Abstract
Background
Lung transplant recipients commonly develop invasive fungal infections (IFIs), but the most effective strategies to prevent IFIs following lung transplantation are not known.
Methods
We prospectively collected clinical data on all patients who underwent lung transplantation at a tertiary care academic hospital from January 2007–October 2014. Standard antifungal prophylaxis consisted of aerosolized amphotericin B lipid complex during the transplant hospitalization. For the first 180 days after transplant, we analyzed prevalence rates and timing of IFIs, risk factors for IFIs, and data from IFIs that broke through prophylaxis.
Results
In total, 156 of 815 lung transplant recipients developed IFIs (prevalence rate, 19.1 IFIs per 100 surgeries, 95% confidence interval [CI] 16.4–21.8%). The prevalence rate of invasive candidiasis (IC) was 11.4% (95% CI 9.2–13.6%), and the rate of non-Candida IFIs was 8.8% (95% CI 6.9–10.8%). First episodes of IC occurred a median of 31 days (interquartile range [IQR] 16–56 days) after transplant, while non-Candida IFIs occurred later, at a median of 86 days (IQR 40–121 days) after transplant. Of 169 IFI episodes, 121 (72%) occurred in the absence of recent antifungal prophylaxis; however, IC and non-Candida breakthrough IFIs were observed, most often representing failures of micafungin (n = 16) and aerosolized amphotericin B (n = 24) prophylaxis, respectively.
Conclusions
Lung transplant recipients at our hospital had high rates of IFIs, despite receiving prophylaxis with aerosolized amphotericin B lipid complex during the transplant hospitalization. These data suggest benefit in providing systemic antifungal prophylaxis targeting Candida for up to 90 days after transplant and extending mold-active prophylaxis for up to 180 days after surgery.
Keywords: invasive fungal infection, lung transplantation, prophylaxis
Lung transplant recipients experienced high rates of invasive fungal infection, despite receiving prophylaxis with aerosolized amphotericin B lipid complex during the transplant hospitalization. Antifungal prophylaxis after lung transplantation should include coverage for invasive candidiasis and prolonged prophylaxis against respiratory molds.
Survival following lung transplantation continues to improve [1], due to advances in donor selection and management, surgical techniques, perioperative care, and immunosuppressive regimens [2–4]. However, despite new antifungal therapies and prophylactic strategies [5–7], lung transplant recipients remain at substantial risk for the development of invasive fungal infections (IFIs) [8–11].
A prospective study completed at 11 US transplant centers from 2001–2006 reported an 8.6% cumulative incidence of IFIs among lung transplant recipients during the first year following transplant surgery [8]. These infections, most often invasive mold infections (IMI) or invasive candidiasis (IC), are associated with high morbidity and mortality [12, 13]. Hospitalizations for patients with IFIs last longer and cost approximately $30 000 more than control patient hospitalizations [14]. Furthermore, lung transplant recipients with invasive aspergillosis have mortality rates that may exceed 50% [15, 16].
Guidelines from the International Society for Heart and Lung Transplantation (ISHLT) [9] and the Infectious Diseases Society of America [5] recommend antifungal prophylaxis after lung transplantation; however, these recommendations are predominantly based on limited and conflicting evidence and consensus opinions of experts. While a recent meta-analysis suggested that antifungal prophylaxis reduces the incidence of invasive aspergillosis after lung transplantation [17], individual studies have been hampered by single-center, nonrandomized, or retrospective designs [18]. Therefore, the most effective strategies for antifungal prophylaxis are not known.
Accordingly, while nearly all transplant centers utilize antifungal prophylaxis, their protocols vary widely [19, 20]. Some centers prescribe universal prophylaxis and begin antifungal prophylaxis immediately after lung transplant surgery for all patients [21]. Other centers use preemptive or targeted prophylaxis and include only recipients at increased risk for IFI, such as patients with cystic fibrosis [20], mold infection in the explanted lungs [22], fungal colonization of the airways [20, 23], primary allograft dysfunction [23], or cytomegalovirus infection [20, 23]. The duration of antifungal prophylaxis also varies considerably, ranging from 3 months to more than 12 months following transplant surgery [24].
An international survey of 58 lung transplant centers reported that 34 (59%) centers employed universal prophylaxis [19]. The majority of these centers (n = 23, 68%) used monotherapy, most often with an aerosolized amphotericin B product (7/23, 30%), systemic voriconazole (7/23, 30%), or itraconazole (6/23, 26%). Of the centers providing universal prophylaxis, 11 (32%) used combination therapy and, in all but 1 case, the prophylaxis regimen contained an aerosolized amphotericin B agent in combination with either voriconazole or itraconazole. Only 4 (12%) centers that used universal prophylaxis extended antifungal coverage beyond 6 months after transplantation.
The objectives of this study were: (1) to describe the epidemiology of IFIs after lung transplant surgery performed at our hospital; and (2) to evaluate the effectiveness of our lung transplant antifungal prophylaxis protocol.
METHODS
Setting and Transplant Protocols
Duke University Hospital is a tertiary-care, 924-bed, academic hospital and transplant center located in central North Carolina. During the study period, we utilized universal antifungal prophylaxis after lung transplant surgeries. Routine prophylaxis consisted of aerosolized amphotericin B lipid complex (ABLC) [25]. Patients received daily doses for 4 days after transplantation, followed by weekly doses for the duration of the index post-transplant hospitalization. Intubated patients received 100 mg, while extubated patients received 50 mg. Recipients who had airway fungal colonization prior to transplant additionally received targeted systemic prophylaxis with a mold-active triazole. Patients not receiving secondary azole prophylaxis who had delayed chest closure after transplant surgery or required postoperative extracorporeal membrane oxygenation (ECMO) received micafungin prophylaxis until chest closure or ECMO decannulation, in addition to standard aerosolized ABLC prophylaxis.
Fungal cultures of donor bronchial tissue were performed prior to implantation. Induction immunosuppression for recipients consisted of basiliximab, methylprednisolone, and either mycophenolate mofetil or azathioprine. Transbronchial biopsies were performed at 1, 3, and 6 months after transplant. Patients with acute cellular rejection typically received corticosteroid protocols and standard antifungal prophylaxis with aerosolized ABLC; however, recipients who required alemtuzumab for refractory rejection additionally received systemic antifungal prophylaxis with posaconazole. Recipients with bronchoalveolar lavage cultures positive for fungal pathogens who did not have probable or proven IFIs did not receive augmented antifungal prophylaxis via protocol, but may have received systemic prophylaxis, depending upon the clinical scenario.
