Arbovirus infections in Brazil, including yellow fever, dengue, zika, and chikungunya, result in considerable morbidity and mortality and are pressing public health concerns. However, our understanding of these outbreaks is hampered by the limited availability of genomic data. In this study, we investigated the genetic diversity and spatial distribution of YFV during the current outbreak by analyzing genomic data from areas in southeastern Brazil not covered by other previous studies. To gain insights into the routes of YFV introduction and dispersion, we tracked the virus by sequencing YFV genomes sampled from nonhuman primates and infected patients from the southeastern region. Our study provides an understanding of how YFV initiates transmission in new Brazilian regions and illustrates that genomics in the field can augment traditional approaches to infectious disease surveillance and control.
KEYWORDS: yellow fever, outbreak, Southeast Brazil, genomic surveillance, outbreak response
ABSTRACT
The recent reemergence of yellow fever virus (YFV) in Brazil has raised serious concerns due to the rapid dissemination of the virus in the southeastern region. To better understand YFV genetic diversity and dynamics during the recent outbreak in southeastern Brazil, we generated 18 complete and nearly complete genomes from the peak of the epidemic curve from nonhuman primates (NHPs) and human infected cases across the Espírito Santo and Rio de Janeiro states. Genomic sequencing of 18 YFV genomes revealed the estimated timing, source, and likely routes of yellow fever virus transmission and dispersion during one of the largest outbreaks ever registered in Brazil. We showed that during the recent epidemic, YFV was reintroduced from Minas Gerais to the Espírito Santo and Rio de Janeiro states multiple times between 2016 and 2019. The analysis of data from portable sequencing could identify the corridor of spread of YFV. These findings reinforce the idea that continued genomic surveillance strategies can provide information on virus genetic diversity and transmission dynamics that might assist in understanding arbovirus epidemics.
IMPORTANCE Arbovirus infections in Brazil, including yellow fever, dengue, zika, and chikungunya, result in considerable morbidity and mortality and are pressing public health concerns. However, our understanding of these outbreaks is hampered by the limited availability of genomic data. In this study, we investigated the genetic diversity and spatial distribution of YFV during the current outbreak by analyzing genomic data from areas in southeastern Brazil not covered by other previous studies. To gain insights into the routes of YFV introduction and dispersion, we tracked the virus by sequencing YFV genomes sampled from nonhuman primates and infected patients from the southeastern region. Our study provides an understanding of how YFV initiates transmission in new Brazilian regions and illustrates that genomics in the field can augment traditional approaches to infectious disease surveillance and control.
INTRODUCTION
Yellow fever (YF) is a vector-borne disease that is endemic in tropical areas of Africa and South America (1). The aetiologic agent is the yellow fever virus (YFV), a single-stranded positive-sense RNA virus belonging to the Flaviviridae family (2). YFV diversity can be classified into four distinct genotypes, which have been named based on their geographical distribution, as follows: East African, West African, South American I, and South American II genotypes (3–6).
In the Americas, YFV transmission can occur via two main epidemiological transmission cycles, the sylvatic (or jungle) and the urban (domestic) cycles. In the sylvatic cycle, nonhuman primates (NHPs) are infected through the bite of mosquito vectors such as Haemagogus spp. and Sabethes spp. (7, 8). However, in the urban cycle, humans can be infected by Aedes sp. mosquito bites (9). YFV infection in humans shows a wide spectrum of disease severity, including asymptomatic infection; mild illness with dengue-like symptoms, including fever, nausea, vomiting, and fatigue; and disease, including fever with jaundice or hemorrhage and death (10).
While eradication is not feasible due to the wildlife reservoir system, large-scale vaccination coverage provides considerable protection against the reurbanization of YFV transmission (11). However, despite the availability of effective vaccines, YF remains an important public health issue in Africa and South America. In late 2016, a severe reemergence of the YFV epidemic occurred in southeastern Brazil. The epidemic has evolved to become the largest observed in the country in decades, reaching areas close to the Atlantic rain forest (11, 12). The YFV 2016 to 2017 epidemic in Brazil accounted for 1,412 epizootics, 777 YF human confirmed cases, most of which were in Southeast Brazil (Minas Gerais, n = 465; Sao Paulo, n = 22; Rio de Janeiro, n = 25; Espírito Santo, n = 252 confirmed cases), and 261 human deaths (13). Following this epidemic, new cases were reported between 2017 and 2018, and in that period 864 epizootics, 1,376 YF human confirmed cases, and 483 human deaths were registered, with the southern states among the most affected by the YFV epidemic (Minas Gerais, n = 532; Sao Paulo, n = 377; Rio de Janeiro, n = 186; Espírito Santo, n = 6 confirmed cases) (14). The epidemic persisted in 2018 and 2019 and accounted for 1,883 NHP notified cases (n = 20 confirmed NHP cases) and 12 human confirmed cases, including 5 human deaths from the state of São Paulo. Most of the confirmed epizootic cases were registered in the southeastern states (95%) (São Paulo, n = 10; Rio de Janeiro, n = 8; and Minas Gerais, n = 1) (13–15).
Although there is currently no evidence that urban transmission has occurred, the outbreak affected areas highly infested by Aedes aegypti and Aedes albopictus where yellow fever vaccination was recently introduced in a routine immunization program. This raises concern that, for the first time in decades, there might be a high risk of YFV urban transmission in Brazil (16). New surveillance and analytical approaches are therefore needed to monitor this threat.
Even so, there is limited information from genomic surveillance studies about the genomic epidemiology and the dissemination dynamics of 2016 to 2019 YFV circulating in Southeast Brazil. Previous studies have shown the spatial and evolutionary dynamics of the current YFV outbreak in different southeastern states (11) and shed light regarding the possible cocirculation of distinct YFV lineages (17). Nevertheless, there is still limited information about the genomic epidemiology of YFV circulating in the states of Espírito Santo and Rio de Janeiro from genomic surveillance studies, and this impairs our understanding of the virus reintroduction, establishment, and dissemination in those regions. Thus, to better understand the reemergence of the recent YFV epidemic in those regions, we analyzed a larger and updated data set of recently released data of the YFV 2016 to 2019 epidemic in Brazil, including 18 newly generated complete genomes from areas not covered by other previous studies from human and NHPs from the southeast states of Espírito Santo and Rio de Janeiro.
(This article was submitted to an online preprint archive [18].)
RESULTS
Molecular diagnostics and genome sequencing from clinical samples.
