Abstract
Background
Triple‐negative apocrine carcinoma (TNAC) of the breast is a very rare type of breast cancer. Furthermore, the clinicopathological features, prognosis, and potential impact of treatment strategies in TNAC remain unclear.
Methods
Data from the Surveillance, Epidemiology, and End Results (SEER) program were used to identify breast cancer patients diagnosed between 2010 and 2016 with TNAC and triple‐negative breast cancer (TNBC, IDC [invasive ductal carcinoma], NOS [not otherwise specified]). Chi‐squared tests were used to examine the categorical variables between the two groups. Overall survival (OS) of TNAC and TNBC was assessed by Kaplan‐Meier analyses and Cox regression. Breast cancer‐specific survival (BCSS) was evaluated by Nelson‐Aalen analyses and competing risk regression.
Results
We identified 31 362 patients from the SEER database, including 366 patients with TNAC and 30 996 patients with TNBC. TNAC was correlated with older age, lower T stage and lower tumor grade. Patients with TNAC had better OS compared with TNBC patients; the 5‐year OS rates were 82.2% vs 73.5% (P < .001). The breast cancer‐related death rate was significantly lower in patients with TNAC than in patients with TNBC, with a 5‐year cumulative incidence of 9.1% vs 22.9% (P < .001). Chemotherapy was significantly associated with improved OS in TNAC patients, but radiotherapy was not associated with OS in TNAC patients. In the multivariable Cox regression, TNAC was still associated with improved OS (HR [hazard ratio], 0.61; 95% CI [confidence interval] 0.45‐0.83; P = .002). In the multivariable competing risk regression, the significantly higher BCSS in patients with TNAC compared patients with TNBC remained (subdistribution HR [SHR], 0.42; 95% CI, 0.27‐0.64; P < .001).
Conclusion
Patients with TNAC had a better prognosis than patients with TNBC, and chemotherapy was associated with survival advantages in TNAC patients.
Keywords: apocrine carcinoma, prognosis, SEER database, triple‐negative breast cancer
Invasive triple‐negative apocrine carcinoma (TNAC) of the breast is a rare type of triple‐negative breast cancer. Furthermore, the clinicopathological features, prognosis of TNAC, and the potential effect of systemic treatments on TNAC remain unclear. The purpose of the present study was to gain better knowledge of clinicopathological features and survival differences in patients with TNAC and TNBC (non‐apocrine breast cancer), by utilizing a population wide database to enroll a large population of breast cancer.

1. INTRODUCTION
Invasive apocrine carcinoma (AC), a pathological type of invasive ductal carcinoma (IDC) of the breast, is defined as a breast tumor composed of epithelium with apocrine differentiation in more than 90% of the tumor cells and accounts for 0.3%‐4% of all breast cancer.1, 2 It is well known that AC tends to represent a unique hormone receptor profile—progesterone receptor (PR)‐negative, estrogen receptor (ER)‐negative, and androgen receptor (AR)‐positive.3 The overexpression of human epidermal growth factor receptor 2 (HER2) is common in AC (~30%),4, 5 but HER2‐negative AC can be phenotyped as triple‐negative breast cancer (TNBC). However, from the management perspective, most AC can be treated as TNBC, thus they are not subjected to standard anti‐HER2 or endocrine treatments. Considering the clinicopathogical features and prognosis, it is reasonable that triple‐negative apocrine carcinoma (TNAC) should be distinguished from TNBC.6, 7
Because TNAC is a rare pathological type of TNBC, the clinicopathological features and prognosis of these patients have only been reported in a limited number of studies—case reports or studies recruiting a small number of patients. As a result, the prognostic values of clinicopathological features and treatments in TNAC patients remain unclear. An observational study of 46 breast cancer patients showed that AC was more often present in older women with lower grade and T stage compared with TNBC, but some AC patients in this study were non‐TNBC.8 Meattini et al showed that TNAC had a favorable overall survival (OS) outcome when compared with other TNBC tumors.9 However, this study provided limited information on the prognosis for TNAC due to its small sample size. Two other studies showed that there was no difference in survival between AC patients and non‐AC patients.10, 11 Consequently, it is important to clarify the clinicopathological features and prognosis of TNAC in a large population.
The purpose of the present study was to investigate the clinicopathological features and survival differences in patients with TNAC and TNBC (IDC, NOS [not otherwise specified]) by utilizing a population‐wide database to enroll a large population of breast cancer patients. OS and breast cancer‐specific survival (BCSS) were compared between the two groups using comprehensive statistical methods with a multivariable Cox model and competing risk regression to adjust for confounding factors. We sought to identify the prognostic factors that might explain the differences in survival between patients with TNAC and TNBC.
