Yasminevirus is an icosahedral double-stranded DNA virus isolated from sewage water by amoeba coculture. Here its structure and replicative cycle in the amoeba Vermamoeba vermiformis are described and genomic and evolutionary studies are reported. This virus belongs to the Klosneuvirinae group of giant viruses, representing the second isolated and cultivated giant virus in this group, and is the first isolated using a coculture procedure. Extended translational machinery pointed to Yasminevirus among the quasiautonomous giant viruses with the most complete translational apparatus of the known virosphere.
KEYWORDS: giant virus, translation, NCLDV, Klosneuvirinae, amoeba
ABSTRACT
The family of giant viruses is still expanding, and evidence of a translational machinery is emerging in the virosphere. The Klosneuvirinae group of giant viruses was first reconstructed from in silico studies, and then a unique member was isolated, Bodo saltans virus. Here we describe the isolation of a new member in this group using coculture with the free-living amoeba Vermamoeba vermiformis. This giant virus, called Yasminevirus, has a 2.1-Mb linear double-stranded DNA genome encoding 1,541 candidate proteins, with a GC content estimated at 40.2%. Yasminevirus possesses a nearly complete translational machinery, with a set of 70 tRNAs associated with 45 codons and recognizing 20 amino acids (aa), 20 aminoacyl-tRNA synthetases (aaRSs) recognizing 20 aa, as well as several translation factors and elongation factors. At the genome scale, evolutionary analyses placed this virus in the Klosneuvirinae group of giant viruses. Rhizome analysis demonstrated that the genome of Yasminevirus is mosaic, with ∼34% of genes having their closest homologues in other viruses, followed by ∼13.2% in Eukaryota, ∼7.2% in Bacteria, and less than 1% in Archaea. Among giant virus sequences, Yasminevirus shared 87% of viral hits with Klosneuvirinae. This description of Yasminevirus sheds light on the Klosneuvirinae group in a captivating quest to understand the evolution and diversity of giant viruses.
IMPORTANCE Yasminevirus is an icosahedral double-stranded DNA virus isolated from sewage water by amoeba coculture. Here its structure and replicative cycle in the amoeba Vermamoeba vermiformis are described and genomic and evolutionary studies are reported. This virus belongs to the Klosneuvirinae group of giant viruses, representing the second isolated and cultivated giant virus in this group, and is the first isolated using a coculture procedure. Extended translational machinery pointed to Yasminevirus among the quasiautonomous giant viruses with the most complete translational apparatus of the known virosphere.
INTRODUCTION
Since 2003, the virology world has been reassessed with the discovery of giant viruses (1). The uniqueness of giant viruses in terms of genomes, particle size, and defense system has changed the field of virology (2). The complexity of giant viruses has gradually been revealed, with structural morphologies and genetic and proteomic contents never seen before in viruses (3, 4). The genome content of giant viruses offers unique features with an unexpected functional diversity of genes that have been subjected to selective pressure similar to those of other microbes (5). Even the translational machinery, which is a hallmark of cellular life and a hallmark of the distinctions between viruses and other microorganisms, has recently been challenged in light of the discovery of new families of giant viruses. For instance, a member of the Mimiviridae family, Tupanvirus, possesses a large translational machinery, including tRNAs, aminoacyl-tRNA synthetases (aaRSs), and factors related to translation maturation or processing and ribosome modification (6). Recently, Schulz et al. identified a group of giant viruses called Klosneuvirus belonging to the Mimiviridae family (7). Based on in silico studies, genomes were assembled from approximately 7,000 metagenomes from a wastewater treatment plant in Austria, and genomic analyses revealed an expanded translational machinery. Based on cultivation-independent (mini)metagenomics on soils, novel lineages were also affiliated with klosneuviruses (8). Beyond these computationally generated genomes, only one isolate from the Klosneuvirus group has been isolated so far, Bodo saltans virus (BsV) (9).
Giant viruses are common in the biosphere and have been isolated from various environmental samples, ecosystems, and geographical locations, as well as from different hosts, including amoebae, invertebrates, and mammals (10). Viruses of the Mimiviridae family have been isolated worldwide, i.e., in England, France, Brazil, Tunisia, South Arabia, South America, and a few other countries. Aside from the geographical location of the samples per se, amoebal hosts remain a major key point in the discovery of new families of giant viruses. For instance, in the genus Acanthamoeba, Acanthamoeba polyphaga and Acanthamoeba castellanii allowed mimiviruses, Marseilleviruses, pandoraviruses, pithoviruses, and Mollivirus sibericum to be isolated, while the change in the amoebal host to Vermamoeba vermiformis has made it possible to isolate specific faustoviruses and kaumoebavirus (11–16).
Here we describe a new giant virus, called Yasminevirus, isolated from V. vermiformis from a sewage water sample in Jeddah, Saudi Arabia. The genome of Yasminevirus was sequenced, and the genome characteristics and content were discerned. Our results revealed an extended arsenal of encoding genes involved in the translational machinery, and evolutionary analyses placed this new virus in the Klosneuvirinae group of giant viruses.
RESULTS
Yasminevirus particle structure and replication cycle.
