Despite cleaning efforts of environmental service teams and substantial compliance with hand hygiene best practices, the microbial burden in patient care settings often exceeds concentrations at which transfer to patients represents a substantial acquisition risk for health care-associated infections (HAIs). Approaches to limit HAI risk have relied on designing health care equipment and furnishings that are easier to clean and/or the use of no-touch disinfection interventions such as germicidal UV irradiation or vapor deposition of hydrogen peroxide. In a clinical trial evaluating the largest fomite in the patient care setting, the bed, a bed was encapsulated with continuously disinfecting antimicrobial copper surfaces, which reduced the bacteria on surfaces by 94% and sustained the microbial burden below the terminal cleaning and disinfection risk threshold throughout the patient’s stay. Such an intervention, which continuously limits microbes on high-touch surfaces, should be studied in a broader range of health care settings to determine its potential long-range efficacy for reducing HAI.
KEYWORDS: health care-associated infections (HAI), terminal cleaning and disinfection (TC&D), self-disinfecting copper, near patient, antimicrobial copper, environmental services, terminal cleaning and disinfection
ABSTRACT
Microbial burden associated with near-patient touch surfaces results in a greater risk of health care-associated infections (HAIs). Acute care beds may be a critical fomite, as traditional plastic surfaces harbor the highest concentrations of bacteria associated with high-touch surfaces in a hospital room’s patient zone. Five high-touch intensive care unit (ICU) bed surfaces encountered by patients, health care workers, and visitors were monitored by routine culture to assess the effect U.S. Environmental Protection Agency (U.S. EPA)-registered antimicrobial copper materials have on the microbial burden. Despite both daily and discharge cleaning and disinfection, each control bed’s plastic surfaces exceeded bacterial concentrations recommended subsequent to terminal cleaning and disinfection (TC&D) of 2.5 aerobic CFU/cm2. Beds with self-disinfecting (copper) surfaces harbored significantly fewer bacteria throughout the patient stay than control beds, at levels below those considered to increase the likelihood of HAIs. With adherence to routine daily and terminal cleaning regimes throughout the study, the copper alloy surfaces neither tarnished nor required additional cleaning or special maintenance. Beds encapsulated with U.S. EPA-registered antimicrobial copper materials were found to sustain the microbial burden below the TC&D risk threshold levels throughout the patient stay, suggesting that outfitting acute care beds with such materials may be an important supplement to controlling the concentration of infectious agents and thereby potentially reducing the overall HAI risk.
IMPORTANCE Despite cleaning efforts of environmental service teams and substantial compliance with hand hygiene best practices, the microbial burden in patient care settings often exceeds concentrations at which transfer to patients represents a substantial acquisition risk for health care-associated infections (HAIs). Approaches to limit HAI risk have relied on designing health care equipment and furnishings that are easier to clean and/or the use of no-touch disinfection interventions such as germicidal UV irradiation or vapor deposition of hydrogen peroxide. In a clinical trial evaluating the largest fomite in the patient care setting, the bed, a bed was encapsulated with continuously disinfecting antimicrobial copper surfaces, which reduced the bacteria on surfaces by 94% and sustained the microbial burden below the terminal cleaning and disinfection risk threshold throughout the patient’s stay. Such an intervention, which continuously limits microbes on high-touch surfaces, should be studied in a broader range of health care settings to determine its potential long-range efficacy for reducing HAI.
INTRODUCTION
Health care-associated infections (HAIs) develop from microbes acquired either endogenously or exogenously during patient care where offending infectious agents were absent or not incubating in the patient at the time of their admission. Morbidity and mortality as a result of HAIs are of significant global concern. The incidence of HAIs ranges from a low in South Korea, 3.7%, to a high in Canada (11.6%) (1). In the United States, of the approximately 2 million patients who will acquire a HAI annually, an estimated 75,000 will lose their lives. For many years, it was postulated that the environment played a minor role, accounting for only 20% to 40% of HAIs (2). In 2013, a study established that upon continuously limiting the bioburden of common patient room objects, a concomitant 58% decrease in the rate of HAI acquisition was similarly observed (3). That study suggested that the built hospital environment can serve as a substantial source from which microbes might significantly contribute to the incidence of HAIs. A limitation of that study was that less than 10% of the most significant fomite in the patient room—the bed—was protected with a continuously disinfecting antimicrobial copper alloy.
The “Five Indications/Five Moments for Hand Hygiene” concept advanced by the World Health Organization (WHO) on minimizing the risk of pathogen transmission does not include hand hygiene before touching furniture or objects in the immediate vicinity of the patient, or the “patient zone,” where items are considered to be contaminated with microbes shed or directly transferred by patients while the health care worker is wearing gloves (https://www.who.int/gpsc/tools/faqs/five_moments/en/). Of the most frequently touched objects in this patient zone, the patient bed is considered a significant microbial reservoir (4–6). Acute care hospital beds used throughout the developed world are traditionally manufactured with injection-molded plastic surfaces. In spite of routine disinfection or terminal cleaning and disinfection (TC&D) efforts to reduce the microbial burden, in situ hospital studies have shown that plastic beds are significant reservoirs for microbes (6–10). In fact, beds have been shown to be among the most touched and heavily microbially burdened objects in medical intensive care unit (ICU) patient rooms (9, 11–20) and are considered high risk for infection transmission to and from patients, visitors, and hospital staff (15, 21). In some of the same studies, when these highly microbially burdened plastic surfaces were modified with a continuously antimicrobial material, namely, an alloy containing greater than 60% (wt/wt) copper, the concentration of bacteria found on surfaces was generally reduced by more than 90% (6, 9, 10). Based on studies that demonstrated that environmental contamination plays an important role in transmission of pathogens responsible for HAIs (22) and that copper-containing surfaces decreased bacterial burden (6, 9, 23, 24), it was postulated that copper materials, in concert with routine terminal cleaning, would serve to keep high-touch bed surfaces significantly cleaner than the plastic designs traditionally used to fabricate acute care beds. Metallic copper surfaces kill bacteria through a multimodal mechanism involving the ability to disrupt bacterial respiration, generate superoxide, and destroy genomic and plasmid DNA in situ (26–29). In this study, the concentrations of bacteria on bed surfaces encountered by patients, health care workers, and visitors were monitored to determine whether U.S. Environmental Protection Agency (U.S. EPA)-registered antimicrobial copper materials, applied to 100% of near-patient bed surfaces, would result in the bed harboring fewer bacteria.
