Abstract
Background
While opioid agonist therapy (OAT) reduces the risk of hepatitis C virus (HCV) acquisition among people who inject drugs (PWID), protective effects may be attenuated in females. We used pooled data from an international collaboration of prospective cohorts to assess sex disparities in HCV incidence among PWID exposed to OAT.
Methods
Independent predictors of HCV infection were identified using Cox regression models with random effects after accounting for the clustering effect of study sites. Unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) are presented in sex‐specific analyses.
Results
Among 701 participants exposed to OAT, HCV incidence was 16.5/100 person-years of observation (PYO) (95% CI, 13.1–20.7) in females and 7.6/100 PYO (95% CI, 6.0–9.5) in males (female:male adjusted HR [aHR], 1.80 [95% CI, 1.37–2.22]; P < .001). Factors associated with HCV acquisition among females exposed to OAT included nonwhite race (aHR, 1.79 [95% CI, 1.25–2.56]; P = .001), unstable housing (aHR, 4.00 [95% CI, 3.62–4.41]; P < .001), daily or more frequent injection (aHR, 1.45 [95% CI, 1.01–2.08]; P = .042), and receptive syringe sharing (aHR, 1.43 [95% CI, 1.33–1.53]; P < .001).
Conclusions
Female PWID exposed to OAT are twice as likely as their male counterparts to acquire HCV. While there is a need for better understanding of sex differences in immune function and opioid pharmacokinetic and pharmacodynamic parameters, structural and behavioral interventions that target women are required to bolster the efficacy of OAT in preventing HCV transmission.
Keywords: sex; hepatitis C virus, people who inject drugs, opioid agonist therapy, harm reduction
Utilizing longitudinal data from 7 international cohorts to examine sex differences in the protective effect of opioid agonist treatment on incident hepatitis C virus among people who inject drugs, we found that females were twice as likely as males to acquire infection.
An estimated 71 million adults worldwide (1%) are living with hepatitis C virus (HCV) [1]. HCV transmission through sexual contact is rare in heterosexual couples (1 per 190 000 sexual contacts) [2] and HCV incidence among human immunodeficiency virus (HIV)–infected men who have sex with men (with presumed sexual acquisition) is also low (0.53% per year) [3], compared to parenterally acquired HCV (ranging from 40% to 100%) [4].
In developed countries, exposure to HCV through injection drug use remains the predominant mode of transmission [5]. There are approximately 16 million people who inject drugs (PWID) worldwide, including an estimated 3.2 million women, with 1 in 2 PWID exposed to HCV [6]. Chronic HCV infection is a major global health burden, associated with severe morbidity, including cirrhosis, hepatocellular cancer, and liver failure, as well as mortality [7].
A recent study reported that female PWID had a 38% higher risk of incident HCV infection, compared to males [8]. The primary risk factor for HCV transmission among PWID is receptive sharing of needles and syringes [6], and several studies indicate females are significantly more likely to engage in receptive syringe sharing (RSS) compared to males [9]. Other risk factors include the sharing of ancillary injecting equipment, such as cookers and cotton filters [10, 11], and drug preparation techniques such as backloading [12]. Some evidence suggests that increased risk of HCV infection among females may also be due to health services not addressing gender-specific needs or services being tailored to males [13]. Although females are at higher risk of acquiring HCV, they are also more likely to spontaneously clear the infection [14].
The advent of direct-acting antiviral treatments for HCV infection has resulted in cure rates of >95% [15]. Despite the efficacy of these treatments, barriers to uptake remain such as the cost of treatment, management of other comorbidities, advanced liver disease, inadequate levels of screening and confirmatory testing, practitioner unfamiliarity with HCV treatment regimens, and non–evidence-based guidelines that render people ineligible for treatment due to current drug or alcohol use [16]. Interventions that prevent transmission of HCV are still required, including access to sterile injection equipment and rapid and accurate point-of-care testing and interventions to reduce risk behavior such as frequency of injection, including effective drug treatment [17].