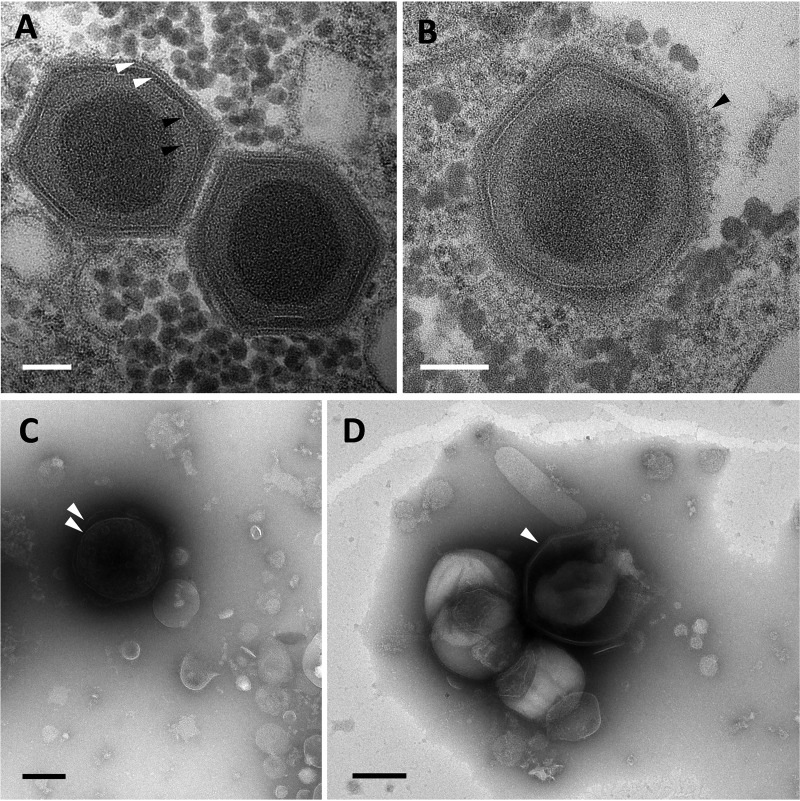
Transmission electron microscopy analyses showed that mature Yasminevirus particles presented an ultrastructure similar to what has been described previously for Bodo saltans virus (BsV) (9). The virion core genome was surrounded by two putative thin membrane layers, which, in turn, were surrounded by a double-layered icosahedral capsid. The average diameter of the virion capsid was observed to be 330 nm (Fig. 1A). The virus capsid was covered by a thin layer of fibers that was difficult to observe (Fig. 1B). For mimivirus, such fibers have been shown to be involved in viral attachment to the host cell membrane (17). Negative staining electron microscopy of Yasminevirus culture supernatant indicated that the virus particles could be observed in two different forms. In some cases, the virus presented an intact double capsid (Fig. 1C), while a break in the capsid often resulted in a morphology similar to that of pacmanvirus (Fig. 1D) (18).
FIG 1.
Morphologic features and ultrastructure of Yasminevirus mature particles. (A and B) Observation of mature Yasminevirus particles by transmission electron microscopy. (A) Transmission electron microscopy image of Yasminevirus particles highlighting the multiple inner layers surrounding the viral core genome. From the inside to the outside, two putative thin membranes, similar to those observed in Bodo saltans virus (black arrowheads), and the viral capsid composed of two visible layers (white arrowheads) can be observed. Scale bar, 100 nm. (B) The outer capsid of Yasminevirus is surrounded by a thin layer of fibrils (arrowhead). Scale bar, 100 nm. (C and D) Observation of mature Yasminevirus particles with negative-staining electron microscopy. Two distinct aspects of virus particles can be observed in a culture supernatant of Yasminevirus. (C) Virus particles with an intact double capsid (arrowhead). (D) Particles with broken capsids, giving rise to a pacmanvirus-like morphology (arrowhead).
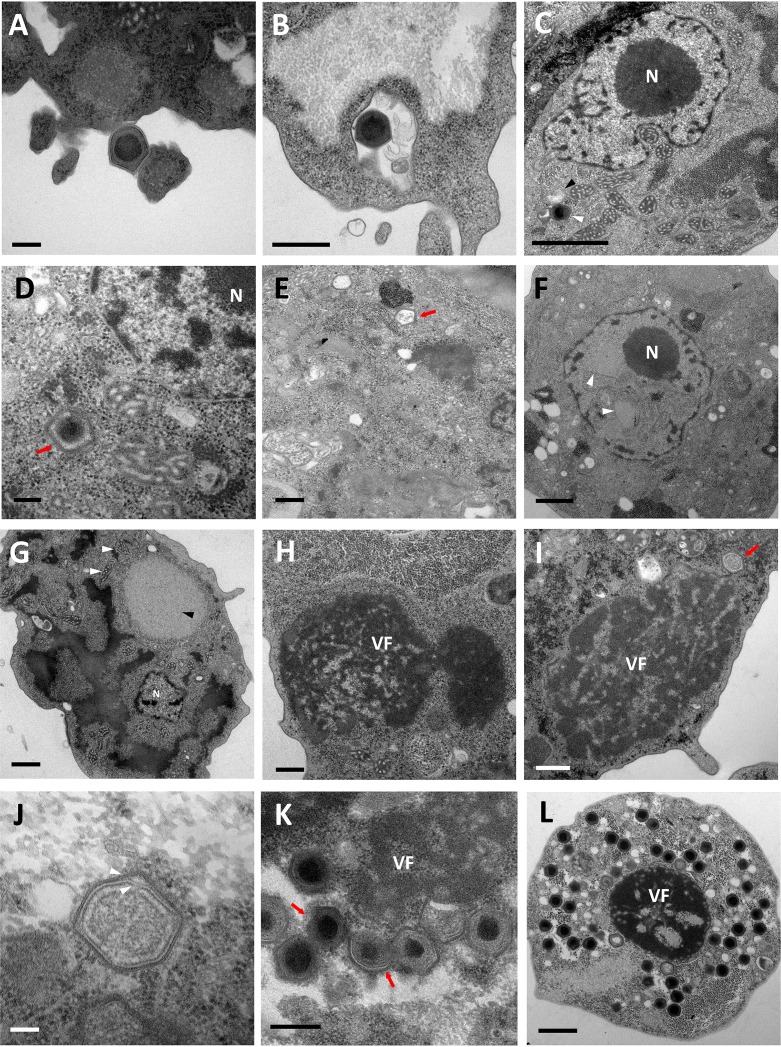
Several amoeba strains were tested as cellular support for virus replication. However, only V. vermiformis showed cytopathic effects after virus inoculation. The cytopathic effect of Yasminevirus is similar to that of amoeba mimiviruses. The viral cycle started with the attachment of a viral infectious particle to the amoeba cytoplasmic membrane (Fig. 2A). Given the size of Yasminevirus particles, their internalization within the host cell seems more likely to occur through phagocytosis, but further analysis is needed to confirm this hypothesis. This mechanism of entry has been proposed for mimivirus (19–21). On the other hand, other giant viruses, such as Marseillevirus, have been shown to exploit the endocytic pathway to infect the host cell (19, 22). At 30 min postinfection (p.i.), we detected virus particles inside the amoeba’s phagosome (Fig. 2B). The Yasminevirus was then able to escape the phagosome (Fig. 2C). Although this event has already been described for some giant viruses, such as pacmanvirus, mimivirus, and Marseillevirus, triggering of the decoating step has been shown to depend on phagosome acidification (18, 19).
FIG 2.
