The role of circulating monocytes as persistent HIV reservoirs during ART is still controversial. Several studies have reported persistent infection of monocytes in virally suppressed individuals; however, others failed to detect HIV in this subset. These discrepancies are likely explained by the diversity of the methods used to isolate monocytes and to detect HIV infection. In this study, we show that only flow cytometry cell sorting yields a highly pure population of monocytes largely devoid of CD4 contaminants. Using this approach in a longitudinal cohort of HIV-infected individuals before and during ART, we demonstrate that HIV is rarely found in monocytes from untreated and treated HIV-infected individuals. This study highlights the importance of using methods that yield highly pure populations of cells as flow cytometry cell sorting to minimize and control for CD4+ T-cell contamination.
KEYWORDS: HIV reservoir, integrated HIV DNA, monocytes
ABSTRACT
Whereas human immunodeficiency virus (HIV) persists in tissue macrophages during antiretroviral therapy (ART), the role of circulating monocytes as HIV reservoirs remains controversial. Three magnetic bead selection methods and flow cytometry cell sorting were compared for their capacity to yield pure CD14+ monocyte populations. Cell sorting by flow cytometry provided the purest population of monocytes (median CD4+ T-cell contamination, 0.06%), and the levels of CD4+ T-cell contamination were positively correlated with the levels of integrated HIV DNA in the monocyte populations. Using cell sorting by flow cytometry, we assessed longitudinally the infection of monocytes and other cell subsets in a cohort of 29 Thai HIV-infected individuals. Low levels of HIV DNA were detected in a minority of monocyte fractions obtained before and after 1 year of ART (27% and 33%, respectively), whereas HIV DNA was readily detected in CD4+ T cells from all samples. Additional samples (2 to 5 years of ART) were obtained from 5 individuals in whom monocyte infection was previously detected. Whereas CD4+ T cells were infected at high levels at all time points, monocyte infection was inconsistent and absent in at least one longitudinal sample from 4/5 individuals. Our results indicate that infection of monocytes is infrequent and highlight the importance of using flow cytometry cell sorting to minimize contamination by CD4+ T cells.
IMPORTANCE The role of circulating monocytes as persistent HIV reservoirs during ART is still controversial. Several studies have reported persistent infection of monocytes in virally suppressed individuals; however, others failed to detect HIV in this subset. These discrepancies are likely explained by the diversity of the methods used to isolate monocytes and to detect HIV infection. In this study, we show that only flow cytometry cell sorting yields a highly pure population of monocytes largely devoid of CD4 contaminants. Using this approach in a longitudinal cohort of HIV-infected individuals before and during ART, we demonstrate that HIV is rarely found in monocytes from untreated and treated HIV-infected individuals. This study highlights the importance of using methods that yield highly pure populations of cells as flow cytometry cell sorting to minimize and control for CD4+ T-cell contamination.
INTRODUCTION
Combination antiretroviral therapy (ART) suppresses human immunodeficiency virus (HIV) replication to undetectable plasma levels and reduces substantially the number of cells carrying proviral DNA in both blood and tissues (1–3). However, ART alone fails to eradicate the virus. HIV persists within a small subset of long-lived CD4+ T cells harboring integrated genomes (4–6), which are often considered the most stable cellular reservoir for HIV in individuals on ART for prolonged periods of time.
In addition to CD4+ T cells, several studies indicate that HIV persists in myeloid cells during ART. Several subsets of macrophages have been shown to act as viral reservoirs for HIV or simian immunodeficiency virus (SIV), including tissue macrophages, such as Kupffer cells in the liver, microglial cells in the brain, alveolar macrophages in the lung, and intestinal macrophages obtained from the gastrointestinal tract (7–18).
Since monocytes circulate in the blood for several days (19) and then migrate to various tissues where they differentiate into macrophages, peripheral monocytes have been proposed as a potential vehicle for HIV dissemination across the myeloid compartment. In particular, infected monocytes may play a key role in viral dissemination to the brain due to their capacity to cross the blood-brain barrier during untreated HIV infection (20–24). However, the role of circulating monocytes as persistent HIV reservoirs during ART is still controversial (25). In vitro studies suggest that freshly isolated blood monocytes are resistant to HIV infection unless they are differentiated into monocyte-derived macrophages (26–28). This observation is mechanistically supported by the relatively low levels of expression of the CD4 receptor (29), blocks in reverse transcription (30–32), nuclear import (33), and high levels of host restriction factors (34, 35) that characterize monocytes.
In vivo, HIV DNA has been detected in circulating monocytes isolated from HIV-infected individuals (36–42). However, it is unclear whether these viral genomes represent genuine HIV DNA integrated in the chromatin of these cells or whether they result from the phagocytosis of infected CD4+ T cells by monocytes (43, 44) or from contamination of the monocyte fraction by CD4+ T cells (45, 46).
In the present study, we used highly pure populations of cells to evaluate the frequency of CD4+ T cells, blood monocytes, and other circulating subsets harboring integrated HIV DNA before and during ART.
RESULTS
Flow cytometry cell sorting provides the purest population of monocytes.
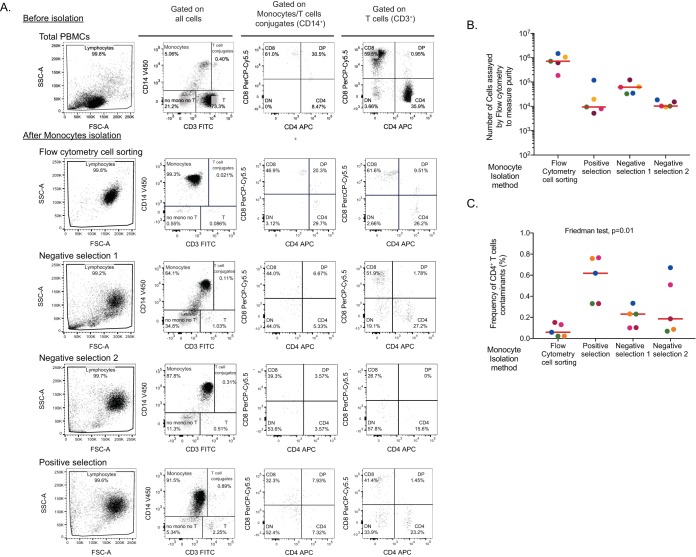
Detection of HIV DNA in purified monocytes could result from contamination by residual CD4+ T cells. Therefore, we aimed to identify a method that would isolate monocytes with minimal levels of CD4+ T-cell contamination. We compared three magnetic bead selection methods for monocytes isolation (one positive- and two negative-selection approaches) and flow cytometry cell sorting of CD14+ cells (gating strategy depicted in Fig. 1) using samples obtained from 5 virally suppressed individuals (Table 1). Following enrichment of monocytes, we evaluated the frequency of cell contaminants in the monocyte fractions (Fig. 2A). Using relatively large numbers of enriched monocytes to assess purity by flow cytometry (Fig. 2B), we found that all methods afforded a relatively pure population of monocytes (<1% CD4+ T-cell contamination, Fig. 2C). Cell sorting by flow cytometry yielded the purest population of monocytes (median CD4+ T-cell contamination, 0.06% [interquartile range {IQR}, 0.02 to 0.14%]), while the positive-selection method resulted in the highest level of contamination (median CD4+ T-cell contamination, 0.6% [IQR, 0.3 to 0.8%]). Of note, monocytes isolated using the two negative-selection methods displayed high levels of autofluorescence (Fig. 2A), which did not allow us to distinguish monocytes (CD3– CD14+) from monocyte-T-cell conjugates (CD3+ CD14+). Therefore, contamination of monocytes by CD4+ T cells may have been underestimated using these methods.
