Abstract
Early diagnosis of and adequate therapy for premalignant lesions in patients with inflammatory bowel disease (IBD) and Barrett's esophagus (BE) has been shown to decrease mortality. Endoscopic examination with histologic evaluation of random and targeted biopsies remains the gold standard for early detection and adequate treatment of neoplasia in both these diseases. Although eventual patient management (including surveillance and treatment) depends upon a precise histologic assessment of the initial biopsy, accurately diagnosing and grading IBD- and BE-associated dysplasia is still considered challenging by many general as well as subspecialized pathologists. Additionally, there are continuing updates in the literature regarding the diagnosis, surveillance, and treatment of these disease entities. This comprehensive review discusses the cancer risk, detailed histopathological features, diagnostic challenges, and updates as well as the latest surveillance and treatment recommendations in IBD- and BE-associated dysplasia.
Keywords: Barrett’s esophagus, inflammatory bowel disease, dysplasia, surveillance
Introduction
Early diagnosis and adequate therapy of premalignant lesions in patients of inflammatory bowel disease (IBD) and Barrett's esophagus (BE) have been shown to decrease mortality due to cancerous complications in these two diseases [1, 2]. Endoscopic examination with histologic evaluation of random and targeted biopsies remains the gold standard for early detection and adequate treatment of neoplasia in both these diseases. Endoscopic therapy, such as radiofrequency ablation and/or endoscopic mucosal resection (EMR)/endoscopic submucosal dissection (ESD), are successfully used in treating dysplasia, intramucosal adenocarcinoma (IMC), and a selected set of submucosal adenocarcinoma (SMC) arising in the setting of BE [2]. In contract, endoscopic intervention of IBD-associated neoplasia recently started for polypoid or endoscopically resectable lesions, while IBD-associated flat dysplasia is still primarily treated by colectomy [1].
Surveillance BE and/or IBD biopsies are considered as “bread and butter type” specimens and constitutes a decent chunk of the surgical pathology caseload in both general and subspecialized gastrointestinal (GI) surgical pathology practices. However, accurately diagnosing and grading IBD- and BE-associated dysplasia is still considered challenging by many general as well as subspecialized GI pathologists. In 2015, a consensus guideline, SCENIC (Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in IBD patients), was published and emphasized the importance of the collaboration of pathologist and endoscopist for the optimal management of IBD patients with neoplasia [1]. Similarly, the new updated 2016 American College of Gastroenterology (ACG) guidelines pertain to the initial diagnosis and subsequent surveillance and treatment recommendations in BE patients [2]. According to the ACG 2016 guidelines, BE is diagnosed when there is extension of salmon-colored mucosa for at least 1 cm proximal to the esophagogastric junction, and this needs to be histologically confirmed by demonstrating intestinal metaplasia (goblet cells) on the biopsy [2]. Overall, these guidelines also call for an increasing use of endoscopic therapy (polypectomy, RFA, EMR, and/or ESD) for IBD- and BE-associated neoplasia that is based on accurate initial histologic diagnosis. Subsequent invasive surgery (if needed), either colectomy or esophagectomy, is then based upon accurate histologic diagnosis on the polypectomy and/or EMR/ESD specimens. Of course, in cases without endoscopically apparent lesions, accurately diagnosing and grading neoplasia in the random biopsies play an essential role in determining the subsequent therapy. To this end, this review discusses the cancer risk, detailed histopathological features, diagnostic challenges, and updates as well as the newest surveillance and treatment recommendations in IBD- and BE-associated dysplasia.
Carcinogenesis and cancer risk for IBD- and BE-associated neoplasia
Carcinogenesis in BE and IBD is believed to occur through genetic aberrations leading to morphological changes manifested as low-grade dysplasia (LGD), high-grade dysplasia (HGD), and then to invasive cancer. Current management has focused on the detection of precancerous changes (dysplasia) in the colorectal epithelium and BE as a marker for increased cancer risk and, thus, as a potential indicator for intervention, either endoscopically or surgically, in IBD and BE patients who are enrolled in the surveillance program. White-light endoscopy of high resolution and/or chromoendoscopy are the recommended surveillance modalities and the biopsy can be random, targeted, or a combination of both [3]. The surveillance biopsy protocols are listed in Table 1.
Table 1.
Time to enter the surveillance program and the surveillance interval for inflammatory bowel disease and Barrett’s esophagus
| Disease | Patients who should enter surveillance program | Time to enter surveillance program | Surveillance interval | Type of endoscopy | Biopsy protocol | Tissue submission |
|---|---|---|---|---|---|---|
| IBD | Pan-colitis or extensive colitis in UC or CD involving one-third of the colon | 8 years after diagnosis | 1- to 3-year interval | High-definition/high-resolution white-light endoscopy with or without chromoendoscopy | Random (four quadrants every 10 cm, about 32 biopsies) and targeted biopsy | Lesional biopsy, random biopsy should be submitted separately |
| IBD patients (<8-year duration) with subsequent PSC diagnosis | At the time of PSC diagnosis | 1-year interval | High-definition/high-resolution white-light endoscopy with or without chromoendoscopy | Random (four quadrants every 10 cm, about 32 biopsies) and targeted biopsy | Lesional biopsy, random biopsy should be submitted separately | |
| PSC patient with subsequent IBD diagnosis | At the time of IBD diagnosis | 1-year interval | High-definition/high-resolution white-light endoscopy with or without chromoendoscopy | Random (four quadrants every 10 cm, about 32 biopsies) and targeted biopsy | Lesional biopsy, random biopsy should be submitted separately | |
| BE | Endoscopically and histologically confirmed BE | 3–5 years after initial diagnosis of BE diagnosis after adequate counseling regarding risks and benefits of surveillance | 3-year interval | High-definition/high-resolution white-light endoscopy with or without chromoendoscopy |
Random (four quadrants every 2 cm) and targeted for routine surveillance Random (four quadrants every 1 cm) and targeted biopsy for high-risk patients with suspected neoplasia |
Lesional biopsy, random biopsy should be submitted separately |
IBD, inflammatory bowel disease; BE, Barrett’s esophagus; UC, ulcerative colitis; CD, Crohn’s disease; PSC, primary sclerosing cholangitis.
Cancer risk in IBD
Two major types of IBD—ulcerative colitis (UC) and Crohn’s disease (CD)—are recognized, and the extent and duration of colitis and the severity of inflammation in the colon over the clinical course are risk factors for neoplastic complications in both UC and CD [4–7]. In addition, the family history of colorectal cancer, young age at diagnosis, and the presence of primary sclerosing cholangitis (PSC) increase the risk of IBD-associated neoplasia [7, 8]. Anti-inflammatory treatment in IBD is associated with a reduced risk for neoplasia [9]. The increased risk of developing carcinoma in the setting of IBD is the single most important reason for surveillance in IBD patients. Dysplasia is considered to be the earliest precursor to cancer. While Crohn and Rosenberg recognized the development of cancer arising in UC as early as 1925, it was not until 1967 that Morson and Pang [10] demonstrated that IBD-associated dysplasia can arise in flat mucosa, can be multifocal, and can be diagnosed on biopsy.
Although earlier literature showed disparity in colorectal-cancer risk between UC and CD, it is now widely accepted that the risk is roughly equal in UC and CD colitis. The absolute cumulative risk is 8% for UC and 7% for CD after 20 years of disease [3]. The cumulative probability of developing cancer in UC is 2% at 10 years, 8% at 20 years, and 18% at 30 years [8, 11]. For IBD, the annual incidence of colorectal cancer is about 0.8% for LGD according to a recent meta-analysis [12]. The progression rate of LGD to cancer also relates to the expertise of the pathologist, with an expert GI pathologist rendering LGD associated with higher risk of progression [12].
Cancer risk in BE
The length of BE, male gender, family history of esophageal cancer, and severity of chronic inflammation over the clinical course are associated with higher risk of BE-associated neoplasia. The presence of dysplasia is the best and most reliable marker of an increased risk of malignancy in BE. There are estimated to be about 17,990 new cases and 15,210 deaths attributable to esophageal cancer in 2013 in the USA [13]. BE is the only known precursor lesion for esophageal adenocarcinoma (EAC)—the most common histological type of esophageal cancer. The incidence of EAC has been reported to be 1.2–5.0 per 1,000 person-years in patients with non-dysplastic BE, 4.4–14.6 per 1,000 person-years in patients with LGD, and 6.6–19.0 per 1,000 person-years in HGD [14–29]. The incidence of HGD or EAC in patients with LGD is 17.3 per 1,000 person-years [15].
Interpretation and grading of IBD- and BE-associated neoplasia
In the landmark paper by Riddell et al. [3], a standardized classification of IBD-associated dysplasia was proposed for the evaluation of biopsy specimens from IBD patients, and this histologic grading system was later adopted for BE-surveillance biopsies [30, 31]. The diagnostic categories include: negative for dysplasia, indefinite for dysplasia, LGD, and HGD. In addition, neoplastic lesions more severe than HGD, such as IMC and SMC, may also be encountered by pathologists on mucosal biopsies. Of note, any dysplasia in IBD and BE should be reviewed by two pathologists, at least one with specialized expertise in GI pathology [2, 3].
Practical approach to IBD- and BE-surveillance biopsies
Typically, the histologic assessment to evaluate IBD- and BE-associated neoplasia starts at low power for the assessment of architectural features, and then cytologic features (such as nuclear enlargement, hyperchromasia, stratification and pleomorphism, loss of polarity, and abnormal mitoses) are assessed at higher power.
Negative for dysplasia
Negative for dysplasia means the epithelial changes are either normal or within the reactive spectrum proportional to the degree of inflammation.
