compared to that of S. didymobotrya (Fresenius) in the first, second, third and fourth hours (p < 0.005; Figure 4).
Figure 4.
Comparison between antipyretic effects of DCM leaf extracts of E. globulus (Labill) and S. didymobotyra (Fresenius) at the dose level of 150 mg/kg body weight on turpentine-induced pyrexia in rats. Means with the same letter are statistically insignificant in each hour of treatment (p > 0.005); bw = bodyweight.
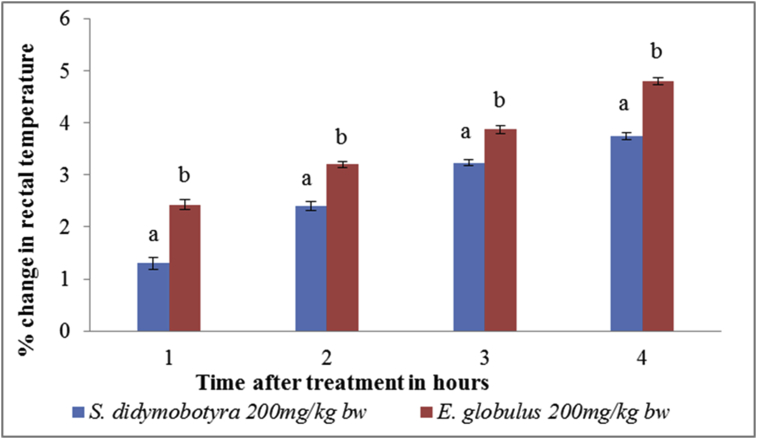
The antipyretic activity of E. globulus (Labill) was significantly higher than that of S. didymobotrya (Fresenius) at the dose level of 200 mg/kg body weight at the 1st, 2nd, 3rd, and 4th hours (p < 0.005; Figure 5).
Figure 5.
Comparison between antipyretic effects of DCM leaf extracts of E. globulus (Labill) and S. didymobotyra (Fresenius) at the dose level of 200 mg/kg body weight on turpentine-induced pyrexia in rats. Means with the same letter are statistically insignificant in each hour of treatment (p > 0.005); bw = bodyweight.
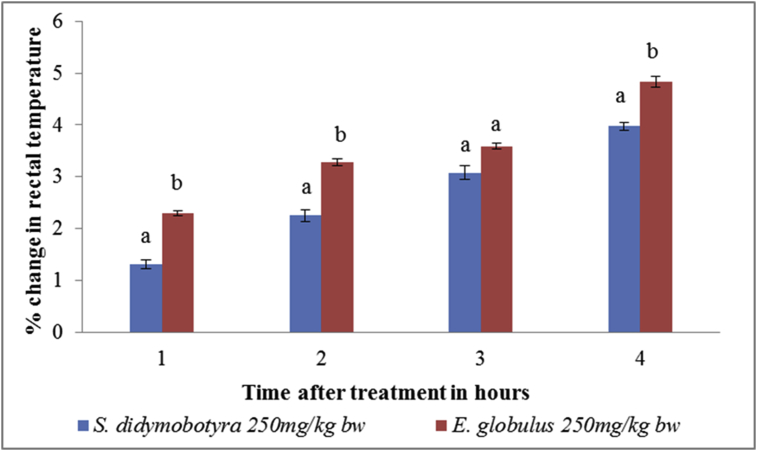
At 250 mg/kg body weight dose level, the antipyretic activity of DCM leaf E. globulus (Labill) was significantly higher compared to that of S. didymobotrya (Fresenius) in the 1st, 2nd, and 4th in rats (p < 0.005; Figure 6). In contrast, the antipyretic effects of E. globulus (Labill) and S. didymobotrya (Fresenius) extracts were not significantly different in the third hour (p > 0.005; Figure 6).
Figure 6.
Comparison between antipyretic effects of DCM leaf extracts of E. globulus (Labill) and S. didymobotyra (Fresenius) at the dose level of 250 mg/kg body weight on turpentine-induced pyrexia in rats. Means with the same letter are statistically insignificant in each hour of treatment (p > 0.005); bw = bodyweight.
4. Discussion
The results of this study demonstrate that E. globulus (Labill) and S. didymobotra (Fresenius) DCM leaf extracts possess antipyretic phytochemicals effective in the reduction of turpentine-induced fever in rats. Upon bio-screening for the antipyretic activity of the DCM leaf extracts of E. globulus (Labill) and S. didymobotra (Fresenius) on turpentine-induced fever in rats, the results showed that fever was remarkably reduced at all dose levels. The DCM leaf extract of E. globulus and S. didymobotra at the dose level of 250 mg/kg body weight, exhibited the highest rectal temperature reduction, which compared well with the reference drug, aspirin.