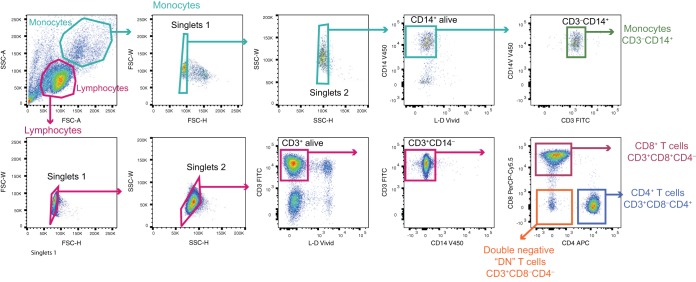
FIG 1.
Gating strategy for cell sorting. Flow cytometry dot plots showing the gating strategy used to isolate CD4+ T cells (CD3+ CD4+ CD8–), CD8+ T cells (CD3+ CD4– CD8+), double-negative (DN) cells (CD3+ CD4– CD8–), and monocytes (CD3– CD14+).
TABLE 1.
Participant characteristics from the leukapheresis study
| Characteristic | Value |
|---|---|
| Age (median [IQR]) (yr) | 55 (55–58) |
| Male (no. [%]) | 5 (100) |
| Absolute CD4 counts (median [IQR]) (cells/μl) | 628 (620–696) |
| No. (%) with VL of <40 HIV RNA copies/mla | 5 (100) |
| Time since HIV diagnosis (median [IQR]) (yr) | 20 (15–21) |
| Time on ART (median [IQR]) (yr) | 10 (9 − 14) |
VL, viral load.
FIG 2.
Assessment of monocyte infection following different methods of isolation. Three magnetic bead selection methods (one positive and two negative selection) and flow cytometry cell sorting of CD14+ cells were used to isolate monocytes. (A) Gating strategy for purity analysis. Flow cytometry dot plots showing the cellular distribution of PBMCs (upper lane) and enriched monocytes following isolation by cell sorting or three magnetic bead selection methods (positive and negative selection). A sample from one representative donor is shown. SSC, side scatter; FITC, fluorescein isothiocyanate; APC, antigen-presenting cell; FSC, forward scatter. (B) Number of monocytes analyzed by flow cytometry. (C) Frequency of CD4+ T-cell contaminants after monocyte enrichment determined by flow cytometry in postenrichment samples.
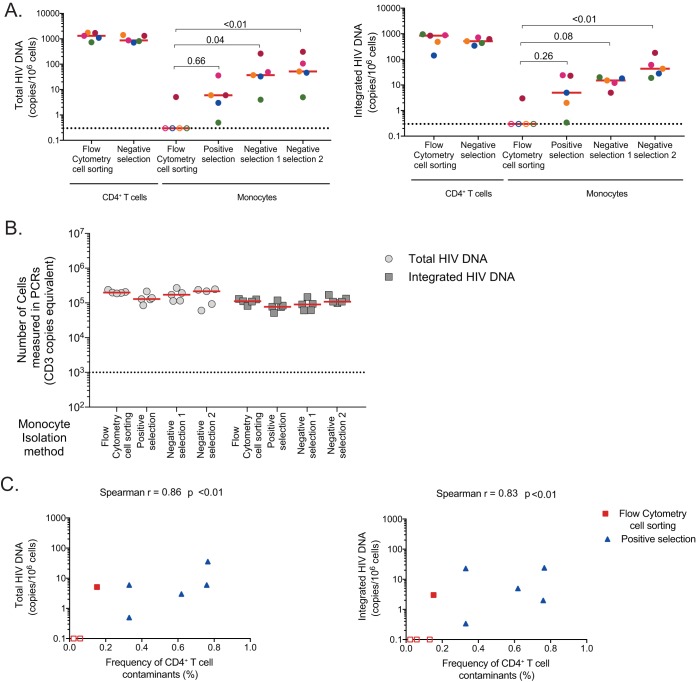
We measured total and integrated HIV DNA levels in enriched CD4+ T cells and monocytes from all 5 donors (Fig. 3A). As expected, high levels of total and integrated HIV DNA were detected in CD4+ T cells enriched by negative selection or by fluorescence-activated cell sorting (FACS). All monocyte fractions obtained by magnetic selection displayed low but detectable levels of total and integrated HIV DNA (Fig. 3A), with negative selections resulting in higher frequencies of infected cells than with positive-selection methods. Interestingly, only 1/5 monocyte samples obtained by flow cytometry cell sorting displayed detectable levels of HIV DNA. HIV DNA levels in monocytes purified by flow cytometry were consistently lower than those measured in monocytes obtained by magnetic selection. Importantly, similar numbers of monocytes were analyzed by PCR following all isolation methods (Fig. 3B), indicating that differences in sensitivity were unlikely to explain these differences.
FIG 3.
Assessment of monocyte infection following different methods of isolation. (A) Frequency of total (left panel) and integrated HIV DNA (right panel) in enriched CD4+ T cells isolated by flow cytometry cell sorting and negative selection, as well as in monocytes isolated using the four methods described for Fig. 2. (B) Number of monocytes analyzed by total and integrated HIV PCR for each sample. C) Correlation between the levels of total (left) or integrated (right) HIV DNA measured in the monocyte fractions and the frequency of residual CD4+ T cells in this subset. Monocytes obtained by flow cytometry cell sorting (in red) and positive magnetic bead selection (in blue) are shown. Due to the high monocyte autofluorescence detected after enrichment with the two negative magnetic bead selection methods, these were excluded from the analysis.
We then evaluated if CD4+ T-cell contaminants could contribute to higher levels of HIV DNA measured in the monocyte fractions. Due to the aforementioned high levels of autofluorescence of monocytes isolated by negative selection, we used only data from cell sorting by flow cytometry and positive magnetic bead selection for this analysis. The frequencies of residual CD4+ T cells measured in the monocyte fraction correlated with the levels of total and integrated HIV DNA measured in this subset (r = 0.86, P < 0.01, and r = 0.83, P < 0.01 for total and integrated HIV DNA, respectively; Fig. 3C), suggesting that the low levels of HIV DNA detected in the monocyte fractions could be attributed to CD4+ T-cell contaminants. Therefore, we used flow cytometry cell sorting to minimize and control for CD4+ T-cell contamination in all subsequent experiments.