Opioid agonist therapy (OAT) has been shown to reduce the incidence of HCV infection among PWID [18–20]. When provided continuously and at therapeutic doses, OAT reduces opioid cravings, injection frequency, and high-risk injection practices [21]. A systematic review and meta-analysis of 12 studies found that current OAT (defined as the use of prescribed methadone or buprenorphine within the previous 6 months) was associated with a 50% reduction in risk of acquiring HCV, compared to no current OAT [22]. Furthermore, a lifetime history of OAT was associated with a 19% reduction in HCV incidence, compared with no history of OAT [22]. This analysis also suggested that the effectiveness of OAT differed between females and males, with a 59% reduction in the effectiveness of the intervention with every 10% increase in female participants [22].
The current study aimed to assess differences in HCV incidence by sex among PWID exposed to OAT and to identify factors independently associated with any decrease in protective efficacy.
METHODS
Study Design
The International Collaboration of Incident HIV and HCV in Injecting Cohorts (InC3) is a collaborative of 10 prospective cohort studies with pooled behavioral and serological data. InC3 methodology has been described in detail elsewhere [23]. Cohorts included in the collaboration originated from 4 countries and include the Amsterdam Cohort Study (ACS), Australian Trial in Acute Hepatitis C (ATAHC), Baltimore Before and After Acute Study in Hepatitis (BBAASH), Boston Acute HCV Study: Transmission, Immunity, Outcomes Network (BAHSTION), Hepatitis C Incidence and Transmission Study–community (HITS-c, Sydney), Hepatitis C Incidence and Transmission Study–prison (HITS-p, Sydney), Hepatitis C Virus Cohort Study (HCVC, Sydney), St Luc Hepatitis Cohort (HepCo, Montreal), Melbourne Injecting Drug User Cohort Study (SuperMIX, Melbourne), and U-Find-Out Study (UFO, San Francisco). Protocols for each study were approved by local institutional research ethics committees. InC3 was determined to be “exempt” from ethical review by the University of New Mexico institutional review board as all data are de-identified.
Study Population
Of the 10 cohorts included in InC3, 3 were excluded from the analysis as the study design did not allow for HCV incidence to be calculated (ATAHC) or data on injection exposure were not available (BAHSTION and BBAASH). HCV-negative participants from the remaining 7 cohorts were included in the current study where the following criteria were met: (1) a history of injection drug use was recorded; (2) at least 1 follow-up serological cohort visit was recorded; (3) biological sex was recorded as male or female; and (4) exposure to OAT in the previous 12 months was recorded at any cohort visit.
Measures
Serological and molecular markers for HCV infection were collected along with self-reported data on sociodemographic characteristics and drug use history and behaviors at 3-month (SuperMix and UFO) and 6-month (ACS, HCVC, HITS-c, HITS-p, and HepCo) intervals. Exposure to OAT was defined as the prescription of OAT (methadone, buprenorphine, or buprenorphine-naloxone) in the previous 12 months on at least 1 cohort visit. Sociodemographic characteristics included race, sexual identity (heterosexual vs homosexual and bisexual), age at baseline, education, recent unstable housing, employment status (full time, part time, casual, or home duties), and recent alcohol intake. Participants whose race was recorded as Asian, black, Hispanic, Indigenous, white-Asian, white-black, and other were classified as nonwhite, and those who reported completing 12 or more years of education were classified as having completed high school.
Injection risk behaviors included number of years since injection initiation, frequency of injection (less than daily vs daily or more frequent), recent RSS, and drug most frequently injected. Drugs injected were classified as either opioids (heroin, pharmaceutical opioids, OAT, or a mixture of heroin and cocaine) or nonopioids (amphetamine, methamphetamine, cocaine, or “other” drugs). Time since injection initiation was calculated using year of first injection and year of baseline visit and subsequently collapsed into tertiles.
HCV Incident Infection
Among HCV-naive participants, HCV incident infection was determined to have occurred if a positive anti-HCV or HCV RNA test was recorded within 2 years of a negative anti-HCV test, or if there was recorded evidence of seroconversion illness (clinically diagnosed jaundice or alanine aminotransferase level >400 IU/L) along with a history of high-risk injection behaviors or the detection of HCV RNA within 3 months of clinical manifestation of acute HCV. Anti-HCV and HCV RNA assays are described elsewhere and varied between, but not within, cohorts [14].
