We recently developed and published a description of a barcoded simian immunodeficiency virus that has a short random sequence inserted directly into the viral genome. This allows for the tracking of individual viral lineages with high fidelity and ultradeep sensitivity. This virus was used to infect 120 rhesus macaques, and we report here the analysis of the barcodes of these animals during primary infection. We found that the vast majority of barcodes were functional in vivo. We then expanded the barcoding approach in a second-generation SIVmac239 stock (SIVmac239M2) with over 16 times the number of barcoded variants of the original stock and a barcoded stock of SIVmac239Opt5M whose sequence had 5 changes from the wild-type SIVmac239 sequence. We also generated 4 barcoded stocks from subtype B and C SHIV clones each containing a human immunodeficiency virus (HIV) type 1 envelope. These virus models are functional and can be useful for studying viral transmission and HIV cure/reservoir research.
KEYWORDS: nonhuman primates, viral models, simian immunodeficiency virus, barcoded virus
ABSTRACT
Genetically barcoded viral populations are powerful tools for evaluating the overall viral population structure as well as assessing the dynamics and evolution of individual lineages in vivo over time. Barcoded viruses are generated by inserting a small, genetically unique tag into the viral genome, which is retained in progeny virus. We recently reported barcoding the well-characterized molecular clone simian immunodeficiency virus (SIV) SIVmac239, resulting in a synthetic swarm (SIVmac239M) containing approximately 10,000 distinct viral clonotypes for which all genetic differences were within a 34-base barcode that could be tracked using next-generation deep sequencing. Here, we assessed the population size, distribution, and authenticity of individual viral clonotypes within this synthetic swarm using samples from 120 rhesus macaques infected intravenously. The number of replicating barcodes in plasma correlated with the infectious inoculum dose, and the primary viral growth rate was similar in all infected animals regardless of the inoculum size. Overall, 97% of detectable clonotypes in the viral stock were identified in the plasma of at least one infected animal. Additionally, we prepared a second-generation barcoded SIVmac239 stock (SIVmac239M2) with over 16 times the number of barcoded variants of the original stock and an additional barcoded stock with suboptimal nucleotides corrected (SIVmac239Opt5M). We also generated four barcoded stocks from subtype B and C simian-human immunodeficiency virus (SHIV) clones. These new SHIV clones may be particularly valuable models to evaluate Env-targeting approaches to study viral transmission or viral reservoir clearance. Overall, this work further establishes the reliability of the barcoded virus approach and highlights the feasibility of adapting this technique to other viral clones.
IMPORTANCE We recently developed and published a description of a barcoded simian immunodeficiency virus that has a short random sequence inserted directly into the viral genome. This allows for the tracking of individual viral lineages with high fidelity and ultradeep sensitivity. This virus was used to infect 120 rhesus macaques, and we report here the analysis of the barcodes of these animals during primary infection. We found that the vast majority of barcodes were functional in vivo. We then expanded the barcoding approach in a second-generation SIVmac239 stock (SIVmac239M2) with over 16 times the number of barcoded variants of the original stock and a barcoded stock of SIVmac239Opt5M whose sequence had 5 changes from the wild-type SIVmac239 sequence. We also generated 4 barcoded stocks from subtype B and C SHIV clones each containing a human immunodeficiency virus (HIV) type 1 envelope. These virus models are functional and can be useful for studying viral transmission and HIV cure/reservoir research.
INTRODUCTION
Nonhuman primates (NHPs) infected with human immunodeficiency virus (SIV) or chimeric SIV-human immunodeficiency virus (SHIV) clones represent the best-established animal model of HIV disease, with its features including transmission, high-titer viral replication, CD4+ T cell depletion, and progressive immunodeficiency eventually leading to AIDS (1–8). The SIVmac239 clone was isolated and cloned 30 years ago (9). It has been used continually since then and represents the quintessential viral clone for NHP models of AIDS (10–14). We have expanded the usefulness of this molecular clone by introducing specific synonymous mutations into individual SIVmac239 clones, producing a synthetic swarm of virus containing 10 genetically distinct viral variants (15–18). This clone (SIVmac239X) was specifically developed for viral transmission studies to precisely and deeply discriminate individual transmitted/founder (T/F) genomes. We recently expanded this original molecularly tagged model with the development of SIVmac239M, a synthetic viral swarm derived from SIVmac239 with ∼10,000 individual lineages that are genetically identical, except for a random, 34-base barcode insert between the vpx and vpr genes of the viral genome (19). The 34-base barcode consists of a random 10-base stretch flanked on each side by 12 complementary bases. These barcodes allow the discrimination of viral lineages and the tracking of infection events by distinct lineages.
Since the barcode is small, it is infrequently discarded by the virus during replication and can be deeply sequenced using next-generation sequencing (NGS) methods, which provide an accurate estimate of the relative proportions of each barcode with a high sensitivity (19). This approach allows for an ultradeep assessment of the viral population that would be impossible if discrimination between lineages was reliant only on the natural variation within a viral swarm. Applications of barcoded viruses include tracking of the distinct viral variants (barcode clonotypes) that are involved in the initial establishment and dissemination of infection, as well as tracking of the variants that contribute to persistence during treatment and recrudescence when treatment is discontinued (19, 20). In that study, the barcode proportion was used to estimate the rate of reactivation from latency, providing a means to accurately assess changes in reservoir size that lead to differences in measured reactivation rates.
Despite the advantages of using SIV to study AIDS virus infection in macaques, the differences in viral structure and target specificity between SIV and human immunodeficiency virus (HIV) can often limit the direct applicability of the findings obtained with nonhuman primates to HIV-infected humans in some areas (7, 21–31). For instance, there are significant differences in the average antibody neutralization profiles between HIV and SIVmac239, which is particularly resistant to neutralization (32). Furthermore, there are sufficient differences between HIV and SIV Envs that evaluation of Env-specific interventions requires generating SIV-specific reagents rather than directly testing HIV Env-targeting therapies in macaques (33–35). Since HIV cannot elicit a sustained productive infection in rhesus macaques (36, 37), there is ongoing significant interest in developing chimeric simian-human immunodeficiency viruses (SHIVs) that contain an HIV type 1 (HIV-1) Env within an SIV backbone.
Recently, there have been efforts to generate SHIVs that contain transmitted/founder (T/F) HIV-1 Envs from viral genomes responsible for establishing clinically relevant HIV infection in humans, rather than using viruses derived by extensive passage in macaques (38–40). We and others have developed several unadapted T/F SHIVs containing a variety of patient-derived HIV-1 Env genes that are capable of replicating in macaques and that show neutralization sensitivities comparable to those of HIV-1 bearing the same envelope (38, 40). In vivo passage-derived clones or site-directed mutations at positions 281 and 375 of the Env gene are also used to increase the binding of HIV Env to rhesus macaque CD4, augmenting viral replication in macaque cells (4, 8).
Adding a barcode to these SHIVs would provide significant resolution of the dynamics of neutralization and transmission and could be used in cure research testing anti-HIV envelope interventions. Four SHIVs were selected for barcoding and included three clones containing either native or minimally modified T/F HIV-1 envelope genes, along with the well-characterized and pathogenic SHIVAD8EO clone (5, 27, 41). Subtype B SHIV1054 contains an unmodified clade B T/F HIV-1 env within an SIVmac239 backbone (38). The two subtype C clones SHIV174 and SHIV224 contain modified T/F HIV-1 env within an SIVmac766 backbone (40).
