Abstract
Background
Long-term tumor control following PDT is a result of its direct effect on tumor and vasculature in combination with induction of inflammatory-reactions upregulating the immune system. When PDT induces necrosis of tumors and vascular system, an immune cascade can be initiated to release all kinds of cytokines including IL1β. This further leads to the activation of inflammatory-cells and hence death of tumor cells.
Methods
Ultraviolet irradiation was used to induce cSCC mice model, gene chip was used to screen inflammatory cytokines, qPCR, ELISA and implanted tumor mice model were used to verify the changes and important role of interleukin-1β, and WB preliminarily explored the production mechanism of interleukin-1β.
Results
Inflammatory cytokines and receptors transcript screening identify IL1r1 as the top4. After ALA-PDT, IL1r1 and IL1β increased in patients’ biopsies, principally in mesenchymal cells. In vivo, the inhibition of ALA-PDT on tumor growth of cutaneous squamous cell carcinoma (cSCC) mice in the group with intralesional injection of anti-IL1β mAb or caspase1-inhibitor was significantly weaker than the control groups. Furthermore, NLRP3-inflammasome and p-p65/p65 were elevated after ALA-PDT mediated IL1β production in cancer-associated-fibroblasts.
Discussion
By means of activating NLRP3-inflammasome with IL1β production in CAFs, PDT stimulates local acute-inflammatory-response, which further promotes PDT effect for cSCC.
Keywords: cancer-associated fibroblast, photodynamic therapy, interleukin-1β, NLRP3-inflammasome, squamous cell carcinoma
Introduction
Importance of an Acute Localized Inflammatory Response in PDT for cSCC
Photodynamic therapy (PDT) is one of the main methods for the treatment of early-stage cancer and palliative treatment of late-stage cancer. It has been approved for clinical use in many countries, including the United States. But PDT destroys cancer and also causes local acute inflammation, which can promote the development of the immune system and effectively control the growth of tumors.1 Previous studies have indicated that PDT increases the cytokines of tumor tissues, accumulates leukocytes and is conducive to tumor destruction. Therefore, PDT has an anti-tumor effect. Togsverd Bo et al demonstrated that the development of cSCC in UV-irradiated hairless mice was slightly delayed by a “non-inflammatory” dose of PDT.2 These observations imply that activating inflammatory process is an event of the essence in PDT.
In addition, the efficacy of PDT is seemingly closely related to the degree and nature of inflammation caused by PDT.3 Animal models have indicated that induction of inflammation and enhancement of anti-tumor immunity following PDT is dependent on therapeutic regimen.4,5 In a research, 6 animals were treated with high-dose PDT, low-dose PDT, or no treatment. After 2 days, the anti-tumor immunity of the high-dose group and the low-dose group was significantly improved.1 However, 60 days after PDT, the most long-term efficacy was seen with high-dose regimen.6 It is believed that the mechanism by which PDT eradicates tumors involves highly complex interactions between local and systemic responses,7,8 but the potential mechanisms of acute inflammatory response and progression after local PDT are still unclear.
Role of IL1β in Acute Inflammatory Response
The two major subfamilies, the apoptotic caspases (caspase-3,-6~10) and the inflammatory caspases (Caspases-1, -4,-5) that form a caspase (cysteine aspartic protease), are widely known for their roles in apoptosis and inflammation. The function of caspase-4,-5 is not fully known and no specific substrates have been identified, but they are believed to be along with caspase-1 are master switches of inflammation.9,10
They are transcribed from the same chromosomal locus, contain an identical amino-terminal CARD, and they all participate in inflammation by modulating the activation of the pro-inflammatory cytokines proil1β and proil18.10 IL1β is an important pro-inflammatory mediator that initiates or amplifies multiple effects related to innate immunity and host responses to tissue injury11 and plays an essential role in the local and systemic inflammation-related responses. Clinical studies concluded that IL1β should be considered a gatekeeper of inflammation.12 A major feature of the PDT-induced local acute inflammatory response is the increase in a number of pro-inflammatory cytokines. These studies imply that IL1β may be the onset of acute inflammation after PDT.
The cytosolic molecular complex of NLRP3 inflammatory bodies is an important regulator of IL1β production.13 NLRP3, also known as NALP3, is one of the major members of NOD-like receptor family. Recent studies have found that cytoplasmic complexes formed by NLRP3 have recently been found to be the most characteristic inflammatory bodies. It is responsible for activating caspase-1, the number of stress or damage pathogens and related stimuli. Upon activation, NLRP3 recruits an adaptor, apoptosis-associated-speck-like protein (ASC) that contains a C-terminal caspase recruitment domain that recruits procaspase-1 to form a multiprotein complex.14 Caspase-1 is then automatically activated. The most abundant cell type in tumors is fibroblasts also known as myofibroblasts or cancer-associated fibroblasts (CAFs).14 Fibroblasts produce biologically active IL1β. Fibroblasts are recognized by a variety of markers such as α-smooth muscle actin (α-SMA), vimentin, and fibroblast activation protein (FAP). However, the differences in morphology and function between fibroblasts and normal cells are still unclear.15
An acute localized inflammatory response plays an important role in PDT effect. A large number of CAFs are contained in cSCC tissue. We suppose that native-activated CAFs secrete pro-inflammatory cytokines to initiate and amplify local acute inflammation, ultimately leading to the anti-tumor immunity in ALA-PDT for cSCC. Here we discuss the mechanism of acute inflammation after PDT, focusing on the CAFs.