2. MATERIALS AND METHODS
2.1. Study population
Patient data were obtained from the Surveillance, Epidemiology, and End Results (SEER) website (http://seer.cancer.gov/) using SEER*stat version 8.3.5. We used SEER data released in March 2019 and extracted data from 2010 to 2016. As the SEER database began to include HER2 status in 2010, we chose 1 January 2010 as the starting point for the study.
The inclusion criteria were as follows: female, over 18 years of age, unilateral breast cancer, pathologic confirmation of AC (ICD‐0‐3 8401) and IDC, NOS (ICD‐0‐3 8500), triple‐negative breast cancer subtype, breast cancer as first and the only diagnosis, diagnosis not obtained from a death certification or autopsy, known survival time and surgery status, and known T and N stage (with T0 and Tis tumors excluded). Finally, 31 362 patients were included; 366 patients were diagnosed with TNAC and 30 996 with TNBC (Table 1).
Table 1.
Stepwise inclusion and exclusion counts
| Removal criterion | TNAC | TNBC | ||
|---|---|---|---|---|
| Removed | Remaining | Removed | Remaining | |
| 2010‐2016 TNAC or TNBC patients | 0 | 454 | 0 | 38 061 |
| Exclude men | 0 | 454 | 40 | 38 021 |
| Exclude patients whose tumor was not the first tumor | 81 | 373 | 6026 | 31 995 |
| Exclude patients without histology or cytology confirmation | 0 | 373 | 7 | 31 988 |
| Exclude patients without survival information/ diagnosed by autopsy/death record only | 0 | 373 | 0 | 31 988 |
| Exclude patients younger than 18 y | 0 | 373 | 1 | 31 987 |
| Exclude patients whose disease is stage T0/Tis | 2 | 371 | 45 | 31 942 |
| Exclude patients with bilateral involvement | 0 | 371 | 8 | 31 934 |
| Exclude patients with unknown T and/or N stage | 5 | 366 | 702 | 31 051 |
| Exclude patients with unknown surgery status | 0 | 366 | 55 | 30 996 |
| Final data set | 0 | 366 | 0 | 30 996 |
The demographic features included age at diagnosis, race, and marital status; the clinicopathological features included tumor grade, breast subtype, laterality, T stage, N stage, metastasis, and American Joint Committee on Cancer (AJCC) stage; and the treatment information included radiation therapy, chemotherapy and surgery. The primary endpoint of the study was BCSS from the date of diagnosis to the date of death from breast cancer, and the secondary outcome was OS from the date of diagnosis to the date of death from any cause. Patients alive at the time of last follow‐up and/or at the end of the analysis period (November 31, 2016) were right censored.
We obtained permission to use data files from the SEER database. Therefore, our study was exempted by the Ethics Committee of Zhujiang Hospital of Southern Medical University.
2.2. Statistical analysis
The clinicopathological features were compared between TNAC and TNBC using the chi‐squared test. Categorical variables were reported as the number of cases and percentages. OS rates were calculated by the Kaplan‐Meier analyses, and survival experiences were compared by using the log‐rank test. Multivariable Cox proportional hazard regression was used to evaluate the prognostic factors for OS, and the results were presented with a hazard ratio (HR) and 95% confidential interval (CI).
For the competing risk regression model, the outcome of interest was defined as breast cancer‐specific death, while death not related to breast cancer was considered a competing risk. The cumulative incidence function for breast cancer‐specific death was performed, considering death not related to breast cancer as a competing risk of death. Nelson‐Aalen cumulative risk curves of the incidence function for breast cancer‐specific death were conducted and compared by Gray's test. Fine and Gray's competing risk regression was used to assess the prognostic factors associated with BCSS, with results presented as a subdistribution HR (SHR) and 95% CI.
All statistical analyses were performed using Stata 15.0 (Stata Corporation, College Station, Texas) and R statistical software 3.5.0 (StataCorp LLC, College Station, Texas). All statistical tests were two‐sided, and the level of significance was set at P < .05.
3. RESULTS
3.1. Patient characteristics of TNAC and TNBC
After applying the inclusion and exclusion criteria, the study cohort included 366 patients diagnosed with TNAC and 30 996 patients diagnosed with TNBC (IDC, NOS) from 2010 to 2016 (Table 1).
Patient demographics, clinicopathological features, and treatment information are shown in Table 2 for TNAC and TNBC. There were no significant differences found in marital status, laterality, N stage, metastasis, and radiation therapy when comparing patients with TNAC and TNBC. However, TNAC patients had an older age at diagnosis (≥50 years, 91.0% vs 70.7%, P < .001) and had a significantly lower black race prevalence (13.9% vs 21.0%, P = .001) than TNBC patients.