HIV infection in circulating cell subsets.
To assess the contributions of different circulating cell subsets to the HIV reservoir, we used flow cytometry cell sorting to isolate CD4+, CD8+, and double-negative (DN; CD4– CD8–) T cells and monocytes (Fig. 1) from 29 individuals enrolled in the SEARCH 011 study before and after 1 year of ART (Table 2). To achieve sufficient sensitivity, only samples for which at least 10,000 cells were assayed (as measured by PCR) were included in the analysis (Fig. 4A). Before ART initiation, all participants displayed detectable levels of integrated HIV DNA in peripheral blood mononuclear cells (PBMCs) and purified CD4+ T cells (Fig. 4B). The majority of the monocyte and double-negative fractions displayed low but detectable levels of HIV DNA (55% and 61% positive samples, respectively), whereas HIV genomes were rarely detected in CD8+ T cells (23% positive samples). After 1 year of ART, integrated HIV DNA was still detected in CD4+ T cells from all participants. The proportions of monocyte and CD8+ T-cell fractions with detectable HIV genomes during ART were similar to those before treatment initiation (7/15 [47%] and 5/27 [18%], respectively). In contrast, the proportion of DN samples with detectable integrated HIV was markedly reduced by ART (61% versus 33%).
TABLE 2.
Participant characteristics from the SEARCH 011 study
| Characteristic | Value |
|---|---|
| Age (median [IQR]) (yr) | 35 (31–38) |
| Male (no. [%]) | 14 (50) |
| Absolute CD4 counts at baseline (median [IQR]) (cells/μl) | 278 (192–368) |
| Absolute CD4 counts after 1 yr of ART (median [IQR]) (cells/μl) | 499 (346–568) |
| log10 VL at baseline (median [IQR]) (HIV RNA copies/ml) | 4.7 (4.4–5.0) |
| VL undetectable after 1 yr of ART (no. [%]) | 29 (100) |
| ART regimen (no. [%]) | |
| NNRTIa | 29 (100) |
NNRTI, nonnucleoside reverse transcriptase inhibitor.
FIG 4.
Levels of HIV DNA in circulating cell-sorted subsets after correction for CD4+ T-cell contamination. PBMCs, as well as isolated CD4+, CD8+, double-negative (DN) T cells (CD8– CD4–), and monocytes were sorted by flow cytometry from the blood of 29 Thai individuals and subjected to integrated HIV DNA quantification. (A) Number of cells analyzed as measured by PCR in PBMCs and in each sorted subset are indicated. Only samples in which at least 10,000 cells (dashed horizontal line) were assayed were included in the succeeding analysis. LOD, limit of detection. (B) Levels of integrated HIV DNA in PBMCs, CD4+ T cells, monocytes, and DN and CD8+ T cells before and after 1 year of ART. Numbers of samples in which HIV DNA was detected (and the corresponding percentages) are indicated. Each individual is color-coded, and horizontal bars indicate median values. (C) Frequencies of residual CD4+ T-cell in CD8+ T-cell, DN, and monocyte enriched populations post-cell sorting. The numbers of samples with measurable residual CD4+ T cells (and the corresponding percentages) are indicated. (D) The levels of HIV DNA in monocytes, DN T cells, and CD8+ T cells were adjusted for CD4+ T-cell contamination. The numbers of samples in which HIV DNA was detected (and the corresponding percentages) are indicated. Samples from each participant are color-coded, and horizontal bars indicate median values. (E) Integrated HIV DNA levels from paired samples obtained at baseline (before ART initiation) and after 1 year of ART in CD4+ T cells, monocytes, DN T cells, and CD8+ T cells. P values were obtained from the Wilcoxon matched-pair signed-rank test. (F) Correlation between the levels of integrated HIV DNA at baseline and after 1 year of ART in CD4+ T cells. (G) Correlations between the frequency of CD4+ T cells harboring integrated HIV DNA and the levels of integrated HIV DNA measured in monocytes (upper left), DN T cells (upper middle), and CD8 T cells (upper right). Similar correlations were repeated after adjusting for CD4+ T-cell contamination (bottom row). (F and G) P values were obtained using the Spearman test. (H) Pie charts representing the contribution of each subset (CD4+ T cells [blue], monocytes [red], DN T cells [green], and CD8+ T cells [yellow]) to the total pool of cells harboring integrated HIV DNA at baseline (before ART, left) and after 1 year on ART (right).
Since CD4+ T-cell contamination could contribute to HIV detection in non-CD4+ T-cell subsets, we assessed the purity of each sorted fraction when enough cells were available (data not shown). Sorted CD4+ T cells were highly pure (median purity, 99.2%), followed by CD8+ T cells (97.3%), DN cells (94.5%), and then monocytes (90.1%), which represented the least pure fractions. Not surprisingly, 81% of the monocyte fractions displayed low levels of CD4+ T-cell contaminants (median, 0.39% [IQR, 0.27 to 0.8%]) (Fig. 4C). Fifty percent of the DN fractions and 25% of the CD8+ T-cell fractions tested were also contaminated by CD4+ T cells (median, 0.35% [IQR, 0.23 to 0.53%] and 0.15% [IQR, 0.12 to 0.18%], respectively). We corrected the levels of integrated HIV DNA in each population by calculating the numbers of HIV genomes attributed to HIV-infected CD4+ T cells in each fraction. We applied the mean frequency of CD4+ T-cell contaminants to each fraction (0.56%, 0.42%, and 0.17% for monocytes, DN cells, and CD8+ T cells, respectively) and used the infection frequency measured in the matched CD4+ T cells to calculate and subtract the contribution of CD4+ T cells to the levels of HIV DNA measured in each subset. After adjustment, only two CD8+ T-cell samples (one before and one after ART initiation) remained positive for HIV DNA, with DNA values close to the limit of detection of the assay (Fig. 4D). All DN fractions obtained from pre-ART samples remained positive after correction (61%), whereas only 20% DN samples displayed detectable levels of HIV DNA during ART. Among DN-positive samples, the median levels of HIV DNA were 174 copies (IQR, 10 to 424 copies) and 11 copies (IQR, 3 to 421 copies) of integrated HIV DNA/106 cells before and after ART initiation, respectively. Monocyte fractions showed the most pronounced changes after adjusting for CD4+ T-cell contamination; only 27% and 33% of the monocyte fractions obtained before and after ART initiation remained positive for HIV DNA after correcting for CD4+ T-cell contamination (Fig. 4D). Among the monocyte fractions with detectable viral genomes, the median levels of HIV DNA were low (35 copies [IQR, 4 to 84 copies] and 18 copies [IQR, 8 to 174 copies] of integrated HIV DNA/106 cells before and after ART initiation, respectively).
There was a significant decrease in the frequency of CD4+ T cells harboring HIV DNA before and after 1 year of suppressive ART (P < 0.0001, Fig. 4E), and the two frequencies were strongly correlated (P < 0.0001, r = 0.9, Fig. 4F). In contrast, we did not observe measurable decays in the levels of HIV DNA in the other cell fractions following ART initiation (Fig. 4E).
