The immature Gag lattice is a critical structural feature of assembling HIV-1 particles, which is primarily important for virion formation and release. While Gag forms a hexameric lattice, driven primarily by the capsid domain, the MA domain additionally trimerizes where three Gag hexamers meet. MA mutants that are defective for trimerization are deficient for Env incorporation and replication, suggesting a requirement for trimerization of the MA domain of Gag in Env incorporation. This study used a gain-of-function, forced viral evolution approach to rescue HIV-1 mutants that are defective for MA trimerization. Compensatory mutations that rescue virus replication do so by restoring Env incorporation and MA trimer formation. This study supports the importance of MA domain trimerization in HIV-1 replication and the potential of the trimer interface as a therapeutic target.
KEYWORDS: envelope, Gag, assembly, human immunodeficiency virus, retrovirus
ABSTRACT
The matrix (MA) domain of HIV-1 Gag plays key roles in virus assembly by targeting the Gag precursor to the plasma membrane and directing the incorporation of the viral envelope (Env) glycoprotein into virions. The latter function appears to be in part dependent on trimerization of the MA domain of Gag during assembly, as disruption of the MA trimer interface impairs Env incorporation. Conversely, many MA mutations that impair Env incorporation can be rescued by compensatory mutations in the trimer interface. In this study, we sought to investigate further the biological significance of MA trimerization by isolating and characterizing compensatory mutations that rescue MA trimer interface mutants with severely impaired Env incorporation. By serially propagating MA trimerization-defective mutants in T cell lines, we identified a number of changes in MA, both within and distant from the trimer interface. The compensatory mutations located within or near the trimer interface restored Env incorporation and particle infectivity and permitted replication in culture. The structure of the MA lattice was interrogated by measuring the cleavage of the murine leukemia virus (MLV) transmembrane Env protein by the viral protease in MLV Env-pseudotyped HIV-1 particles bearing the MA mutations and by performing crystallographic studies of in vitro-assembled MA lattices. These results demonstrate that rescue is associated with structural alterations in MA organization and rescue of MA domain trimer formation. Our data highlight the significance of the trimer interface of the MA domain of Gag as a critical site of protein-protein interaction during HIV-1 assembly and establish the functional importance of trimeric MA for Env incorporation.
IMPORTANCE The immature Gag lattice is a critical structural feature of assembling HIV-1 particles, which is primarily important for virion formation and release. While Gag forms a hexameric lattice, driven primarily by the capsid domain, the MA domain additionally trimerizes where three Gag hexamers meet. MA mutants that are defective for trimerization are deficient for Env incorporation and replication, suggesting a requirement for trimerization of the MA domain of Gag in Env incorporation. This study used a gain-of-function, forced viral evolution approach to rescue HIV-1 mutants that are defective for MA trimerization. Compensatory mutations that rescue virus replication do so by restoring Env incorporation and MA trimer formation. This study supports the importance of MA domain trimerization in HIV-1 replication and the potential of the trimer interface as a therapeutic target.
INTRODUCTION
The assembly of HIV-1 particles is driven by a variety of protein-protein, protein-lipid, and protein-RNA interactions, primarily mediated by the viral Pr55Gag polyprotein (referred to here as Gag). Gag possesses the ability to form virus-like particles (VLPs) in the absence of any other viral proteins or viral nucleic acid. The major domains of Gag, listed from the N to C terminus, are matrix (MA), capsid (CA), nucleocapsid (NC), and p6. CA-NC and NC-p6 are separated by spacer peptides SP1 and SP2, respectively. VLP assembly and release require contributions from each Gag domain: MA directs Gag trafficking to the plasma membrane (PM) by specifically binding to phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2]; CA-CA interactions promote the formation of an extensive lattice of Gag hexamers; NC binds to the HIV-1 genomic RNA (or other nucleic acids if the viral genome is unavailable) and promotes CA multimerization; finally, p6 recruits the endosomal sorting complex required for transport (ESCRT) machinery to promote membrane scission between the budding VLP and the host cell (1, 2).
While Gag alone can generate VLPs, for a particle to be infectious requires the expression of the viral enzymes, regulatory proteins, and the envelope (Env) glycoprotein. Env is translated as a 160-kDa transmembrane (TM) precursor protein, gp160, in the endoplasmic reticulum, where it is heavily glycosylated (3). gp160 traffics through the Golgi apparatus where it is processed to the mature Env glycoproteins gp120 and gp41, which remain noncovalently associated as a heterotrimeric complex containing three molecules each of gp120 and gp41. gp120 is surface exposed and binds the receptor (CD4) and coreceptor (CXCR4 or CCR5) to trigger membrane fusion during virus entry. gp41 is composed of three domains: an ectodomain, transmembrane domain, and long cytoplasmic tail (CT). The ectodomain and transmembrane domain comprise the fusion machinery. The CT contains motifs that promote trafficking from the trans-Golgi compartment and recycling endosomes to the PM (4, 5), endocytosis from the PM (6–8), and recycling through retromer (9).
The HIV-1 Env CT is over 150 amino acids long, a length that is typical of many lentiviruses but unusually long compared to the lengths of most other retroviruses. This greater length may permit lentiviruses to acquire multiple functions in the CT; however, it is also a bulky protein domain that must be accommodated by the underlying Gag lattice during HIV-1 particle assembly (10). It has been demonstrated that the gp41 CT mediates trapping and clustering of Env trimers at the site of virus assembly (11–13) and that the CT promotes the retention of Env in detergent-stripped Gag VLPs (14), consistent with trapping of the CT by the Gag lattice. Although the structure of the CT in HIV-1 virions is unknown, several models have been proposed (reviewed in references 15 and 16). Recent nuclear magnetic resonance (NMR) studies of an in vitro-purified CT in a micellar system revealed that the N-terminal portion of the CT is disordered and presumably exposed to the cytoplasm, while the C-terminal region, which contains three α-helices known as lentiviral lytic peptides (LLPs), is tightly membrane associated (17). As both the CT of Env and the MA domain of Gag occupy space on the cytoplasmic face of the PM, MA likely assembles in a specific way to accommodate the long CT. In contrast to the well-characterized hexameric organization of the CA domain in intact HIV-1 virions, the arrangement of MA in virions remains to be defined. Early structures of lentiviral MA proteins solved by X-ray crystallography indicated a trimeric arrangement (18, 19). The formation of MA and Gag trimers has been demonstrated in intact virions by using a cross-linking assay (20), and analysis of MA protein assembled in vitro on PI(4,5)P2-containing membranes revealed a hexamer-of-trimers arrangement (21). In the latter model, a central aperture is present in the MA domain lattice; this opening in the lattice could help accommodate the long gp41 CT.
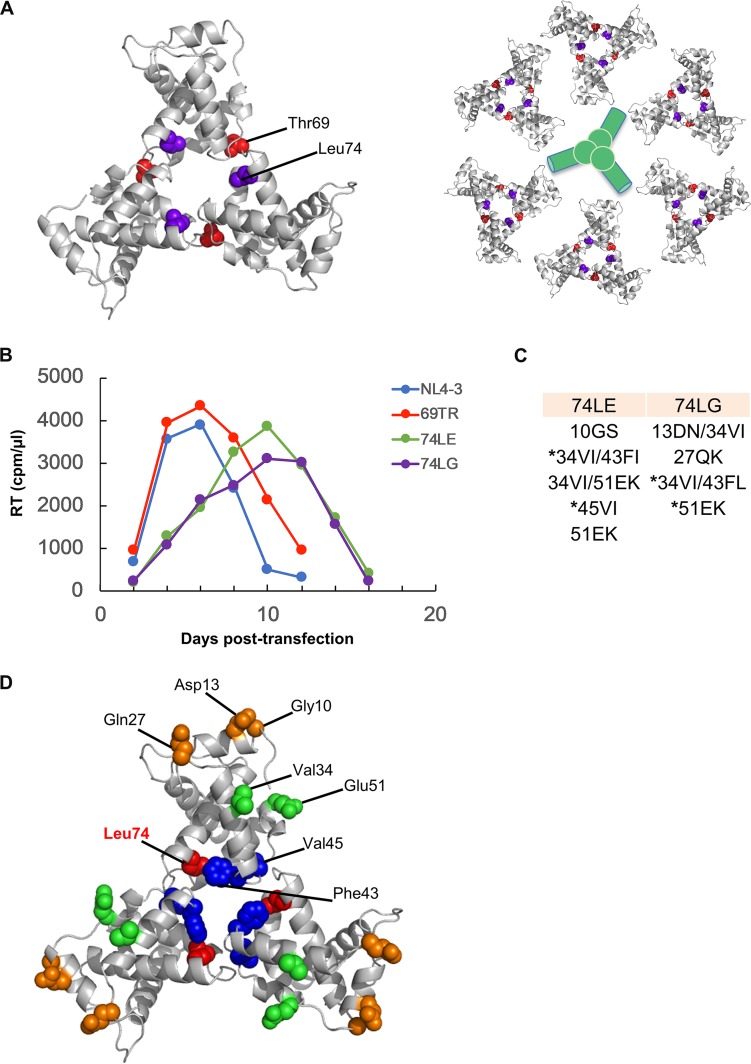
Evidence confirming dependence of HIV-1 Env incorporation on both MA and the Env CT has been obtained from many biochemical and genetic studies (10, 22). The gp41 CT contains amino acid residues that allow Env to interface with the cellular factors that direct trafficking of Env to sites of viral assembly (5). In addition, a small deletion in the CT has been shown to inhibit Env incorporation into particles, and this mutation can be rescued by a single amino acid change in MA (23). Similarly, Env incorporation can be inhibited by deletion or mutation of MA (24–31). These Env incorporation-defective MA mutants can be rescued by truncation of the Env CT (26, 28) or by compensatory changes in MA (29, 31, 32); in particular, a wide variety of Env incorporation-defective mutations were shown to be rescued by a mutation near the MA trimer interface (31). Furthermore, MA domain trimerization has been shown to be important for Env incorporation; mutation of residues at the trimer interface, such as Thr69 and Leu74 (Fig. 1A), prevents formation of a wild-type (WT) MA trimer and blocks Env incorporation without affecting virus particle assembly (20). These data suggest a model wherein trimerization of the MA domain of Gag promotes Env incorporation by relieving potential steric hindrance between the Env CT and MA (20).
