Recombinant trimeric proteins based on HIV-1 env genes are being developed for vaccine trials in humans. A feature of these proteins is their mimicry of the envelope glycoprotein structure on virus particles that is targeted by neutralizing antibodies, i.e., antibodies that prevent cells from becoming infected. One vaccine concept under exploration is that recombinant trimers may be able to elicit virus-neutralizing antibodies when delivered as immunogens. A commonly used design is designated SOSIP.664, a term reflecting the sequence changes that are used to stabilize the trimers and allow their production in practically useful amounts. Here, we show that these stabilizing changes act to increase trimer yield during the biosynthesis process within the producer cell but have little impact on the properties of purified trimers.
KEYWORDS: HIV-1 vaccine, Env trimers
ABSTRACT
Soluble recombinant native-like (NL) envelope glycoprotein (Env) trimers of various human immunodeficiency virus type 1 (HIV-1) genotypes are being developed as vaccine candidates aimed at the induction of broadly neutralizing antibodies (bNAbs). The prototypic design, designated BG505 SOSIP.664, incorporates an intersubunit disulfide bond (SOS) to covalently link the gp120 and gp41 ectodomain (gp41ECTO) subunits and a point substitution, I559P (IP), to further stabilize the gp41ECTO components. Without the SOS and IP changes, proteolytically cleaved trimers tend to disintegrate into their constituent gp120 and gp41ECTO subunits. We show, however, that NL trimers lacking the SOS and/or IP change can be affinity purified in amounts sufficient for analyses of their antigenicity and thermal stability. In general, these trimer variants have properties highly comparable to those of the fully stabilized SOSIP.664 version. We conclude that the major effect of the SOS and IP changes is to substantially increase trimer stability during and after the expression process, thereby allowing useful amounts to be produced. However, once the trimers have been purified, the SOS and IP changes have only subtle impacts on thermostability and the antigenicity of bNAb and other epitopes.
IMPORTANCE Recombinant trimeric proteins based on HIV-1 env genes are being developed for vaccine trials in humans. A feature of these proteins is their mimicry of the envelope glycoprotein structure on virus particles that is targeted by neutralizing antibodies, i.e., antibodies that prevent cells from becoming infected. One vaccine concept under exploration is that recombinant trimers may be able to elicit virus-neutralizing antibodies when delivered as immunogens. A commonly used design is designated SOSIP.664, a term reflecting the sequence changes that are used to stabilize the trimers and allow their production in practically useful amounts. Here, we show that these stabilizing changes act to increase trimer yield during the biosynthesis process within the producer cell but have little impact on the properties of purified trimers.
INTRODUCTION
The envelope glycoprotein (Env) trimer is targeted by neutralizing antibodies (NAbs) that arise during infection by human immunodeficiency virus type 1 (HIV-1) and is a focus of vaccine development programs (1–9). A subset of NAbs active against multiple circulating HIV-1 strains, referred to as broadly neutralizing antibodies (bNAbs), target relatively conserved epitopes on the trimer surface. Immunogens that present multiple bNAb epitopes are being evaluated for their abilities to induce similar antibodies in test animals and humans (1–11). The Env trimer is metastable, which reflects its need to undergo receptor-mediated conformational changes during the process of virus-cell fusion but creates challenges when making useful amounts as a recombinant protein (7, 10, 11). Generally, trimers are produced in secreted (i.e., soluble) form to improve yields and simplify purification procedures. However, proteolytically cleaved soluble trimers are quite fragile and require stabilization by protein engineering (7, 12). The prototype of the native-like (NL) soluble trimer design, designated SOSIP.664, includes an engineered intersubunit disulfide bond (SOS) between gp120 and the gp41 ectodomain (gp41ECTO), a gp41-stabilizing substitution (I559P [IP]), and a truncation at gp41ECTO residue 664 (7, 12). Further SOSIP trimer design improvements have been described (7, 13–17).
The SOSIP.664 and related trimers of various genotypes present all known bNAb epitopes, except those in the membrane-proximal external region (MPER) that is not included in the design (7, 12). When tested in various species, these trimers generally induce autologous NAbs, but heterologous tier-2 NAbs are induced only weakly and/or inconsistently (13, 14, 17–20). Given an increasingly detailed body of information about how bNAbs emerge during HIV-1 infection, it is not surprising that a protein immunogen does not induce bNAbs efficiently by itself (1, 3, 8, 21–23). It has been suggested, however, that SOSIP trimers are not in the correct conformation to induce bNAbs, i.e., that the SOS and IP changes have detrimental effects on epitope presentation (24–28). The methods and data interpretation underlying these assertions have been questioned (see references 26 and 29 and the comment by J. P. Moore at https://www.nature.com/articles/s41586-019-1101-y#article-comments). Here, we show that affinity-purified NL trimers of the prototypic BG505 genotype are highly similar antigenically, irrespective of whether they include the SOS and/or IP changes. Thus, stabilization modifications are required to allow the secretion of practically useful amounts of NL trimers from transfected cells, but once the trimers have been released, they can be purified and analyzed. We suggest, therefore, that the limited ability of a single genotype of SOSIP trimer to induce bNAbs is due to the long-recognized disconnect between antigenicity and immunogenicity and not to poor antigenicity per se. We propose that SOSIP trimers can continue to serve as an immunogen design platform harnessed to knowledge of how the human immune system generates bNAbs.
RESULTS
Expression and affinity purification of BG505 trimer variants.
The BG505 (clade A) SOSIP.664 construct and the variants used in this study have all been described previously (30). In some cases, the Env proteins contain a C-terminal His tag to facilitate immunoassays (see Materials and Methods) (13, 31). To facilitate comparison with our earlier study, we have adopted the terminology used there to describe each construct (Table 1). The standard BG505-based construct, usually referred to as SOSIP.664, is referred to here as SOSIP.R6; the variant lacking the I559P change (i.e., Ile-559) is SOS.R6; the one lacking the SOS bond (i.e., Ala-501 and Thr-605) is IP.R6; and when both stabilizing changes are absent, the construct name is WT.R6. The R6 terminology indicates the presence of the RRRRRR sequence in place of the REKR cleavage site at residues 508 to 511 to optimize the furin protease cleavage of the expressed gp140 proteins (32).
TABLE 1.