Materials and Methods
Animal and Cell Line
Female SKH-1 hairless mice (6–8 weeks old, Shanghai Public Health Clinical, China) were used. As described previously, cSCC was induced on the back of mice by using solar-simulated UV irradiation (Sigma, Shanghai, China). After the mice were sacrificed, the cSCC tumors were excised and enzymatically decomposed into single cell suspensions for experiments. Then, mSCC cells and mCAFs were washed in PBS and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 20% fetal bovine serum (FBS), 1% penicillin/streptomycin antibiotics (all:Gibco, Billings, MT, USA) at 37°C in an atmosphere of 5% CO2. By differential paces of sticking to wall, purification of mSCC cells and mCAFs reached to 95%. (Ethical statement: the mSCC cell line approved by huadong hospital affiliated to fudan University Ethics Committee no. 2015K007). Human dermal fibroblasts (HDF) were cultured in fibroblast medium (Sciencecell, CA, USA). Mouse fibroblast cell line (NIH/3T3) (ATCC®CRL-1658™) and the human SCC cell line (A431) were cultured in DMEM supplemented with 10% FBS (both:Gibco, Billings, MT, USA). The mSCC cell line (PECA) was cultured in RPMI1640 supplemented with 10% FBS (both:Gibco, Billings, MT, USA). PECA cell line was obtained from the Cell Lines Service (Germany). Cell lines including HDF, NIH/3T3, A431 were obtained from the ATCC. Animal experiments have been approved by huadong hospital affiliated to fudan University Ethics Committee no. 2015K007, and all the experiments have followed the NC3Rs ARRIVE guidelines.
Microarray Analysis
SCC tumor samples of mice models mentioned above were obtained from mice before ALA-PDT (n=3), 3 hrs after ALA-PDT (n=3) and 6 hrs after ALA-PDT (n=3). The gene expression profiling on SCC tumor before, 3 hrs after and 6 hrs after ALA-PDT was assessed using Affymetrix GeneChip® Mouse Transcriptome Assay 1.0 (Affymetrix, Santa Clara, CA, USA) and the data analyses were performed at the Gene-Cloud of Biotechnology Information (Genminix Informatics Ltd. Co, Gminix). Normalization of the arrays was performed using RMA assay, and Chipster software (CSC IT Center for Science, Espoo, Finland). Mean signal level before ALA-PDT was used as the control for each sample after ALA-PDT. Sequence specificity of Affymetrix probes was verified by BLAST search.
AlA-PDT
In vitro, CAFs, HDF, NIH/3T3, SCC cells, A431, PECA were incubated with ALA (0.5mM) in full serum-free medium for 5 hrs at 37°C, washed twice with PBS and exposed to the light at dose of 0.5J/cm2 (632.8nm, 10mW/cm2). In vivo, 8% ALA cream was topically applied on mSCC for 3hrs in darkroom. Mice were irradiated by helium-neon laser (632.8nm) at 100mW/cm2 and 15J/cm2 as previously described.15
Quantitative RT-PCR
Samples of human SCC (n=5) were obtained from Shanghai skin disease Hospital from surgery of SCC patients before and 3hrs after ALA-PDT. Samples of mSCC (n=5) were obtained from mouse models before and 6hrs after ALA-PDT. Levels of mRNA of IL1β, ILIR1 mRNA were detected by SYBR-green-qPCR (Life Technologies, Carlsbad, USA).
SCC Implantation Tumor Model Assay
A total of 20 female SKH-1 hairless mice were randomly divided into 4 groups and a mixture of SCC cells (5×106) and NIH/3T3 cells (1×106) were injected subcutaneously. The groups were as follows:
Anti-IL1β mAb group, an intratumor injection was given 50μL(1 μg/μL) anti-IL1β mAb (B122) (BioLegend, san Diego, CA 92121) each mouse;
IgG control group, an intratumor injection was given 50μL(1 μg/μL) IgG (hTK888) (BioLegend, san Diego, CA 92121) each mouse;
Caspase1 inhibitor group, an intratumor injection was given 50 mg/kg belnacasan (Selleck, Houston, TX) dissolved in 2% DMSO+30% PEG300+ddH2O solution per mouse;
2% DMSO+30% PEG300+ddH2O solution group, an intratumor injection was given 50μL 2% DMSO+30% PEG300+ddH2O solution each mouse.
When the mice tumor had grown to 5mm in about 10 days, the treatment was started. The intratumor injections were administered the day before PDT, during PDT and 24 hrs after PDT. The size of tumors was measured by vernier caliper every 2–3 days and tumor volume was calculated with the formula V=(length × width2)/2.
Detection of IL1β from fibroblasts so as to evaluate the release of IL1β in response to ALA-PDT, fibroblasts were incubated with 0.5mM of ALA for 5hrs and cell supernatant was collected at 0.5J/cm2. After centrifugation, the supernatants were analyzed using an ELISA-based IL1β(R&D) assay kits at specific time points (0hrs, 3hrs, 6hrs,12 hrs, 24hrs) after PDT according to the manufacturers instructions.