Additionally, we generated new barcoded stocks of wild-type SIVmac239 (designated SIVmac239M2) that contains over 16-fold more variants than our original SIVmac239M stock. We also barcoded a version of SIVmac239 (SIVmac239Opt5M) whose sequence contains 5 nucleotide changes from the sequence of the original clone to correct the suboptimal nucleotides generated during the original cloning of wild-type SIVmac239 (42). These newly generated barcoded stocks have population distributions similar to the distribution of SIVmac239M, and each stock contains viruses with thousands of unique barcodes.
Characterizing a newly developed barcoded virus stock involves quantifying the number of distinct barcode lineages in the stock and assessing how many of them are replication competent in vivo. Here we used the data obtained from a comprehensive analysis of all barcodes detected in 120 animals infected with SIVmac239M to develop and refine our predictive and analytical tools to accurately determine the number and replication competence of barcodes in a given stock. The results obtained with multiple different barcoded SIV and SHIV stocks demonstrate that this approach is broadly adaptable to other viral clones of interest. We anticipate that these virus stocks will be useful in studies involving transmission, viral evolution, and reservoir/cure research.
RESULTS
Effect of inoculum dose on acute infection dynamics with SIVmac239M.
One hundred twenty macaques were intravenously infected with one of six different infectious doses of SIVmac239M: 100, 200, 500, 2,200, 10,000, or 220,000 infectious units (IU). Viral RNA was quantified and sequenced from plasma during primary infection to identify the barcodes actively replicating in vivo. We first examined the viral growth curves during primary infection in a subset of animals (n = 72) with sufficiently frequent sampling to allow accurate assessments of the viral load dynamics (Fig. 1A). Animals infected with a higher dose typically displayed an earlier peak viral load, while animals infected with lower doses typically reached peak viremia later. While the time to detectable viremia decreased with increasing infectious dose, the viral growth rate during ramp-up viremia was highly consistent between animals regardless of the inoculum dose (average overall growth rate, 1.7 day−1; analysis of variance [ANOVA], P = 0.9) (Fig. 1B).
FIG 1.
Viral load and barcode assessment in vivo. One hundred twenty rhesus macaques were intravenously infected with one of six different doses of SIVmac239M. (A) The average plasma viral loads from the animals were plotted, with the standard error being shown. The limit of detection was 15 viral RNA (vRNA) copies/ml. (B) The calculated growth rates for animals with at least two viral load measurements during ramp-up showed no difference between groups (ANOVA, P = 0.9). (C) Sequence analysis of viral RNA from plasma obtained within 2 weeks of infection revealed that the number of detectable barcodes was dose dependent. (D) The inoculating dose also shaped the distribution of barcodes within each animal, as assessed by plotting the most abundant barcode as a percentage of the total.
We next assessed the relationship between the number of detectable barcodes at peak viremia and the challenge dose across all 120 infected animals (Fig. 1C). As expected, the number of detectable barcodes increased with the challenge dose. The average number of detectable barcodes at peak viremia was 12 at the 100-IU dose, 29 at the 200-IU dose, 68 at the 500-IU dose, 308 at the 2,200-IU dose, 698 at the 10,000-IU dose, and 2,439 at the 220,000-IU dose. Although there was variability in the number of detectable barcodes between animals, the average number of detectable barcodes was ∼7-fold less than the input dose for animals infected with a dose of 2,200 IU dose or lower. In animals challenged with a higher dose (10,000 IU and 220,000 IU), the difference between the measured input dose and the number of detectable barcodes increased to 14- and 90-fold, respectively, as the dose equaled and then exceeded the number of barcodes within the stock.
An increase in the infectious dose was also associated with a decrease in the relative proportion of the most prevalent barcode (Fig. 1D). The results from animals inoculated with 200 IU, for example, indicated that a single barcode variant could account for anywhere from 20 to 90% of the viral population during ramp-up viremia. Although the dominance of the population by a single barcode variant does not preclude the usefulness of a low-dose input, it is important to acknowledge that the fewer evenly distributed individual barcodes that there are in a population, the more challenging it is to discriminate between unique viral events. Importantly, across the entire study, the least prevalent barcode was frequently found near the limit of detection, suggesting that the number of detectable variants is a minimum estimate of the barcodes found in each animal. This effect is more pronounced in the animals receiving a higher dose (2,200, 10,000, or 220,000 IU), where the amount of the least-abundant detectable clone in each animal was within 3% of the limit of detection. Overall, these data demonstrate that altering the infectious dose affects the time to detection, the number of detectable barcodes, and the distribution of barcodes in a predictable manner without altering the viral growth rate.
Identifying replication-competent barcodes.
Previously, the distribution of barcodes found in the SIVmac239M stock was defined based on the number of replicate runs in which a barcode was detected following low-template sequencing (19). Although this low-template sequencing approach allowed for the clear identification of all possible barcodes, the measured frequency of each barcode was limited to the number of replicates in which each barcode was found, rather than the relative frequency compared to that of all other barcodes in the stock. To better characterize the distribution of each barcode within the virus stock, high-template PCR and next-generation sequencing were performed by analyzing 5 × 105 cDNA templates in the high-template PCR. For the SIVmac239M stock, using this approach we identified 9,531 barcodes with proportional frequencies ranging from 5 × 10−3 to 5 × 10−7. The proportion of each barcode identified was then compared to the number of replicates in which each barcode was found using the low-template approach (Fig. 2). For viral barcodes found in only a few low-template replicates, the measured frequencies in the distribution were variable, with the amounts of 1,233 barcodes falling below the limit of detection for the individual assay, though these may still represent authentic but extremely low-frequency barcodes. Together, this comparison provides evidence that the use of both approaches (the high-replicate/low-template approach to define all possible barcodes and the high-template PCR to define the relative proportion of each barcode) provides a useful assessment of the viral barcode population structure.
FIG 2.
Evaluation of clonotypes found in the SIVmac239M stock. The relative proportion of each barcode from the high-template PCR (n = 9,531 barcodes) (y axis) is plotted against the number of replicate aliquots in which that barcode was found using the multiple, low-template PCR assay (x axis). The density of the barcodes for any single point is colored using a log-scale heat map. There were 1,233 additional barcodes that were identified in the low-template analysis but that were present at levels below the detection limit (red line) in the high-template assay.
Using the results from the high-template run, we then compared the barcodes identified in the SIVmac239M stock to those found replicating in 120 macaques during primary infection following intravenous infection. Of the 9,531 barcodes within the detectable, high-template distribution of the stock (Fig. 3A), 8,757 (92%) were detected as viral RNA in animals during acute infection (Fig. 3B). Additionally, of the 1,233 barcodes detected only in the low-template sequencing, 57 were found in animals (Fig. 3B). To determine which barcodes were potentially not found in animals due to a barcode that impacted the viral replication capacity, we used a simulation based on the distribution of barcodes in the stock to generate a theoretical distribution of barcodes that should have been identified, given the number of animals sampled and the number of detectable barcodes found in each animal (Fig. 3C). The difference between the simulation and the experimental data was 259 barcodes (3.0% of the total barcodes) that were detectable in the simulation but that were not found in animals. These barcodes likely represent the few nonfunctional clones that cannot replicate equivalently to other barcoded variants but that, theoretically, should have been inoculated into at least 1 of the 120 animals. Overall, these data demonstrate that the vast majority of clones in the SIVmac239M stock are replication competent in vivo and that the barcodes found within the animals are drawn from this distribution, with a limited preference for or selection against viruses containing particular barcodes.