The Duke University Institutional Review Board approved this investigation and research.
Analysis Plan
Data were prospectively collected on 820 consecutive lung transplant surgeries performed at our hospital from January 2007 through October 2014. We retrospectively analyzed lung transplant donor and recipient demographics and clinical data, including recipient data from the first 180 days following each transplant surgery. Data sources included the electronic medical record and pharmacy database at our hospital, as well as the United Network for Organ Sharing single-center database.
Infectious disease clinicians analyzed data on all patients in this cohort to determine which patients developed IFIs during the first 180 days after lung transplantation. IFIs were included if they met criteria for proven or probable invasive fungal disease, as defined by the revised European Organization for Research and Treatment of Cancer/Mycoses Study Group [26]. We did not include cases of possible invasive fungal disease or airway fungal colonization.
The date of diagnosis was defined as the date that the first positive culture or tissue specimen revealing an IFI on histopathology was obtained. A patient had multiple episodes of IFI if a subsequent IFI was caused by a different fungal genus or was diagnosed at least 60 days after the most recent prior positive culture or histopathology specimen. There were 5 patients who experienced an episode of IFI within 180 days of 2 separate lung transplant surgeries. In these instances, we attributed the IFI to the transplant surgery immediately preceding the IFI diagnosis and excluded the other surgery from analysis.
We determined the overall prevalence rate of IFIs during the 8-year study period and then stratified by date of transplant, type of IFI (IC or non-Candida IFI), causative pathogen, and site of infection. We used unadjusted log-binomial regression to calculate prevalence rates, prevalence rate ratios (PRRs), and 95% confidence intervals (CIs) for the study time periods. We also used univariate log-binomial regression to investigate preoperative and perioperative risk factors for overall IFI, IC, and non-Candida IFI.
We analyzed the antifungal therapy given prior to IFI diagnoses and defined a breakthrough infection as an IFI that occurred while on antifungal therapy for at least 48 hours in the 30 days prior to diagnosis. For IC to qualify as a breakthrough infection, a patient was required to fail systemic antifungal prophylaxis (ie, aerosolized ABLC was not considered to provide prophylaxis against IC). For an IMI to qualify as a breakthrough infection, a patient was required to fail mold-active prophylaxis (ie, fluconazole was not considered to provide prophylaxis against IMI).
Data were analyzed using SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Invasive Fungal Infection
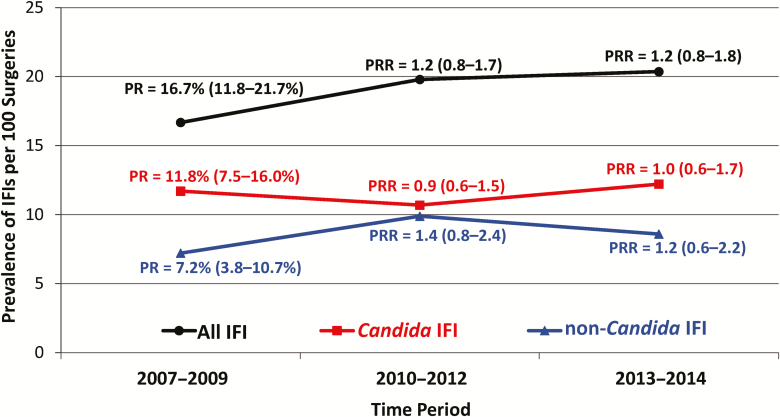
A total of 156 out of 815 lung transplant recipients developed an IFI within 180 days after transplant surgery, giving an overall prevalence rate of 19.1 IFIs per 100 surgeries (95% CI 16.4–21.8%). During the study time period, the 180-day prevalence rate did not change significantly for overall IFIs, IC, or non-Candida IFIs (Figure 1).
Figure 1.
Prevalence rates of IFIs, stratified by Candida and non-Candida IFIs, among 815 lung transplant recipients who underwent lung transplantation from 2007 through 2014. Prevalence rates were calculated for the first 180 days after transplantation. For each time period, prevalence rates were compared to 2007–2009 prevalence rates. Prevalence rates, prevalence rate ratios, and 95% confidence intervals are given. Abbreviations: IFI, invasive fungal infection; PR, prevalence rate; PRR, prevalence rate ratio.
Among the 156 patients who developed IFIs, we identified 169 total IFI episodes. There were 9 patients who had episodes of both IC and non-Candida IFI; 3 patients who had 2 episodes of IC following the same transplant surgery; and 1 patient who had 2 episodes of non-Candida IFI. There were 96 (57%) episodes of IC and 73 (43%) episodes of non-Candida IFI, of which 68 (93%) represented IMI.
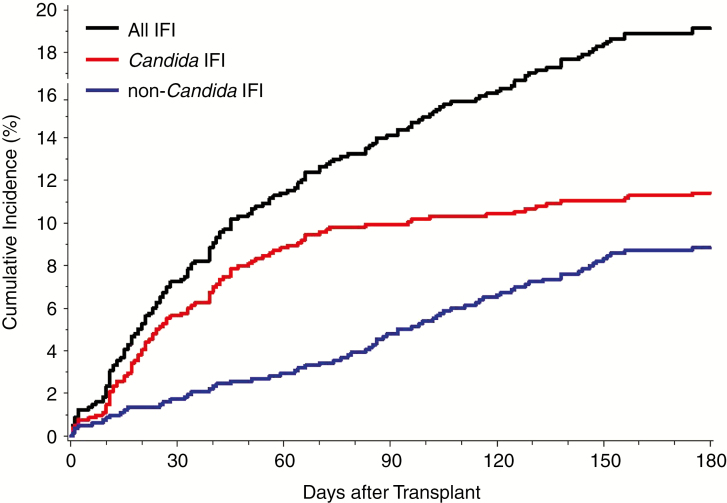
The median duration from the date of transplant surgery to the date of first IFI was 43 days (interquartile range [IQR] 19–94 days). Of the 156 patients with IFIs, 115 (74%) developed IFI in the first 90 days after transplant surgery (Figure 2). Of 169 episodes of IFI, 121 (72%) occurred in the absence of recent antifungal prophylaxis, but the other 48 (28%) IFIs broke through prophylactic therapy.
Figure 2.
Cumulative incidence of IFIs, stratified by Candida and non-Candida IFIs, among 815 lung transplant recipients who underwent lung transplantation from 2007 through 2014. IFIs were limited to the first 180 days after transplantation. For each patient, only the first IFI for each category was considered. Abbreviation: IFI, invasive fungal infection.