ELISA Analysis
The cells were grown in 10 cm plates until 70–75% confluence was reached. Then, their media were only replaced by DMEM. These cells were then incubated for another 24 hrs at 37°C. Then, CAFs had the ALA-PDT or not and whereupon their media were collected in 3hrs/6hrs/12hrs/24hrs/48hrs, filter-sterilized, aliquoted, and stored at 80°C. After this treatment, the CAFs were washed and the media were replaced with DMEM supplemented with 20% FBS. The cells were then allowed to secrete their own cytokines for 3 hrs, after which the medium was collected, filter-sterilized, aliquoted, and stored at −80°C. Secretion of IL1 was quantified with ELISA kits obtained from R&D Systems (catalog #DY401, Minneapolis, MN). The assay was performed the manufacturer’s instructions. Samples were assayed in triplicate. Error bars represent ± one standard deviation.
Western Blot Analysis
Production of NLRP3, ASC, Casp-1,IL1β, Phospho-NF-κB p65, NF-κB p65 in CAFs was determined by Western blotting using anti-NLRP3 antibody (NBP2-12446, Novus, 1:500), anti-ASC antibody (sc-271054, Santa Cruz Biotechnology, 1:200), anti- Casp-1 antibody (sc-56036, Santa Cruz Biotechnology, 1:200) and anti-IL1β antibody (ab9722, Abcam, 1:10000), anti- Phospho-NF-κB p65 antibody (3033s, Cell and Signaling, 1:1000), and anti-NF-κB p65 antibody (8242s, Cell and Signaling, 1:1000). Anti-GAPDH (AC-15) (Sigma-Aldrich, St Louis, MO, USA) was used as the loading control. Sample lysates and Western blots were collected as previously described.16 The results normalized to GAPDH were quantified by using Image J. (NIH, Bethesda, MD, USA).
Immuno-Histochemical Studies
At the designated time points after the above treatment, fresh isolated tissue or cell was stored in formalin, and 5μm sections were de-waxed (30 mins 56°C, 2 × 10 mins xylene), then hydrated, antigen demembrance and blocked. Afterwards, the first-order anti-IL1beta antibody of 1 μg/mL was stained at room temperature for 30 mins. The slides were washed in PBS, incubated for 30 mins with goat anti-rabbit IgG secondary antibody in the blocking solution. The slides were filled with strept avidin-biotin complex for 30 mins washed in PBS, stained with DAB stain and hematoxylin counterstain, and observed under a light microscope. (All: Boster, China)
Statistical analysis of all quantitative data was recorded as mean S.D. Comparisons between two groups were carried out by Student’s t test. Analysis was performed by SPSS 13.0 (SPSS, Chicago, IL), and statistical significance was defined as P<0.05.
Result
IL1r1 and IL1β Expressing Elevated After ALA-PDT
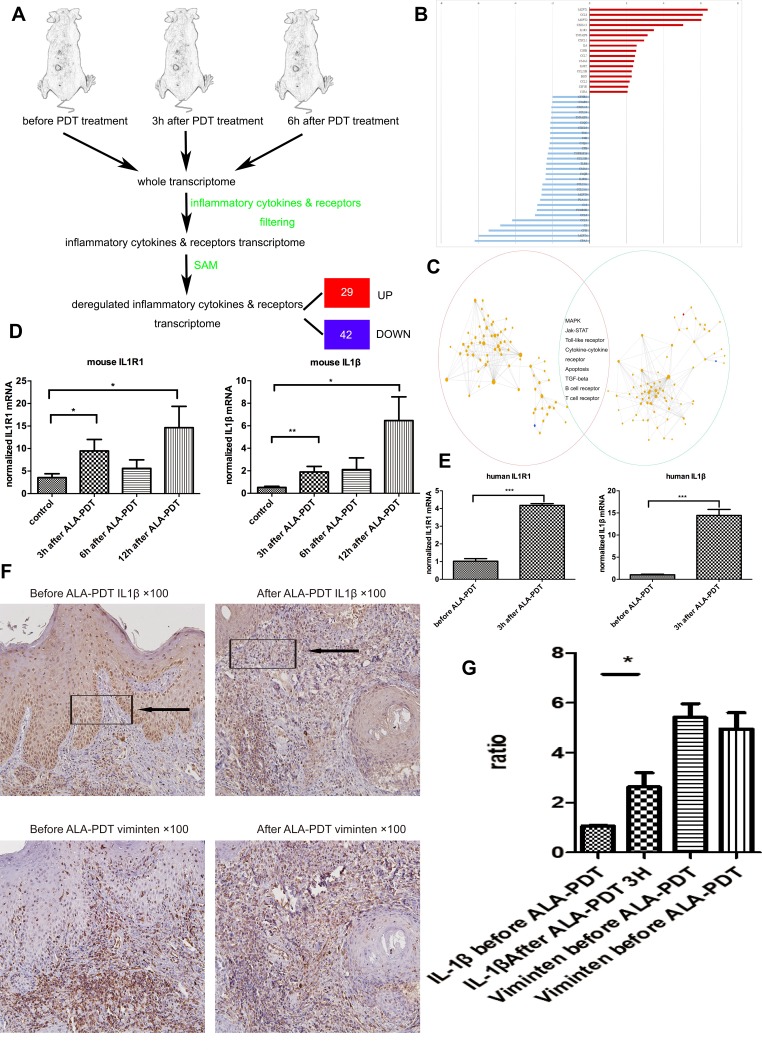
To better understand the factors regulating the production of pro-inflammatory cytokines and anti-inflammatory cytokines after ALA-PDT, DNA microarray technology were used to identify transcripts involved in inflammatory cytokines and receptors. UV-induced SCC mice model (SCC mice) with histological and clinical features similar to those reported in humans was used here.17 The entire murine genome was filtered to select only involved in inflammatory cytokines and receptors. Using the Significance Analysis of Microarrays method, it was showed that 29 genes were significantly up-regulated and 42 were down-regulated after PDT for cSCC (Figure 1A and B). The KEGG database were used to analyze pathways associated with the inflammatory after ALA-PDT. There were 133 pathways at 3 hrs after PDT and 140 pathways at 6 hrs identified. To further study the internal links between pathways, we built the pathway interaction networks based on the KEGG database on the website (http://www.gcbi.com.cn). The presence of several inflammation-associated pathways in both interaction networks identified led to follow-up studies (Figure 1C). We found several significant genes to perform validate experiment including IL1r1 and its ligand IL1β (data not show except for IL1r1, Figure 1D). Consulted researches and in the analysis of the comparison of the two pathway network graph (Figure 1C) identifies up-regulated gene IL1r1 as a key feature of dysregulated inflammatory receptor in PDT for cutaneous squamous cell carcinoma.