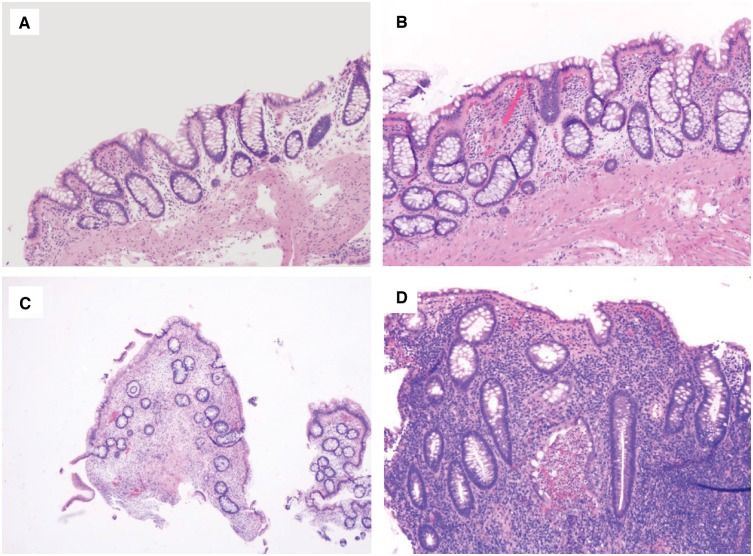
In an IBD colonic-surveillance biopsy, negative for dysplasia encompasses the following scenarios: (i) normal colonic mucosa (Figure 1A), (ii) chronic quiescent colitis (Figure 1B) or chronic inactive colitis (Figure 1C) after treatment and (iii) chronic active colitis with epithelial changes within the reactive/regenerative spectrum (Figure 1D). In the latter, the epithelium may show nuclear enlargement and hyperchromasia, but these changes are in proportion to the inflammation and there is surface nuclear maturation.
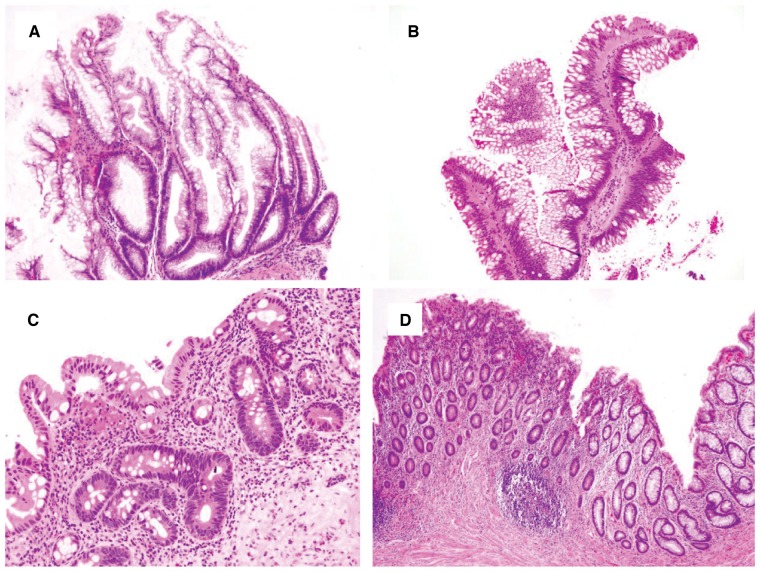
Figure 1.
Colonic mucosa, negative for dysplasia. (A) Normal colonic mucosa in inflammatory bowel disease surveillance as a result of effective treatment (H&E stain, magnification 200×). (B) Chronic quiescent colitis (H&E stain, magnification 200×). (C) Chronic inactive colitis. The biopsy shows crypt distortion and mild chronic inflammation, but without significant epithelial injury or neutrophilic inflammation (H&E stain, magnification 40×). (D) Chronic active colitis with reactive changes. The nuclear change is proportional to the inflammation. All these biopsies should be interpreted as negative for dysplasia (H&E stain, magnification 200×).
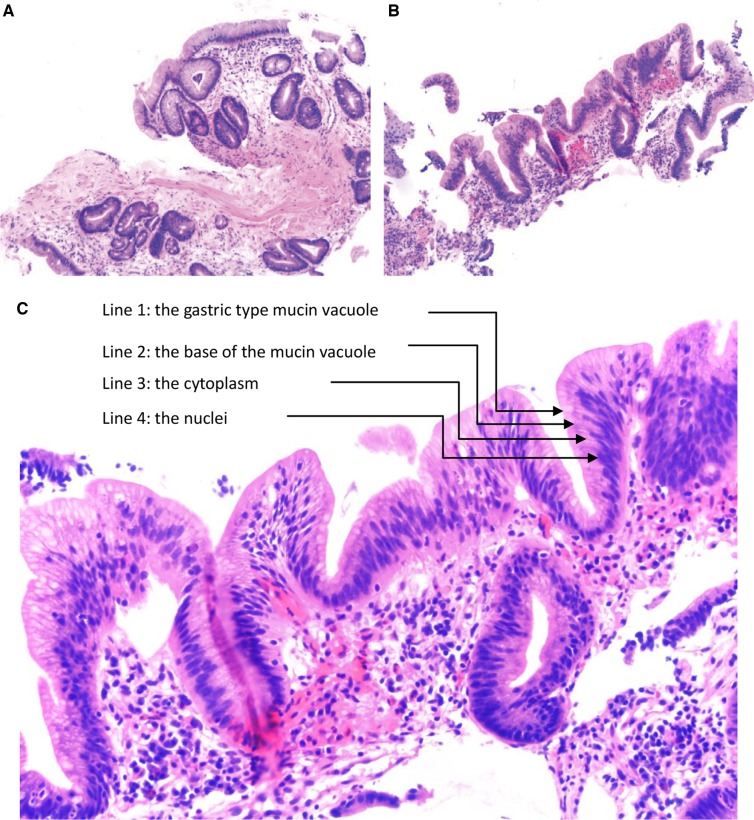
In a BE-surveillance biopsy, negative for dysplasia encompasses the following scenarios: (i) the surface epithelium shows maturation, the nuclei are bland and uniform with smooth contour, the cells have low nuclear/cytoplasmic ratio, and there is no architectural complexity or cytologic atypia (Figure 2A) and (ii) the basal glands may show mild regenerative atypia referred to as the “baseline atypia” of Barrett’s mucosa, but there is surface maturation (Figure 2B).
Figure 2.
Barrett’s esophagus (BE), negative for dysplasia. (A) BE biopsy showing intestinal metaplasia and surface maturation (H&E stain, magnification 200×). (B) BE shows epithelial reactive changes (H&E stain, magnification 200×). The nuclear change is proportional to the inflammation. (C) The presence of “four lines” in this biopsy indicates a diagnosis of negative for dysplasia (H&E stain, magnification 400×).
The most common diagnostic challenge is to make an interpretation of negative for dysplasia in cases with prominent reactive atypia in the presence of active inflammation. One recent study indicated that BE-surveillance biopsies with maintained cell polarity and surface gastric-type mucin vacuoles can be safely diagnosed as negative for dysplasia even with mild to moderate nuclear enlargement. Visual appreciation of “four lines,” with line 1 being the gastric-type mucin vacuole, line 2 being the base of the mucin vacuole, line 3 being the cytoplasm, and line 4 being the nuclei (Figure 2C), is a really helpful histologic feature seen in “negative for dysplasia” biopsies. In contrast, LGD typically does not maintain its four lines, with loss of apical mucin vacuole and the cytoplasm line due to nuclear stratification [32]. Also, surface-only nuclear stratification, upper mucosa-limited atypia (“top-heavy” atypia), and villiform architecture support reactive changes [33].
Most IBD- (90% at the colonoscopy level) and BE-surveillance biopsies (90.4% at the endoscopy level) are negative for dysplasia [32, 34]. These patients can be followed with recommended surveillance intervals of 1–3 years for IBD and 3–5 years for BE. Two consecutive negative colonoscopies in IBD-surveillance biopsies indicate a very low risk for colorectal cancer [35] and a potentially longer surveillance interval in this selected population may be safe.
LGD
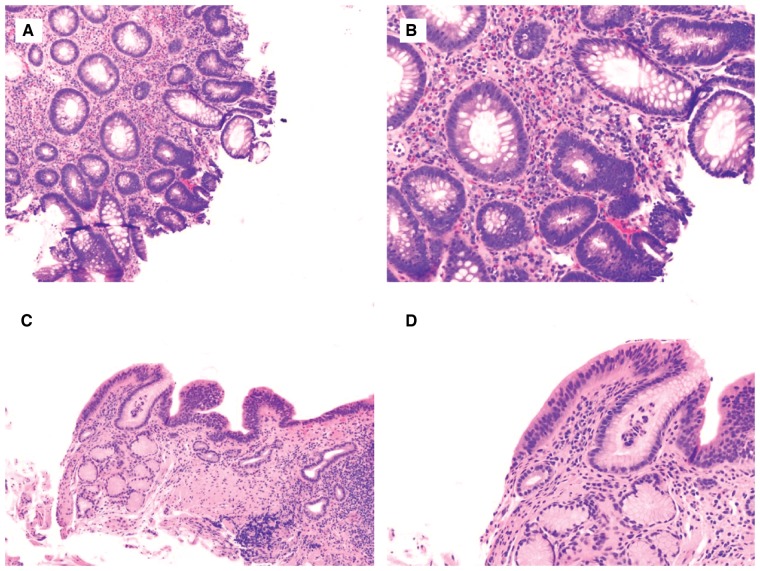
LGD is characterized by some glandular crowding, with mildly decreased lamina propria along with nuclear hyperchromasia, nuclear enlargement, and an increased nuclear-to-cytoplasmic ratio. In many but not all cases, there is also nuclear stratification, in which nuclei become elongated and pencillate in morphology, resembling colonic adenoma (Figure 3A–D). However, the nuclei maintain their polarity with respect to the basement membrane and typically do not occupy the superficial aspect of the epithelium. The diagnostic frequency of LGD in IBD patients at the colonoscopy level is 11.1%. Overall, the rate of flat LGD in IBD at the colonoscopy level is 2.3% and at the biopsy level is 0.09% [36]. Similarly, LGD in BE has a diagnostic frequency of 2.5% at the endoscopy level [32].
Figure 3.