Design and properties of BG505 trimer variants
| Constructa | Purificationb | Yield (mg/liter)c | % NL trimersd | Tm (°C)e |
|---|---|---|---|---|
| SOSIP.R6 | PGT151 | 3–4 | 94 | 66.5 |
| SOS.R6 | PGT151 | 0.5–0.8 | 91 | 66.6 |
| IP.R6 | PGT151 | 0.8–1 | 86 | 65.8 |
| WT.R6 | PGT151 | 0.1–0.2 | 100 | 66.4 |
| SOSIP.R6 | 2G12/SEC | 2–2.5 | >95 | ND |
| SOS.R6 | 2G12/SEC | 0.8–1 | 56 | ND |
| IP.R6 | 2G12/SEC | 1.2–1.8 | 29 | ND |
| WT.R6 | 2G12/SEC | 0.2–0.4 | 17 | ND |
| SOSIP.R6 | PGT145 | 3–4 | >95 | ND |
| SOS.R6 | PGT145 | 0.6–0.8 | >95 | ND |
| IP.R6 | PGT145 | 0.8–1 | >95 | ND |
| WT.R6 | PGT145 | 0.1–0.15 | >95 | ND |
The constructs are summarized in Fig. 1 and reference 30. The proteins were expressed by transient transfection of ExpiCHO cells (PGT151 or 2G12/SEC purification) or 293F cells (PGT145 purification). All the proteins contained a C-terminal His tag, except the PGT145-purified trimers that were visualized by nsEM.
The affinity column-based chromatography methods used to purify trimers are described in the text.
The total amounts of trimers purified from the transfection culture supernatants via the stated affinity column are shown. The yields for the PGT145-purified trimers are for the nontagged versions.
The purified trimers were assessed by nsEM and classified as NL or nonnative. The ratio of NL to total trimers (100%) was then determined.
The Tm values determined by DSC are listed. ND, not done (insufficient yields).
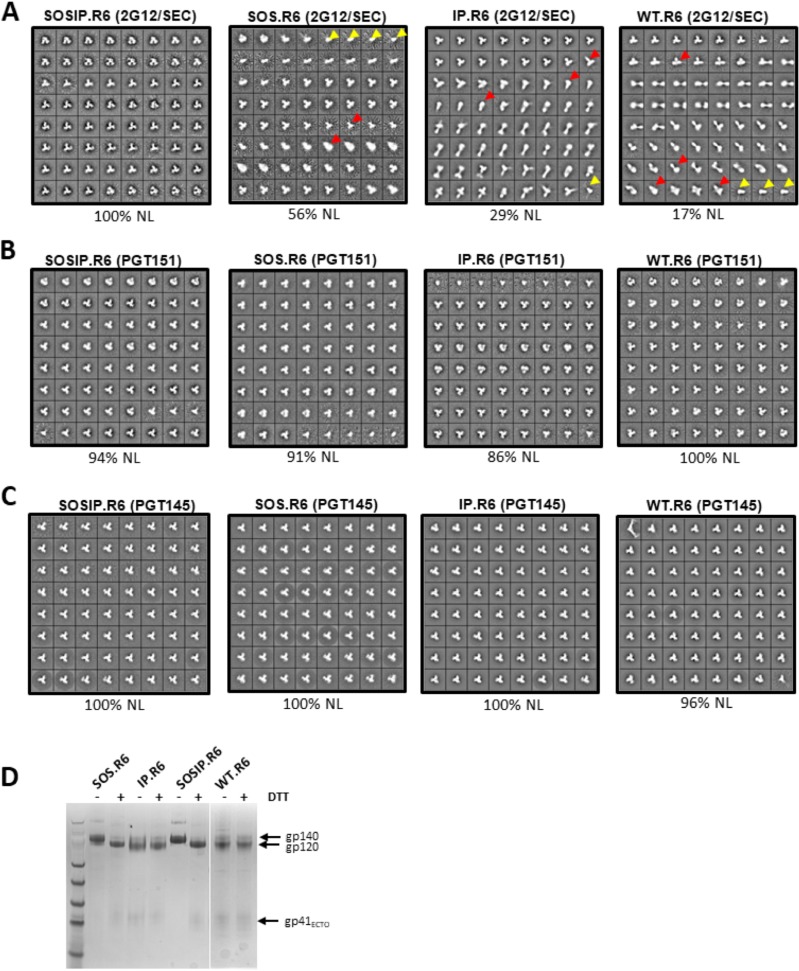
The four Env constructs described above were all expressed by transient transfection of ExpiCHO cells, together with furin. The resulting supernatants were then processed via the 2G12/SEC or PGT151 affinity column-based method to isolate trimer-containing fractions. Unlike the 2G12 column, the PGT151 column specifically purifies NL trimers. Alternatively, Env proteins were expressed in 293F cells and purified by affinity chromatography using the trimer-selective PGT145 bNAb. The total amounts of trimers purified by each method for each construct were quantified by the bicinchoninic acid (BCA) method. The 2G12/SEC procedure yielded larger amounts of the non-SOSIP trimers than did the PGT151 or PGT145 column (Table 1). The implication is that only a subset of the trimers derived from the SOS.R6, IP.R6, and WT.R6 constructs are in NL form and hence PGT151 or PGT145 reactive. Visualization of the various batches of purified Env proteins using negative-stain electron microscopy (nsEM) confirmed this supposition. Thus, the 2G12/SEC method yielded 100% NL trimers only for the SOSIP.R6 construct and ever-decreasing proportions for SOS.R6 (56%), IP.R6 (29%), and WT.R6 (17%) (Fig. 1A and Table 1). In each case, the remainder of the Env proteins were in various nonnative forms, mostly trimeric but with some monomers also visible. In contrast, similar proportions (86 to 100%) of the PGT151- or PGT145-purified Env proteins from each construct were NL trimers (Fig. 1B and C and Table 1). SDS-PAGE gels under nonreducing and reducing conditions confirmed that the PGT151-purified trimers from each of the four constructs were fully cleaved. On the gels, the trimers from the IP.R6 and WT.R6 constructs dissociated into gp120 and not gp140 proteins under both reducing and nonreducing conditions, which is attributable to the absence of the intersubunit SOS bond (Fig. 1D).
FIG 1.