Figure 1.
The main framework to construct the gene pathways dependency network. (A) Steps in identifying the genes regulated by ALA-PDT. (B) The 46 regulators most highly ranked based on their p-value in the microarray are depicted. (C) The KEGG database were used to analyze pathways associated with the inflammatory after ALA-PDT. 133 pathways at 3 hrs(red cycle) after PDT and 140 pathways at 6 hrs (green cycle) identified. Then built the pathway interaction based on the KEGG database on the website and identified the eight signaling pathways (http://www.gcbi.com.cn). (D) Gene expression fold changes of two target genes IL1R1 and IL1β as determined by microarray and real-time q-PCR experiments. The direction and magnitude of fold changes obtained from the real-time q-PCR technique were comparable to those obtained from the microarray technique. P < 0.05 for gene expression fold changes quantified by real-time PCR experiments as determined by two-tailed unpaired Student’s t-test. (E–G) IL1β expression was increased 3hrs after ALA-PDT especially in fibroblasts (Vimentin positive cells).
Notes: Data were presented as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001.
To ensure the sequencing data of SCC mice were true and reliable in human, patients tissue were collected before ALA-PDT and 3 hrs after ALA-PDT in hospital, and verified IL1r1 and IL1β up-regulated after ALA-PDT by q-PCR (Figure 1E). Immunohistochemistry was used to observe the expression of IL1β in the patients’ tissues. Interestingly it found that the increasing of IL1β were mostly existed in mesenchymal cells (vimentin+) after ALA-PDT in clinic patients (Figure 1F) . It is supposed the mesenchymal cells mainly fibroblasts secreted more IL1β after photodynamic therapy stimulation.
IL1β Activity Is Critical for the Therapeutic Efficacy
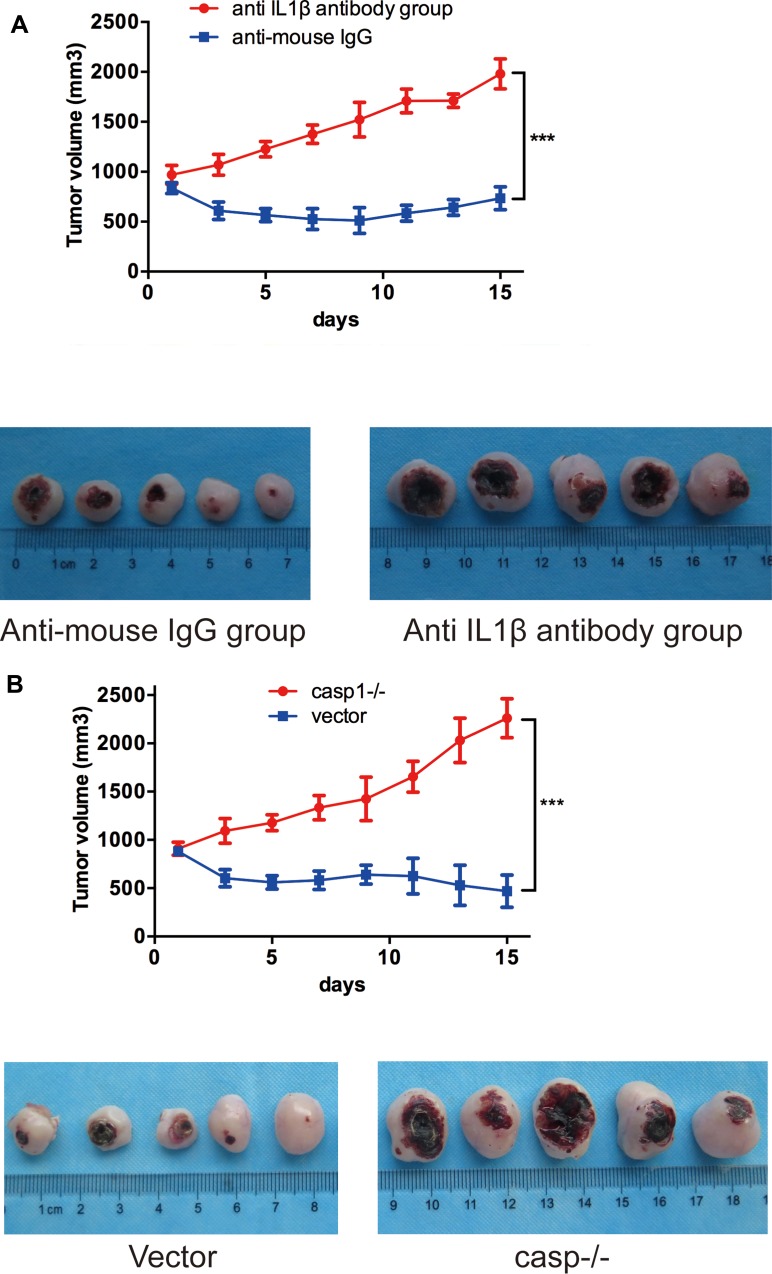