Low-grade dysplasia in inflammatory bowel disease (IBD)- and Barrett’s esophagus (BE)-surveillance biopsies. (A) and (B) IBD colonic biopsy shows focal area resembling tubular adenoma, consisting of glands lined by enlarged, stratified, hyperchromatic nuclei (H&E stain; (A) magnification 100×; (B) magnification 200×). The changes extend to the surface epithelium (lack of surface maturation). There is no architectural complexity, loss of nuclear polarity, or significant pleomorphism—features to suggest HGD. (C) and (D) Similar features are present in a BE-surveillance biopsy, supporting the diagnosis of low-grade dysplasia (H&E stain; (C) magnification 40×; (D) magnification 100×).
The key features for diagnosing LGD that have moderate to good inter-observer agreement are (i) abrupt transition with a distinct area of glands that demonstrate a lack of surface maturation, (ii) mucin depletion, (iii) nuclear enlargement and hyperchromasia, and (iv) an increase in mitosis [36]. In addition, some variants of IBD-associated dysplasia and subtypes of BE-associated dysplasia will be discussed later in this paper.
HGD
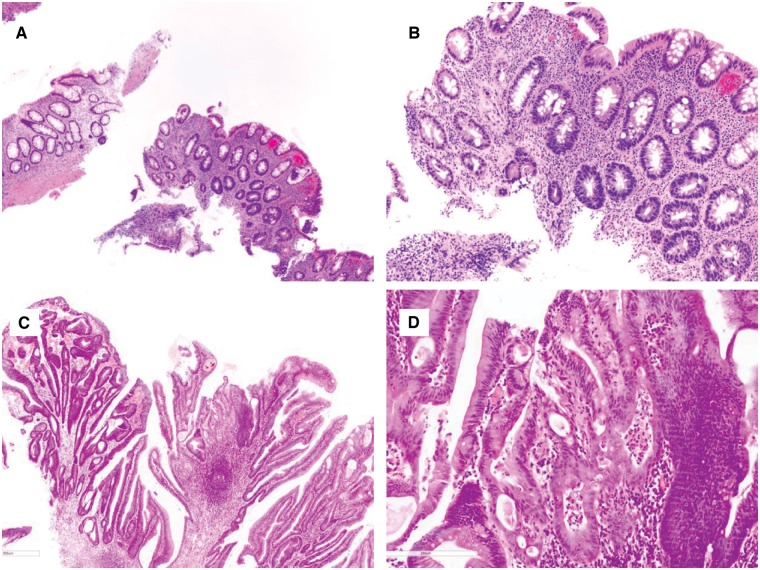
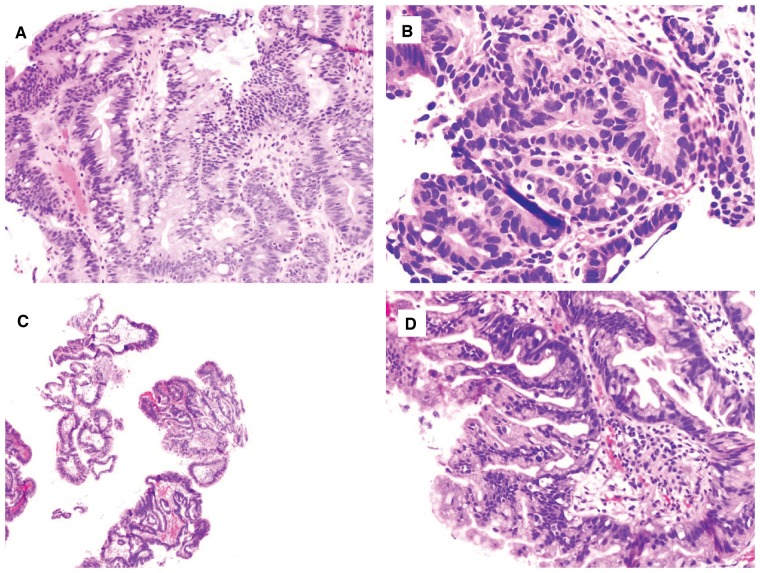
HGD is characterized by greater architectural complexity (glandular crowding and cribriforming or significant papillary architecture) and cytological atypia (loss of nuclear polarity, greater nuclear pleomorphism, and enlargement) compared to LGD, in both IBD- and BE-surveillance biopsies (Figures 4 and 5).
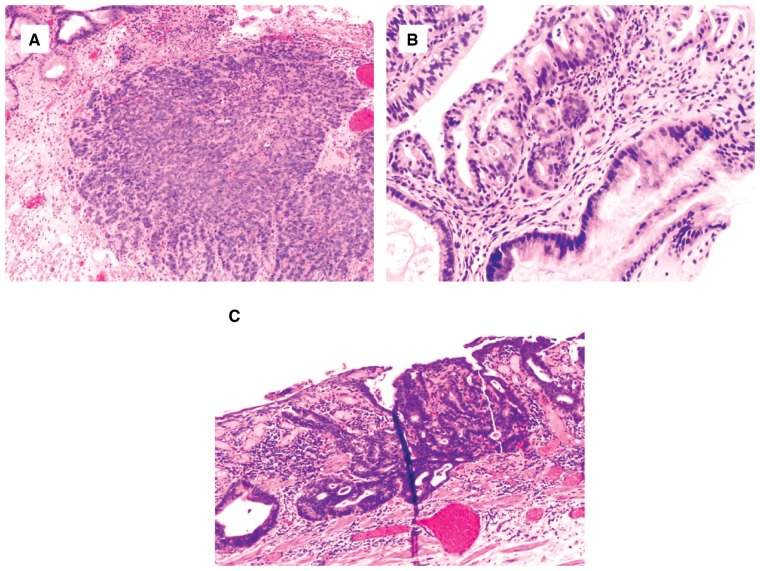
Figure 4.
Chronic colitis with high-grade dysplasia. (A) and (B) This biopsy shows an area consisting of atypical glands lined by enlarged dark nuclei without surface maturation (H&E stain; (A) magnification 100×; (B) magnification 200×). The adjacent fragment provides an internal negative control. (C) and (D) This colon biopsy shows architectural and cytologic features of high-grade dysplasia (H&E stain; (C) magnification 40×; (D) magnification 200×). There is focal cribriforming, nuclear pleomorphism, and loss of nuclear polarity.
Figure 5.
Barrett’s esophagus with high-grade dysplasia. (A) and (B) This biopsy shows focus of atypical and crowded glands lined by cells containing enlarged and hyperchromatic nuclei, extending to the surface ((A) H&E stain, magnification 200×). There is a high nuclear/cytoplasmic ratio and nuclear pleomorphism ((B) H&E stain, magnification 400×). (C) and (D) This biopsy shows proliferation of glands with marked architectural complexity and enlarged and hyperchromatic nuclei. There is no surface maturation (H&E stain; (C) magnification 40×; (D) magnification 200×).
Unlike the thin, pencillate nuclei of LGD, the nuclei in HGD tend to be somewhat rounder, oval-shaped, or irregular, with more profound atypia. There are often increased atypical mitotic figures in HGD. A diagnosis of HGD can be made based on the presence of either marked cytological or marked architectural complexity. Overall, HGD in IBD is relatively uncommon, with a 0.4% frequency at the colonoscopy level [32]. Similarly, HGD in BE has a diagnostic frequency of 1.3% at the endoscopy level [32].
Histologic features of negative for dysplasia, LGD, and HGD in IBD- and BE-surveillance biopsies are summarized in Table 2.
Table 2.
Histological features of negative for dysplasia, low-grade dysplasia, and high-grade dysplasia in inflammatory bowel disease and Barrett’s esophagus surveillance biopsies
| Histologic feature | Negative for dysplasia | Low-grade dysplasia | High-grade dysplasia |
|---|---|---|---|
| Surface maturation | Present | Absent | Absent |
| Architectural and cytological abnormalities | Normal to within reactive spectrum, i.e. proportional to inflammation or injury | Abnormal in either or both, mild to moderate | Abnormal in either or both, profound |
| A distinct focus of glands | Absent | Present | Present |
| Architectural abnormalities | Absent to mild (in the presence of inflammation and ulceration) | Crowding but still have moderate amount of lamina propria between glands | Crowding with diminished amount of lamina propria between glands |
| Cribriforming glands and/or papillation of surface epithelium | Absent or minimal (in the presence of inflammation) | Absent or minimal (in the presence of inflammation) | Present in many cases |
| Degree of nuclear stratification | Absent or minimal (in the presence of inflammation) | Halfway to the luminal surface of epithelium | Full-thickness stratification to the luminal surface of epitheliu |
| Loss of mucin | Absent or mild (in the presence of inflammation) | Present | More profound |
| Dystrophic goblet cells | Absent or focal | May be present | May be present |
| Nuclear enlargement | Absent to mild (in the presence of inflammation) | Present | Present, profound |
| Nuclear hyperchromasia | Absent to mild (in the presence of inflammation) | Present | Present |
| Nuclear pleomorphism | Absent | Absent | Present in most cases |
| Nuclear polarity | Maintained | Maintained | Lost |
| Increased mitosis | Absent or present | Present | Present |
| Abnormal mitosis | Absent | May be present in few cases | May be present in few cases |
| Apoptosis in glands | Absent or present (in the presence of inflammation) | May be present even in the absence of inflammation | May be present even in the absence of inflammation |
| Nucleolar enlargement | Absent or present (a single prominent nucleolus in the presence of inflammation) | Usually absent | Absent or present |
| Inflammation | Absent or present | Usually minimal inflammation | Usually minimal inflammation but can be prominent |
Indefinite for dysplasia
Indefinite for dysplasia is applied to epithelium with cytomorphological features worrisome for dysplasia in the presence of significant inflammation, erosions, and/or adjacent ulceration, so that it is difficult to discern whether this is true dysplasia or exuberant reactive/regenerative changes. Reactive changes associated with marked inflammation can closely resemble dysplasia, including loss of mucin, nuclear enlargement and hyperchromasia, nuclear overlapping, and architectural complexity. Riddell proposed further stratification of this diagnosis into “indefinite for dysplasia, probably positive” and “indefinite for dysplasia, probably negative,” which would theoretically call for surveillance within 3–6 months for BE and within 6–9 months for IBD [3]; however, this practice of subtyping varies within different institutions. Overall, indefinite for dysplasia in IBD is not common, with a frequency of 1.6% at the colonoscopy level [34], and is more frequently diagnosed in a BE-surveillance biopsy with a rate of 4.3%–8.6% at the endoscopy level [32].