Characterization of the Yasminevirus replication cycle with transmission electron microscopy. (A) Yasminevirus particle attachment to the Vermamoeba vermiformis cytoplasmic membrane before its internalization by phagocytosis. Scale bar, 200 nm. (B) Virus particles inside the phagosome. Scale bar, 500 nm. (C) Once in the cytoplasm, the virus seems to escape the phagosome and loses its outer capsid (black arrowhead). The Yasminevirus particle is indicated by a white arrowhead. Scale bar, 1 μm. N, nucleus. (D) The virus particle loses its internal capsid before the genome release step. Scale bar, 200 nm. (E) Empty particles after the release of the viral content into the amoeba cytoplasm (arrow). Scale bar, 500 nm. (F) The eclipse phase is marked by a remodeling of the cell nucleus by the appearance of cleared areas inside the nucleus (arrowheads). Scale bar, 1 μm. (G) The early stages of infection are then characterized by the appearance of a bright area in the cytoplasm of the host cell, which represents the early virus factory (black arrowhead). The mitochondria recruited around the virus factory are highlighted with white arrowheads. Scale bar, 1 μm. (H) At a later stage of infection, the virus factory (VF) starts to appear denser and occupies a larger part of the host cell cytoplasm. Scale bar, 500 nm. (I) Assembly of virions, the first virus progeny (arrow). Scale bar, 500 nm. (J) Immature virion on which the double proteinaceous capsid is completely assembled (arrowheads). Scale bar, 100 nm. (K) In some cases, genome acquisition could occur before the complete assembly of the virion (arrows). Viral particles that have already acquired their genomes have an electron-dense region at the center of the capsids. Scale bar, 400 nm. (L) At the end of the viral cycle, mature virions occupy the whole host cell cytoplasm up until host cell lysis. Scale bar, 1 μm.
Afterwards, the next step was characterized by a loss of the double capsid (Fig. 2C and D) and then the release of the virus content into the host cell cytoplasm (Fig. 2E). This phenomenon has been observed in some cells between 1 and 2 h p.i. It prepares the eclipse phase, during which the viral particles disappear from the cytoplasm (Fig. 2F). This step was also marked by a remodeling of the host cell nucleus and by the appearance of cleared areas inside the nucleus (Fig. 2F). Such remodeling probably indicates that Yasminevirus manipulates the host cell nucleus to trigger the expression of its genome during the early stage of infection, but further extensive analysis is required before affirming this possibility. Indeed, it has been demonstrated that Marseilleviruses initiate the replication of their genomes by transiently hijacking the host nuclear transcription complex to the early virus factories. This finding is consistent with the absence of a transcription complex in the proteome of Marseilleviruses, which is necessary to initiate the viral cycle (23).
The first early virus factory was observed at 12 h p.i. It consisted of a bright area that appeared in the cytoplasm next to the nucleus of the host cell (Fig. 2G). This area then changed to an electron-dense diffuse structure giving rise to the mature virus factory in which Yasminevirus particle assembly occurred (Fig. 2H). The first synthesized viral progenies are shown in Fig. 2I and J. Later on in the cycle, the mature virions started to appear and accumulated in the periphery of the host cell (Fig. 2K). Interestingly, we observed a curious phenomenon regarding the dynamics of virion morphogenesis. Previous studies have demonstrated that for mimivirus, capsid assembly and genome acquisition occur successively in a defined order (24). It has been shown that after the formation of the complete capsid, the genome enters the capsid through the portal opposite the stargate and condenses inside, and then the entrance portal is sealed (24, 25). For Yasminevirus, we propose that genome acquisition could occur even before the formation of the complete capsid. We clearly observed partially assembled virions that had already acquired genomes, as shown in Fig. 2K. At the end of the viral cycle, the mature virions occupied the whole amoeba cytoplasm before cell lysis (approximately 24 h p.i.) (Fig. 2L).