Table 2.
Patient and tumor characteristics in the triple‐negative AC (TNAC) and IDC (TNBC) groups
| Characteristic | TNAC (N = 366) | TNBC (N = 30 996) | P‐value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | <.001 | ||||
| 18‐49 | 33 | 9.0 | 9072 | 29.3 | |
| ≥50 | 333 | 91.0 | 21 924 | 70.7 | |
| Race | .001 | ||||
| White | 265 | 72.4 | 21 881 | 70.6 | |
| Black | 51 | 13.9 | 6521 | 21.0 | |
| Other | 48 | 13.1 | 2406 | 7.8 | |
| Unknown | 2 | 0.5 | 188 | 0.6 | |
| Marital status | .500 | ||||
| Married | 185 | 50.5 | 16 622 | 53.6 | |
| Unmarried | 161 | 44.0 | 12 757 | 41.2 | |
| Unknown | 20 | 5.5 | 1617 | 5.2 | |
| Laterality | .167 | ||||
| Left | 170 | 46.4 | 15 925 | 51.4 | |
| Right | 196 | 53.6 | 15 067 | 48.6 | |
| Unknown | 0 | 0.0 | 4 | 0.0 | |
| Tumor grade | <.001 | ||||
| I/II | 242 | 66.1 | 5193 | 16.7 | |
| III/IV | 113 | 30.9 | 24 878 | 80.3 | |
| Unknown | 11 | 3.0 | 925 | 3.0 | |
| T stage | <.001 | ||||
| T1 | 224 | 61.2 | 13 265 | 42.8 | |
| T2 | 108 | 29.5 | 13 139 | 42.4 | |
| T3 | 23 | 6.3 | 2657 | 8.6 | |
| T4 | 11 | 3.0 | 1935 | 6.2 | |
| N stage | .296 | ||||
| N0 | 250 | 68.3 | 19 755 | 63.7 | |
| N1 | 83 | 22.7 | 7816 | 25.2 | |
| N2 | 20 | 5.5 | 1919 | 6.2 | |
| N3 | 13 | 3.6 | 1506 | 4.9 | |
| Metastasis | .070 | ||||
| M0 | 355 | 97.0 | 29 417 | 94.9 | |
| M1 | 11 | 3.0 | 1579 | 5.1 | |
| AJCC stage | <.001 | ||||
| I | 181 | 49.5 | 11 052 | 35.7 | |
| II/III | 174 | 47.5 | 18 365 | 59.2 | |
| IV | 11 | 3.0 | 1579 | 5.1 | |
| Radiation therapy | .090 | ||||
| None/unknown | 168 | 45.9 | 15 609 | 50.4 | |
| Done | 198 | 54.1 | 15 387 | 49.6 | |
| Chemotherapy | <.001 | ||||
| None/unknown | 134 | 36.6 | 7247 | 23.4 | |
| Done | 232 | 53.4 | 23 729 | 76.6 | |
| Surgery | <.001 | ||||
| None | 10 | 2.7 | 2487 | 8.0 | |
| Done | 356 | 97.3 | 28 509 | 92.0 | |
Abbreviations: AJCC, American Joint Committee on Cancer; TNAC, triple‐negative apocrine carcinoma; TNBC, triple‐negative breast cancer.
A higher rate of grade I/II tumors (66.1% vs 16.7%, P < .001) was observed in TNAC patients than in TNBC patients. Moreover, TNAC patients presented with a greater frequency of T1 stage (61.2% vs 42.8%, P < .001). Consequently, a higher proportion of TNAC patients had AJCC stage I disease compared with TNBC patients (49.5% vs 35.7%, P < .001). Treatments were also significantly different between TNAC and TNBC patients. The surgery rate was higher in patients with TNAC compared to patients with TNBC (97.3% vs 92.0%, P < .001). In addition, chemotherapy was used less frequently in patients with TNAC than in patients with TNBC (59.8% vs 73.6%, P < .001).
3.2. Survival analysis
In the current study, the median follow‐up time was 33 months for the TNAC group and 30 months for the TNBC group. There were 1423 (4.6%) breast cancer‐related deaths observed in the TNBC group and 23 (6.3%) in the TNAC group. Deaths from other causes were identified in 4305 (13.9%) patients and 19 (5.2%) patients in the TNBC and TNAC groups, respectively.