The results above suggest that IL1β may play important roles in PDT. Intuitively, large amount of anti-IL1β-mAb was injected intratumorally neutralizing the IL1β secreted by ALA-PDT to verify its efficacy. The average size of anti-IL1β mAb-treated tumors increased to 1446±123.5 mm3 while the controls increased to ~626.4±38.97 mm3 (Figure 2A). The results indicated that the tumor growth was much faster in the anti-IL1β-mAb-treated group compared to the controls, suggesting that IL1β could play crucial roles in the ALA-PDT efficacy. The experiment was repeated three times and the differences are statistically significant.
Figure 2.
(A, B) Accelerated solid tumor growth in anti-IL1β-antibody-group and caspase1-inhibitor-group(casp−/− ). Growth curve of SCC tumor (A, n=10; B, n=10) tumors in SKH-1 mice after ALA-PDT.
Note: ***p<0.001.
The experiment was repeated by inhibiting the upstream regulation sequence of IL1β gene, caspase1, which plays pivotal role in the secretion of biologically active IL1β. Here the average size of caspase1-inhibited tumors increased to 1486±165.7 mm3 while the control increased to 611.4±43.18 mm3 (Figure 2B). The experiment was repeated three times and the differences are statistically significant. In a word, neutralizing IL1β or inhibiting the activity of caspase1 attenuated efficacy of ALA-PDT delaying SCC development in SCC tumor-bearing mice.
ALA-PDT Induce IL1β Production in CAFs
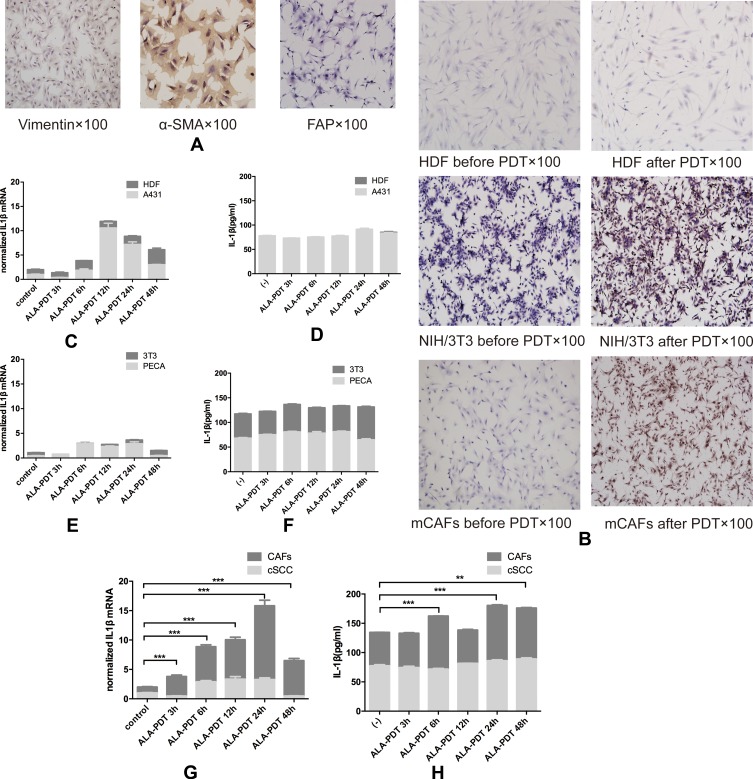
The mCAFs were isolated from the UV-induced SCC tumor in mice and activation marker α-SMA, vimentin and FAP was expressed in mCAFs we got (Figure 3A). In vitro, mCAFs, HDF, NIH/3T3, mSCC, A431 and PECA were irradiated by the light at a dose of 0.5 J/cm2 after incubating with ALA (0.5 mM) in full serum-free medium for 5 hrs at 37°C. After processing mCAFs secreted more IL1β but non-activated fibroblasts including HDF and NIH/3T3 did not (Figure 3B and C–H). All of tumor cell including mSCC, A431, PECA expressed and secreted ILβ before and after treatment. The difference was not statistically significant.
Figure 3.