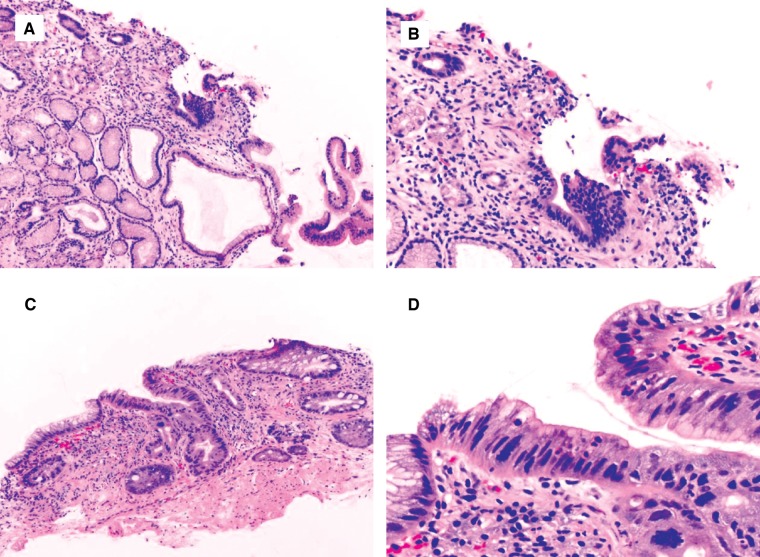
The key features of diagnosing indefinite for dysplasia are glands with cytomorphological features of dysplasia in the presence of active inflammation; a few highly atypical glands in active inflammation; a few highly atypical glands without surface epithelium, due to either tangential section, mechanical sloughing, or erosion; a few dark glands with crushing artifact; or detached atypical epithelium (Figures 6 and 7).
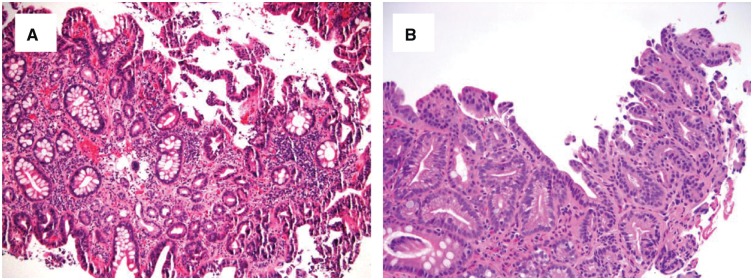
Figure 6.
Chronic colitis with epithelial changes indefinite for dysplasia, likely positive for dysplasia. This biopsy shows focal hypermucinous changes in a minute fragment ((A) H&E stain, magnification 40×; (B) H&E stain, magnification 100×). This feature is worrisome for, but not diagnostic of, dysplasia, and thus is best interpreted as indefinite for dysplasia.
Figure 7.
Barrett’s esophagus with epithelial changes indefinite for dysplasia. (A) and (B) This biopsy shows a few distinct glands lined by epithelial cells with dark and enlarged nuclei (H&E stain; (A) magnification 100×; (B) magnification 200×). There is no surface present and thus it is best interpreted as indefinite for dysplasia. (C) and (D) This biopsy show a distinct single gland lined by epithelial cells with dark, enlarged, and pleomorphic nuclei (H&E stain; (C) magnification 200×; (D) magnification 400×). This case may be interpreted as indefinite for dysplasia due to the extremely limited nature of the biopsy.
A diagnosis of indefinite for dysplasia in IBD- and BE-surveillance biopsies carries an intermediate risk for progression to cancer and serves to increase the surveillance frequency [37–39]. Choi et al. [40] reported that 13% of cases of indefinite for dysplasia in IBD biopsies were found to have LGD and 2% had HGD or carcinoma at a follow-up of 28 months.
Doing recut levels, applying the “four-line” rule in BE biopsies, and using ancillary p53 stain in occasional cases (discussed below) can help with an accurate diagnosis or favor one over the other.
HGD with marked architectural distortion cannot rule out carcinoma or features suspicious for carcinoma
Not uncommonly, we encounter the scenario in which there are features of HGD and only focal or equivocal features suspicious for invasion. In these cases, we may use the terminology “high-grade dysplasia with marked architectural distortion cannot rule out carcinoma (HGD MAD)” [41] or “HGD with features suspicious for carcinoma (HGD/S)” [42], especially if the lesion is clinically, endoscopically, and radiographically worrisome for an invasive component. These terms are more often used for a BE-surveillance biopsy.
The reported key features for diagnosing HGD/MAD or HGD-S overlap and include a cribriform growth pattern, at least three dilated glands with intraluminal necrotic debris, glandular crowding (“back-to-back” gland pattern), ulcers and a large number of neutrophils within HGD, and the incorporation of HGD into a benign squamous epithelium [41–43] (Figure 8).
Figure 8.
Barrett’s esophagus with features suspicious for carcinoma. (A) This biopsy shows features of high-grade dysplasia (HGD). In addition, there are a few glands with eosinophilic luminal debris (H&E stain, magnification 100×). (B) This biopsy shows HGD. In addition, there is one single cell infiltrating into the lamina propria (H&E stain, magnification 100×). (C) This biopsy shows HGD. In addition, there are a few glands with eosinophilic luminal debris and incorporation of neoplastic glands onto the squamous epithelium (H&E stain, magnification 200×). (D) This biopsy shows HGD. In addition, there is back-to-back glandular crowding (H&E stain, magnification 100×). All these features are worrisome for, but not diagnostic of, intramucosal adenocarcinoma.
Intramucosal adenocarcinoma
This diagnosis is usually made in the setting of BE-associated neoplasia. The criteria include more than one focus of a single cell infiltrating the lamina propria, sheets of cells obliterating the lamina propria, a never-ending/anastomosing gland pattern, and abortive or angulated glands infiltrating the lamina propria (Figure 9).
Figure 9.
Barrett’s esophagus with intramucosal adenocarcinoma (IMC). IMC is defined as invasion of neoplastic cells beyond the basement membrane of the glands but not beyond the muscularis mucosae. It can exhibit (A) a solid growth pattern (H&E stain, magnification 100×), (B) invasion of individual neoplastic cells in the lamina propria (H&E stain, magnification 200×), or (C) an anastomosing glandular pattern (H&E stain, magnification 100×).
Submucosal adenocarcinoma
A diagnosis of SMC on a mucosal biopsy requires neoplastic glands surrounded by unequivocal stromal desmoplasia.
Histologic variants and special issues in IBD- and BE-associated neoplasia
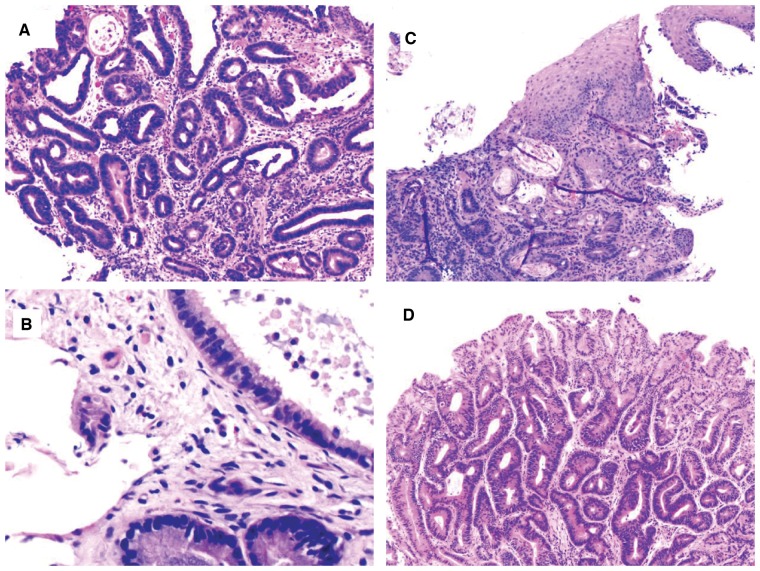
Histologic variants of IBD-associated dysplasia
While many IBD-associated dysplastic lesions histologically resemble sporadic adenomas, with pseudostratified and pencillate nuclei extending to the surface epithelium, IBD patients often have dysplastic lesions that are uniquely dissimilar to sporadic adenomas [3, 37, 44]. Subtyping IBD-associated dysplasia has been recently attempted by a group of GI pathologists and included seven categories: conventional adenoma-like dysplasia, terminal epithelial differentiation, sessile serrated polyp/adenoma (SSP/A)-like dysplasia (Figure 10A), traditional serrated adenoma (TSA)-like dysplasia (Figure 10B), hypermucinous dysplasia, goblet cell-depleted dysplasia, and serrated dysplasia not otherwise specified [45]. Diagnostic agreement for these categories ranged from <50% for TSA-like to 92% for goblet cell-depleted dysplasia. Terminal epithelial differentiation dysplasia and goblet cell-depleted dysplasia are rare and are characterized by flat configuration, non-crowded crypt pattern, and cytoplasmic features that either simulate the repertoire of normal colonic epithelial cells (terminal epithelial differentiation) (Figure 10C) or are devoid of goblet cells (goblet-cell-depleted dysplasia) (Figure 10D). The key to the diagnosis of terminal epithelial differentiation and goblet-cell-depleted dysplasia is nuclear enlargement and hyperchromasia without surface maturation. Some of these categories may include lesions previously diagnosed as indefinite for dysplasia. There are sparse data on SSP/A in IBD; in one recent study of 13 IBD patients with index SSP/A followed over a median of 6 years, 61.5% developed metachronous visible dysplasia or additional SSP/A, suggesting that SSP/A should be treated as a variant of dysplasia that needs complete endoscopic removal and continuous surveillance [46]. This novel classification has laid the groundwork for future studies on the biology and natural history of these lesions. For example, attempts of subtyping IBD-associated colorectal carcinoma (CRC) showed that about 53% are conventional, 22% mucinous, 15% low-grade tubuloglandular, 8% serrated, and 2% special subtypes. Interestingly and not surprisingly, this study revealed that conventional-type CRC was associated with conventional adenoma-like dysplasia; mucinous adenocarcinoma with conventional villous adenoma-like dysplasia; low-grade tubuloglandular adenocarcinoma with goblet cell-depleted dysplasia [47]. These associations suggest that the genetic basis underlying the variation in dysplasia morphology persists in the form of distinct carcinoma subtypes.