To explore whether patients with TNAC and TNBC had different OS rates, we first compared the Kaplan‐Meier curves and the 5‐year OS rates of patients with TNAC and TNBC. The 5‐year OS rate for all patients was 73.6% (95% CI, 72.9%‐74.2%), with a 5‐year OS rate of 82.2% (95% CI, 76.1%‐86.8%) for patients with TNAC and 73.5% (95% CI, 72.8%‐74.1%) for patients with TNBC. The Kaplan‐Meier curves and log‐rank test showed that TNAC patients had better OS rates compared with TNBC patients (Figure 1, P < .001). To further investigate the impacts of chemotherapy and radiation therapy on OS in TNAC patients, a Kaplan‐Meier analysis was applied to the calculated OS rates. From Figure 2A, chemotherapy was significantly associated with improved TNAC OS (P = .005), with a 5‐year OS rate of 89.3% (95% CI, 8.27%‐93.5%) for the chemotherapy group and 71.7% (95% CI, 59.9%‐80.5%) for the non‐chemotherapy group. However, radiation therapy was not associated with OS in TNAC patients (P = .808, Figure 2B).
Figure 1.
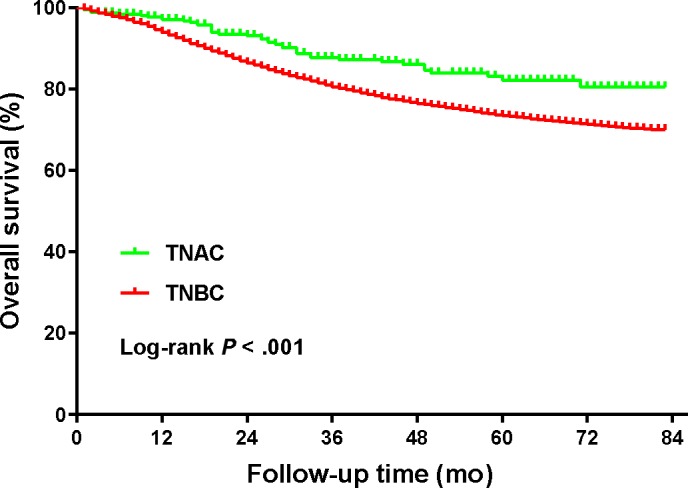
Comparison of overall survival between patients with TNAC and patients with TNBC (31 362 patients in total)
Figure 2.
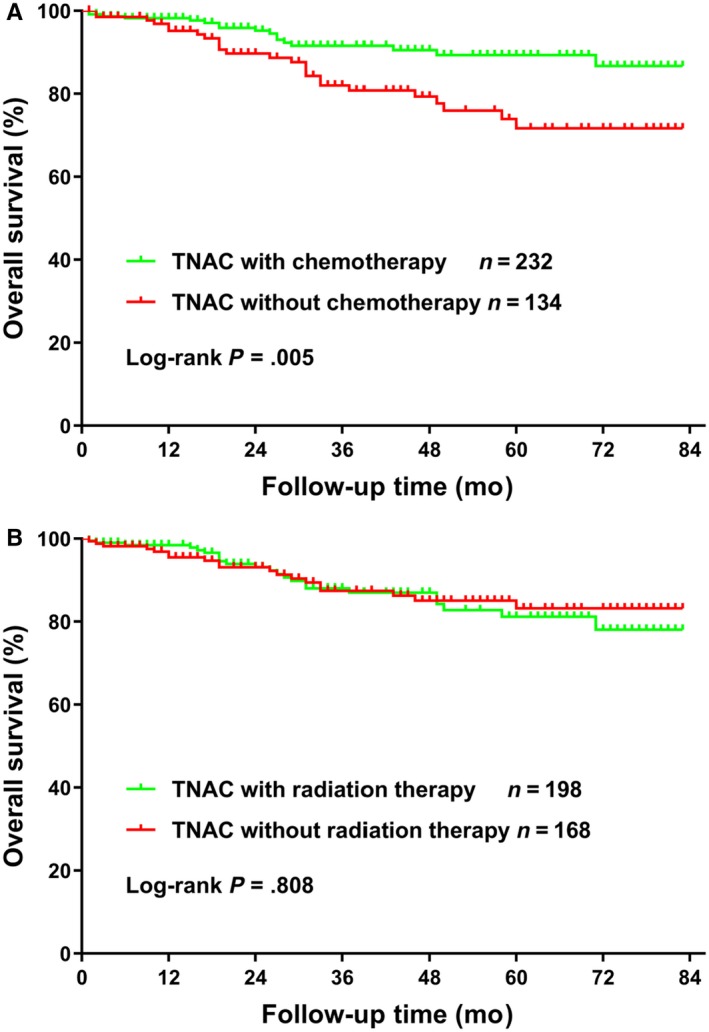
Comparison of overall survival for 366 TNAC patients with or without chemotherapy (A) or radiation therapy (B)
Considering deaths unrelated to breast cancer, the cumulative incidence of breast cancer‐related death in all patients over 5 years was 22.7% (95% CI, 22.0%‐23.5%), with a 5‐year cumulative incidence of 9.1% (95% CI, 5.6%‐14.8%) for TNAC and 22.9% (95% CI, 22.2%‐23.7%) for TNBC. As shown in Figure 3, TNAC patients had a lower cumulative incidence of breast cancer‐related death than TNBC patients (P < .001).