RESULTS
Beds encapsulated with continuously antimicrobial surfaces harbored significantly fewer microbes than equivalent control beds in the patient care setting.
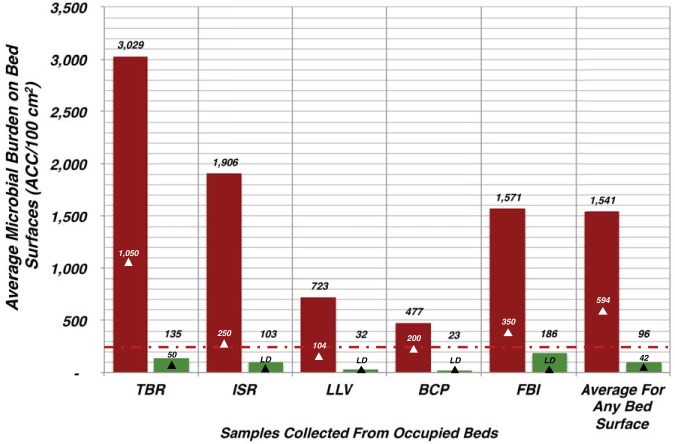
In addition to thorough bed cleaning after patient discharge, as part of Highpoint Health’s daily cleaning protocols, the high-touch bed surfaces were routinely disinfected. In the preintervention phase of this pragmatic crossover trial, control patient beds were found to significantly accumulate higher concentrations of aerobic CFU across all areas sampled (Fig. 1). The tops of the bed rails (TBR) on the control were the mostly heavily soiled, harboring an average of 3,029 aerobic CFU (ACC)/100 cm2. The other areas sampled from occupied control beds accumulated fewer microbes, but their average concentrations were all between 2× and 8× higher than the suggested HAI risk threshold target of <250 ACC/100 cm2 for TC&D (Fig. 1). The interventional beds harbored significantly lower concentrations of bacteria (P < 0.0001) than levels observed in samples taken from control bed surfaces (Fig. 1). The most heavily burdened area on the copper beds was the interior patient-facing surface of the bed footboard (FBI; average of 186 ACC/100 cm2); however, this concentration was still significantly lower than that on the comparative location on the plastic footboard (1,571 ACC/100 cm2) (Fig. 1). Overall, the cumulative average ACC/100 cm2 on the copper beds was 94% lower than that on the control beds (Fig. 1).
FIG 1.
Active care beds encapsulated with an antimicrobial surface harbored significantly fewer microbes than corresponding control beds. The microbial burden affiliated with the surfaces from occupied single-patient ICU rooms described in the Fig. 4 legend was collected and determined as described. The average concentration for each of the near-patient areas sampled is shown (red colored bars for the control [N = 70 beds] and green colored bars [N = 43 beds] for surfaces encapsulated with copper). The white triangles indicate the median concentrations for the control surfaces, while the black triangles indicate the median values for the copper-encapsulated near-patient bed surfaces evaluated. The designations associated with the black triangles for ISR, LLV, BCP, and FBI denote the median values recovered below the limit of detection of the culture assay. The differences between control and interventional groups were all found to be significant (P < 0.0001). The red dashed line denotes the discharge/terminal cleaning and disinfection (TC&D) risk threshold target of less than 250 ACC/100 cm2. TBR, tops of the bed rails; ISR, inside patient-facing surface of intermediate rail of bed closest to room doors; LLV, bed rail lift/release lever; BCP, external elevation control panel; FBI, interior patient-facing surface of the bed footboard.
Encapsulation of near-patient bed surfaces with antimicrobial copper resulted in a significantly lower likelihood of bacterial concentrations exceeding levels recommended subsequent to TC&D.