Figure 10.
Variant of chronic colitis-associated dysplasia. (A) Chronic colitis with sessile serrated polyp/adenoma (H&E stain, magnification 100×). Biopsy from this polyp from the patient’s colon involved in chronic colitis reveals a lesion resembling a sessile serrated polyp. (B) Chronic colitis with low-grade dysplasia, resembling a traditional serrated adenoma (H&E stain, magnification 100×). (C) Chronic colitis with low-grade dysplasia, terminal epithelial differentiation type (H&E stain, magnification 200×). This biopsy shows an area consisting of “normally appearing” colon glands and surface epithelium. But there is hyperchromasia and nuclear enlargement, and no active inflammation. (D) Chronic colitis with low-grade dysplasia, goblet-cell-depleted type (H&E stain, magnification 40×). This flat area reveals uniform epithelial cells lacking goblet cells.
Despite these research efforts, there is no clinical utility to further subtype IBD-associated dysplasia or IBD-associated CRC. But, an awareness of the existence of these variants may help one either diagnose or make a decision to send such rare and unusual cases out for an expert consultation for optimal patient management.
Special issues with IBD-associated dysplasia
The term “dysplasia-associated lesion or mass” (DALM), in theory, denotes a lesion that arises in association with inflammation, has greater architectural complexity, has a greater villous component, and is more likely to have skip areas of dysplasia within the lesion as well as dysplasia in the surrounding mucosa [48]. As terminology has evolved, “adenoma-like” and “non-adenoma-like” have been coined and are applied, respectively, to lesions that appear polypoid or non-polypoid endoscopically. These terms have recently fallen out of favor, as they have not been shown to be practically worthwhile for guiding management of IBD-associated dysplasia [37, 48, 49]. Thus, in current pathology practice, these terms are not used any more.
Endoscopic/surgical management and surveillance of IBD dysplasia
Detection of dysplasia traditionally has relied on both the examination of the mucosa with targeted biopsies of visible lesions and extensive random biopsies to identify invisible dysplasia. Current US guidelines recommend obtaining at least 32 random biopsy specimens from all segments of the colon as the foundation of endoscopic surveillance (Table 1) [9, 50]. However, with advances in colonoscopic technology (i.e. high-resolution white-light colonoscopy, chromoendoscopy, and narrow-band imaging), the current management guidelines for patients with IBD endorse a combination of endoscopic assessment of lesion resectability and the histologic grading of dysplasia. The IBD colon-surveillance-biopsy protocol still varies greatly within different institutions. Common practice includes targeted biopsies and random biopsies with high-definition white-light colonoscopy or chromoendoscopy [51]. In the consensus statement, the term “endoscopically resectable” indicates that the lesion has distinct margins, appears to be completely removed on visual inspection after endoscopic resection, histology confirms the complete removal of the lesion, and there is no dysplasia in the mucosa adjacent to the lesion [1]. Thus, detection of an endoscopically visible lesion and determination of endoscopic resectability of such a lesion by the endoscopist becomes critical for the adequate management of IBD with dysplasia. The role of endoscopist in this process includes (i) generating a detailed endoscopic report and (ii) submitting endoscopically resected lesion(s), biopsy from mucosa adjacent to the lesion, and random biopsies in separate jars with accurate labeling. The role of pathologists in this process includes (i) confirming the presence of dysplasia in the endoscopically resected lesion and the margin status, (ii) assessing for dysplasia in the biopsy adjacent to the lesion, and (iii) assessing for dysplasia in the random biopsies: the so-called “flat dysplasia.”
If a dysplastic lesion, either LGD or HGD, is removed completely endoscopically and confirmed histologically, the patient can continue surveillance without colectomy, provided that there is no dysplasia in the biopsy at the base of the lesion and there is no dysplasia in the random biopsies. In cases with HGD on random (i.e. endoscopically invisible) biopsy, colectomy is usually recommended. In cases with focal LGD on random biopsy, the patient may undergo intensified surveillance with a shortened interval of 3–6 months or be referred to an IBD center for a repeat colonoscopy with high resolution and/or chromoendoscopy. In patients with multifocal LGD on random biopsy or persistent LGD, colectomy is recommended. Terminology for reporting the findings on IBD-surveillance colonic biopsy of IBD patients and subsequent management are summarized in Table 3.
Table 3.
Terminology for reporting findings in surveillance colonic biopsy of inflammatory bowel disease patients
| Location of biopsy | Endoscopic finding | Pathologic diagnosis | Implications |
|---|---|---|---|
| Outside of colitis region | Polyp or sessile lesions | Sporadic adenoma, hyperplastic polyp, or sessile serrated polyp | Complete removal with routine IBD annual surveillance |
| Inside of colitis region | Polyp (resectable) | Polypoid LGD or HGD | Complete removal with intensified surveillance |
| Polyp (unresectable) on conventional colonoscopy | Polypoid LGD or HGD (should be confirmed by another GI pathologist) | IBD expert referral with chromoendoscopy or colonoscopy of high resolution:
|
|
| Visible but unresectable mass/lesion (elevated, flat, depressed) or invisible on conventional colonoscopy | LGD, HGD, or invasive adenocarcinoma (should be confirmed by another GI pathologist) | Focal LGD: intensified surveillance or referral to an IBD center for a repeat colonoscopy with high resolution and/or chromoendoscopy or colectomy (depending on clinical and endoscopic suspicion) HGD: colectomy Invasive adenocarcinoma: colectomy | |
| Sessile lesion | Sessile serrated polyp | Complete removal with routine IBD annual surveillance |
IBD, inflammatory bowel disease; LGD, low-grade dysplasia; HGD, high-grade dysplasia.
Histologic variants of BE-associated dysplasia
Crypt dysplasia.
Loss of surface maturation is a key feature to support the diagnosis of dysplasia in most BE-surveillance biopsies. However, certain practical dilemmas do exist. “Deep crypt dysplasia or basal crypt dysplasia” is a situation that low-grade or even HGD is present but limited to the deep crypts, while the surface maturation is maintained [52, 53]. Studies have shown that, in deep crypt dysplasia, conventional dysplasia actually is present in about 47% of cases, and the former may share similar molecular signature, such as aneuploidy and mutated p53 expression pattern, similar to conventional dysplasia [52]. Therefore, it is important to recognize this variant of dysplasia. When in doubt, deeper sections should be performed and it may be reasonable to at least label the case with “indefinite for dysplasia” so that close follow-up could be indicated.
Dysplasia type. Attempts have been made to type BE-associated dysplasia by several researchers as intestinal-type (adenomatous-type) and gastric foveolar-type (non-adenomatous-type) [33, 54, 55]. Adenomatous-type dysplasia is composed of glands lined by tall columnar cells with pencillate, hyperchromatic, variably stratified nuclei and dense eosinophilic cytoplasm, resembling colorectal adenoma (Figure 11A). In contrast, foveolar-type dysplasia is characterized by cells with mucinous to eosinophilic to amphophilic cytoplasm and round to oval, non-stratified, basally located nuclei, many of which have prominent nucleoli (Figure 11B). The glands in foveolar-type dysplasia have a propensity to be smaller, more uniform, and closer to each other. While loss of nuclear polarity is an objective criterion used to separate low- from high-grade adenomatous dysplasia, this criterion cannot be used to separate low- from high-grade gastric dysplasia, as their nuclei are round and non-stratified, and do not have a longitudinal axis. Instead, the distinction between low- and high-grade foveolar dysplasia primarily relies on the nuclear size three to four times or more the size of a mature lymphocyte as a major criterion for HGD [33, 54]. In some cases, nuclear pleomorphism and glandular cribriforming and marked crowding may also help to diagnose foveolar-type HGD [33]. Inter- and intra-observer agreement in the assessment of foveolar and adenomatous dysplasia varies greatly [33]. In addition, up to 27% of BE-associated dysplasia is hybrid-type [56]. Thus, in current pathology practice, there is no need to type BE-associated dysplasia; however, the awareness of this variant is needed to accurately diagnose dysplasia and not confuse it with reactive changes.
Figure 11.
Two types of Barrett’s esophagus-associated dysplasia. (A) Adenomatous-type low-grade dysplasia (H&E stain, magnification 100×). The dysplasia consists of hyperchromatic pencillate nuclei, resembling nuclear changes in colonic adenoma. (B) Gastric foveolar-type low-grade dysplasia (H&E stain, magnification 100×). This area is composed of glands lined with epithelial cells with a round and basally located nucleus. The nucleus contains open chromatin and is inconspicuous to the variably prominent nucleolus. The cells contain apical mucin. The morphology does not resemble “colonic adenoma.”
Endoscopic/surgical management and surveillance of BE dysplasia
According to the latest ACG 2016 recommendations, endoscopic therapy is considered as the preferred treatment modality for BE-LGD (endoscopic surveillance being an acceptable alternative) and patients with confirmed BE-HGD should be managed with endoscopic therapy [2]. Patients with nodularity in the BE segment should undergo EMR of the nodular lesion(s) as the initial diagnostic and therapeutic maneuver, and further therapy should be based upon histological assessment of the EMR specimen. If the EMR specimen demonstrates HGD or IMC, endoscopic ablative therapy of the remaining BE should be performed. Overall, the guidelines call for the increased use of endoscopic therapy in the form of ablation and/or EMR/ESD.
