Abstract
The recruitment of RNA polymerase II (Pol II) to core promoters is highly regulated during rapid induction of genes. In response to heat shock, heat shock transcription factor 1 (HSF1) is activated and occupies heat shock gene promoters. Promoter‐bound HSF1 recruits general transcription factors and Mediator, which interact with Pol II, but stress‐specific mechanisms of Pol II recruitment are unclear. Here, we show in comparative analyses of HSF1 paralogs and their mutants that HSF1 interacts with the pericentromeric adaptor protein shugoshin 2 (SGO2) during heat shock in mouse cells, in a manner dependent on inducible phosphorylation of HSF1 at serine 326, and recruits SGO2† to the HSP70 promoter. SGO2‐mediated binding and recruitment of Pol II with a hypophosphorylated C‐terminal domain promote expression of HSP70, implicating SGO2 as one of the coactivators that facilitate Pol II recruitment by HSF1. Furthermore, the HSF1‐SGO2 complex supports cell survival and maintenance of proteostasis in heat shock conditions. These results exemplify a proteotoxic stress‐specific mechanism of Pol II recruitment, which is triggered by phosphorylation of HSF1 during the heat shock response.
Keywords: heat shock protein, heat shock transcription factor, mouse, RNA polymerase II, shugoshin
Subject Categories: Protein Biosynthesis & Quality Control, Transcription
Shugoshin 2 is a new co‐factor for heat shock factor 1 and facilitates recruitment of RNA polymerase II to stress‐inducible promoters.
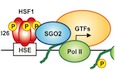
Introduction
Transcription by RNA polymerase II (Pol II) is regulated at multiple processes including formation of the preinitiation complex (PIC), initiation, pausing, and elongation. The formation of the PIC containing Pol II and general transcription factors (GTFs), which include TFIIA, TFIIB, TFIID, TFIIE, TFIIF, and TFIIH, at gene promoters is one of the major regulatory processes in eukaryotic gene expression. This process is facilitated by transcriptional activators that bind to specific DNA sequences near target genes and recruit coactivators including chromatin remodeling complexes and histone‐modifying enzymes, which open the structure of chromatin through regulating its post‐translational modifications (Fuda et al, 2009; Sainsbury et al, 2015; Venkatesh & Workman, 2015). Coactivators, such as Mediator, also interact directly with components of the transcription machinery and hence facilitate the recruitment of Pol II (Hantsche & Cramer, 2017; Soutourina, 2018), although some activators can directly interact with and stabilize them. The activator‐dependent recruitment of the PIC including Pol II is highly regulated, especially in inducible genes, the expression of which is rapidly and robustly upregulated in response to external stimuli (Weake & Workman, 2010).
The heat shock response (HSR) is an adaptive mechanism against proteotoxic stresses that adjust proteostasis capacity primarily via the induction of heat shock proteins (HSPs), or chaperones, which facilitate protein folding (Lindquist, 1986; Balch et al, 2008; Morimoto, 2008). The HSR is mainly regulated at the level of transcription by evolutionarily conserved heat shock transcription factors (HSFs) that bind to HSR elements (HSEs) in eukaryotes (Wu, 1995; Morimoto, 1998; Nakai, 2016; Gomez‐Pastor et al, 2018). In contrast to the single HSF gene in yeast, worm, and fruit fly, four HSF genes (HSF1–HSF4) exist in vertebrates. Among these, HSF1 regulates HSP expression during heat shock in mammals and lizards, whereas it is dispensable in avians, in which HSF3 takes the place of HSF1 (Fujimoto & Nakai, 2010; Takii et al, 2017). HSF1 mostly remains as an inert monomer in unstressed conditions. In response to heat shock, it is converted to an active trimer that binds to HSEs, and its transcriptional activity is enhanced by post‐translational modifications including phosphorylation (Björk & Sistonen, 2010; Nakai, 2016; Gomez‐Pastor et al, 2018). HSF1‐mediated induction of HSPs as well as non‐HSPs is associated with elevated proteostasis capacity and cell survival.
Transcriptional mechanisms of the HSR have been extensively studied. In Drosophila, GAGA‐associated factor constitutively binds to the HSP70 promoter, which allows for the establishment of an open chromatin environment and paused Pol II at the promoter‐proximal region (Jonkers & Lis, 2015; Duarte et al, 2016). In response to heat shock, trimerized HSF quickly occupies HSEs in the promoter (Guertin & Lis, 2010). Promoter‐bound HSF directly recruits Mediator (Park et al, 2001) and TATA box‐binding protein, a core component of TFIID (Mason & Lis, 1997), which facilitate PIC formation and recruitment of Pol II. It also recruits other coactivators, including P‐TEFb and TIP60, that promote rapid loss of nucleosomes and the release of stalled Pol II, which is another highly regulated process (Jonkers & Lis, 2015). In mammals, constitutively bound HSF1 in complex with replication protein A (RPA) promotes the establishment of paused Pol II and an open chromatin environment (Fujimoto et al, 2012). It also recruits PARP13‐PARP1 complex to prepare for the response (Fujimoto et al, 2018). During heat shock, activated HSF1 heavily occupies HSEs in the HSP70 promoter and dramatically induces its transcription (Vihervaara et al, 2013; Takii et al, 2015; Mahat et al, 2016), by recruiting various kinds of coactivators including ASC‐2 (Hong et al, 2004), MLL1 (Chen et al, 2014), and BRG1 (Sullivan et al, 2001; Corey et al, 2003), and by causing the redistribution of PARP1 (Fujimoto et al, 2018). These coactivators promote establishment of an active chromatin state and may thereby facilitate PIC formation during heat shock (Corey et al, 2003; Takii et al, 2015; Fujimoto et al, 2018). However, the molecular mechanisms by which the stress‐inducible activator HSF1 directly facilitates PIC formation and the recruitment of Pol II are not well known in mammalian cells.
Here, we generated HSF1 mutants lacking transcriptional activity using an evolutionary approach and identified activity‐dependent components of HSF1 complexes bound to the HSP70 promoter in vitro in mouse cell extracts. We revealed that the expression of HSPs during heat shock is markedly enhanced by a component of the HSF1 transcription complexes, shugoshin 2 (SGO2), which regulates chromosome segregation during meiosis in mice (Watanabe, 2005; Gutiérrez‐Caballero et al, 2012; Marston, 2015). Remarkably, HSF1‐SGO2 facilitated PIC formation in the HSR by directly recruiting Pol II to promoters. Furthermore, the HSF1‐SGO2 complex maintained proteostasis capacity in heat shock conditions.
Results
AsHSF1 mutants lacking transcriptional activity
It is important to identify HSF1 mutants with different potential to activate HSP genes in order to uncover coregulators of HSF1. To this end, we used an evolutionary approach involving comparative analysis of HSF1 paralogs in vertebrates. We confirmed that the overexpression of anole lizard (Anolis sagrei) HSF1 (AsHSF1) as well as human HSF1 (hHSF1) in HSF1‐null mouse embryonic fibroblasts (MEF) restored the heat shock‐induced expression of HSP70 mRNA, whereas that of chicken HSF1 (cHSF1) did not (Appendix Fig S1A) (Takii et al, 2017). We investigated the ability of various cHSF1 mutants, parts of which were swapped with corresponding AsHSF1 regions, to induce HSP70 mRNA in this experimental system. It was revealed that substitution of the cHSF1 DNA‐binding domain (DBD) with that of AsHSF1 (cHSF1‐ch2 and ‐ch3) moderately restored the induction of HSP70 mRNA during heat shock, and further substitution of region X (cHSF1‐ch4) fully restored the induction (Appendix Fig S1B). Furthermore, substitution of only Ser21 in cHSF1 DBD with the corresponding Pro17 in AsHSF1 DBD (cHSF1‐S21P) modestly restored the induction (Fig 1A and Appendix Fig S1C and D). This proline residue is located at the N‐terminal end of the DBD and is conserved in eukaryotic HSFs except cHSF1 (Nakai & Morimoto, 1993) (Appendix Fig S1E). Moreover, the 24 amino acids containing the evolutionarily conserved site d or f (called the d‐region) in region X are deleted in cHSF1 (Nakai & Morimoto, 1993; Fujimoto et al, 2010) (Appendix Fig S1F), and insertion of the AsHSF1 d‐region (cHSF1‐S21P/ins‐d) restored the full induction (Fig 1A and Appendix Fig S1G). In contrast, substitution of Pro17 in AsHSF1 with serine and deletion of the d‐region containing 24 amino acids (AsHSF1‐P17S/Δd) reduced the induction of HSP70 mRNA (Fig 1B). hHSF1 mutants possessing the same mutations (hHSF1‐P16S/Δd) also have reduced potential to induce HSP70 mRNA, although the reduction level was modest (Fig 1C).
Figure 1. AsHSF1 mutants lacking transcriptional activity in mouse cells.
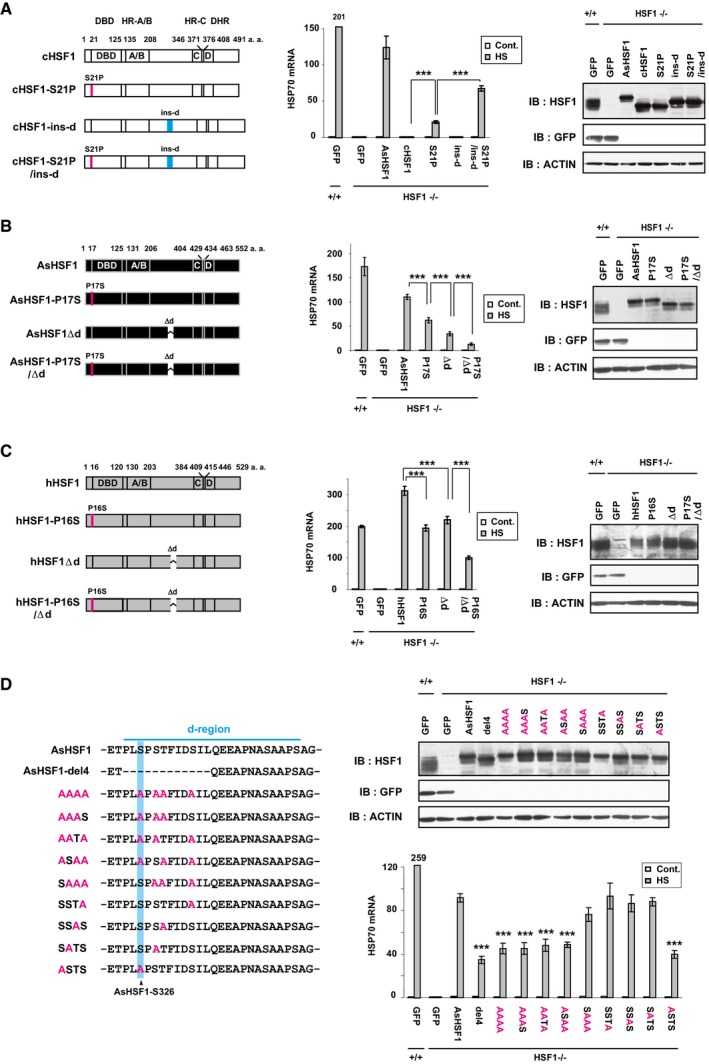
- Potential of cHSF1 mutants to induce HSP70 expression. HSF1−/− MEF cells were infected with adenovirus expressing wild‐type cHSF1, cHSF1‐S21P, cHSF1‐ins‐d (d‐region of AsHSF1 inserted), cHSF1‐S21P/ins‐d (left), or GFP as a control. These HSF1+/+ cells were untreated (Cont.) or treated with heat shock at 42°C for 30 min (HS), and HSP70 mRNA levels were quantified by RT–qPCR. The levels relative to that in control Ad‐GFP‐infected HSF1+/+ cells were shown (middle). Extracts from cells before heat shock were subjected to immunoblotting using anti‐HSF1 or anti‐β‐actin antibody (right).
- Potential of AsHSF1 mutants to induce HSP70 expression. Cells were infected with adenovirus expressing wild‐type AsHSF1, AsHSF1‐P17S, AsHSF1Δd, or AsHSF1‐P17S/Δd (left). HSP70 mRNA levels were quantified by RT–qPCR before and after heat shock (middle). Extracts were subjected to immunoblotting (right).
- Potential of hHSF1 mutants to induce HSP70 expression. Cells were infected with adenovirus expressing wild‐type hHSF1, hHSF1‐P16S, hHSF1Δd, or hHSF1‐P16S/Δd (left). HSP70 mRNA levels were quantified by RT–qPCR (middle), and extracts were subjected to immunoblotting (right).
- Alanine substitution of serine and threonine in the AsHSF1 d‐region. Serine and threonine were substituted with alanine (red) in the N‐terminal half of the d‐region, which was required to induce HSP70 expression (left; Appendix Fig S1H). Cells were infected with adenovirus expressing wild‐type and mutated AsHSF1 (upper right), and HSP70 mRNA levels were quantified by RT–qPCR before and after heat shock (lower right). Amino acids corresponding to AsHSF1‐S326 are highlighted in blue.