Liver, spleen, kidney, and blood samples from 14 NHPs and liver and serum samples from 4 human infected cases collected from areas not covered by other previous studies in the states of Rio de Janeiro and Espírito Santo, Southeast Brazil, between January 2017 and April 2018, were tested for YFV RNA using the reverse transcription quantitative PCR (RT-qPCR) assay (19, 20) at the Flavivirus Laboratory at FIOCRUZ Rio de Janeiro (LABFLA/FIOCRUZ).
Most confirmed cases in NHPs were from animals of the Alouatta genus (42.9%; 6 of 14), followed by Callithrix (35.7%; 5 of 14), Sapajus (7.1%; 1 of 14), and Leontopithecus rosalia (14.3%; 2 of 14). PCR cycle threshold (CT) values were on average 12.23 (range, 7.2 to 22.4) (Table 1).
TABLE 1.
Epidemiological data for the sequenced samplesa
| ID | CT value | Sample type | Host | Species | State | Municipality | Collection date (day/month/year) | Age | Sex | Residence |
|---|---|---|---|---|---|---|---|---|---|---|
| RJ182 | 8.2 | Liver | NHP | Alouatta sp. | RJ | São Sebastião do Alto | 09/03/2017 | NA | M | |
| RJ193 | 10.2 | Liver | NHP | Alouatta sp. | RJ | São Sebastião do Alto | 27/03/2017 | 1 | M | |
| RJ141 | 22.4 | Serum | Human | ES | Ibatiba | 24/01/2017 | 16 | M | Rural | |
| RJ183 | 11.2 | Serum | Human | RJ | São Sebastião do Alto | 12/03/2017 | 25 | M | Rural | |
| RJ194 | 6.5 | Liver | NHP | Alouatta sp. | RJ | São Sebastião do Alto | 27/03/2017 | 15 | F | |
| RJ147 | 21.9 | Whole blood | NHP | Alouatta sp. | ES | Domingos Martins | 31/01/2017 | NA | NA | |
| RJ173 | 15 | Whole blood | NHP | Cebus sp. | ES | Itarana | 09/02/2017 | NA | NA | |
| RJ184 | 14.4 | Liver | Human | ES | Cariacica | 13/03/2017 | 65 | M | Rural | |
| RJ213 | 8.1 | Liver | NHP | Callithrix sp. | RJ | Valença | 22/01/2018 | 5 | F | |
| RJ186 | 10.9 | Liver | NHP | Alouatta sp. | ES | Guarapari | 06/03/2017 | NA | NA | |
| RJ177 | 11.5 | Serum | Human | ES | Brejetuba | 16/02/2017 | 46 | M | Urban | |
| RJ188 | 9.9 | Whole blood | NHP | Callithrix sp. | ES | Cariacica | 08/03/2017 | NA | NA | |
| RJ201 | 13.4 | Liver | NHP | Callithrix sp. | RJ | Nova Iguaçu | 28/11/2017 | 2 | F | |
| RJ219 | 11.2 | Kidney | NHP | Callithrix sp. | RJ | Angra dos Reis | 05/02/2018 | NA | NA | |
| RJ189 | 13.7 | Whole blood | NHP | Alouatta sp. | ES | Serra | 20/03/2017 | NA | F | |
| RJ216 | 7.2 | Liver | NHP | Callithrix sp. | RJ | Duas Barras | 25/01/2018 | 10 | F | |
| LABFLA09 | 22.1 | Liver | NHP | Leontopithecus Rosalia | RJ | Silva Jardim | 24/04/2018 | NA | NA | |
| LABFLA10 | 11.76 | Liver | NHP | Leontopithecus Rosalia | RJ | Silva Jardim | 24/04/2018 | NA | NA |
ID, study identifier; CT, RT-qPCR quantification cycle threshold value; RJ, Rio de Janeiro; ES, Espírito Santo; Municipality, Municipality of residence; F, female; M, male; NA, not available.
The median age of human patients was 38 years (range, 16 to 65 years). A total of 75% of the affected subjects lived in rural areas (Table 1). Only one subject lived in an urban area, with a history of travel to rural areas. To investigate the source and transmission of YFV and the genetic diversity of the virus circulating in humans and NHPs across the Rio de Janeiro and Espírito Santo states, we used the MinION handheld nanopore sequencer to generate 18 complete and near complete genomic sequences (average coverage = 89.9%; Table 2) using a previously described MinION sequencing protocol (11, 21) that allowed rapid data generation through fast sample preparation and library construction (1 day) as an interesting approach to get rapid critical information (such as lineage identification and pathogen transmission dynamics) useful for surveillance services and decision makers.
TABLE 2.
Sequencing statistics for the 18 new obtained sequences
| Study identifier | NCBI accession no. | No. of mapped reads | Avg depth coverage | No. of bases covered >10× | No. of bases covered >25× | Reference covered (%) |
|---|---|---|---|---|---|---|
| RJ182 | MK882607 | 21,104 | 961.34 | 10,220 | 10,220 | 99.31 |
| RJ193 | MK882613 | 2,953 | 133.99 | 10,215 | 9,697 | 95.95 |
| RJ141 | MK882601 | 11,776 | 523.89 | 10,175 | 9,955 | 96.17 |
| RJ183 | MK882608 | 1,453 | 67.88 | 9,934 | 8,599 | 82.52 |
| RJ194 | MK882615 | 1,146 | 55.27 | 9,381 | 7,964 | 79.84 |
| RJ147 | MK882602 | 3,319 | 148.16 | 8,461 | 7,651 | 71.19 |
| RJ173 | MK882599 | 1,361 | 63.57 | 9,017 | 7,628 | 74.68 |
| RJ184 | MK882609 | 1,241 | 57.04 | 9,480 | 8,109 | 78.23 |
| RJ213 | MK882618 | 2,520 | 116.01 | 10,206 | 9,674 | 93.01 |
| RJ186 | MK882610 | 4,007 | 190.77 | 9,460 | 9,445 | 90.36 |
| RJ177 | MK882604 | 22,538 | 1,057.4 | 10,227 | 10,219 | 99.31 |
| RJ188 | MK882611 | 74,369 | 3,227.15 | 10,237 | 10,231 | 99.31 |
| RJ201 | MK882617 | 8,679 | 399.91 | 9,490 | 9,454 | 90.34 |
| RJ219 | MK882621 | 8,894 | 405.58 | 10,205 | 9,957 | 96.2 |
| RJ189 | MK882612 | 4,840 | 219.19 | 9,695 | 9,146 | 89.4 |
| RJ216 | MK882619 | 6,807 | 313.58 | 10,220 | 9,709 | 93.1 |
| LABFLA09 | MK882600 | 312,871 | 4,637.05 | 10,210 | 9,975 | 89.97 |
| LABFLA10 | MK882603 | 470,582 | 5,028.42 | 9,693 | 9,871 | 99.35 |
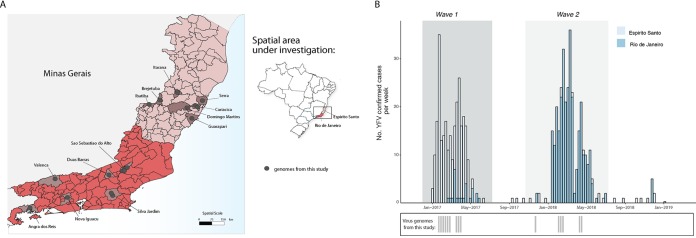
YF samples sequenced in this study were geographically widespread across 6 municipalities of Rio de Janeiro and 7 municipalities of Espírito Santo (Fig. 1A).