(A to C) nsEM visualization of 2G12/SEC-, PGT151-, and PGT145-purified trimers. Env proteins secreted from transiently transfected ExpiCHO cells were purified by the 2G12/SEC (A) or PGT151 (B) affinity chromatography method or secreted from transiently transfected 293F cells and purified by PGT145 affinity chromatography (C). The percentage of total trimer in NL form shown by nsEM imaging is indicated below each image. In the 2G12/SEC-purified SOSIP.R6, SOS.R6, IP.R6, and WT.R6 preparations, non-NL trimers (indicated by the red arrowheads) were estimated to be present at 0%, 25%, 68%, and 78% and monomers (yellow arrowheads) at 0%, 19%, 3%, and 5%, respectively. (D) SDS-PAGE gels under nonreducing and reducing conditions of PGT151-purified trimers from each of the four constructs. DTT, dithiothreitol.
In summary, the elimination of the SOS and/or IP changes from the standard construct substantially impacts the proportion of them that are in NL form (Fig. 1 and Table 1). Nonetheless, NL trimers can be purified from each of the four constructs on a PGT151 or PGT145 affinity column in amounts sufficient to allow their visualization by nsEM.
Antigenicity of PGT151-purified trimers.
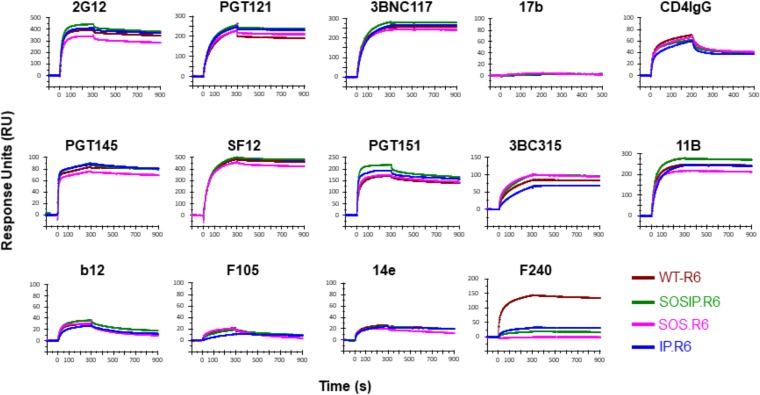
We used surface plasmon resonance (SPR) to assess the antigenicity of the four PGT151-purified NL trimer preparations against a panel of bNAbs representing 7 distinct epitope clusters and also CD4-IgG and non-NAbs (see Materials and Methods for their epitopes). The trimers were immobilized on the chip surface via their C-terminal His tags, and the test monoclonal antibodies (MAbs) were then allowed to flow over them (Fig. 2). There were only modest differences in bNAb and CD4-IgG binding among the four trimers. The variation in extents of binding at the end of the association was <30%; binding to IP.R6 or SOS.R6 was somewhat lower than to SOSIP.R6, and the kinetic profiles were superimposable (Fig. 2). The N241/289 glycan hole neutralization epitope for the autologous NAb 11B was also similarly antigenic on the four trimers; SOS.R6 was the least reactive, but the binding signal for the trimer was only ∼20% lower than for SOSIP.R6. In contrast, neither the CD4 binding site epitope for non-NAb F105 nor the V3 epitope for non-NAb 14e was well exposed on any of the trimers. The F240 non-NAb to a gp41ECTO cluster 1 epitope reacted strongly with only the WT.R6 trimer (Fig. 2). The variable reactivity for F240 occurs because the epitope overlaps the location of the intersubunit SOS bond, which is not present in the WT.R6 trimer. We conclude that the PGT151-purified trimers adopt similar conformations, judging by their antibody reactivity profiles. Of note is that bNAb binding to the SOSIP.R6 trimer was never lower than to its WT.R6 counterpart. We were unable to purify sufficient His-tagged SOS.R6, IP.R6, and WT.R6 trimers via the PGT145 affinity column for an antigenicity analysis.
FIG 2.
Antigenicity of PGT151-purified trimers by SPR. The indicated BG505 trimer variants were immobilized on the chip surface via their C-terminal His tags. The binding of the indicated antibodies or CD4-IgG, added at 500 nM, was monitored in RU (y axis) over time (s) (x axis). Note that the scales on the y axes are not identical, with top values of 100 to 500 RU.
Thermal stability of PGT151-purified trimers.
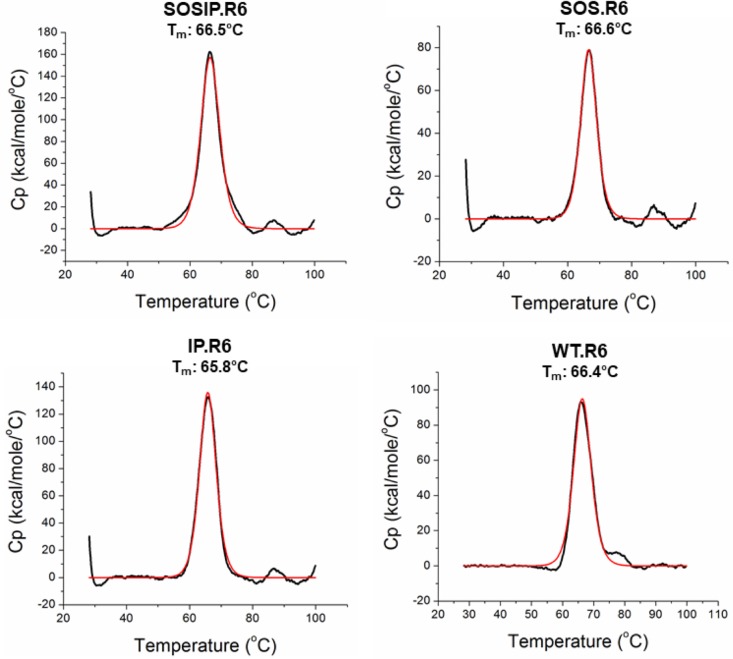
The four PGT151-purified NL trimer preparations were analyzed by differential scanning calorimetry (DSC) to assess their thermal stabilities (Fig. 3). The midpoint melting temperatures (Tm) were highly similar, ranging from 65.8 to 66.6°C (Table 1). Thus, once assembled, secreted, and purified, the various NL-trimer preparations are highly stable, irrespective of whether the SOS and/or IP stabilizing changes are present. The DSC analysis required more of the SOS.R6, IP.R6, and WT.R6 trimers than we could purify via the PGT145 affinity column.