Genomic characteristics of Yasminevirus.
The genome of Yasminevirus has been assembled into two scaffolds totaling 2,126,343 bp, with a GC content estimated at 40.2%. A total of 1,541 protein-coding DNA sequences (CDS) were predicted, totaling the coding proportion of the genome at ∼88.5% (1,882,542 bp) (Fig. 3). Functional annotation retrieved 518 proteins (∼33.6%) with assigned functions and 1,023 hits as hypothetical proteins (∼66.4%). The presence of translational machinery is a key feature of living organisms and a distinction separating the living world from viruses (26). The complexity of the protein translation process requires different partners, such as rRNAs and associated ribosomal proteins, tRNAs, aminoacyl-tRNA synthetases (aaRSs), and translation factors (TF), which associate various proteins involved in polypeptide chain synthesis. Although tRNAs were first found in other viruses, such as phycodnavirus (27), followed by the identification of partial components of the translational apparatus in mimiviruses and Cafeteria roenbergensis virus (28–30), Yasminevirus possesses a nearly complete translational machinery, expanding the previous repertoire of reported translation components (Fig. 4). Yasminevirus possesses a large number of tRNAs (70) that are associated with 45 codons and recognize 20 amino acids, 20 aaRSs recognizing 20 aa, as well as several translation and elongation factors (see Data Set S1 in the supplemental material). When comparing Yasminevirus to Klosneuvirinae, we observed that Yasminevirus possessed an extended arsenal of translation components (Fig. 4). So far, only Tupanvirus is comparable to Yasminevirus. Despite this exceptional viral translational machinery, one critical factor is still missing as Yasminevirus has no ribosomal proteins. In the absence of these pivotal ribosomal proteins, one might speculate on the extent of the dependence of Yasminevirus on the host translational machinery. Because of the specificity in protein synthesis, Yasminevirus completes the picture of the viral translational machinery, thus promoting a hot debate about the origin and evolution of these quasiautonomous viruses.
FIG 3.
Graphical circular map of the genome of Yasminevirus. From outside to center: scaffolds (orange/brown), genes on the forward strand (dark brown), genes on the reverse strand (blue), tRNA (green), and GC skew (light green and purple).
FIG 4.
Translation system components in Yasminevirus. tRNA, aminoacyl-tRNA synthetase (aaRS), and translation factors (TF) are compared in the Klosneuvirinae group and in Tupanvirus.
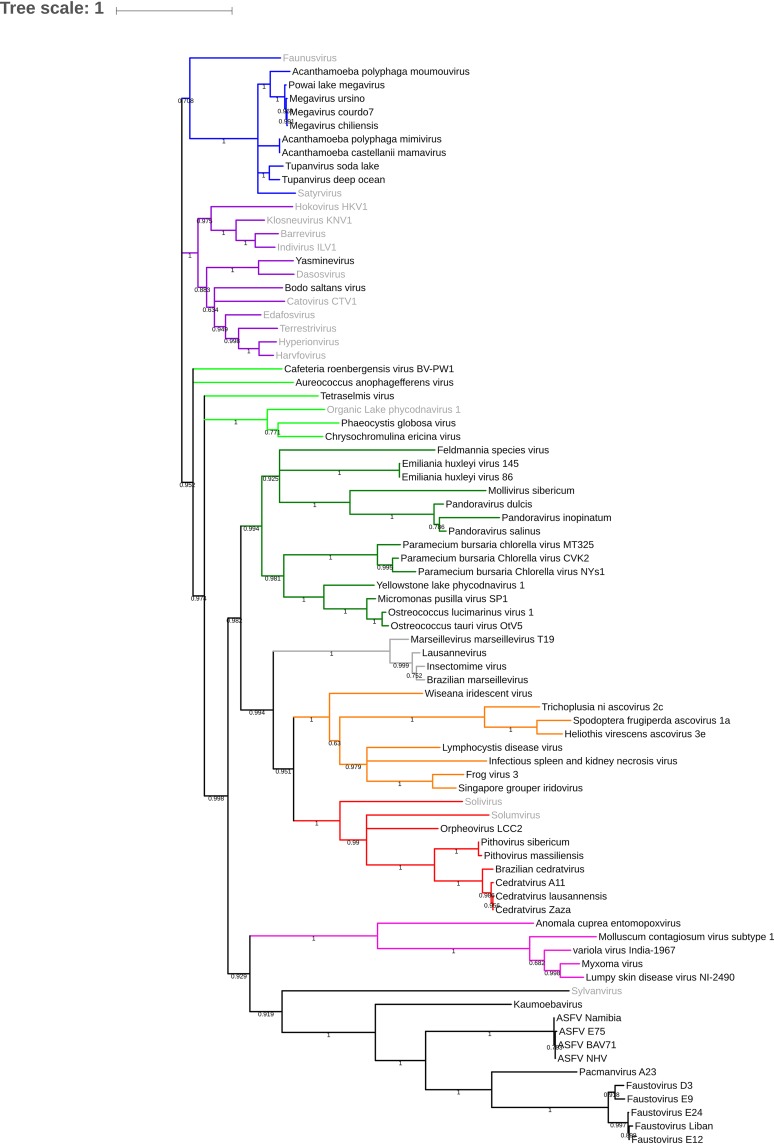
The rhizome of Yasminevirus was constructed in order to study the mosaicism of its genome (Fig. 5). Approximately 34.6% of the genome of Yasminevirus was determined to be composed of genes with their closest homologues in other viruses, followed by ∼13.2% in Eukaryota, ∼7.2% in Bacteria, and less than 1% in Archaea. Open reading frames (ORFs) with no detectable homology to other ORFs (ORFans) represented 44.4% of the total predicted ORFs. Interestingly, a large proportion of viral hits were represented only by Klosneuvirinae, totaling ∼87.9%, with Terrestrivirus being the most prevalent. Among those of eukaryotic origin, Metazoa (4.6%), fungi (2.7%), Viridiplantae (1.2%) and Stramenopiles (0.9%) were the most represented groups, whereas bacteria were mainly represented by Gammaproteobacteria (1.3%), Alphaproteobacteria (0.5%), Bacteroidetes (0.9%), and Firmicutes (0.9%). By including all kingdoms and basing the analysis on the best hits, Yasminevirus was identified to be closely related to the members of the Klosneuvirinae group. This was confirmed using a classical phylogenetic approach based on the DNA polymerase B gene as a gene marker (Fig. 6). According to the phylogenetic tree based on a single gene marker (RNApolB), Yasminevirus belongs to the Klosneuvirinae group. To decipher the taxonomy relationship at the full-genome scale, we carried out a whole-genome comparative study by comparing Yasminevirus within Klosneuvirinae. Orthologous genes were identified, and the shared genes are summarized in Fig. 7 and Table 1. According to the large proportion of orthologous genes shared with Klosneuvirinae, we confirmed that Yasminevirus belongs to this group of viruses, with the highest proportion of shared genes shared with Terrestrivirus. The results based on the whole genome of Klosneuvirinae confirmed the taxonomic position of Yasminevirus on the phylogenetic tree as a new member of the Klosneuvirinae group of giant viruses.