Several preoperative and perioperative patient characteristics were associated with increased prevalence rates of IFI (Table 1). Preoperative variables that were statistically significant predictors of IFI included diabetes mellitus (PRR 1.4, 95% CI 1.1–2.0); low functional status, as measured by the Karnofsky Performance Status Scale [27] (PRR 1.5, 95% CI 1.1–2.1); high lung allocation score [28] (PRR 1.1, 95% CI 1.1–2.1); and an indication for transplant of primary graft failure of a prior lung transplantation (PRR 2.4, 95% CI 1.1–5.0). Perioperative characteristics that were statistically significant predictors of IFI included female donor status (PRR 1.3, 95% CI 1.0–1.8), ECMO requirement within the first 7 postoperative days (PRR 1.4, 95% CI 1.0–2.2), ventilator support requirement for more than 48 hours after surgery (PRR 2.0, 95% CI 1.5–2.6), and hemodialysis requirement prior to discharge from the index transplant hospitalization (PRR 2.3, 95% CI 1.4–3.9). Stratification of these results by type of IFI did not reveal meaningful differences between patients who developed IC and non-Candida IFI (data not shown), except for bilateral lung transplantation, which was a significant predictor of IC (PRR 1.9, 95% CI 1.1–3.1; P = .02) but not associated with non-Candida IFI (PRR 0.8, 95% CI 0.5–1.2; P = .25).
Table 1.
Preoperative and Perioperative Characteristics of 815 Patients Who Underwent Lung Transplantation Surgery From 2007 Through 2014
| Characteristic | Invasive Fungal Infection (n = 156) |
No Invasive Fungal Infection (n = 659) |
Prevalence Rate Ratio for Invasive Fungal Infection (95% CI) | P Value |
|---|---|---|---|---|
| Preoperative characteristics | ||||
| Age at transplant, median (IQR), years | 61.5 (47.0–67.0) | 61.0 (48.0–66.0) | 1.0 (0.9–1.1)a | .67 |
| Male sex, n (%) | 94 (60.3) | 405 (61.5) | 1.0 (0.7–1.3) | .78 |
| Race/ethnicity, n (%) | ||||
| White | 139 (89.1) | 600 (91.0) | 0.8 (0.5–1.3) | .45 |
| Black | 12 (7.7) | 51 (7.7) | 1.0 (0.6–1.7) | .98 |
| Hispanic | 4 (2.6) | 7 (1.1) | 1.9 (0.9–4.3) | .11 |
| Asian | 1 (0.6) | 1 (0.2) | 2.6 (0.7–10.6) | .18 |
| Underlying pulmonary disease, n (%) | ||||
| Idiopathic pulmonary fibrosis | 72 (46.2) | 296 (44.9) | 1.0 (0.8–1.4) | .78 |
| Chronic obstructive pulmonary disease | 28 (17.9) | 137 (20.8) | 0.9 (0.6–1.2) | .43 |
| Cystic fibrosis | 20 (12.8) | 89 (13.5) | 1.0 (0.6–1.5) | .82 |
| Primary graft failure (re-transplant) | 4 (2.6) | 5 (0.8) | 2.4 (1.1–5.0) | .02 |
| Other disease | 32 (20.5) | 132 (20.0) | 1.0 (0.7–1.5) | .89 |
| Diabetes mellitus, n (%) | 47 (30.1) | 140 (21.2) | 1.4 (1.1–2.0) | .02 |
| Obese (BMI > 30 kg/m2), n (%) | 1 (0.6) | 14 (2.1) | 0.3 (0.1–2.3) | .27 |
| Underweight (BMI < 20 kg/m2), n (%) | 37 (23.7) | 120 (18.2) | 1.3 (0.9–1.8) | .11 |
| Prior lung transplant, n (%) | 13 (8.3) | 40 (6.1) | 1.3 (0.8–2.1) | .29 |
| Karnofsky Performance Status Score ≤30%, n (%) | 33 (21.2) | 92 (14.0) | 1.5 (1.1–2.1) | .02 |
| Lung allocation score, median (IQR) | 45.2 (37.4–60.0) | 42.3 (35.8–53.6) | 1.1 (1.0–1.2) a | .01 |
| Perioperative characteristics | ||||
| Hospitalized at time of transplant, n (%) | 29 (18.6) | 92 (14.0) | 1.3 (0.9–1.9) | .14 |
| Ventilator at time of transplant, n (%) | 9 (5.8) | 35 (5.3) | 1.1 (0.6–2.0) | .82 |
| ECMO at time of transplant, n (%) | 5 (3.2) | 20 (3.0) | 1.0 (0.5–2.3) | .91 |
| Female donor, n (%)b | 66 (42.3) | 223 (33.9) | 1.3 (1.0–1.8) | .05 |
| CMV mismatch (recipient CMV negative), n (%) | 47 (30.1) | 151 (22.9) | 1.3 (1.0–1.8) | .06 |
| Ischemic time, median (IQR), hoursc | 6.7 (5.5–8.1) | 6.6 (5.5–7.9) | 1.0 (1.0–1.1)a | .68 |
| Bilateral lung transplantation, n (%) | 118 (75.6) | 483 (73.3) | 1.1 (0.8–1.5) | .55 |
| Open chest in first 7 days after surgery, n (%) | 8 (5.1) | 26 (3.9) | 1.2 (0.7, 2.3) | .50 |
| ECMO in first 7 days after surgery, n (%) | 24 (15.4) | 67 (10.2) | 1.4 (1.0–2.1) | .05 |
| Ventilator support longer than 48 hours, n (%) | 62 (39.7) | 142 (21.5) | 2.0 (1.5–2.6) | <.0001 |
| Dialysis required prior to hospital discharge, n (%) | 9 (5.8) | 12 (1.8) | 2.3 (1.4–3.9) | .001 |
| Acute cellular rejection in first 180 days, n (%)d | 100 (64.1) | 427 (64.8) | 1.0 (0.7–1.3) | .87 |
Results are stratified by development of invasive fungal infection in the first 180 days after surgery. Bold values indicate the variables that have statistically significant P values.
Abbreviations: BMI, body mass index; CI, confidence interval; CMV, cytomegalovirus; ECMO, extracorporeal membrane oxygenation; IFI, invasive fungal infection; IQR, interquartile range.
aPrevalence rate ratios and CIs are given for 10-year increases in age, 10-point increases in lung allocation score, and 1-hour increases in ischemic time.
bDonor sex was excluded from the analysis for 1 patient without an IFI, who received donor lungs from 1 female and 1 male donor during the same transplant surgery.
cMissing data for ischemic time were excluded from the analysis for 4 patients without IFIs.
dBiopsy-confirmed acute cellular rejection that occurred within 180 days of transplantation.