Fever (pyrexia) is defined as a complicated physiologic response caused by infection or aseptic stimuli. The body temperature elevation occurs when PGE2 accumulate in the hypothalamus pre-optic region. The neurons firing rate in the hypothalamus control thermoregulation and is usually altered by increased synthesis of PGE2. Research has reported that most antipyretic drugs exert their action by inhibiting cyclooxygenase enzymatic activity and consequently reducing PGE2 levels within the hypothalamic region [20]. However, other different mechanisms in the management of pyrexia cannot be ruled out.
This study was carried out to evaluate the antipyretic effects of dichloromethane leaf extracts of E. globulus (Labill) and S. didymobotra (Fresenius) in rats. The antipyretic potential was done using turpentine as pyrexia inducing agent [21,22]. The findings from the present study were in agreement with other studies on antipyretic potential medicinal plants in animal models. Similar work by [23] demonstrated antipyretic effects of aqueous and methanolic root extracts of Asparagus racemosus in yeast-induced pyrexia. Besides, similar findings were reported by [11] on the antipyretic effect of Urtica dioica (L.) aqueous leaf extract against brewer's yeast-induced fever in animal models. A study by [24] demonstrated the antipyretic effects of selected medicinal plants in albino rats. Further, Mangifera indica and Ocimum gratissimum extracts reduced brewer's yeast-induced pyrexia in animals [25].
Generally, the NSAIDs produce their antipyretic action via prostaglandin biosynthesis inhibition within the pre-optic region of the hypothalamus [26]. It is therefore, possible that the DCM leaf extracts of E. globulus and S. didymobotra could have a similar mechanism of action to that of aspirin through inhibition of prostaglandin biosynthesis in the hypothalamus.
The antipyretic effects of DCM leaf extracts of E. globulus and S. didymobotra at different dose levels exhibited a dose-independent response on turpentine-induced fever in Swiss albino rats. Lower dosages of 25 mg/kg and 50 mg/kg body weight were not as effective as 200 and 250 mg/kg body weight; this could be attributed to rapid metabolism, clearance and inactivation of active principles due to their low concentration at lower dosage levels. Similar findings were reported by [27] on dichloromethane-methanolic leaf and stem bark extracts of Ximenia americana in rats model possess antipyretic potential. This study used dose ranges that were within the dose ranges used by [28] while evaluating the antipyretic activity of Pseudocedrela kotschyi ethanolic leaf extract used dose levels of 50, 100 and 150 mg/kg body weights in rats.
The DCM leaf extracts of E. globulus and S. didymobotra at all the dose levels (25, 50, 100, 150, 200 and 250 mg/kg body weight), never lower rectal temperature in the 1st and 2nd hours as effectively as in the 3rd and 4th hours. These findings be could attributed to the biotransformation of bioactive components in the extract to become antipyretic [29].
The DCM leaf extract of E. globulus at the 200 mg/kg body weight was marginally effective than aspirin, while the DCM leaf extract of S. didymobotra at 250 mg/kg body weight dose level was equally effective compared to aspirin. These findings suggest a better or a similar prostaglandin synthesis inhibition by the active components in the plant's extracts. There is therefore, a possibility of the plant extracts working effectively by blocking alternative mechanisms during fever inhibition.
These plants are endowed with several bioactive compounds such as Terpinolene, Alpha-pinene, Borneol, Globulol and Terpineols. Studies have associated these compounds with antipyretic activity. According to [30], globulol has antipyretic potential in animal models. The GC-MS results revealed the presence of terpineols which are components of essential oils. In a study by [31], terpineols isolated from Artemisia caerulescens subsp. Gallica possesses antipyretic activity in animal models.
The GC-MS results also revealed the presence of alpha-pinene which possesses antipyretic activity in animal models. In a study carried out by [32], alpha-pinene isolated from Black cumin seed had significant antipyretic effects in mice model. In a study done by [33], revealed that Borneol possesses antipyretic activity in animal models.
5. Conclusion
In conclusion, this study has revealed that these plants are endowed the many bioactive compounds such as terpenoids and flavonoids which possess antipyretic activity in rats.
Declarations
Author contribution statement
Mworia Joseph Kiambi, Mathew Piero Ngugi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Cromwell Mwiti Kibiti, Joseph Ngeranwa Ngari: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors wish to thank Kenyatta University for availing the laboratories for animal breeding and experimentation and the International Centre of Insect Physiology and Ecology for allowing us use their laboratory to carry out GC-MS analysis of the plant extracts.