Time to HCV infection was estimated using a hierarchy of successive serological (anti-HCV), virological (HCV RNA), and clinical (symptoms and liver function tests) data. Among participants with a documented seroconversion illness, date of infection was determined as 6 weeks prior to the onset of symptoms. Participants who recorded a positive HCV RNA test following a negative anti-HCV test during acute HCV detection had an estimated date of infection of 28 days prior to the positive test. Participants with a positive RNA test following a negative anti-HCV test and with no acute HCV detection were estimated to have seroconverted at the midpoint between the last negative anti-HCV test and first positive anti-HCV test or positive HCV RNA test.
Statistical Analysis
The primary study outcome was HCV incident infection while exposed to OAT. Participants who seroconverted were censored at the first HCV-positive visit date, whereas participants who did not record a positive HCV antibody or RNA test were censored at the last recorded visit where laboratory results were available. Time from entry in each study was used to calculate person-years of observation (PYO). Sex-specific crude HCV incidence rates were determined using Kaplan-Meier methods. Cox proportional hazards regression models with random effects were used to identify the independent predictors of incident HCV infection after accounting for the clustering effect of study sites and adjusting for missing data in each variable. Sociodemographic variables included in bivariate analysis included race, sexual identity, education, recent unstable housing, employment status, and recent alcohol intake, while injection-related variables included years since injecting initiation, drug most frequently injected, injection frequency, and recent RSS. The current study only included variables where <50% of data were missing, with variables relating to recent imprisonment and utilization of needle and syringe programs excluded from the analysis (51% and 54% missing, respectively). Those variables with <50% of missing data were all considered in our analysis. We handled the missing observations based on recommended statistical principles [24, 25]. A separate missing category was created and included in our analysis for variables with >5% missing data, while missing data was included in the reference category for variables with <5% missing data. Sex-specific sensitivity analyses were conducted to fit the final multivariable models using only available data to assess the impact of the missing data on our analyses. Data were analyzed using Stata version 14.2 software (StataCorp, College Station, Texas).
RESULTS
Sample Characteristics
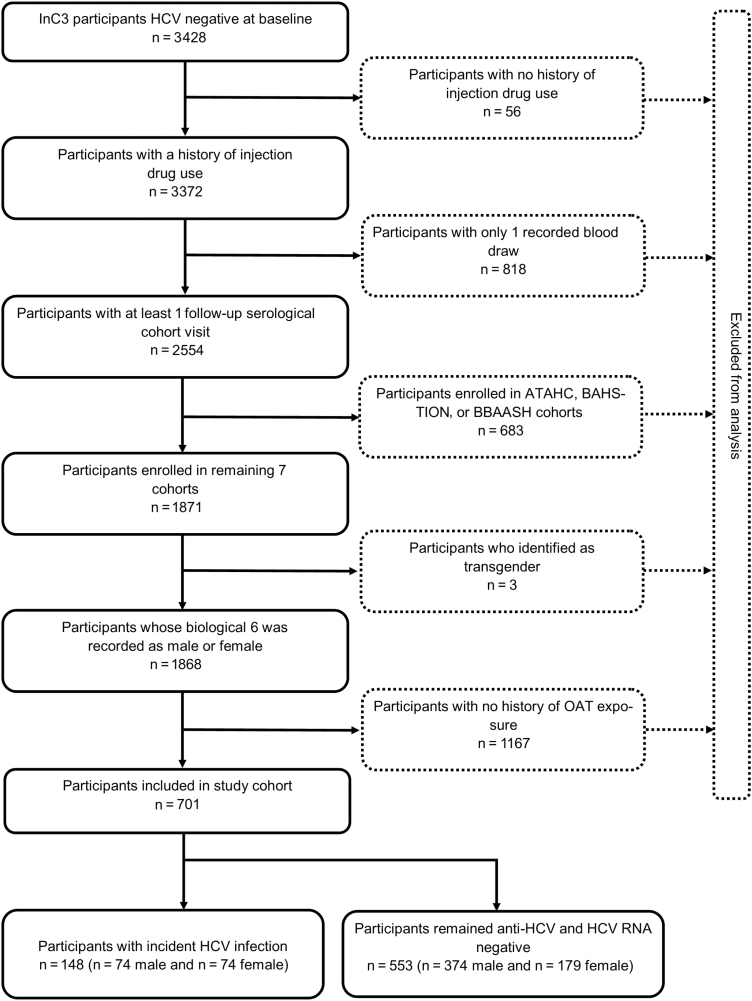
A total of 701 participants from 7 cohorts were eligible for inclusion (Figure 1). As shown in Table 1, the majority were male (64%) and white (75%). The median age was 26 years (interquartile range [IQR], 17–49 years) and the median number of years since injection initiation was 7 (IQR, 1–34 years). Forty percent had completed high school and 29% reported recent unstable housing. One in 5 (22%) participants reported daily or more frequent injection and 18% reported recent RSS. Seven participants tested HIV positive at baseline, 4 of whom were subsequently exposed to HCV.