Invasive Candidiasis
There were 93 patients who developed IC (prevalence rate, 11.4 infections per 100 surgeries, 95% CI 9.2–13.6%; Figure 1). Among 96 episodes, Candida albicans/C. dubliniensis (not further identified) was the most common pathogen (n = 70, 73%), followed by C. tropicalis (n = 13, 14%) and C. glabrata (n = 12, 13%; Table 2). The bloodstream (n = 47, 49%) was the most common site of infection. There were 43 (45%) episodes of IC that involved the pleural space and 15 (16%) that were deep incisional surgical site infections. There were 14 (15%) episodes of IC that involved multiple Candida species and 27 (28%) that included infections at noncontiguous sites. In particular, 20 (43%) bloodstream infections involved additional sites, of which 14 (70%) involved the pleural space.
Table 2.
Causative Pathogens and Sites of Infection Among 93 Patients Who Developed 96 Episodes of Invasive Candidiasis in the First 180 Days After Lung Transplantations Performed From 2007 Through 2014
| Site of Infection | All Candida Species (n = 96) |
C. albicans/ C. dubliniensisa (n = 70) |
C. tropicalis
(n = 13) |
C. glabrata
(n = 12) |
C. parapsilosis
(n = 7) |
C. krusei
(n = 5) |
Otherb (n = 3) |
Multiple Candida Species (n = 14) |
|---|---|---|---|---|---|---|---|---|
| Blood | 47 (49) | 28 (40) | 9 (69) | 8 (67) | 5 (71) | 3 (60) | 3 (100) | 10 (71) |
| Pleura | 43 (45) | 37 (53) | 8 (62) | 3 (25) | 1 (14) | 1 (20) | 1 (33) | 7 (50) |
| Incisional SSI | 15 (16) | 12 (17) | 1 (8) | 2 (17) | 0 (0) | 1 (20) | 0 (0) | 1 (7) |
| Peritoneum | 11 (11) | 8 (11) | 3 (23) | 4 (33) | 0 (0) | 3 (60) | 0 (0) | 6 (43) |
| Tracheobronchial | 5 (5) | 5 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Otherc | 4 (4) | 2 (3) | 0 (0) | 1 (8) | 1 (14) | 0 (0) | 0 (0) | 0 (0) |
| Multiple sites | 27 (28) | 19 (27) | 8 (62) | 6 (50) | 0 (0) | 3 (60) | 1 (33) | 10 (71) |
Data are presented as no. (%). Episodes of invasive candidiasis associated with multiple sites of infection or species of Candida were included in multiple categories.
Abbreviations: C., Candida; SSI, surgical site infection.
a C. albicans/C. dubliniensis was not further identified.
bOther Candida species include C. lusitaniae (n = 2) and C. zeylanoides (n = 1).
cOther sites of infection include nonincisional site soft tissue (n = 2), lung (n = 1), and pericardium (n = 1).
The median duration from the date of transplant to the date of first episode of IC was 31 days (IQR, 16–56 days). Most first episodes (n = 81, 87%) occurred within 90 days of the transplant surgery (Figure 2).
Despite systemic antifungal prophylaxis, 18 (19%) episodes of breakthrough IC occurred (Table 3); 16 (89%) of these patients received micafungin prophylaxis. Most micafungin failures (n = 11, 69%) involved infections that included sites other than the bloodstream, including 4 Candida empyemas.
Table 3.
Episodes of Breakthrough Invasive Candidiasis (n = 18) That Occurred Despite Antifungal Prophylaxis Among 815 Lung Transplant Recipients During the First 180 Days After Lung Transplantation Performed From 2007 Through 2014
| Antifungal Agent | Pathogen | Site of Infection | Possible Explanation for Breakthrough Infection |
|---|---|---|---|
| Micafungin, 16/18 (89) | C. albicans,a 8/16 (50) | Blood, 9/16 (56) | Poor site penetration,b 11/16 (69) |
| C. glabrata, 4/16 (25) | Pleura, 4/16 (25) | Postoperative ECMO, 6/16 (38) | |
| C. krusei, 4/16 (25) | Surgical incision, 4/16 (25) | Postoperative open chest, 4/16 (25) | |
| C. parapsilosis, 2/16 (13) | Peritoneum, 3/16 (19) | Proven antifungal resistance, 1/16 (6) | |
| C. tropicalis, 2/16 (13) | Other soft tissue, 2/16 (13) | None known, 2/16 (13) | |
| … | Other, 1/16 (6) | … | |
| Voriconazole, 3/18 (17) | C. albicans,a 1/3 (33) | Blood, 1/3 (33) | Subtherapeutic drug level, 1/3 (33) |
| C. glabrata, 1/3(33) | Peritoneum, 1/3 (33) | None known,c 2/3 (67) | |
| C. krusei, 1/3 (33) | Pleura, 1/3 (33) | … | |
| C. parapsilosis, 1/3 (33) | Other soft tissue, 1/3 (33) | … | |
| Posaconazole, 1/18 (6) | C. zeylanoides, 1/1 (100) | Blood, 1/1 (100) | None known, 1/1 (100) |
Data are presented as no./No. (%). Episodes of IC associated with multiple antifungal agents, pathogens, sites of infection, or explanations for breakthrough infection were included in multiple categories.
Abbreviations: C., Candida; ECMO, extracorporeal membrane oxygenation; IC, invasive candidiasis.
a C. albicans/C. dubliniensis was not further identified.
b“Poor site penetration” for micafungin includes all episodes of IC that had sites of infection other than the bloodstream.
cBoth patients who received voriconazole without a known explanation for the breakthrough infection did not have serum drug levels monitored prior to the development of invasive fungal infection.
Non-Candida Invasive Fungal Infection
There were 72 patients who developed non-Candida IFIs (prevalence rate, 8.8 infections per 100 surgeries, 95% CI 6.9–10.8%; Figure 1). Aspergillus was the most common pathogen and caused 42 (58%) of 73 episodes (Table 4). Nearly all episodes (n = 67, 92%) represented lung parenchymal (n = 63) and/or tracheobronchial infections (n = 11). There were 12 (16%) episodes involving multiple organisms, and only 3 (4%) infections were proven to be disseminated with involvement of noncontiguous sites.
Table 4.