Figure 3.
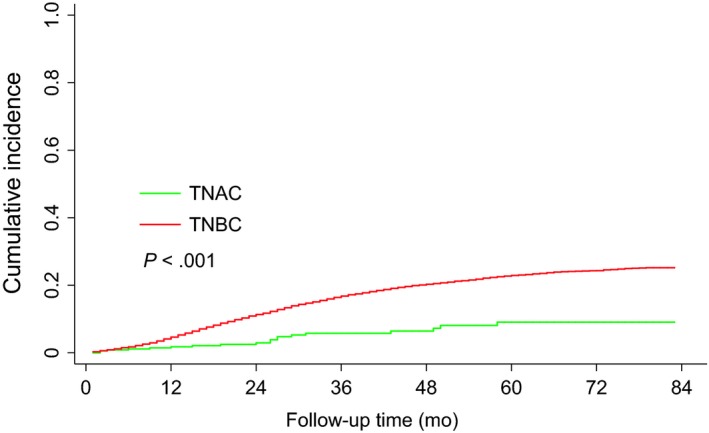
Comparison of the cumulative probability of breast cancer‐specific death between patients with TNAC and patients with TNBC (31 362 patients in total)
To adjust for potential confounding factors, including age, race, marital status, tumor grade, T stage, N stage, metastasis, radiation therapy, chemotherapy and surgery, a multivariable Cox regression model was performed. Consistent with the results of the univariable analysis (Table 3), TNAC patients had better OS rates compared with TNBC patients (HR, 0.61; 95% CI, 0.45‐0.83; P = .002). In the Cox regression model, older age (P < .001), unmarried status (P < .001), high‐grade tumor (P < .001), advanced T stage (P < .001), advanced N stage (P < .001), and metastasis (P < .001) were independent factors associated with worse OS. However, other races (P < .001), radiation therapy (P < .001), chemotherapy (P < .001), and surgery (P < .001) were independent protective factors for OS.
Table 3.
Univariable and multivariable analysis of overall survival (OS)
| Characteristic | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value* | HR (95% CI) | P‐value* | |
| Age ≥ 50 y | 1.26 (1.18‐1.33) | <.001 | 1.28 (1.20‐1.36) | <.001 |
| Race | ||||
| White | 1 (reference) | 1 (reference) | ||
| Black | 1.25 (1.17‐1.33) | <.001 | 1.03 (0.97‐1.10) | .311 |
| Other | 0.79 (0.71‐0.88) | <.001 | 0.75 (0.67‐0.84) | <.001 |
| Unknown | 0.25 (0.12‐0.53) | <.001 | 0.22 (0.11‐0.47) | <.001 |
| Marital status | ||||
| Married | 1 (reference) | 1 (reference) | ||
| Unmarried | 1.66 (1.57‐1.79) | <.001 | 1.28 (1.21‐1.35) | <.001 |
| Unknown | 1.33 (1.19‐1.50) | <.001 | 1.16(1.04‐1.31) | .012 |
| Tumor grade | ||||
| I/II | 1 (reference) | 1 (reference) | ||
| III/IV | 1.27 (1.18‐1.36) | <.001 | 1.15 (1.07‐1.24) | <.001 |
| Unknown | 1.77 (1.53‐2.05) | <.001 | 1.06 (0.87‐1.17) | .937 |
| T stage | ||||
| T1 | 1 (reference) | 1 (reference) | ||
| T2 | 2.24 (2.09‐2.40) | <.001 | 1.93 (1.79‐2.07) | <.001 |
| T3 | 4.92 (4.52‐5.36) | <.001 | 2.92 (2.65‐3.20) | <.001 |
| T4 | 10.66 (9.82‐11.57) | <.001 | 3.67 (3.33‐4.05) | <.001 |
| N stage | ||||
| N0 | 1 (reference) | 1 (reference) | ||
| N1 | 2.70 (2.54‐2.87) | <.001 | 1.99 (1.86‐2.12) | <.001 |
| N2 | 4.22 (3.88‐4.59) | <.001 | 2.92 (2.67‐3.19) | <.001 |
| N3 | 7.41 (6.83‐8.03) | <.001 | 3.28 (2.99‐3.61) | <.001 |
| Metastasis | 12.02 (11.25‐12.84) | <.001 | 3.74 (3.45‐4.06) | <.001 |
| AC Histology | 0.58 (0.43‐0.78) | <.001 | 0.61 (0.45‐0.83) | .002 |
| Radiation therapy | 0.63 (0.60‐0.66) | <.001 | 0.80 (0.76‐0.85) | <.001 |
| Chemotherapy | 0.65 (0.62‐0.69) | <.001 | 0.45 (0.42‐0.47) | <.001 |
| Surgery | 0.16 (0.15‐0.17) | <.001 | 0.44 (0.41‐0.48) | <.001 |
Abbreviations: AC, apocrine carcinoma; CI, confidential interval; HR, hazard ratios; SHR, subdistribution hazard ratio.