To determine if the detection of HIV DNA in the non-CD4+ T-cell fractions could be attributed to contamination by CD4+ T cells, we correlated the frequency of cells harboring HIV DNA in CD4+ T cells with the levels of HIV DNA detected in the other fractions before and after adjusting for CD4+ T-cell contaminants (Fig. 4G). Before correction, the levels of HIV DNA measured in monocytes were strongly correlated with the frequency of cells harboring HIV DNA in CD4+ T cells (r = 0.6, P < 0.001). However, this correlation did not persist after adjusting for CD4+ T-cell contamination, suggesting that the presence of HIV DNA in monocytes is likely attributed to the insufficient purity of the monocyte fraction in the majority of the samples. In contrast, the strong positive correlation between the levels of HIV DNA in DN and CD4+ T cells resisted the correction for CD4+ T-cell contaminants (r = 0.9, P < 0.0001 in both cases). HIV DNA was too rarely detected in CD8+ T cells to conclude on this subset.
We then calculated the relative contribution of each subset to the pool of cells harboring integrated HIV DNA before and after ART. CD4+ T cells were by far the most important contributor to the pool of cells carrying integrated viral genomes before and after ART initiation (mean contributions, 98.3% and 99.11%, respectively), whereas monocytes and DN cells marginally contributed to the pool of infected cells (mean contributions of monocytes and DN before and after ART, 0.2% and 0.87%, and 1.5% and 0.02%, respectively; Fig. 4H).
Longitudinal assessment of HIV infection in sorted cell subsets.
Since we identified a few participants in whom HIV DNA was still detected in monocytes after correction for CD4+ T-cell contamination, we sought to determine if this potential viral reservoir was stable over time. Additional longitudinal blood samples (2 to 3 years of ART) were collected from a few individuals (n = 5) in whom integrated HIV DNA was detected in the monocyte fraction either before or after ART (Fig. 5). Only samples with a minimum of 10,000 cells analyzed by PCR were included in the analysis (Fig. 5A). Integrated HIV DNA was detected at high levels in CD4+ T cells at all time points in all 5 participants (Fig. 5B, left). In contrast, although HIV DNA was repeatedly detected in the monocyte fractions of one participant even after adjustment for CD4+ T-cell contamination, monocyte infection was inconsistent and absent in at least one longitudinal sample from the 4 other participants (Fig. 5B, right). Of note, the detection of integrated HIV DNA in monocyte was not more frequent before than after ART initiation.
FIG 5.
Longitudinal assessment of HIV infection in CD4+ T cells and monocytes. (A) Number of cells at each time point measured by PCR in sorted CD4+ T cells (left) and monocytes (right) in five individuals in whom HIV DNA was previously detected (either at baseline or after 1 year of ART) in the monocyte fraction. (B) Levels of integrated HIV DNA in CD4+ T cells (left) and monocytes (right) were assessed longitudinally in five individuals in whom HIV DNA was previously detected (either at baseline or after 1 year of ART) in the monocyte fraction. The levels of HIV DNA in monocytes were adjusted for CD4+ T-cell contamination. Only samples in which a minimum of 10,000 cells were analyzed were included. Each individual’s data are color-coded.
DISCUSSION
In this study, we measured the relative contributions of different circulating populations to the pool of HIV-infected cells. Our data confirm that CD4+ T cells are the main contributor to the circulating HIV reservoir, before and after ART initiation, while DN (CD4– CD8–) and blood monocyte subsets harbor a minute fraction of HIV genomes (47).
ART initiation led to a modest decay (3.2-fold) in the levels of integrated HIV DNA in CD4+ T cells. The levels before and after ART strongly correlated, indicating that the levels of HIV DNA before ART predict the size of the reservoir after 1 year of therapy. While total HIV DNA has been shown to decrease with ART in multiple studies (3, 48–50), whether the levels of integrated HIV DNA are affected by ART is still unclear (48, 51–54). In this study, we quantified integrated HIV DNA in isolated CD4+ T cells, which avoids the confounding factor of an increase in the proportion of CD4+ T cells when HIV DNA measures are performed in PBMCs.
In accordance with other studies, we found that DN cells often harbored detectable levels of integrated HIV DNA in viremic participants (61%), whereas this proportion was reduced after ART initiation (20%) (55, 56). Productive HIV infection leads to downregulation of the CD4 molecule from the cell surface in vitro and in vivo (55, 57–60), suggesting that the HIV-infected DN cells we detected in this study may be CD4+ T cells that have lost expression of this receptor. DN cells still harbored HIV during ART in a few individuals, which is consistent with previous studies (61). These rare cells may still actively produce HIV proteins, as recently proposed (57). Since our analysis was limited to samples collected after 1 year of ART, whether DN cells can represent a stable long-term reservoir for HIV remains to be investigated.
Integrated HIV DNA was detected in a significant proportion of the monocyte fractions, both before and after 1 year of ART. The levels of HIV DNA detected in monocytes were associated with the levels measured in CD4+ T cells, highlighting the importance of using methods that yield highly pure populations of monocytes, such as flow cytometry cell sorting, which minimizes CD4+ T-cell contamination and allows to control for it.
Contrary to what we observed for DN cells, there was no change in the proportion of monocyte fractions harboring HIV DNA after ART initiation (∼30%). Furthermore, and in contrast to CD4+ T cells and DN cells, the frequency of infected monocytes was extremely low (18 cells/million) and not affected by ART. Indeed, in 3 of the 5 participants from whom 3 to 4 longitudinal samples were available, infection of monocytes was not detected at the pre-ART time point. Overall, our results indicate that infection of monocytes is extremely rare and inconsistent over time. We cannot exclude that the rare detection of infected monocytes is attributed to insufficient purity of the cell population.
Several studies have detected HIV DNA in monocytes using different enrichment methods such as magnetic selection approaches (22, 38–40, 42, 62, 63), plastic adherence (37), or flow cytometry cell sorting (25, 36). In the majority of these studies, the levels of CD4+ T-cell contamination were assessed by flow cytometry or through the detection of rearranged T-cell receptor (TCR) by PCR in the enriched monocyte population. Despite the relatively high purity reported in these studies, our results indicate that even a minimal contamination by CD4+ T cells can result in a false-positive detection of HIV DNA in purified monocytes, which are prone to stick to T cells. Even by using a stringent flow cytometry cell sorting strategy in which we excluded cell doublets and CD3+ cells from the monocyte gate, residual levels of CD4+ T cells were observed. A combination of different enrichment strategies, such as depletion of CD3+ T cells, positive or negative selection of monocytes, and cell sorting by flow cytometry, may improve the purity of the monocyte population (25). Our results emphasize the importance of assessing purity when isolating monocytes for HIV reservoir studies. Although the quantification of rearranged TCR by PCR may represent a sensitive and qualitative method to control for T-cell contamination, it will not distinguish between genuine CD4+ T-cell contamination and the phagocytosis of dying CD4+ T cells or capture of CD4+ T cells by myeloid cells (43, 44). In contrast, extracellular staining for CD4+ T cells offers the advantage of detecting cell doublets between T cells and monocytes but may not be sensitive enough unless half the sorted cells are used to perform this control.