FIG 1.
Location of mutations that induce MA trimerization defects and selection of second-site mutations capable of rescuing trimer-defective mutants in MT-4 cells. (A) The structure of the MA trimer, solved by X-ray crystallography (18) (left side), and the hexamer-of-trimer model based on MA assembly on 2D membranes (21) (right side). Thr69 (red) and Leu74 (purple) are present at the trimer interface and have previously been shown to impair MA trimerization (20). MA trimer structure generated from PDB accession number 1HIW using PyMOL. Hypothetical site of Env trimer accommodation is indicated in green. (B) MT-4 cells were transfected with a WT pNL4-3 molecular clone or mutant derivatives bearing substitutions at positions 69 and 74. At 2-day intervals the cells were split, and samples of medium were assayed for RT activity. Cells were harvested from the peaks of viral replication for 74LE and 74LG, and viral DNA was amplified and sequenced to identify second-site mutations. (C) Second-site mutations identified in selection experiments. An asterisk indicates those mutants that were selected for further studies. (D) Location of second-site mutations in the MA trimer structure. The putative compensatory mutations identified by propagation of the trimerization-defective mutants 74LG and 74LE are highlighted on the MA trimer crystal structure of PDB accession number 1HIW. Leu74 is shown in red. Compensatory mutations at the trimer interface are shown in blue, and those at the putative Env interface are in orange. Val34 and Glu51, located between the two interfaces, are shown in green.
Protease (PR)-mediated Gag cleavage serves as a trigger for activation of HIV-1 Env-mediated fusion. The inability of Env on the immature particle to catalyze membrane fusion is reversed by truncating the long gp41 CT (33, 34), suggesting that interactions between the gp41 CT and the immature Gag lattice suppress fusion activity. Other retroviruses have also evolved strategies to suppress the fusogenic activity of the Env glycoprotein complex on viral particles until the virion undergoes maturation. For example, in the case of several other retroviruses, e.g., murine leukemia virus (MLV) (35), Mason-Pfizer monkey virus (M-PMV) (36), and equine infectious anemia virus (EIAV) (37), the Env CT is directly cleaved by the viral PR to activate fusogenicity. We previously described HIV-1 Env mutants that escape the inhibitory activity of an entry inhibitor by acquiring PR cleavage sites in the gp41 CT (38); these mutants thus recapitulate the strategy used by MLV, M-PMV, and EIAV to link Env activation with particle maturation. When MLV Env is used to pseudotype HIV-1 particles, the HIV-1 PR is able to cleave the CT of the transmembrane Env protein p15(E) to p12(E), removing the so-called R peptide to activate fusion. We previously reported an HIV-1 MA mutant in which MLV R peptide cleavage was blocked (39). The latter finding suggests that changes in the structure of the MA lattice underlying the viral envelope can prevent the viral PR from accessing the CT of MLV Env. Although the precise structural perturbations in the MA domain of Gag responsible for blocking PR access to p15(E) remain to be defined, PR-mediated MLV R peptide cleavage in HIV-1 particles pseudotyped with MLV Env provides a tool with which to probe the structure of the HIV-1 MA lattice in virions.
In this study, we adopted a gain-of-function approach in which trimerization-defective MA mutants that are defective for Env incorporation and, consequently, replication incompetent were propagated sequentially in the MT-4 and SupT1 T-cell lines to select for compensatory mutations. Changes at or close to the MA trimer interface and at the putative interface between MA and the Env CT were identified. Compensatory mutations located in the vicinity of the trimer interface rescued Env incorporation and virus replication. MA trimerization-defective and rescue mutants exhibited alterations in amphotropic MLV (A-MLV) processing, indicating a structural perturbation of MA arrangement. Structural analysis of an in vitro-assembled MA lattice suggested that the compensatory mutations restored a trimeric arrangement of MA. This work underscores the importance of the MA trimer interface for Env incorporation into particles.
RESULTS
Propagation of trimerization-defective MA mutants in T cells selects for compensatory mutations.
We previously reported that disruption of trimerization of the MA domain of Gag, imposed by mutations at the crystallographic MA trimer interface, was associated with severe defects in virus replication, particle infectivity, and Env incorporation (20). To examine further the requirement for MA trimerization in HIV-1 replication, we pursued a gain-of-function approach by attempting to select for compensatory mutations that would rescue the defects in Env incorporation and particle infectivity imposed by MA trimer interface mutations. Three replication-incompetent viruses, which have been previously described to be highly defective in MA domain trimer formation (69TR, 74LG, and 74LE), were selected for these studies. In initial experiments, HIV-1(NL4-3) molecular clones encoding these MA mutants were transfected into the SupT1 T-cell line. In multiple independent experiments, no replication was observed for any of the three mutants over a period of several months in culture (20; data not shown). In a further attempt to identify compensatory mutations, we performed serial passage experiments in the MT-4 T-cell line, which is more permissive for HIV-1 replication than SupT1 cells (40, 41) (Fig. 1B). Consistent with previously published data (20), the 69TR virus replicated with kinetics similar to those of the WT in several independent experiments, and viral DNA sequencing indicated that no additional mutations were acquired by this mutant. These results indicate that approximately 3-fold reductions in virus particle infectivity and Env incorporation (20) do not result in a delay in virus replication in MT-4 cells. Because of this combination of replication phenotypes (no delay in MT-4 cells and lethality in SupT1 cells), we were unable to take the viral reversion approach for the 69TR mutant. In contrast, the 74LE and 74LG mutants exhibited delayed replication relative to that of the WT in the MT-4 cell line (Fig. 1B). MA- and Env-coding viral DNA was amplified by PCR at the time of peak virus replication and sequenced. Several second-site mutations in MA were identified (Fig. 1C). Notably, changes in Phe43 and Glu51 were found for both 74LE and 74LG viruses. The 34VI substitution, which we have identified previously (32, 41), was also found in combination with other mutations. Thus, viruses with defects in MA domain trimer formation acquired additional changes upon serial passage in cell culture.
Structural analysis of second-site MA mutations.