FIG 3.
Comparing the SIVmac239M stock with the barcodes identified in vivo. (A) Histogram depicting the number and relative proportions of barcodes found in the SIVmac239M stock, measured using the high-template PCR (n = 9,531). (B) The barcodes that were found in all 120 animals (n = 8,757) are overlaid on the viral stock distribution, such that the visible red bar graphs represent barcodes found in the stock but not in the animals. (C) A simulation of the barcodes that would theoretically be detected in any of the 120 infected animals was performed, and the results are overlaid with the barcodes that were experimentally found in all 120 animals, such that the visible orange bar graphs represent barcodes that were found in the simulation but that were not found in animals (n = 259). The limit of detection for the high-template distribution was −6.5 log10. Sequences not detected (ND) in the stock following high-template sequencing are indicated.
Because the usefulness of the barcoded virus model is dependent upon an accurate assessment of the relative proportion of each barcode, we sought to confirm the reproducibility of our next-generation sequencing approach. In our approach, the Illumina adaptors are synthesized directly with the primers used in the PCR, allowing for direct sequencing without additional manipulation post-PCR. To assess the accuracy of barcode detection and quantification in animals, the viruses in plasma samples with high viremia from 2 animals (animals M295 and V184) infected with 10,000 IU of SIVmac239M were sequenced following 10 independent PCRs, each of which included 5 × 105 cDNA templates per reaction mixture. The mean proportion and standard error for each barcode were determined (Fig. 4). For animal M295, we found a total of 1,154 barcodes across all 10 replicates, with an average of 1,017 barcodes per replicate (Fig. 4A). For animal V184, there were 561 barcodes detected in total, with the mean being 504 barcodes per replicate (Fig. 4B). The barcodes that were not detected in all 10 replicates were all found within 1 log of the limit of detection in both animals. These results indicate that a single sequencing run captured, on average, 88% (M295) or 90% (V184) of the barcodes found in all 10 replicates. For infrequent barcodes near the limit of detection, the probability of detecting each barcode was reduced and limited by the sample input and the underlying population distribution. Next, plasma from M295 was evaluated using the Primer-ID approach to genetically tag individual cDNA molecules prior to PCR, as previously described (43). This approach has been shown to accurately assess template proportions without PCR-biased amplification. Results from three replicate Primer-ID assays were compared to those for the 10 replicates described above. Using our NGS approach, a total of 801 different barcodes were observed in all 10 replicates analyzed; of these, 105 barcodes appeared in all 3 replicates analyzed using the Primer-ID approach (Fig. 4C). While the use of the Primer-ID approach reduced the depth of coverage, the average barcode proportion was highly correlated between the Primer-ID approach and our standard MiSeq analyses (Pearson’s product moment correlation rho = 0.91). Together, these data confirm that barcode proportionality is not biased during PCR and that it can be accurately assessed using a single high-template PCR and sequencing run.
FIG 4.

Accuracy and reproducibility of single high-template PCR sequencing. Viral RNA was extracted from plasma from two animals, animals V184 (A) and M295 (B), with high viremia and subjected to high-template PCR and sequencing using 10 independent replicates. The mean log10 barcode proportion was plotted for each detectable barcode (x axis, black circles), with the 95% confidence interval of each barcode being plotted on the y axis (cyan and magenta lines). A single sequencing run captured, on average, 90% (V184) or 88% (M295) of the barcodes found in all 10 replicates. (C) To compare the Primer-ID approach and our NGS approach, the mean proportion (log10) of M295 plasma barcodes was plotted (x axis) against the entire range (log10) of each barcode appearing in all 10 MiSeq sequencing replicates (gray line). Superimposed are the mean (red dot) and range (red line) of each barcode appearing in all Primer-ID replicates (y axis).
Generation of barcoded SIV and SHIV stocks.
Although the initially produced SIVmac239M stock contained nearly 10,000 unique barcodes, there are experimental scenarios in which higher numbers of barcoded viruses would be useful. Therefore, we generated a new stock of barcoded wild-type SIVmac239 (SIVmac239M2) (Fig. 5A) containing a much larger number of unique barcodes. We also produced an SIVmac239Opt5M stock (Fig. 5A) in which the T860C mutation in the primer binding site, the C3465T (reverse transcriptase S205L) and C4689T (integrase A54V) mutations in Pol, and the A8855G mutation in Env/Tat/Rev (Rev K40R and Env R751G) were corrected to the previously determined optimal base, as reported earlier (44). We also introduced a G6802A mutation in Env (V67M) that we found consistently reverts naturally in vivo. The four SHIVs selected for barcoding contained HIV-1 envelopes in an SIV backbone and included a native T/F HIV-1 Env (SHIV1054) (38), the well-characterized, pathogenic SHIVAD8EO clone (41) (Fig. 5B), and two subtype C clones minimally modified to enhance rhesus CD4 binding (SHIV174 and SHIV224) (40) (Fig. 5C).
FIG 5.
Schematic summary of each barcoded SIV and SHIV. (A) The SIVmac239M2 and SIVmac239Opt5M (red) viral stocks were produced in a manner identical to that used for the original generation of SIVmac239M. PBS, phosphate-buffered saline. (B) SHIV1054 was generated in the SIVmac239 backbone, while the barcode was inserted directly into the SHIVAD8EOM clone. (C) The two subtype C clones were generated in the SIVmac766 backbone using the indicated restriction sites. The TZM-bl cell titer for each viral stock is shown. For the SHIV clones, the amino acids found at positions 281 and 375 of Env are shown. (D) The replication in primary rhesus CD4+ T cells of each barcoded virus is shown. Culture supernatants were collected over 14 days, with medium replacement occurring at each collection time point to maintain the cultures in a 2-ml total volume. The viral p27 Gag protein was quantified by ELISA.
SIV and SHIV genomes were successfully barcoded using either MluI or AgeI as the insert restriction site, which allowed for the introduction of the 34-nucleotide barcode dimer between the vpx and vpr genes of the SIV backbone (Fig. 5A to C). The flanking region of the insert is distinct in some clones to allow for discrimination between SHIVs by sequencing only the barcoded region. Viral stocks were generated by transfection, and each barcoded stock was designated by the addition of the letter M to the clone name (e.g., SHIV1054M). Each barcoded SHIV stock was infectious, based on the findings of the TZM-bl cell reporter assay, with infectivity titers ranging from 1.0 × 104 to 1.6 × 105 IU/ml (Fig. 5A to C). The replication of the barcoded viral stocks was confirmed in vitro using rhesus CD4+ T cells (Fig. 5D).
Characterization of barcoded SIV and SHIV stocks.