We next searched for amino acids in the AsHSF1 d‐region required for transcriptional activity and found that deletion of the N‐terminal 12 amino acids (AsHSF1‐del4), but not other amino acids, partially reduced HSP70 expression (Appendix Fig S1H). This short region contains 4 serine or threonine residues, one of which (AsHSF1‐Ser326) corresponds to hHSF1‐Ser326, whose phosphorylation is accompanied by transcriptional activation (Guettouche et al, 2005). It turned out that a mutation of AsHSF1‐Ser326 is sufficient for reduced HSP70 expression during heat shock (Fig 1D). In conclusion, our evolutionary approach uncovered amino acid residues of AsHSF1, Pro17 and the d‐region including Ser326, which are required to induce HSP70 expression in mouse cells.
Identification of coregulators recruited to AsHSF1 complexes in the HSP70 promoter
We examined the in vivo binding of ectopically expressed AsHSF1 mutants (AsHSF1‐P17S, AsHSF1Δd, and AsHSF1‐P17S/Δd) to the HSP70 promoter in HSF1‐null MEF cells and found that their occupancy was elevated dramatically during heat shock like that of wild‐type AsHSF1 (Appendix Fig S2A and B). In contrast, Pol II occupancy on the pausing and coding regions was enhanced at lower levels during heat shock in cells expressing AsHSF1 mutants than that in cells expressing wild‐type AsHSF1 (Appendix Fig S2C). Consistently, histone H3 occupancy was higher in the same cells. These results suggested that AsHSF1 mutants cannot recruit coregulators, which enhance Pol II occupancy, to the HSP70 promoter during heat shock.
To identify coregulators of AsHSF1, we analyzed complexes bound in vitro to AsHSF1 and three AsHSF1 mutants on HSP70 promoter using a biotin‐labeled DNA pull‐down assay (Foulds et al, 2013) (Fig 2A). A human HSPA1A (HSP70‐1) promoter fragment (wt70P) containing the proximal and distal HSE sequences (pHSE and dHSE) or its HSE‐mutated promoter fragment (mu70P; Appendix Fig S2D) was incubated with a nuclear extract of MEF cells infected with each adenovirus expression vector, and bead‐bound proteins were resolved by SDS–PAGE (Appendix Fig S2E) and identified by mass spectrometry (MS). A total of 1,870 proteins were identified in AsHSF1:wt70P complex, and identified peptide numbers of the 676 proteins were higher than those in AsHSF1:mu70P complex (difference of peptide numbers > 1; HSE‐dependent components; Fig 2B and C). This assay successfully identified already known coregulators of HSF1, such as p300, CBP, ATF1, CREB (Takii et al, 2015), GCN5 (Zelin et al, 2012), TRIM28 (Bunch et al, 2014), CHD4 (Khaleque et al, 2008), STUB/CHIP (Dai et al, 2003), and a set of Mediator (Kim & Gross, 2013) as well as HSF2 and HSF4 (Ostling et al, 2007; Fujimoto et al, 2008), in addition to many proteins with various functions (Fig 2C). Among HSE‐dependent components, we further selected 179 proteins whose differences in peptide numbers between AsHSF1:wt70P and AsHSF1:mu70P complexes are higher than those between AsHSF1‐P17S/Δd:wt70P and AsHSF1‐P17S/Δd:mu70P complexes (difference of peptide numbers > 1). Numbers of identified peptides of these proteins, including p300, CBP, STUB/CHIP, CHD4, and TRIM28, were correlated with the transcriptional activity of AsHSF1 (activity‐dependent components; Fig 2D and Table EV1).
Figure 2. Identification of coregulators recruited to AsHSF1 complexes on the HSP70 promoter.

- Schematics of the in vitro assay for the detection of AsHSF1 complexes on the HSP70 promoter. The human HSPA1A (HSP70‐1) promoter fragment (wt70P, −448 to +13) containing pHSE and dHSE or its HSE‐mutated promoter fragment (mu70P) was immobilized on magnetic beads and incubated with nuclear extract of heat‐shocked HSF1−/− MEF cells expressing AsHSF1 or its mutant. Bead‐bound proteins were resolved by SDS–PAGE and identified by MS.
- Numbers of HSE‐dependent, activity‐dependent, or activity‐independent components of AsHSF1 complexes. The HSE‐dependent 676 components consist of proteins, whose peptide numbers identified in AsHSF1:wt70P complex were higher than those in AsHSF1:mu70P complex (difference of peptide numbers > 1; a full list is shown in Table EV1). The activity‐dependent 179 components consisted of proteins, whose differences in peptide numbers between AsHSF1:wt70P and AsHSF1:mu70P complexes are higher than those between AsHSF1‐P17S/Δd:wt70P and AsHSF1‐P17S/Δd:mu70P complexes (difference of peptide numbers > 1).
- HSE‐dependent components of AsHSF1 complexes on the HSP70 promoter. Among 676 HSE‐dependent components shown in (B), 85 transcription‐related and 115 other proteins, whose difference of peptide numbers was more than three, are classified (difference of peptide numbers > 3). Gray bars indicate known coregulators of HSF1.
- HSE‐ and activity‐dependent components of AsHSF1 complexes on HSP70 promoter. Major 21 proteins out of 179 HSE‐ and activity‐dependent components are listed (difference of peptide numbers > 5). Numbers of identified peptides from AsHSF1:wt70P and AsHSF1:mu70P complexes and their differences are shown (left). Relative enrichment is indicated as a heatmap with color scale in each complex (right).
SGO2 enhances HSP70 expression during heat shock
We examined the impact of the HSE‐ and activity‐dependent components on the HSR in mouse cells and found that knockdown of five components including MED12, SGO2, PHF6, MCCC1, and MORF4L2, out of 10 newly identified components, significantly reduced the expression of HSP70 mRNA during heat shock (Fig 3A and Appendix Fig S3A). Knockdown of the other five components enhanced the induced HSP70 expression or had no effect like that of p300 (Takii et al, 2015). Among them, we analyzed the roles of SGO2, which regulates chromosome segregation during meiosis in mice (Llano et al, 2008; Orth et al, 2011). We found that SGO2 protein was substantially expressed in interphase cells including cells arrested at G1/S phase of the cell cycle (Appendix Fig S3B). Proportions of G1 and G2/M were nearly constant in cells infected with Ad‐sh‐mSGO2 for 3 days (Appendix Fig S3B), excluding a possibility that the reduced HSP70 expression was due to aberrant cell cycle progression.
Figure 3. SGO2 enhances HSP70 expression during heat shock.

- Functional screening of major components in AsHSF1 complexes. We selected 10 proteins out of major 21 HSE‐ and activity‐dependent components (Fig 2D), by removing known coregulators, an enzyme, and some translation‐related proteins. MEF cells were infected with adenovirus expressing shRNAs for genes of these HSE‐ and activity‐dependent components, and treated with heat shock at 42°C for 30 min. HSP70 mRNA levels were quantified by RT–qPCR, and induction levels relative to that in SCR‐treated cells (%) are shown.
- Stability of SGO2 during heat shock. MEF cells were treated with heat shock at 42°C for the indicated periods. Nuclear and cytoplasmic extracts from these cells were prepared and subjected to immunoblotting of SGO2 and HSF1, as well as a nuclear protein lamin B1 and HSP90, which localizes predominantly in the cytoplasm. SGO2 localized in the nucleus, and HSF1 accumulated in the nucleus during heat shock. A star indicates non‐specific bands.
- Expression of HSP mRNAs in SGO2‐knockdown cells during heat shock. Cells were infected for 72 h with adenovirus expressing shRNA for SGO2 (KD1 or KD2) or scrambled RNA (SCR), and treated with heat shock at 42°C for 20, 40, and 60 min. mRNA levels of HSP70 (HSPA1A/B), HSP40 (DNAJB1), and HSP25 (HSPB1) were quantified by RT–qPCR, and the levels relative to that in control SCR‐treated cells (fold induction) are shown (upper left). HSP70 mRNA levels in control conditions are shown separately (upper right). Cell extracts were prepared using NP40‐lysis buffer, and subjected to immunoprecipitation and immunoblotting using antibody for SGO2 or SGO1 (lower).
- Expression of HSPs in SGO2‐knockdown cells during heat shock. SGO2‐knockdown cells were treated with heat shock at 42°C for 30 min and recovered for the indicated periods (HS + Rec.). Cell extracts were prepared using NP40‐lysis buffer and subjected to immunoblotting.
- Expression of HSP70 mRNAs during treatment with proteotoxic stress inducers. SGO2‐knockdown cells were treated with heat shock at 42°C for 30 min (HS), 10 μM MG132 for 3 h, 5 mM AZC for 3 h, and 50 μm sodium arsenite for 6 h (As). HSP70 mRNA levels were quantified by RT–qPCR.
- Expression of HSP70 protein in HSF1−/− MEF cells stably expressing AsHSF1. SGO2 was knocked down in MEF‐AsHSF1 cells (clone #9). These cells were untreated (Cont.) or treated with heat shock at 42°C for 30 min and then recovered for 6 h (HS). Cell extracts were prepared and subjected to immunoblotting. Ectopically expressed AsHSF1 is stabilized during heat shock (Takii et al, 2017).
Shugoshin 2 was stable in the nucleus during heat shock at 42°C for 5 and 10 min, although it decreased thereafter (Fig 3B). We found that knockdown of SGO2 reduced mRNA levels of HSP70, HSP40, and HSP25 at 20, 40, and 60 min after heat shock (Fig 3C). To estimate accumulation of HSPs, we also examined protein levels during recovery for 1, 3, or 6 h after heat shock at 42°C for 30 min, and showed reduction of HSP levels in SGO2‐knockdown cells (Fig 3D). The basal expression of HSP mRNAs was unaffected by SGO2 knockdown (Fig 3C). We confirmed the reduced HSP70 expression in other mouse cell lines including C2C12, F9, and Neuro‐2a cells (Appendix Fig S3C). Furthermore, the induction of HSP70 mRNA during treatment with other proteotoxic stress inducers, including a proteasome inhibitor MG132, a proline analog l‐azetidine‐2‐carboxylic acid (AZC), and sodium arsenite (As), was also reduced by SGO2 knockdown (Fig 3E). To investigate whether the transcriptional role of SGO2 is specific to proteotoxic stress, we examined the expression of inducible genes in response to DNA damage and elevation of cAMP level. It was revealed that forskolin‐induced expression of AREG and CXCL1 mRNAs and doxorubicin‐induced expression of LHX15 and RAP2B mRNAs were unaffected by SGO2 knockdown (Fig EV1). Knockdown of SGO1, another member of the shugoshin family, did not alter the expression of HSP70 mRNA during heat shock (Appendix Fig S3D). These results indicated that mouse SGO2 is not generally required for transcription, but distinctively enhances the expression of HSPs during proteotoxic stresses.
Figure EV1. Effect of SGO2 knockdown on expression of inducible genes by other stimuli.
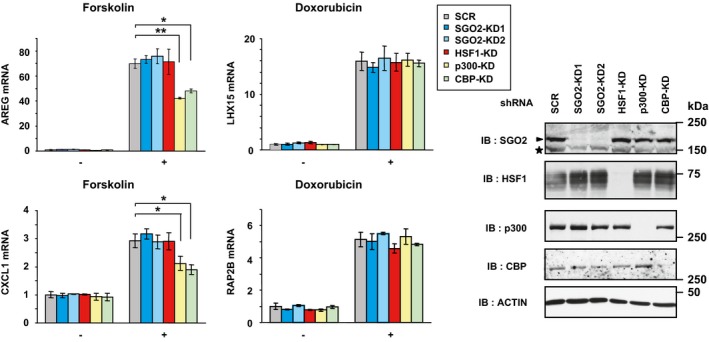
MEF cells were infected for 72 h with adenovirus expressing shRNA for SGO2 (SGO2‐KD1 or SGO2‐KD2), HSF1, p300, CBP, or scrambled RNA (SCR). Amphiregulin (AREG) and CXCL1 mRNA levels during treatment with forskolin (20 μM) for 1 h were quantified by RT–qPCR, and the levels relative to those in control SCR‐treated cells are shown (left upper). The forskolin‐mediated induction was reduced by p300 or CBP knockdown (Takii et al, 2015), but not by SGO2 or HSF1 knockdown. The mRNA levels of LHX15 and RAP2B, HSF1‐independent doxorubicin‐inducible gene products (Fujimoto et al, 2018), during the treatment with doxorubicin (0.5 μM) for 24 h were also quantified by RT–qPCR (left lower). Cell extracts were prepared using NP40‐lysis buffer and subjected to immunoblotting (right). Error bars, SD (n = 3). Asterisks indicate *P < 0.05 or **P < 0.01 by Student's t‐test. An arrowhead indicates SGO2 band, and an asterisk indicates non‐specific band in SGO2 blot.
A lizard HSF1 mutant, AsHSF1‐P17S/Δd, had little potential to induce HSP70 mRNA during heat shock, whereas a human HSF1 mutant, hHSF1‐P16S/Δd, had a reduced but measurable potential (Fig 1B and C). This observation led us to examine the effects of SGO2 knockdown on HSP70 expression in MEF cells expressing AsHSF1. The protein levels of HSP70, HSP40, and HSP25 were induced dramatically in these cells during recovery for 6 h after heat shock. Surprisingly, they were hardly induced by SGO2 knockdown (Fig 3F). This result further supported the assertion that mouse SGO2 plays an important role in the HSR.