Episodes of Non-Candida Invasive Fungal Infection (n = 73) That Occurred Within 180 Days After Lung Transplantation in the First 180 Days After Lung Transplantation Performed From 2007 Through 2014
| Fungal Pathogen | Number of Non-Candida IFI Episodes (n = 73) |
|---|---|
| Invasive Aspergillosis | 42 (58) |
| Aspergillus fumigatus | 27/42 (64) |
| Aspergillus flavus | 8/42 (19) |
| Aspergillus ochraceous | 4/42 (10) |
| Aspergillus niger | 3/42 (7) |
| Aspergillus terreus | 3/42 (7) |
| Other Aspergillus spp. | 1/42 (2) |
| Multiple Aspergillus spp. | 4/42 (10) |
| Fusarium spp. | 6 (8) |
| Paecilomyces spp. | 6 (8) |
| Hormographiella spp. | 2 (3) |
| Scedosporium apiospermum | 2 (3) |
| Scopulariopsis spp. | 2 (3) |
| Arthrographis sp. | 1 (1) |
| Dematiaceous mold | 5 (7) |
| Mucorales | 4 (5) |
| Unspecified mold | 3 (4) |
| Multiple mold genera | 7 (10) |
| Trichosporon spp. | 4 (5) |
| Cryptococcus spp. | 2 (3) |
| Blastomyces dermatitidis | 1 (1) |
Data are presented as no. (%) or no./No. (%). Episodes of non-Candida invasive fungal infection associated with multiple fungal pathogens were included in multiple categories.
Abbreviations: IFI, invasive fungal infection; sp., species; spp., multiple species.
The median duration from the date of transplant to the date of first episode of non-Candida IFI was 86 days (IQR 40–121 days). Just over half of first episodes (n = 39, 54%) occurred within 90 days of the transplant surgery (Figure 2).
There was 1 patient who developed early reactivation of Blastomycosis infection and was found to have yeast consistent with Blastomyces dermatitidis on a native lung explant histopathologic evaluation. Bronchial tissue cultures from only 2 donors grew potentially pathogenic fungi other than Candida. There was 1 recipient who developed an IFI from Trichosporon 16 days after receiving transplanted lungs from a donor who had Trichosporon growth on a bronchial culture.
Despite antifungal prophylaxis, 30 (41%) non-Candida breakthrough IFIs occurred, including 26 (38%) of 68 IMIs (Table 5). Most patients (n = 24, 80%) classified as prophylaxis failures broke through aerosolized ABLC; however, 7 (29%) ABLC failures had discontinued prophylaxis more than 1 week (median, 17 days; range, 13–22 days) prior to their IFI diagnosis. Pathogens frequently resistant to amphotericin B caused over half (n = 14, 58%) of ABLC prophylaxis failures [29, 30], and 3 (13%) patients who failed ABLC developed infections outside of the respiratory tract.
Table 5.
Episodes of Breakthrough Non-Candida Invasive Fungal Infection (n = 30) That Occurred Despite Antifungal Prophylaxis Among 815 Lung Transplant Recipients During the First 180 Days After Lung Transplantation Performed From 2007 Through 2014
| Antifungal Agent | Pathogen (genus) | Site of Infection | Possible Explanation for Breakthrough Infection |
|---|---|---|---|
| i-ABLC, 24/30 (80) | Aspergillus, 6/24 (25) | Lung, 21/24 (88) | Poor activity against pathogen, 14/24 (58) |
| Fusarium, 3/24 (13) | Blood, 1/24 (4) | Prophylaxis stopped,a 8/24 (33) | |
| Paecilomyces, 3/24 (13) | Bone, 1/24 (4) | Poor site penetration,b 3/24 (13) | |
| Ochroconis, 2/24 (8) | Pleural, 1/24 (4) | Pre-existing infection,c 1/24 (4) | |
| Trichosporon, 2/24 (8) | Tracheo-bronchial, 1/24 (4) | None known, 4/24 (17) | |
| Mucorales, 2/24 (8) | … | … | |
| Other, 8/24 (33) | … | … | |
| Micafungin, 7/30 (23) | Cunninghamella, 2/7 (29) | Lung, 6/7 (86) | Poor site penetration,b 5/7 (71) |
| Blastomyces, 1/7 (14) | Blood, 1/7 (14) | Poor activity against pathogen, 4/7 (57) | |
| Fusarium, 1/7 (14) | Bone, 1/7 (14) | Pre-existing infection,c 1/7 (14) | |
| Hyphomycete, 1/7 (14) | Liver/spleen, 1/7 (14) | None known, 1/7 (14) | |
| Ochroconis, 1/7 (14) | … | … | |
| Unspecified mold, 1/7 (14) | … | … | |
| Voriconazole, 7/30 (23) | Aspergillus, 2/7 (29) | Lung, 5/7 (71) | Poor activity against pathogen, 4/7 (57) |
| Scopulariopsis, 2/7 (29) | Tracheo-bronchial, 2/7 (29) | Subtherapeutic drug level, 1/7 (14) | |
| Mucorales, 2/7 (29) | Bone, 1/7 (14) | None known,d 2/7 (29) | |
| Scedosporium, 1/7 (14) | … | … | |
| Itraconazole, 1/30 (3) | Aspergillus, 1/1 (100) | Lung, 1/1 (100) | None known,d 1/1 (100) |
| Posaconazole, 1/30 (3) | Fusarium, 1/1 (100) | Lung, 1/1 (100) | Short duration of prophylaxis, 1/1 (100) |
Data are presented as no./No. (%). Episodes of non-Candida IFI associated with multiple antifungal agents, pathogens, sites of infection, or explanations for breakthrough infection were included in multiple categories.
Abbreviations: i-ABLC, aerosolized amphotericin B lipid complex; IFI, invasive fungal infection.
a“Prophylaxis stopped” indicates that antifungal prophylaxis was given within 30 days of the IFI diagnosis but was discontinued prior to the diagnosis.
b“Poor site penetration” for i-ABLC includes all episodes of non-Candida IFI that had sites of infection outside of the respiratory tract. For micafungin, this designation includes all episodes that had sites of infection other than the bloodstream.
c“Pre-existing infection” refers to a Blastomyces infection diagnosed on the first post-transplant day in a patient who also had yeast consistent with Blastomyces dermatitidis seen on a native lung explant histopathologic evaluation.
dThere was 1 patient who received voriconazole and 1 patient who received itraconazole without a known explanation for the breakthrough infection who did not have serum drug levels monitored prior to the development of an IFI.
All 42 infections caused by Aspergillus represented invasive pulmonary aspergillosis (IPA), including 8 (19%) patients who broke through mold-active prophylaxis. Of the 34 patients who developed IPA and were not classified as prophylaxis failures, 32 (94%) patients had been discharged from the index transplant hospitalization and, per protocol, were no longer receiving aerosolized ABLC prophylaxis.