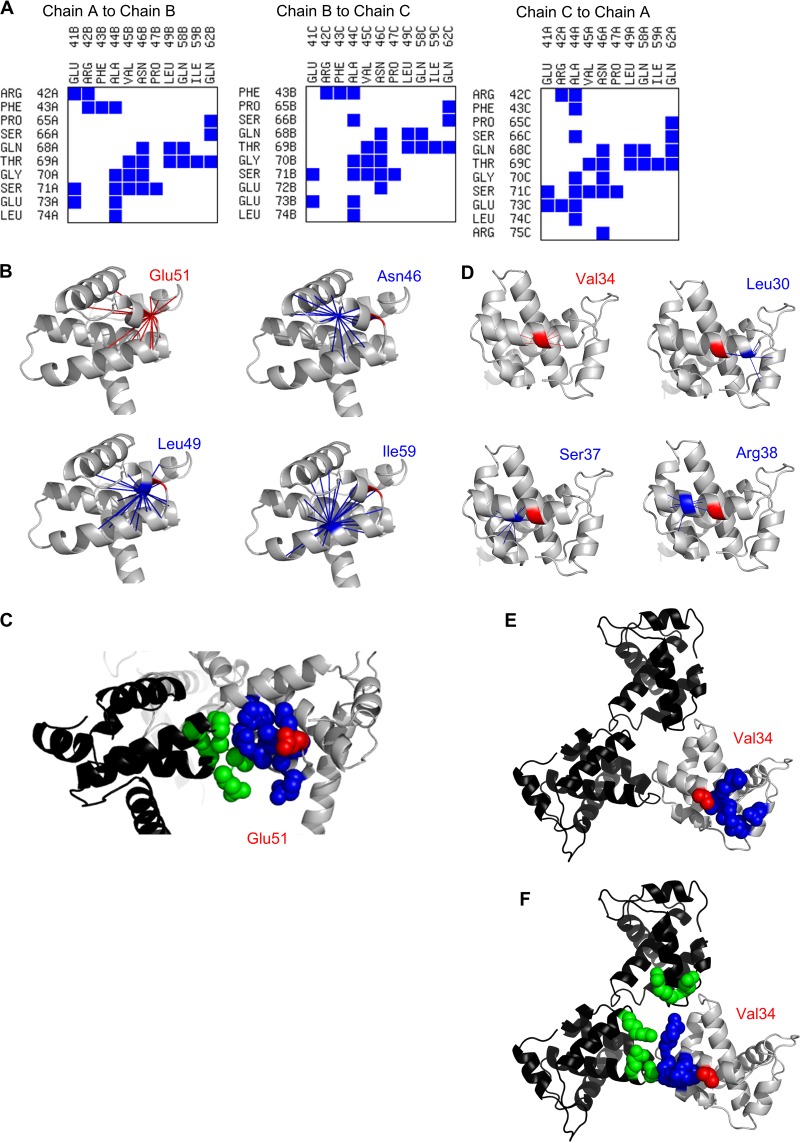
The potential mechanisms of rescue by the second-site mutations were investigated by contact map analysis (CMA) of the MA trimer. Phe43 and Val45 are located at the trimer interface and thus might reasonably be expected to influence MA trimerization (Fig. 1D and 2A). In contrast, Gly10, Asp1,3 and Gln27 are located near the proposed interface between the MA trimer and the Env CT (Fig. 1A and D) and therefore might influence the incorporation of Env into the MA lattice. Val34 and Glu51 are located near neither the trimer interface nor the putative Env interface. CMA of interactions within MA monomers was performed to identify networks of residues that are in close enough proximity to one another that changes in one position could influence more distant sites. It should be noted that these analyses are not intended to predict the consequence of any specific mutation but, rather, to determine whether the residues are positioned such that long-range indirect interactions with critical interfaces are possible. These analyses suggested that Glu51 has the potential to influence the trimer interface indirectly (Fig. 2B and C) via interactions with Asn46, Leu49, and Ile59. Similar analysis of Val34 suggested direct and indirect interactions with Leu30, leading to interactions with Leu12 and Glu16 (Fig. 2D and E); Leu12, Glu16, Leu30, and Val34 have all been shown to be important for Env incorporation (26, 30, 31, 42). Val34 may also interact directly with Ser37 and Arg38, leading to indirect interactions with Glu41, Arg42, and Pro47; all of these residues are found at the trimer interface (Fig. 2A, D, and F). These putative diverse interactions of Val34 may contribute to the capacity of mutations at this position to inhibit Env incorporation (29) or to completely or partially rescue diverse Env incorporation defects induced by mutations in MA and the gp41 CT (26, 32). Collectively, the mutations identified in these passaging experiments are all located at, or have the potential to influence, at least one of the two regions of the MA trimer known to be essential for Env incorporation.
FIG 2.
Contact map analysis for MA trimer. (A) Trimer interface contacts were identified in the MA trimer crystal structure of PDB accession number 1HIW using the Contacts of Structural Units (CSU) online tool (http://ligin.weizmann.ac.il/space) and are indicated with blue squares. (B) Interactions of Glu51 within chain A of the MA trimer were identified using CMView, version 1.1 (60). Glu51 and the contacts it makes are shown by radiating red lines. The contacts of three residues implicated in the trimer interface and in contact with Glu51 are shown in blue. Neither positive nor negative consequences are inferred from the data, only that the residues are in close enough proximity that an interaction is possible. (C) Trimer interface interactions potentially affected by Glu51 substitutions. Chain A of the MA trimer is shown in gray, and chain C is in black. Glu51 of chain A is shown in red; residues in chain A that are contacted directly (Asn46, Leu49, and Ile59) or indirectly (Leu40, Pro47, Gly48, Leu50, Glu54, Gly55, and Cys56) by Glu51 are shown in blue. Trimer interface residues of chain C are shown in green (Ser66, Gln68, Thr69, Gly70, Ser71, and Leu74). (D) Interactions of Val34 within chain A of the MA trimer were identified using CMView, as described in panel B. (E) Env interface residues potentially affected by Val34 substitutions. Chain A of the MA trimer is shown in gray, and chain B (top) and chain C (left) are shown in black. Val34 of chain A is shown in red; residues in chain A that are contacted directly (Leu30, Lys31, His32, and Ile33) or indirectly (Leu12 and Glu16) by Val34 are shown in blue. (F) Trimer interface interactions potentially affected by Val34 substitutions. Chain A of the MA trimer is shown in gray, and chain B (top) and chain C (left) are shown in black. Val34 of chain A is shown in red; residues in chain A that are contacted directly (Ser37 and Arg38) or indirectly (Glu41, Arg42, and Pro47) by Val34 are shown in blue. Trimer interface residues are indicated in green: Glu41 and Arg42 of chain B and Arg42, Ser71 and Glu73 of chain C.
Compensatory mutations rescue replication competency of MA trimerization-defective MA mutants.
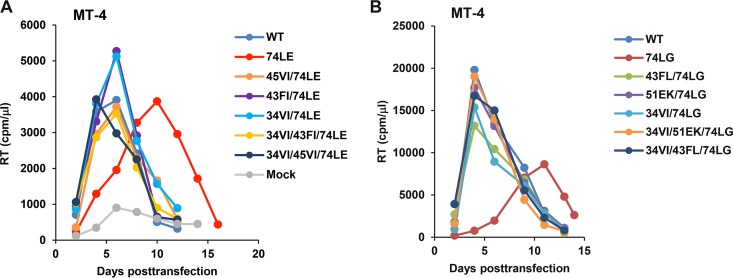
To verify the ability of the compensatory mutations to rescue MA trimerization-defective mutants, we repeated MT-4 passaging experiments with 74LE and 74LG, alone and in combination with a set of putative compensatory mutations. For this analysis, we focused on mutations predicted to act, directly or indirectly, on the trimer interface: 34VI, 43FI, 43FL, 45VI, and 51EK. A set of single, double, and triple mutants containing the changes indicated in Fig. 1C were generated. Although 45VI and 51EK, unlike 43FI and 43FL, were not originally selected with 34VI for the 74LE and 74LG mutants, respectively, in MT-4 cells (Fig. 1C), we additionally generated the 34VI/45VI/74LE and 34VI/51EK/74LG triple mutants. As expected, the two original trimerization-defective mutants, 74LE and 74LG, exhibited the most severely impaired replication kinetics (Fig. 3). All 74LE- and 74LG-derived viruses, containing either single or double compensatory changes, replicated with near-WT kinetics in MT-4 cells. Single mutants 34VI, 43FL, 43FI, 45VI, and 51EK, were also fully replication competent in this T-cell line (data not shown).
FIG 3.
Replication kinetics of 74LE (A) and 74LG (B) viruses containing second-site mutations in MT-4 cells. Cells were transfected with the WT or mutant HIV-1 molecular clones as indicated. At 2-day intervals the cells were split, and samples of medium were assayed for RT activity.
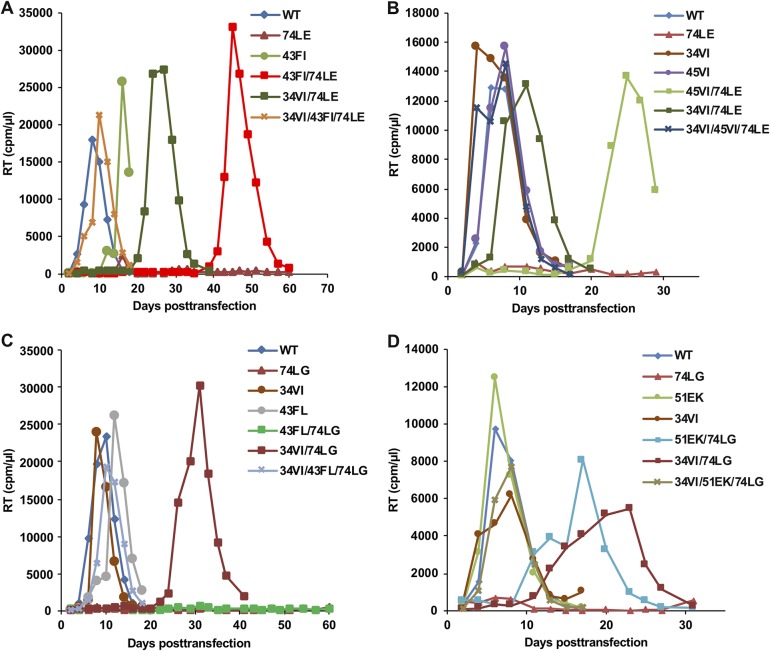
To assess the replication fitness of the single, double, and triple mutants in a more stringent context, we transfected the mutant molecular clones into the SupT1 T-cell line (Fig. 4). As observed previously, 74LE and 74LG were replication incompetent. In the set of 74LE-derived mutants (Fig. 4A and B), two triple mutants, 34VI/43FI/74LE and 34VI/45VI/74LE, exhibited near-WT replication kinetics. Double mutants replicated with some delay or were replication incompetent; the length of the delay and whether or not replication ever occurred varied between experimental replicates, likely reflecting the stochastic nature of acquisition of additional compensatory mutations. Some of the mutants that exhibited a delay in replication were sequenced to identify any additional changes. For example, 43FI/74LE and 45VI/74LE acquired 34VI, and 34VI/74LE reverted to 34VI/74LK. The 74LG- and 74LE-derived mutants behaved similarly. The double mutants replicated efficiently in MT-4 cells but were delayed relative to replication of the WT or were completely replication defective in SupT1 cells. The triple mutants 34VI/43FL/74LG and 34VI/51EK/74LG restored the replication kinetics to WT levels (Fig. 4C and D). Sequencing of the replicating double mutants identified the emergence of 51EK with 34VI/74LG and 34VI with 51EK/74LG (data not shown).