There are several limitations to our study. First, we isolated monocytes solely on the basis of CD14 expression. Classical monocytes (CD14+ CD16–) represent ∼85% of the circulating pool of monocytes, whereas the remaining ∼15% consist of the intermediate (CD14+ CD16+) and nonclassical (CD14low CD16+) monocyte subsets (64). It is known that the minor CD16+ monocyte subset preferentially harbors HIV in infected individuals on ART compared to other monocyte populations (65, 66), although it remains controversial (25). Importantly, this subset may play a critical role in the neuropathogenesis of HIV infection (21, 67). In our study, we sorted all CD14+ monocytes, which includes this potentially HIV-enriched population among a large proportion of noninfected monocytes. The use of additional markers such as CD16 may have increased the likelihood of detecting rare HIV-infected monocytes.
Another limitation of our study is the use of HIV DNA as a marker for the HIV reservoir since most HIV genomes detected by PCR are defective and do not represent replication competent HIV (68). Nonetheless, these PCR-based assays are well suited to detect very low infection frequencies in highly pure populations of cells. Since the majority of the monocyte fractions we analyzed were negative for HIV DNA, we predict that replication-competent HIV would not have been recovered from these samples, as recently shown by Cattin et al. (25).
Finally, we did not analyze the coreceptor usage of the viral genomes detected in monocytes. Since monocytes express relatively low levels of CCR5 and high levels of CXCR4, it is possible that monocyte infection is restricted to HIV-positive individuals in whom X4 strains are present. Interestingly, HIV CRF01_AE, which is the major HIV clade circulating in Thailand, has been associated with a more rapid disease progression and an earlier coreceptor switch in a cohort of CRF01_AE-infected individuals than with HIV subtype B (69, 70). Since HIV CRF01_AE leads to a more rapid depletion of CD4+ T cells and to a relatively rapid switch in coreceptor usage than do other clades, this may lead to higher levels of infection of CD14+ circulating monocytes in individuals infected with this clade. A study formally comparing the infection frequencies of monocytes between different geographical regions would be needed to answer this interesting question.
In conclusion, our results show that HIV infection of monocytes is a rare event during ART. Our results highlight the importance of using highly pure populations of sorted cells, such as monocytes isolated by flow cytometry cell sorting, in order to minimize and control for CD4+ T-cell contamination.
MATERIALS AND METHODS
Study participants.
To compare different methods of monocyte and CD4+ T-cell isolation, large numbers of PBMCs were obtained by leukapheresis from 5 HIV-infected and virally suppressed individuals on stably suppressive ART (>6.5 years). The characteristics of these participants are summarized in Table 1. To longitudinally assess monocyte infection, blood samples from 29 Thai adult participants from the SEARCH 011 study (ClinicalTrials.gov identifier NCT00782808) were obtained prior to and after >12 months of ART. The characteristics of the SEARCH 011 participants have been described before (22, 71) and are summarized in Table 2. All participants signed informed consent forms approved by the institutional review boards of the McGill University Health Centre, Centre Hospitalier de l’Université de Montréal, Chulalongkorn University, the Walter Reed Army Institute of Research (WRAIR), and the University of California at San Francisco (UCSF).
Isolation of CD4+ T cells and monocytes by magnetic selection.
PBMCs were isolated from the leukapheresis or blood draw product by Ficoll Hypaque density gradient centrifugation and cryopreserved in liquid nitrogen until use. Total CD4+ T cells were isolated from PBMCs by negative magnetic selection (EasySep human CD4+ T-cell enrichment kit, catalog no. 19052; Stemcell Technologies). Three different methods of monocyte magnetic bead selection were compared in this study, as follows: (i) CD14 MicroBeads (positive-selection kit, number 130-050-201; Miltenyi Biotec), (ii) human monocyte enrichment kit without CD16 depletion (negative-selection kit, catalog no. 19058, “negative selection 1”; Stemcell Technologies), and (iii) EasySep human monocyte isolation kit (negative-selection kit, catalog no. 19359, “negative selection 2”; Stemcell Technologies).
The purity of the enriched CD4+ T cells and monocyte fractions was assessed by flow cytometry using the following panel of antibodies: CD3-AF700, CD4-APC, CD8-PerCP-Cy5.5, CD14-V450, and Aqua-ViViD for LIVE/DEAD staining (all from BD Biosciences).
Cell sorting.
Cryopreserved PBMCs were thawed and stained using the following antibody panel: CD3-FITC, CD4-APC, CD8-PerCP-Cy5.5, CD14-V450, and Aqua-ViViD for LIVE/DEAD staining (all from BD Biosciences). After 30 min of staining at 4°C, cells were washed and immediately sorted on a FACSAria II cell sorter (BD Biosciences) to obtain CD4+ (CD3+ CD14– CD4+ CD8–), CD8+ (CD3+ CD14– CD4– CD8+), and double-negative (DN; CD3+ CD14– CD4– CD8–) T cells, and monocytes (CD3– CD14+). Sorting from leukapheresis samples was performed using the 4-way purity settings, where only drops free of contaminating particles were sorted. Since cell availability was a limiting factor for PBMCs isolated from blood draws, we balanced purity and yield. Following cell sorting, quality control (QC) of the sorted sample was performed to assess the purity of the populations. The sorted cell fractions were centrifuged at 12,000 × g for 5 min, and dry pellets were stored at –80°C until use.
Quantification of total and integrated HIV DNA.
The frequency of enriched or sorted cell subsets harboring total and/or integrated HIV DNA was measured by real-time nested PCR, as previously described (52) with long terminal repeat (LTR)-gag-specific (for total) and Alu-gag-specific (for integrated) primers. The results were normalized to the number of copies of the CD3 gene. To achieve sufficient sensitivity, only samples for which at least 10,000 cells were assayed were included in the analysis.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism (version 7.0). Continuous variables are reported as medians with interquartile ranges (IQR). Comparisons between isolation methods were compared using a Kruskal-Wallis one-way analysis of variance (ANOVA). Comparisons within a given cell subset before and after ART were performed using the Wilcoxon test for paired continuous variables. Correlations were tested using the Spearman test.
ACKNOWLEDGMENTS
We are grateful to all the study participants for their time and participation in this study. The study team is grateful to the individuals who participated in this study, the staff at the Thai Red Cross AIDS Research Centre and the Department of Retrovirology, U.S. Army Medical Component, Armed Forces Research Institute of Medical Sciences (AFRIMS). We thank Josée Girouard, Mario Legault, and the Réseau SIDA Maladies Infectieuses (FRQ-S) for recruitment and clinical assistance with the study participants. We thank Kim Kusser (Flow Cytometry Core Facility, VGTI, Port St. Lucie, FL, USA), Dominique Gauchat and Annie Gosselin (Flow Cytometry Core Facility, CR-CHUM, Montréal, Quebec, Canada), and Olfa Debbeche (biosafety level 3 [BSL-3] Core Facility, CR-CHUM, Montréal, Quebec, Canada). We also thank Rémi Fromentin, Petronela Ancuta, and Amélie Cattin for helpful discussions.