DISCUSSION
We analyzed the epidemiology of IFI following lung transplant surgery at our hospital over an 8-year time period and evaluated the effectiveness of our antifungal prophylaxis protocol. The overall prevalence rate of IFIs in the first 180 days after transplant was 19.1%. This rate was stable over the study time period, but was notably higher than the incidence rates of IFIs reported in prior single-center studies [31, 32] and the 8.6% 1-year cumulative incidence of IFIs reported by a network of 11 US lung transplant centers [8].
The 180-day prevalence rate of 11.4% for IC was also higher than expected [8, 13, 31, 32]. The bloodstream and pleural space were the most common sites of Candida infections, and bloodstream infections were frequently complicated by disseminated infections at multiple sites, most commonly involving the pleural space.
Interestingly, almost 20% of patients with IC broke through systemic prophylaxis, nearly always with micafungin. Micafungin breakthrough IFIs in the setting of invasive disease outside of the bloodstream raise the question of inadequate tissue penetration of echinocandins, particularly into the pleural space [33–35]. In addition, micafungin breakthroughs among patients requiring EMCO may also reflect decreased drug exposure related to micafungin extraction by the ECMO circuit [36]. A few patients broke through triazole prophylaxis, at times in the setting of subtherapeutic or unknown serum drug levels.
The 180-day prevalence rate of non-Candida IFIs of 8.8% was also higher than previously reported rates [8, 12, 16, 31, 32]. The majority of these infections represented pulmonary IMIs caused by Aspergillus, and most cases of IPA occurred more than 30 days after the discontinuation of antifungal prophylaxis. However, non-Candida IFIs occurred despite antifungal prophylaxis in 30 patients. The elevated rate of breakthrough IFIs, primarily indicative of aerosolized ABLC prophylaxis failure, emphasizes the potential for molds resistant to amphotericin B to cause IFIs in this patient population.
Data from this study emphasize 3 primary potential explanations for the high IFI prevalence at our center. First, the lack of universal systemic coverage targeting Candida very likely contributed to elevated rates of IC. Current ISHLT guidelines recommend the consideration of fluconazole or an echinocandin for universal prophylaxis to decrease the risk of IC during the first 2 to 4 weeks after lung transplant [9]. Our data support IC prophylaxis but raise concerns regarding the efficacy of echinocandins (in this case, micafungin). Furthermore, most episodes of IC in our study occurred more than 4 weeks after transplant, and our time-to-event analysis suggests that systemic coverage for Candida should extend for up to 90 days post-transplant.
Second, providing prophylaxis against IMI through the end of the index transplant hospitalization may have provided an inadequate duration of mold-active coverage. Almost half of the IMIs in this study had onset during months 4 through 6 following transplant. The high number of delayed IMIs that occurred after the completion of aerosolized ABLC prophylaxis supports the ISHLT recommendation to continue postoperative mold prophylaxis for 4 to 6 months [9].
Third, high rates of breakthrough pulmonary mold infections indicate that aerosolized ABLC may provide incomplete protection as a prophylactic agent. Pathogens known to have unfavorable minimum inhibitory concentrations for amphotericin B—which aerosolized ABLC would, therefore, not be expected to prevent—caused over half of the ABLC breakthrough infections [29, 30]. Exposure of the transplanted graft to the environment over time may inevitably select for a breakthrough infection with a pathogen that is resistant to the prophylactic agent being used [37]. The lung transplant field would benefit from a carefully randomized and comparative study that examines the protective effect of prolonged post-transplant aerosolized amphotericin and mold-active azole exposure.
In addition to antifungal prophylaxis strategies, we investigated other local factors that could have contributed to the high prevalence of IFIs at our hospital. The multitude of different pathogens that caused IFIs and the stable prevalence of IFIs over time argued against a common-source outbreak of hospital-acquired fungal infections. At our hospital, we previously identified postoperative ECMO use and delayed sternal closure as risk factors for IC. This study reveals additional novel risk factors for IFIs, including low functional status, high lung allocation score, primary graft failure requiring re-transplantation, prolonged postoperative mechanical ventilation, postoperative hemodialysis, and, for IC, bilateral lung transplantation. These potential risk factors, if validated in subsequent studies, could be incorporated into risk-stratified IFI prophylaxis protocols.
We also evaluated potential donor-related risk factors for IFIs. Ischemia-reperfusion injury has been postulated as a potential risk factor for IFI [23, 38, 39], but in our study, ischemic time was not increased for patients who developed IFIs. Also, donor airway colonization with potentially pathogenic fungi other than Candida was exceedingly rare and was linked to only 1 case of recipient IFI. Female donor status was associated with increased IFI prevalence and merits further study at other transplant centers.
Based on the results of our study, we chose to add universal systemic prophylaxis against IC to our postoperative protocol. We considered utilizing monotherapy with a mold-active triazole, such as voriconazole or delayed-release posaconazole, to target both IC and IMIs. However, we had several concerns about universal prophylaxis with these agents, including tolerability [40–42]; drug interactions [43–45], particularly with calcineurin inhibitors; the need for therapeutic drug monitoring [46, 47]; acquisition costs; an increased risk of skin cancers with prolonged voriconazole therapy [48]; and a possible shift towards non-Aspergillus and multidrug-resistant IMIs occurring in patients receiving prophylaxis with these agents at our institution [37]. Due to these concerns, we elected to use fluconazole for universal prophylaxis for 90 days after transplantation. We also considered extending the duration of aerosolized ABLC beyond the transplant hospitalization. However, this agent is expensive and poorly covered by third-party payers when used for outpatient prophylaxis, which hindered the implementation of universal prophylaxis with aerosolized ABLC following hospital discharge.
This study was unique, due to the large number of IFIs described and the detailed nature of the analysis: we described IC and non-Candida IFIs separately, time-trended prevalence rates over 8 years, and stratified IFIs by site of infection and pathogen. These data emphasize the potential for high rates of IC after lung transplantation if antifungal prophylaxis includes only aerosolized amphotericin B, an agent commonly used as monotherapy prophylaxis. In addition, our data indicate that the diagnosis of candidemia in this population should prompt careful evaluation for invasive infections at other sites, particularly in the pleural space. Our findings also suggest that micafungin may not provide adequate prophylaxis against IC at sites other than the bloodstream or for patients who require ECMO. Finally, early discontinuation of mold prophylaxis after conclusion of the index transplant hospitalization may increase the risk of later-onset IMI; however, aerosolized amphotericin B may have poor activity against some molds that cause IFI in the lung transplant population and, in these cases, may provide ineffective prophylaxis regardless of duration.