FIG 3.
Thermostability of PGT151-purified trimers by DSC. Thermal denaturation curves for the four trimer variants are shown. The resulting Tm values are listed in Table 1. Cp, heat capacity.
DISCUSSION
The engineered SOS and IP sequence changes integral to the SOSIP design confer stability on cleaved trimers in expression cultures. Without the sequence mutations, a substantial proportion of the trimers either do not assemble properly or cannot survive long enough postsecretion to accumulate in the supernatant. However, once synthesized and secreted, enough fully native-like trimers that lack the SOS and IP changes can be affinity purified by the conformationally selective PGT151 or PGT145 bNAbs and then analyzed. The analyses show that the four PGT151-purified trimers are all highly thermally stable, with Tm values of 65.8 to 66.6°C, irrespective of whether the SOS and IP changes are present. We have not attempted to assess longer-term stability, as there is no practical utility to removing these stabilizing changes because of the reduced yields. Only nsEM could be used to study the PGT145-purified His-tagged trimers due to yield constraints.
Antigenically, the PGT151-purified trimers that contain or lack the SOS and IP changes are quite similar in respect to their presentation of bNAb and most non-NAb epitopes; the WT.R6 variant, which lacks both the SOS and IP changes, is not antigenically superior to the fully stabilized SOSIP.R6 trimer. The purified trimers, irrespective of the affinity chromatography method used, are also indistinguishable at the level of resolution provided by nsEM imaging. Cryo-electron microscopy (cryo-EM) analyses at much higher resolution provide strong additional support for the similarity between full-length membrane-associated trimers and soluble SOSIP trimer structures (29, 33, 34). The SOS and IP changes, when added to fusion-competent, full-length Env proteins, do compromise fusion function, because they impede the full sequence of CD4-induced conformational changes that are involved in the process (35). Even so, the SOS bond is compatible with membrane fusion provided it is broken by the addition of a reducing agent at an appropriate time (36). It has been reported elsewhere that the SOS and IP changes perturb the structure of trimers and create entities that are not appropriate mimics of “native Env proteins” (24–28). However, much of the methodology used to draw such conclusions is questionable. Immunoprecipitation and/or staining assays based on MAb reactivity with Env proteins on the surfaces of transfected cells are highly problematic, as there is abundant evidence that a substantial fraction of cell surface Env is in nonnative and antigenically distinctive forms (37–39). A recent biophysics-based study reinforced and expanded upon these longstanding concerns (40).
It has been asserted that SOSIP.664 trimers differ from the native (virion-associated) form of the Env trimer, as judged by a single-molecule fluorescence resonance energy transfer (smFRET) assay of conformational flexibility (26). Specifically, smFRET reports that probe-modified BG505 SOSIP.664 trimers occupy three conformational states, but predominantly state 2, whereas the Env proteins on BG505 HIV-1 virions are mostly in state 1. Both forms of Env occasionally can adopt a “fully open” state 3 conformation that resembles the CD4 receptor-bound structure (26). However, a different conclusion about the conformational state of SOSIP.664 trimers was drawn based on an alternative spectroscopy technique to assess protein conformational transitions, double electron-electron resonance (DEER) spectroscopy (41). In summary, the DEER experiments showed that BG505 SOSIP.664 trimers were in a single conformational state with respect to the trimer apex and the distance between the probes in the V1 and V4 loops (41). Moreover, all of the distances measured using DEER were consistent with the X-ray crystallography and cryo-EM structures of various SOSIP trimers (41–45). Concerns have now been expressed about the validity of the smFRET methodology used to study SOSIP trimers (see references 26 and 29 and the comment by J. P. Moore at https://www.nature.com/articles/s41586-019-1101-y#article-comments). Additional studies by research groups experienced in smFRET and related biophysics techniques are clearly warranted.
The immune system responds to adjuvanted solution phase proteins over a period measured in hours or days, whereas biophysics methods measure conformational transitions over a time scale of micro- to milliseconds, while antigenicity assays report over a time scale measured in seconds. Any conformation that an HIV-1 Env trimer can adopt on that time scale will certainly be sampled by the immune system under the in vivo conditions relevant to vaccine delivery. The SOSIP.664 trimer and its later-generation derivatives consistently present bNAb epitopes from every known cluster except the absent MPER with high affinity, as shown by multiple techniques (7, 9, 15, 46–48). However, while the absence of a bNAb epitope(s) from an immunogen is clearly problematic, the presence of one does not mandate that it will induce similar antibodies; antigenicity does not predict immunogenicity. The key difficulty in HIV-1 vaccine design at present is not the presentation of bNAb epitopes to the immune system, it is finding ways to persuade the host to respond to those epitopes. Virion Env induces bNAbs in only a minority of HIV-1-infected people after many years of varying antigenic stimuli resulting from neutralization escape and multiple cycles of somatic hypermutation (1–9, 49, 50). It is highly unlikely that any purified HIV-1 Env protein, whether a SOSIP trimer or another, could mimic those processes to induce bNAbs by itself. Various groups are already pursuing persuasive pathways to bNAb induction that harness both structural biology and immunology insights while using SOSIP trimers as a design platform (1–9, 50, 51).
MATERIALS AND METHODS
Env construct design, protein production, and trimer purification.
(i) 2G12- or PGT151-purified trimers. The BG505 SOSIP trimer construct and the variants used in this study have all been described previously (Table 1) (30). All the constructs contain an 8-amino-acid Gly-Ser linker and then an 8-histidine tag immediately after residue 664 in gp41ECTO to facilitate SPR procedures (47). The Env proteins were expressed by transient transfection of ExpiCHO cells in serum-free medium, with a cotransfected furin expression construct to maximize gp140 cleavage (31, 52). Env proteins were purified from the resulting culture supernatants using a 2G12 or a PGT151 bNAb affinity column, as previously described (12, 31, 52). The proteins eluted from the 2G12 column were further processed using a Superdex 200 26/60 size exclusion chromatography (SEC) column to isolate the trimer fractions (12, 30).