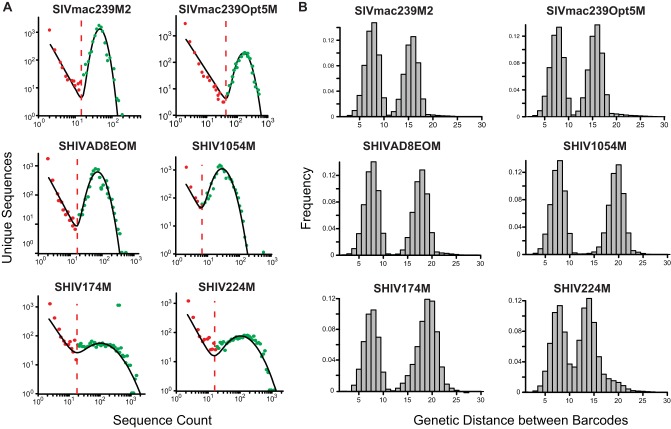
To characterize the barcodes in each stock, viral RNA was extracted from each stock and cDNA was synthesized, PCR amplified, and sequenced using the low-template approach (5,000 input templates) with 80 replicates per sample. The resulting sequences from each replicate sequencing run were analyzed independently using a mixed-model distribution to differentiate authentic barcodes from PCR-generated erroneous barcodes as described previously (19). The model was fitted to every replicate sequencing run using the mle function in R (v3.3.3), and an appropriate cutoff was used to separate sequences (Fig. 6A). Using this approach, we detected between 2,788 and 145,528 barcodes per stock.
FIG 6.
Sequence distributions and genetic distance between barcodes in new stocks. (A) A mixed-model distribution (black line) was fitted to each of the 80 low-template sequence data replicates, after which the minimum between distributions was used to designate a cutoff (dashed lines). Sequences identified to the left of the cutoff (red) were considered PCR-generated errors and discarded, while sequences to the right of the cutoff (green) were retained. Representative plots are shown for the 80 replicates analyzed. (B) For each new stock, the Hamming distance between each pair of barcodes was plotted. The median distance between barcodes in the same orientation was 8 nucleotides for each SHIV stock. The second peak, which had Hamming distances ranging from 15 to 20 nucleotides, arose from a comparison of barcodes in opposite orientations.
The genetic distance between each barcode was then determined for all 9 barcoded stocks. Because the barcode insert can ligate in either direction, we calculated the genetic distance between all barcodes inserted in the same orientation. We found that for each virus stock, the median genetic distance between any two barcodes was 8 nucleotides, with an average of 7.57 nucleotides (range, 7.46 to 7.76 nucleotides) (Fig. 6B). These distributions are consistent with the findings for the original SIVmac239M stock, which has a median of 8 bases and a mean of 7.5 bases between barcodes (19). Together, these data confirm that most viral barcodes are sufficiently genetically distinct to allow for the unequivocal identification of individual barcodes using our sequencing approach.
To determine the distribution of the identified barcodes in each new viral stock, we performed high-template sequencing utilizing 5 × 105 input cDNA copies per reaction mixture. The overall proportional distributions were similar to the distribution for SIVmac239M, with variation being seen only in the number of detectable viral barcodes (Fig. 7). As was seen with SIVmac239M, the total number of barcodes detected with the low-template/high-replicate approach identified more barcodes than the high-template sequencing approach, which detected an average of 85.7% of the low-input barcodes across all 6 viral stocks, a value similar to the 88.5% detected in SIVmac239M (Fig. 3). Overall, based on the results obtained from the extensive in vivo analyses performed with SIVmac239M and on the comparable barcode distributions observed between the SIVmac239M stock and our 6 new stocks, we anticipate that with the new barcoded viruses, virions establishing infection will be drawn randomly from the stock distribution, with the resultant number of detectable genomes in vivo correlating with the challenge dose and with a minimal overrepresentation of any given barcode occurring.
FIG 7.
Distribution of all barcodes in each SHIV stock. Histograms depicting the number of barcodes found in each viral stock, based on the relative proportion following high-template PCR and sequencing, are shown. Sequences not detected (ND) in the high-template sequences are indicated. The number of barcodes in each group is shown.
One key advantage to generating viral stocks with a high barcode count is that individual founder genomes can be accurately assessed even with a large viral challenge dose. Limiting the number of founders to those with single, unique barcodes could provide insight into the sites of initial viral replication and the dynamics of early replication in different tissues. Using a simulation of a set number of infection events drawn randomly from the barcode frequency distribution of SIVmac239M, we determined how many individual infection events would originate from a unique founder barcode and how frequently each of duplicate barcodes could cause infection events (Fig. 8). As expected, the number and overall distribution of barcodes in the stock determined how frequently barcodes were unique or duplicated for any given number of infection events. For SIVmac239M, at 100 infection events, on average, one barcode would initiate two infection events. At 500 infection events, on average, 23 barcodes would initiate two separate events and 1 barcode would initiate three distinct events. For SIVmac239M2, which contained the greatest number of unique barcodes, infection with 100 or 200 events would each originate from a unique barcode, but at 500 infection events, on average, a single barcode would infect more than one target cell initially. Remarkably for this stock, even at 2,000 infection events, only 1% (on average, 23 barcodes) would originate from duplicate founders (Fig. 8). These data provide the framework for the strategic application of the barcoded virus model system to track the dynamics of individual founder lineages throughout the course of infection with unprecedented sensitivity and accuracy.
FIG 8.
Simulation of the number of unique founder barcodes. The distributions of the SIVmac239M and SIVmac239M2 stocks were used to assess the proportion of genomes predicted to initiate infection with a unique barcoded founder or with multiple founders of the same barcode. The average proportion following 100 simulations is plotted for each single or multiple founder genome at 100, 200, 500, and 2,000 theoretical infection events. The dashed lines equal 1/total number of genomes.
DISCUSSION
Barcoding of viruses via a small, random-sequence, genetic insert has provided new opportunities for assessing the dynamics of individual viral lineages within a population. This approach is useful for studying viral dynamics during primary infection, immune evasion, and suppressive therapy and subsequent viral rebound following analytical treatment interruption (ATI). In our previous publication, assessment of the barcoded viruses that contribute to post-ATI rebound viremia provided insight into both the number and the rate of independent recrudescence events that lead from latency to productive infection (19), information which may be invaluable to the field of HIV cure research. Here, we sought to expand our validation of the barcoded virus approach by examining the viral dynamics of individual barcode clonotypes during primary infection. To this end, 120 rhesus macaques were infected intravenously with various doses of SIVmac239M. We correlated the infectious dose with the time to viremia, the number of clonotypes detected, and the distribution of barcodes in plasma. Unsurprisingly, a lower infectious dose corresponded to the detection of fewer barcode variants and an increase in the relative contribution of some individual barcode variants to the plasma population. This has strong implications for using the barcoded virus in models of viral dynamics in general and viral recrudescence in particular, since a highly prevalent barcode during acute infection can skew the population structure and mask multiple reactivation events from latent viruses with the same prevalent barcode. Thus, the infectious dose is an essential parameter to consider in order to ensure that sufficient diversity can be both achieved and measured.
Importantly, the viral growth rate during primary infection, as assessed by the slope of the increase in plasma viremia, was equivalent regardless of the infectious dose, suggesting that although the dose affects the number of initially infected cells, exponential viral replication quickly produces comparable growth rates. At extremely low doses, the growth of individual viral lineages may vary widely as initial growth is established, but once growth is established, plasma viral growth rates are consistent across groups. Following a high-infectious-dose challenge, the variation in the relative proportion of different individual clonotype lineages is reduced, as measured by the percentage of the most frequent barcode. In these animals receiving a high dose, although early stochastic events were still present, multiple copies of each viral lineage were present, thereby increasing the probability that at least one founder of each clonotype would be able to initiate immediate exponential growth. Therefore, by assessing the viral dynamics of primary infection using a barcoded virus, we can conclude that heterogeneity between individual clonotypes is due to stochastic events during early viral growth which affect the overall population structure.