Figure 1.
Participant inclusion flowchart. Abbreviations: ATAHC, Australian Trial in Acute Hepatitis C; BAHSTION, Boston Acute HCV Study: Transmission, Immunity, Outcomes Network; BBAASH, Baltimore Before and After Acute Study in Hepatitis; HCV, hepatitis C virus; HIV, human immunodeficiency virus; InC3, International Collaboration of Incident HIV and HCV in Injecting Cohorts, OAT, opioid agonist therapy.
Table 1.
Baseline Characteristics of Study Participants, Overall and by Sex (N = 701)
| Variable | Total Sample (N = 701) | Male (n = 448) | Female (n = 253) |
|---|---|---|---|
| Race | |||
| Asian | 46 (7) | 32 (7) | 14 (6) |
| Black | 2 (<1) | 2 (<1) | 0 (0) |
| Hispanic | 6 (1) | 5 (1) | 1 (<1) |
| Indigenous | 41 (6) | 15 (3) | 26 (10) |
| White | 525 (75) | 338 (75) | 187 (74) |
| Other | 40 (6) | 26 (6) | 14 (5) |
| Missing | 41 (6) | 30 (7) | 11 (5) |
| Sexual identity | |||
| Heterosexual | 416 (59) | 282 (63) | 134 (53) |
| Homosexual or bisexual | 56 (8) | 18 (4) | 38 (15) |
| Missing | 229 (33) | 148 (33) | 81 (32) |
| Age, y, median (IQR) | 26 (17–49) | 27 (18–48) | 25 (17–45) |
| Education | |||
| Completed high school | 283 (40) | 183 (41) | 100 (40) |
| Did not complete high school | 262 (37) | 158 (35) | 104 (41) |
| Missing | 156 (22) | 107 (24) | 49 (19) |
| Unstable housinga | |||
| No | 256 (37) | 168 (38) | 88 (35) |
| Yes | 202 (29) | 138 (31) | 64 (25) |
| Missing | 243 (35) | 142 (32) | 101 (40) |
| Unemployeda | |||
| No | 180 (26) | 126 (28) | 54 (21) |
| Yes | 182 (26) | 113 (25) | 69 (27) |
| Missing | 339 (48) | 209 (47) | 130 (51) |
| Alcohol intakea | |||
| No | 145 (21) | 93 (21) | 52 (21) |
| Yes | 257 (37) | 178 (40) | 79 (31) |
| Missing | 299 (43) | 177 (40) | 122 (48) |
| Years since injecting initiation, median (IQR) | 7 (1–34) | 8 (1–34) | 7 (1–27) |
| Drug most frequently injecteda | |||
| Nonopioids | 61 (9) | 38 (9) | 23 (9) |
| Opioids | 331 (47) | 224 (50) | 107 (42) |
| Missing | 309 (44) | 186 (42) | 123 (49) |
| Injection frequencya | |||
| Less than daily | 405 (58) | 271 (60) | 134 (53) |
| Daily | 154 (22) | 104 (23) | 50 (20) |
| Missing | 142 (20) | 73 (16) | 69 (27) |
| Receptive syringe sharinga | |||
| No | 339 (48) | 230 (51) | 109 (43) |
| Yes | 127 (18) | 85 (19) | 42 (17) |
| Missing | 235 (34) | 133 (30) | 102 (40) |
| HIV status | |||
| Negative | 491 (70) | 308 (69) | 183 (72) |
| Positive | 7 (1) | 7 (2) | 0 (0) |
| Unknown | 1 (<1) | 1 (<1) | 0 (0) |
| Missing | 202 (29) | 132 (29) | 70 (28) |
| Participating sites | |||
| ACS (Amsterdam) | 126 (18) | 87 (19) | 39 (15) |
| HCVC (Sydney) | 133 (19) | 86 (19) | 47 (19) |
| HITS-c (Sydney) | 67 (10) | 49 (11) | 18 (7) |
| HITS-p (Sydney) | 140 (20) | 71 (16) | 69 (27) |
| HepCo (Montreal) | 81 (12) | 56 (13) | 25 (10) |
| SuperMIX Study (Melbourne) | 79 (11) | 54 (12) | 25 (10) |
| UFO Study (San Francisco) | 75 (11) | 45 (10) | 30 (12) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: ACS, Amsterdam Cohort Study; HCVC, Hepatitis C Virus Cohort Study; HepCo, St Luc Hepatitis Cohort; HITS-c, Hepatitis C Incidence and Transmission–community study; HITS-p, Hepatitis C Incidence and Transmission–prison study; HIV, human immunodeficiency virus; IQR, interquartile range; SuperMIX Study, Networks 2 and Melbourne Injecting Drug User Cohort Study; UFO, U-Find-Out Study.