P values from the Cox proportional hazard regression model.
Furthermore, considering deaths unrelated to breast cancer, a multivariable Gray's competing risk regression model was performed to adjust for potential confounding factors. Consistent with the results of the univariable analysis, TNAC patients still had better BCSS rates compared with TNBC patients (SHR, 0.42; 95% CI, 0.27‐0.64; P < .001) (Table 4). As shown in Table 4, unmarried status (P < .001), high‐grade tumor (P < .001), advanced T stage (P < .001), advanced N stage (P < .001), and metastasis (P < .001) were significant risk factors for BCSS. In contrast, other races (P < .001), radiation therapy (P < .001), chemotherapy (P < .001), and surgery (P < .001) were associated with improved BCSS.
Table 4.
Univariable and multivariable analysis of breast cancer‐specific survival (BCSS)
| Characteristic | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value* | HR (95% CI) | P‐value* | |
| Age ≥ 50 y | 0.97 (0.91‐1.03) | .332 | 1.07 (0.99‐1.15) | .068 |
| Race | ||||
| White | 1 (reference) | 1 (reference) | ||
| Black | 1.28 (1.19‐1.37) | <.001 | 1.02 (0.94‐1.11) | .667 |
| Other | 0.83 (0.73‐0.94) | .004 | 0.78 (0.68‐0.89) | <.001 |
| Unknown | 0.15 (0.05‐0.47) | .001 | 0.11 (0.03‐0.38) | <.001 |
| Marital status | ||||
| Married | 1 (reference) | 1 (reference) | ||
| Unmarried | 1.45 (1.36‐1.54) | <.001 | 1.12 (1.04‐1.20) | <.001 |
| Unknown | 1.16 (1.01‐1.33) | .042 | 1.09 (0.95‐1.26) | .23 |
| Tumor grade | ||||
| I/II | 1 (reference) | 1 (reference) | ||
| III/IV | 1.46 (1.34‐1.60) | <.001 | 1.19 (1.08‐1.32) | <.001 |
| Unknown | 2.07 (1.75‐2.46) | <.001 | 1.02 (0.82‐1.27) | .864 |
| T stage | ||||
| T1 | 1 (reference) | 1 (reference) | ||
| T2 | 2.78 (2.55‐3.02) | <.001 | 2.06 (1.88‐2.26) | <.001 |
| T3 | 6.78 (6.12‐7.49) | <.001 | 3.27(2.91‐3.67) | <.001 |
| T4 | 13.57 (12.27‐15.01) | <.001 | 3.76 (3.30‐4.29) | <.001 |
| N stage | ||||
| N0 | 1 (reference) | 1 (reference) | ||
| N1 | 3.50 (3.26‐3.77) | <.001 | 2.28 (2.10‐2.48) | <.001 |
| N2 | 5.62 (5.11‐6.18) | <.001 | 3.45 (3.09‐3.87) | <.001 |
| N3 | 10.22 (9.31‐11.22) | <.001 | 4.01 (3.53‐4. 55) | <.001 |
| Metastasis | 12.42 (11.48‐13.45) | <.001 | 3.61 (3.24‐4.01) | <.001 |
| AC Histology | 0.35 (0.23‐0.55) | <.001 | 0.42 (0.27‐0.64) | <.001 |
| Radiation therapy | 0.72 (0.68‐0.76) | <.001 | 0.85 (0.79‐0.92) | .001 |
| Chemotherapy | 1.06 (0.98‐1.13) | .138 | 0.69 (0.63‐0.76) | <.001 |
| Surgery | 0.17 (0.15‐0.18) | <.001 | 0.48 (0.43‐0.53) | <.001 |
Abbreviations: AC, apocrine carcinoma; CI, confidential interval; HR, hazard ratios; SHR, subdistribution hazard ratio.