FIG 5.
The rhizome of Yasminevirus. Viruses are depicted in red, Archaea in pink, Eukaryota in blue, and Bacteria in green. ORFans are depicted in orange. All protein sequences were used as queries in a BLASTp search against the nonredundant (nr) protein database from NCBI. BLAST results were filtered to keep the best hits, and taxonomic affiliation was retrieved from the NCBI database. The best hit was selected and integrated in a circular genomic data image (37).
FIG 6.
Phylogenetic tree of Yasminevirus based on DNA polymerase B proteins of nucleocytoplasmic large DNA viruses (NCLDVs). Colors have been assigned for the different virus groups as follows: blue for Mimivirus and extended Mimiviridae; green for pandoraviruses, Mollivirus sibericum, and Phycodnaviridae; black for groups of Asfarviridae, faustoviruses, pacmanvirus, and kaumoebavirus; gray for Marseilleviridae; red for orpheovirus, cedratvirus, and pithoviruses; and purple for asco-iridoviridae.
FIG 7.
Orthologous genes shared between Yasminevirus and other members within Klosneuvirinae.
TABLE 1.
Orthologous genes shared by Yasminevirus and viruses in the Klosneuvirinae group
| Virus | No. of orthologous genes shared by: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Barrevirus | Bodo saltans virus | Catovirus | Dasosvirus | Edafosvirus | Harvfovirus | Hokovirus | Hyperionvirus | Indivirus | Klosneuvirus | Terrestrivirus | |
| Barrevirus | |||||||||||
| Bodo saltans virus | 100 | ||||||||||
| Catovirus | 123 | 211 | |||||||||
| Dasosvirus | 16 | 42 | 43 | ||||||||
| Edafosvirus | 77 | 139 | 219 | 34 | |||||||
| Harvfovirus | 79 | 116 | 190 | 34 | 260 | ||||||
| Hokovirus | 98 | 160 | 193 | 36 | 135 | 107 | |||||
| Hyperionvirus | 120 | 177 | 282 | 52 | 334 | 705 | 157 | ||||
| Indivirus | 180 | 187 | 256 | 34 | 169 | 135 | 185 | 195 | |||
| Klosneuvirus | 179 | 215 | 315 | 43 | 247 | 195 | 241 | 280 | 338 | ||
| Terrestrivirus | 135 | 206 | 307 | 48 | 247 | 248 | 192 | 359 | 223 | 322 | |
| Yasminevirus | 111 | 207 | 258 | 59 | 199 | 182 | 195 | 254 | 215 | 277 | 377 |
DISCUSSION
Fifteen years after the discovery of Mimivirus as the first giant virus (1), the isolation of giant viruses from various environments remains a gold mine contributing to the understanding of the characteristics of these fascinating viruses and their ecology and evolution. Metagenomics-based genome reconstructions have led to the discovery of new virus families, such as the subfamily Klosneuvirinae (7, 8). The isolation of members of this computer-generated group is a major challenge in confirming and detailing in silico hypotheses. The study of Yasminevirus placed this giant virus as a new member within Klosneuvirinae. So far, only one isolate, Bodo saltans virus, has been described in this family (9), and Yasminevirus is the first isolate identified using a coculture procedure with the free-living amoeba Vermamoeba vermiformis. Phylogenetic and phylogenomic comparisons within Klosneuvirinae confirmed that Yasminevirus belonged to this group, with a high proportion of orthologous genes shared by all the viruses. Previously, Klosneuvirinae have been proposed as the third family in the Mimiviridae group and are divided into four lineages called Klosneuvirus, Catovirus, Hokovirus, and Indivirus (7). Yasminevirus represents an additional lineage related to the Klosneuvirinae viruses. Based on the mosaicity of the genome of Yasminevirus in our rhizome representation, Yasminevirus shared a higher number of genes with Terrestrivirus than other Klosneuvirinae viruses, with a proportion of genes at approximately ∼8.7% as best hits. Regarding lineages A, B, and C of Mimiviridae, only a small proportion of genes were detected (below 1%), whereas Tupanvirus shared 2% of the genes as best hits. These results are in agreement with the phylogenetic position of Yasminevirus determined using the DNA polymerase B marker gene. The proportion of ORFans in Yasminevirus remains the highest within Klosneuvirinae, reaching ∼44% of the predicted protein models.