FIG 4.
Replication kinetics of 74LE (A and B) and 74LG (C and D) viruses containing compensatory mutations in SupT1 cells. Cells were transfected with the WT and mutant HIV-1 molecular clones as indicated. The cells were split every other day, and aliquots of medium were collected for RT analysis.
From these data, we confirm that trimer interface mutations can severely impair HIV-1 replication and demonstrate that these mutants can be rescued by compensatory changes at or near the trimer interface. Full rescue of replication in SupT1 cells required 34VI in combination with a trimer interface mutation.
Compensatory mutations restore Env incorporation and infectivity.
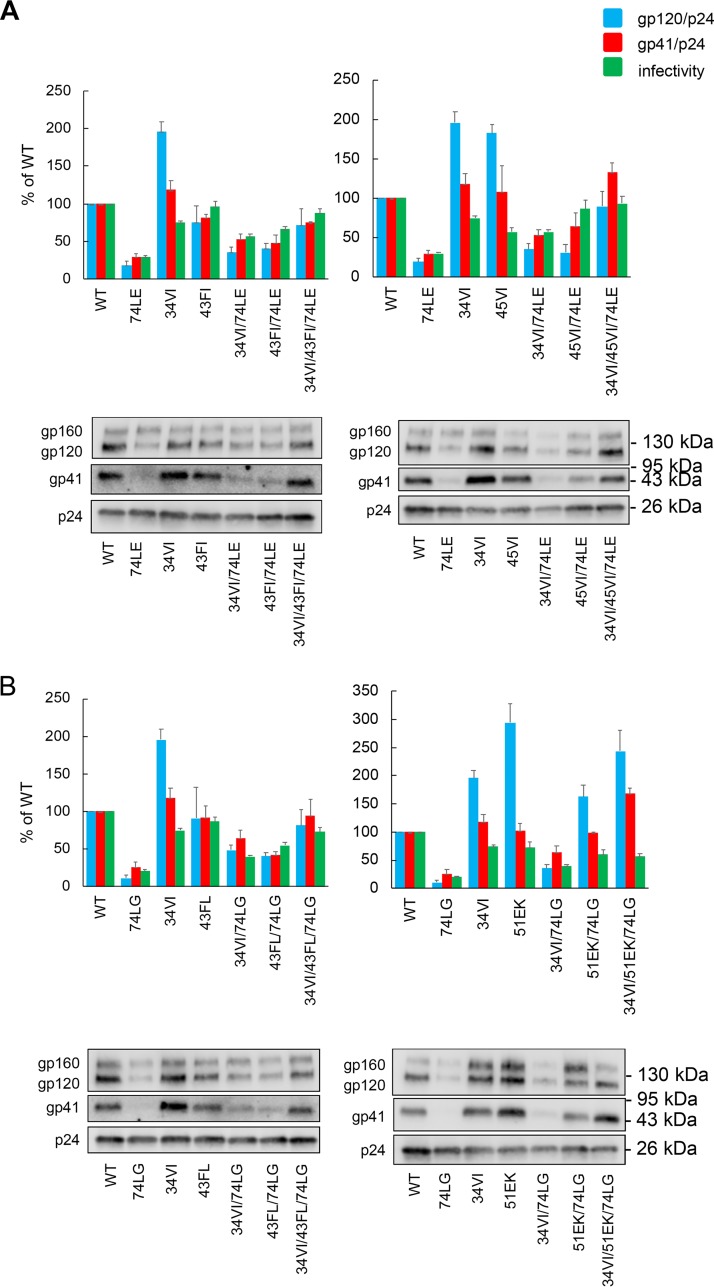
Having established that compensatory mutations could restore the replicative fitness of the MA domain trimerization-defective mutants, we examined the impact of the compensatory mutations on Env incorporation and virus infectivity (Fig. 5). As previously reported (20), when produced in HeLa cells, the single mutants 74LE and 74LG significantly impaired both Env incorporation (as measured by the ratio of virion-associated gp120/p24 and gp41/p24) and infectivity. The double mutants, which were demonstrated to rescue replication in MT-4 cells, appeared, in most cases, to partially restore Env incorporation and infectivity. The further addition of 34VI to create the triple mutants capable of replication in SupT1 cells increased Env incorporation to WT or near-WT levels. Thus, the effect of the MA mutations on virus replication in SupT1 cells correlated with the levels of Env incorporation and particle infectivity.
FIG 5.
Characterization of 74LE-derived (A) and 74LG-derived (B) rescue mutants. HeLa cells were transfected with the HIV-1 mutants indicated; after 48 h virus was harvested and assayed for infectivity by TZM-bl assay, and levels of virion-associated p24 (CA), gp120, and gp41 were quantified (see Materials and Methods). Ratios of gp120 to p24 and of gp41 to p24 were quantified and plotted. A representative gel for Env incorporation analysis is shown, with the positions of the Env precursor gp160, the surface glycoprotein gp120, the transmembrane glycoprotein gp41, and p24 (CA) indicated. The positions of molecular mass markers are shown on the right. Averages from at least three independent experiments are indicated ± standard errors of the means.
Compensatory mutations alter the arrangement of MA in virions and restore MA trimer formation in vitro.
The panel of compensatory mutations that we describe above as restoring replication, Env incorporation, and infectivity were analyzed for MA trimerization using the glutaraldehyde cross-linking assay we developed previously (20). We observed that none of the compensatory mutations was able to measurably rescue MA trimerization in virions in the cross-linking assay (data not shown), suggesting either that the compensatory mutations are not restoring MA trimerization or that the cross-linking assay is highly sensitive to subtle shifts in the structure of the WT trimer. Consistent with the latter possibility, a variety of other potential pairs of MA trimer interface residues failed to cross-link in this assay, despite appearing to be in close proximity in the crystal structure (data not shown).
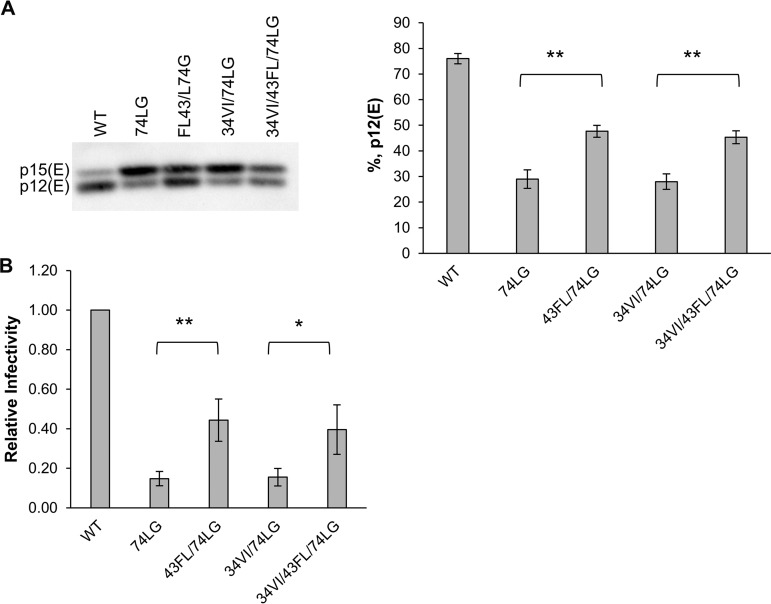
As described in the introduction, PR-mediated cleavage of the MLV TM Env protein p15(E) to p12(E) in HIV-1 virions pseudotyped with MLV Env provides a tool with which to probe MA structure (39). Due to our unsuccessful efforts to cross-link MA trimers in revertant virions, we quantified p15(E) cleavage to examine the arrangement of MA in mutant virions. In virus particles containing WT MA, approximately 75% of p15(E) was converted to p12(E) (Fig. 6A). In contrast, p15(E) cleavage was markedly reduced in 74LG virions, with p12(E) levels at ∼30% (Fig. 6A). These results suggest that changes in the organization of the MA lattice in 74LG mutant virions impair the ability of PR to cleave off the R peptide from p15(E). In 43FL/74LG and 34VI/43FL/74LG virions, p12(E) levels were ∼45 to 50%, whereas p15(E) processing efficiency in 34VI/74LG particles was similar to that in 74LG virions. Infectivity data correlated closely with p15(E) processing efficiency (Fig. 6B): 74LG and 34VI/74LG particles were poorly infectious, whereas 43FL/74LG and 34VI/43FL/74LG virions exhibited significantly increased infectivity relative to that of 74LG particles. These data suggest that the 43FL mutation partially reverses MA structural defects imposed by 74LG, while the 34VI substitution does not cause structural changes measurable using this approach. Consistent with these results, we also observed that the 34VI/51EK/74LG mutant displays significantly increased levels of p15(E) cleavage relative to that of 74LG. Similarly, 74LE virions exhibit a severe defect in p15(E) processing, which is partially rescued in the 34VI/45VI/74LE mutant (data not shown).