aIn the previous 3 or 6 months depending on cohort interview intervals.
HCV Incidence by Sex
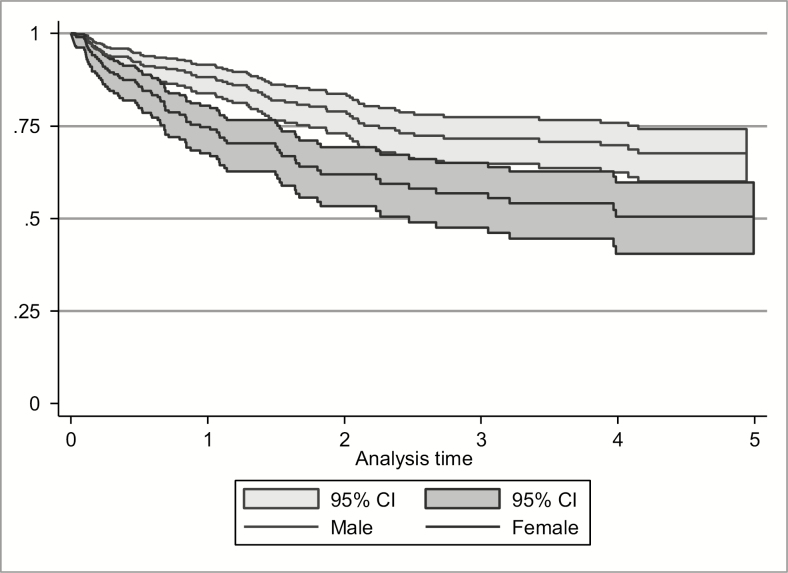
A total of 3003 observed visits were recorded by the 701 participants exposed to OAT included here. Over 1427 PYO, 148 incident HCV infections were recorded, resulting in HCV incidence (per 100 PYO) of 16.5 (95% confidence interval [CI], 13.1–20.7) and 7.6 (95% CI, 6.0–9.5) among females and males, respectively (Figure 2). The unadjusted female-to-male hazard ratio (HR) was 1.90 (95% CI, 1.37–2.61; P < .001), and the adjusted HR (aHR) was 1.80 (95% CI, 1.37–2.22; P < .001).
Figure 2.
Kaplan-Meier survival estimates of hepatitis C virus incidence by sex among participants exposed to opioid agonist therapy. Abbreviation: CI, confidence interval.
Factors Associated With HCV Incident Infection by Sex
In bivariate analysis, incident HCV infection was significantly higher among females exposed to OAT who were nonwhite (HR, 2.39 [95% CI, 2.25–2.52]; P < .001), did not complete high school (HR, 2.05 [95% CI, 1.08–3.90]; P = .029), reported recent unstable housing (HR, 5.13 [95% CI, 4.42–5.96]; P < .001), had an injection history of between 6 and 10 years (HR, 1.35 [95% CI, 1.06–1.72]; P = .014), injected daily or more frequently (HR, 2.65 [95% CI, 1.97–3.57]; P < .001) and reported recent RSS (HR, 1.71 [95% CI, 1.47–1.97]; P < .001). In multivariable analysis (Table 2), incident HCV infection was significantly higher among females exposed to OAT who were nonwhite (aHR, 1.79 [95% CI, 1.25–2.56]; P = .001) and who reported recent unstable housing (aHR, 4.00 [95% CI, 3.62–4.41]; P < .001), recent daily or more frequent injection (aHR, 1.45 [95% CI, 1.01–2.08]; P = .042), and recent RSS (aHR, 1.43 [95% CI, 1.33–1.53]; P < .001).