(ii) PGT145-purified trimers. The same BG505-based constructs were used as for PGT151-purified trimers, except that the nsEM images were obtained using trimers that did not contain a C-terminal His tag. The Env proteins were expressed by transient transfection of 293F cells (with cotransfected furin), and the trimers were purified with a PGT145 bNAb affinity column as described previously (31, 52). We used 293F cells rather than ExpiCHO cells to avoid possible problems associated with PGT145 columns under some circumstances (53). However, the smaller scale of 293F cell cultures prevented us from purifying enough of the unstable His-tagged SOS.R6, IP.R6, and WT.R6 trimers for the DSC and SPR analyses.
The amounts of trimers purified by each method were quantified using the BCA protein quantitation method (ThermoFisher). Native PAGE and SDS-PAGE analyses were performed as described previously (30).
Negative-stain electron microscopy.
Samples were diluted to 0.01 mg/ml in 1× Tris-buffered saline (TBS), pH 7.4, and 3 μl was deposited on plasma-cleaned (argon/oxygen) carbon-coated copper mesh grids (Electron Microscopy Sciences). A 2% (wt/vol) solution of uranyl formate was used to stain the grids for 50 s. The grids were transferred to a ThermoFisher Tecnai Spirit (120 keV) with a TVIPS complementary metal oxide semiconductor (CMOS) (4,000 by 4,000) camera for data collection, which was automated using Leginon software (54). Data were collected at ×52,000 magnification, −1.5-μm defocus, a pixel size of 2.05 Å, and a total dose of 25 e−/Å2. Micrographs were stored in the Appion database (55). DoGpicker (56) was used to pick particles, which were then stacked with a box size of 160 pixels and aligned with iterative multivariate statistical analysis/multireference alignment (MSA/MRA) (57). Any classes with contaminants or double particles were removed from further analysis. Classes showing monomers or native-like or non-native-like phenotypes were selected for and quantified. The percentage of each phenotype was calculated by dividing by the total number of particles.
Differential scanning calorimetry.
A MicroCal VP-Capillary DSC calorimeter (Malvern Instruments) was used for thermal denaturation of 125 μg of purified trimers (all at ∼0.9 mg/ml), which had been buffer exchanged into 1× phosphate-buffered saline (PBS), pH 7.4. The temperature range used was 25 to 100°C with a scan rate of 60° per hour. Data were analyzed with Origin 7.0 software and fitted using the non-two-state model.
Antibodies.
MAbs were obtained as gifts or purchased from the following sources: F105, John Mascola; 3BNC117 and 3BC315, Michel Nussenzweig; PGT121, PGT145, PGT151, b12, and F240, International AIDS Vaccine Initiative; CD4-IgG, Michael Farzan; 2G12, Polymun Scientific; 14e and 17b, James Robinson; 11B, Rogier Sanders; and SF12, Pamela Bjorkman. The epitope clusters recognized by the bNAbs are as follows: 2G12, outer-domain N-glycans (2); PGT121, V3 base and glycan (2); 3BNC117, CD4 binding site (2); 3BC315, gp41 and interprotomer (2); PGT145, V2 apex (2); SF12, the center of what was previously known as the “silent face” (58); PGT151, the gp120-gp41 interface (2); b12, the CD4 binding site (2). MAb 11B is a BG505-specific autologous NAb (N241/289 glycan hole) (59). The non-NAbs tested were F240 (gp41 cluster I) (60), 14e (V3), and 17b (CD4-induced site) (61). CD4-IgG is an engineered protein with neutralization activity, based on soluble CD4, that recognizes the CD4 binding site (62).
Surface plasmon resonance.
SPR analyses were performed with His-tagged trimers immobilized by anti-His antibody covalently linked to CM5 chips, as previously described (14, 47, 48). All experiments were conducted at 25°C. The HBS-EP running buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% surfactant P20) was used in all the experiments at a flow rate of 50 μl/min. Briefly, for binding analysis of IgGs of MAbs at a single concentration (500 nM), His-tagged variants of the BG505 trimers were immobilized to a response of ligand (RL) value of 280 ± 3.7 (mean ± standard error of the mean [SEM]) response units [RU] on anti-His CM5 sensor chips. MAbs were injected for 300 s and allowed to dissociate for 600 s. At the end of each cycle, the sensor chip surface was regenerated with a single pulse of 10 mM glycine (pH 2.0) for 60 s at a flow rate of 75 μl/min. The data presented are representative of 2 or 3 replicates.
ACKNOWLEDGMENTS
This research was supported by NIH grant P01 AI 110657 and by Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery grant OPP1115782.
We are grateful to the various MAb donors.
REFERENCES
- 1.Andrabi R, Bhiman JN, Burton DR. 2018. Strategies for a multi-stage neutralizing antibody-based HIV vaccine. Curr Opin Immunol 53:143–151. doi: 10.1016/j.coi.2018.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton DR, Hangartner L. 2016. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu Rev Immunol 34:635–659. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escolano A, Dosenovic P, Nussenzweig MC. 2017. Progress toward active or passive HIV-1 vaccination. J Exp Med 214:3–16. doi: 10.1084/jem.20161765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes BF, Burton DR. 2017. Developing an HIV vaccine. Science 355:1129–1130. doi: 10.1126/science.aan0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haynes BF, Mascola JR. 2017. The quest for an antibody-based HIV vaccine. Immunol Rev 275:5–10. doi: 10.1111/imr.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwong PD, Mascola JR. 2018. HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity 48:855–871. doi: 10.1016/j.immuni.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Sanders RW, Moore JP. 2017. Native-like Env trimers as a platform for HIV-1 vaccine design. Immunol Rev 275:161–182. doi: 10.1111/imr.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamatatos L, Pancera M, McGuire AT. 2017. Germline-targeting immunogens. Immunol Rev 275:203–216. doi: 10.1111/imr.