In order to have a clear understanding of the functionality of the barcoded viruses in our stock, it was essential to determine what proportion of the stock variants could actively replicate in vivo. Upon analyzing the barcode variants detected in all 120 infected animals, we found that the majority of barcodes identified in the stock were replication competent and detectable in vivo. Based on a computational simulation, there were only 259 barcodes that were of sufficiently high proportion in the stock distribution that they theoretically should have initiated infection but that were not identified in any of the 120 infected animals. These clones are likely either nonfunctional or may display a diminished replicative capacity due to the particular barcode insert. However, because the overwhelming majority of the barcoded variants in the stock could be detected in vivo, we conclude that, overall, the barcode insert does not significantly impact replicative fitness. Given how compact that lentivirus genomes are, it is likely that the barcode is negatively selected over time. Our previous work (19) suggests that selection against the barcode is negligible over the course of several months of active viral replication. Overall, these data demonstrate that the accurate characterization of any viral stock should be predictive of the number of detectable, replicating barcodes in animals infected with a given dose without the need to infect substantial numbers of animals.
Because the relative proportion of viral variants provides such crucial information on the dynamics of the viral population, we sought to confirm that the proportion of viral barcodes detected by deep sequencing accurately represents the viral population of the sample and can be consistently reproduced. We therefore tested in 10 independent PCR replicates viruses from the plasma of two infected macaques specifically to assess the reproducibility of the measured barcode proportions. We found high levels of concordance in both the total number of barcodes detected per run and their relative proportion across 4 logs of the viral population. When the Primer-ID assay was performed as a secondary method of assessing the reproducibility of the barcode proportion, we found a high correlation between tbe Primer-ID method and our NGS method. Overall, we conclude that the high-template-based PCR minimizes the effects of early stochastic differences in amplification rates. While the samples used here contained high viral loads, care should be taken when using low-template PCR, which is likely more susceptible to early stochastic events that may alter the observed barcode proportions.
While the barcoded SIVmac239 model can be used in many NHP models of HIV disease, preclinical trials of Env-targeted interventions require HIV-1 Env as the viral target. In NHP models, this is achieved through the use of a chimeric SIV genome bearing an HIV Env (SHIV). We generated four barcoded virus stocks from clinically relevant subtype C and B HIV-1 Envs derived from both T/F viruses and a commonly used lab-adapted strain of HIV. All SHIV clones were successfully barcoded between their vpx and vpr genes, and the resulting stocks contained between 2,500 and 40,000 barcode variants, with no single variant being significantly overrepresented in any of the viral stocks. Since the development and characterization of the barcoded SHIVs were nearly identical to those of SIVmac239M, we anticipate that a limited number of barcoded lineages will be nonfunctional and that SHIV barcode variants will be relatively equivalent. These new SHIV stocks will be valuable tools to evaluate Env-targeting approaches aimed at reducing or eliminating viral reservoirs or blocking primary infection. Furthermore, given the strong dose dependence of SIVmac239M on the number of detectable barcodes, the desired number and proportion of barcodes that originate from a single founder genome can be controlled by careful consideration of the inoculating dose and the stock virus population structure. Here, we demonstrated the utility of generating and characterizing barcoded viral genomes in order to fine-tune studies seeking to define viral population dynamics and the benefit of adapting this technique for variety of SIV and SHIV strains. Finally, the methods used to develop and analyze these viral stocks are not restricted to lentiviruses and may be directly transferable to other viral clones.
MATERIALS AND METHODS
Ethics statement.
One hundred twenty Indian-origin rhesus macaques (Macaca mulatta) were included in the study. The animals were housed at the National Institutes of Health or the Oregon National Primate Research Center and cared for in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) standards in an AAALAC-accredited facility, and all procedures were performed according to protocols approved by the Institutional Animal Care and Use Committees of the National Cancer Institute (assurance number A4149-01) and the Oregon National Primate Research Center (assurance number A3304-01).
SIVmac239M infection.
One hundred twenty macaques were intravenously infected with transfection-derived SIVmac239M produced in transfected HEK-293T cells, and blood was sampled frequently during primary infection to assess plasma viremia (SIV RNA viral load) and perform barcode sequencing. The stock infectivity titer was determined using TZM-bl cells, as reported previously (19). We infected 36 animals with 2.2 × 105 IU, 26 animals with 1 × 104 IU, 4 animals with 2.2 × 103 IU, 30 animals with 5 × 102 IU, 22 animals with 2 × 102 IU, and 2 animals with 1 × 102 IU.
Animal sample collection and viral load measurement.
Whole blood was collected from sedated animals. Plasma for viral RNA quantification was prepared from blood collected in EDTA or acid citrate dextrose (ACD) Vacutainer tubes (BD). Following separation from whole blood by centrifugation, plasma aliquots were stored at −80°C. Viral RNA was measured using a quantitative real-time PCR for determination of the plasma viral load as described previously (4). The limit of detection for the assay was 15 viral RNA copies/ml.
Sequencing.
Plasma viral RNA was quantified by reverse transcription-PCR prior to sequencing on an Illumina MiSeq sequencer as previously described (19). Briefly, viral RNA from plasma or a viral stock was extracted using a QIAamp viral RNA minikit (Qiagen). cDNA was synthesized using SuperScript III reverse transcriptase (Thermo Fisher) and gene-specific primer vpr.cDNA3 (5′-CAG GTT GGC CGA TTC TGG AGT GGA TGC-3′), and the amount of cDNA template used for input normalization was determined by quantitative PCR with primers VpxF1 (5′-CTA GGG GAA GGA CAT GGG GCA GG-3′ at positions 6082 to 6101) and VprR1 (5′-CCA GAA CCT CCA CTA CCC ATT CATC-3′ at positions 6220 to 6199) and with a labeled probe (ACC TCC AGA AAA TGA AGG ACC ACA AAG GG). Prior to MiSeq sequencing, PCR was performed with a set amount of input template (up to 500,000 copies/sample) using the vpxF1 and vprR1 primers, containing an 8-nucleotide index sequence for multiplexing, a spacer (4 random nucleotides), and the Illumina adaptor sequence (P5 and P7). PCR was performed using high-fidelity Platinum Taq polymerase (Thermo Fisher) with the following reaction conditions: 94°C for 2 min and 40 cycles of 94°C for 15 s, 60°C for 1.5 min, and 68°C for 30 s, with a final extension of 68°C for 5 min. The amplified DNA was purified and quantified prior to MiSeq sequencing (Illumina). In one experiment, cDNA was genetically labeled using the cDNA primer PrimerID.cDNA (5′-GTG ACT GGA GTT CAG ACG TGT GCT CTT CCG ATC TNN NNN NNN NCA GTC CAG AAC CTC CAC TAC CCA TTC ATC-3′). PCR was then performed as described above using the VpxF1 primer and the genetic tag primer PrimerID.F (5′-GTG ACT GGA GTT CAG ACG TGT GCT C-3′). Following sequencing, only individual cDNA molecules based on unique Primer-ID sequences were used to determine the proportion of barcoded lineages.
Sequence analysis.