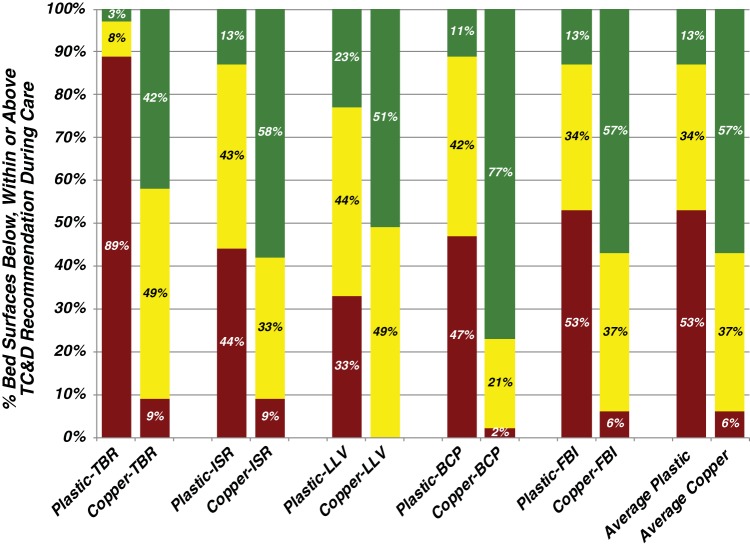
The most heavily burdened locations on the control bed were the tops of the bed rails (TBR). Of the samples recovered, 89% were found to exceed the benchmark TC&D risk threshold (Fig. 2). In contrast, only 9% of the samples recovered from interventional copper beds exceeded the TC&D risk threshold, with 42% found to be free of detectable bacteria (Fig. 2). All of the other areas of the control bed warrant concern of being significant fomites, as they all routinely harbored concentrations of bacteria that well exceeded the risk threshold (Fig. 2). Considering the data collectively, 53% of the surfaces sampled from occupied control beds routinely harbored concentrations of bacteria above the TC&D risk threshold of <250 ACC/100 cm2 (Fig. 2). In comparison, the corresponding areas investigated on the encapsulated copper beds were found to be free of bacteria for the majority (57%) of the samples evaluated.
FIG 2.
Encapsulation of near-patient surfaces with antimicrobial copper resulted in a significantly lower likelihood that any bed surface exceeded the TC&D recommendation during care. The concentrations recovered from the five near-patient areas sampled from each occupied bed were categorized based on their concentrations. Those that resulted in an undetectable level of bacteria are colored green, those with a concentration of between 1 and 250 ACC/100 cm2 are in yellow, and those exceeding the terminal cleaning and disinfection risk threshold target of 250 ACC/100 cm2 are in red. The likelihood that any bed surface sampled within one of the risk concentrations is the result of the average value encountered for all the near-patient bed surfaces evaluated (N = 70 control beds; N = 43 encapsulated copper beds) as described in Materials and Methods. The prefix of “Plastic” or “Copper” and then one of the following abbreviations indicates the material from which the item’s surface was fabricated. TBR, tops of the bed rails; ISR, inside patient-facing surface of intermediate rail of bed closest to room doors; LLV, bed rail lift/release lever; BCP, external elevation control panel; FBI, interior patient-facing surface of the bed footboard.
The microbial burden affiliated with the patient bed is dependent on length of stay.
Upon patient discharge, the standard of care requires that all patient rooms be subjected to terminal cleaning and disinfection. In the Highpoint Health Hospital (HPH) ICU, touch surfaces within each room are cleaned with a U.S. EPA-registered hospital disinfectant qualified to reduce the microbial burden by at least 4 orders of magnitude. Additionally, each patient room is subjected to a daily cleaning regimen, in which visibly soiled near-patient bed surfaces are cleaned and treated with a disinfecting concentration of quaternary ammonium-based agents. In spite of rigorous adherence to this infection control best practice, an analysis based on patient length of stay (LOS) indicated that the microbial concentration associated with the beds increased as a function of the patient’s length of stay (Fig. 3). The microbial burden data recovered from each bed were segregated and categorized into the following three groups: less than 5 days length of stay (N = 64 beds; 34 control plastic, 30 interventional copper), between 5 and 10 days LOS (N = 40 beds; 30 control plastic, 10 interventional copper), and greater than 10 days LOS (N = 9 beds; 6 control plastic, 3 interventional copper). The first category was selected because the average LOS is 4.5 days for patients in U.S. hospitals and 3.5 days for patients cared for in an ICU (30). The other two groups offer a snapshot as to microbial risk that near-patient surroundings, like the bed, may contribute to the overall patient HAI risk as a function of LOS.
FIG 3.
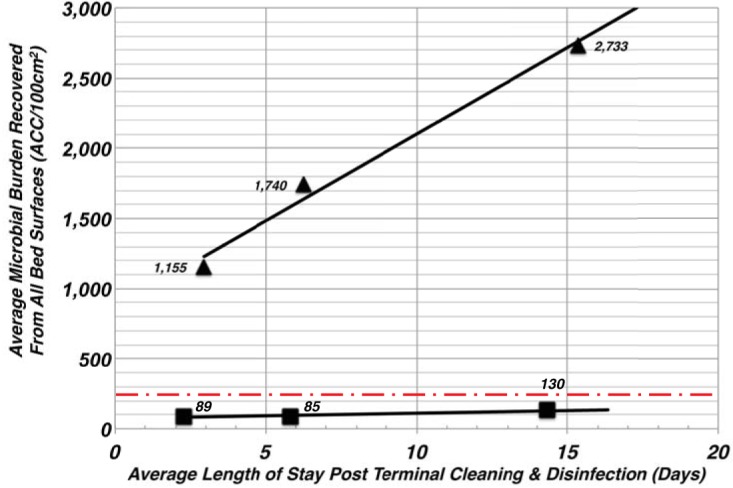
The microbial burden affiliated with the patient bed is dependent on length of stay. The microbial burdens affiliated with each bed area were separated into one of three categories based on the LOS of the patient. The efficiency with which interventional bed surfaces were able to control the microbial burden was assessed against the values from the control beds using pairwise comparisons using the Mann-Whitney test with a significance level (P) assessed as less than 5% (P < 0.05) using Prism 8 software. The number of patients considered in the analysis was 70 for the control and 43 for the interventional beds. The values comparing control to interventional groups were found to be all significant at P < 0.0001, as were the first two control values to the third grouping (>10 days); for the <5 days to >10 days grouping, the P value was 0.0003. Solid triangles represent the average microbial burden for control beds, and the filled squares represent that for the interventional or copper-encapsulated beds. The red dashed line denotes the discharge/terminal cleaning & disinfection (TC&D) risk threshold target of less than 250 ACC/100 cm2.