Surprisingly, the size of the Yasminevirus genome was 2.1 Mb, which is the largest length in the family of isolated Klosneuvirinae. So far, only Hyperionvirus could be larger, and its genome has been estimated to reach 2.38 Mb based on in silico reconstruction. Another characteristic and a discrepancy between Yasminevirus and all other Klosneuvirinae viruses is the GC content. Indeed, the GC content within the Klosneuvirinae family ranged from 21% to 37%, whereas the GC content of Yasminevirus was slightly higher, at ∼41% (7, 8, 31). Notably, Yasminevirus possesses a large set of components of the translational machinery, as described for other members of Klosneuvirinae. Nevertheless, Yasminevirus encodes the most complete arsenal of translational machinery components among the Klosneuvirinae, and a single giant virus of the Mimiviridae family (Tupanvirus) competes with Yasminevirus in this regard (6, 32). Yasminevirus also possesses an 18S-like region with low sequence similarity with copy 2 of Tupanvirus, as described for the Mimiviridae family. The unexpected translation system of Yasminevirus challenges the notion of the dependence of translation on the host. One hypothesis could be the ability of such viruses to override the host machinery for protein translation using a set of viral proteins. Such a strategy could allow viruses to broaden their host range, as fewer protein factors would be required to ensure sufficient production of viral proteins (8).
MATERIALS AND METHODS
Virus isolation.
Samples collected from sewage water were inoculated on a V. vermiformis (strain CDC19) monolayer in starvation medium at 30°C, as previously described for the isolation of faustovirus and kaumoebavirus (13, 14).
Virus production.
Yasminevirus was produced in starvation medium using V. vermiformis (strain CDC19) as a cell support. After complete lysis of the cells, the culture was centrifuged at 3,000 × g for 10 min to pellet residual amoebae and cysts. The virus was then concentrated from a large volume of culture supernatant (approximately 2 liters) by ultracentrifugation at 22,000 × g for 1 h. The pellet was resuspended in 15 ml of sterile phosphate-buffered saline (PBS). The resulting supernatant was digested with 75 μl of Turbo DNase (Ambion) at 37°C for 1 h and then loaded onto a discontinuous sucrose gradient composed of two sucrose layers, 66% and 30%. Finally, the virus was purified by ultracentrifugation at 30,000 × g for 1 h (MLS50 Beckman Coulter rotor), and the viral pellet was harvested below the 66% sucrose layer and resuspended in 1 ml of sterile PBS.
Viral cycle.
A suspension of V. vermiformis was prepared by successive cycles of centrifugation (3,000 × g for 10 min) and resuspension in starvation medium. Ten milliliters of amoebae at 106 cells/ml was inoculated with Yasminevirus at a multiplicity of infection (MOI) of 10 in a 25-cm2 culture flask. After 1 h of incubation at 30°C for virus adsorption, the supernatant was collected and successive centrifugation cycles at 3000 × g for 10 min were performed to remove extracellular Yasminevirus particles. The cells were resuspended in 10 ml of fresh medium for a second incubation at 30°C for 24 h. This time point was considered hour zero (H0). At H0, H1, H2, H6, H8, H12, H16, and H24, a 1-ml aliquot was collected for transmission electron microscopy (see below). A culture flask containing only amoebae was used as a negative control.
Transmission electron microscopy.
Infected cells were washed with PBS and then fixed with 2% glutaraldehyde–0.1 M cacodylate for at least 1 h at 4°C. The pellets were washed 3 times with 0.1 M cacodylate buffer and then fixed with 1% osmium tetroxide in 0.1 M potassium ferricyanide solution for 1 h. Increasing ethanol concentrations (30%, 50%, 75%, and 100%) were used for sample dehydration before embedding in an Epon 812 resin. Finally, sections (70 nm) were stained with 5% uranyl acetate and lead citrate for examination under a Tecnai G2 transmission electron microscope (FEI, Germany) at 200 kilo-electronvolts (keV).
Genome sequencing and analyses.
The Yasminevirus genomes were sequenced using an Illumina MiSeq instrument (Illumina Inc., San Diego, CA) with the paired-end application for short-read sequencing and a MinION sequencing device (Oxford Nanopore Technologies) following the instructions of the manufacturer for long-read sequences. The sequence reads were assembled de novo using SPAdes software version 3.10.1 (33). The gene predictions were performed with Prokka (34). tRNA sequences were identified using the ARAGORN tool. The functional annotations were inferred by BLAST searches against the GenBank NCBI nonredundant (nr) protein sequence database (E value < 1 × 10−3). Finally, the genome annotation was manually revised and curated. The predicted ORFs that were smaller than 50 amino acids and had no hits in any database were ruled out. All predicted ORFs between 50 and 100 amino acids were confirmed by generating a three-dimensional (3D) model using the PHYRE2 Protein Fold Recognition Server (35). Phylogenetic analyses were carried out based on the B DNA polymerase gene marker. The predicted amino acid sequences were obtained from the NCBI GenBank and aligned using Muscle in the Mega software program (36). Trees were constructed using the maximum likelihood evolution method with 1,000 replicates. For comparisons within the Klosneuvirinae group, groups of orthologues were determined using the Proteinortho tool with 1e−3 and 50% as the E value and coverage thresholds, respectively. To study the mosaicism of the Yasminevirus genome, all protein-coding sequences were BLAST searched against the nr database, and the results were filtered to retain the best hits. After selection, the best hit was chosen and integrated into a circular genomic data image as a rhizome representation (37). Taxonomic affiliation was retrieved from the NCBI database.