FIG 1.
Spatial and temporal distribution of YF cases from the Espírito Santo and Rio de Janeiro states during 2017 and 2019. (A) Map of the states of Espírito Santo (ES) and Rio de Janeiro (RJ), located in the southeastern region of Brazil and its municipalities. Circles indicate where samples from this study were collected. (B) Time series of human (H) and nonhuman primate YFV cases in ES and RJ states confirmed by serology, reverse transcription quantitative PCR (RT-qPCR), or virus isolation. Below, the dates of sample collection of the virus genomes generated in this study are shown in gray bars.
Fig. 1B shows the number of YFV confirmed cases in the Espírito Santo and Rio de Janeiro states. Epidemiological data revealed two distinct YFV epidemic waves. The first epidemic wave (wave 1) is represented by the YFV cases mainly registered in the state of Espírito Santo during the first semester of 2017 (January to April; n = 252 cases), although some sporadic cases were reported in the following year (Fig. 1B). The second wave (wave 2) is represented by YFV cases registered in the state of Rio de Janeiro during the first semester of 2018 (February to May; n = 220 cases) (Fig. 1B). Although the majority of cases in Rio de Janeiro occurred between February and March 2018, there was also a reemergence of YFV in that state detected around March 2017 during epidemic wave 1 that mainly affected the state of Espírito Santo.
Genetic history of YFV in southeastern Brazil.
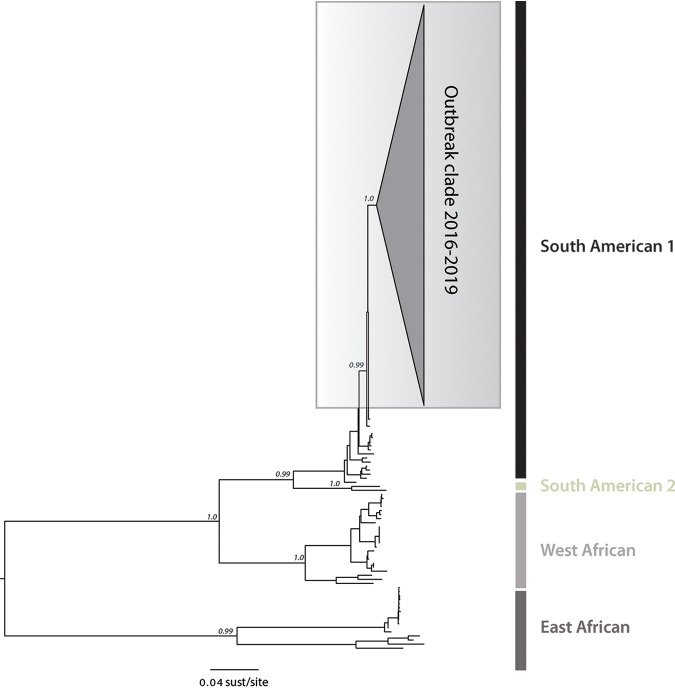
To investigate the phylogenetic relationship of YFV strains circulating in the southeastern states of Espírito Santo and Rio de Janeiro, we estimated a maximum likelihood (ML) phylogenetic tree for a data set of 181 reference sequences comprising the four YFV lineages. Our ML phylogeny revealed that, as suspected, the newly generated YFV sequences belong to the South American I (SA1) lineage with high statistical support (bootstrap = 100%), clustering with other Brazilian isolates from the 2016 to 2019 epidemic (Fig. 2).
FIG 2.
Molecular phylogenetics of the Brazilian YFV epidemic. Maximum likelihood phylogeny of complete YFV genomes showing the outbreak clade (gray triangle) within the South American I (SA1) genotype. The scale bar is in units of substitutions per site (sust/site).
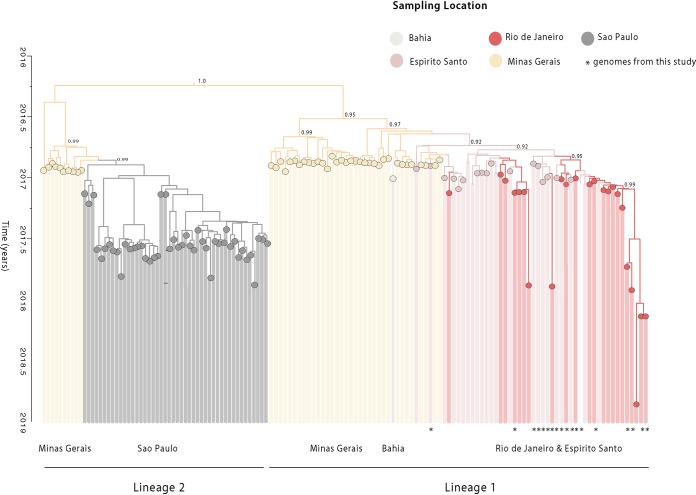
Subsequently, to investigate the dynamics of the YFV epidemic within the Southeast Region, genetic analyses were conducted on a second data set (data set 2; n = 137), including recently published sequences from the YFV 2016 to 2019 epidemic in Brazil, belonging to the SA1 lineage. The time scale of our phylogenetic estimates was consistent with recent studies (17, 22, 23) and confirmed the presence of two distinct lineages circulating in the current YFV epidemic, named hereafter as SA1 lineage 1 and SA1 lineage 2 (Fig. 3). SA1 lineage 1 comprises sequences from the northern and eastern regions of the Minas Gerais, Bahia, Espírito Santo, and Rio de Janeiro states, and the time of the most recent common ancestor (TMRCA) of this lineage was dated back to September 2016 (95% Bayesian credible interval [BCI]; July to November 2016). The SA1 lineage 2 comprises sequences from the southern municipalities of Minas Gerais and sequences from the southeastern state of Sao Paulo, and the TMRCA of this lineage was dated back to around July 2016 (95% BCI; June to December 2016) (Fig. 3). Our time-scaled phylogeny showed that the sequences generated in this study clustered together with high support (posterior probability [pp] = 90%) within SA1 lineage 1 (Fig. 3).