FIG 6.
Effects of MA mutations on PR-mediated cleavage of MLV p15(E) in MLV Env-pseudotyped particles. (A) Virions produced in 293T cells were collected by ultracentrifugation, lysed, and analyzed by Western blotting. A representative gel is shown (left). The percentage of p12(E) in virions was calculated as [p12(E)/(p12(E) + p15(E)] × 100 (right). Values are means ± standard deviations (n = 3 independent experiments). (B) TZM-bl cells were infected with A-MLV Env pseudotyped virions, and at 24 h postinfection luciferase signals were analyzed and normalized to virus-associated RT units. The WT value was set at 1. Values are means ± standard deviations (n = 4 independent experiments). *, P < 0.05; **, P < 0.01.
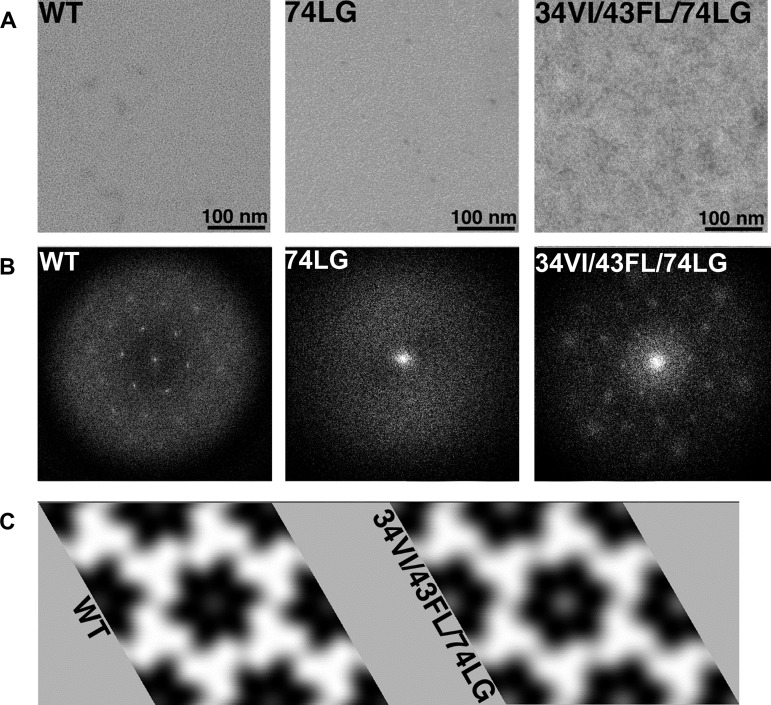
As a direct approach to evaluating MA trimerization, the effects of selected MA mutations on the organization of MA assembled on two-dimensional (2D) membrane monolayers were determined. For this analysis, a replication-incompetent, trimerization-defective mutant (74LG) and a replication-competent triple mutant (34VI/43FL/74LG) were examined (Fig. 7). As previously reported (21), WT MA assembled on membranes and gave a calculated diffraction pattern (power spectrum) with clear hexagonal reflections (Fig. 7B, left panel). When these reflections were indexed, Fourier filtered, and back-transformed, they showed that WT MA formed a hexameric lattice of trimers, as observed previously (Fig. 7C, left panel) (21). In contrast, the 74LG proteins showed no evidence of organized lattice formation, and no crystalline reflections were observed in power spectra of membrane-bound 74LG proteins (Fig. 7B, center panel). Importantly, as indicated by the pattern of reflections (Fig. 7B, right panel), the replication-competent triple mutant formed an ordered lattice that when indexed, filtered, and back-transformed at a resolution of 25 Å, closely resembled that of the WT (Fig. 7C, right panel).These data support the hypothesis that the compensatory mutations rescue replication by restoring MA trimer formation. Higher-resolution approaches will be needed to discern any subtle structural differences between the WT and the triple mutant, but it is clear from these results that restoration of MA trimer formation accompanied the rescue in virus replication and Env incorporation conferred by the second-site MA mutations.
FIG 7.
Analysis of membrane-bound MA proteins. MA proteins were assembled onto lipid monolayers, lifted onto EM grids, stained, and imaged. (A) Boxed images of protein-containing areas, with size bars as indicated. (B) Calculated diffraction patterns (power spectra). As shown, both WT and 34VI/43FL/74LG proteins gave power spectra with hexagonal reflections, while the 74LG protein failed to yield ordered two-dimensional (2D) arrays in multiple trials. (C) To obtain 2D projection images of the WT and 34VI/43FL/74LG proteins, Fourier transforms of ordered regions in micrographs were indexed, Fourier filtered, and back-transformed as described in Materials and Methods. In each case, assemblies are shown perpendicular to the membrane from beneath, protein regions are in white, and protein-free regions are dark. As observed previously, matrix proteins appear as interconnected trimers around hexamer holes. Note that the two assemblies appear nearly indistinguishable except for a slight difference in unit cell sizes (WT, a = b = 92.5 Å; 34VI/43FL/74LG, a = b = 92.4 Å) that is not significant at this resolution (25 Å).
DISCUSSION
In this study, we used a gain-of-function approach to investigate the role of MA domain trimerization in HIV-1 Env incorporation and virus infectivity. MA mutants that were defective for MA and Gag trimer formation were propagated in T-cell lines for selection of second-site compensatory mutations that restored Env incorporation and viral replication. Our results suggest that compensatory mutations can rescue virus replication defects through structural changes in the MA lattice and restore MA trimer formation in vitro.
In our initial SupT1 selection experiments, we were unable to generate any compensatory changes for severely defective trimer interface mutants; we therefore propagated the defective viruses in the highly permissive MT-4 T-cell line. For two of the most defective mutants, 74LE and 74LG, a number of mutations in MA, present as single changes or as combinations of two changes, were identified. Based on their location on the current MA lattice model, three groups of mutations were identified. Two of the mutated residues, Phe43 and Val45, are located at the putative trimer interface, consistent with the presumed mechanism that the original defects were caused by perturbation of interactions at the trimer interface. Additionally, we found mutations (Gly10, Asp13, and Gln27) that are distant from the trimer interface but close to a surface thought to interface with Env. It is unlikely that these mutations influence the trimerization of the MA domain, but they are near residues that are known to influence Env incorporation, such as Leu7, Ser8, Leu12, Glu16, and Leu30 (26, 28, 31). The final group of compensatory mutations, 34VI and 51EK, was found between the two interfaces. 34VI has been previously demonstrated to rescue Env incorporation in the context of other defective MA (32) and gp41 CT (23) mutants. It is possible that 34VI and 51EK influence interactions at the MA-MA and MA-gp41 interfaces indirectly; however, at this time, we have no direct evidence that this is the case.
To verify that the identified second-site mutations are capable of rescuing MA trimer interface defects, the mutations were introduced into the trimer-defective 74LE and 74LG clones and characterized in cell culture experiments. In highly permissive MT-4 cells, all second-site mutations were individually able to restore WT-like replication kinetics of the 74LE or 74LG virus. In contrast, replication of 74LE or 74LG virus containing only single compensatory changes was attenuated in SupT1 cells. In these cells, combinations of two mutations were required for rescue of the defective viruses: 34VI coupled with a mutation that modifies the MA trimer interface, specifically, 43FI, 43FL, 45VI, or 51EK. Consistent with replicative capacity in SupT1 cells, the triple mutant viruses incorporated a WT level of Env and displayed rescued infectivity, in some cases to WT levels. Thus, detrimental effects of MA trimer interface mutations can be compensated by a number of changes at or near the interface.