Sequences were demultiplexed based on exact matches to the Illumina P5 index sequence (1 of 40). All sequences for each unique index read were then aligned to the first 28 bases of the vpr gene, allowing 2 nucleotide mismatches. The 34 bases directly upstream of the start codon for vpr, corresponding to the barcode, were extracted. Since the number of template cDNA copies was quantified using quantitative reverse transcription-PCR, the limit of detection for animal samples was set at the theoretical number of sequences resulting from a single template copy (1/template input). In rare cases, two barcodes differing by only a single nucleotide were identified in an animal. Since we cannot rule out the possibility of PCR error in these cases, all minor barcode sequences (less than 0.5% of the total sequence reads) that differed by a single nucleotide from another prevalent barcode in the test samples were excluded. For multiplexed samples, index hopping was occasionally observed. In this case, barcodes were identified at a proportion of 0.2% or less in one index run and were also found in another sample as a prevalent lineage. These contaminating sequences were also excluded. Sequence analysis was performed using the barcoded analysis tool (BAT; http://bit.ly/2OsiMiB), a custom web-based tool written in R (v3.3.3) to analyze NGS data for barcoded viruses.
Simulation of barcode distribution.
A probability distribution was generated for each barcoded virus using the relative proportion of all detected barcodes in the viral stock. A simulation was conducted to determine how many theoretical draws from the viral stock were required to obtain the number of barcodes observed in each animal infected with SIVmac239M. This simulation was repeated 100 times, and the average number of draws required was compared to experimental data. A probability distribution that models the expected distribution of barcodes at a given infectious dose was generated for each barcoded viral stock.
Construction of barcoded libraries.
Plasmid libraries for SIVmac239M2 were generated directly from the pSIVmac239Nef Open clone, and the SIVmac239Opt5M library was generated from the same SIVmac239 clone, but its sequence contained 5 mutations from the wild-type sequence to correct the suboptimal nucleotides generated during the original cloning process. The mutations included a T860C mutation in the primer binding site, a C3465T mutation (reverse transcriptase S205L) and a C4689T mutation (integrase A54V) in Pol, and an A8855G mutation in Env/Tat/Rev (Rev K40R and Env R751G), as reported earlier (44). We also generated a G6802A mutation in Env (V67M) that we found also consistently reverts naturally in vivo and is likely a suboptimal nucleotide in the wild-type clone.
The generation of barcoded libraries for SIVmac239M2, SIVmac239Opt5M, SHIVAD8EO, and SHIV1054 was performed identically to the generation of SIVmac239M (19), since each clone contained the SIVmac239 backbone (5, 27, 38, 41). Briefly, an MluI restriction site was inserted between the vpx and vpr genes of each plasmid, which was then digested with MluI and ligated with 34-base dimerized oligonucleotides comprised of a stretch of 10 random bases flanked on either side by complementary bases designed to ensure primer dimerization. These constructs were digested again with MluI prior to transformation into Stbl4 cells (Thermo Fisher) to limit the production of plasmid clones without a barcode insert. Bacteria were grown in 1-liter cultures, and plasmid libraries were isolated by CsCl purification.
To generate barcoded plasmid libraries for SHIVCAP174 and SHIVCAP224, which are cloned into the SIVmac766 backbone (4, 40), primers were designed to insert an AgeI instead of an MluI restriction site between vpx and vpr. Barcode insertion was performed as described above, using unique flanking sequences around the 10-base barcode to enable discrimination between different barcoded SHIVs. Plasmid libraries were expanded as described above.
Generation of viral stocks of barcoded viruses.
Plasmid libraries containing the barcoded viral genomes were transfected into HEK-293T cells using the Mirus Trans-IT 293 transfection reagent. The culture medium (Dulbecco modified Eagle medium with the GlutaMAX supplement [Gibco]) was amended with supplemental growth medium at 24 h posttransfection, the supernatant was collected at 48 h posttransfection and passed through a 0.45-μm-pore-size filter, and aliquots were prepared and stored at −80°C. Viral infectivity was determined using a TZM-bl cell reporter assay, as described previously (19). For each new barcoded stock, between 1,000 and 3,000 1-ml aliquots were generated.
Sequence analysis of barcoded stocks.
Each barcoded virus was sequenced using 80 independent replicate PCRs, with each reaction mixture containing an input value of 5,000 viral template copies with >100× coverage. Sequences generated from this low-template PCR either arose from an authentic barcode input template or were derived from a PCR-generated error. Low-template sequencing allows for a clear distinction between authentic barcodes, which have a log-normal distribution, and PCR-generated errors, which have a power law distribution. The mle function in R (v3.3.3) was used to fit a mixed distribution model to each replicate as described previously (19). The script for these analyses can be found online (http://bit.ly/2OsiMiB) under Barcoded Stock Characterization Code.
In vitro replication.
Rhesus macaque peripheral blood mononuclear cells were isolated from whole blood using SepMate tubes (StemCell Technologies) with Lymphoprep (StemCell Technologies) density gradient medium and centrifugation. CD4+ T cells were enriched by negative selection (CD4+ T cell isolation kit; Miltenyi). Enriched rhesus CD4+ T cells were activated with anti-CD2/CD3/CD28 beads (T cell activation kit; Miltenyi) per the manufacturer’s instructions and cultured with 100 U/ml interleukin-2 (IL-2) in RPMI medium (Gibco) supplemented with 10% fetal bovine serum, 1% l-glutamine, and 1% penicillin-streptomycin (RPMI complete) at a density of 2 × 106 to 3 × 106 cells/ml. After 72 h, activation beads were removed with a magnet, according to the kit instructions. Cells were maintained in RPMI complete with 100 U/ml IL-2 for the duration of the experiment. For each experiment, individual SHIVs were used to infect 1 × 106 activated rhesus CD4+ T cells from three naive animals mixed equally prior to use. Using a multiplicity of infection of 0.01 to 0.02 in a total volume of 1.5 ml, infections were performed by spinoculation at 800 × g for 2 h at room temperature, and then the cells were incubated at 37°C for 2 h. At the end of this incubation period, the cultures were washed 3 times (5 to 10 ml) in RPMI complete to remove excess virus and then incubated at 37°C. Culture supernatants were collected over 14 days, with medium replacement occurring at each collection time point to maintain the cultures in a 2-ml total volume. Viral p27 protein was quantified by enzyme-linked immunosorbent assay (ELISA; Advanced Bioscience Laboratories) with a limit of detection of 62.5 pg/ml.
ACKNOWLEDGMENTS
We thank various cores and personnel from the AIDS and Cancer Virus Program at the Frederick National Laboratory, including Vicky Coalter, Adam Wiles, Rodney Wiles, Donald Johnson, and Jacob Kiser within the Nonhuman Primate Research Support Core for assistance with study coordination and processing of nonhuman primate blood; Laura Newman and Leslie Lipkey within the Viral Evolution Core for viral genome sequencing; and the Quantitative Molecular Diagnostics Core for viral load measurements. We also thank Matthew Breed, Joshua Kramer, and the Laboratory Animal Sciences Program staff for expert animal care.
The TZM-bl cell line was obtained through the NIH AIDS Reagent Program.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E and funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, grants R37AI054292, UM1AI126611-02, and P51OD011092.