Phosphorylated HSF1 at Ser326 recruits SGO2 to HSP70 promoter
We confirmed that HSF1 was co‐precipitated with SGO2 from an extract of heat‐shocked cells, but not from that of unstressed cells (Fig 4A). Therefore, we performed ChIP assays using primer sets for the mouse HSPA1A (HSP70.3) locus (Fig 4B). HSF1 constitutively bound to pHSE in the HSPA1A promoter (we refer to this as the HSP70 promoter later) at a low level, but not to dHSE, and its binding dramatically increased at both pHSE and dHSE during heat shock (Fig 4C) (Takii et al, 2015). SGO2 did not occupy any region within the HSP70 locus in unstressed condition. Remarkably, SGO2 occupied pHSE, but not dHSE, at its highest level at 5 min after heat shock, and its occupancy decreased slightly afterward (Fig 4C). Simultaneously, SGO2 moderately occupied the downstream pausing region, where Pol II is stalled. HSF1 knockdown abolished the heat shock‐induced occupancy of SGO2 on the pHSE and pausing region, whereas SGO2 knockdown slightly reduced HSF1 binding at 5 min after heat shock and had no effect at 30 min (Fig 4D and Appendix Fig S4A). SGO2 also occupied the HSP40 and HSP25 promoters during heat shock, and its occupancy was blocked by HSF1 knockdown (Fig 4D). These results indicated that HSF1 recruits SGO2 to HSP promoters during heat shock. A further implication is that HSF1 may recruit different coactivators depending on the location of its binding site.
Figure 4. Phosphorylated HSF1 at Ser326 recruits SGO2 to the HSP70 promoter.

- Interaction of HSF1 with SGO2. Extracts in NP‐40 lysis buffer were prepared from control MEF cells (C) and cells treated with heat shock at 42°C for 30 min (HS). Complexes co‐immunoprecipitated using SGO2 antibody were blotted with HSF1 or SGO2 antibody. An arrowhead indicates SGO2 band.
- Schematic view of the mouse HSPA1A locus. Shaded boxes indicate DNA regions (1–9) amplified by RT–qPCR.
- Occupancy of HSF1 and SGO2 on the HSPA1A locus in control MEF cells (Cont.) and cells treated with heat shock at 42°C for 5, 10, and 30 min (HS). ChIP‐qPCR on each region shown in (B) was performed.
- HSF1‐dependent recruitment of SGO2 to HSP promoters. MEF cells, which were infected with Ad‐sh‐mSGO2‐KD1, Ad‐sh‐mHSF1‐KD2, or Ad‐sh‐SCR, were treated with heat shock at 42°C for 0, 5, and 30 min. ChIP‐qPCR analyses on promoters of HSP70.3 (HSPA1A), HSP40 (DNAJB1), and HSP25 (HSPB1) were performed (left). Extracts of cells were subjected to immunoblotting (right).
- HSF1 mutants lacking the d‐region did not interact with SGO2. HSF1‐null cells were co‐infected with adenovirus expressing hHSF1 mutants and HA‐tagged mSGO2, and treated with heat shock at 42°C for 30 min. Complexes co‐immunoprecipitated with hHSF1 were blotted with HA or HSF1 antibody.
- HSF1‐S326 mutants did not interact with SGO2. HSF1‐null cells were co‐infected with adenovirus expressing hHSF1‐S326 mutants and HA‐tagged mSGO2, and untreated (Cont.) or treated with heat shock at 42°C for 30 min (HS). Complexes co‐immunoprecipitated with hHSF1 were blotted with HA, HSF1, or HSF1‐phospho‐S326 (S326P) antibody.
- SGO2 occupancy in the presence of HSF1‐S326 mutants. Cells, in which endogenous HSF1 was substituted with each mutant, were untreated (Cont.) or treated with heat shock at 42°C for 5 min (HS). ChIP‐qPCR analyses of HSF1 and SGO2 in the pHSE were performed (left). Extracts of cells were subjected to immunoblotting (right). Red arrows indicate phosphorylated hHSF1 bands.
- HSP70 expression in the presence of hHSF1 mutants. Cells, in which endogenous HSF1 was substituted with each mutant, were untreated (Cont.) or treated with heat shock at 42°C for 30 min (HS). HSP70 mRNA levels were quantified by RT–qPCR.
Because many components of Mediator including MED12 were identified in AsHSF1 complex (Fig 2C and D), we examined dependency of MED12 recruitment on SGO2 and found that SGO2 knockdown partially reduced occupancy of MED12 during heat shock (Appendix Fig S4B). In contract, SGO2 occupancy was not affected by MED12 knockdown (Appendix Fig S4C).
We next tested whether SGO2 interacts with hHSF1 mutants lacking transcriptional activity. We found that overexpressed mSGO2 is co‐precipitated with wild‐type hHSF1 and hHSF1‐P16S at a lower level but not with two hHSF1 mutants lacking the d‐region (Fig 4E). This result showed the similar tendency as that obtained by MS analysis (Fig. 2D). ChIP assays showed that substitution of endogenous HSF1 with hHSF1Δd or hHSF1‐P16S/Δd abolished occupancy of SGO2 on pHSE in the HSP70 promoter (Appendix Fig S4D). Considering that AsHSF1‐S326 in the d‐region is required for transcriptional activity (Fig 1D) and phosphorylation of mouse HSF1‐S326 was confirmed by MS (Appendix Fig S4E), we examined the interaction of hHSF1‐S326 mutants with SGO2. It turned out that mSGO2 was co‐precipitated with phosphorylated hHSF1, but not with hHSF1‐S326A or hHSF1‐S326G, during heat shock (Fig 4F). The substitution with hHSF1‐S326 mutants abolished SGO2 occupancy on the HSP70 promoter (Fig 4G) and reduced the induction of HSP70 expression during heat shock (Fig 4H). mSGO2 was also co‐precipitated with mouse HSF1 (mHSF1), but not with mHSF1Δd, mHSF1‐S326A, or mHSF1‐S326G, during heat shock (Fig EV2A), and substitution with mHSF1‐S326 mutants reduced the induction of HSP70 expression (Fig EV2B). These results demonstrated that phosphorylated HSF1 at Ser326 recruits SGO2 to HSP70 promoter during heat shock.
Figure EV2. Interaction of mHSF1 with mSGO2 and HSP70 expression in the presence of mHSF1 mutants.

- Mouse HSF1‐S326 mutants did not interact with SGO2. HSF1‐null cells were co‐infected with adenovirus expressing mHSF1 mutants (HSF1 mutant lacking the d‐region or HSF1‐S326 mutant) and HA‐tagged mSGO2, and untreated (C) or treated with heat shock at 42°C for 30 min (HS). Complexes co‐immunoprecipitated with mHSF1 were blotted with HA or HSF1 antibody.
- HSP70 expression in the presence of mHSF1 mutants. HSF1−/− MEF cells were infected with adenovirus expressing each mouse HSF1 mutant and were untreated (Cont.) or treated with heat shock at 42°C for 30 min (HS). HSP70 mRNA levels were quantified by RT–qPCR (left). Extracts of cells were subjected to immunoblotting (right). Error bars, SD (n = 3). Asterisks indicate *P < 0.05 or **P < 0.01 by Student's t‐test.
We then performed GST pull‐down assays using mSGO2 deletion mutants and identified a minimal region (amino acids 879–885) that is required for interaction with hHSF1 (Appendix Fig S4F). We overexpressed hemagglutinin (HA)‐tagged mSGO2 and deletion mutants, mSGO2Δ879–885 and mSGO2Δ871–1,029, in MEF cells, in addition to mSGO2‐N55I and mSGO2‐149AAAA, which are deficient in the interaction with protein phosphatase 2A (PP2A) and mitotic arrest deficient 2 (MAD2), respectively (Kitajima et al, 2006; Orth et al, 2011). Immunoprecipitation analysis showed that hHSF1 interacts with mSGO2, mSGO2‐N55I, and mSGO2‐149AAAA, but not with mSGO2Δ879–885 and mSGO2Δ871–1,029 (Fig EV3A). Furthermore, replacement of endogenous SGO2 with the latter two mutants reduced their occupancy on the HSP70 promoter and the induction of HSP70 mRNA during heat shock, but substitution with mSGO2‐N55I and mSGO2‐149AAAA did not affect them (Fig EV3B and C). These results confirmed that HSF1‐mediated SGO2 recruitment promotes HSP70 expression during heat shock. Given that the association of SGO2 with PPA2 and MAD2 regulates chromosome segregation (Gutiérrez‐Caballero et al, 2012; Marston, 2015), the role of SGO2 in the HSR may be independent of chromosome segregation.
Figure EV3. Replacement of endogenous SGO2 with its interaction mutants.
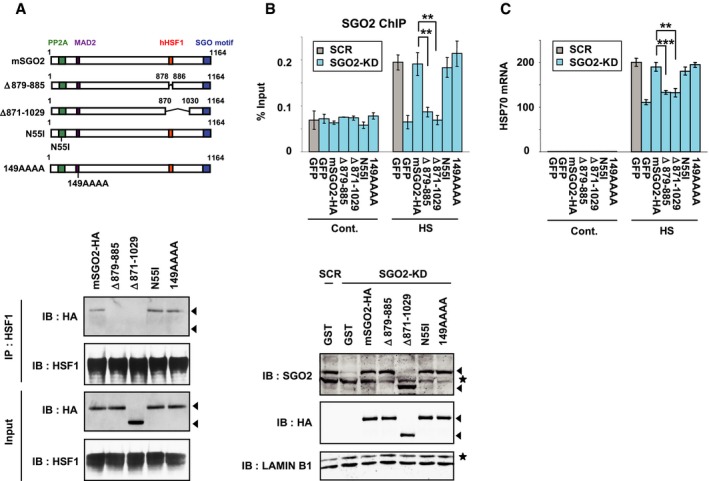
- Interaction of mSGO2 deletion mutants with hHSF1. MEF cells were co‐infected with adenovirus expressing hHSF1‐Flag and HA‐tagged mSGO2 deletion mutants, including mSGO2Δ879–885, mSGO2Δ871–1,029 (no interaction with hHSF1), mSGO2‐N55I (no interaction with PP2A), and mSGO2‐149AAAA (the “AHLP” motif was replaced with “AAAA”, no interaction with MAD2) (Kitajima et al, 2006; Orth et al, 2011). These cells were treated with heat shock at 42°C for 30 min. Complexes co‐immunoprecipitated with hHSF1 were blotted with HA or HSF1 antibody. Arrowheads indicate band positions of mSGO2 mutants in the third blot. Arrowheads in the top blot indicate expected band positions of mSGO2 mutants.
- SGO2 occupancy in the presence of mSGO2 mutants. MEF cells, in which endogenous SGO2 was substituted with each HA‐tagged mSGO2 mutant or GFP, were untreated (Cont.) or treated with heat shock at 42°C for 5 min (HS). ChIP‐qPCR analyses in the pHSE of HSP70 promoter were performed (upper). Extracts of cells were prepared in buffer C and subjected to immunoblotting using SGO2, HA or lamin B1 antibody (lower). Arrowheads indicate band positions of mSGO2 mutants in SGO2 and HA blots, and asterisks indicate non‐specific bands.
- HSP70 expression in the presence of mSGO2 mutants. Cells, in which endogenous SGO2 was substituted with each HA‐tagged mSGO2 mutant, were untreated (Cont.) or treated with heat shock at 42°C for 30 min (HS). HSP70 mRNA levels were quantified by RT–qPCR.
SGO2 interacts with Pol II containing a hypophosphorylated CTD
To understand the mechanisms by which SGO2 enhances HSP70 expression, we identified 22 proteins (identified peptide number > 5 by MS) that were co‐precipitated with overexpressed mSGO2‐HA in unstressed MEF cells (Fig 5A, Appendix Fig S5A, and Table EV2). Surprisingly, we found that three RNA polymerase (Pol II) core components, RPB1 (20 peptides were identified), RPB2 (13 peptides), and RPB3 (six peptides) (Wild & Cramer, 2012), were major SGO2‐interacting proteins, in addition to five subunits of the PP2A complex that binds to and dephosphorylates cohesin (Kitajima et al, 2006). Heat shock did not affect identified peptide numbers of the co‐precipitated Pol II core components, although it reduced those of TOPO2B and TPX2 (Fig 5A). We confirmed the co‐precipitation of RPB1 as well as PP2A with SGO2 in NP‐40 extracts of both unstressed and heat‐shocked cells (Fig 5B). Among 274 SGO2‐interacting proteins (identified peptide number > 2), 30 proteins were also components of AsHSF1 complexes (Table EV2). However, HSF1 or any component of Mediator was not identified in this analysis.
Figure 5. SGO2 interacts with hypophosphorylated Pol II .