Buried intestinal metaplasia and neoplasia after endoscopic ablation of BE and BE-associated dysplasia
Endoscopic ablation of BE-associated dysplasia has advanced significantly in the past two decades with the initial use of photodynamic therapy to the current use of radiofrequency ablation [57, 58]. Ablation usually results in the replacement of the columnar epithelium with the squamous epithelium (referred to as the neosquamous epithelium). The neosquamous epithelium has been shown to be devoid of the molecular alterations characteristic of BE [59]. However, one of the complications of ablation is that residual Barrett’s epithelium and/or dysplasia may remain under the neosquamous epithelium and remain invisible to the endoscopist’s eye—so-called “buried BE” or “buried neoplasia.” In a recent review, the prevalence of buried BE was 14% after photodynamic therapy and 0.9% after radiofrequency ablation [60]. However, the post-ablation biopsies are often superficial and may miss deeper foci of buried BE, and hence this percentage may be an underestimation. In addition, there are reports of progression of buried BE and BE-associated dysplasia after endoscopic therapy to cancer in some instances [61, 62]. Buried dysplasia may be difficult to diagnose, as the features used by pathologists to diagnose dysplasia such as the assessment of surface maturation cannot be evaluated easily in buried glands covered by the post-ablated neosquamous epithelium. Thus, these post-ablation patients should be followed by continued surveillance and biopsies taken from the original length of BE in addition to the biopsy from the esophagogastric junction [2].
Histologic evaluation of endoscopically resected BE-associated neoplasia
Specimen preparation. The techniques of EMR and ESD are commonly used to treat superficial neoplasms in the esophagus. All lesions received in surgical pathology via endoscopy are pinned out flat on a cork or foam board in most institutes/hospitals. All EMR and ESD specimens have peripheral and deep resection margins and they should be handled as follows using the protocol as previously reported [63]:
If the specimen is oriented, a diagram or a picture can be helpful.
Measure the specimen and identify the lesion.
Measure the lesion and determine the nearest peripheral margin.
If the specimen is oriented, it is treated similarly to an oriented skin ellipse.
Three different ink colors are typically sufficient; always use black on the deep margin; blue and orange (or yellow) are applied to opposing peripheral edges.
If no orientation is provided, a single ink color can be used for the peripheral margin.
Cut 2- to 3-mm sections perpendicular to the long axis and lay sections “on edge” in cassettes.
Determine the deepest extent of the lesion.
Document the distances of the lesion to the nearest peripheral and deep margins in the gross description.
Submit peripheral margins perpendicular or, if the lesion is >1 cm away, en-face is acceptable.
Entirely submit the specimens if the specimen is not large.
For larger dissections, use appropriate judgement—or consult the pathologist.
*Submit the nearest peripheral margins (perpendicular quadrants).
*Submit the entire lesion if it fits in 10 cassettes.
*If the lesion is too large for 10 cassettes, use the general rule (one section per centimeter of the most suspicious areas) and include those sections with the closest deep margin and deepest invasion.
*If no definitive invasive component is identified on initial blocks, the entire lesion must be entirely submitted to microscopically evaluate for micro-invasion.
Histologic evaluation. Histologic evaluation and accurate diagnosis of BE-associated neoplasm in EMR and ESD specimens play an essential role in the subsequent management of the patients. Key features to look for are: depth of invasion (mucosa, submucosa, or beyond), grade of adenocarcinoma, lymphovascular invasion, as well as the status of resection margins. For SMC, the depth of submucosal invasion needs to be measured from the deepest layer of the muscularis mucosae into the submucosa and reported in μm. A cancer template should be used to report the tumor parameters (https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates).
Intramucosal adenocarcinoma. Generally speaking, IMC is not used in colonic lesions or biopsy for the most part, as invasion of the lamina propria does not increase the risk for nodal metastasis. In contrast, IMC in BE has a nodal metastasis rate of 3.4% [64]. The key feature for diagnosing IMC is to recognize the invasion of the neoplastic cells through the basement membrane into the lamina propria but not beyond the muscularis mucosae. Microscopically, the tumor could be categorized as well differentiated (more than 95% gland formation), moderately differentiated (50%–95% gland formation), or poorly differentiated carcinoma (less than 50% gland formation). Just like HGD, endoscopic therapy is the preferred treatment for IMC. However, a careful search for a signet ring cell component, poor differentiation component, and lymphovascular invasion is important, as IMC with these features may be treated with esophagectomy due to the high risk of nodal metastasis [64]. The status of margins (both deep and lateral mucosal edges) should also be reported.
Submucosally invasive adenocarcinoma. The treatment of SMC of the esophagus used to be esophagectomy because of the high rate of nodal metastasis. However, this practice has undergone significant changes, as we know more about the biology of SMC. One recent review article revealed a “rule of doubling” for the frequency of lymph-node metastases in patients with SMC: tumor invasion into each progressively deeper one-third of the submucosal layer corresponds to a 2-fold increase in the risk of nodal metastases (9.9% in SMC invading the superficial one-third of the submucosa, 22.0% in SMC invading the mid one-third of the submucosa, and 40.7% in SMC invading the deep one-third of the submucosa) [65]. In a recent study, lymphovascular invasion, poor differentiation, and a depth of invasion >500 μm into the submucosa have been shown to be associated with lymph node metastasis. For low-risk SMC, which is defined as invasion <500 μm into the submucosa, has no lymphovascular invasion, and is well or moderately differentiated, there is no reported lymph node metastasis [66].
The key feature for diagnosing SMC is to recognize invasion of the neoplastic cells through the muscularis mucosae. Optimal embedding and orientation, identification of submucosally located structures, and recognition of desmoplasia are essential. When a diagnosis of SMC is made, the depth of invasion should be reported, either in thirds (superficial, mid-, or deep thirds of the submucosa) or in micrometers of the distance from the deepest layer of the muscularis mucosae to the deepest tumor invasion into the submucosa (as measured by using an ocular micrometer). As with IMC, efforts should be taken to identify lymphovascular invasion and other components such as a signet ring cell and neuroendocrine carcinoma associated with an adverse outcome. In addition, the status of margins should be reported.
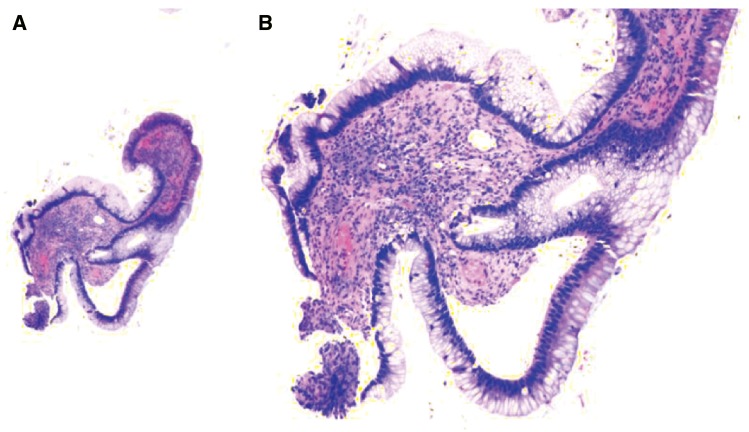
Diagnosis of SMC can be challenging in EMR/ESD specimens, as duplicated muscularis mucosae is very common in BE as a reparative/regenerative response and is present in about 92% of BE resections [67]. The deeper layer is the original muscularis mucosae and the superficial layer is the newly formed (neo) muscularis mucosae. In EMR or ESD specimens, the deeper layer of the muscularis mucosae is not always seen or well preserved, which is a potential pitfall for overstaging IMC as SMC. Identification of submucosally located structures such as esophageal mucus glands and large caliber vessels (Figure 12) and recognition of desmoplasia might be helpful.
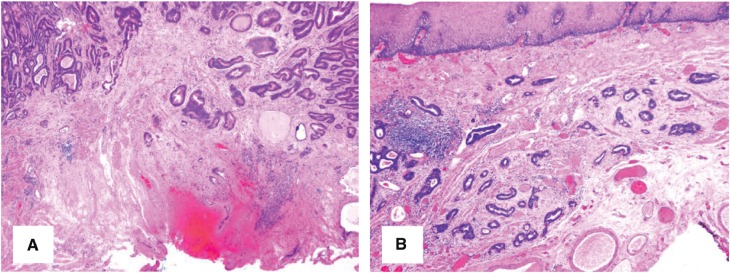
Figure 12.
Barrett’s esophagus with submucosally invasive adenocarcinoma (SMC) in an endoscopic submucosal dissection specimen. (A) SMC is characterized by invasion of neoplastic cells in the submucosa with desmoplasia (H&E stain, magnification 20×). (B) In some cases, the stromal change may be myxoid (H&E stain, magnification 20×). The large vessels help to confirm the submucosal location of the neoplastic glands.
Ancillary tests for IBD- and BE-associated neoplasia
Histomorphological assessment of H&E-stained IBD colonic biopsies and BE-surveillance biopsies remains the standard practice. Judicious use of ancillary tests in rare difficult cases and under certain circumferences based on initial histomorphological assessment and suspicion is justifiable. But universal use of such tests should not be advocated.
Although numerous studies have been performed to identify such magic biomarkers, none of them has been validated in prospective studies and the quality of these studies varied significantly, as most of them used histologic diagnoses as the gold standard. The most useful biomarkers are p53 and DNA content [68–73]. In one reasonably sized study, 57% IBD-associated dysplasia and colorectal cancer had increased p53 expression (defined as 8% nuclei with strong immunoreactivity) [73]. In a subsequent study by the same group, the authors demonstrate strong nuclear p53 expression in biopsy with indefinite for dysplasia is associated with neoplasia progression [38]. A judicious use of p53 is in cases with profound cytologic atypia worrisome for dysplasia but there is active inflammation or erosion. Strong and diffuse p53 immunoreactivity may help to diagnose or favor dysplasia in this scenario. Immunohistochemistry for p53 may also be helpful in confirming rare variants such as hypermucinous change and goblet-cell-depleted lesion as dysplasia. A basal pattern of p53 nuclear staining pattern is also reported in IBD-surveillance biopsies [71]. Cases with this staining pattern should be labeled as indefinite for dysplasia if the histomorphology is suspicious but not sufficient for a definitive diagnosis of dysplasia.