The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
REFERENCES
- 1.Lopker MJ, Del Prete GQ, Estes JD, Li H, Reid C, Newman L, Lipkey L, Camus C, Easlick JL, Wang S, Decker JM, Bar KJ, Learn G, Pal R, Weiss DE, Hahn BH, Lifson JD, Shaw GM, Keele BF. 2016. Derivation and characterization of pathogenic transmitted/founder molecular clones from simian immunodeficiency virus SIVsmE660 and SIVmac251 following mucosal infection. J Virol 90:8435–8453. doi: 10.1128/JVI.00718-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt F, Keele BF, Del Prete GQ, Voronin D, Fennessey CM, Soll S, Kane M, Raymond A, Gifford RJ, KewalRamani V, Lifson JD, Bieniasz PD, Hatziioannou T. 2019. Derivation of simian tropic HIV-1 infectious clone reveals virus adaptation to a new host. Proc Natl Acad Sci U S A 116:10504–10509. doi: 10.1073/pnas.1818059116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, Mansfield KG, Tomaras GD, Haynes BF, Kolodkin-Gal D, Letvin NL, Hahn BH, Shaw GM, Barouch DH. 2010. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol 84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Wang S, Kong R, Ding W, Lee FH, Parker Z, Kim E, Learn GH, Hahn P, Policicchio B, Brocca-Cofano E, Deleage C, Hao X, Chuang GY, Gorman J, Gardner M, Lewis MG, Hatziioannou T, Santra S, Apetrei C, Pandrea I, Alam SM, Liao HX, Shen X, Tomaras GD, Farzan M, Chertova E, Keele BF, Estes JD, Lifson JD, Doms RW, Montefiori DC, Haynes BF, Sodroski JG, Kwong PD, Hahn BH, Shaw GM. 2016. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc Natl Acad Sci U S A 113:E3413–E3422. doi: 10.1073/pnas.1606636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gautam R, Nishimura Y, Lee WR, Donau O, Buckler-White A, Shingai M, Sadjadpour R, Schmidt SD, LaBranche CC, Keele BF, Montefiori D, Mascola JR, Martin MA. 2012. Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: implications for use in vaccine studies. J Virol 86:8516–8526. doi: 10.1128/JVI.00644-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatziioannou T, Del Prete GQ, Keele BF, Estes JD, McNatt MW, Bitzegeio J, Raymond A, Rodriguez A, Schmidt F, Mac Trubey C, Smedley J, Piatak M Jr, KewalRamani VN, Lifson JD, Bieniasz PD. 2014. HIV-1-induced AIDS in monkeys. Science 344:1401–1405. doi: 10.1126/science.1250761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatziioannou T, Evans DT. 2012. Animal models for HIV/AIDS research. Nat Rev Microbiol 10:852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Prete GQ, Keele BF, Fode J, Thummar K, Swanstrom AE, Rodriguez A, Raymond A, Estes JD, LaBranche CC, Montefiori DC, KewalRamani VN, Lifson JD, Bieniasz PD, Hatziioannou T. 2017. A single gp120 residue can affect HIV-1 tropism in macaques. PLoS Pathog 13:e1006572. doi: 10.1371/journal.ppat.1006572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 10.Fennessey CM, Keele BF. 2013. Using nonhuman primates to model HIV transmission. Curr Opin HIV AIDS 8:280–287. doi: 10.1097/COH.0b013e328361cfff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Prete GQ, Lifson JD, Keele BF. 2016. Nonhuman primate models for the evaluation of HIV-1 preventive vaccine strategies: model parameter considerations and consequences. Curr Opin HIV AIDS 11:546–554. doi: 10.1097/COH.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans DT, Silvestri G. 2013. Nonhuman primate models in AIDS research. Curr Opin HIV AIDS 8:255–261. doi: 10.1097/COH.0b013e328361cee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veazey RS, Lackner AA. 2017. Nonhuman primate models and understanding the pathogenesis of HIV infection and AIDS. ILAR J 58:160–171. doi: 10.1093/ilar/ilx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Rompay K. 2017. Tackling HIV and AIDS: contributions by non-human primate models. Lab Anim (NY) 46:259–270. doi: 10.1038/laban.1279. [DOI] [PubMed] [Google Scholar]
- 15.Del Prete GQ, Park H, Fennessey CM, Reid C, Lipkey L, Newman L, Oswald K, Kahl C, Piatak M Jr, Quinones OA, Alvord WG, Smedley J, Estes JD, Lifson JD, Picker LJ, Keele BF. 2014. Molecularly tagged simian immunodeficiency virus SIVmac239 synthetic swarm for tracking independent infection events. J Virol 88:8077–8090. doi: 10.1128/JVI.01026-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deleage C, Immonen TT, Fennessey CM, Reynaldi A, Reid C, Newman L, Lipkey L, Schlub TE, Camus C, O'Brien S, Smedley J, Conway JM, Del Prete GQ, Davenport MP, Lifson JD, Estes JD, Keele BF. 2019. Defining early SIV replication and dissemination dynamics following vaginal transmission. Sci Adv 5:eaav7116. doi: 10.1126/sciadv.aav7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayala VI, Trivett MT, Barsov EV, Jain S, Piatak M Jr, Trubey CM, Alvord WG, Chertova E, Roser JD, Smedley J, Komin A, Keele BF, Ohlen C, Ott DE. 2016. Adoptive Transfer of engineered rhesus simian immunodeficiency virus-specific CD8+ T cells reduces the number of transmitted/founder viruses established in rhesus macaques. J Virol 90:9942–9952. doi: 10.1128/JVI.01522-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hensley-McBain T, Berard AR, Manuzak JA, Miller CJ, Zevin AS, Polacino P, Gile J, Agricola B, Cameron M, Hu SL, Estes JD, Reeves RK, Smedley J, Keele BF, Burgener AD, Klatt NR. 2018. Intestinal damage precedes mucosal immune dysfunction in SIV infection. Mucosal Immunol 11:1429–1440. doi: 10.1038/s41385-018-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fennessey CM, Pinkevych M, Immonen TT, Reynaldi A, Venturi V, Nadella P, Reid C, Newman L, Lipkey L, Oswald K, Bosche WJ, Trivett MT, Ohlen C, Ott DE, Estes JD, Del Prete GQ, Lifson JD, Davenport MP, Keele BF. 2017. Genetically-barcoded SIV facilitates enumeration of rebound variants and estimation of reactivation rates in nonhuman primates following interruption of suppressive antiretroviral therapy. PLoS Pathog 13:e1006359. doi: 10.1371/journal.ppat.1006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinkevych M, Fennessey CM, Cromer D, Tolstrup M, Sogaard OS, Rasmussen TA, Keele BF, Davenport MP. 2018. Estimating initial viral levels during simian immunodeficiency virus/human immunodeficiency virus reactivation from latency. J Virol 92:e01667-17. doi: 10.1128/JVI.01667-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar N, Chahroudi A, Silvestri G. 2016. Animal models to achieve an HIV cure. Curr Opin HIV AIDS 11:432–441. doi: 10.1097/COH.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borducchi EN, Liu J, Nkolola JP, Cadena AM, Yu WH, Fischinger S, Broge T, Abbink P, Mercado NB, Chandrashekar A, Jetton D, Peter L, McMahan K, Moseley ET, Bekerman E, Hesselgesser J, Li W, Lewis MG, Alter G, Geleziunas R, Barouch DH. 2018. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563:360–364. doi: 10.1038/s41586-018-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Julg B, Liu PT, Wagh K, Fischer WM, Abbink P, Mercado NB, Whitney JB, Nkolola JP, McMahan K, Tartaglia LJ, Borducchi EN, Khatiwada S, Kamath M, LeSuer JA, Seaman MS, Schmidt SD, Mascola JR, Burton DR, Korber BT, Barouch DH. 2017. Protection against a mixed SHIV challenge by a broadly neutralizing antibody cocktail. Sci Transl Med 9:eaao4235. doi: 10.1126/scitranslmed.aao4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julg B, Tartaglia LJ, Keele BF, Wagh K, Pegu A, Sok D, Abbink P, Schmidt SD, Wang K, Chen X, Joyce MG, Georgiev IS, Choe M, Kwong PD, Doria-Rose NA, Le K, Louder MK, Bailer RT, Moore PL, Korber B, Seaman MS, Abdool Karim SS, Morris L, Koup RA, Mascola JR, Burton DR, Barouch DH. 2017. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci Transl Med 9:eaal1321. doi: 10.1126/scitranslmed.aal1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Ghneim K, Sok D, Bosche WJ, Li Y, Chipriano E, Berkemeier B, Oswald K, Borducchi E, Cabral C, Peter L, Brinkman A, Shetty M, Jimenez J, Mondesir J, Lee B, Giglio P, Chandrashekar A, Abbink P, Colantonio A, Gittens C, Baker C, Wagner W, Lewis MG, Li W, Sekaly RP, Lifson JD, Burton DR, Barouch DH. 2016. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science 353:1045–1049. doi: 10.1126/science.aag0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, Nason MC, Montefiori D, Moldt B, Poignard P, Diskin R, Bjorkman PJ, Eckhaus MA, Klein F, Mouquet H, Cetrulo Lorenzi JC, Gazumyan A, Burton DR, Nussenzweig MC, Martin MA, Nishimura Y. 2014. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med 211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, Mankoff Z, Schmidt SD, Lifson JD, Mascola JR, Nussenzweig MC, Martin MA. 2016. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautam R, Nishimura Y, Gaughan N, Gazumyan A, Schoofs T, Buckler-White A, Seaman MS, Swihart BJ, Follmann DA, Nussenzweig MC, Martin MA. 2018. A single injection of crystallizable fragment domain-modified antibodies elicits durable protection from SHIV infection. Nat Med 24:610–616. doi: 10.1038/s41591-018-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pauthner MG, Nkolola JP, Havenar-Daughton C, Murrell B, Reiss SM, Bastidas R, Prevost J, Nedellec R, von Bredow B, Abbink P, Cottrell CA, Kulp DW, Tokatlian T, Nogal B, Bianchi M, Li H, Lee JH, Butera ST, Evans DT, Hangartner L, Finzi A, Wilson IA, Wyatt RT, Irvine DJ, Schief WR, Ward AB, Sanders RW, Crotty S, Shaw GM, Barouch DH, Burton DR. 2019. Vaccine-induced protection from homologous tier 2 SHIV challenge in nonhuman primates depends on serum-neutralizing antibody titers. Immunity 50:241–252.e246. doi: 10.1016/j.immuni.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, McKee K, Lord DM, Wei RR, Deng G, Louder M, Schmidt SD, Mankoff Z, Wu L, Asokan M, Beil C, Lange C, Leuschner WD, Kruip J, Sendak R, Kwon YD, Zhou T, Chen X, Bailer RT, Wang K, Choe M, Tartaglia LJ, Barouch DH, O'Dell S, Todd J-P, Burton DR, Roederer M, Connors M, Koup RA, Kwong PD, Yang Z-Y, Mascola JR, Nabel GJ. 2017. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 358:85–90. doi: 10.1126/science.aan8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puffer BA, Pohlmann S, Edinger AL, Carlin D, Sanchez MD, Reitter J, Watry DD, Fox HS, Desrosiers RC, Doms RW. 2002. CD4 independence of simian immunodeficiency virus Envs is associated with macrophage tropism, neutralization sensitivity, and attenuated pathogenicity. J Virol 76:2595–2605. doi: 10.1128/jvi.76.6.2595-2605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mason RD, Welles HC, Adams C, Chakrabarti BK, Gorman J, Zhou T, Nguyen R, O’Dell S, Lusvarghi S, Bewley CA, Li H, Shaw GM, Sheng Z, Shapiro L, Wyatt R, Kwong PD, Mascola JR, Roederer M. 2016. Targeted isolation of antibodies directed against major sites of SIV Env vulnerability. PLoS Pathog 12:e1005537. doi: 10.1371/journal.ppat.1005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Javaherian K, Langlois AJ, Schmidt S, Kaufmann M, Cates N, Langedijk JP, Meloen RH, Desrosiers RC, Burns DP, Bolognesi DP. 1992. The principal neutralization determinant of simian immunodeficiency virus differs from that of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A 89:1418–1422. doi: 10.1073/pnas.89.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robert-Guroff M, Aldrich K, Muldoon R, Stern TL, Bansal GP, Matthews TJ, Markham PD, Gallo RC, Franchini G. 1992. Cross-neutralization of human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus isolates. J Virol 66:3602–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 37.Shibata R, Sakai H, Kawamura M, Tokunaga K, Adachi A. 1995. Early replication block of human immunodeficiency virus type 1 in monkey cells. J Gen Virol 76:2723–2730. doi: 10.1099/0022-1317-76-11-2723. [DOI] [PubMed] [Google Scholar]
- 38.Del Prete GQ, Ailers B, Moldt B, Keele BF, Estes JD, Rodriguez A, Sampias M, Oswald K, Fast R, Trubey CM, Chertova E, Smedley J, LaBranche CC, Montefiori DC, Burton DR, Shaw GM, Markowitz M, Piatak M Jr, KewalRamani VN, Bieniasz PD, Lifson JD, Hatziioannou T. 2014. Selection of unadapted, pathogenic SHIVs encoding newly transmitted HIV-1 envelope proteins. Cell Host Microbe 16:412–418. doi: 10.1016/j.chom.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Brien SP, Swanstrom AE, Pegu A, Ko S-Y, Immonen TT, Del Prete GQ, Fennessey CM, Gorman J, Foulds KE, Schmidt SD, Doria-Rose N, Williamson C, Hatziioannou T, Bieniasz PD, Li H, Shaw GM, Mascola JR, Koup RA, Kwong PD, Lifson JD, Roederer M, Keele BF. 2019. Rational design and in vivo selection of SHIVs encoding transmitted/founder subtype C HIV-1 envelopes. PLoS Pathog 15:e1007632. doi: 10.1371/journal.ppat.1007632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shingai M, Donau OK, Schmidt SD, Gautam R, Plishka RJ, Buckler-White A, Sadjadpour R, Lee WR, LaBranche CC, Montefiori DC, Mascola JR, Nishimura Y, Martin MA. 2012. Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV-1 strains. Proc Natl Acad Sci U S A 109:19769–19774. doi: 10.1073/pnas.1217443109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexander L, Denekamp L, Czajak S, Desrosiers RC. 2001. Suboptimal nucleotides in the infectious, pathogenic simian immunodeficiency virus clone SIVmac239. J Virol 75:4019–4022. doi: 10.1128/JVI.75.8.4019-4022.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou S, Jones C, Mieczkowski P, Swanstrom R. 2015. Primer ID validates template sampling depth and greatly reduces the error rate of next-generation sequencing of HIV-1 genomic RNA populations. J Virol 89:8540–8555. doi: 10.1128/JVI.00522-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fennessey CM, Reid C, Lipkey L, Newman L, Oswald K, Piatak M Jr, Roser JD, Chertova E, Smedley J, Gregory Alvord W, Del Prete GQ, Estes JD, Lifson JD, Keele BF. 2015. Generation and characterization of a SIVmac239 clone corrected at four suboptimal nucleotides. Retrovirology 12:49. doi: 10.1186/s12977-015-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]