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward AB, Wilson IA. 2017. The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunol Rev 275:21–32. doi: 10.1111/imr.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey AK, Cupo A, Ozorowski G, Sharma VK, Behrens AJ, Go EP, Ketas TJ, Yasmeen A, Klasse PJ, Sayeed E, Desaire H, Crispin M, Wilson IA, Sanders RW, Hassell T, Ward AB, Moore JP. 2018. cGMP production and analysis of BG505 SOSIP.664, an extensively glycosylated, trimeric HIV-1 envelope glycoprotein vaccine candidate. Biotechnol Bioeng 115:885–899. doi: 10.1002/bit.26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitaker N, Hickey JM, Kaur K, Xiong J, Sawant N, Cupo A, Lee WH, Ozorowski G, Medina-Ramirez M, Ward AB, Sanders RW, Moore JP, Joshi SB, Volkin DB, Dey AK. 2019. Developability assessment of physicochemical properties and stability profiles of HIV-1 BG505 SOSIP.664 and BG505 SOSIP.v4.1-GT1.1 gp140 envelope glycoprotein trimers as candidate vaccine antigens. J Pharm Sci 108:2264–2277. doi: 10.1016/j.xphs.2019.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, Kim HJ, Blattner C, de la Pena AT, Korzun J, Golabek M, de Los Reyes K, Ketas TJ, van Gils MJ, King CR, Wilson IA, Ward AB, Klasse PJ, Moore JP. 2013. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog 9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Taeye SW, Ozorowski G, Torrents de la Pena A, Guttman M, Julien JP, van den Kerkhof TL, Burger JA, Pritchard LK, Pugach P, Yasmeen A, Crampton J, Hu J, Bontjer I, Torres JL, Arendt H, DeStefano J, Koff WC, Schuitemaker H, Eggink D, Berkhout B, Dean H, LaBranche C, Crotty S, Crispin M, Montefiori DC, Klasse PJ, Lee KK, Moore JP, Wilson IA, Ward AB, Sanders RW. 2015. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell 163:1702–1715. doi: 10.1016/j.cell.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torrents de la Pena A, Julien JP, de Taeye SW, Garces F, Guttman M, Ozorowski G, Pritchard LK, Behrens AJ, Go EP, Burger JA, Schermer EE, Sliepen K, Ketas TJ, Pugach P, Yasmeen A, Cottrell CA, Torres JL, Vavourakis CD, van Gils MJ, LaBranche C, Montefiori DC, Desaire H, Crispin M, Klasse PJ, Lee KK, Moore JP, Ward AB, Wilson IA, Sanders RW. 2017. Improving the immunogenicity of native-like HIV-1 envelope trimers by hyperstabilization. Cell Rep 20:1805–1817. doi: 10.1016/j.celrep.2017.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon YD, Pancera M, Acharya P, Georgiev IS, Crooks ET, Gorman J, Joyce MG, Guttman M, Ma X, Narpala S, Soto C, Terry DS, Yang Y, Zhou T, Ahlsen G, Bailer RT, Chambers M, Chuang G-Y, Doria-Rose NA, Druz A, Hallen MA, Harned A, Kirys T, Louder MK, O'Dell S, Ofek G, Osawa K, Prabhakaran M, Sastry M, Stewart-Jones GBE, Stuckey J, Thomas PV, Tittley T, Williams C, Zhang B, Zhao H, Zhou Z, Donald BR, Lee LK, Zolla-Pazner S, Baxa U, Schön A, Freire E, Shapiro L, Lee KK, Arthos J, Munro JB, Blanchard SC, Mothes W, Binley JM, McDermott AB, Mascola JR, Kwong PD. 2015. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat Struct Mol Biol 22:522–531. doi: 10.1038/nsmb.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steichen JM, Kulp DW, Tokatlian T, Escolano A, Dosenovic P, Stanfield RL, McCoy LE, Ozorowski G, Hu X, Kalyuzhniy O, Briney B, Schiffner T, Garces F, Freund NT, Gitlin AD, Menis S, Georgeson E, Kubitz M, Adachi Y, Jones M, Mutafyan AA, Yun DS, Mayer CT, Ward AB, Burton DR, Wilson IA, Irvine DJ, Nussenzweig MC, Schief WR. 2016. HIV vaccine design to target germline precursors of glycan-dependent broadly neutralizing antibodies. Immunity 45:483–496. doi: 10.1016/j.immuni.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torrents de la Peña A, Sanders RW. 2018. Stabilizing HIV-1 envelope glycoprotein trimers to induce neutralizing antibodies. Retrovirology 15:63. doi: 10.1186/s12977-018-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, Arendt H, Kim HJ, Lee JH, Pugach P, Williams M, Debnath G, Moldt B, van Breemen MJ, Isik G, Medina-Ramirez M, Back JW, Koff WC, Julien JP, Rakasz EG, Seaman MS, Guttman M, Lee KK, Klasse PJ, LaBranche C, Schief WR, Wilson IA, Overbaugh J, Burton DR, Ward AB, Montefiori DC, Dean H, Moore JP. 2015. HIV-1 vaccines. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng C, Pancera M, Bossert A, Schmidt SD, Chen RE, Chen X, Druz A, Narpala S, Doria-Rose NA, McDermott AB, Kwong PD, Mascola JR. 2015. Immunogenicity of a prefusion HIV-1 envelope trimer in complex with a quaternary-structure-specific antibody. J Virol 90:2740–2755. doi: 10.1128/JVI.02380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, Eroshkin AM, Guenaga J, Kaushik K, Kulp DW, Liu J, McCoy LE, Oom AL, Ozorowski G, Post KW, Sharma SK, Steichen JM, de Taeye SW, Tokatlian T, Torrents de la Pena A, Butera ST, LaBranche CC, Montefiori DC, Silvestri G, Wilson IA, Irvine DJ, Sanders RW, Schief WR, Ward AB, Wyatt RT, Barouch DH, Crotty S, Burton DR. 2017. Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 46:1073–1088 e1076. doi: 10.1016/j.immuni.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonsignori M, Zhou T, Sheng Z, Chen L, Gao F, Joyce MG, Ozorowski G, Chuang G-Y, Schramm CA, Wiehe K, Alam SM, Bradley T, Gladden MA, Hwang K-K, Iyengar S, Kumar A, Lu X, Luo K, Mangiapani MC, Parks RJ, Song H, Acharya P, Bailer RT, Cao A, Druz A, Georgiev IS, Kwon YD, Louder MK, Zhang B, Zheng A, Hill BJ, Kong R, Soto C, Mullikin JC, Douek DC, Montefiori DC, Moody MA, Shaw GM, Hahn BH, Kelsoe G, Hraber PT, Korber BT, Boyd SD, Fire AZ, Kepler TB, Shapiro L, Ward AB, Mascola JR, Liao H-X, Kwong PD, Haynes BF. 2016. Maturation pathway from germline to broad HIV-1 neutralizer of a CD4-mimic antibody. Cell 165:449–463. doi: 10.1016/j.cell.