FIG 3.
Time-scaled phylogenetic tree of the current YF epidemic in Brazil. Molecular clock phylogeny obtained by combining the 18 new YFV complete genomes generated here (starred tips) plus publicly available data (n = 137) of the YFV 2016 to 2019 epidemic in Brazil (11, 12, 17, 22, 23). Numbers in nodes represent clade posterior probability of >0.90. Branch colors represent different sampling locations.
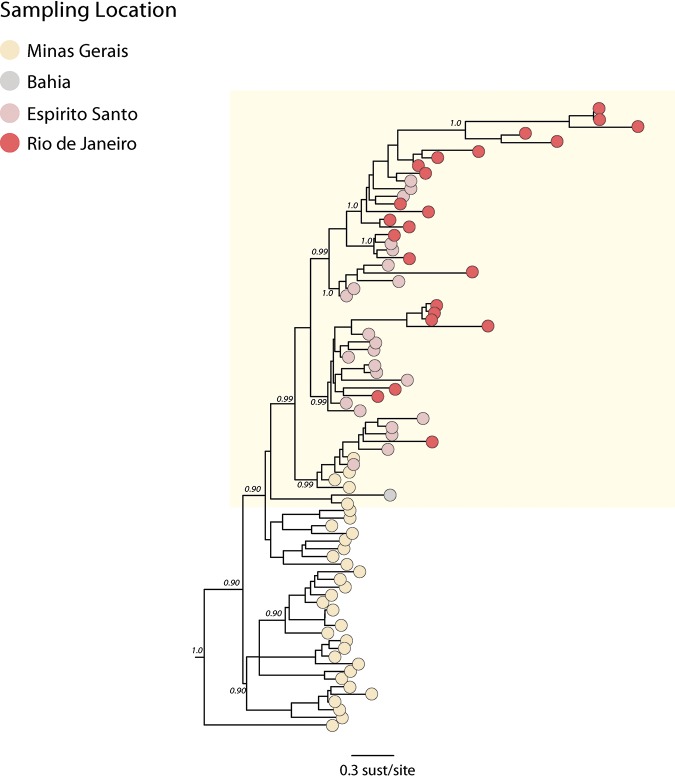
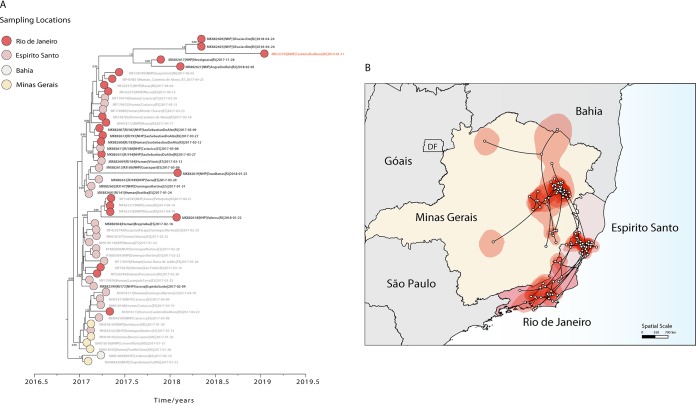
In order to understand the transmission and the spatiotemporal evolution of SA1 lineage 1, we analyzed a subset of 80 (data set 3) sequences representing all of the available sequences from this lineage (Fig. 4). We performed a regression of genetic divergence from root to tip against sampling dates that confirmed sufficient temporal signal (r2 = 0.70) in this data set. A time-scaled phylogenetic analysis using a Bayesian Markov chain Monte Carlo (MCMC) framework (24) was then performed to investigate the time of introduction of YFV into the Espírito Santo and Rio de Janeiro states (Fig. 5A). Figure 5A shows a zoom of our Bayesian time-scaled phylogeny highlighting SA1 lineage 1 comprising the 2017 to 2019 YFV strains from the Minas Gerais, Bahia, Espírito Santo, and Rio de Janeiro states. Our analysis showed that samples from Espírito Santo were intermixed with sequences from Rio de Janeiro. This suggests that the YFV epidemic in Espírito Santo and Rio de Janeiro was not caused by a single introduction event, as observed in Sao Paulo (17, 22), but resulted from multiple introductions over time.
FIG 4.
Molecular clock phylogeny including the clade comprising the new isolates plus all the YFV strains from the 2017 to 2019 outbreak belonging to the SA1 lineage 1 clade. Numbers along branches represent clade posterior probability of >0.90. Colors represent different locations.
FIG 5.
Spatiotemporal dynamics of YFV SA1 lineage 1. (A) Molecular clock phylogeny including the clade comprising the 2017 to 2019 YFV strains from Minas Gerais, Bahia, Espírito Santo, and Rio de Janeiro states belonging to SA1 lineage 1. Numbers along branches represent clade posterior probability of >0.90. YFV isolates from Casimiro de Abreu, sampled in January 2019, are highlighted in red. Colors represent different locations. (B) Reconstructed spatiotemporal continuous diffusion of the YFV SA1 lineage 1 outbreak clade. Phylogenetic branches are mapped in space according to the location of phylogenetic nodes (circles). Lines show the cross-state movement of the virus from Minas Gerais followed by movement to the states of Espírito Santo and Rio de Janeiro. Shaded regions show 95% credible regions of internal nodes.
We next used a continuous diffusion model to investigate how SA1 lineage 1 has been spreading over space and time. We found evidence that YFV disseminated through southeastern Brazilian states using two distinct paths with an average dispersal rate of 0.12 km/day (95% high posterior density [HPD], 0.09 to 0.14 km/day). From the northern region of Minas Gerais state, YFV spread to the south region of Bahia state around January 2017 (95% BCI, December 2016 to February 2017) (Fig. 5), and from the eastern region of Minas Gerais state, YFV moved toward Espírito Santo state (pp = 0.99) with introductions estimated around January 2017 (95% BCI, November 2016 to January 2017) (Fig. 5A and B). Since its introduction in the Espírito Santo state, the virus has spread through the neighboring state (Fig. 5). Our analyses revealed that YFV was likely introduced in Rio de Janeiro state several times between January (95% BCI, December 2016 to February 2017) and March 2017 (February 2017 to May 2017), spreading southward from the border with Espírito Santo state and reaching Angra dos Reis municipality, which is located in the southern region of Rio de Janeiro. Our data further suggest that after its first introduction in Rio de Janeiro, the virus persisted until 2019 as indicated by the isolate MK533792 sampled in the municipality of Casimiro de Abreu in January 2019 (12) (Fig. 5A), reinforcing the need for maintaining continuous surveillance and high vaccination coverage in the southeastern region.