Data availability.
Yasminevirus A1 was deposited in the EMBL-EBI database under accession number PRJEB27730.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the French Government under the Investissements d’avenir (Investments for the Future) program managed by the Agence Nationale de la Recherche (ANR; French National Agency for Research) (reference, Méditerranée Infection 10-IAHU-03), by the Région Provence-Alpes-Côte d’Azur, and by European funding FEDER PRIMI. This research was also supported by a grant from the Institut Universitaire de France (IUF; Paris, France) allocated to Anthony Levasseur.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.La Scola B, Audic S, Robert C, Jungang L, De Lamballerie X, Drancourt M, Birtles R, Claverie JM, Raoult D. 2003. A giant virus in amoebae. Science 299:2033. doi: 10.1126/science.1081867. [DOI] [PubMed] [Google Scholar]
- 2.Colson P, La Scola B, Levasseur A, Caetano-Anollés G, Raoult D. 2017. Mimivirus: leading the way in the discovery of giant viruses of amoebae. Nat Rev Microbiol 15:243–254. doi: 10.1038/nrmicro.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forterre P. 2017. Viruses in the 21st century: from the curiosity-driven discovery of giant viruses to new concepts and definition of life. Clin Infect Dis 65:S74–S79. doi: 10.1093/cid/cix349. [DOI] [PubMed] [Google Scholar]
- 4.Claverie JM, Abergel C. 2016. Giant viruses: update, enigmas, controversies and perspectives. Med Sci (Paris) 32:1087–1096. doi: 10.1051/medsci/20163212012. [DOI] [PubMed] [Google Scholar]
- 5.Levasseur A, Andreani J, Delerce J, Bou Khalil J, Robert C, La Scola B, Raoult D. 2016. Comparison of a modern and fossil pithovirus reveals its genetic conservation and evolution. Genome Biol Evol 8:2333–2339. doi: 10.1093/gbe/evw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrahão J, Silva L, Silva LS, Khalil JYB, Rodrigues R, Arantes T, Assis F, Boratto P, Andrade M, Kroon EG, Ribeiro B, Bergier I, Seligmann H, Ghigo E, Colson P, Levasseur A, Kroemer G, Raoult D, La Scola B. 2018. Tailed giant Tupanvirus possesses the most complete translational apparatus of the known virosphere. Nat Commun 9:749. doi: 10.1038/s41467-018-03168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz F, Yutin N, Ivanova NN, Ortega DR, Lee TK, Vierheilig J, Daims H, Horn M, Wagner M, Jensen GJ, Kyrpides NC, Koonin EV, Woyke T. 2017. Giant viruses with an expanded complement of translation system components. Science 356:82–85. doi: 10.1126/science.aal4657. [DOI] [PubMed] [Google Scholar]
- 8.Schulz F, Alteio L, Goudeau D, Ryan EM, Yu FB, Malmstrom RR, Blanchard J, Woyke T. 2018. Hidden diversity of soil giant viruses. Nat Commun 9:4881. doi: 10.1038/s41467-018-07335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeg CM, Chow CT, Suttle CA. 2018. The kinetoplastid-infecting Bodo saltans virus (BsV), a window into the most abundant giant viruses in the sea. Elife 7:e33014. doi: 10.7554/eLife.33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer MG. 2016. Giant viruses come of age. Curr Opin Microbiol 31:50–57. doi: 10.1016/j.mib.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Philippe N, Legendre M, Doutre G, Couté Y, Poirot O, Lescot M, Arslan D, Seltzer V, Bertaux L, Bruley C, Garin J, Claverie JM, Abergel C. 2013. Pandoraviruses: amoeba viruses with genomes up to 25 Mb reaching that of parasitic eukaryotes. Science 341:281–286. doi: 10.1126/science.1239181. [DOI] [PubMed] [Google Scholar]
- 12.Legendre M, Bartoli J, Shmakova L, Jeudy S, Labadie K, Adrait A, Lescot M, Poirot O, Bertaux L, Bruley C, Couté Y, Rivkina E, Abergel C, Claverie JM. 2014. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology. Proc Natl Acad Sci U S A 111:4274–4279. doi: 10.1073/pnas.1320670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reteno DG, Benamar S, Khalil JB, Andreani J, Armstrong N, Klose T, Rossmann M, Colson P, Raoult D, La Scola B. 2015. Faustovirus, an asfarvirus-related new lineage of giant viruses infecting amoebae. J Virol 89:6585–6594. doi: 10.1128/JVI.00115-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajrai L, Benamar S, Azhar E, Robert C, Levasseur A, Raoult D, La Scola B. 2016. Kaumoebavirus, a new virus that clusters faustoviruses and Asfarviridae. Viruses 8:278. doi: 10.3390/v8110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legendre M, Lartigue A, Bertaux L, Jeudy S, Bartoli J, Lescot M, Alempic JM, Ramus C, Bruley C, Labadie K, Shmakova L, Rivkina E, Couté Y, Abergel C, Claverie JM. 2015. In-depth study of Mollivirus sibericum, a new 30,000-y-old giant virus infecting Acanthamoeba. Proc Natl Acad Sci U S A 112:E5327–E5335. doi: 10.1073/pnas.1510795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pagnier I, Valles C, Raoult D, La Scola B. 2015. Isolation of Vermamoeba vermiformis and associated bacteria in hospital water. Microb Pathog 80:14–20. doi: 10.1016/j.micpath.