This work was supported by the Canadian Institutes for Health Research (CIHR, grant 364408), the Canadian HIV Cure Enterprise Team Grant HIG-133050 from the CIHR in partnership with CANFAR and IAS, and the Réseau SIDA et Maladies Infectieuses du Fonds de Recherche du Quebec-Santé (FRQS), amfAR grant 109316 with support from FAIR, and the U.S. National Institutes of Health grant R01-NS061696 (V.G.V.). M.M. is supported by a BP-DGR AGAUR Postdoctoral Fellowship. N.C. is supported by a Research Scholar Career Award of the Quebec Health Research Fund (FRQ-S, number 253292).
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of the Army, the U.S. Department of Defense, or any of the institutions. The investigators have adhered to the policies for the protection of human subjects as prescribed in AR-70.
Authors have received honoraria for participating in advisory meetings for ViiV Healthcare (J.A. and V.G.V.), Gilead (J.A.), Merck (J.A. and V.G.V.), Roche (J.A.), and AbbVie (J.A.).
We declare no conflicts of interest.
REFERENCES
- 1.Murray JM, Zaunders JJ, McBride KL, Xu Y, Bailey M, Suzuki K, Cooper DA, Emery S, Kelleher AD, Koelsch KK, PINT Study Team . 2014. HIV DNA subspecies persist in both activated and resting memory CD4+ T cells during antiretroviral therapy. J Virol 88:3516–3526. doi: 10.1128/JVI.03331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrigue I, Pellegrin I, Hoen B, Dumon B, Harzic M, Schrive MH, Séréni D, Fleury H. 2000. Cell-associated HIV-1-DNA quantitation after highly active antiretroviral therapy-treated primary infection in patients with persistently undetectable plasma HIV-1 RNA. AIDS 14:2851–2855. doi: 10.1097/00002030-200012220-00006. [DOI] [PubMed] [Google Scholar]
- 3.Besson GJ, Lalama CM, Bosch RJ, Gandhi RT, Bedison MA, Aga E, Riddler SA, McMahon DK, Hong F, Mellors JW. 2014. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin Infect Dis 59:1312–1321. doi: 10.1093/cid/ciu585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong JK, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 5.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 6.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. 1998. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A 95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igarashi T, Brown CR, Endo Y, Buckler-White A, Plishka R, Bischofberger N, Hirsch V, Martin MA. 2001. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc Natl Acad Sci U S A 98:658–663. doi: 10.1073/pnas.021551798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. 1986. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 9.Orenstein JM, Fox C, Wahl SM. 1997. Macrophages as a source of HIV during opportunistic infections. Science 276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 10.Hufert FT, Schmitz J, Schreiber M, Schmitz H, Racz P, von Laer DD. 1993. Human Kupffer cells infected with HIV-1 in vivo. J Acquir Immune Defic Syndr 6:772–777. [PubMed] [Google Scholar]
- 11.Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DFJ, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL. 2006. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol 12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- 12.Jambo KC, Banda DH, Kankwatira AM, Sukumar N, Allain TJ, Heyderman RS, Russell DG, Mwandumba HC. 2014. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol 7:1116–1126. doi: 10.1038/mi.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yukl SA, Sinclair E, Somsouk M, Hunt PW, Epling L, Killian M, Girling V, Li P, Havlir DV, Deeks SG, Wong JK, Hatano H. 2014. A comparison of methods for measuring rectal HIV levels suggests that HIV DNA resides in cells other than CD4+ T cells, including myeloid cells. AIDS 28:439–442. doi: 10.1097/QAD.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costiniuk CT, Salahuddin S, Farnos O, Olivenstein R, Pagliuzza A, Orlova M, Schurr E, De Castro C, Bourbeau J, Routy J-P, Ancuta P, Chomont N, Jenabian M-A. 2018. HIV persistence in mucosal CD4+ T cells within the lungs of adults receiving long-term suppressive antiretroviral therapy. AIDS 32:2279–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, Engle EL, Forsyth E, Bullock BT, Mac Gabhann F, Wietgrefe SW, Haase AT, Zink MC, Mankowski JL, Clements JE, Gama L. 2017. Brain macrophages in simian immunodeficiency virus-infected, antiretroviral-suppressed macaques: a functional latent reservoir. mBio 8:e01186-17. doi: 10.1128/mBio.01186-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honeycutt JB, Thayer WO, Baker CE, Ribeiro RM, Lada SM, Cao Y, Cleary RA, Hudgens MG, Richman DD, García JV. 2017. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat Med 23:638–643. doi: 10.1038/nm.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiNapoli SR, Ortiz AM, Wu F, Matsuda K, Twigg H III, Hirsch VM, Knox K, Brenchley JM. 2017. Tissue-resident macrophages can contain replication-competent virus in antiretroviral-naive, SIV-infected Asian macaques. JCI Insight 2:e91214. doi: 10.1172/jci.insight.91214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cribbs SK, Lennox J, Caliendo AM, Brown LA, Guidot DM. 2015. Healthy HIV-1-infected individuals on highly active antiretroviral therapy harbor HIV-1 in their alveolar macrophages. AIDS Res Hum Retroviruses 31:64–70. doi: 10.1089/AID.2014.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, Macallan D, Yona S. 2017. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med 214:1913–1923. doi: 10.1084/jem.20170355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. 1997. Unique monocyte subset in patients with AIDS dementia. Lancet 349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 21.Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW. 2014. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res 12:85–96. doi: 10.2174/1570162X12666140526114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valcour VG, Shiramizu BT, Sithinamsuwan P, Nidhinandana S, Ratto-Kim S, Ananworanich J, Siangphoe U, Kim JH, de Souza M, Degruttola V, Paul RH, Shikuma CM, Southeast Asia Research Collaboration with the University of Hawaii 001 Protocol Team . 2009. HIV DNA and cognition in a Thai longitudinal HAART initiation cohort: the SEARCH 001 cohort study. Neurology 72:992–998. doi: 10.1212/01.wnl.0000344404.12759.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valcour VG, Ananworanich J, Agsalda M, Sailasuta N, Chalermchai T, Schuetz A, Shikuma C, Liang C-Y, Jirajariyavej S, Sithinamsuwan P, Tipsuk S, Clifford DB, Paul R, Fletcher JLK, Marovich MA, Slike BM, DeGruttola V, Shiramizu B, SEARCH 011 Protocol Team . 2013. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One 8:e70164. doi: 10.1371/journal.pone.0070164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valcour VG, Shiramizu BT, Shikuma CM. 2010. HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J Leukoc Biol 87:621–626. doi: 10.1189/jlb.0809571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cattin A, Salinas TRW, Gosselin A, Planas D, Shacklett B, Cohen ÉA, Ghali MP, Routy J-P, Ancuta P. 2019. HIV-1 is rarely detected in blood and colon myeloid cells during viral-suppressive antiretroviral therapy. AIDS 33:1293. doi: 10.1097/QAD.0000000000002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collman R, Hassan NF, Walker R, Godfrey B, Cutilli J, Hastings JC, Friedman H, Douglas SD, Nathanson N. 1989. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med 170:1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naif HM, Li S, Alali M, Sloane A, Wu L, Kelly M, Lynch G, Lloyd A, Cunningham AL. 1998. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol 72:830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rich EA, Chen IS, Zack JA, Leonard ML, O'Brien WA. 1992. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J Clin Invest 89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. 1999. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A 96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Triques K, Stevenson M. 2004. Characterization of restrictions to human immunodeficiency virus type 1 infection of monocytes. J Virol 78:5523–5527. doi: 10.1128/jvi.78.10.5523-5527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamond TL, Roshal M, Jamburuthugoda VK, Reynolds HM, Merriam AR, Lee KY, Balakrishnan M, Bambara RA, Planelles V, Dewhurst S, Kim B. 2004. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J Biol Chem 279:51545–51553. doi: 10.1074/jbc.M408573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien WA. 1994. HIV-1 entry and reverse transcription in macrophages. J Leukoc Biol 56:273–277. doi: 10.1002/jlb.56.3.273. [DOI] [PubMed] [Google Scholar]
- 33.Neil S, Martin F, Ikeda Y, Collins M. 2001. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J Virol 75:5448–5456. doi: 10.1128/JVI.75.12.5448-5456.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng G, Greenwell-Wild T, Nares S, Jin W, Lei KJ, Rangel ZG, Munson PJ, Wahl SM. 2007. Myeloid differentiation and susceptibility to HIV-1 are linked to APOBEC3 expression. Blood 110:393–400. doi: 10.1182/blood-2006-10-051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. 2011. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu T, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins JI, Corey L. 2002. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14+ monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol 76:707–716. doi: 10.1128/jvi.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonza S, Mutimer HP, Oelrichs R, Jardine D, Harvey K, Dunne A, Purcell DF, Birch C, Crowe SM. 2001. Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. AIDS 15:17–22. doi: 10.1097/00002030-200101050-00005. [DOI] [PubMed] [Google Scholar]
- 38.Lambotte O, Taoufik Y, de Goër MG, Wallon C, Goujard C, Delfraissy JF. 2000. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J Acquir Immune Defic Syndr 23:114–119. doi: 10.1097/00126334-200002010-00002. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, Zhu H, Wilcox CK, van't Wout A, Andrus T, Llewellyn N, Stamatatos L, Mullins JI, Corey L, Zhu T. 2008. Blood monocytes harbor HIV type 1 strains with diversified phenotypes including macrophage-specific CCR5 virus. J Infect Dis 197:309–318. doi: 10.1086/524847. [DOI] [PubMed] [Google Scholar]
- 40.Fulcher JA, Hwangbo Y, Zioni R, Nickle D, Lin X, Heath L, Mullins JI, Corey L, Zhu T. 2004. Compartmentalization of human immunodeficiency virus type 1 between blood monocytes and CD4+ T cells during infection. J Virol 78:7883–7893. doi: 10.1128/JVI.78.15.7883-7893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almodóvar S, del C Colón M, Maldonado IM, Villafañe R, Abreu S, Meléndez I, Domínguez C, Cuevas W, Collins TM, Lorenzo E. 2007. HIV-1 infection of monocytes is directly related to the success of HAART. Virology 369:35–46. doi: 10.1016/j.virol.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Gibellini D, Borderi M, De Crignis E, Cicola R, Cimatti L, Vitone F, Chiodo F, Re MC. 2008. HIV-1 DNA load analysis in peripheral blood lymphocytes and monocytes from naive and HAART-treated individuals. J Infect 56:219–225. doi: 10.1016/j.jinf.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Baxter AE, Russell RA, Duncan CJA, Moore MD, Willberg CB, Pablos JL, Finzi A, Kaufmann DE, Ochsenbauer C, Kappes JC, Groot F, Sattentau QJ. 2014. Macrophage infection via selective capture of HIV-1-infected CD4+ T cells. Cell Host Microbe 16:711–721. doi: 10.1016/j.chom.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calantone N, Wu F, Klase Z, Deleage C, Perkins M, Matsuda K, Thompson EA, Ortiz AM, Vinton CL, Ourmanov I, Loré K, Douek DC, Estes JD, Hirsch VM, Brenchley JM. 2014. Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells. Immunity 41:493–502. doi: 10.1016/j.immuni.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Josefsson L, Stockenstrom von S, Faria NR, Sinclair E, Bacchetti P, Killian M, Epling L, Tan A, Ho T, Lemey P, Shao W, Hunt PW, Somsouk M, Wylie W, Douek DC, Loeb L, Custer J, Hoh R, Poole L, Deeks SG, Hecht F, Palmer S. 2013. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc Natl Acad Sci U S A 110:E4987–E4996. doi: 10.1073/pnas.1308313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Stockenstrom S, Odevall L, Lee E, Sinclair E, Bacchetti P, Killian M, Epling L, Shao W, Hoh R, Ho T, Faria NR, Lemey P, Albert J, Hunt P, Loeb L, Pilcher C, Poole L, Hatano H, Somsouk M, Douek D, Boritz E, Deeks SG, Hecht FM, Palmer S. 2015. Longitudinal genetic characterization reveals that cell proliferation maintains a persistent HIV type 1 DNA pool during effective HIV therapy. J Infect Dis 212:596–607. doi: 10.1093/infdis/jiv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. 2004. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol 78:1160–1168. doi: 10.1128/jvi.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koelsch KK, Liu L, Haubrich R, May S, Havlir D, Günthard HF, Ignacio CC, Campos-Soto P, Little SJ, Shafer R, Robbins GK, D'Aquila RT, Kawano Y, Young K, Dao P, Spina CA, Richman DD, Wong JK. 2008. Dynamics of total, linear nonintegrated, and integrated HIV-1 DNA in vivo and in vitro. J Infect Dis 197:411–419. doi: 10.1086/525283. [DOI] [PubMed] [Google Scholar]
- 49.Hocqueloux L, Avettand-Fénoêl V, Jacquot S, Prazuck T, Legac E, Mélard A, Niang M, Mille C, Le Moal G, Viard J-P, Rouzioux C, AC32 (Coordinated Action on HIV Reservoirs) of the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) . 2013. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother 68:1169–1178. doi: 10.1093/jac/dks533. [DOI] [PubMed] [Google Scholar]