DISCUSSION
In this study, we generated and analyzed 18 new YFV complete and nearly complete genomic sequences from samples from humans and nonhuman primates collected in several municipalities not covered by other previous studies in the Espírito Santo and Rio de Janeiro states in 2017 and 2018.
Although previous studies have already shown the spatial and evolutionary dynamics of the current YFV outbreak in Brazil (11, 12, 17, 22), the shortage of genomic data from the Espírito Santo and Rio de Janeiro states hampered the ability to shed light on the reemergence and establishment of YFV transmission in those regions. Trying to determine in a large scale the corridor of spread of YFV and the geographic hot spots is key to predicting and preventing other possible spillover events. The generated genomic data provide a more detailed understanding of the introduction and progression of YFV SA1 lineage 1 and reveal the timing, source, and likely routes of yellow fever virus transmission and dispersion during the largest outbreak in Brazil in decades.
According to the Ministry of Health epidemiological bulletin, YFV reemergence in the states of Espírito Santo and Rio de Janeiro was confirmed in January and February of 2017, respectively (13–15).
Our estimates indicated that YFV strains from the epidemic first emerged in the state of Espírito Santo from Minas Gerais around January 2017 (95% BCI, November 2016 to January 2017), which is consistent with epidemiological data (13–15). From the state of Espírito Santo, YFV spread southward to the great metropolitan area of Rio de Janeiro state. Moreover, our data indicated that the circulation of YFV in Rio de Janeiro may have resulted from multiple and independent introduction events from Espírito Santo state, highlighting a complex dispersion dynamic of the current YFV outbreak in Brazil, which occurred between January (95% BCI, December 2016 to February 2017) and March 2017 (February 2017 to May 2017). Our data further suggest that after its first introduction in Rio de Janeiro, the virus persisted until 2019 as indicated by the isolate MK533792 sampled in the municipality of Casimiro de Abreu in January 2019 (12). This estimation suggests that YFV might have persisted in Rio de Janeiro state for approximately 24 months. This suggests that Rio de Janeiro state possibly possesses the ecological conditions to maintain YFV outside the period of transmission (December to May) (12). Ultimately, given the abundance of sylvatic competent vectors (12) and nonhuman primates (22, 23), these data could indicate that there is some potential for the establishment of an enzootic transmission cycle of yellow fever in Mata Atlântica.
Epidemiological data also indicated two distinct YFV epidemic waves (13, 14). The first epidemic wave is represented by the YFV cases mainly registered in the Minas Gerais and Espírito Santo states during the first semester of 2017, while the second wave is represented by the YFV cases registered in Rio de Janeiro state during the first semester of 2018. Transmission of YFV in areas with susceptible NHP species typically occurs in time periods characterized by environmental conditions suitable to support higher mosquito abundance (12, 25).
As previously suggested (17), we found evidence regarding the circulation of two distinct YFV lineages that might have been spread at distinct evolutionary and diffusion rates. Using YFV genetic data, we estimate that YFV SA1 lineage 1 spread at a rate of 0.12 km/day (95% HPD, 0.09 to 0.14 km/day), which is slightly lower than previous estimates (11, 17). This might be due to the larger data set analyzed in this study, which might explain differences in the rate of YFV spread among different areas as well as different lineages.
These findings reinforce the idea that continued genomic surveillance strategies are needed to assist in the monitoring and understanding of arbovirus epidemics, which might help to attenuate the public health impacts of infectious diseases. In the present research article, we aimed to provide genomic information and reinforce the idea of the use of epidemiological and genomic data generated by a portable, easy to set up sequencing system as an approach to get rapid critical information (such as lineage identification and pathogen transmission dynamics) that could be used by surveillance services and decision makers.
In this study, we also demonstrate that by analyzing heterochronous data sets with samples collected in different time points and/or locations, phylodynamics becomes a powerful tool to prevent and identify viral lineage movement and to describe trends in epidemic spread (11, 26, 27).
Continued surveillance in humans and nonhuman primates (NHP) in nonepidemic periods in the Southeast Region will be important in order to quantify the risk of new outbreaks and the establishment of new YFV transmission cycles in the region. In conclusion, our study shows that genomic data generated by portable sequencing technology can be employed to assist public health services in monitoring and understanding the diversity of circulating mosquito-borne viruses.
MATERIALS AND METHODS
Sample collection.
Human and nonhuman primate samples were collected under the guidelines of a national strategy of YF surveillance for molecular diagnostics by the Flavivirus Laboratory (LABFLA) at Oswaldo Cruz Foundation (Fiocruz) in Rio de Janeiro, Brazil, which is a Brazilian Ministry of Health Regional Reference Laboratory for arboviruses. The majority of samples were linked to a digital record that collated epidemiological and clinical data, such as date of sample collection, municipality of residence, neighborhood of residence, demographic characteristics (age and sex), and date of onset of clinical symptoms.
Ethical statement.
The project was supported by the Pan American World Health Organization (PAHO) and the Brazilian Ministry of Health (MoH) as part of the arboviral genomic surveillance efforts within the terms of Resolution 510/2016 of CONEP (Comissão Nacional de Ética em Pesquisa, Ministério da Saúde; National Ethical Committee for Research, Ministry of Health). The diagnostic of YFV infection at LABFLA was approved by the Ethics Committee of the Oswaldo Cruz Institute (CAAE90249218.6.1001.54248).
RT-qPCR.
Total RNA was extracted from tissue and serum samples using a MagMAX pathogen RNA/DNA kit (Life Technologies, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. Viral RNA was detected using two previously published RT-qPCR techniques (19, 20).
cDNA synthesis and whole-genome nanopore sequencing.
Sequencing was attempted on the 18 selected RT-PCR-positive samples regardless of CT value as previously described (11, 21, 27). All positive samples were submitted to a cDNA synthesis protocol (11, 21) using a ProtoScript II first strand cDNA synthesis kit. Then, a multiplex tiling PCR was attempted using the previously published YFV primer scheme and 30 cycles of PCR using Q5 high-fidelity DNA polymerase (NEB) as previously described (21). Amplicons were purified using 1× AMPure XP beads (Beckman Coulter), and cleaned-up PCR product concentrations were measured using a Qubit double-stranded DNA (dsDNA) high-sensitivity (HS) assay kit on a Qubit 3.0 fluorimeter (Thermo Fisher). DNA library preparation was performed using the Ligation sequencing kit (Oxford Nanopore Technologies) and the Native barcoding kit (NBD103; Oxford Nanopore Technologies, Oxford, UK). A sequencing library was generated from the barcoded products using the genomic DNA sequencing kit SQK-MAP007/SQK-LSK208 (Oxford Nanopore Technologies). The sequencing library was loaded onto a R9.4 flow cell (Oxford Nanopore Technologies).