Several different approaches were applied to characterize the mechanism of rescue of Env incorporation by the compensatory changes. Use of the cross-linking assay we described previously (20) did not reveal any rescue of MA trimer formation in the replication-competent triple mutants. This suggests either that the mechanism of rescue is not related to MA domain trimerization or that the cross-linking assay is sensitive to small changes in the arrangement of the MA trimer that affect the distance between the two Lys residues that serve as substrates for glutaraldehyde cross-linking. Two additional approaches that were applied to the 34VI/43FL/74LG mutant support the latter scenario. A series of experiments with MLV Env-pseudotyped virions revealed that PR-mediated cleavage of the MLV R peptide was significantly impaired in the 74LG mutant (and other MA trimer-defective mutants) but was partially restored in 34VI/43FL/74LG virions. Loss of MLV Env R peptide cleavage has been previously associated with mutations in HIV-1 MA (39) and presumably indicates structural alterations in the MA protein or lattice. Intriguingly, the enhancement of MLV Env processing efficiency was associated with the 43FL mutation, which is located at the trimer interface, but not with 34VI. We also observed that the 74LE mutation disrupted R peptide cleavage and that this defect was partially rescued by 45VI, which is also located at the MA trimer interface. The second approach used to interrogate the structure of the MA lattice was a low-resolution 2D crystallographic analysis of the oligomeric organization of MA. These experiments revealed a loss of the ordered MA trimer structure with the 74LG trimer-defective mutant and the restoration of a trimeric structure that closely resembled that of the WT with the 34VI/43FL/74LG triple mutant. These data demonstrate that a combination of 34VI/43FL rescues Env incorporation of the 74LG mutant virus by restoring the ability to form WT-like MA trimers. Combining these structural data with MLV R peptide processing, we propose that the 43FL mutation primarily contributes to structural changes in the MA lattice, whereas 34VI compensates for residual defects in HIV-1 Env incorporation by a currently unknown mechanism.
We have also demonstrated rescue of the 74LG MA trimerization defect in the 34VI/43FL/74LG triple mutant in recently published work, using recombinant proteins for an in vitro MA UV cross-linking assay and to demonstrate MA-CT interaction (43). That study demonstrates that 34VI/43FL/74LG recovers MA trimerization and CT interaction, which had been lost in the 74LG mutant; MA trimerization and CT interaction had previously been demonstrated for the WT MA protein (44). These data further support the ability of the compensatory mutations described here to rescue the interactions and functions of HIV-1 MA following perturbation of the MA trimer interface.
We favor a model in which the structural configuration of the MA domain lattice is a primary determinant of Env incorporation, potentially acting in concert with direct Env-MA interactions. According to this model, a large aperture at the center of the Gag lattice may be required to accommodate the long CT of gp41, in particular, the putatively disordered region between the membrane-spanning domain and the lentiviral lytic peptide sequences (17). Mutation of residues that define the perimeter of the aperture (Leu7, Ser8, Leu12, Trp15, Glu16, Leu30, and Glu98) or perturbation of the MA lattice (Ala44, Thr69, and Leu74) thus results in a loss of Env incorporation. This perturbation could either be a complete loss of MA domain trimerization, resulting in a reduction in the size of the central aperture, or a more subtle perturbation of the lattice, altering the specific characteristics of the aperture (e.g., size, shape, hydrophobicity, or charge) such that it no longer accommodates the gp41 CT. This subtle perturbation may block Env incorporation entirely via a steric mechanism or by a mechanism that disrupts both the steric accommodation of the CT by the MA domain lattice and direct MA-Env interactions. We have previously described MA mutations, most notably 34VI and 62QR, that suppress Env incorporation defects (23, 29, 31, 32). However, 34VI acts by a (still ill-defined) mechanism that is unlikely to directly involve MA domain trimerization, and while the 62QR mutation is located at the trimer interface, it rescues mutations that do not affect trimer formation. In contrast, the mutations we report here likely rescue Env incorporation-defective trimer interface mutations by restoring MA trimer formation. Thus, these mutations are mechanistically novel and distinct from the suppressor mutations that we along with others have described previously. Although we previously reported that mutations that block trimerization of the MA domain of Gag impair Env incorporation, it was formally possible that these mutations could also disrupt aspects of MA structure unrelated to trimer formation and that these structural perturbations could be responsible for impaired Env incorporation. We show here, using a gain-of-function approach, that rescue of trimerization-defective MA mutants corresponds to restoration of trimer formation. These findings add support to the hypothesis that MA trimer formation is required for Env incorporation.
Overall, our data are consistent with the important role of the MA domain trimer interface in promoting MA trimerization to facilitate Env incorporation into virions during assembly; a high proportion of compensatory mutations capable of rescuing MA trimerization defects are located at the trimer interface or have demonstrable potential to influence the trimer interface. In addition, our data provide greater insight into the mechanisms underlying the loss of Env incorporation and might contribute to our understanding how HIV-1 could acquire resistance to mutation- or inhibitor-induced perturbation of the MA domain trimer interface.
MATERIALS AND METHODS
Cells lines and antibodies.
HeLa and TZM-bl cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 5% (vol/vol) fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (Gibco). TZM-bl is a HeLa-derived indicator cell line that expresses firefly luciferase following infection by HIV (45–49). 293T cells were cultured in DMEM, supplemented with 10% (vol/vol) FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. Anti-HIV-1 IgG is pooled patient serum obtained from the NIH AIDS Reagent Program. HIV-1 gp41 was detected with the 2F5 monoclonal antibody (50) or the 10E8 monoclonal antibody (51). HIV-1 gp120 was detected with the 16H3 monoclonal antibody (52). Amphotropic (A)-MLV p15(E) and p12(E) were detected with a rabbit p15(E) antiserum (a gift from David Ott).
Plasmids.
HIV-1 particles were generated using the full-length proviral clone pNL4-3 (53). Point mutations were introduced by first subcloning the BssHI-SpeI fragment from pNL4-3 into pBluescript (Stratagene). Mutations were introduced using the Quick-change method (Stratagene) according to the manufacturer’s instructions, and the mutant BssHI-SphI fragment was recloned into pNL4-3. All mutations were confirmed by DNA sequencing (Macrogen). MA mutant clones lacking Env expression were generated by insertion of BssHII-SpeI fragments from the corresponding pNL4-3 mutant clones into the pNL4-3/KFS plasmid (26, 54). A-MLV Env was expressed from pSV-A-MLV-env (obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH, from Nathaniel Landau and Dan Littman) (55).
Virus replication.
HIV-1 replication was assayed by rate of spreading infection in SupT1 cells as reported previously (56). Virus replication was monitored by measuring reverse transcriptase (RT) activity as described previously (57). When necessary, genomic DNA was extracted using a QIAamp kit (Qiagen) according to the manufacturer’s protocol; viral sequences were amplified by PCR and sequenced (Macrogen).
Infectivity.
pNL4-3 clones or Env(−) pNL4-3/KFS derivatives along with the pSV-A-MLV-env plasmid were transfected into HeLa or 293T cells using PolyJet (Signagen) or Lipofectamine 2000, according to the manufacturer’s instructions. Virus-containing supernatants were harvested 48 to 72 h posttransfection and assayed for RT activity as described previously (57). TZM-bl cells were infected with the supernatants in the presence of dextran, the media were replenished, and the luciferase signal was measured at 24 h postinfection using Britelite Plus (Perkin-Elmer). Infectivity was defined as the level of luciferase expressed by TZM-bl cells divided by the total amount of virus (based on RT activity) with which they were infected. Statistics were calculated using GraphPad Prism. Unpaired t tests were performed, and two-tailed P values of <0.05 and <0.01 were considered statistically significant.
Env incorporation into virions.
Virions were harvested 48 h posttransfection by filtering the supernatant through a 0.45-μm-pore-size membrane and then pelleting the sample by centrifugation at 76,000 × g for 1 h at 4°C. Virions were resuspended in 2× Laemmli buffer (120 mM Tris-Cl [pH 6.8], 4% SDS, 20% glycerol, 10% β-mercaptoethanol, 0.02% bromophenol blue) and analyzed by Western blotting. Protein samples were separated by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (Immobilon, Millipore). Membranes were probed with primary antibody overnight at 4°C, washed, and then incubated for 1 h with species-specific horseradish peroxidase-conjugated secondary antibody. After the final washes, bands were detected by chemiluminescence (Chemidoc XRS+; Bio-Rad) and with a Sapphire Biomolecular Imager (Azure Biosystems). Quantification was performed using ImageStudio Lite (Li-Cor Biosciences). To calculate Env incorporation, volumes (average pixel intensity multiplied by the area covered by the band) was determined for each gp41 band and divided by the volume for the corresponding CA band.
A-MLV Env processing efficiency.
293T cells were cotransfected with pNL4-3/KFS clones encoding WT or mutant MA and pSV-A-MLV-env at a 10:1 ratio using Lipofectamine 2000 according to the manufacturer’s instructions. At 48 to 72 h posttransfection, the viral supernatants were filtered, and A-MLV Env-pseudotyped virions were collected by ultracentrifugation and resuspended in lysis buffer (50 mM Tris-HCl [pH 7.5], 300 mM NaCl, 0.5% Triton X-100, 10 mM iodoacetamide, and protease inhibitor cocktail tablets [Roche]). The samples were subjected to 12% SDS-PAGE in tricine-SDS running buffer. Protein products were transferred to a polyvinylidene fluoride (PVDF) membrane (Immobilon, Millipore) by wet electroblotting. The membrane was incubated with 5% nonfat milk in Tris-buffered saline and 0.05% Tween 20 detergent for 30 min at room temperature and then with a rabbit p15(E) antiserum overnight at 4°C. The membrane was washed, incubated for 45 min with anti-rabbit horseradish peroxidase-conjugated secondary antibody, and washed again, and the protein bands were revealed by SuperSignal Femto reagent (Thermo Scientific), followed by analysis with ImageLab software (Bio-Rad). To calculate percentage of A-MLV Env processing, the intensity of the p12(E) band was divided by total intensity of the p12(E) plus p15(E) band and multiplied by 100. Statistics were calculated using GraphPad Prism. Unpaired t tests were performed, and two-tailed P values of <0.05 and <0.01 were considered statistically significant.