It was found that for each of the three LOS categories, the microbial concentrations recovered from the plastic beds were significantly greater than those of the copper beds; the cumulative aerobic CFU (ACC)/100 cm2 increased on the plastic beds by 133% over the LOS to a 3-fold greater concentration of microbes than that observed on the copper beds. Correspondingly, the average ACC on the plastic beds was 5× to 11× greater than the HAI risk threshold (<250 ACC/100cm2) for the three LOS categories, while the average ACC recovered from each of the copper beds was well below the HAI risk threshold for each LOS category. At less than 5 days LOS, the average concentration observed on the plastic control beds was 1,155 ACC/100 cm2, compared to 89 ACC/100cm2 for the interventional copper beds. At more than 10 days LOS, the average concentrations on the plastic beds was 2,733 ACC/100 cm2, compared to 130 ACC/100 cm2 on the self-disinfecting interventional beds. Overall, comparing the concentrations between the control and interventional groups, the statistical differences between the control and copper-encapsulated bed groupings were found to be significant (P < 0.0001).
DISCUSSION
In this study, the infection control properties of hospital beds were enhanced by encapsulating all of their near-patient surfaces with U.S. EPA-registered copper coating and foil. The in situ effectiveness of copper as an antimicrobial intervention for controlling the microbial burden on bed surfaces was previously established by 3 distinct clinical trials conducted in ICUs at three tertiary medical centers, a pediatric ICU, and a medical/surgical unit at a community hospital (8–10). A limitation to each study was that only a small percentage of the bed surfaces was protected with the continuously disinfecting agent. Here, we addressed that limitation and evaluated beds that had all of their critical (near-patient) touch surfaces encapsulated with U.S. EPA-registered antimicrobial copper materials. On average, the microbial burden on the bed surfaces tested was reduced by over 94% compared to levels observed on the equivalent control plastic beds.
Prior to this study, there was a great deal of speculation about copper’s long-term durability and the ability to keep copper surfaces clean and tarnish free in an active patient care setting. As an antimicrobial agent, copper does not lose its bactericidal effectiveness through surface oxidation, unlike silver (31). Throughout the 11 months that the copper materials were in use, all of the interventional bed surfaces held up well to the rigors of patient care, daily cleaning, and TC&D with U.S. EPA-registered quaternary ammonium-based disinfectants and/or sodium hypochlorite-based solutions used under infection control precautions subsequent to patient care.
The Joint Commission’s infection control (IC) standard IC 02.02.01 presently requires hospitals to reduce the risk of infections associated with medical equipment, devices, and supplies (32). A benchmark proposed to consider environmental surfaces as clean or benign after terminal cleaning is a concentration of <2.5 CFU per cm2 of ACC and <1 CFU/cm2 for health care-associated pathogens, e.g., Staphylococcus aureus (33). Should the concentration of microbes recovered from surfaces immediately subsequent to TC&D exceed this concentration, there exists a higher risk of nosocomial transmission of the microbe from colonized near-patient surfaces to patients, health care workers, or visitors. With revisions to IC 02.02.01, infection control leaderships of health care facilities are increasingly looking for approaches to minimize the potential for citation by the Joint Commission. Seemingly straightforward methods used in the past to remove visible bioburden and dried blood from instruments and surfaces fail to address the root cause, namely, that even if surfaces look “hospital clean,” they may not be sufficiently debulked of potentially hazardous microbes. One of the provocative observations from this trial was that while discharge cleaning significantly reduced the intrinsic microbial burden, the ACC concentrations on terminally cleaned interventional (copper) bed surfaces were 85 to 90% lower than those on similarly cleaned control (plastic) bed surfaces. Additionally, despite daily cleaning of the beds, microbial burden for patients cared for in control beds increased significantly as a function of their length of stay. In contrast, the burden recovered from the self-disinfecting copper beds was sustained significantly below the TC&D risk threshold throughout the patients’ stay without regard to LOS.
That microbial burden increased as a function of length of stay in control beds was not unexpected, in that Cohen and colleagues learned that the mean number of people entering a patient room was 5.5 per h, with nursing staff accounting for 45% of those entries (34). In the course of a 15-h waking day, this amounts to approximately 83 people per day entering the patient’s room. Consequently, as the significance of the findings is considered here, the observation that the antimicrobial action of copper on near-patient surfaces kept these areas at microbial burden levels below the TC&D risk threshold may suggest infection control dividends of fewer infections and better outcomes. Furthermore, it is anticipated that encapsulated copper surfaces will have a higher likelihood of reducing the inherent risk associated with near-patient surfaces than will adjunct, intermittent, and discontinuous disinfection technologies entering the marketplace, such as UV irradiation and vapor phase hydrogen peroxide, or indicator glow markers, covertly placed on near-patient contact locations (35–40), which are intended to identify surfaces that environmental services (EVS) workers endeavored to clean, rather than whether the surfaces are, in fact, microbiologically clean.
Keeping the health care environment clean is not just an esthetic that the public expects but is fundamental to keeping patients safe. An issue raised with any augment to infection control practices is how much any intervention will add to the base cost of the current practices for infection control. As a reference, Lucado and colleagues reported in 2010 that the cost to treat HAIs results in an additional 19.2 days of care and nearly $43,000 more than stays without infections (41). While the evidence is limited, there are data that show that debulking of the built environment leads to reductions in infections (3).