Generation of consensus sequences.
Consensus sequences for each barcoded sample were generated following a previously published approach (21). Briefly, raw files were basecalled using Albacore, demultiplexed and trimmed using Porechop, and then mapped with bwa to a reference genome (GenBank accession number JF912190). Nanopolish variant calling was applied to the assembly to detect single-nucleotide variants to the reference genome. Consensus sequences were generated; nonoverlapped primer binding sites and sites for which coverage was <20× were replaced with ambiguity code N. Sequencing statistics can be found in Table 1.
Collation of YFV complete genome data sets.
Genotyping was first conducted using the phylogenetic yellow fever typing tool available at http://www.krisp.org.za/tools.php. The genome sequences generated here were combined with a data set comprising previously published genomes from the 2016 to 2019 YFV epidemic in Brazil (11, 12, 17, 22, 23). Two complete or nearly complete YFV genome data sets were generated. Data set 1 (n = 199) comprised the data reported in this study (n = 18) plus (n = 181) complete or almost complete YFV genomic sequences (>10,000 bp) retrieved from NCBI in June 2019 and covering all four existing genotypes. Subsequently, to investigate the dynamic of the YFV infection within the Southeast Region, genetic analyses were conducted on a smaller data set (data set 2) including a larger and updated data set of recently released data of the YFV 2016 to 2019 epidemic in Brazil belonging to the SA1 lineage (n = 137). Thus, to understand the transmission and the spatiotemporal evolution of YFV SA1 lineage 1 from this data set, we generated a subset (data set 3) that included all identified sequences from that lineage (n = 80). Maximum likelihood (ML) phylogenetic trees were estimated using RAxML (28) under a GTR + Γ4 nucleotide substitution model. Statistical support for phylogenetic nodes was estimated using a ML bootstrap approach with 1,000 replicates.
In order to investigate the temporal signal in our YFV data sets 2 and 3, we regressed root-to-tip genetic distances from this ML tree against sample collection dates using TempEst v.1.5.1 (http://tree.bio.ed.ac.uk) (29).
Dated phylogenetics.
To estimate time-calibrated phylogenies dated from time-stamped genome data, we conducted phylogenetic analysis using a Bayesian software package (24). Here, we used the GTR + Γ4 nucleotide substitution model and Bayesian Skygrid tree prior (30) with an uncorrelated relaxed clock with a lognormal distribution (31). We employed a stringent model selection analysis using both path sampling (PS) and stepping stone (SS) procedures to estimate the most appropriate molecular clock model for the Bayesian phylogenetic analysis. We tested (i) the strict molecular clock model, which assumes a single rate across all phylogeny branches, and (ii) the more flexible uncorrelated relaxed molecular clock model with a lognormal rate distribution (uncorrelated lognormal [UCLN]). Both SS and PS estimators indicated the strict molecular clock (Bayes factor = 4.3) as the best-fitted model to the data set under analysis. Analyses were run in duplicate in BEAST v.1.10.4 (24) for 50 million MCMC steps, sampling parameters, and trees every 5,000th step. A noninformative continuous time Markov chain reference prior on the molecular clock rate was used (32). Convergence of MCMC chains was checked using Tracer v.1.7.1 (33). Maximum clade trees were summarized using TreeAnnotator after discarding 10% as burn-in.
Phylogeographic analyses.
To investigate the spread of YFV in Southeast Brazil, we analyzed in more detail SA1 lineage 1, which includes the n = 80 sequences (Fig. 4). We used a Skygrid coalescent tree prior (30) and a continuous phylogeographic model that uses a relaxed random walk to model the spatial diffusion of lineages. Dispersal velocity variation among lineages was modelled using a Cauchy distribution (34, 35). Virus diffusion through time and space was summarized using 1,000 phylogenies sampled at regular intervals from the posterior distribution (after exclusion of burn-in). Sampling locations of each georeferenced YFV sequence from the Espírito Santo and Rio de Janeiro states are listed in Table S1 in the supplemental material. Georeferenced and time-stamped sequences were analyzed in BEAST v.1.10.4 (24) using the BEAGLE library (36) to enhance computational speed.
Data availability.
New sequences were deposited in GenBank under accession numbers MK882599 to MK882604, MK882607 to MK882613, MK882615, MK882617 to MK882619, and MK882621 (see Table 2).
Supplementary Material
ACKNOWLEDGMENTS
We thank all personnel from Health Surveillance System in Rio de Janeiro that coordinated surveillance and helped with data collection and assembly. We also thank Ronaldo Lapa e Solange Regina Conceição from the Instituto Oswaldo Cruz (Fiocruz, Rio de Janeiro) for their support.
This work was supported by the ZiBRA2 project supported by the Brazilian Ministry of Health (SVS-MS) and the Pan American Organization (OPAS) and founded by Decit/SCTIE/MoH and CNPq (440685/2016-8 and 440856/2016-7); by CAPES (88887.130716/2016-00, 88881.130825/2016-00, and 88887.130823/2016-00); and by EU’s Horizon 2020 program through ZIKAlliance (PRES-005-FEX-17-4-2-33). The Flavivirus Laboratory activities was also supported by Faperj (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro) under grant number E-26/2002.930/2016, by the International Development Research Centre (IDRC) Canada over the grant 108411-001 and Horizon 2020 through ZikaPlan and ZikAction under the grant’s agreement numbers 734584 and 734857. M.G. is supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
M.G., M.C.L.D.M., V.F., L.C.J.A., and A.M.B.D.F. conceived and designed the study. M.G., M.C.L.D.M., V.F., M.A.M.-G., A.F., J.X., J.G.D.J., C.D.D.S.R., C.C.D.S., P.C.S., S.A.S., F.L.LC., F.D.B.N., and J.T. performed investigations. M.G., M.C.L.D.M., V.F., T.G., J.T., N.R.F., A.P.M.R., D.G.R., A.L.D.A., W.K.O., R.F.D.C.S., C.F.C.D.A., T.D.O., C.A.F., S.F.A., R.V.C., A.C., L.C.J.A., and A.M.B.D.F. curated the data. M.G., V.F., T.G., J.T., N.R.F., L.C.J.A., and A.M.B.D.F. performed formal analysis. M.G., M.C.L.D.M., V.F., L.C.J.A., and A.M.B.D.F. wrote the original draft of the paper and M.G., M.C.L.DM., T.D.O., P.C.S., A.P.M.R., D.G.R., R.V.C., N.R.F., and A.M.B.D.F. revised the paper. A.P.M.R., D.G.R., A.L.D.A., W.K.O., R.F.D.C.S., C.F.C.D.A., C.A.F., S.F.A., R.V.C., A.C., and A.M.B.D.F. provided resources.