- Identification of SGO2‐interacting proteins. MEF cells expressing mSGO2‐HA were heat‐shocked at 42°C for 0, 5, or 10 min. Nuclear extracts of these cells were prepared, and proteins immunoprecipitated with preimmune (P.I.) or anti‐SGO2 serum were identified by MS. Differences between numbers of identified peptides from the SGO2 complexes and those from control complexes were calculated, and 22 proteins, whose differences of identified peptide numbers in non‐heat shock condition were more than five, are listed (difference of peptide numbers > 5; a full list is shown in Table EV2). Relative enrichment is indicated as a heatmap with color scale in each complex.
- Interaction between SGO2 and Pol II. Extracts in NP‐40 lysis buffer were prepared from control MEF cells (C) and cells treated with heat shock at 42°C for 10 min (HS). Complexes co‐immunoprecipitated using preimmune (P.I.) or anti‐SGO2 serum (SGO2) were subjected to immunoblotting using antibodies for total CTD, CTD phospho‐S5, HSF1, PP2A, or SGO2. Positions of hyperphosphorylated (IIo) and hypophosphorylated (IIa) Pol II are indicated by arrowheads. Asterisks indicate non‐specific bands. Arrowheads in the Ser‐P blot indicate positions of hyperphosphorylated (IIo) and hypophosphorylated (IIa) Pol II.
- Phosphorylation of Pol II in vitro. Nuclear extracts were incubated in the presence of 3 mM ATP at 30°C for the indicated periods and were subjected to immunoblotting. An arrowhead in the bottom blot indicates lamin B1 band.
- SGO2 does not interact with in vitro phosphorylated Pol II. Nuclear extracts were incubated in the presence of 3 mM ATP at 30°C for 4 or 15 h and were subjected to immunoprecipitation and immunoblotting. An arrowhead in the bottom blot indicates SGO2 band.
- SGO2 interacts with unphosphorylated Pol II CTD, but not a phosphorylated form. Purified GST‐mCTD52 protein was phosphorylated by incubation with a nuclear extract (NE) from HeLa cells in the presence of 3 mM ATP at 30°C for 15 h. Addition of ATP or the NE alone is not sufficient for phosphorylation of GST‐mCTD52. Addition of 50 mM EDTA blocked the phosphorylation. The phosphorylated or unphosphorylated GST‐mCTD52 was mixed at 4°C for 3 h with a nuclear extract from MEF cells overexpressing mSGO2‐HA. The complexes pulled down with GST‐mCTD52 were subjected to immunoblotting using antibody for HA, CTD phospho‐S5 or phosphor‐S2, or GST.
The C‐terminal domain (CTD) of RPB1 is composed of 52 repeats of the consensus heptad Tyr1‐Ser2‐Pro3‐Thr4‐Ser5‐Pro6‐Ser7, whose phosphorylation principally on Ser2, Ser5, and Ser7 is regulated during transcription steps. Pol II with an unphosphorylated CTD is recruited to the promoter and highly phosphorylated during elongation (Phatnani & Greenleaf, 2006; Harlen & Churchman, 2017). Remarkably, SGO2 interacted with hypophosphorylated Pol II (IIa), but not with hyperphosphorylated Pol II (IIo; Fig 5B). Hypophosphorylated Pol II in the nuclear fraction was partially hyperphosphorylated in vitro in the presence of ATP, and this form (IIo) was not co‐precipitated with SGO2 (Fig 5C and D). Furthermore, we examined the interaction of mSGO2‐HA with the mouse CTD containing 52 heptad repeats fused to GST (GST‐mCTD52) using a GST pull‐down assay. It was revealed that GST‐mCTD52 interacts with mSGO2‐HA, whereas in vitro phosphorylated GST‐mCTD52 does not (Fig 5E). These results demonstrated that SGO2 interacts with Pol II containing a hypophosphorylated CTD.
Protein complexes that associate with Pol II have been identified, and some components such as RPAP2, RPAP3, and RPAP4 facilitate the nuclear import and biogenesis of Pol II (Boulon et al, 2010; Forget et al, 2010, 2013). However, knockdown of SGO2, which is located in the nucleus, did not affect the amount of nuclear‐localized Pol II (Appendix Fig S5B). SGO2 was extracted from nuclei with increasing NaCl concentrations (300–700 mM), whereas Pol II was more resistant to salt extraction (Appendix Fig S5C). SGO2 knockdown did not alter the amount of Pol II in the nuclear pellet (Appendix Fig S5D), suggesting that SGO2 is not involved in non‐specific Pol II loading onto chromatin.
HSF1‐SGO2 complex facilitates Pol II recruitment to the HSP70 promoter
We investigated the time‐dependent profiles of Pol II occupancy within the HSP70 locus during heat shock using ChIP assays. In unstressed condition, Pol II occupied the promoter‐proximal pausing region at a very low level in unstressed MEF cells (Jonkers & Lis, 2015). Pol II occupancy in that region was elevated dramatically at 10 min after heat shock (Fig 6A) (Takii et al, 2015). Simultaneously, Pol II occupancy elevated substantially in other regions (regions 6, 7, and 8) of the gene body. At 30 min after heat shock, it was elevated much more in region 8 near the 3′‐end (Vihervaara et al, 2017). We found that SGO2 knockdown markedly reduced the elevated occupancy of Pol II in the gene body including the pausing region and region 8 at 10 and 30 min after heat shock (Fig 6A). It did not affect phosphorylation levels of the Pol II CTD (Appendix Fig S6A). Remarkably, Pol II was hardly recruited to the pausing region during heat shock and recovery in cells expressing AsHSF1 (Appendix Fig S6B).
Figure 6. HSF1‐SGO2 complex facilitates Pol II recruitment to HSP70 during heat shock.
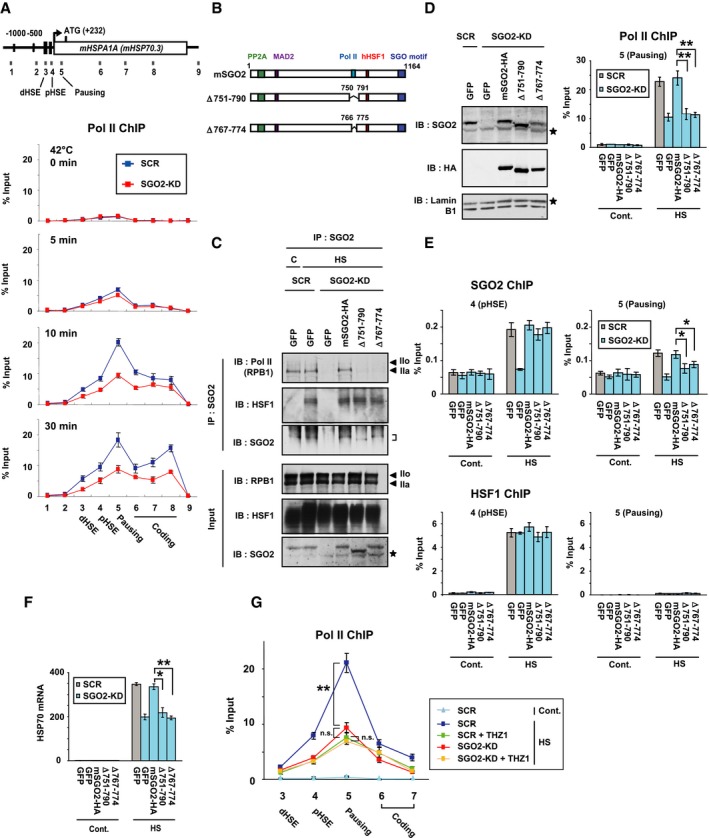
-
AOccupancy of Pol II on the HSP70 (HSPA1A) locus during heat shock. Scrambled RNA‐treated (SCR) and SGO2‐knockdown (SGO2‐KD) cells were treated with heat shock at 42°C for 0, 5, 10, and 30 min. ChIP‐qPCR on each region was performed (see Fig 4B). The occupancy level of paused Pol II was very low in unstressed MEF cells.
-
BSchematic representation of mSGO2 deletion mutants lacking the Pol II interacting region. PP2A‐, MAD2‐, and HSF1‐interacting domains as well as a conserved SGO motif are also shown.
-
CInteraction of mSGO2 deletion mutants with Pol II. Endogenous SGO2 was substituted with HA‐tagged mSGO2 or its deletion mutants, and untreated (C) or treated with heat shock at 42°C for 10 min (HS). Complexes co‐immunoprecipitated with SGO2 were blotted with antibody for total CTD (Pol II), HSF1, or SGO2. Positions of hyperphosphorylated (IIo) and hypophosphorylated (IIa) Pol II are indicated by arrowheads. Asterisks indicate non‐specific bands. A position of SGO2 bands is indicated on the right.
-
D, EPol II occupancy in the presence of mSGO2 mutants. Cells, in which endogenous SGO2 was substituted with each mutant, were untreated (Cont.) or treated with heat shock at 42°C for 10 min (HS). ChIP‐qPCR analyses of Pol II (D), SGO2, and HSF1 (E) in the pausing region (region 5) and the pHSE (region 4) in the HSP70 locus were performed. Extracts of cells were subjected to immunoblotting (D). Asterisks indicate non‐specific bands.
-
FHSP70 expression in the presence of mSGO2 mutants. Cells, in which endogenous SGO2 was substituted with each mutant, were untreated (Cont.) or treated with heat shock at 42°C for 30 min (HS). HSP70 mRNA levels were quantified by RT–qPCR.
-
GEffect of SGO2 knockdown on Pol II occupancy in the presence of THZ1. Scrambled RNA‐treated (SCR) and SGO2‐knockdown (SGO2‐KD) cells were untreated (Cont.) or treated with heat shock at 42°C for 10 min (HS). Some cells were pre‐treated with 1 μM THZ1 for 2 h before heat shock. ChIP‐qPCR analyses in the regions 3 to 7 in the HSP70 locus were performed.
To examine whether SGO2‐Pol II interaction facilitates Pol II recruitment to HSP70, we identified the amino acid residues of SGO2 (amino acids: 751–790) that are required for the interaction (Appendix Fig S6C). Pol II was not co‐precipitated with SGO2 deletion mutants SGO2Δ751–790 and SGO2Δ767–774, whereas HSF1 was co‐precipitated with these mutants (Fig 6B and C). Interaction of SGO2 with PP2A was not affected by deletion of the Pol II interacting region as well as the HSF1 interacting region (Δ879–885) (Appendix Fig S6D). Substitution of endogenous SGO2 with the interaction mutants reduced Pol II occupancy in the pausing region during heat shock, although occupancy levels of SGO2 mutants were the same as that of wild‐type SGO2 in the pHSE (Fig 6D and E). HSF1 occupancy level was unaffected by the substitution with SGO2 mutants. Consistently, HSP70 mRNA levels were reduced in these cells during heat shock (Fig 6F). To test the hypothesis that forced recruitment of SGO2 is sufficient to activate a reporter gene, we analyzed properties of N‐terminal, middle, and C‐terminal region of mSGO2, which were fused to GAL4 DNA‐binding domain. It was revealed that the middle region containing Pol II interacting region (amino acids 751–790), but not the N‐terminal and C‐terminal regions, has potential to activate the luciferase reporter gene (Fig EV4). The middle region lacking the interacting region has little potential to activate the gene. These results indicated that the HSF1‐SGO2 complex facilitates the recruitment of Pol II to the HSP70 promoter during heat shock. It is worth noting that occupancy of the SGO2 mutants in the pausing region decreased even in the presence of the pHSE‐bound HSF1 (Fig 6E), suggesting that SGO2 occupancy in the pausing region relies partly on its interaction with Pol II as well as that with HSF1 (Appendix Fig S4A).
Figure EV4. SGO2 has potential to activate a reporter gene.

- Schematic representation of mSGO2 N‐terminal (GAL4‐N), middle (GAL4‐M), or C‐terminal (GAL4‐C) region fused to GAL4 DNA‐binding domain (amino acids 1–147; GAL4DBD). The middle region lacking the region required for interaction with Pol II (GAL4‐mutM) is also shown.
- HEK293 cells were transfected with the reporter plasmid ptk‐galp3‐luc and the expression plasmid for HA‐tagged each region (wild‐type or mutated) fused to GAL4 DBD. The cells in each dish were divided into two groups and were subjected to luciferase assay (left) and Western blotting using HA antibody (right). Error bars, SD (n = 3). Asterisks indicate **P < 0.01 by Student's t‐test. Arrowheads indicate positions of truncated SGO2 proteins fused to GAL4 DBD.
To strength our proposal, we treated cells with a CDK7 inhibitor THZ1, which suppresses initiation and pausing (Kwiatkowski et al, 2014; Steurer et al, 2018). THZ1 treatment markedly decreased heat shock‐induced expression of HSP70 mRNA (Appendix Fig S6E). Phosphorylation of the Pol II CTD at Ser5 was inhibited by THZ1 treatment, but total levels of hyper‐ and hypophosphorylated Pol II were not largely changed (Appendix Fig S6F). Occupancy levels of Pol II in the promoter‐proximal regions were similarly reduced by THZ1 treatment and by SGO2 knockdown during heat shock (Fig 6G). In contrast, the occupancy levels of Pol II in THZ1‐treated cells were no longer reduced by SGO2 knockdown. These results were consistent with our proposal that SGO2 facilitates Pol II recruitment, which is an upstream step of initiation and pausing.