Based on Association for the Health Care Environment (AHE) estimates for the appropriate time to clean and disinfect a patient room, cleaning costs are estimated at $12 to $13 per single patient room. Thus, any addition to the standard of care required to enhance infection control requires a consideration of the cost of the intervention with respect to its impact on the HAI rate. Additional methods evaluated extensively by the infection control community, like the process of daily cleaning, are two “no-touch” technologies: hydrogen peroxide vapor deposition (HPV) within patient care settings (42) and UV C (100 nm to 280 nm spectrum energy)-based systems (43, 44). Both methods have been shown to be effective in reducing burden within the built environment (35, 36, 42, 45–47), but each is limited as an intervention by being a discontinuous antimicrobial technology, with each adding significantly to the cost of terminal cleaning and disinfection of patient rooms.
Despite terminal and daily cleaning of the HPH ICU beds, 47% of the standard (plastic) bed surfaces had ACC levels above the proposed benchmark standard for cleanliness, while over 90% of the copper bed surfaces achieved the benchmark standard, at an ACC level that was, on average, 94% lower than that observed on plastic rails. The cost to achieve and maintain this level of cleanliness is based on encapsulating the bed with antimicrobial copper, which was approximately $2,200 per bed amortized over 5 years, or about $1.20 per bed per day. This represents less than 10% of the application costs for other adjunct cleaning options, such as implementing an additional daily cleaning (∼$12 to $13/room), UV irradiation (∼$10/room), or hydrogen peroxide vapor phase deposition (∼$100/room), inclusive of labor, supplies, and equipment amortization considered for each method. Not only was the copper intervention superior in effectiveness at controlling burden within the built environment, but because it is the only adjunct to act continuously, actively killing bacteria 24 h/day and only adding a modest increase to the environmental services/infection control budget, it is anticipated that the value delivered by this intervention to the infection control bundle warrants further studies to assess its impact on HAI rates, ultimately leading to consideration for its adoption.
MATERIALS AND METHODS
Study design and setting.
The intent of this study was to assess, in an active patient care setting, the ability of a self-disinfecting patient bed to lower its incidence of serving as a microbial reservoir. To that end, we designed a pragmatic crossover study in which the control and interventional arms of the study were conducted in the medical intensive care unit (ICU) at the Highpoint Health Hospital (HPH) for 23 months. The study design employed periodic environmental monitoring of the intrinsic bacterial burden associated with high-touch bed surfaces. Occupied control beds were monitored from 24 April 2017 through 23 July 2018, whereas occupied interventional beds were monitored from 23 April 2018 through 4 March 2019. A mixture of control and interventional beds were present between 23 April 2018 and 23 July 2018, as the copper beds were introduced when rooms became available subsequent to patient discharge from a control bed. The study was presented to the institutional review board and deemed exempt.
HPH is a 62-licensed-bed acute care hospital located in Lawrenceburg, IN, which draws patients from the surrounding rural areas, as well as from the Southwest community of metropolitan Cincinnati, OH. The ICU has eight single-patient rooms arranged in an “L” configuration with a centralized nursing station. Rooms 2 to 7 are directly visible, and rooms 1 and 8 are to the periphery of the nursing station. The average daily census of the unit for the study period was 5.2 (65%). Researchers recorded the occupancy status of each room when beds were sampled. Throughout the study, select hospital staff members directly observed hand hygiene and utilized the Qualaris monitor system. Observed compliance rates were routinely greater than 93%.
Composition of control and interventional beds.
The control ICU beds utilized for this study were Hill-Rom TotalCare SpO2RT beds. The exterior finishes of each of the frequently touched bed surfaces (Fig. 4A to E) were fabricated from injection-molded polypropylene. The pressure-sensitive touch panels that control bed functions, including rail articulations, were fabricated from a medical-grade polyester film label. In the interventional arm of this study, the TotalCare SpO2RT bed was modified by Bed Techs, Inc. (Greendale, IN), such that 100% of the near-patient bed surfaces were encapsulated with a U.S. EPA-registered antimicrobial material fabricated and applied according to the specifications of LuminOre CopperTouch (U.S. EPA registration no. 89266-2; Carlsbad, CA). The interventional bed control panels employed a pressure-sensitive film fabricated from an antimicrobial copper foil (MR Label Co.; Cincinnati, OH) using materials with U.S. EPA registration from the Copper Development Association, McLean, VA (U.S. EPA registration no. 82012-2) (Fig. 4H to I).
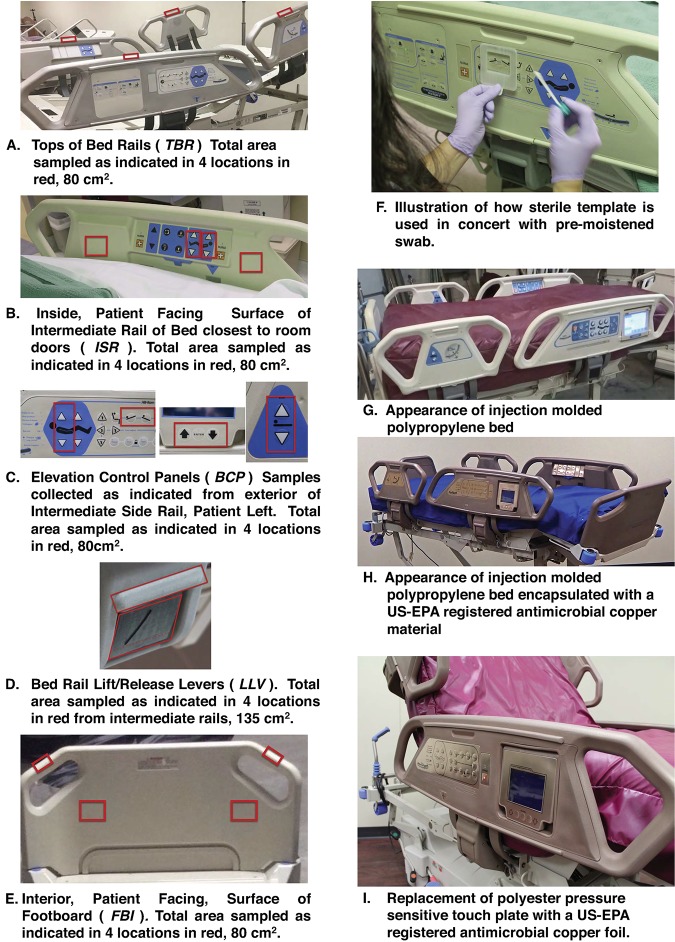
FIG 4.