(A) Surface markers of mCAFs including vimentin, α-SMA and FAP positive. (B) CAFs and NIH/3T3 secreted more IL1β than HDF through IHC. Tumor cells including A431 (C, D), PECA (E, F) and cSCC (G, H) expressed more IL1β after ALA-PDT with time, however, the changes have no statistical significance. Fibroblasts including HDF (C, D) and NIH/3T3 (E, F) expressed more IL1β 48hrs after ALA-PDT, but the difference has not statistical significance. mCAFs expressed more IL1β with time and secreted more IL1β 6hrs, 24hrs and 48hrs after ALA-PDT.
Notes: **p<0.01; ***p<0.001.
NLRP3 Inflammasome Mediates Interleukin-1β Production of Cancer-Associated Fibroblasts After ALA-PDT in an NF-κB-Dependent Manner
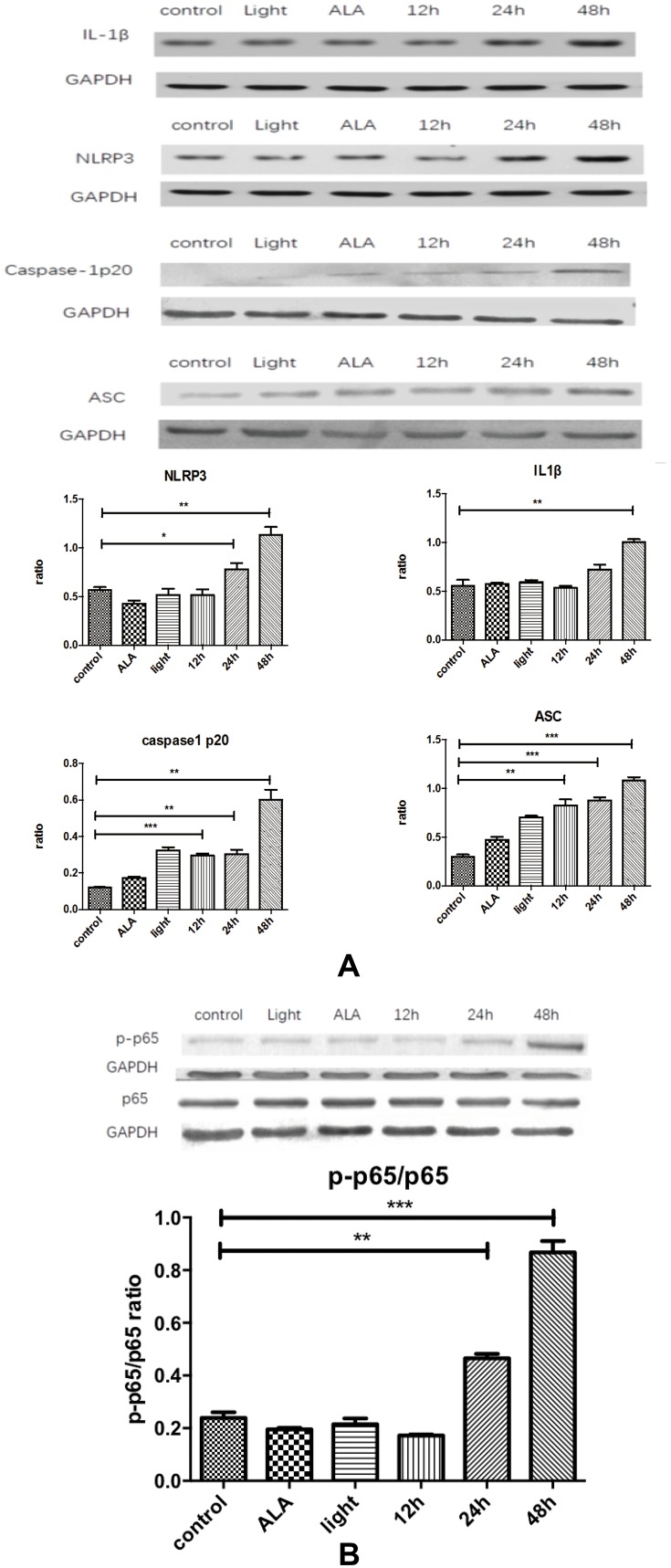
For the purpose of providing mechanistic insights into the IL1β response to ALA-PDT within the tumor microenvironment, we selected several relatively important pathways obtained from the pathways interaction network analysis crossing comparison of genetic regulatory network (GRN) of IL1β. We got the cells before and after treatment through the process mentioned above. The expression of NLRP3, ASC, caspase1, caspase1 p20, and pP65/P65 in mCAFs processed significantly increased (Figure 4). It was determined that ALA-PDT stimulated NLRP3 inflammasome activating and NF-ΚB signaling pathway mediating IL1β production in CAFs.
Figure 4.
Production of IL1β occurs through activating NLRP3 inflammasome in an NF-κB-dependent manner. (A) Compared with the control, IL1β, NLRP3, caspase-1p20 and ASC all showed increased expression with time, esp in the 48hrs. (B) p-p65/p65 ratio increases with time. Control: CAFs were incubated with DMEM and without exposed to the light; Light: CAFs were incubated with DMEM for 5hrs at 37°C and exposed to the light; ALA: CAFs were incubated with ALA (0.5 mM) for 5h at 37°C, but not exposed to the light;12hrs/24hrs/48hrs: CAFs were incubated with ALA (0.5 mM) and exposed to the light. We collected the cell samples in 12hrs/24hrs/48hrs.
Notes: *p<0.05; **p<0.01; ***p<0.001.