HSF1‐SGO2 complex maintains proteostasis capacity
We investigated whether SGO2‐mediated transcription is associated with proteostasis capacity and cell survival. We found that cell survival was markedly reduced by knockdown of SGO2 as well as HSF1 during extreme heat shock at 45°C (Fig 7A). It was also reduced by replacement of endogenous SGO2 with its interaction mutants, or by replacement of endogenous HSF1 with hHSF1‐S326A or hHSF1‐S326G (Appendix Fig S7A). Reduction in cell survival was accompanied by elevated accumulation of insoluble ubiquitylated proteins within the cells (Appendix Fig S7B).
Figure 7. HSF1‐SGO2 complex maintains proteostasis capacity.
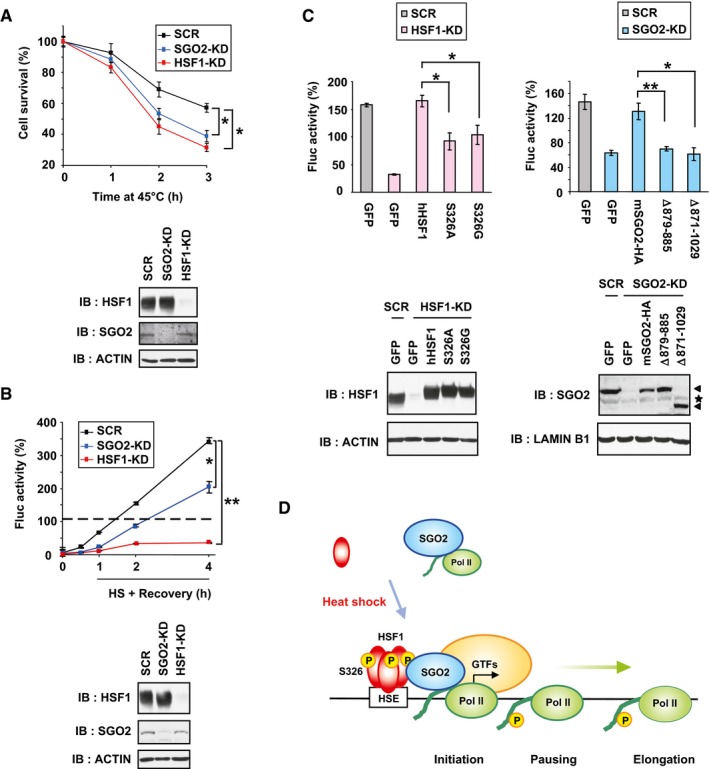
- SGO2‐knockdown cells were sensitive to heat shock. MEF cells were infected for 72 h with Ad‐sh‐mSGO2‐KD1, Ad‐sh‐mHSF1‐KD, or Ad‐sh‐SCR. After incubating these cells at 45°C for 1, 2, or 3 h, number of viable cells excluding trypan blue was counted (upper). Extracts of cells were subjected to immunoblotting (lower).
- SGO2 promotes refolding of the luciferase sensor protein during recovery from heat shock. MEF‐Fluc cells were infected with Ad‐sh‐mSGO2‐KD1, Ad‐sh‐mHSF1‐KD or Ad‐sh‐SCR, and treated with heat shock at 42°C for 2 h (HS). These cells were then recovered at 37°C (Recovery) for the indicated periods, and luciferase activity values were calculated. The value of control cells maintained at 37°C was set to 100% (dotted line; upper). Extracts of cells were subjected to immunoblotting (lower).
- HSF1‐SGO2 complex promotes refolding of the luciferase sensor protein. MEF‐Fluc cells were infected with Ad‐sh‐mSGO2‐KD1, Ad‐sh‐mHSF1‐KD, or Ad‐sh‐SCR for 24 h and then infected with each adenoviral expression vector for 48 h. These cells were treated with heat shock at 42°C for 2 h and then recovered at 37°C for 2 h. Luciferase activity values were calculated, and relative activities are shown (upper). Extracts of cells were subjected to immunoblotting (lower). Arrowheads indicate SGO2 bands, and a position of non‐specific bands is indicated by an asterisk.
- Schematic model of Pol II recruitment by HSF1‐SGO2 complex during heat shock. In response to heat shock, S326‐phosphorylated trimeric HSF1 heavily occupies the HSEs in the HSP70 promoter and recruits SGO2. HSF1‐SGO2 complex facilitates recruitment of unphosphorylated (or hypophosphorylated) Pol II. The Pol II CTD is phosphorylated during initiation and elongation, and hyperphosphorylated Pol II dissociates from HSF1‐SGO2.
Ectopically expressed luciferase is a sensor protein of proteostasis capacity in mammalian cells (Schröder et al, 1993; Donnelly et al, 2014). We generated MEF cells stably expressing luciferase (MEF‐Fluc) and performed luciferase refolding assays. Treatment of MEF‐Fluc cells with heat shock at 42°C for 2 h was sufficient to denature luciferase, and refolding was then monitored by measuring luminescence at 37°C until 4 h (Fig 7B). It was revealed that luciferase refolding was severely impaired by HSF1 knockdown and moderately impaired by SGO2 knockdown. Replacement with interaction mutants of both HSF1 and SGO2 also impaired luciferase refolding moderately (Fig 7C). We furthermore monitored proteostasis capacity using an aggregation‐prone polyglutamine polyQ81‐GFP protein (Fujimoto et al, 2005). The ratio of cells with polyQ81‐GFP inclusion in SGO2‐knockdown cells was same as that in scrambled RNA‐treated cells in unstressed conditions, but it was more enhanced during recovery from mild heat shock at 41°C for 30 min, like that in HSF1‐knockdown cells (Fig EV5A). Insoluble polyQ81‐GFP protein accumulated at consistently higher levels in SGO2‐knockdown cells than in scrambled RNA‐treated cells (Fig EV5B). These results demonstrated that the HSF1‐SGO2 complex supports cell survival and maintains proteostasis capacity during heat shock.
Figure EV5. SGO2 suppresses polyglutamine aggregates during recovery from moderate heat shock.

-
A, BSGO2‐knockdown cells were infected with Ad‐polyQ81‐GFP for 2 h and then maintained for 22 h. These cells were untreated (Cont.) or treated with moderate heat shock at 41°C for 30 min (HS) and recovered for 2 h (R2) or 4 h (R4). Percentages of cells with polyQ81‐GFP inclusions (arrows) (A) and accumulation of insoluble polyQ81‐GFP (B) were shown. Error bars, SD (n = 3). Asterisks indicate *P < 0.05 or **P < 0.01 by Student's t‐test.
Discussion
The recruitment of Pol II to the core promoter and the release of paused Pol II for productive elongation are two prominent regulatory processes, which are triggered by the activation of transcription factors during rapid transcriptional induction of genes in response to stimuli (Jonkers & Lis, 2015). In addition to the latter regulatory process, the mechanisms of Pol II recruitment in inducible genes have been studied intensively. Activated transcription factors not only recruit coactivators, such as chromatin remodeling factors and histone‐modifying enzymes, to alter chromatin structure and make it more accessible to itself and other factors but also act directly on the assembly of GTFs and Mediator, which physically interact with and recruit Pol II (Weake & Workman, 2010; Soutourina, 2018). HSF directly recruits some components of GTFs and Mediator and facilitates PIC formation during heat shock in budding yeast and Drosophila (Mason & Lis, 1997; Park et al, 2001; Anandhakumar et al, 2016). Here, we showed that the HSF1‐interacting protein SGO2 directly facilitates the recruitment of Pol II in mouse cells (Fig 7D). SGO2 is quickly recruited to pHSE, but not to dHSE, in the HSP70 promoter during the HSR in a manner dependent on HSF1, and it promotes the induction of HSP70 expression (Figs 3 and 4). Thus, SGO2 is one of the coactivators that facilitate Pol II recruitment by HSF1. This function of SGO2 may be specific to the HSR, because it does not affect the expression of several inducible genes in response to DNA damage and the elevation of cAMP level (Fig EV1). But, it is still possible that SGO2 could be involved in transcription of genes such as rapidly inducible genes on other conditions. Because SGO2 also facilitates recruitment of MED12 (Appendix Fig S4B), it will be of great importance to elucidate cooperative mechanisms between SGO2 and Mediator for facilitating Pol II recruitment. Notably, SGO2 is indispensable for the induction of HSP70 expression especially in HSF1‐null mouse cells expressing lizard HSF1 (Fig 3F and Appendix Fig S6B). Considering that HSF1 in one species can induce the HSR in cells from other eukaryotic species (Clos et al, 1993; Liu et al, 1997; Inouye et al, 2003; Takii et al, 2017), our observations suggest the existence of proteotoxic‐specific mechanisms of Pol II recruitment in eukaryotes.
Shugoshins, SGO1 and SGO2, were originally identified as protectors of centromeric cohesion during meiosis and mitosis (Kitajima et al, 2004), and this function is mediated by the recruitment of PP2A to centromeres (Kitajima et al, 2006; Riedel et al, 2006). Subsequent studies have revealed that shugoshins fundamentally act as pericentromeric adaptors for proteins including PP2A and MAD2 during M phase of the cell cycle (Gutiérrez‐Caballero et al, 2012; Marston, 2015). Here, we showed that at least three core components of Pol II, including RPB1, RPB2, and RPB3, are major mouse SGO2‐interacting proteins (Fig 5A), and HSF1‐SGO2 recruits Pol II in mouse cells as described above. SGO2‐null mice develop normally and display no overt phenotype, and SGO2 may have no crucial role during mitosis in mouse cells (Appendix Fig S3B) (Llano et al, 2008; Orth et al, 2011). Of note, human SGO1 also recruits Pol II to kinetochores and promotes mitosis‐specific centromeric transcription (Liu et al, 2015). Recently, fission yeast SGO2, a homolog of human SGO1, was reported to localize on the chromatin including subtelomeres during interphase and regulate chromatin condensation and the transcription of subtelomeric genes (Tashiro et al, 2016). Considering that mouse SGO2 binds to chromatin (Appendix Fig S5C) and interacts with Pol II irrespective of proteotoxic stress (Fig 5A), it could play similar roles in transcription. Taken together, shugoshins play important roles in chromatin structure and transcription during interphase, in addition to their original roles as pericentromeric adaptors during M phase.
Once Pol II is recruited to the HSP70 promoter, it must dissociate from the HSF1‐SGO2 complex to escape from the promoter. In general, changes in the phosphorylation state of the Pol II CTD are coupled with transcription steps. Pol II with an unmodified CTD in the PIC escapes from promoters by Ser5 phosphorylation during initiation, and paused Pol II is highly phosphorylated at Ser5 and Ser7. Phosphorylation at Ser2 then triggers transition of the paused Pol II to productive elongation (Phatnani & Greenleaf, 2006; Harlen & Churchman, 2017). The phosphorylation of Ser5 lowers the affinity of Pol II for Mediator and promotes promoter escape (Jeronimo & Robert, 2014; Wong et al, 2014; Ebmeier et al, 2017). Likewise, the affinity of SGO2 with Pol II or recombinant CTD is reduced by hyperphosphorylation (multiple phosphorylation at Ser5 and Ser2; Fig 5). Thus, hyperphosphorylation of the CTD causes Pol II dissociation from the HSF1‐SGO2 complex and may facilitate promoter escape of Pol II.
In response to heat shock, HSF1 is converted to a DNA‐binding trimer and acquires transcriptional activity. These activation processes are enhanced by post‐translational modifications including phosphorylation (Björk & Sistonen, 2010; Nakai, 2016; Gomez‐Pastor et al, 2018). HSF1 possesses a potent transcriptional activation domain in the C‐terminus (Green et al, 1995; Shi et al, 1995; Zuo et al, 1995), which is usually masked by the evolutionarily conserved regulatory domain in vertebrates (the minimal region between amino acids 221 and 310 in hHSF1) (Green et al, 1995; Takii et al, 2017). Transcriptional activity is induced by unmasking the activation domain, which interacts with the chromatin remodeling factor BRG1 (Sullivan et al, 2001; Corey et al, 2003), and probably with GTFs and Mediator (Mason & Lis, 1997; Park et al, 2001). Although HSF1 is hyperphosphorylated during the HSR (Baler et al, 1993; Sarge et al, 1993), multisite phosphorylation in the regulatory domain or activation domain has no effect on the acquisition of transcriptional activity and may not be involved in the unmasking event (Newton et al, 1996; Budzyński et al, 2015). In contrast, phosphorylation of HSF1 at a single site such as Ser230, Ser326, or Ser419 enhances heat shock‐induced transcriptional activity (Holmberg et al, 2001; Guettouche et al, 2005; Kim et al, 2005). In particular, phosphorylation of HSF1‐S326, which is located upstream of the activation domain, is associated with not only heat shock‐induced transcriptional activity but also enhanced activity in cancer cells (Dai et al, 2012; Tang et al, 2015). Our evolutionary approach determined that HSF1‐S326 in the conserved d‐region is a critical regions for heat shock‐induced transcriptional activity in vertebrates (Fig 1). Furthermore, phosphorylated HSF1 at Ser326 recruits SGO2, which interacts with Pol II (Figs 4 and 5). Thus, we propose a mechanism for phosphorylation‐dependent enhancement of HSF1 transcriptional activity during the HSR. In addition, we showed that the Pro16 in hHSF1 is involved in transcriptional activity. Because this residue is positioned at a surface of the winged helix‐turn‐helix DBD (Neudegger et al, 2016), it could be required for the recruitment of some coactivators too.