Sampling locations of the patient bed used to assess the microbial burden. Total aerobic CFU were determined as described from 4 locations from each of 5 areas associated with the patient’s bed (A to E) using a premoistened swab and sterile template (F). Control beds were fabricated from injected molded polypropylene (G), and the interventional study beds were encapsulated using a U.S. EPA-registered antimicrobial copper coating (H) and U.S. EPA-registered antimicrobial copper foil touch pads (I). TBR, tops of the bed rails; ISR, inside patient-facing surface of intermediate rail of bed closest to room doors; BCP, external elevation control panel; LLV, bed rail lift/release lever; FBI, interior patient-facing surface of the bed footboard.
Sample collection procedure and burden evaluation.
The total aerobic CFU within an area of 100 cm2 (ACC/100 cm2) was measured from four locations from within each of the five high-touch areas located on the patient bed (Fig. 4A to E). Daily cleaning of ICU rooms, including all of the near-patient bed surfaces, was consistently conducted between 7 a.m. and 11 a.m. using a U.S. EPA-registered hospital-grade disinfectant with a quaternary ammonium compound as its active agent. Sample collection occurred between 11:00 a.m. and 2:00 p.m. Reproducible sampling was facilitated by using a sterile Teflon template positioned in proximity to sampling locations on the bed (Fig. 4F) and a Puritan ESK environmental sampling kit employing the 7.9-cm premoistened sterile polyester-tipped swab (1.73 cm) supplied within the kit. Microbes were liberated from the bed surfaces by swabbing the sterile template-outlined area with uniform pressure, using a back-and-forth motion to cover the entire surface. Once the sample was taken, the swab was immediately placed into 4 ml of neutralizing buffer provided in the kit to limit the activity of any residual quaternary ammonium agents that may have been transferred during sampling as prescribed by Boyd and Sehulster (48). Burden was assessed by plating 100 μl of each neutralized sample onto Trypticase soy agar supplemented with 5% sheep erythrocytes (BD-BBL), with inoculated plates developing at 37°C for 48 h. The number of aerobic colonies observed on each plate was recorded and reported as aerobic CFU (ACC) per 100 cm2. In total, 558 samples were collected from patient-occupied beds, 350 samples were taken from control beds, and 215 from the interventional beds.
Statistical analysis.
The efficiency with which interventional bed surfaces were able to control the microbial burden was assessed against the values from the control beds with a nonparametric pairwise comparison using the Mann-Whitney test with a significance level (P) assessed as less than 5% (P < 0.05) using Prism 8 software. Descriptive statistics were also calculated in order to assess the average burden (ACC/100 cm2) for each of the groups. The microbial burden on the bed rails (Fig. 3) was correlated against the patient’s length of stay (LOS). Patients in the control and interventional groups were stratified into one of the following three group based on LOS: fewer than 5 days, 5 to 10 days and more than 10 days. The average LOS for each group was plotted on the abscissa, and the mean bioburden is represented on the ordinate axis. Significance (P < 0.05) between the two groups was assessed using the nonparametric Mann-Whitney test.
ACKNOWLEDGMENTS
The work described here was supported by an unrestricted research grant from Bed Techs, Inc. The views, opinions and/or findings presented here are those of the author(s) alone.
We especially thank Peter Sharpe and Michael Wilson for their helpful discussions and diligence in their tireless pursuit of encapsulating beds with copper, and NSF International, especially Christine Greene, director, STAT EPID and EVS Protects.