- 50.Laanani M, Ghosn J, Essat A, Mélard A, Seng R, Gousset M, Panjo H, Mortier E, Girard P-M, Goujard C, Meyer L, Rouzioux C, Agence Nationale de Recherche sur le Sida PRIMO Cohort Study Group . 2015. Impact of the timing of initiation of antiretroviral therapy during primary HIV-1 infection on the decay of cell-associated HIV-DNA. Clin Infect Dis 60:1715–1721. doi: 10.1093/cid/civ171. [DOI] [PubMed] [Google Scholar]
- 51.Pinzone MR, Graf E, Lynch L, McLaughlin B, Hecht FM, Connors M, Migueles SA, Hwang W-T, Nunnari G, O'Doherty U. 2016. Monitoring integration over time supports a role for cytotoxic T lymphocytes and ongoing replication as determinants of reservoir size. J Virol 90:10436–10445. doi: 10.1128/JVI.00242-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandergeeten C, Fromentin R, Merlini E, Lawani MB, DaFonseca S, Bakeman W, McNulty A, Ramgopal M, Michael N, Kim JH, Ananworanich J, Chomont N. 2014. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J Virol 88:12385–12396. doi: 10.1128/JVI.00609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mexas AM, Graf EH, Pace MJ, Yu JJ, Papasavvas E, Azzoni L, Busch MP, Di Mascio M, Foulkes AS, Migueles SA, Montaner LJ, O'Doherty U. 2012. Concurrent measures of total and integrated HIV DNA monitor reservoirs and ongoing replication in eradication trials. AIDS 26:2295–2306. doi: 10.1097/QAD.0b013e32835a5c2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu JJ, Wu TL, Liszewski MK, Dai J, Swiggard WJ, Baytop C, Frank I, Levine BL, Yang W, Theodosopoulos T, O'Doherty U. 2008. A more precise HIV integration assay designed to detect small differences finds lower levels of integrated DNA in HAART treated patients. Virology 379:78–86. doi: 10.1016/j.virol.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaiser P, Joos B, Niederöst B, Weber R, Günthard HF, Fischer M. 2007. Productive human immunodeficiency virus type 1 infection in peripheral blood predominantly takes place in CD4/CD8 double-negative T lymphocytes. J Virol 81:9693–9706. doi: 10.1128/JVI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marodon G, Landau NR, Posnett DN. 1999. Altered expression of CD4, CD54, CD62L, and CCR5 in primary lymphocytes productively infected with the human immunodeficiency virus. AIDS Res Hum Retroviruses 15:161–171. doi: 10.1089/088922299311583. [DOI] [PubMed] [Google Scholar]
- 57.DeMaster LK, Liu X, VanBelzen DJ, Trinité B, Zheng L, Agosto LM, Migueles SA, Connors M, Sambucetti L, Levy DN, Pasternak AO, O'Doherty U. 2015. A subset of CD4/CD8 double-negative T cells expresses HIV proteins in patients on antiretroviral therapy. J Virol 90:2165–2179. doi: 10.1128/JVI.01913-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piguet V, Schwartz O, Le Gall S, Trono D. 1999. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol Rev 168:51–63. doi: 10.1111/j.1600-065x.1999.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 59.Glushakova S, Munch J, Carl S, Greenough TC, Sullivan JL, Margolis L, Kirchhoff F. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J Virol 75:10113–10117. doi: 10.1128/JVI.75.21.10113-10117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pardons M, Baxter AE, Massanella M, Pagliuzza A, Fromentin R, Dufour C, Leyre L, Routy J-P, Kaufmann DE, Chomont N. 2019. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog 15:e1007619. doi: 10.1371/journal.ppat.1007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheney KM, Kumar R, Purins A, Mundy L, Ferguson W, Shaw D, Burrell CJ, Li P. 2006. HIV type 1 persistence in CD4−/CD8− double negative T cells from patients on antiretroviral therapy. AIDS Res Hum Retroviruses 22:66–75. doi: 10.1089/aid.2006.22.66. [DOI] [PubMed] [Google Scholar]
- 62.Ndhlovu LC, D'Antoni ML, Ananworanich J, Byron MM, Chalermchai T, Sithinamsuwan P, Tipsuk S, Ho E, Slike BM, Schuetz A, Zhang G, Agsalda-Garcia M, Shiramizu B, Shikuma CM, Valcour V, SEARCH 011 Study Group . 2015. Loss of CCR2 expressing non-classical monocytes are associated with cognitive impairment in antiretroviral therapy-naive HIV-infected Thais. J Neuroimmunol 288:25–33. doi: 10.1016/j.jneuroim.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kallianpur KJ, Valcour VG, Lerdlum S, Busovaca E, Agsalda M, Sithinamsuwan P, Chalermchai T, Fletcher JLK, Tipsuk S, Shikuma CM, Shiramizu BT, Ananworanich J, SEARCH 011 Study Group . 2014. HIV DNA in CD14+ reservoirs is associated with regional brain atrophy in patients naive to combination antiretroviral therapy. AIDS 28:1619–1624. doi: 10.1097/QAD.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong KL, Tai JJ-Y, Wong W-C, Han H, Sem X, Yeap W-H, Kourilsky P, Wong S-C. 2011. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 118:e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 65.Ellery PJ, Tippett E, Chiu Y-L, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM. 2007. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol 178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 66.Jaworowski A, Kamwendo DD, Ellery P, Sonza S, Mwapasa V, Tadesse E, Molyneux ME, Rogerson SJ, Meshnick SR, Crowe SM. 2007. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis 196:38–42. doi: 10.1086/518443. [DOI] [PubMed] [Google Scholar]
- 67.Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V. 2005. Circulating proviral HIV DNA and HIV-associated dementia. AIDS 19:45–52. doi: 10.1097/00002030-200501030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho Y-C, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DIS, Lai J, Blankson JN, Siliciano JD, Siliciano RF. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song H, Ou W, Feng Y, Zhang J, Li F, Hu J, Peng H, Xing H, Ma L, Tan Q, Li D, Wang L, Wu B, Shao Y. 2019. Disparate impact on CD4 T cell count by two distinct HIV-1 phylogenetic clusters from the same clade. Proc Natl Acad Sci U S A 116:239–244. doi: 10.1073/pnas.1814714116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Xue Y, Zhou L, Lin Y, Yu X, Wang X, Zhen X, Zhang W, Ning Z, Yue Q, Fu J, Shen F, Gai J, Xu Y, Mao J, Gao X, Shen X, Kang L, Vanham G, Cheng H, Wang Y, Zhuang M, Zhuang X, Pan Q, Zhong P. 2014. Evidence that HIV-1 CRF01_AE is associated with low CD4+T cell count and CXCR4 co-receptor usage in recently infected young men who have sex with men (MSM) in Shanghai, China. PLoS One 9:e89462. doi: 10.1371/journal.pone.0089462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valcour VG, Sithinamsuwan P, Nidhinandana S, Thitivichianlert S, Ratto-Kim S, Apateerapong W, Shiramizu BT, Desouza MS, Chitpatima ST, Watt G, Chuenchitra T, Robertson KR, Paul RH, McArthur JC, Kim JH, Shikuma CM. 2007. Neuropsychological abnormalities in patients with dementia in CRF 01_AE HIV-1 infection. Neurology 68:525–527. doi: 10.1212/01.wnl.0000253196.78193.c7. [DOI] [PubMed] [Google Scholar]