References
- 1.Anochie I.P. Mechanisms of fever in humans. Int. J. Microbiol. Immunol. Res. 2013 May;2(5):37–43. [Google Scholar]
- 2.Freedman R.R., Blacker C.M. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil. Steril. 2002 Mar 1;77(3):487–490. doi: 10.1016/s0015-0282(01)03009-6. [DOI] [PubMed] [Google Scholar]
- 3.Childs C., Vail A., Protheroe R., King A.T., Dark P.M. Differences between brain and rectal temperatures during routine critical care of patients with severe traumatic brain injury. Anaesthesia. 2005 Aug;60(8):759–765. doi: 10.1111/j.1365-2044.2005.04193.x. [DOI] [PubMed] [Google Scholar]
- 4.Prajitha N., Athira S.S., Mohanan P.V. Pyrogens, a polypeptide produces fever by metabolic changes in hypothalamus: mechanisms and detections. Immunol. Lett. 2018 Oct 15 doi: 10.1016/j.imlet.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 5.MurakaMi M. Lipid mediators in life science. Exp. Anim. 2011;60(1):7–20. doi: 10.1538/expanim.60.7. [DOI] [PubMed] [Google Scholar]
- 6.Sabina E.P., Nasreen A., Vedi M., Rasool M. Analgesic, antipyretic and ulcerogenic effects of piperine: an active ingredient of pepper. J. Pharm. Sci. Res. 2013 Oct 1;5(10):203. [Google Scholar]
- 7.Naidu R.K., Pham T.M. Springer; New York, NY: 2015. Pain Management. In Basic Clinical Anesthesia; pp. 265–296. [Google Scholar]
- 8.Osterweil A.C. University of California; Berkeley: 2005. Flesh Cinema: the Corporeal Avant-Garde, 1959–1979. [Google Scholar]
- 9.Veronica S.A., Cheruiyot K.S., Bosibori M.J., Munene I.M., Murugi N.J., Piero N.M. Antiinflammatory, analgesic and antipyretic effects of dichloromethane stem bark extract of Acacia mellifera. J. Phytopharmacol. 2017 Sep;6(4):239–246. [Google Scholar]
- 10.Vijay P., Vijayvergia R. Analgesic, anti-inflammatory and antipyretic activity of Cissus quadrangularis. J. Pharm. Sci. Technol. 2010;2(1):111–118. [Google Scholar]
- 11.Joshi B.C., Mukhija M., Kalia A.N. Pharmacognostical review of Urtica dioica L. Int. J. Green Pharm. 2014;8(4):201–209. [Google Scholar]
- 12.Mueller M.S., Mechler E. Georg Thieme Verlag; 2005. Medicinal Plants in Tropical countries. Traditional Use-Experience-Facts; pp. 1–168. [Google Scholar]
- 13.Semenya S., Potgieter M., Tshisikhawe M., Shava S., Maroyi A. Medicinal utilization of exotic plants by Bapedi traditional healers to treat human ailments in Limpopo province, South Africa. J. Ethnopharmacol. 2012;144(3):646–655. doi: 10.1016/j.jep.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi M.J., Mallikarjun C., Kian W.G. Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets: a study in diet induced hyperlipidemic rabbits. Asian J. Pharm. Sci. 2015 Feb 1;10(1):40–56. [Google Scholar]
- 15.WU X.Y., Animal Care and Use Committee of The American Society of Mammalogists Effect of pentobarbital and isoflurane on acute stress response in rat. Physiol. Behav. 2015;145:118–121. doi: 10.1016/j.physbeh.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Jiang R., Zhang Z., Zhang L. Antipyretic and anti-inflammatory effects of asiaticoside in lipopolysaccharide-treated rat through up-regulation of heme oxygenase-1. Phytother. Res. 2013 Aug;27(18):1136–1141. doi: 10.1002/ptr.4838. [DOI] [PubMed] [Google Scholar]
- 17.Khan A., Rahman M., Islam S. Antipyretic activity of Peperomia pellucida leaves in rabbit. Turk. J. Biol. 2008 Feb 19;32(1):37–41. [Google Scholar]
- 18.Gaddam S.K., Cruz J., Robertson C. Humana Press; Totowa, NJ: 2013. Erythropoietin and Cytoprotective Cytokines in Experimental Traumatic Brain Injury. InTissue-Protective Cytokines; pp. 141–162. [DOI] [PubMed] [Google Scholar]
- 19.Farré M., Asperger D., Kantiani L., González S., Petrovic M., Barceló D. Assessment of the acute toxicity of triclosan and methyl triclosan in wastewater based on the bioluminescence inhibition of Vibrio fischeri. Anal. Bioanal. Chem. 2008 Apr 1;390(8):1999–2007. doi: 10.1007/s00216-007-1779-9. [DOI] [PubMed] [Google Scholar]