REFERENCES
- 1.World Health Organization. 2010. The burden of health care-associated infection worldwide: a summary. World Health Organization, Geneva, Switzerland: https://www.who.int/gpsc/country_work/summary_20100430_en.pdf. [Google Scholar]
- 2.Weinstein RA. 1991. Epidemiology and control of nosocomial infections in adult intensive care units. Am J Med 91:179S–184S. doi: 10.1016/0002-9343(91)90366-6. [DOI] [PubMed] [Google Scholar]
- 3.Salgado CD, Sepkowitz KA, John JF, Cantey JR, Attaway HH, Freeman KD, Sharpe PA, Michels HT, Schmidt MG. 2013. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect Control Hosp Epidemiol 34:479–486. doi: 10.1086/670207. [DOI] [PubMed] [Google Scholar]
- 4.Creamer E, Humphreys H. 2008. The contribution of beds to healthcare-associated infection: the importance of adequate decontamination. J Hosp Infect 69:8–23. doi: 10.1016/j.jhin.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Russotto V, Cortegiani A, Fasciana T, Iozzo P, Raineri SM, Gregoretti C, Giammanco A, Giarratano A. 2017. What healthcare workers should know about environmental bacterial contamination in the intensive care unit. Biomed Res Int 2017:6905450. doi: 10.1155/2017/6905450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt MG, Attaway HH, Fairey SE, Steed LL, Michels HT, Salgado CD. 2013. Copper continuously limits the concentration of bacteria resident on bed rails within the ICU. Infect Control Hosp Epidemiol 34:530–533. doi: 10.1086/670224. [DOI] [PubMed] [Google Scholar]
- 7.Attaway HH III, Fairey S, Steed LL, Salgado CD, Michels HT, Schmidt MG. 2012. Intrinsic bacterial burden associated with intensive care unit hospital beds: effects of disinfection on population recovery and mitigation of potential infection risk. Am J Infect Control 40:907–912. doi: 10.1016/j.ajic.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Hinsa-Leasure SM, Nartey Q, Vaverka J, Schmidt MG. 2016. Copper alloy surfaces sustain terminal cleaning levels in a rural hospital. Am J Infect Control 44:e195–e203. doi: 10.1016/j.ajic.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt MG, Attaway HH, Sharpe PA, John J Jr, Sepkowitz KA, Morgan A, Fairey SE, Singh S, Steed LL, Cantey JR, Freeman KD, Michels HT, Salgado CD. 2012. Sustained reduction of microbial burden on common hospital surfaces through introduction of copper. J Clin Microbiol 50:2217–2223. doi: 10.1128/JCM.01032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt MG, von Dessauer B, Benavente C, Benadof D, Cifuentes P, Elgueta A, Duran C, Navarrete MS. 2016. Copper surfaces are associated with significantly lower concentrations of bacteria on selected surfaces within a pediatric intensive care unit. Am J Infect Control 44:203–209. doi: 10.1016/j.ajic.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Arinder P, Johannesson P, Karlsson I, Borch E. 2016. Transfer and decontamination of S. aureus in transmission routes regarding hands and contact surfaces. PLoS One 11:e0156390. doi: 10.1371/journal.pone.0156390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oumokhtar B, El Ouali Lalami A, Benaicha N, Arhoune B, Bono W. 2017. Environmental surfaces in healthcare setting: a great potential risk of pathogens transmission. Biomed Res 28:2398–2401. [Google Scholar]
- 13.Catalano M, Quelle LS, Jeric PE, Di Martino A, Maimone SM. 1999. Survival of Acinetobacter baumannii on bed rails during an outbreak and during sporadic cases. J Hosp Infect 42:27–35. doi: 10.1053/jhin.1998.0535. [DOI] [PubMed] [Google Scholar]
- 14.Boyce JM. 2007. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect 65(Suppl 2):50–54. doi: 10.1016/S0195-6701(07)60015-2. [DOI] [PubMed] [Google Scholar]
- 15.Yuen JW, Chung TW, Loke AY. 2015. Methicillin-resistant Staphylococcus aureus (MRSA) contamination in bedside surfaces of a hospital ward and the potential effectiveness of enhanced disinfection with an antimicrobial polymer surfactant. Int J Environ Res Public Health 12:3026–3041. doi: 10.3390/ijerph120303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dancer SJ, White LF, Lamb J, Girvan EK, Robertson C. 2009. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC Med 7:28. doi: 10.1186/1741-7015-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wille I, Mayr A, Kreidl P, Brühwasser C, Hinterberger G, Fritz A, Posch W, Fuchs S, Obwegeser A, Orth-Höller D, Lass-Flörl C. 2018. Cross-sectional point prevalence survey to study the environmental contamination of nosocomial pathogens in intensive care units under real-life conditions. J Hosp Infect 98:90–95. doi: 10.1016/j.jhin.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Cheng VC, Chau PH, Lee WM, Ho SK, Lee DW, So SY, Wong SC, Tai JW, Yuen KY. 2015. Hand-touch contact assessment of high-touch and mutual-touch surfaces among healthcare workers, patients, and visitors. J Hosp Infect 90:220–225. doi: 10.1016/j.jhin.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Huslage K, Rutala WA, Sickbert-Bennett E, Weber DJ. 2010. A quantitative approach to defining “high-touch” surfaces in hospitals. Infect Control Hosp Epidemiol 31:850–853. doi: 10.1086/655016. [DOI] [PubMed] [Google Scholar]
- 20.Smith SJ, Young V, Robertson C, Dancer SJ. 2012. Where do hands go? An audit of sequential hand-touch events on a hospital ward. J Hosp Infect 80:206–211. doi: 10.1016/j.jhin.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Guh A, Carling P. 2010. Options for evaluating environmental cleaning. http://www.cdc.gov/HAI/toolkits/Evaluating-Environmental-Cleaning.html. Accessed 9 October 2019.