HSF1 regulates proteostasis capacity in both unstressed and stressed cells, and it inhibits progression of age‐related diseases including neurodegenerative disease and cancer (Labbadia & Morimoto, 2015). We showed that SGO2, in association with HSF1, supports cell survival and maintains proteostasis capacity during heat shock (Fig 7). SGO2 had no effect on aggregation of the aggregation‐prone polyQ81 protein in unstressed condition, but markedly inhibited it in febrile‐range thermal conditions (Fig EV5). Notably, there are species‐specific differences in the functions of shugoshins (Gutiérrez‐Caballero et al, 2012), and mouse SGO2 shares only 51% amino acid sequence identity with human SGO2. It will be of interest to elucidate the precise roles of SGO1 and SGO2 in transcription in other species including human, as the interaction of HSF1 with SGO2 could be an attractive candidate for the treatment of patients suffering from neurodegenerative diseases or cancer.
Materials and Methods
Plasmids and adenoviral vectors
To generate expression vectors for chimeras between AsHSF1 and cHSF1, cDNA fragments of AsHSF1 mutants, mHSF1 mutants, and hHSF1 mutants, which have a C‐terminal Flag tag (Nakai & Morimoto, 1993; Takii et al, 2017), were generated by PCR‐mediated site‐directed mutagenesis and inserted into pShuttle‐CMV vectors (Stratagene) at KpnI/XhoI sites (Fujimoto et al, 2018). To generate an expression vector for mouse SGO2 protein tagged with HA at the C‐terminus, a mSGO2 cDNA fragment (flanked by KpnI and XhoI sites) was amplified by reverse transcription–polymerase chain reaction (RT–PCR) using total RNA isolated from mouse embryonic fibroblasts (MEF) and inserted into a pShuttle‐CMV vector. Sequences of these expression vectors were verified using a 3500 Genetic Analyzer (Applied Biosystems). Viral DNA containing the cDNA for each HSF1 mutant was generated in accordance with the manufacturer's instructions for an AdEasy adenoviral vector system (Agilent Technologies). Viruses were infected into HEK293 cells, and the virus particles were enriched by CsCl gradient centrifugation and stored at −80°C until use.
To generate adenovirus vectors expressing short hairpin RNAs against mouse SGO2, SGO1, MED12, MCM5, and ILF3, oligonucleotides containing each target sequence were annealed and inserted into pCR2.1‐hU6 at the BamHI/HindIII sites, and then XhoI/HindIII fragments containing hU6‐shRNA were inserted into a pShuttle‐CMV vector (Stratagene) using the primers listed in Appendix Table S1 (Fujimoto et al, 2018).
Cell cultures, treatments, and adenovirus infection
Immortalized HSF1+/+ (clone #10) or HSF1−/− (clone #4) MEF cells (Takii et al, 2015) were maintained at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Sigma‐Aldrich). To overexpress each HSF1 mutant, HSF1−/− cells were infected with an adenovirus expressing each mutant (5 × 106 to 2 × 107 pfu/ml) in DMEM without FBS for 2 h and maintained with normal medium for 46 h. Simultaneously, HSF1+/+ cells were infected with Ad‐GFP as a control in the same way. Cells were treated with heat shock at 41, 42, or 45°C for the indicated periods, 10 μM MG132 (Sigma‐Aldrich) for 3 h, 5 mM AZC (Tokyo Chemical Industry) for 3 h, and 50 μM sodium arsenite (Sigma‐Aldrich) for 6 h, 20 μM forskolin (Wako Pure Chemical Industries, Ltd) for 1 h, and 0.5 μM doxorubicin (Enzo Life Sciences) for 24 h.
To knockdown HSF1, SGO2, or SGO1, MEF cells were infected with Ad‐sh‐mHSF1‐KD, Ad‐sh‐mSGO2‐KD1, Ad‐sh‐mSGO2‐KD2, Ad‐sh‐mSGO1‐KD1, or Ad‐sh‐mSGO1‐KD2 (1 × 108 pfu/ml) for 2 h and maintained in normal medium for 70 h. To replace endogenous HSF1 with exogenous hHSF1 or its phosphorylation site mutants, cells were infected with Ad‐sh‐mHSF1‐KD (1 × 108 pfu/ml) for 2 h and maintained in normal medium for 22 h. The cells were then infected with Ad‐hHSF1‐S326A‐Flag or Ad‐hHSF1‐S326G‐Flag (2 × 107 pfu/ml) for 2 h and maintained with normal medium for a further 46 h. Replacement of SGO2 with its mutant was performed in the same way.
To establish HSF1−/− MEF cells stably expressing AsHSF1 (MEF‐AsHSF1), HSF1−/− cells (clone #4) were co‐transfected with pcDNA3.1‐AsHSF1‐HA (Takii et al, 2017) and pLoxPuro (Arakawa et al, 2001), and stable transformants were isolated in medium containing 1.0 μg/ml puromycin.
Assessment of mRNA
Total RNA was isolated from cells using TRIzol (Ambion). First‐strand cDNA was synthesized using PrimeScript II Reverse Transcriptase and oligo dT primer in accordance with the manufacturer's instructions (TAKARA). RT–PCR was performed using the primers summarized in Appendix Table S2. Real‐time quantitative PCR (qPCR) was performed using StepOnePlus (Applied Biosystems) with the Power SYBR Green PCR Master Mix (Applied Biosystems) using primers listed in Appendix Table S3 for heat shock genes including mouse HSP70, HSP40, and HSP25 (Takii et al, 2015), doxorubicin‐inducible genes including LHX15 and RAP2B (Zhang et al, 2013; Dimitrova et al, 2014), and forskolin‐inducible genes including AREG and CXCL1 (Kasper et al, 2011; Pasqualucci et al, 2011). Relative quantities of mRNAs were normalized against β‐actin mRNA levels. All reactions were performed in triplicate with samples derived from three experiments.
Western blotting
Cells were lysed in NP‐40 lysis buffer (150 mM NaCl, 1.0% Nonidet P‐40, 50 mM Tris, pH 8.0) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, and 1 μg/ml pepstatin) and centrifuged at 16,000 g for 10 min. The supernatants were collected and stored at −80°C until use. Aliquots of protein (40 μg) were subjected to SDS–PAGE and then transferred onto nitrocellulose membranes. To detect SGO2 in cell extracts prepared using NP40‐lysis buffer, it was immunoprecipitated from cell extracts (4 mg) using 2.5 μl antiserum for mSGO2 (anti‐mSGO2‐2) and then subjected to SDS–PAGE. Alternatively, cells were lysed in buffer C (0.42 M NaCl, 20 mM HEPES, pH 7.9, 25% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA) containing protease inhibitors to efficiently extract SGO2 from chromatin. Precision Plus Protein Dual‐Color Standards (Bio‐Rad) were used to estimate protein sizes. The membranes were blotted using rabbit antibodies for vertebrate HSF1 (anti‐cHSF1x) (Takii et al, 2017), mammalian HSF1 (anti‐mHSF1j; ABE1044; Merck Millipore) (Takii et al, 2015), HSF1 phospho‐S326 (ab76076; Abcam), mouse SGO2 (anti‐mSGO2‐2; this study), mouse SGO1 (anti‐mSGO1‐1; this study), mouse MED12 (anti‐mMED12ΔQ‐2; this study), human HSP90 (anti‐hHSP90d), human HSP40 (anti‐hHSP40‐1), mouse HSP25 (anti‐mHSP27c) (Takaki et al, 2007), and mouse lamin B1 (ab16048; Abcam), mouse antibodies for HSP70 (W27; Santa Cruz), β‐actin (AC‐15; Sigma) and GFP (GF200; Nacalai), and rat antibody for HA (3F10; Roche). We generated rabbit antisera against mouse SGO2 (anti‐mSGO2‐2) and SGO1 (anti‐mSGO1‐1) by immunizing rabbits with bacterially expressed recombinant GST‐mSGO2 (amino acids 1–400) and GST‐mSGO1 (amino acids 109–481), respectively. We also generated rabbit antiserum against mouse MED12 (anti‐mMED12ΔQ‐2) by immunizing with recombinant GST‐mMED12ΔQ (amino acids 1,923–2,190 lacking amino acids 2,055–2,130). Peroxidase‐conjugated goat anti‐rabbit, anti‐mouse IgG, or anti‐rat IgG (Cappel) were used as second antibodies. Chemiluminescent signals from ECL detection reagents (GE Healthcare) were captured on X‐ray film (Super RX; Fujifilm).
ChIP assay
The chromatin immunoprecipitation (ChIP) assay was performed using a kit in accordance with the manufacturer's instructions (EMD Millipore). The antibodies used for ChIP assays were as follows: anti‐HSF1 (αmHSF1j; Millipore ABE1044), anti‐mSGO2 (anti‐mSGO2‐2; this study), anti‐mSGO1 (anti‐mSGO1‐1; this study), anti‐Pol II CTD repeats (8WG16, Abcam ab817), anti‐Pol II CTD phosphor‐S5 (4H8, Cell Signaling 2629), anti‐Pol II CTD phosphor‐S5 (Abcam ab5131), anti‐Pol II CTD phosphor‐S2 (Abcam ab5095), anti‐mMED12 (anti‐mMED12ΔQ‐2; this study), and anti‐H3 (Abcam ab1791). Real‐time qPCR of ChIP‐enriched DNAs in mouse HSPA1A (HSP70.3) locus was performed (Fujimoto et al, 2012). PCR using primers for regions 3–9, but not that using primers for regions 1 and 2, amplified both HSPA1A and HSPA1B. Percentage input was determined by comparing the cycle threshold value of each sample to a standard curve generated from a 5‐point serial dilution of genomic input and compensated by values obtained using normal IgG. IgG‐negative control immunoprecipitations for all sites yielded < 0.05% input. All reactions were performed in triplicate with samples derived from three experiments.
DNA pull‐down assay
A DNA fragment of human HSPA1A (HSP70‐1) promoter (−448 to +13) was amplified by PCR using the pHSP70‐luc reporter plasmid as a template (Takii et al, 2010) and inserted into pcDNA3.1/Neo vector (Invitrogen) at the KpnI/XhoI site to generate phHSP70pro. Both pHSE and dHSE in the HSP70 promoter were mutated by PCR‐mediated site‐directed mutagenesis using mutated internal primers and generated pmuhHSP70pro. The mutations were confirmed by DNA sequencing. A 479 bp biotin‐labeled human HSPA1A promoter fragment (−448 to +13) was made by PCR using LA Tag DNA polymerase (Takara Bio. Inc.), phHSP70pro or pmuhHSP70pro plasmid as a template, and biotin‐labeled oligonucleotide primers (Foulds et al, 2013). The primers used are hHsp70pro F‐bio, biotin‐5′‐TCT GGT ACC TGT AAC GTG GCC GGG CGG TG‐3′, and hHsp70pro R‐bio, biotin‐5′‐AGC CTC GAG CAG GCT AGC CGT TAT CCG GA‐3′ (underlines, KpnI and XhoI sites; italics, promoter sequences). The DNA fragments (wt70P and mu70P) were separated by agarose gel electrophoresis (2% agarose) and purified using Promega Wizard SV Gel and PCR Cleanup System (Fisher Scientific).
Dynabeads M‐280 streptavidin (60 μl: Invitrogen) were mixed with 500 μl buffer containing 1 M NaCl, 1 mM EDTA, and 10 mM Tris–HCl (pH 7.5), and rotated for 15 min at room temperature. The beads were washed three times using Magnetic Particle Concentrator (Invitrogen) with a washing buffer (20 mM HEPES [pH 7.9], 100 mM KCl, 1 mM MgCl2, 0.2 mM EDTA, 10% glycerol, and 0.02% Nonidet P‐40), suspended in 500 μl washing buffer containing 2 mg/ml bovine serum albumin, mixed with biotin‐labeled DNA fragments (4 μg), and rotated overnight at 4°C. The bead‐immobilized DNAs were then washed three times with washing buffer, and supernatants were removed.
HSF1−/− MEF cells (stock #4) were infected with adenovirus expression wild‐type AsHSF1 or each mutant (AsHSF1‐P17S, AsHSF1Δd, or AsHSF1‐P17S/Δd) in DMEM without FBS for 2 h, maintained with normal medium for 46 h, and then treated with heat shock at 42°C for 30 min. Nuclear extracts (each 8 ml) were prepared from these cells (Dignam et al, 1983), which were cultured in forty 100 mm dishes per each product, and dialyzed overnight at 4°C in phosphate‐buffered saline (PBS) containing 1 mM EDTA and 10% glycerol. After centrifugation at 17,250 g for 20 min, supernatants were added with Nonidet P‐40 (final 0.02%), poly dI‐dC (40 μg; Amersham Biosciences), and DTT (final 1 mM), and then rotated with the bead‐immobilized DNAs described above for 3 h at 4°C. The beads were washed four times with washing buffer and resuspended in 30 μl 2× SDS–sample buffer. After boiling, 6 μl protein sample was loaded on 8% SDS–PAGE, and proteins on gels were visualized with a silver stain kit (Nacalai Tesque, Japan). Precision Plus Protein Dual‐Color Standards (Bio‐Rad) were used to estimate protein sizes. The other 24 μl protein sample was loaded on 12% SDS–PAGE for preparation of gel slices for MS.