Studies on biomarkers for BE-associated neoplasia have been done similarly to those for IBD, but with more robust data, particularly on p53 [74]. Immunostain for p53 appears to increase diagnostic stratification in BE-surveillance biopsies and decrease the proportion of indefinite for dysplasia diagnoses [75]. Faint scattered p53 positivity within nuclei typically correlates with a wild-type gene status, whereas either strong nuclear positivity or complete absence (null pattern) of staining correlates best with TP53 mutations. Routine use of p53 in BE surveillance to diagnose BE dysplasia or predict BE neoplasia progression is not advocated at this point, as there are some issues with p53 immunostain such as the standardization of test and establishment of cutoffs to call the stain positive or not (stain intensity and scattered vs diffuse staining) [76]. One recent paper did report that one gland at least 50% nuclei-positive for strong p53 or more in the initial/index biopsy is associated with progression to HGD/adenocarcinoma [77], but this requires further validation. According to recommendations from the Rodger Haggitt GI pathology society (GIPS), “a diagnosis of dysplasia remains a morphologic diagnosis, ancillary stains are not recommended for diagnosing dysplasia in BE at this time. Although p53 is a promising marker for identifying high-risk BE patients, existing data are insufficient to recommend p53 staining for routine use as a prognostic marker at present” [76].
With the advances in molecular-testing and gene-sequencing technology, more promising biomarkers may emerge in the near future. However, currently, H&E stain remains the gold standard. In both IBD- and BE-surveillance biopsies, the wild-type p53 staining pattern does not exclude the diagnosis of dysplasia.
Conclusions
IBD and BE are chronic inflammatory diseases of the colon and the esophagus, respectively. Both are associated with increased risk of adenocarcinoma and the risk is increased with the length of diseased segment, histological severity of inflammation, and family history of cancer. A surveillance program with endoscopic examination and biopsy has been in place for decades to reduce the incidence and mortality of cancer in patients with IBD and BE. Interpretation of IBD- and BE-surveillance biopsies are similarly challenging, to say the least. However, clinical-outcome-based studies are increasingly being published and support the role of histological stratification of biopsies into negative for dysplasia, LGD, and HGD based on a set of histomorphological features. In addition, subtle histological variants of dysplasia are increasingly being recognized in IBD as well as in BE. The current literature also supports a category of indefinite for dysplasia in both IBD- and BE-surveillance biopsies to convey an intermediate risk of neoplastic progression. Emerging evidence appears to support judicious use of immunostain for p53 in difficult cases, although histological examination of H&E stain still remains the gold standard.
Endoscopic treatment, such as ablation and EMR/ESD, for BE-associated dysplasia and early adenocarcinoma has matured and is currently used in many large medical centers. This type of specimen may pose diagnostic challenges to the pathologist and the pathologist should be aware of certain pitfalls, such as “buried BE/ buried neoplasia” and duplication of muscularis mucosae, and not overstage IMC as SMC in BE.
Conflicts of interest
None declared.
References
- 1. Laine L, Kaltenbach T, Barkun A. et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015;148:639–51. [DOI] [PubMed] [Google Scholar]
- 2. Shaheen NJ, Falk GW, Iyer PG. et al. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol 2016;111:30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riddell RH, Goldman H, Ransohoff DF. et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol 1983;14:931–68. [DOI] [PubMed] [Google Scholar]
- 4. Gillen CD, Walmsley RS, Prior P. et al. Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut 1994;35:1590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta RB, Harpaz N, Itzkowitz S. et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology 2007;133:1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rutter M, Saunders B, Wilkinson K. et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004;126:451–9. [DOI] [PubMed] [Google Scholar]
- 7. Shah SC, ten Hove JR, Castaneda D. et al. High risk of advanced colorectal neoplasia in patients with primary sclerosing cholangitis associated with inflammatory bowel disease. Clin Gastroenterol Hepatol 2018;16:1106–13. [DOI] [PubMed] [Google Scholar]
- 8. Itzkowitz SH, Harpaz N.. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology 2004;126:1634–48. [DOI] [PubMed] [Google Scholar]
- 9. Farraye FA, Odze RD, Eaden J. et al. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010;138:746–74. [DOI] [PubMed] [Google Scholar]
- 10. Morson BC, Pang LS.. Rectal biopsy as an aid to cancer control in ulcerative colitis. Gut 1967;8:423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eaden JA, Abrams KR, Mayberry JF.. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fumery M, Dulai PS, Gupta S. et al. Incidence, risk factors, and outcomes of colorectal cancer in patients with ulcerative colitis with low-grade dysplasia: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2017;15:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Howlader N, Noone AM, Krapcho M. et al. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute, 2013. [Google Scholar]
- 14. Desai TK, Krishnan K, Samala N. et al. The incidence of esophageal adenocarcinoma in non-dysplastic oesophagus: a meta-analysis. Gut 2012;61:970–6. [DOI] [PubMed] [Google Scholar]
- 15. Singh S, Manickam P, Amin AV. et al. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:897–909. [DOI] [PubMed] [Google Scholar]
- 16. Rastogi A, Puli S, El-Serag HB. et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394–8. [DOI] [PubMed] [Google Scholar]
- 17. Sharma P, Falk GW, Weston AP. et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2006;4:566–72. [DOI] [PubMed] [Google Scholar]
- 18. Shaheen NJ, Crosby MA, Bozymski EM. et al. Is there publication bias in the reporting of cancer risk in Barrett's esophagus? Gastroenterology 2000;119:333–8. [DOI] [PubMed] [Google Scholar]
- 19. de Jonge PJ, van Blankenstein M, Looman CW. et al. Risk of malignant progression in patients with Barrett's oesophagus: a Dutch nationwide cohort study. Gut 2010;59:1030–6. [DOI] [PubMed] [Google Scholar]
- 20. Yousef F, Cardwell C, Cantwell MM. et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett's esophagus: a systematic review and meta-analysis. Am J Epidemiol 2008;168:237–49. [DOI] [PubMed] [Google Scholar]
- 21. Hvid-Jensen F, Pedersen L, Drewes AM. et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365:1375–83. [DOI] [PubMed] [Google Scholar]
- 22. Curvers WL, ten Kate FJ, Krishnadath KK. et al. Low-grade dysplasia in Barrett's esophagus: overdiagnosed and underestimated. Am J Gastroenterol 2010;105:1523–30. [DOI] [PubMed] [Google Scholar]
- 23. Dulai GS, Shekelle PG, Jensen DM. et al. Dysplasia and risk of further neoplastic progression in a regional Veterans Administration Barrett's cohort. Am J Gastroenterology 2005;100:775–83. [DOI] [PubMed] [Google Scholar]
- 24. Conio M, Blanchi S, Lapertosa G. et al. Long-term endoscopic surveillance of patients with Barrett's esophagus: incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterology 2003;98:1931–9. [DOI] [PubMed] [Google Scholar]
- 25. Schnell TG, Sontag SJ, Chejfec G. et al. Long-term nonsurgical management of Barrett's esophagus with high-grade dysplasia. Gastroenterology 2001;120:1607–19. [DOI] [PubMed] [Google Scholar]
- 26. Srivastava A, Hornick JL, Li X. et al. Extent of low-grade dysplasia is a risk factor for the development of esophageal adenocarcinoma in Barrett's esophagus. Am J Gastroenterol 2007;102:483–93. [DOI] [PubMed] [Google Scholar]
- 27. Lim CH, Treanor D, Dixon MF. et al. Low-grade dysplasia in Barrett's esophagus has a high risk of progression. Endoscopy 2007;39:581–7. [DOI] [PubMed] [Google Scholar]
- 28. Shaheen NJ, Sharma P, Overholt BF. et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277–88. [DOI] [PubMed] [Google Scholar]
- 29. Azizi S, Al-Rubaye H, Turmi MAA. et al. Detecting dysplasia using white light endoscopy or chromoendoscopy in ulcerative colitis patients without primary sclerosing cholangitis: a systematic review and meta-analysis. Int J Surg 2018;52:180–8. [DOI] [PubMed] [Google Scholar]
- 30. Reid BJ, Haggitt RC, Rubin CE. et al. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol 1988;19:166–78. [DOI] [PubMed] [Google Scholar]
- 31. Montgomery E, Bronner MP, Goldblum JR. et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol 2001;32:368–78. [DOI] [PubMed] [Google Scholar]
- 32. Waters KM, Salimian KJ, Voltaggio L. et al. Refined criteria for separating low-grade dysplasia and nondysplastic Barrett esophagus reduce equivocal diagnoses and improve prediction of patient outcome: a 10-year review. Am J Surg Pathol 2018;42:1723–9. [DOI] [PubMed] [Google Scholar]
- 33. Serra S, Ali R, Bateman AC. et al. Gastric foveolar dysplasia: a survey of reporting habits and diagnostic criteria. Pathology 2017;49:391–6. [DOI] [PubMed] [Google Scholar]
- 34. Mooiweer E, van der Meulen-de Jong AE, Ponsioen CY. et al. Incidence of interval colorectal cancer among inflammatory bowel disease patients undergoing regular colonoscopic surveillance. Clin Gastroenterol Hepatol 2015;13:1656–61. [DOI] [PubMed] [Google Scholar]