We declare no competing interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Barrett AD, Monath TP. 2003. Epidemiology and ecology of yellow fever virus. Adv Virus Res 61:291–315. doi: 10.1016/S0065-3527(03)61007-9. [DOI] [PubMed] [Google Scholar]
- 2.Monath TP, Vasconcelos PF. 2015. Yellow fever. J Clin Virol 64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Bryant JE, Holmes EC, Barrett AD. 2007. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog 3:e75. doi: 10.1371/journal.ppat.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mutebi JP, Wang H, Li L, Bryant JE, Barrett AD. 2001. Phylogenetic and evolutionary relationships among yellow fever virus isolates in Africa. J Virol 75:6999–7008. doi: 10.1128/JVI.75.15.6999-7008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes MR, Palacios G, Cardoso JF, Martins LC, Sousa EC Jr, de Lima CP, Medeiros DB, Savji N, Desai A, Rodrigues SG, Carvalho VL, Lipkin WI, Vasconcelos PF. 2012. Genomic and phylogenetic characterization of Brazilian yellow fever virus strains. J Virol 86:13263–13271. doi: 10.1128/JVI.00565-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang GJ, Cropp BC, Kinney RM, Trent DW, Gubler DJ. 1995. Nucleotide sequence variation of the envelope protein gene identifies two distinct genotypes of yellow fever virus. J Virol 69:5773–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dégallier N, Travassos da Rosa AP, Hervé JP, Travassos da Rosa JFS, Vasconcelos PFC, Silva C. 1992. A comparative study of yellow fever in Africa and South America. J Braz Assoc Advanc Sci 44:143–151. [Google Scholar]
- 8.Mondet B, da Rosa AP, Vasconcelos PF. 1996. The risk of urban yellow fever outbreaks in Brazil by dengue vectors. Aedes aegypti and Aedes albopictus. Bull Soc Pathol Exot 89:107–113. [PubMed] [Google Scholar]
- 9.Souza RPD, Petrella S, Coimbra TLM, Maeda AY, Rocco IM, Bisordi I, Silveira VR, Pereira LE, Suzuki A, Silva SJDS, Silva FG, Salvador FS, Tubaki RM, Menezes RT, Pereira M, Bergo ES, Hoffmann RC, Spinola RMF, Tengan CH, Siciliano MM. 2011. Isolation of yellow fever virus (YFV) from naturally infected Haemagogus (Conopostegus) leucocelaenus (diptera, cukicudae) in São Paulo State, Brazil, 2009. Rev Inst Med Trop Sao Paulo 53:133–139. doi: 10.1590/S0036-46652011000300004. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro AF, Cavalin RF, Abdul Hamid Suleiman JM, Alves da Costa J, Januaria de Vasconcelos M, Sant’Ana Málaque CM, Sztajnbok J. 2019. Yellow fever: factors associated with death in a hospital of reference in infectious diseases, São Paulo, Brazil, 2018. Am J Trop Med Hyg 101:180–188. doi: 10.4269/ajtmh.18-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faria NR, Kraemer MUG, Hill SC, Goes de Jesus J, Aguiar RS, Iani FCM, Xavier J, Quick J, Du Plessis L, Dellicour S, Thézé J, Carvalho RDO, Baele G, Wu CH, Silveira PP, Arruda MB, Pereira MA, Pereira GC, Lourenço J, Obolski U, Abade L, Vasylyeva TI, Giovanetti M, Yi D, Weiss DJ, Wint GRW, Shearer FM, Funk S, Nikolay B, Fonseca V, Adelino TER, Oliveira MAA, Silva MVF, Sacchetto L, Figueiredo PO, Rezende IM, Mello EM, Said RFC, Santos DA, Ferraz ML, Brito MG, Santana LF, Menezes MT, Brindeiro RM, Tanuri A, Dos Santos FCP, Cunha MS, Nogueira JS, Rocco IM, et al. 2018. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science 361:894–899. doi: 10.1126/science.aat7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abreu FVS, Delatorre E, dos Santos AAC, Ferreira-de-Brito A, de Castro MG, Ribeiro IP, Furtado ND, Vargas WP, Ribeiro MS, Meneguete P, Bonaldo MC, Bello G, Lourenço-de-Oliveira R. 2019. Combination of surveillance tools reveals that yellow fever virus can remain in the same Atlantic Forest area at least for three transmission seasons. Mem Inst Oswaldo Cruz 114:e190076. doi: 10.1590/0074-02760190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brazilian Ministry of Health. 2017. Epidemiological update yellow fever. Brazilian Ministry of Health, Brasília, Brazil: http://portalarquivos2.saude.gov.br/images/pdf/2017/setembro/06/2017_027.pdf. [Google Scholar]
- 14.Brazilian Ministry of Health. 2018 Epidemiological update yellow fever. Brazilian Ministry of Health, Brasília, Brazil. [Google Scholar]
- 15.Secretaria de Vigilância em Saúde. 2019. Monitoramento do Período Sazonal da Febre Amarela Brasil–2018/2019. Ministério da Saúde, Brasília, Brazil. [Google Scholar]
- 16.Shearer FM, Moyes CL, Pigott DM, Brady OJ, Marinho F, Deshpande A, Longbottom J, Browne AJ, Kraemer MUG, O'Reilly KM, Hombach J, Yactayo S, de Araújo VEM, da Nóbrega AA, Mosser JF, Stanaway JD, Lim SS, Hay SI, Golding N, Reiner RC. 2017. Global yellow fever vaccination coverage from 1970 to 2016: an adjusted retrospective analysis. Lancet Infect Dis 17:1209–1217. doi: 10.1016/S1473-3099(17)30419-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delatorre E, de Abreu FVS, Ribeiro IP, Gómez MM, Dos Santos AAC, Ferreira-de-Brito A, Neves M, Bonelly I, de Miranda RM, Furtado ND, Raphael LMS, da Silva LFF, de Castro MG, Ramos DG, Romano APM, Kallás EG, Vicente ACP, Bello G, Lourenço-de-Oliveira R, Bonaldo MC. 2019. Distinct YFV lineages co-circulated in the central-western and southeastern Brazilian regions from 2015 to 2018. Front Microbiol 24:1079. doi: 10.3389/fmicb.2019.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovanetti M, de Mendonça MCL, Fonseca V, Mares-Guia MA, Fabri A, Xavier J, de Jesus JG, Gräf T, dos Santos Rodrigues CD, dos Santos CC, Sampaio SA, Chalhoub FLL, de Bruycker Nogueira F, Theze J, Romano APM, Ramos DG, de Abreu AL, Oliveira WK, do Carmo Said RF, de Alburque CFC, de Oliveira T, Fernandes CA, Aguiar SF, Chieppe A, Sequeira PC, Faria NR, Cunha RV, Alcantara LCJ, de Filippis AMB. 2019. Yellow fever virus spread in Rio de Janeiro and Espírito Santo, 2016–2019: phylodynamic assessment to improve intervention strategies. bioRxiv doi: 10.1101/711994. [DOI]