Mass spectrometry
Proteins were fractionated by SDS–PAGE on 12% acrylamide gels. Gel bands were cut out and subjected to in‐gel digestion with trypsin or chymotrypsin. Resulting peptides were dissolved in a solution containing 0.1% trifluoroacetic acid and 2% acetonitrile and analyzed by an LTQ Orbitrap Velos Pro mass spectrometer (Thermo Fisher Scientific, Waltham, MA) coupled with a nanoLC instrument (Advance, Michrom BioResources, Auburn, CA) and HTC‐PAL autosampler (CTC Analytics, Zwingen, Switzerland). Peptide separation was performed with an in‐house pulled fused silica capillary (internal diameter, 0.1 mm; length, 15 cm; tip internal diameter, 0.05 mm) packed with 3‐μm C18 L‐column (Chemicals Evaluation and Research Institute, Japan). The mobile phases consisted of 0.1% formic acid and 100% acetonitrile. Peptides were eluted with a gradient of 5–35% acetonitrile for 40 min at a flow rate of 200 nl/min. Collision‐induced dissociation (CID) spectra were acquired automatically in the data‐dependent scan mode with the dynamic exclusion option. Full MS spectra were obtained with Orbitrap in the mass/charge (m/z) range of 300–2,000 with a resolution of 60,000 at m/z 400. The 12 most intense precursor ions for in the full MS spectra were selected for subsequent ion‐trap MS/MS analysis with the automated gain control (AGC) mode. The AGC was set to 1.00 × 106 for full MS and 1.00 × 104 for CID MS/MS. The normalized collision energy values were set to 35%. Lock mass function was activated to minimize mass error during analysis.
Co‐immunoprecipitation
To detect interaction of endogenous proteins in MEF cell extracts, cells were lysed in NP‐40 lysis buffer and centrifuged at 16,000 g for 10 min. The supernatants containing 10 mg proteins were incubated with 2.5 μl rabbit antiserum for SGO2 (anti‐mSGO2‐2) on ice for 1 h and mixed with 20 μl protein A‐Sepharose beads (GE Healthcare) by rotating at 4°C for 1 h. The complexes were washed five times with NP‐40 lysis buffer and were subjected to immunoblotting using antibodies for SGO2 (anti‐mSGO2‐2), HSF1 (anti‐mHSF1j), anti‐Pol II CTD repeats (8WG16; Abcam ab817), anti‐Pol II CTD phosphor‐S5 (4H8; Cell Signaling 2629), and anti‐PP2A, A subunit, alpha isoform (Cell Signaling 2260). To detect interaction of ectopically expressed proteins, HSF1‐null MEF cells or SGO2‐knockdown cells were co‐infected for 48 h with adenovirus expressing hHSF1‐Flag (or its mutants) and mSGO2‐HA (or its mutants). In some experiments, HSF1‐null cells were co‐infected similarly with adenovirus expressing mHSF1‐Flag (or its mutants) and mSGO2‐HA (or its mutants). Cell extracts in NP‐40 lysis buffer containing 4 mg proteins were subjected to immunoprecipitation using HSF1 (anti‐mHSF1j) or SGO2 (anti‐mSGO2‐2) antibody, and then immunoblotting using antibodies for HSF1, HSF1‐S326P, SGO2, RPB1, or HA.
Identification of SGO2‐interacting proteins
Mouse embryonic fibroblast cells (stock #10; seven 100 mm dishes per each sample) were infected for 48 h with an adenovirus expressing HA‐tagged mSGO2 (Ad‐mSGO2‐HA) and were untreated or treated with heat shock at 42°C for 5 or 10 min. Nuclear extracts (each 1.75 ml) were prepared in buffer C (20 mM HEPES [pH 7.9], 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, and 0.5 mM dithiothreitol) (Dignam et al, 1983) and were diluted with 1.8 volume of dilution buffer (50 mM Tris–HCl [pH 8.0] and 1.35% NP‐40) to generate nuclear extracts containing 150 mM NaCl and 1% NP‐40. These nuclear extracts were incubated with 2.5 μl rabbit antiserum for SGO2 (anti‐mSGO2‐2) on ice for 3 h and mixed with 20 μl protein A‐Sepharose beads (GE Healthcare) by rotating at 4°C for 1 h. The complexes were washed five times with NP‐40 lysis buffer and were added with 30 μl 2× SDS–PAGE sample buffer. Aliquots (3 μl) were separated by 8% SDS–PAGE and subjected to the silver staining or immunoblotting. The other 24 μl protein sample was loaded on 12% SDS–PAGE for preparation of gel slices for MS.
To phosphorylate Pol II in vitro, nuclear extracts were prepared in a buffer containing 50 mM HEPES‐KOH, pH 7.9, 25% glycerol, 500 mM NaCl, and 12.5 mM MgCl2, and were diluted with 1.5 volume of H2O to generate extracts containing 20 mM HEPES‐KOH, pH 7.9, 10% glycerol, 200 mM NaCl, and 5 mM MgCl2. The nuclear extracts were added with ATP to a final concentration of 3 mM and were then incubated at 30°C for 4 or 15 h (Egloff et al, 2010). Immunoprecipitation was performed using these nuclear extracts containing 1% NP‐40 as described above.
Statistical analysis
Data were analyzed using Student's t‐test or analysis of variance (ANOVA). Asterisks in figures indicate that differences were significant (P < 0.05). Error bars represent the standard deviations for more than three independent experiments.
Author contributions
RT and AN designed the project; RT, MF, AK, PS, and AN performed the experiments; RT, MM, and KIN performed proteome analysis; and RT and AN wrote the manuscript. All authors discussed the results and commented on the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Table EV1
Table EV2
Review Process File
Acknowledgements
We are grateful to Dr. Tatsuya Hitano for valuable comments, and Dr. Yuki Yamaguchi and Tohru Natsume for advice on our initial analysis. We thank Mizuho Oda and Emiko Koba for their expert technical assistance. This work was supported by JSPS KAKENHI grant numbers 18H02625 (to A.N.), 19K07402, 16K08625 (to R.T.), the Uehara Memorial Foundation (to A.N.), Kato Memorial Bioscience Foundation, Takeda Science Foundation (to R.T.), the Yamaguchi University “Pump‐Priming Program” (to A.N.), and the Cooperative Research Project Program of the Medical Institute of Bioregulation, Kyushu University (to A.N. and R.T.).
The EMBO Journal (2019) 38: e102566
Correction added on 4 November 2019, after first online publication: HSF1 was replaced with SGO2.
References
- Anandhakumar J, Moustafa YW, Chowdhary S, Kainth AS, Gross DS (2016) Evidence for multiple mediator complexes in yeast independently recruited by activated heat shock factor. Mol Cell Biol 36: 1943–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Lodygin D, Buerstedde JM (2001) Mutant loxP vectors for selectable marker recycle and conditional knock‐outs. BMC Biotechnol 1: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW (2008) Adapting proteostasis for disease intervention. Science 319: 916–919 [DOI] [PubMed] [Google Scholar]
- Baler R, Dahl G, Voellmy R (1993) Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol Cell Biol 13: 2486–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk JK, Sistonen L (2010) Regulation of the members of the mammalian heat shock factor family. FEBS J 277: 4126–4139 [DOI] [PubMed] [Google Scholar]
- Boulon S, Pradet‐Balade B, Verheggen C, Molle D, Boireau S, Georgieva M, Azzag K, Robert MC, Ahmad Y, Neel H et al (2010) HSP90 and its R2TP/prefoldin‐like cochaperone are involved in the cytoplasmic assembly of RNA polymerase II. Mol Cell 39: 912–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzyński MA, Puustinen MC, Joutsen J, Sistonen L (2015) Uncoupling stress‐inducible phosphorylation of heat shock factor 1 from its activation. Mol Cell Biol 35: 2530–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunch H, Zheng X, Burkholder A, Dillon ST, Motola S, Birrane G, Ebmeier CC, Levine S, Fargo D, Hu G et al (2014) TRIM28 regulates RNA polymerase II promoter‐proximal pausing and pause release. Nat Struct Mol Biol 21: 876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen J, Yu J, Yang G, Temple E, Harbinski F, Gao H, Wilson C, Pagliarini R, Zhou W (2014) Identification of mixed lineage leukemia 1 (MLL1) protein as a coactivator of heat shock factor 1 (HSF1) protein in response to heat shock protein 90 (HSP90) inhibition. J Biol Chem 289: 18914–18927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos J, Rabindran S, Wisniewski J, Wu C (1993) Induction temperature of human heat shock factor is reprogrammed in a Drosophila cell environment. Nature 364: 252–255 [DOI] [PubMed] [Google Scholar]
- Corey LL, Weirich CS, Benjamin IJ, Kingston RE (2003) Localized recruitment of a chromatin‐remodeling activity by an activator in vivo drives transcriptional elongation. Genes Dev 17: 1392–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Zhang C, Wu Y, McDonough H, Whaley RA, Godfrey V, Li HH, Madamanchi N, Xu W, Neckers L et al (2003) CHIP activates HSF1 and confers protection against apoptosis and cellular stress. EMBO J 22: 5446–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Santagata S, Tang Z, Shi J, Cao J, Kwon H, Bronson RT, Whitesell L, Lindquist S (2012) Loss of tumor suppressor NF1 activates HSF1 to promote carcinogenesis. J Clin Invest 122: 3742–3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11: 1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova N, Zamudio JR, Jong RM, Soukup D, Resnick R, Sarma K, Ward AJ, Raj A, Lee JT, Sharp PA et al (2014) LincRNA‐p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol Cell 54: 777–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly N, Passerini V, Dürrbaum M, Stingele S, Storchová Z (2014) HSF1 deficiency and impaired HSP90‐dependent protein folding are hallmarks of aneuploid human cells. EMBO J 33: 2374–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte FM, Fuda NJ, Mahat DB, Core LJ, Guertin MJ, Lis JT (2016) Transcription factors GAF and HSF act at distinct regulatory steps to modulate stress‐induced gene activation. Genes Dev 30: 1731–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebmeier CC, Erickson B, Allen BL, Allen MA, Kim H, Fong N, Jacobsen JR, Liang K, Shilatifard A, Dowell RD et al (2017) Human TFIIH kinase CDK7 regulates transcription‐associated chromatin modifications. Cell Rep 20: 1173–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff S, Szczepaniak SA, Dienstbier M, Taylor A, Knight S, Murphy S (2010) The integrator complex recognizes a new double mark on the RNA polymerase II carboxyl‐terminal domain. J Biol Chem 285: 20564–20569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget D, Lacombe AA, Cloutier P, Al‐Khoury R, Bouchard A, Lavallée‐Adam M, Faubert D, Jeronimo C, Blanchette M, Coulombe B (2010) The protein interaction network of the human transcription machinery reveals a role for the conserved GTPase RPAP4/GPN1 and microtubule assembly in nuclear import and biogenesis of RNA polymerase II. Mol Cell Proteomics 9: 2827–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget D, Lacombe AA, Cloutier P, Lavallée‐Adam M, Blanchette M, Coulombe B (2013) Nuclear import of RNA polymerase II is coupled with nucleocytoplasmic shuttling of the RNA polymerase II‐associated protein 2. Nucleic Acids Res 41: 6881–6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds CE, Feng Q, Ding C, Bailey S, Hunsaker TL, Malovannaya A, Hamilton RA, Gates LA, Zhang Z, Li C et al (2013) Proteomic analysis of coregulators bound to ERα on DNA and nucleosomes reveals coregulator dynamics. Mol Cell 51: 185–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuda NJ, Ardehali MB, Lis JT (2009) Defining mechanisms that regulate RNA polymerase II transcription in vivo . Nature 461: 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Takaki E, Hayashi T, Kitaura Y, Tanaka Y, Inouye S, Nakai A (2005) Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem 280: 34908–34916 [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Oshima K, Shinkawa T, Wang BB, Inouye S, Hayashida N, Takii R, Nakai A (2008) Analysis of HSF4 binding regions reveals its necessity for gene regulation during development and heat shock response in mouse lenses. J Biol Chem 283: 29961–29970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Hayashida N, Katoh T, Oshima K, Shinkawa T, Prakasam R, Tan K, Inouye S, Takii R, Nakai A (2010) A novel mouse HSF3 has the potential to activate nonclassical heat‐shock genes during heat shock. Mol Biol Cell 21: 106–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Nakai A (2010) The heat shock factor family and adaptation to proteotoxic stress. FEBS J 277: 4112–4125 [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Takaki E, Takii R, Prakasam R, Hayashida N, Iemura S, Natsume T, Nakai A (2012) RPA assists HSF1 access to nucleosomal DNA by recruiting histone chaperone FACT. Mol Cell 48: 182–194 [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Takii R, Katiyar A, Srivastava P, Nakai A (2018) Poly(ADP‐Ribose) polymerase 1 promotes the human heat shock response by facilitating heat shock transcription factor 1 binding to DNA. Mol Cell Biol 38: e0005–e0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Pastor R, Burchfiel ET, Thiele DJ (2018) Regulation of heat shock transcription factors and their roles in physiology and disease. Nat Rev Mol Cell Biol 19: 4–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M, Schuetz TJ, Sullivan EK, Kingston RE (1995) A heat shock‐responsive domain of human HSF1 that regulates transcription activation domain function. Mol Cell Biol 15: 3354–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin MJ, Lis JT (2010) Chromatin landscape dictates HSF binding to target DNA elements. PLoS Genet 6: e1001114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettouche T, Boellmann F, Lane WS, Voellmy R (2005) Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem 6: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez‐Caballero C, Cebollero LR, Pendás AM (2012) Shugoshins: from protectors of cohesion to versatile adaptors at the centromere. Trends Genet 28: 351–360 [DOI] [PubMed] [Google Scholar]