- 20.Saper C.B., Romanovsky A.A., Scammell T.E. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat. Neurosci. 2012 Aug;15(8):1088. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maina G.S., Maina M.B., Muriithi N.J., Kiambi M.J., Kelvin J.K., Umar A., John M.K., Ann N.W., Piero N.M., David M.N. Antipyretic properties of dichloromethane: methanolic leaf and root bark extracts of carissa edulis in rats. Asian J. Biomed. Pharmaceut. Sci. 2015;5(43):12–20. [Google Scholar]
- 22.Hussain M., Waqas H.M., Hussain I., Majeed A., Raza S.M., Janbaz K.H. Pharmacological validation of the folkloric uses of Cyperus rotundus L. in different ailments: an in vivo and in vitro research. Pak. J. Pharm. Sci. 2018 Jan 1;31(1):465–475. research 1998 Jan 1 (Vol. 115). [PubMed] [Google Scholar]
- 23.Vasundra D.P., Divya P.S. Antipyretic activity of ethanol and aqueous extract of root of Asparagus racemosus in yeast induced pyrexia. Asian J. Pharmaceut. Clin. Res. 2013;6(3):190–193. [Google Scholar]
- 24.Bekhit A.A., Farghaly A.M., Shafik R.M., Elsemary M.M., El-Shoukrofy M.S., Bekhit A.E., Ibrahim T.M. Synthesis, evaluation and modeling of some triazolothienopyrimidinones as anti-inflammatory and antimicrobial agents. Future Med. Chem. 2017 Jun;9(9):881–897. doi: 10.4155/fmc-2016-0242. [DOI] [PubMed] [Google Scholar]
- 25.Tarkang P.A., Okalebo F.A., Siminyu J.D., Ngugi W.N., Mwaura A.M., Mugweru J., Agbor G.A., Guantai A.N. Pharmacological evidence for the folk use of Nefang: antipyretic, anti-inflammatory and antinociceptive activities of its constituent plants. BMC Complement Altern. Med. 2015 Dec;15(1):174. doi: 10.1186/s12906-015-0703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raju G.S., RahmanMoghal M.M., Hossain M.S., Hassan M.M., Billah M.M., Ahamed S.K., Rana S.M. Assessment of pharmacological activities of two medicinal plant of Bangladesh: Launaea sarmentosa and Aegialitis rotundifolia roxb in the management of pain, pyrexia and inflammation. Biol. Res. 2014 Dec;47(1):55. doi: 10.1186/0717-6287-47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaichu D.M., Mawia A.M., Gitonga G.M., Ngugi M.P., Mburu D.N. Phytochemical screening and antipyretic activities of dichloromethane-methanolic leaf and stem bark extracts of Ximenia americana in rat models. J. Herbmed. Pharmacol. 2017;6 [Google Scholar]
- 28.Akuodor G.C., Essien A.D., Essiet G.A., David-Oku E., Akpan J.L., Udoh F.V. Evaluation of antipyretic potential of Pseudocedrela kotschyi Schweint. Harms (Meliaceae) Eur. J. Med. Plants. 2013 Jan 1;3(1):105. [Google Scholar]
- 29.Altemimi A., Lakhssassi N., Baharlouei A., Watson D., Lightfoot D. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017 Dec;6(4):42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muko K.N., Ohiri P.C., Ezugwu C.O. Antipyretic and analgesic activities of sphenoceutrum Jollyanum. Nigerian J. Nat. Prod. Med. 1998;2(1):52–53. [Google Scholar]
- 31.Moran A., Martin M.L., Montero M.J., de Urbina A.O., Sevilla M.A., San Roman L. Analgesic, antipyretic and anti-inflammatory activity of the essential oil of Artemisia caerulescens subsp. gallica. J. Ethnopharmacol. 1989 Dec 1;27(3):307–317. doi: 10.1016/0378-8741(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 32.Khan Aisha Saleem. Medicinally Important Trees. Springer; Cham: 2017. Antipyretic and analgesic activities of some economically important woody plants; pp. 159–185. [Google Scholar]
- 33.Kumar Ravendra, Sethi Sonali, Prakash Om, Pant Anil Kumar, Kumar Mahesh, Valery A. Isidorov, and Lech Szczepaniak. "Chemical composition of rhizome oleoresin and anti-inflammatory, antinociceptive and antipyretic activity of oleoresins of Alpinia allughas Roscoe. from tarai region of Uttarakhand. Indones. J. Pharm. 2017;28(3):136. [Google Scholar]