Discussion
Early studies have confirmed that the toxic effects of PDT do not completely eliminate tumor cells. Even if a high-dose regimen is used to destroy tumor cells to a large extent, tumor clonogenicity cannot be decreased.18 These observations indicate additional indirect mechanisms including local acute inflammation exists which is conducive to the success of PDT. Acute inflammatory response is triggered by direct injury of cells in the illuminated area, including tumor cells and interstitial cells. The complex inflammations are host responses develop to removal of necrotic and damaged cells contribute to the reconstruction of the vascular system, acceleration of its recovery and finally maintenance of tissue homeostasis. Through secreting a variety of growth factors, cytokines and proteases, CAFs are considered to be critical effectors of tumor progression.15,18–22 Previous studies have focused more on the effect of chronic inflammation induce by CAFs on tumor metastasis and evolution, and less on the role of acute inflammation in anti-tumor therapy.19,21,23 Recent published papers have linked CAF biology to the recruitment and regulation of innate and adaptive immune cells.24 Also, Togsverd-Bo K had confirmed non-inflammatory-low-dose PDT has little influence on cSCC, whereas the extent and nature of acute local inflammation caused by PDT is related to long-term efficacy.1,2 Clinical observation has reported that induction of robust local inflammatory response is conducive to the anti-tumor effects of PDT and promotes the development of systemic immunity.25 But underlying mechanism of initiation and progression of this inflammatory response remains obscure. Gene chip was used to obtain the different expressions of inflammatory cytokines and receptors for better understanding the factors regulating the pro-inflammatory cytokines and anti-inflammatory cytokine production after ALA-PDT. It was found that IL1r1 is at once one of the largest inflammatory factors and its related pathways at 3 and 6 hrs have also consistently altered, which implies the weight it bears in PDT. Here further confirmed the rise of IL1r1 and IL1β expression in the mice model and patient biopsy samples by q-PCR (Figure 1). IL1β that initiates and/or amplifies multiple effects related to innate immunity and host responses to tissue damage and microbial invasion, is a critical pro-inflammatory mediator for local and systemic inflammation. Upon completion of assembly of the NLRP3-inflammatory corpuscle complex, caspase-1 is automatically cleaved and activated, and facilitate the pro-il1β cleaving into its active form and regulating its secretion.13 After binding of IL1β to the cognate receptor IL1R1, it stimulates the production of inflammatory mediators and the biological responses such as vascularity, lipogenesis, lipid metabolism and inflammation through various pathways such as JNK, MAPK and NF-κB. In vivo, intralesional injection of anti-IL1β mAb or caspase 1 inhibitor has only a slight effect on tumor development in mice (Figure 2). In other words, IL1β activity is a key to therapeutic effects because neutralization reduces the cure rate of PDT-treated tumors. Further inhibiting caspase1, the key enzyme for IL1β activation, the effect is weakened more significantly.
Clinically, tumor tissues undergo brittle changes following PDT. Immunohistochemistry reveals that mesenchymal cell mainly CAFs have significantly increased expression of IL1β after treatment (Figure 3). Several studies drew a conclusion that fibroblasts, as the main cellular component of the tumor microenvironment, play a key synergistic role in facilitating occurrence and development toward clinically identifiable tumor by cooperating with carcinogenic cells.20–22 In fact, paracrine activation of tumor stroma (CAFs) produces a microenvironment rich in pro-inflammatory cytokines, and local inflammation selectively stimulates tumorigenesis.19–21,23 Recently, CAF has been demonstrated by several studies to be an indispensable mediator of inflammation in squamous cell carcinogenesis. These imply that CAFs have a key role in creating chronic inflammatory microenvironment, and maintaining a balanced network of inflammatory cytokines. The mCAFs isolated from cSCC tumor and corroborated it through immunohistochemistry and morphological characteristics. Further, CAFs had expressed and secreted a certain amount of IL1β after PDT (Figure 3).
Generally, IL1β production is critically regulated by NLRP3 inflammasome.13 By collecting CAFs before and after PDT, it can be found that the activation of NLRP3 inflammasomes produces caspase1p20, and the NF-κB pathway has also been activated synchronically (Figure 4). These results preliminary demonstrate that the NLRP3 inflammasome mediating IL1β production in CAFs contributes to ALA-PDT effect for cSCC, in an NF-κB-dependent manner. Recently, the correlation mechanisms between inflammation and cancer have been studied in depth and the new experimental results indicate that there are various relationships between them. For instance, NLRP3 inflammasome as the core part of inflammatory reaction, secretes IL1β which has a dual function of tumor promotion and suppression. Lewis et al26 reported that IL1 is up-regulated in various tumor types such as breast, colon, lung, head and neck cancers, and melanomas, and patients with high expression of IL1 generally have poor prognosis, so they considered IL1 as a factor in the tumor progression. On the other hand, Wei et al reported that in human liver cancer, the expression of all NLRP3 inflammatory components was either completely absent or significantly down-regulated but tissue adjacent to carcinoma.27 In summary, the present study preliminarily delves into the CAFs activating NLRP3 inflammasomes to produce IL1β and promote the treatment of cSCC with PDT. However, the concentration–response relationship of IL1β with dual roles of promotion and suppression still remains unclear. The reason is that in vitro it is difficult to create the complex microenvironment of the body. More work is needed to uncover the potential for complex and balanced cytokines networks.