P values from the competing risk regression model.
4. DISCUSSION
The diagnosis of AC of the breast has been controversial because of the lack of strict diagnostic criteria.2 In the current study, we identified AC patients coded by ICD‐O‐3 8401/3, and the diagnosis is morphologically exactly AC, which is not a carcinoma with apocrine features, differentiation, or type. TNAC is an extremely rare type of triple‐negative breast cancer,1, 9 so it is quite difficult to obtain a large enough number of these patients in clinical practice. Based on a large population from the SEER database, a retrospective study was performed to explore the clinicopathological features and prognostic factors of TNAC patients. Our study demonstrated that patients with TNAC had better OS when compared with TNBC patients in a multivariable Cox regression analysis. After taking deaths not related to breast cancer into consideration, TNAC patients had significant BCSS benefits compared with TNBC patients. Until now, because there is a shortage of precise prognostic data, TNAC is often grouped with other TNBCs, which usually rely on broad‐spectrum and highly efficient multidrug chemotherapeutic regimens.12, 13 However, based on the current study, there is reason to believe that treating TNAC like other TNBCs is inappropriate. For example, the most significant distinguishing feature of TNAC is its preference for older women (P < .001), a population less likely to tolerate aggressive multidrug chemotherapies.
Similar to our study, a retrospective study of the SEER database from 2003 to 2013 found that OS and BCSS were both worse in AC patients than in patients with IDC in univariable analysis, but it was not found to be an independent prognostic factor in multivariable analysis.14 Furthermore, this study investigated the prognosis of molecular subtypes and showed that patients with TNAC presented better OS and BCSS than patients with TNBC in a univariable analysis. However, this study could not analyze TNAC well as the SEER database did not start recording HER2 status until 2010. The recent SEER data including HER2 status provide a unique opportunity to explore the prognosis of patients with TNAC vs other TNBC and compare the effect of chemotherapy and radiotherapy in this situation. Comparatively, our study included a large population of only TNAC and TNBC patients, and multivariable Cox regression and competing risk models were used for statistical analysis. Thus, the power of the analysis in our study is convincing.
In addition, the current study found that TNAC patients were associated with an older age, a lower proportion of black race, a lower tumor grade, and a lower T stage than TNBC patients. Most of these findings were consistent with previous studies. Mills et al found that patients with AC more often present in older women with lower grade and T stage,8 which is consistent with our findings. According to two retrospective studies, the proportion of lymph node metastasis was significantly lower in patients with AC than in patients with IDC,11, 15 while our results showed no difference in N stage. In the current study, the results showed that TNAC patients were less likely to receive chemotherapy, while chemotherapy was associated with improved survival in TNAC patients. This result was in accordance with a previously published study.16
Inevitably, there were several limitations to the present study. First, this is a retrospective study of the SEER database, so the selection biases might limit the validity of this study. Second, the HER2 status was not included in the SEER data until 2010. Thus, the follow‐up period was limited. Third, it has been widely accepted that AC is defined by a combination of morphologic (apocrine morphology in > 90% of tumor cells) and immunohistochemical (ER‐ and PR‐negative and AR‐positive) characteristics.17 However, the AR status was not an essential criterion for the diagnosis of AC; therefore, its expression was not recorded routinely in the SEER database. Additionally, the SEER data do not offer detailed information on chemoradiotherapy regimens, biological targeted therapy, and so on. This information might also affect the survival of breast cancer patients. Finally, some patients did not have clear information about receiving chemotherapy and/or radiation therapy in this study due to the limitations of SEER database coding. We divided this population into patients with treatment and without treatment, so this might reduce the statistical power of the categorical variables. These limitations may have contributed to study bias and undermined the power of the analysis.
5. CONCLUSIONS
In the present study, we found that TNAC has unique clinicopathological characteristics. After investigation, our results showed that patients with TNAC have better survival outcomes compared to patients with TNBC. Chemotherapy was associated with improved survival in TNAC. However, radiotherapy was not associated with improved prognosis in TNAC. These findings not only enhance the comprehension of the clinicopathological features and prognostic factors of this rare carcinoma but also provide a basis for the development of therapeutic guidelines for TNAC.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENT
This study was supported by Foshan Science and Technology Bureau Foundation (2017AG100171).