Structural analysis.
Models of MA were generated in MacPyMOL (Schrödinger) using the coordinates of Protein Data Bank accession number 1HIW (18). Trimer interface contacts were identified using the online tool CMA (Contact Map Analysis) and further analyzed using Contacts of Structural Units (CSU) software (http://ligin.weizmann.ac.il/space) (58, 59). Interactions within single chains were identified using CMView, version 1.1 (60). Results generated in CMView were visualized using MacPyMOL. Prior to use of CMA, the residue numbering of PDB accession number 1HIW was adjusted to conform to that used throughout the paper, using the tool renumbered_PDB (https://github.com/ikagiampis/renumbered_PDB).
Electron microscopy.
Myristylated WT, 74LG, and 34VI/43FL/74LG MA proteins were purified as described previously (44). Proteins were incubated at ∼1 mg/ml in 8 μl of 25 mM sodium acetate (pH 6.0), 300 mM NaCl, 1.25 mM β-mercaptoethanol, 5% polyethylene glycol (molecular weight [MW], 6,000) at 4°C for 18 to 48 h beneath lipid monolayers composed of 60% egg phosphatidylcholine (Avanti), 20% cholesterol (Sigma), 20% dioleoyl-phosphatidylserine (Avanti) (275 ng of total lipid in 1.1 μl of 1:1 chloroform-hexane). Under these conditions, WT two-dimensional (2D) crystals appeared similar to those observed by Alfadhli et al. (21). After incubations, samples were lifted onto lacey electron microscopy (EM) grids (Ted Pella) for 1 min, rinsed for 30 s with distilled water, wicked, stained 1 min with 1.33% uranyl acetate, wicked, and dried.
Samples were imaged on a Delong LVEM5 at 5 to 6 keV; an FEI Tecnai transmission electron microscope (TEM) equipped with an AMT XR16-ActiveVu charge-coupled-device (CCD) camera at 80 keV, 4.05 Å/pixel, and defocus values of −1,000 to −3,000 nm or under low-dose conditions on a FEI Tecnai TEM equipped with an Eagle 4 megapixel CCD at 120 keV, 3.19 Å/pixel, and defocus values of −200 to −1,500 nm to give contrast transfer function (CTF) first zeroes beyond 2 nm. For simple representation of power spectra, images were boxed, and power spectra were calculated using ImageJ software. For analysis of 2D crystals, ordered areas in low-dose images were boxed, Fourier transformed, indexed, and unbent using the 2dx image processing package (61). Resulting amplitudes and phases (aph) files were subjected to space group analysis (21), yielding best fits of p6 symmetry for both WT and 34VI/43FL/74LG proteins, with real space dimensions for WT of a = b = 92.5 ± 5.6 Å, γ = 120°, and a = b = 92.4 ± 5.1 Å, γ = 120°, for the 34VI/43FL/74LG protein. Merged WT and 34VI/43FL/74LG aph files were complete to 25 Å, filtered to that resolution limit, and back-transformed assuming p6 symmetry to yield Fourier-filtered images in which proteins appear white and protein-free areas are dark.
ACKNOWLEDGMENTS
We thank members of the Freed laboratory and S. Welbourn for helpful discussion and critical review of the manuscript. We acknowledge K. Waki for the pNL4-3 subclone used for MA mutagenesis. The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-Ig from NABI and NHLBI; TZM-bl from John C. Kappes, Xiaoyun Wu, and Tranzyme, Inc.; HIV-1 anti-gp41 monoclonal antibody (10E8) from Mark Connors; HIV-1 anti-gp120 MAb (16H3) from Barton Haynes and Hua-Xin Liao; pSV-A-MLV-env from Nathaniel Landau and Dan Littman.
This work was supported by the Intramural Research Programs of the Center for Cancer Research, National Cancer Institute, NIH (E.O.F. and V.N.K.), and by the Intramural AIDS Targeted Antiviral Program (E.O.F.). E.B. and A.A. were supported by NIH grant R01 GM060170.
REFERENCES
- 1.Freed EO. 2015. HIV-1 assembly, release and maturation. Nat Rev Microbiol 13:484–496. doi: 10.1038/nrmicro3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundquist WI, Kräusslich HG. 2012. HIV-1 assembly, budding, and maturation. Cold Spring Harb Perspect Med 2:a006924. doi: 10.1101/cshperspect.a006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Checkley MA, Luttge BG, Freed EO. 2011. HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J Mol Biol 410:582–608. doi: 10.1016/j.jmb.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi M, Chu H, Chen X, Choi J, Wen X, Hammonds J, Ding L, Hunter E, Spearman P. 2015. A tyrosine-based motif in the HIV-1 envelope glycoprotein tail mediates cell-type– and Rab11-FIP1C–dependent incorporation into virions. Proc Natl Acad Sci U S A 112:7575–7580. doi: 10.1073/pnas.1504174112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi M, Williams JA, Chu H, Chen X, Wang JJ, Ding L, Akhirome E, Wen X, Lapierre LA, Goldenring JR, Spearman P. 2013. Rab11-FIP1C and Rab14 direct plasma membrane sorting and particle incorporation of the HIV-1 envelope glycoprotein complex. PLoS Pathog 9:e1003278. doi: 10.1371/journal.ppat.1003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boge M, Wyss S, Bonifacino JS, Thali M. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J Biol Chem 273:15773–15778. doi: 10.1074/jbc.273.25.15773. [DOI] [PubMed] [Google Scholar]
- 7.Ohno H, Aguilar RC, Fournier MC, Hennecke S, Cosson P, Bonifacino JS. 1997. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238:305–315. doi: 10.1006/viro.1997.8839. [DOI] [PubMed] [Google Scholar]
- 8.Rowell JF, Stanhope PE, Siliciano RF. 1995. Endocytosis of endogenously synthesized HIV-1 envelope protein. Mechanism and role in processing for association with class II MHC. J Immunol 155:473–488. [PubMed] [Google Scholar]
- 9.Groppelli E, Len AC, Granger LA, Jolly C. 2014. Retromer regulates HIV-1 envelope glycoprotein trafficking and incorporation into virions. PLoS Pathog 10:e1004518. doi: 10.1371/journal.ppat.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tedbury PR, Freed EO. 2014. The role of matrix in HIV-1 envelope glycoprotein incorporation. Trends Microbiol 22:372–378. doi: 10.1016/j.tim.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buttler CA, Pezeshkian N, Fernandez MV, Aaron J, Norman S, Freed EO, Van Engelenburg SB. 2018. Single molecule fate of HIV-1 envelope reveals late-stage viral lattice incorporation. Nat Commun 9:1861. doi: 10.1038/s41467-018-04220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy NH, Chan J, Lambele M, Thali M. 2013. Clustering and mobility of HIV-1 Env at viral assembly sites predict its propensity to induce cell-cell fusion. J Virol 87:7516–7525. doi: 10.1128/JVI.00790-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muranyi W, Malkusch S, Müller B, Heilemann M, Kräusslich HG. 2013. Super-resolution microscopy reveals specific recruitment of HIV-1 envelope proteins to viral assembly sites dependent on the envelope C-terminal tail. PLoS Pathog 9:e1003198. doi: 10.1371/journal.ppat.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyma DJ, Kotov A, Aiken C. 2000. Evidence for a stable interaction of gp41 with Pr55Gag in immature human immunodeficiency virus type 1 particles. J Virol 74:9381–9387. doi: 10.1128/jvi.74.20.9381-9387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva ES, Mulinge M, Bercoff DP. 2013. The frantic play of the concealed HIV envelope cytoplasmic tail. Retrovirology 10:54. doi: 10.1186/1742-4690-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steckbeck JD, Kuhlmann AS, Montelaro RC. 2013. C-terminal tail of human immunodeficiency virus gp41: functionally rich and structurally enigmatic. J Gen Virol 94:1–19. doi: 10.1099/vir.0.046508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy RE, Samal AB, Vlach J, Saad JS. 2017. Solution structure and membrane interaction of the cytoplasmic tail of HIV-1 gp41 Protein. Structure 25:1708–1718.e5. doi: 10.1016/j.str.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill CP, Worthylake D, Bancroft DP, Christensen AM, Sundquist WI. 1996. Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: implications for membrane association and assembly. Proc Natl Acad Sci U S A 93:3099–3104. doi: 10.1073/pnas.93.7.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao Z, Belyaev AS, Fry E, Roy P, Jones IM, Stuart DI. 1995. Crystal structure of SIV matrix antigen and implications for virus assembly. Nature 378:743–747. doi: 10.1038/378743a0. [DOI] [PubMed] [Google Scholar]