- 22.Rutala WA, Weber DJ. 2016. Monitoring and improving the effectiveness of surface cleaning and disinfection. Am J Infect Control 44:e69–e76. doi: 10.1016/j.ajic.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 23.Marais F, Mehtar S, Chalkley L. 2010. Antimicrobial efficacy of copper touch surfaces in reducing environmental bioburden in a South African community healthcare facility. J Hosp Infect 74:80–82. doi: 10.1016/j.jhin.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Mikolay A, Huggett S, Tikana L, Grass G, Braun J, Nies DH. 2010. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl Microbiol Biotechnol 87:1875–1879. doi: 10.1007/s00253-010-2640-1. [DOI] [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Warnes SL, Caves V, Keevil CW. 2012. Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environ Microbiol 14:1730–1743. doi: 10.1111/j.1462-2920.2011.02677.x. [DOI] [PubMed] [Google Scholar]
- 27.Warnes SL, Green SM, Michels HT, Keevil CW. 2010. Biocidal efficacy of copper alloys against pathogenic enterococci involves degradation of genomic and plasmid DNAs. Appl Environ Microbiol 76:5390–5401. doi: 10.1128/AEM.03050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warnes SL, Highmore CJ, Keevil CW. 2012. Horizontal transfer of antibiotic resistance genes on abiotic touch surfaces: implications for public health. mBio 3:e00489-12. doi: 10.1128/mBio.00489-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warnes SL, Keevil CW. 2016. Lack of involvement of fenton chemistry in death of methicillin-resistant and methicillin-sensitive strains of Staphylococcus aureus and destruction of their genomes on wet or dry copper alloy surfaces. Appl Environ Microbiol 82:2132–2136. doi: 10.1128/AEM.03861-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter A, Johnson L, Coustasse A. 2014. Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick) 33:128–135. doi: 10.1097/HCM.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 31.Michels HT, Noyce JO, Keevil CW. 2009. Effects of temperature and humidity on the efficacy of methicillin-resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Lett Appl Microbiol 49:191–195. doi: 10.1111/j.1472-765X.2009.02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Joint Commission. 5 September 2018. Accreditation and certification—4-1-1 on survey enhancements: new scoring revisions for IC.02.02.01 now in effect. The Joint Commission Online, Oak Brook, IL. [Google Scholar]
- 33.Dancer SJ. 2014. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev 27:665–690. doi: 10.1128/CMR.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen B, Hyman S, Rosenberg L, Larson E. 2012. Frequency of patient contact with health care personnel and visitors: implications for infection prevention. Jt Comm J Qual Patient Saf 38:560–565. doi: 10.1016/S1553-7250(12)38073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson DJ, Knelson LP, Moehring RW, Lewis SS, Weber DJ, Chen LF, Triplett PF, Blocker M, Cooney RM, Schwab JC, Lokhnygina Y, Rutala WA, Sexton DJ, CDC Prevention Epicenters Program . 2018. Implementation lessons learned from the benefits of enhanced terminal room (BETR) disinfection study: process and perceptions of enhanced disinfection with ultraviolet disinfection devices. Infect Control Hosp Epidemiol 39:157–163. doi: 10.1017/ice.2017.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber DJ, Rutala WA, Anderson DJ, Chen LF, Sickbert-Bennett EE, Boyce JM. 2016. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: focus on clinical trials. Am J Infect Control 44:e77–e84. doi: 10.1016/j.ajic.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carling PC. 2008. Evaluating the thoroughness of environmental cleaning in hospitals. J Hosp Infect 68:273–274. doi: 10.1016/j.jhin.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Carling PC, Briggs J, Hylander D, Perkins J. 2006. An evaluation of patient area cleaning in 3 hospitals using a novel targeting methodology. Am J Infect Control 34:513–519. doi: 10.1016/j.ajic.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Carling PC, Briggs JL, Perkins J, Highlander D. 2006. Improved cleaning of patient rooms using a new targeting method. Clin Infect Dis 42:385–388. doi: 10.1086/499361. [DOI] [PubMed] [Google Scholar]
- 40.Rupp ME, Adler A, Schellen M, Cassling K, Fitzgerald T, Sholtz L, Lyden E, Carling P. 2013. The time spent cleaning a hospital room does not correlate with the thoroughness of cleaning. Infect Control Hosp Epidemiol 34:100–102. doi: 10.1086/668779. [DOI] [PubMed] [Google Scholar]
- 41.Lucado J, Paez K, Andrews R, Steiner C. 2010. Adult hospital stays with infections due to medical care, 2007. HCUP Statistical Brief #94. Agency for Healthcare Research and Quality, Rockville, MD. [PubMed] [Google Scholar]
- 42.Passaretti CL, Otter JA, Reich NG, Myers J, Shepard J, Ross T, Carroll KC, Lipsett P, Perl TM. 2013. An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug-resistant organisms. Clin Infect Dis 56:27–35. doi: 10.1093/cid/cis839. [DOI] [PubMed] [Google Scholar]
- 43.Raggi R, Archulet K, Haag CW, Tang W. 2018. Clinical, operational, and financial impact of an ultraviolet-C terminal disinfection intervention at a community hospital. Am J Infect Control 46:1224–1229. doi: 10.1016/j.ajic.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 44.de Kraker MEA, Harbarth S, Dancer SJ. 2018. Shining a light on ultraviolet-C disinfection: no golden promises for infection prevention. Am J Infect Control 46:1422–1423. doi: 10.1016/j.ajic.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 45.Rutala WA, Kanamori H, Gergen MF, Sickbert-Bennett EE, Sexton DJ, Anderson DJ, Laux J, Weber DJ, CDC Prevention Epicenters Program. 2018. Antimicrobial activity of a continuous visible light disinfection system. Infect Control Hosp Epidemiol 39:1250–1253. doi: 10.1017/ice.2018.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bache SE, Maclean M, Gettinby G, Anderson JG, MacGregor SJ, Taggart I. 2018. Universal decontamination of hospital surfaces in an occupied inpatient room with a continuous 405 nm light source. J Hosp Infect 98:67–73. doi: 10.1016/j.jhin.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Otter JA, Yezli S, Salkeld JA, French GL. 2013. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control 41:S6–S11. doi: 10.1016/j.ajic.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Boyd WW, Sehulster L. 2004. Microbiological assay of environmental and medical-device surfaces, p 13.10.1–13.10.12. In Isenberg HD. (ed), Clinical microbiology procedures handbook, 2nd ed ASM Press, Washington, DC. [Google Scholar]