- 19.Drosten C, Göttig S, Schilling S, Asper M, Panning M, Schmitz H, Günther S. 2002. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J Clin Microbiol 40:2323–2330. doi: 10.1128/jcm.40.7.2323-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domingo C, Patel P, Yillah J, Weidmann M, Méndez JA, Nakouné ER, Niedrig M. 2012. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J Clin Microbiol 50:4054–4060. doi: 10.1128/JCM.01799-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quick J, Grubaugh ND, Pullan ST, Claro IM, Smith AD, Gangavarapu K, Oliveira G, Robles-Sikisaka R, Rogers TF, Beutler NA, Burton DR, Lewis-Ximenez LL, de Jesus JG, Giovanetti M, Hill SC, Black A, Bedford T, Carroll MW, Nunes M, Alcantara LC Jr, Sabino EC, Baylis SA, Faria NR, Loose M, Simpson JT, Pybus OG, Andersen KG, Loman NJ. 2017. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat Protoc 12:1261–1276. doi: 10.1038/nprot.2017.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza RP, Hill SC, Thézé J, Claro I, Aguiar RS, Dellicour D, Abade L, Santos FCP, Cunha MS, Nogueira JS, Salles FCS, Rocco IM, Maeda AY, Vasami FGS, Du Plessis L, Silveira PP, Giovanetti M, de Goes J, Quick J, Fernandes N, Guerra J, Réssio MRA, Cirqueira CS, Iglezias SD, Delgado JD, Macedo FLL, Timenetsky M, de Paula R, Spinola R, Deus JT, Mucci LF, Tubaki RM, Menezes RMT, Ramos PL, Abreu AL, Cruz LN, Loman N, Bispo A, Pybus OG, Alcantara LVJ, Sabino EC, Faria NR. 2019. Genomic surveillance of yellow fever virus epidemic waves in São Paulo, Brazil, 2017–2018. bioRxiv doi: 10.1101/645341. [DOI]
- 23.Cunha MS, da Costa AC, de Azevedo Fernandes NCC, Guerra JM, Dos Santos FCP, Nogueira JS, D'Agostino LG, Komninakis SV, Witkin SS, Ressio RA, Maeda AY, Vasami FGS, Kaigawa UMA, de Azevedo LS, de Souza Facioli PA, Macedo FLL, Sabino EC, Leal É, de Souza RP. 2019. Epizootics due to yellow fever virus in São Paulo State, Brazil: viral dissemination to new areas (2016–2017). Sci Rep 9:5474. doi: 10.1038/s41598-019-41950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A. 2018. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol 4:vey016. doi: 10.1093/ve/vey016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Possas C, Lourenço-de-Oliveira R, Tauil PL, Pinheiro FP, Pissinatti A, Cunha RVD, Freire M, Martins RM, Homma A. 2018. Yellow fever outbreak in Brazil: the puzzle of rapid viral spread and challenges for immunisation. Mem Inst Oswaldo Cruz 113:e180278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardy JL, Loman NJ. 2017. Towards a genomics-informed, real-time, global pathogen surveillance system. Nat Rev Genet 19:9–20. doi: 10.1038/nrg.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faria NR, Quick J, Claro IM, Thézé J, de Jesus JG, Giovanetti M, Kraemer MUG, Hill SC, Black A, da Costa AC, Franco LC, Silva SP, Wu CH, Raghwani J, Cauchemez S, Du Plessis L, Verotti MP, de Oliveira WK, Carmo EH, Coelho GE, Santelli A, Vinhal LC, Henriques CM, Simpson JT, Loose M, Andersen KG, Grubaugh ND, Somasekar S, Chiu CY, Muñoz-Medina JE, Gonzalez-Bonilla CR, Arias CF, Lewis-Ximenez LL, Baylis SA, Chieppe AO, Aguiar SF, Fernandes CA, Lemos PS, Nascimento BLS, Monteiro HAO, Siqueira IC, de Queiroz MG, de Souza TR, Bezerra JF, Lemos MR, Pereira GF, Loudal D, et al. 2017. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature 546:406–410. doi: 10.1038/nature22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rambaut A, Lam TT, Max Carvalho L, Pybus OG. 2016. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen). Virus Evol 2:vew007. doi: 10.1093/ve/vew007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro B, Rambaut A, Drummond AJ. 2006. Choosing appropriate substitution models for the phylogenetic analysis of protein-coding sequences. Mol Biol Evol 23:7–9. doi: 10.1093/molbev/msj021. [DOI] [PubMed] [Google Scholar]
- 31.Drummond AJ, Ho SY, Phillips MJ, Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol 4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira MAR, Suchard MA. 2008. Bayesian analysis of elapsed times in continuous-time Markov chains. Can J Stat 36:355–368. doi: 10.1002/cjs.5550360302. [DOI] [Google Scholar]
- 33.Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemey P, Rambaut A, Welch JJ, Suchard MA. 2010. Phylogeography takes a relaxed random walk in continuous space and time. Mol Biol Evol 27:1877–1885. doi: 10.1093/molbev/msq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pybus OG, Suchard MA, Lemey P, Bernardin FJ, Rambaut A, Crawford FW, Gray RR, Arinaminpathy N, Stramer SL, Busch MP, Delwart EL. 2012. Unifying the spatial epidemiology and molecular evolution of emerging epidemics. Proc Natl Acad Sci U S A 109:15066–15071. doi: 10.1073/pnas.1206598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayres DL, Darling A, Zwickl DJ, Beerli P, Holder MT, Lewis PO, Huelsenbeck JP, Ronquist F, Swofford DL, Cummings MP, Rambaut A, Suchard MA. 2012. BEAGLE: an application programming interface and high-performance computing library for statistical phylogenetics. Syst Biol 61:170–173. doi: 10.1093/sysbio/syr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
New sequences were deposited in GenBank under accession numbers MK882599 to MK882604, MK882607 to MK882613, MK882615, MK882617 to MK882619, and MK882621 (see Table 2).