- 20.Tedbury PR, Novikova M, Ablan SD, Freed EO. 2016. Biochemical evidence of a role for matrix trimerization in HIV-1 envelope glycoprotein incorporation. Proc Natl Acad Sci U S A 113:E182–E190. doi: 10.1073/pnas.1516618113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alfadhli A, Barklis RL, Barklis E. 2009. HIV-1 matrix organizes as a hexamer of trimers on membranes containing phosphatidylinositol-(4,5)-bisphosphate. Virology 387:466–472. doi: 10.1016/j.virol.2009.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson MC. 2011. Mechanisms for Env glycoprotein acquisition by retroviruses. AIDS Res Hum Retroviruses 27:239–247. doi: 10.1089/AID.2010.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami T, Freed EO. 2000. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and α-helix 2 of the gp41 cytoplasmic tail. J Virol 74:3548–3554. doi: 10.1128/jvi.74.8.3548-3554.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandano L, Stevenson M. 2012. A highly conserved residue in the C-terminal helix of HIV-1 matrix is required for envelope incorporation into virus particles. J Virol 86:2347–2359. doi: 10.1128/JVI.06047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen LA, Honda A, Varnai P, Brown FD, Balla T, Donaldson JG. 2007. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol Biol Cell 18:2244–2253. doi: 10.1091/mbc.e06-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freed EO, Martin MA. 1995. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J Virol 69:1984–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YM, Tang XB, Cimakasky LM, Hildreth JE, Yu XF. 1997. Mutations in the matrix protein of human immunodeficiency virus type 1 inhibit surface expression and virion incorporation of viral envelope glycoproteins in CD4+ T lymphocytes. J Virol 71:1443–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mammano F, Kondo E, Sodroski J, Bukovsky A, Göttlinger HG. 1995. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J Virol 69:3824–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ono A, Huang M, Freed EO. 1997. Characterization of human immunodeficiency virus type 1 matrix revertants: effects on virus assembly, Gag processing, and Env incorporation into virions. J Virol 71:4409–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tedbury PR, Mercredi PY, Gaines CR, Summers MF, Freed EO. 2015. Elucidating the mechanism by which compensatory mutations rescue an HIV-1 matrix mutant defective for Gag membrane targeting and envelope glycoprotein incorporation. J Mol Biol 427:1413–1427. doi: 10.1016/j.jmb.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tedbury PR, Ablan SD, Freed EO. 2013. Global rescue of defects in HIV-1 envelope glycoprotein incorporation: implications for matrix structure. PLoS Pathog 9:e1003739. doi: 10.1371/journal.ppat.1003739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freed EO, Martin MA. 1996. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J Virol 70:341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyma DJ, Jiang J, Shi J, Zhou J, Lineberger JE, Miller MD, Aiken C. 2004. Coupling of human immunodeficiency virus type 1 fusion to virion maturation: a novel role of the gp41 cytoplasmic tail. J Virol 78:3429–3435. doi: 10.1128/jvi.78.7.3429-3435.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murakami T, Ablan S, Freed EO, Tanaka Y. 2004. Regulation of human immunodeficiency virus type 1 Env-mediated membrane fusion by viral protease activity. J Virol 78:1026–1031. doi: 10.1128/jvi.78.2.1026-1031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson LE, Sowder R, Copeland TD, Smythers G, Oroszlan S. 1984. Quantitative separation of murine leukemia virus proteins by reversed-phase high-pressure liquid chromatography reveals newly described gag and env cleavage products. J Virol 52:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brody BA, Rhee SS, Sommerfelt MA, Hunter E. 1992. A viral protease-mediated cleavage of the transmembrane glycoprotein of Mason-Pfizer monkey virus can be suppressed by mutations within the matrix protein. Proc Natl Acad Sci U S A 89:3443–3447. doi: 10.1073/pnas.89.8.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice NR, Henderson LE, Sowder RC, Copeland TD, Oroszlan S, Edwards JF. 1990. Synthesis and processing of the transmembrane envelope protein of equine infectious anemia virus. J Virol 64:3770–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waheed AA, Ablan SD, Roser JD, Sowder RC, Schaffner CP, Chertova E, Freed EO. 2007. HIV-1 escape from the entry-inhibiting effects of a cholesterol-binding compound via cleavage of gp41 by the viral protease. Proc Natl Acad Sci U S A 104:8467–8471. doi: 10.1073/pnas.0701443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiernan RE, Freed EO. 1998. Cleavage of the murine leukemia virus transmembrane env protein by human immunodeficiency virus type 1 protease: transdominant inhibition by matrix mutations. J Virol 72:9621–9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novikova M, Adams LJ, Fontana J, Gres AT, Balasubramaniam M, Winkler DC, Kudchodkar SB, Soheilian F, Sarafianos SG, Steven AC, Freed EO. 2018. Identification of a structural element in HIV-1 Gag required for virus particle assembly and maturation. mBio 9:e01567-18. doi: 10.1128/mBio.01567-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murakami T, Freed EO. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc Natl Acad Sci U S A 97:343–348. doi: 10.1073/pnas.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joshi A, Ablan SD, Soheilian F, Nagashima K, Freed EO. 2009. Evidence that productive human immunodeficiency virus type 1 assembly can occur in an intracellular compartment. J Virol 83:5375–5387. doi: 10.1128/JVI.00109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alfadhli A, Staubus AO, Tedbury PR, Novikova M, Freed EO, Barklis E. 2 August 2019. Analysis of HIV-1 matrix-envelope cytoplasmic tail interactions. J Virol. doi: 10.1128/JVI.01079-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alfadhli A, Mack A, Ritchie C, Cylinder I, Harper L, Tedbury PR, Freed EO, Barklis E. 2016. Trimer enhancement mutation effects on HIV-1 matrix protein binding activities. J Virol 90:5657–5664. doi: 10.1128/JVI.00509-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol 74:8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 72:2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Platt EJ, Bilska M, Kozak SL, Kabat D, Montefiori DC. 2009. Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J Virol 83:8289–8292. doi: 10.1128/JVI.00709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeuchi Y, McClure MO, Pizzato M. 2008. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J Virol 82:12585–12588. doi: 10.1128/JVI.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46:1896–1905. doi: 10.1128/aac.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purtscher M, Trkola A, Gruber G, Buchacher A, Predl R, Steindl F, Tauer C, Berger R, Barrett N, Jungbauer A, Katinger H. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses 10:1651–1658. doi: 10.1089/aid.1994.10.1651. [DOI] [PubMed] [Google Scholar]
- 51.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao F, Scearce RM, Alam SM, Hora B, Xia S, Hohm JE, Parks RJ, Ogburn DF, Tomaras GD, Park E, Lomas WE, Maino VC, Fiscus SA, Cohen MS, Moody MA, Hahn BH, Korber BT, Liao H, Haynes BF. 2009. Cross-reactive monoclonal antibodies to multiple HIV-1 subtype and SIVcpz envelope glycoproteins. Virology 394:91–98. doi: 10.1016/j.virol.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 59:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freed EO, Delwart EL, Buchschacher GL, Panganiban AT. 1992. A mutation in the human immunodeficiency virus type 1 transmembrane glycoprotein gp41 dominantly interferes with fusion and infectivity. Proc Natl Acad Sci U S A 89:70–74. doi: 10.1073/pnas.89.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landau NR, Page KA, Littman DR. 1991. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J Virol 65:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freed EO, Orenstein JM, Buckler-White AJ, Martin MA. 1994. Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J Virol 68:5311–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willey RL, Smith DH, Lasky LA, Theodore TS, Earl PL, Moss B, Capon DJ, Martin MA. 1988. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol 62:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sobolev V, Eyal E, Gerzon S, Potapov V, Babor M, Prilusky J, Edelman M. 2005. SPACE: a suite of tools for protein structure prediction and analysis based on complementarity and environment. Nucleic Acids Res 33:W39–W43. doi: 10.1093/nar/gki398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sobolev V, Sorokine A, Prilusky J, Abola E, Edelman M. 1999. Automated analysis of interatomic contacts in proteins. Bioinformatics 15:327–332. doi: 10.1093/bioinformatics/15.4.327. [DOI] [PubMed] [Google Scholar]
- 60.Vehlow C, Stehr H, Winkelmann M, Duarte JM, Petzold L, Dinse J, Lappe M. 2011. CMView: interactive contact map visualization and analysis. Bioinformatics 27:1573–1574. doi: 10.1093/bioinformatics/btr163. [DOI] [PubMed] [Google Scholar]
- 61.Gipson B, Zeng X, Zhang ZY, Stahlberg H. 2007. 2dx-User-friendly image processing for 2D crystals. J Struct Biol 157:64–72. doi: 10.1016/j.jsb.2006.07.020. [DOI] [PubMed] [Google Scholar]