Acknowledgment
The abstract of this paper was presented at the Kunming 24th Annual Meeting of Chinese Society of Dermatology as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts”; E-poster Hyperlink: http://csd2018.meetingchina.org/uploads/2018/07/20180706161732_12897.pdf.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No.81572671).
Ethical Statement
This study was approved by Huadong Hospital affiliated to Fudan University Ethics Committee (approval no. 2015K007). All patients provided written informed consent before participating in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Henderson BW, Gollnick SO, Snyder JW, et al. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004;64:2120–2126. doi: 10.1158/0008-5472.CAN-03-3513 [DOI] [PubMed] [Google Scholar]
- 2.Togsverd-Bo K, Lerche CM, Philipsen PA, et al. Artificial daylight photodynamic therapy with “non-inflammatory” doses of hexyl aminolevulinate only marginally delays SCC development in UV-exposed hairless mice. Photochem Photobiol Sci. 2013;12:2130–2136. doi: 10.1039/c3pp50152c [DOI] [PubMed] [Google Scholar]
- 3.Brackett CM, Gollnick SO. Photodynamic therapy enhancement of anti-tumor immunity. Photochem Photobiol Sci. 2011;10:649–652. doi: 10.1039/c0pp00354a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kousis PC, Henderson BW, Maier PG, et al. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res. 2007;67:10501–10510. doi: 10.1158/0008-5472.CAN-07-1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Huang JH, Wang Z, et al. Endoplasmic reticulum stress-mediated autophagy contributes to 5-ethylamino- 9 - diethylaminobenzo [a] phenoselenazinium - mediated photodynamic therapy via the PERK-eIF2α pathway. Onco Targets Ther. 2018;24:4315–4325. doi: 10.2147/OTT.S163366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gollnick SO. Photodynamic therapy and antitumor immunity. J Natl Compr Canc Netw. 2012;10 Suppl 2:S40–S43. doi: 10.6004/jnccn.2012.0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Firczuk M, Nowis D, Golab J. PDT-induced inflammatory and host responses. Photochem Photobiol Sci. 2011;10:653–663. doi: 10.1039/c0pp00308e [DOI] [PubMed] [Google Scholar]
- 8.Korbelik M. PDT-associated host response and its role in the therapy outcome. Lasers Surg Med. 2006;38:500–508. doi: 10.1002/(ISSN)1096-9101 [DOI] [PubMed] [Google Scholar]
- 9.Jimenez Fernandez D, Lamkanfi M. Inflammatory caspases: key regulators of inflammation and cell death. Biol Chem. 2015;396:193–203. doi: 10.1515/hsz-2014-0253 [DOI] [PubMed] [Google Scholar]
- 10.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038 [DOI] [PubMed] [Google Scholar]
- 11.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612 [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217. doi: 10.1002/eji.201141550 [DOI] [PubMed] [Google Scholar]
- 13.Abderrazak A, Syrovets T, Couchie D, et al. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015;4:296–307. doi: 10.1016/j.redox.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stehlik C, Lee SH, Dorfleutner A, et al. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol. 2003;171:6154–6163. doi: 10.4049/jimmunol.171.11.6154 [DOI] [PubMed] [Google Scholar]
- 15.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Ji J, Zhang H, et al. Stimulation of dendritic cells by DAMPs in ALA-PDT treated SCC tumor cells. Oncotarget. 2015;6:44688–44702. doi: 10.18632/oncotarget.v6i42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole CA, Davies RE, Forbes PD, et al. Comparison of action spectra for acute cutaneous responses to ultraviolet radiation: man and albino hairless mouse. Photochem Photobiol. 1983;37:623–631. doi: 10.1111/php.1983.37.issue-6 [DOI] [PubMed] [Google Scholar]
- 18.Henderson BW, Waldow SM, Mang TS, et al. Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer Res. 1985;45:572–576. [PubMed] [Google Scholar]
- 19.Mueller L, Goumas FA, Affeldt M, et al. Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am J Pathol. 2007;171:1608–1618. doi: 10.2353/ajpath.2007.060661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth-bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 21.Erez N, Truitt M, Olson P, et al. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041 [DOI] [PubMed] [Google Scholar]
- 22.Bussard KM, Mutkus L, Stumpf K, et al. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016;18:84. doi: 10.1186/s13058-016-0740-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Servais C, Erez N. From sentinel cells to inflammatory culprits: cancer-associated fibroblasts in tumour-related inflammation. J Pathol. 2013;229:198–207. doi: 10.1002/path.4103 [DOI] [PubMed] [Google Scholar]
- 24.Harper J, Sainson RC. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Semin Cancer Biol. 2014;25:69–77. doi: 10.1016/j.semcancer.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 25.Theodoraki MN, Lorenz K, Lotfi R, et al. Influence of photodynamic therapy on peripheral immune cell populations and cytokine concentrations in head and neck cancer. Photodiagnosis Photodyn Ther. 2017;19:194–201. doi: 10.1016/j.pdpdt.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 26.Lewis AM, Varghese S, Xu H, et al. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med. 2006;4:48. doi: 10.1186/1479-5876-4-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Q, Mu K, Li T, et al. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest. 2014;94:52–62. doi: 10.1038/labinvest.2013.126 [DOI] [PubMed] [Google Scholar]