- 35. Ten Hove JR, Shah SC, Shaffer SR. et al. Consecutive negative findings on colonoscopy during surveillance predict a low risk of advanced neoplasia in patients with inflammatory bowel disease with long-standing colitis: results of a 15-year multicentre, multinational cohort study. Gut 2019;68:615–22. [DOI] [PubMed] [Google Scholar]
- 36. Ten Kate FJC, Nieboer D, ten Kate FJW. et al. Improved progression prediction in Barrett’s esophagus with low-grade dysplasia using specific histologic criteria. Am J Surg Pathol 2018;42:918–26. [DOI] [PubMed] [Google Scholar]
- 37. Harpaz N, Polydorides AD.. Colorectal dysplasia in chronic inflammatory bowel disease: pathology, clinical implications, and pathogenesis. Arch Pathol Lab Med 2010;134:876–95. [DOI] [PubMed] [Google Scholar]
- 38. Horvath B, Liu G, Wu X. et al. Overexpression of p53 predicts colorectal neoplasia risk in patients with inflammatory bowel disease and mucosa changes indefinite for dysplasia. Gastroenterol Rep (Oxf) 2015;3:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lai KK, Horvath B, Xie H. et al. Risk for colorectal neoplasia in patients with inflammatory bowel disease and mucosa indefinite for dysplasia. Inflamm Bowel Dis 2015;21:378–84. [DOI] [PubMed] [Google Scholar]
- 40. Choi WT, Rabinovitch PS, Wang D. et al. Outcome of “indefinite for dysplasia” in inflammatory bowel disease: correlation with DNA flow cytometry and other risk factors of colorectal cancer. Hum Pathol 2015;46:939–47. [DOI] [PubMed] [Google Scholar]
- 41. Downs-Kelly E, Mendelin JE, Bennett AE. et al. Poor interobserver agreement in the distinction of high-grade dysplasia and adenocarcinoma in pretreatment Barrett’s esophagus biopsies. Am J Gastroenterol 2008;103:2333–40. [DOI] [PubMed] [Google Scholar]
- 42. Zhu W, Appelman HD, Greenson JK. et al. A histologically defined subset of high-grade dysplasia in Barrett mucosa is predictive of associated carcinoma. Am J Clin Pathol 2009;132:94–100. [DOI] [PubMed] [Google Scholar]
- 43. Patil DT, Goldblum JR, Ribicki L. et al. Prediction of adenocarcinoma in esophagectomy specimens based upon analysis of preresection biopsies of Barrett esophagus with at least high-grade dysplasia: a comparison of 2 systems. Am J Surg Pathol 2012;36:13441. [DOI] [PubMed] [Google Scholar]
- 44. Kilgore SP, Sigel JE, Goldblum JR.. Hyperplastic-like mucosal changes in Crohn’s disease: an unusual form of dysplasia? Mod Pathol 2000;13:797–801. [DOI] [PubMed] [Google Scholar]
- 45. Harpaz N, Goldblum JR, Shepherd N. et al. Novel classification of dysplasia in IBD [abstract]. Mod Pathol 2017;30(Suppl 2):172A. [Google Scholar]
- 46. Jackson WE, Achkar J-P, Macaron C. et al. The significance of sessile serrated polyps in inflammatory bowel disease. Inflamm Bowel Dis 2016;22:2213–20. [DOI] [PubMed] [Google Scholar]
- 47. Iwaya M, Ota H, Tateishi Y. et al. Relationship between carcinoma subtype and overlying dysplasia in IBD-associated colorectal carcinoma. Mod Pathol 2018;753:271. [Google Scholar]
- 48. Torres C, Antonioli D, Odze RD.. Polypoid dysplasia and adenomas in inflammatory bowel disease: a clinical, pathologic, and follow-up study of 89 polyps from 59 patients. Am J Surg Pathol 1998;22:275–84. [DOI] [PubMed] [Google Scholar]
- 49. Chiu K, Riddell RH, Schaeffer DF.. DALM, rest in peace: a pathologist’s perspective on dysplasia in inflammatory bowel disease in the post-DALM era. Mod Pathol 2018;31:1180–90. [DOI] [PubMed] [Google Scholar]
- 50. Farraye FA, Odze RD, Eaden J. et al. AGA medical position statement on the diagnosis and management of neoplasia in inflammatory bowel disease. Gastroenterology 2010;138:738–45. [DOI] [PubMed] [Google Scholar]
- 51. Bharadwaj S, Narula N, Tandon P. et al. Role of endoscopy in inflammatory bowel disease. Gastroenterol Rep (Oxf) 2018;6:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lomo LC, Blount PL, Sanchez CA. et al. Crypt dysplasia with surface maturation: a clinical, pathologic, and molecular study of a Barrett’s esophagus cohort. Am J Surg Pathol 2006;30:423–35. [DOI] [PubMed] [Google Scholar]
- 53. Coco DP, Goldblum JR, Hornick JL. et al. Interobserver variability in the diagnosis of crypt dysplasia in Barrett esophagus. Am J Surg Pathol 2011;35:45–54. [DOI] [PubMed] [Google Scholar]
- 54. Mahajan D, Bennett AE, Liu X. et al. Grading of gastric foveolar-type dysplasia in Barrett’s esophagus. Mod Pathol 2010;23:1–11. [DOI] [PubMed] [Google Scholar]
- 55. Brown IS, Whiteman DC, Lauwers GY.. Foveolar type dysplasia in Barrett Esophagus. Mod Pathol 2010;23:834–43. [DOI] [PubMed] [Google Scholar]
- 56. Patil DT, Bennett AE, Mahajan D. et al. Distinguishing Barrett gastric foveolar dysplasia from reactive cardiac mucosa in gastroesophageal reflux disease. Hum Pathol 2013;44:1146–53. [DOI] [PubMed] [Google Scholar]
- 57. Mino-Kenudson M, Ban S, Ohana M. et al. Buried dysplasia and early adenocarcinoma arising in Barrett’s esophagus after porfimer-photodynamic therapy. Am J Surg Pathol 2007;31:403–9. [DOI] [PubMed] [Google Scholar]
- 58. Korst RJ, Santana-Joseph S, Rutledge JR. et al. Patterns of recurrent and persistent intestinal metaplasia after successful radiofrequency ablation of Barrett’s esophagus. J Thorac Cardiovasc Surg 2013;145:1529–34. [DOI] [PubMed] [Google Scholar]
- 59. Pouw RE, Gondrie JJ, Rygiel AM. et al. Properties of the neosquamous epithelium after radiofrequency ablation of Barrett’s esophagus containing neoplasia. Am J Gastroenterol 2009;104:1366–73. [DOI] [PubMed] [Google Scholar]
- 60. Gray NA, Odze RD, Spechler SJ.. Buried metaplasia after endoscopic ablation of Barrett’s esophagus: a systemic review. Am J Gastroenterol 2011;106:1899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Odze RD, Lauwers GY.. Histopathology of Barrett’s esophagus after ablation and endoscopic mucosal resection therapy. Endoscopy 2008;40:1008–15. [DOI] [PubMed] [Google Scholar]
- 62. Castela J, Serrano M, Ferro SM. et al. Buried Barrett’s esophagus with high-grade dysplasia after radiofrequency ablation. Clin Endosc 2019;52:269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lewis JT, Wang KK, Abraham SC.. Muscularis mucosae duplication and the musculo-fibrous anomaly in endoscopic mucosal resections for Barrett’s esophagus: implications for staging of adenocarcinoma. Am J Surg Pathol 2008;32:566–71. [DOI] [PubMed] [Google Scholar]
- 64. Li ZG, Zhu H, Shi H. et al. Lymphovascular invasion and nodal metastasis in intramucosal adenocarcinoma of the esophagus and esophagastric junction. J Dig Dis 2015;16:197–204. [DOI] [PubMed] [Google Scholar]
- 65. Yin F, Hernandez Gonzalo D, Lai J. et al. Histopathology of Barrett’s esophagus and early-stage esophageal adenocarcinoma: an updated review. Gastrointest Disord 2018;1:147–63. [Google Scholar]
- 66. Manner H, Wetzka J, May A. et al. Early-stage adenocarcinoma of the esophagus with mid to deep submucosal invasion (pT1b sm2-3): the frequency of lymph-node metastasis depends on macroscopic and histological risk patterns. Dis Esophagus 2017;30:1–11. [DOI] [PubMed] [Google Scholar]
- 67. Abraham SC, Krasinskas AM, Correa AM. et al. Duplication of the muscularis mucosae in Barrett esophagus: an underrecognized feature and its implication for staging of adenocarcinoma. Am J Surg Pathol 2007;31:1719–25. [DOI] [PubMed] [Google Scholar]
- 68. Burmer GC, Rabinovitch PS, Haggitt RC. et al. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology 1992;103:1602–10. [DOI] [PubMed] [Google Scholar]
- 69. Yin J, Harpaz N, Tong Y. et al. p53 point mutations in dysplastic and cancerous ulcerative colitis lesions. Gastroenterology 1993;104:1633–9. [DOI] [PubMed] [Google Scholar]
- 70. Harpaz N, Peck AL, Yin J. et al. p53 protein expression in ulcerative colitis-associated colorectal dysplasia and carcinoma. Hum Pathol 1994;25:1069–74. [DOI] [PubMed] [Google Scholar]
- 71. Noffsinger AE, Belli JM, Miller MA. et al. A unique basal pattern of p53 expression in ulcerative colitis is associated with mutation in the p53 gene. Histopathology 2001;39:482–92. [DOI] [PubMed] [Google Scholar]
- 72. Lashner BA, Shapiro BD, Husain A. et al. Evaluation of the usefulness of testing for p53 mutations in colorectal cancer surveillance for ulcerative colitis. Am J Gastroenterol 1999;94:456–62. [DOI] [PubMed] [Google Scholar]
- 73. Xie H, Xiao SY, Pai R. et al. Diagnostic utility of TP53 and cytokeratin 7 immunohistochemistry in idiopathic inflammatory bowel disease-associated neoplasia. Mod Pathol 2014;27:303–13. [DOI] [PubMed] [Google Scholar]
- 74. Stachler MD, Camarda ND, Deitrick C. et al. Detection of mutations in Barrett’s esophagus before progression to high-grade dysplasia or adenocarcinoma. Gastroenterology 2018;155:156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van der Wel MJ, Duits LC, Pouw RE. et al. Improved diagnostic stratification of digitized Barrett’s oesophagus biopsies by p53 immunohistochemical staining. Histopathology 2018;72:1015–23. [DOI] [PubMed] [Google Scholar]
- 76. Srivastava A, Appelman H, Goldsmith JD. et al. The use of ancillary stains in the diagnosis of Barrett esophagus and Barrett esophagus-associated dysplasia: recommendation from the Rodger C. Haggitt gastrointestinal pathology society. Am J Surg Pathol 2017;41:e8–21. [DOI] [PubMed] [Google Scholar]
- 77. Younes M, Brown K, Lauwers GY. et al. p53 protein accumulation predicts malignant progression in Barrett’s metaplasia: a prospective study of 275 patients. Histopathology 2017;71:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]