The primary limitations of this study were its single-center design and retrospective analysis. We reported that IFI rates at our center were higher than expected based upon prior, published IFI rates; however, a comparison of IFI rates across transplant centers is inherently difficult due to differences in factors such as immunosuppression, prophylaxis, and environmental risks. Our IFI rates and other findings may not be generalizable to transplant centers with different fungal epidemiology or important differences in the perioperative and postoperative care of lung transplant recipients. Also, our definition of a breakthrough infection included any IFI that occurred within 30 days of prior antifungal exposure. Some breakthrough infections that occurred after the discontinuation of prophylaxis were likely due to inadequate drug concentrations, rather than drug failure.
In summary, we analyzed the epidemiology of IFIs following lung transplant surgeries at a large transplant center. High rates of IC confirm the need for systemic antifungal prophylaxis targeting Candida and suggest benefit in providing coverage for up to the first 90 days after transplant. Many patients developed IMIs after completing prescribed prophylaxis, which emphasizes the potential benefit of extending mold-active coverage for up to 180 days after transplant surgery. Based on these data and international guidelines, transplant centers that do not provide systemic Candida prophylaxis or that prescribe short durations of mold prophylaxis should reevaluate their antifungal prophylaxis protocol in the context of local epidemiology. However, several important questions about antifungal prophylaxis remain unanswered. Is universal prophylaxis superior to preemptive prophylaxis? Is combination therapy better than monotherapy? Which antifungal agents are most effective, and what is the ideal duration of prophylaxis? Can we use a risk-stratified approach to prophylaxis in the lung transplant population? A prospective, randomized, multicenter trial is warranted to help answer these questions.
Notes
Financial support. This work was supported by the Health Resources and Services Administration (contract number 234-2005-370011C).
Potential conflicts of interest. A. W. B. was supported by the Transplant Infectious Disease Interdisciplinary Research Training Grant (grant number 5T32AI100851-02) from the National Institutes of Health (NIH). J. R. P. was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH (grant numbers R01-AI73896, P01-AI04533, and R01-AI93257). B. D. A. was supported by the NIAID of the NIH (grant numbers K23-AI52222 and K24-AI072522); has served as a consultant and received a research grant to her institution from Lediant Pharmaceuticals; and has served as a consultant and site investigator for Astellas, Scynexis, F2G, and Cidara Pharmaceuticals. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: thirty-second official adult lung and heart-lung transplantation report–2015; focus theme: early graft failure. J Heart Lung Transplant 2015; 34:1264–77. [DOI] [PubMed] [Google Scholar]
- 2. Gray AL, Mulvihill MS, Hartwig MG. Lung transplantation at Duke. J Thorac Dis 2016; 8:E185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yeung JC, Cypel M, Waddell TK, van Raemdonck D, Keshavjee S. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques–non-heart-beating donor lung retrieval and ex vivo donor lung perfusion. Thorac Surg Clin 2009; 19:261–74. [DOI] [PubMed] [Google Scholar]
- 4. Fuehner T, Kuehn C, Welte T, Gottlieb J. ICU care before and after lung transplantation. Chest 2016; 150:442–50. [DOI] [PubMed] [Google Scholar]
- 5. Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis 2016; 63:e1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soysal A. Prevention of invasive fungal infections in immunocompromised patients: the role of delayed-release posaconazole. Infect Drug Resist 2015; 8:321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 2016; 387:760–9. [DOI] [PubMed] [Google Scholar]
- 8. Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010; 50:1101–11. [DOI] [PubMed] [Google Scholar]
- 9. Husain S, Sole A, Alexander BD, et al. The 2015 international society for heart and lung transplantation guidelines for the management of fungal infections in mechanical circulatory support and cardiothoracic organ transplant recipients: Executive summary. J Heart Lung Transplant 2016; 35:261–82. [DOI] [PubMed] [Google Scholar]
- 10. Gabardi S, Kubiak DW, Chandraker AK, Tullius SG. Invasive fungal infections and antifungal therapies in solid organ transplant recipients. Transpl Int 2007; 20:993–1015. [DOI] [PubMed] [Google Scholar]
- 11. Solé A, Salavert M. Fungal infections after lung transplantation. Transplant Rev 2008; 22:89–104. [DOI] [PubMed] [Google Scholar]
- 12. Neofytos D, Treadway S, Ostrander D, et al. Epidemiology, outcomes, and mortality predictors of invasive mold infections among transplant recipients: a 10-year, single-center experience. Transpl Infect Dis 2013; 15:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andes DR, Safdar N, Baddley JW, et al. ; Transplant-Associated Infection Surveillance Network (TRANSNET) Investigators The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Transpl Infect Dis 2016; 18:921–31. [DOI] [PubMed] [Google Scholar]
- 14. Dodds Ashley E, Drew R, Johnson M, et al. Cost of invasive fungal infections in the era of new diagnostics and expanded treatment options. Pharmacotherapy 2012; 32:890–01. [DOI] [PubMed] [Google Scholar]
- 15. Solé A, Morant P, Salavert M, Pemán J, Morales P; Valencia Lung Transplant Group Aspergillus infections in lung transplant recipients: risk factors and outcome. Clin Microbiol Infect 2005; 11:359–65. [DOI] [PubMed] [Google Scholar]
- 16. Mehrad B, Paciocco G, Martinez FJ, Ojo TC, Iannettoni MD, Lynch JP 3rd. Spectrum of Aspergillus infection in lung transplant recipients: case series and review of the literature. Chest 2001; 119:169–75. [DOI] [PubMed] [Google Scholar]
- 17. Pilarczyk K, Haake N, Heckmann J, et al. Is universal antifungal prophylaxis mandatory in adults after lung transplantation? A review and meta-analysis of observational studies. Clin Transplant 2016; 30:1522–31. [DOI] [PubMed] [Google Scholar]
- 18. Patel TS, Eschenauer GA, Stuckey LJ, Carver PL. Antifungal prophylaxis in lung transplant recipients. Transplantation 2016; 100:1815–26. [DOI] [PubMed] [Google Scholar]
- 19. Neoh CF, Snell GI, Kotsimbos T, et al. Antifungal prophylaxis in lung transplantation–a world-wide survey. Am J Transplant 2011; 11:361–6. [DOI] [PubMed] [Google Scholar]
- 20. Husain S, Zaldonis D, Kusne S, Kwak EJ, Paterson DL, McCurry KR. Variation in antifungal prophylaxis strategies in lung transplantation. Transpl Infect Dis 2006; 8:213–8. [DOI] [PubMed] [Google Scholar]
- 21. Neoh CF, Snell G, Levvey B, Morrissey CO, Stewart K, Kong DC. Antifungal prophylaxis in lung transplantation. Int J Antimicrob Agents 2014; 44: 194–202. [DOI] [PubMed] [Google Scholar]
- 22. Vadnerkar A, Clancy CJ, Celik U, et al. Impact of mold infections in explanted lungs on outcomes of lung transplantation. Transplantation 2010; 89: 253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Society of Transplantation Infectious Disease Community of Practice. Fungal infections. Am J Transplant 2004; 4(Suppl 10):110–34. [DOI] [PubMed] [Google Scholar]
- 24. He SY, Makhzoumi ZH, Singer JP, Chin-Hong PV, Arron ST. Practice variation in Aspergillus prophylaxis and treatment among lung transplant centers: a national survey. Transpl Infect Dis 2015; 17:14–20. [DOI] [PubMed] [Google Scholar]
- 25. Drew RH, Dodds Ashley E, Benjamin DK Jr, Duane Davis R, Palmer SM, Perfect JR. Comparative safety of amphotericin B lipid complex and amphotericin B deoxycholate as aerosolized antifungal prophylaxis in lung-transplant recipients. Transplantation 2004; 77:232–7. [DOI] [PubMed] [Google Scholar]
- 26. De Pauw B, Walsh TJ, Donnelly JP, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Snyder J, Salkowski N, Lamb K, et al. Karnofsky performance score and its use in risk adjustment of transplant outcomes in the United States. Scientific Registry of Transplant Recipients. Available at https://www.srtr.org/media/1111/atc2012_kasiske_snyder_part_ii.pdf Accessed 12 February 2018. [Google Scholar]
- 28. United Network for Organ Sharing. A guide to calculating the lung allocation score. Available at https://www.unos.org/wp-content/uploads/unos/lung_allocation_score.pdf. Accessed 12 February 2018.