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, NISC Comparative Sequencing Program, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelsoe G, Haynes BF. 2018. What are the primary limitations in B-cell affinity maturation, and how much affinity maturation can we drive with vaccination? Breaking through immunity’s glass ceiling. Cold Spring Harb Perspect Biol 10:a029397. doi: 10.1101/cshperspect.a029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alsahafi N, Anand SP, Castillo-Menendez L, Verly MM, Medjahed H, Prevost J, Herschhorn A, Richard J, Schon A, Melillo B, Freire E, Smith AB III, Sodroski J, Finzi A. 2018. SOSIP changes affect human immunodeficiency virus type 1 envelope glycoprotein conformation and CD4 engagement. J Virol 92:e01080-18. doi: 10.1128/JVI.01080-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castillo-Menendez LR, Nguyen HT, Sodroski J. 2018. Conformational differences between functional human immunodeficiency virus envelope glycoprotein trimers and stabilized soluble trimers. J Virol 93:e01709-18. doi: 10.1128/JVI.01709-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu M, Ma X, Castillo-Menendez LR, Gorman J, Alsahafi N, Ermel U, Terry DS, Chambers M, Peng D, Zhang B, Zhou T, Reichard N, Wang K, Grover JR, Carman BP, Gardner MR, Nikic-Spiegel I, Sugawara A, Arthos J, Lemke EA, Smith AB III, Farzan M, Abrams C, Munro JB, McDermott AB, Finzi A, Kwong PD, Blanchard SC, Sodroski JG, Mothes W. 2019. Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET. Nature 568:415–419. doi: 10.1038/s41586-019-1101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen HT, Alsahafi N, Finzi A, Sodroski JG. 2019. Effects of the SOS (A501C/T605C) and DS (I201C/A433C) disulfide bonds on HIV-1 membrane envelope glycoprotein conformation and function. J Virol 93:e00304-19. doi: 10.1128/JVI.00304-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alsahafi N, Debbeche O, Sodroski J, Finzi A. 2015. Effects of the I559P gp41 change on the conformation and function of the human immunodeficiency virus (HIV-1) membrane envelope glycoprotein trimer. PLoS One 10:e0122111. doi: 10.1371/journal.pone.0122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan J, Peng H, Chen B, Harrison SC. 2019. Cryo-EM structure of full-length HIV-1 Env bound with the Fab of antibody PG16. BioRxiv doi: 10.1101/730333. [DOI] [PMC free article] [PubMed]
- 30.Ringe RP, Sanders RW, Yasmeen A, Kim HJ, Lee JH, Cupo A, Korzun J, Derking R, van Montfort T, Julien JP, Wilson IA, Klasse PJ, Ward AB, Moore JP. 2013. Cleavage strongly influences whether soluble HIV-1 envelope glycoprotein trimers adopt a native-like conformation. Proc Natl Acad Sci U S A 110:18256–18261. doi: 10.1073/pnas.1314351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringe RP, Yasmeen A, Ozorowski G, Go EP, Pritchard LK, Guttman M, Ketas TA, Cottrell CA, Wilson IA, Sanders RW, Cupo A, Crispin M, Lee KK, Desaire H, Ward AB, Klasse PJ, Moore JP. 2015. Influences on the design and purification of soluble, recombinant native-like HIV-1 envelope glycoprotein trimers. J Virol 89:12189–12210. doi: 10.1128/JVI.01768-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binley JM, Sanders RW, Master A, Cayanan CS, Wiley CL, Schiffner L, Travis B, Kuhmann S, Burton DR, Hu SL, Olson WC, Moore JP. 2002. Enhancing the proteolytic maturation of human immunodeficiency virus type 1 envelope glycoproteins. J Virol 76:2606–2616. doi: 10.1128/jvi.76.6.2606-2616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JH, Ozorowski G, Ward AB. 2016. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science 351:1043–1048. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torrents de la Pena A, Rantalainen K, Cottrell CA, Allen JD, van Gils MJ, Torres JL, Crispin M, Sanders RW, Ward AB. 2019. Similarities and differences between native HIV-1 envelope glycoprotein trimers and stabilized soluble trimer mimetics. PLoS Pathog 15:e1007920. doi: 10.1371/journal.ppat.1007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abrahamyan LG, Markosyan RM, Moore JP, Cohen FS, Melikyan GB. 2003. Human immunodeficiency virus type 1 Env with an intersubunit disulfide bond engages coreceptors but requires bond reduction after engagement to induce fusion. J Virol 77:5829–5836. doi: 10.1128/jvi.77.10.5829-5836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binley JM, Cayanan CS, Wiley C, Schulke N, Olson WC, Burton DR. 2003. Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions. J Virol 77:5678–5684. doi: 10.1128/jvi.77.10.5678-5684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crooks ET, Tong T, Osawa K, Binley JM. 2011. Enzyme digests eliminate nonfunctional Env from HIV-1 particle surfaces, leaving native Env trimers intact and viral infectivity unaffected. J Virol 85:5825–5839. doi: 10.1128/JVI.00154-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pancera M, Wyatt R. 2005. Selective recognition of oligomeric HIV-1 primary isolate envelope glycoproteins by potently neutralizing ligands requires efficient precursor cleavage. Virology 332:145–156. doi: 10.1016/j.virol.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 39.Herrera C, Spenlehauer C, Fung MS, Burton DR, Beddows S, Moore JP. 2003. Nonneutralizing antibodies to the CD4-binding site on the gp120 subunit of human immunodeficiency virus type 1 do not interfere with the activity of a neutralizing antibody against the same site. J Virol 77:1084–1091. doi: 10.1128/jvi.77.2.1084-1091.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray K, Mengistu M, Orlandi C, Pazgier M, Lewis GK, DeVico AL. 2019. Concurrent exposure of neutralizing and non-neutralizing epitopes on a single HIV-1 envelope structure. Front Immunol 10:1512. doi: 10.3389/fimmu.2019.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stadtmueller BM, Bridges MD, Dam KM, Lerch MT, Huey-Tubman KE, Hubbell WL, Bjorkman PJ. 2018. DEER spectroscopy measurements reveal multiple conformations of HIV-1 SOSIP envelopes that show similarities with envelopes on native virions. Immunity 49:235–246 e234. doi: 10.1016/j.immuni.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. 2013. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB. 2013. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang GY, Ofek G, Stewart-Jones GB, Stuckey J, Bailer RT, Joyce MG, Louder MK, Tumba N, Yang Y, Zhang B, Cohen MS, Haynes BF, Mascola JR, Morris L, Munro JB, Blanchard SC, Mothes W, Connors M, Kwong PD. 2014. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gristick HB, von Boehmer L, West AP Jr, Schamber M, Gazumyan A, Golijanin J, Seaman MS, Fatkenheuer G, Klein F, Nussenzweig MC, Bjorkman PJ. 2016. Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site. Nat Struct Mol Biol 23:906–915. doi: 10.1038/nsmb.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crispin M, Ward AB, Wilson IA. 2018. Structure and immune recognition of the HIV glycan shield. Annu Rev Biophys 47:499–523. doi: 10.1146/annurev-biophys-060414-034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yasmeen A, Ringe R, Derking R, Cupo A, Julien JP, Burton DR, Ward AB, Wilson IA, Sanders RW, Moore JP, Klasse PJ. 2014. Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits. Retrovirology 11:41. doi: 10.1186/1742-4690-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derking R, Ozorowski G, Sliepen K, Yasmeen A, Cupo A, Torres JL, Julien JP, Lee JH, van Montfort T, de Taeye SW, Connors M, Burton DR, Wilson IA, Klasse PJ, Ward AB, Moore JP, Sanders RW. 2015. Comprehensive antigenic map of a cleaved soluble HIV-1 envelope trimer. PLoS Pathog 11:e1004767. doi: 10.1371/journal.ppat.1004767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kadelka C, Liechti T, Ebner H, Schanz M, Rusert P, Friedrich N, Stiegeler E, Braun DL, Huber M, Scherrer AU, Weber J, Uhr T, Kuster H, Misselwitz B, Cavassini M, Bernasconi E, Hoffmann M, Calmy A, Battegay M, Rauch A, Yerly S, Aubert V, Klimkait T, Boni J, Kouyos RD, Gunthard HF, Trkola A, Swiss H. 2018. Distinct, IgG1-driven antibody response landscapes demarcate individuals with broadly HIV-1 neutralizing activity. J Exp Med 215:1589–1608. doi: 10.1084/jem.20180246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGuire AT. 2019. Targeting broadly neutralizing antibody precursors: a naive approach to vaccine design. Curr Opin HIV AIDS 14:294–301. doi: 10.1097/COH.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 51.Tokatlian T, Read BJ, Jones CA, Kulp DW, Menis S, Chang JYH, Steichen JM, Kumari S, Allen JD, Dane EL, Liguori A, Sangesland M, Lingwood D, Crispin M, Schief WR, Irvine DJ. 2019. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 363:649–654. doi: 10.1126/science.aat9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pugach P, Ozorowski G, Cupo A, Ringe R, Yasmeen A, de Val N, Derking R, Kim HJ, Korzun J, Golabek M, de Los Reyes K, Ketas TJ, Julien JP, Burton DR, Wilson IA, Sanders RW, Klasse PJ, Ward AB, Moore JP. 2015. A native-like SOSIP.664 trimer based on an HIV-1 subtype B env gene. J Virol 89:3380–3395. doi: 10.1128/JVI.03473-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cupo A, Cruz Portillo VM, Gelfand P, Yasmeen A, Klasse PJ, Moore JP. 2019. Optimizing the production and affinity purification of HIV-1 envelope glycoprotein SOSIP trimers from transiently transfected CHO cells. PLoS One 14:e0215106. doi: 10.1371/journal.pone.0215106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B. 2005. Automated molecular microscopy: the new Leginon system. J Struct Biol 151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Lander GC, Stagg SM, Voss NR, Cheng A, Fellmann D, Pulokas J, Yoshioka C, Irving C, Mulder A, Lau PW, Lyumkis D, Potter CS, Carragher B. 2009. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J Struct Biol 166:95–102. doi: 10.1016/j.jsb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voss NR, Yoshioka CK, Radermacher M, Potter CS, Carragher B. 2009. DoG Picker and TiltPicker: software tools to facilitate particle selection in single particle electron microscopy. J Struct Biol 166:205–213. doi: 10.1016/j.jsb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogura T, Iwasaki K, Sato C. 2003. Topology representing network enables highly accurate classification of protein images taken by cryo electron-microscope without masking. J Struct Biol 143:185–200. doi: 10.1016/j.jsb.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Schoofs T, Barnes CO, Suh-Toma N, Golijanin J, Schommers P, Gruell H, West AP Jr, Bach F, Lee YE, Nogueira L, Georgiev IS, Bailer RT, Czartoski J, Mascola JR, Seaman MS, McElrath MJ, Doria-Rose NA, Klein F, Nussenzweig MC, Bjorkman PJ. 2019. Broad and potent neutralizing antibodies recognize the silent face of the HIV envelope. Immunity 50:1513–1529 e1519. doi: 10.1016/j.immuni.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCoy LE, van Gils MJ, Ozorowski G, Messmer T, Briney B, Voss JE, Kulp DW, Macauley MS, Sok D, Pauthner M, Menis S, Cottrell CA, Torres JL, Hsueh J, Schief WR, Wilson IA, Ward AB, Sanders RW, Burton DR. 2016. Holes in the glycan shield of the native HIV envelope are a target of trimer-elicited neutralizing antibodies. Cell Rep 16:2327–2338. doi: 10.1016/j.celrep.2016.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavacini LA, Duval M, Robinson J, Posner MR. 2002. Interactions of human antibodies, epitope exposure, antibody binding and neutralization of primary isolate HIV-1 virions. AIDS 16:2409–2417. doi: 10.1097/00002030-200212060-00005. [DOI] [PubMed] [Google Scholar]
- 61.Thali M, Moore JP, Furman C, Charles M, Ho DD, Robinson J, Sodroski J. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol 67:3978–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, Chiang JJ, Haworth KG, Decker JM, Alpert MD, Bailey CC, Neale ES Jr, Fellinger CH, Joshi VR, Fuchs SP, Martinez-Navio JM, Quinlan BD, Yao AY, Mouquet H, Gorman J, Zhang B, Poignard P, Nussenzweig MC, Burton DR, Kwong PD, Piatak M Jr, Lifson JD, Gao G, Desrosiers RC, Evans DT, Hahn BH, Ploss A, Cannon PM, Seaman MS, Farzan M. 2015. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]