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues RAL, dos Santos Silva LK, Dornas FP, de Oliveira DB, Magalhães TFF, Santos DA, Costa AO, de Macêdo Farias L, Magalhães PP, Bonjardim CA, Kroon EG, La Scola B, Cortines JR, Abrahão JS. 2015. Mimivirus fibrils are important for viral attachment to the microbial world by a diverse glycoside interaction repertoire. J Virol 89:11812–11819. doi: 10.1128/JVI.01976-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreani J, Yaacoub J, Khalil B, Sevvana M, Benamar S, F Di P, Bitam I, Colson P, Klose T, Rossmann MG, Raoult D, La Scola B. 2017. Pacmanvirus, a new giant icosahedral virus at the crossroads between Asfarviridae and faustoviruses. J Virol 91:e00212-17. doi: 10.1128/JVI.00212-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrade A, Rodrigues RAL, Oliveira GP, Andrade KR, Bonjardim CA, La Scola B, Kroon EG, Abrahão JS. 2017. Filling knowledge gaps for mimivirus entry, uncoating, and morphogenesis. J Virol 91:e01335-17. doi: 10.1128/JVI.01335-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzan-Monti M, La Scola B, Barrassi L, Espinosa L, Raoult D. 2007. Ultrastructural characterization of the giant volcano-like virus factory of Acanthamoeba polyphaga Mimivirus. PLoS One 2:e328. doi: 10.1371/journal.pone.0000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutsa Y, Zauberman N, Sabanay I, Minsky A. 2010. Vaccinia-like cytoplasmic replication of the giant. Proc Natl Acad Sci U S A 107:5978–5982. doi: 10.1073/pnas.0912737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arantes TS, Rodrigues RAL, Dos Santos Silva LK, Oliveira GP, de Souza HL, Khalil JYB, de Oliveira DB, Torres AA, da Silva LL, Colson P, Kroon EG, da Fonseca FG, Bonjardim CA, La Scola B, Abrahão JS. 2016. The large Marseillevirus explores different entry pathways by forming giant infectious vesicles. J Virol 90:5246–5255. doi: 10.1128/JVI.00177-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabre E, Jeudy S, Santini S, Legendre M, Trauchessec M, Couté Y, Claverie JM, Abergel C. 2017. Noumeavirus replication relies on a transient remote control of the host nucleus. Nat Commun 8:15087–15012. doi: 10.1038/ncomms15087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuznetsov YG, Klose T, Rossmann M, McPherson A. 2013. Morphogenesis of mimivirus and its viral factories: an atomic force microscopy study of infected cells. J Virol 87:11200–11213. doi: 10.1128/JVI.01372-13. (Erratum, 88:3055, 2014, 10.1128/JVI.03772-13.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zauberman N, Mutsafi Y, Halevy DB, Shimoni E, Klein E, Xiao C, Sun S, Minsky A. 2008. Distinct DNA exit and packaging portals in the virus Acanthamoeba polyphaga mimivirus. PLoS Biol 6:e114. doi: 10.1371/journal.pbio.0060114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lwoff A. 1957. The concept of virus the third Marjory Stephenson memorial lecture. J Gen Microbiol 17:239–253. doi: 10.1099/00221287-17-2-239. [DOI] [PubMed] [Google Scholar]
- 27.Van Etten JL, Meints RH. 1999. Giant viruses infecting algae. Annu Rev Microbiol 53:447–494. doi: 10.1146/annurev.micro.53.1.447. [DOI] [PubMed] [Google Scholar]
- 28.Claverie JM, Abergel C. 2010. Mimivirus: the emerging paradox of quasi-autonomous viruses. Trends Genet 26:431–437. doi: 10.1016/j.tig.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Claverie JM, Ogata H. 2009. Ten good reasons not to exclude giruses from the evolutionary picture. Nat Rev Microbiol 7:615. doi: 10.1038/nrmicro2108-c3. [DOI] [PubMed] [Google Scholar]
- 30.Fischer MG, Allen MJ, Wilson WH, Suttle CA. 2010. Giant virus with a remarkable complement of genes infects marine zooplankton. Proc Natl Acad Sci U S A 107:19508–19513. doi: 10.1073/pnas.1007615107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilhelm SW, Bird JT, Bonifer KS, Calfee BC, Chen T, Coy SR, Gainer PJ, Gann ER, Heatherly HT, Lee J, Liang X, Liu J, Armes AC, Moniruzzaman M, Rice JH, Stough JM, Tams RN, Williams EP, LeCleir GR. 2017. A student’s guide to giant viruses infecting small eukaryotes: from Acanthamoeba to Zooxanthellae. Viruses 17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arslan D, Legendre M, Seltzer V, Abergel C, Claverie JM. 2011. Distant Mimivirus relative with a larger genome highlights the fundamental features of Megaviridae. Proc Natl Acad Sci U S A 108:17486–17491. doi: 10.1073/pnas.1110889108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 35.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Yasminevirus A1 was deposited in the EMBL-EBI database under accession number PRJEB27730.