- 29. Kanafani ZA, Perfect JR. Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis 2008; 46:120–8. [DOI] [PubMed] [Google Scholar]
- 30. Sanglard D. Emerging threats in antifungal-resistant fungal pathogens. Front Med (Lausanne) 2016; 3:11 Available at https://www.ncbi.nlm.nih.gov/pubmed/27014694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chong PP, Kennedy CC, Hathcock MA, Kremers WK, Razonable RR. Epidemiology of invasive fungal infections in lung transplant recipients on long-term azole antifungal prophylaxis. Clin Transplant 2015; 29:311–8. [DOI] [PubMed] [Google Scholar]
- 32. Pinney MF, Rosenberg AF, Hampp C, Schain D, Akindipe O, Baz M. Invasive fungal infections in lung transplant recipients not receiving routine systemic antifungal prophylaxis: 12-year experience at a university lung transplant center. Pharmacotherapy 2011; 31:537–45. [DOI] [PubMed] [Google Scholar]
- 33. Pfeiffer CD, Garcia-Effron G, Zaas AK, Perfect JR, Perlin DS, Alexander BD. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol 2010; 48:2373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Welte R, Eller P, Lorenz I, Joannidis M, Bellmann R. Anidulafungin pharmacokinetics in ascites fluid and pleural effusion of critically ill patients. Antimicrob Agents Chemother 2018; 62 Available at https://www.ncbi.nlm.nih.gov/pubmed/29439960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamada N, Kumada K, Kishino S, Mochizuki N, Ohno K, Ogura S. Distribution of micafungin in the tissue fluids of patients with invasive fungal infections. J Infect Chemother 2011; 17:731–4. [DOI] [PubMed] [Google Scholar]
- 36. Watt KM, Cohen-Wolkowiez M, Williams DC, et al. Antifungal extraction by the extracorporeal membrane oxygenation circuit. J Extra Corpor Technol 2017; 49:150–9. [PMC free article] [PubMed] [Google Scholar]
- 37. Lamoth F, Chung SJ, Damonti L, Alexander BD. Changing epidemiology of invasive mold infections in patients receiving azole prophylaxis. Clin Infect Dis 2017; 64:1619–21. [DOI] [PubMed] [Google Scholar]
- 38. Hsu JL, Khan MA, Sobel RA, et al. Aspergillus fumigatus invasion increases with progressive airway ischemia. PLOS One 2013; 8:e77136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verleden GM, Vos R, van Raemdonck D, Vanaudenaerde B. Pulmonary infection defense after lung transplantation: does airway ischemia play a role? Curr Opin Organ Transplant 2010; 15:568–71. [DOI] [PubMed] [Google Scholar]
- 40. Dolton MJ, Ray JE, Chen SC, Ng K, Pont LG, McLachlan AJ. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob Agents Chemother 2012; 56:4793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dolton MJ, McLachlan AJ. Voriconazole pharmacokinetics and exposure-response relationships: assessing the links between exposure, efficacy and toxicity. Int J Antimicrob Agents 2014; 44:183–93. [DOI] [PubMed] [Google Scholar]
- 42. Neofytos D, Avdic E, Magiorakos AP. Clinical safety and tolerability issues in use of triazole derivatives in management of fungal infections. Drug Healthc Patient Saf 2010; 2:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andes D, Azie N, Yang H, et al. Drug-drug interaction associated with mold-active triazoles among hospitalized patients. Antimicrob Agents Chemother 2016; 60:3398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brüggemann RJ, Alffenaar JW, Blijlevens NM, et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis 2009; 48:1441–58. [DOI] [PubMed] [Google Scholar]
- 45. Billaud EM, Guillemain R, Berge M, et al. Pharmacological considerations for azole antifungal drug management in cystic fibrosis lung transplant patients. Med Mycol 2010; 48(Suppl 1):S52–9. [DOI] [PubMed] [Google Scholar]
- 46. Seyedmousavi S, Mouton JW, Verweij PE, Brüggemann RJ. Therapeutic drug monitoring of voriconazole and posaconazole for invasive aspergillosis. Expert Rev Anti Infect Ther 2013; 11:931–41. [DOI] [PubMed] [Google Scholar]
- 47. Dolton MJ, Ray JE, Marriott D, McLachlan AJ. Posaconazole exposure-response relationship: evaluating the utility of therapeutic drug monitoring. Antimicrob Agents Chemother 2012; 56:2806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singer JP, Boker A, Metchnikoff C, et al. High cumulative dose exposure to voriconazole is associated with cutaneous squamous cell carcinoma in lung transplant recipients. J Heart Lung Transplant 2012; 31:694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]