Wu W, Wu M, Peng G, Shi D, Zhang J. Prognosis in triple‐negative apocrine carcinomas of the breast: A population‐based study. Cancer Med. 2019;8:7523–7531. 10.1002/cam4.2634
Contributor Information
Degang Shi, Email: degang_shi@yahoo.com.
Jian Zhang, Email: blacktiger@139.com.
REFERENCES
- 1. Vranic S, Schmitt F, Sapino A, et al. Apocrine carcinoma of the breast: a comprehensive review. Histol Histopathol. 2013;28:1393‐1409. [DOI] [PubMed] [Google Scholar]
- 2. Vranic S, Tawfik O, Palazzo J, et al. EGFR and HER‐2/neu expression in invasive apocrine carcinoma of the breast. Mod Pathol. 2010;23:644‐653. [DOI] [PubMed] [Google Scholar]
- 3. Sapp M, Malik A, Hanna W. Hormone receptor profile of apocrine lesions of the breast. Breast J. 2003;9:335‐336. [DOI] [PubMed] [Google Scholar]
- 4. Liu X, Feng C, Liu J, et al. The importance of EGFR as a biomarker in molecular apocrine breast cancer. Hum Pathol. 2018;77:1‐10. [DOI] [PubMed] [Google Scholar]
- 5. Vranic S, Feldman R, Gatalica Z. Apocrine carcinoma of the breast: A brief update on the molecular features and targetable biomarkers. Bosnian J Basic Med Sci. 2017;17:9‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsutsumi Y. Apocrine carcinoma as triple‐negative breast cancer: novel definition of apocrine‐type carcinoma as estrogen/progesterone receptor‐negative and androgen receptor‐positive invasive ductal carcinoma. Jpn J Clin Oncol. 2012;42:375‐386. [DOI] [PubMed] [Google Scholar]
- 7. Moriya T, Sakamoto K, Sasano H, et al. Immunohistochemical analysis of Ki‐67, p53, p21, and p27 in benign and malignant apocrine lesions of the breast: its correlation to histologic findings in 43 cases. Mod Pathol. 2000;13:13‐18. [DOI] [PubMed] [Google Scholar]
- 8. Mills AM, Gottlieb CE, Wendroth SM, Brenin CM, Atkins KA. Pure apocrine carcinomas represent a clinicopathologically distinct androgen receptor‐positive subset of triple‐negative breast cancers. Am J Surg Pathol. 2016;40:1109‐1116. [DOI] [PubMed] [Google Scholar]
- 9. Meattini I, Pezzulla D, Saieva C, et al. Triple negative apocrine carcinomas as a distinct subtype of triple negative breast cancer: a case‐control study. Clin Breast Cancer. 2018;18:e773‐e780. [DOI] [PubMed] [Google Scholar]
- 10. Takeuchi H, Tsuji K, Ueo H, Kano T, Maehara Y. Clinicopathological feature and long‐term prognosis of apocrine carcinoma of the breast in Japanese women. Breast Cancer Res Treat. 2004;88:49‐54. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka K, Imoto S, Wada N, Sakemura N, Hasebe K. Invasive apocrine carcinoma of the breast: clinicopathologic features of 57 patients. Breast J. 2008;14:164‐168. [DOI] [PubMed] [Google Scholar]
- 12. Reis‐Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108‐118. [DOI] [PubMed] [Google Scholar]
- 13. Weigelt B, Horlings HM, Kreike B, et al. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141‐150. [DOI] [PubMed] [Google Scholar]
- 14. Zhang N, Zhang H, Chen T, Yang Q. Dose invasive apocrine adenocarcinoma has worse prognosis than invasive ductal carcinoma of breast: evidence from SEER database. Oncotarget. 2017;8:24579‐24592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuo K, Fukutomi T, Hasegawa T, Akashi‐Tanaka S, Nanasawa T, Tsuda H. Histological and immunohistochemical analysis of apocrine breast carcinoma. Breast Cancer. 2002;9:43‐49. [DOI] [PubMed] [Google Scholar]
- 16. Altundag K. De‐escalating systemic chemotherapy might be considered for pure triple negative apocrine breast cancer patients. J BUON. 2019;24:864. [PubMed] [Google Scholar]
- 17. D'Arcy C, Quinn CM. Apocrine lesions of the breast: part 2 of a two‐part review. Invasive apocrine carcinoma, the molecular apocrine signature and utility of immunohistochemistry in the diagnosis of apocrine lesions of the breast. J Clin Pathol. 2019;72:7‐11. [DOI] [PubMed] [Google Scholar]