- Hantsche M, Cramer P (2017) Conserved RNA polymerase II initiation complex structure. Curr Opin Struct Biol 47: 17–22 [DOI] [PubMed] [Google Scholar]
- Harlen KM, Churchman LS (2017) The code and beyond: transcription regulation by the RNA polymerase II carboxy‐terminal domain. Nat Rev Mol Cell Biol 18: 263–273 [DOI] [PubMed] [Google Scholar]
- Holmberg CI, Hietakangas V, Mikhailov A, Rantanen JO, Kallio M, Meinander A, Hellman J, Morrice N, MacKintosh C, Morimoto RI et al (2001) Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. EMBO J 20: 3800–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Kim SH, Heo MA, Choi YH, Park MJ, Yoo MA, Kim HD, Kang HS, Cheong J (2004) Coactivator ASC‐2 mediates heat shock factor 1‐mediated transactivation dependent on heat shock. FEBS Lett 559: 165–170 [DOI] [PubMed] [Google Scholar]
- !?A3B2 twb=0.42w?>Inouye S, Katsuki K, Izu H, Fujimoto M, Sugahara K, Yamada S, Shinkai Y, Oka Y, Katoh Y, Nakai A (2003) Activation of heat shock genes is not necessary for protection by heat shock transcription factor 1 against cell death due to a single exposure to high temperatures. Mol Cell Biol 23: 5882–5895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo C, Robert F (2014) Kin28 regulates the transient association of mediator with core promoters. Nat Struct Mol Biol 21: 449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers I, Lis JT (2015) Getting up to speed with transcription elongation by RNA polymerase II. Nat Rev Mol Cell Biol 16: 167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Thomas MC, Zambetti GP, Brindle PK (2011) Double null cells reveal that CBP and p300 are dispensable for p53 targets p21 and Mdm2 but variably required for target genes of other signaling pathways. Cell Cycle 10: 212–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleque MA, Bharti A, Gong J, Gray PJ, Sachdev V, Ciocca DR, Stati A, Fanelli M, Calderwood SK (2008) Heat shock factor 1 represses estrogen‐dependent transcription through association with MTA1. Oncogene 27: 1886–1893 [DOI] [PubMed] [Google Scholar]
- Kim SA, Yoon JH, Lee SH, Ahn SG (2005) Polo‐like kinase 1 phosphorylates heat shock transcription factor 1 and mediates its nuclear translocation during heat stress. J Biol Chem 280: 12653–12657 [DOI] [PubMed] [Google Scholar]
- Kim S, Gross DS (2013) Mediator recruitment to heat shock genes requires dual Hsf1 activation domains and mediator tail subunits Med15 and Med16. J Biol Chem 288: 12197–12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima TS, Kawashima SA, Watanabe Y (2004) The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427: 510–517 [DOI] [PubMed] [Google Scholar]
- Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, Watanabe Y (2006) Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441: 46–52 [DOI] [PubMed] [Google Scholar]
- Kwiatkowski N, Zhang T, Rahl PB, Abraham BJ, Reddy J, Ficarro SB, Dastur A, Amzallag A, Ramaswamy S, Tesar B et al (2014) Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature 511: 616–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI (2015) The biology of proteostasis in aging and disease. Annu Rev Biochem 84: 435–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S (1986) The heat‐shock response. Annu Rev Biochem 55: 1151–1191 [DOI] [PubMed] [Google Scholar]
- Liu XD, Liu PC, Santoro N, Thiele DJ (1997) Conservation of a stress response: human heat shock transcription factors functionally substitute for yeast HSF. EMBO J 16: 6466–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Qu Q, Warrington R, Rice A, Cheng N, Yu H (2015) Mitotic transcription installs SGO1 at centromeres to coordinate chromosome segregation. Mol Cell 59: 426–436 [DOI] [PubMed] [Google Scholar]
- Llano E, Gómez R, Gutiérrez‐Caballero C, Herrán Y, Sánchez‐Martín M, Vázquez‐Quiñones L, Hernández T, de Alava E, Cuadrado A, Barbero JL et al (2008) Shugoshin‐2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev 22: 2400–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahat DB, Salamanca HH, Duarte FM, Danko CG, Lis JT (2016) Mammalian heat shock response and mechanisms underlying its genome‐wide transcriptional regulation. Mol Cell 62: 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston AL (2015) Shugoshins: tension‐sensitive pericentromeric adaptors safeguarding chromosome segregation. Mol Cell Biol 35: 634–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason PB Jr, Lis JT (1997) Cooperative and competitive protein interactions at the hsp70 promoter. J Biol Chem 272: 33227–33233 [DOI] [PubMed] [Google Scholar]
- Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12: 3788–3796 [DOI] [PubMed] [Google Scholar]
- Morimoto RI (2008) Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev 22: 1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A (ed) (2016) Heat shock factor. Tokyo, Japan: Springer; [Google Scholar]
- Nakai A, Morimoto RI (1993) Characterization of a novel chicken heat shock transcription factor, heat shock factor 3, suggests a new regulatory pathway. Mol Cell Biol 13: 1983–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudegger T, Verghese J, Hayer‐Hartl M, Hartl FU, Bracher A (2016) Structure of human heat‐shock transcription factor 1 in complex with DNA. Nat Struct Mol Biol 23: 140–146 [DOI] [PubMed] [Google Scholar]
- Newton EM, Knauf U, Green M, Kingston RE (1996) The regulatory domain of human heat shock factor 1 is sufficient to sense heat stress. Mol Cell Biol 16: 839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth M, Mayer B, Rehm K, Rothweiler U, Heidmann D, Holak TA, Stemmann O (2011) Shugoshin is a Mad1/Cdc20‐like interactor of Mad2. EMBO J 30: 2868–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostling P, Björk JK, Roos‐Mattjus P, Mezger V, Sistonen L (2007) Heat shock factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1. J Biol Chem 282: 7077–7086 [DOI] [PubMed] [Google Scholar]
- Park JM, Werner J, Kim JM, Lis JT, Kim YJ (2001) Mediator, not holoenzyme, is directly recruited to the heat shock promoter by HSF upon heat shock. Mol Cell 8: 9–19 [DOI] [PubMed] [Google Scholar]
- Pasqualucci L, Dominguez‐Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, Kasper LH, Lerach S, Tang H, Ma J et al (2011) Inactivating mutations of acetyltransferase genes in B‐cell lymphoma. Nature 471: 189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phatnani HP, Greenleaf AL (2006) Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev 20: 2922–2936 [DOI] [PubMed] [Google Scholar]
- Riedel CG, Katis VL, Katou Y, Mori S, Itoh T, Helmhart W, Gálová M, Petronczki M, Gregan J, Cetin B et al (2006) Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441: 53–61 [DOI] [PubMed] [Google Scholar]
- Sainsbury S, Bernecky C, Cramer P (2015) Structural basis of transcription initiation by RNA polymerase II. Nat Rev Mol Cell Biol 16: 129–143 [DOI] [PubMed] [Google Scholar]
- Sarge KD, Murphy SP, Morimoto RI (1993) Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA‐binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol 13: 1392–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder H, Langer T, Hartl FU, Bukau B (1993) DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat‐induced protein damage. EMBO J 12: 4137–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Kroeger PE, Morimoto RI (1995) The carboxyl‐terminal transactivation domain of heat shock factor 1 is negatively regulated and stress responsive. Mol Cell Biol 15: 4309–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutourina J (2018) Transcription regulation by the mediator complex. Nat Rev Mol Cell Biol 19: 262–274 [DOI] [PubMed] [Google Scholar]
- Steurer B, Janssens RC, Geverts B, Geijer ME, Wienholz F, Theil AF, Chang J, Dealy S, Pothof J, van Cappellen WA et al (2018) Live‐cell analysis of endogenous GFP‐RPB1 uncovers rapid turnover of initiating and promoter‐paused RNA polymerase II. Proc Natl Acad Sci USA 115: E4368–E4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EK, Weirich CS, Guyon JR, Sif S, Kingston RE (2001) Transcriptional activation domains of human heat shock factor 1 recruit human SWI/SNF. Mol Cell Biol 21: 5826–5837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki E, Fujimoto M, Nakahari T, Yonemura S, Miyata Y, Hayashida N, Yamamoto K, Vallee RB, Mikuriya T, Sugahara K et al (2007) Heat shock transcription factor 1 is required for maintenance of ciliary beating in mice. J Biol Chem 282: 37285–37292 [DOI] [PubMed] [Google Scholar]
- Takii R, Inouye S, Fujimoto M, Nakamura T, Shinkawa T, Prakasam R, Tan K, Hayashida N, Ichikawa H, Hai T et al (2010) Heat shock transcription factor 1 inhibits expression of IL‐6 through activating transcription factor 3. J Immunol 184: 1041–1048 [DOI] [PubMed] [Google Scholar]
- Takii R, Fujimoto M, Tan K, Takaki E, Hayashida N, Nakato R, Shirahige K, Nakai A (2015) ATF1 modulates the heat shock response by regulating the stress‐inducible HSF1‐transcription complex. Mol Cell Biol 35: 11–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takii R, Fujimoto M, Matsuura Y, Wu F, Oshibe N, Takaki E, Katiyar A, Akashi H, Makino T, Kawata M et al (2017) HSF1 and HSF3 cooperatively regulate the heat shock response in lizards. PLoS ONE 12: e0180776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Dai S, He Y, Doty RA, Shultz LD, Sampson SB, Dai C (2015) MEK guards proteome stability and inhibits tumor‐suppressive amyloidogenesis via HSF1. Cell 160: 729–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro S, Handa T, Matsuda A, Ban T, Takigawa T, Miyasato K, Ishii K, Kugou K, Ohta K, Hiraoka Y et al (2016) Shugoshin forms a specialized chromatin domain at subtelomeres that regulates transcription and replication timing. Nat Commun 7: 10393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh S, Workman JL (2015) Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol 16: 178–189 [DOI] [PubMed] [Google Scholar]
- Vihervaara A, Sergelius C, Vasara J, Blom MA, Elsing AN, Roos‐Mattjus P, Sistonen L (2013) Transcriptional response to stress in the dynamic chromatin environment of cycling and mitotic cells. Proc Natl Acad Sci USA 110: E3388–E3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihervaara A, Mahat DB, Guertin MJ, Chu T, Danko CG, Lis JT, Sistonen L (2017) Transcriptional response to stress is pre‐wired by promoter and enhancer architecture. Nat Commun 8: 255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y (2005) Sister chromatid cohesion along arms and at centromeres. Trends Genet 21: 405–412 [DOI] [PubMed] [Google Scholar]
- Weake VM, Workman JL (2010) Inducible gene expression: diverse regulatory mechanisms. Nat Rev Genet 11: 426–437 [DOI] [PubMed] [Google Scholar]
- Wild T, Cramer P (2012) Biogenesis of multisubunit RNA polymerases. Trends Biochem Sci 37: 99–105 [DOI] [PubMed] [Google Scholar]
- Wong KH, Jin Y, Struhl K (2014) TFIIH phosphorylation of the Pol II CTD stimulates mediator dissociation from the preinitiation complex and promoter escape. Mol Cell 54: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C (1995) Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol 11: 441–469 [DOI] [PubMed] [Google Scholar]
- Zelin E, Zhang Y, Toogun OA, Zhong S, Freeman BC (2012) The p23 molecular chaperone and GCN5 acetylase jointly modulate protein‐DNA dynamics and open chromatin status. Mol Cell 48: 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, He Y, Lee KH, Dubois W, Li Z, Wu X, Kovalchuk A, Zhang W, Huang J (2013) Rap2b, a novel p53 target, regulates p53‐mediated pro‐survival function. Cell Cycle 12: 1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Rungger D, Voellmy R (1995) Multiple layers of regulation of human heat shock transcription factor 1. Mol Cell Biol 15: 4319–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Table EV1
Table EV2
Review Process File