Table 2.
Factors Associated With Hepatitis C Virus Acquisition Among People Who Inject Drugs Exposed to Opioid Agonist Therapy, by Sex
| Females | Males | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Unadjusted | Adjusteda | Unadjusted | Adjusteda | ||||||||
| HR | (95% CI) | P Value | HR | (95% CI) | P Value | HR | (95% CI) | P Value | HR | (95% CI) | P Value | |
| Race | ||||||||||||
| White (reference) | … | 1 | … | … | 1 | … | … | 1 | … | … | 1 | … |
| Nonwhite | 2.39 | (2.25–2.52) | < .001 | 1.79 | (1.25–2.56) | .001 | 1.20 | (.98–1.46) | .077 | 1.40 | (1.06–1.84) | .017 |
| Sexual identity | ||||||||||||
| Heterosexual (reference) | … | 1 | … | … | … | … | … | 1 | … | … | … | … |
| Homosexual or bisexual | 0.98 | (.68–1.42) | .925 | … | … | … | 1.06 | (.09–12.66) | .966 | … | … | … |
| Education | ||||||||||||
| Completed high school (reference) | … | 1 | … | … | … | … | … | 1 | … | … | … | … |
| Did not complete high school | 2.05 | (1.08–3.90) | .029 | … | … | … | 1.73 | (1.24–2.42) | .001 | … | … | … |
| Unstable housingb | ||||||||||||
| No (reference) | … | 1 | … | … | 1 | … | … | 1 | … | … | 1 | … |
| Yes | 5.13 | (4.42–5.96) | < .001 | 4.00 | (3.62–4.41) | < .001 | 3.99 | (3.25–4.89) | < .001 | 3.29 | (2.03–5.32) | < .001 |
| Unemployedb | ||||||||||||
| No (reference) | … | 1 | … | … | … | … | … | 1 | … | … | … | … |
| Yes | 0.82 | (.61–1.11) | .194 | … | … | … | 1.26 | (.69–2.28) | .452 | … | … | … |
| Alcohol intakeb | ||||||||||||
| No (reference) | … | 1 | … | … | … | … | … | 1 | … | … | … | … |
| Yes | 0.68 | (.12–3.92) | .668 | … | … | … | 0.66 | (.33–1.31) | .232 | … | … | … |
| Years since injecting initiation at baseline (quartiles) | ||||||||||||
| >10 (reference) | … | 1 | … | … | … | … | … | 1 | … | … | … | … |
| 6–10 | 1.35 | (1.06–1.72) | .014 | … | … | … | 1.18 | (.90–1.56) | .233 | … | … | … |
| <6 | 1.40 | (.74–2.64) | .301 | … | … | … | 0.96 | (.57–1.62) | .867 | … | … | … |
| Drug most frequently injectedb | ||||||||||||
| Nonopioids (reference) | … | 1 | … | … | … | … | … | 1 | … | … | 1 | … |
| Opioids | 1.33 | (.70–2.53) | .379 | … | … | … | 0.60 | (.45–.82) | .001 | 0.60 | (.48–.75) | < .001 |
| Injection frequencyb | ||||||||||||
| Less than daily (reference) | … | 1 | … | … | 1 | … | … | 1 | … | … | 1 | … |
| Daily or more frequent injection | 2.65 | (1.97–3.57) | < .001 | 1.45 | (1.01–2.08) | .042 | 4.02 | (2.27–7.09) | < .001 | 3.79 | (3.43–4.18) | < .001 |
| Receptive syringe sharingb | ||||||||||||
| No (reference) | … | 1 | … | … | 1 | … | … | 1 | … | … | … | … |
| Yes | 1.71 | (1.47–1.97) | < .001 | 1.43 | (1.33–1.53) | < .001 | 2.25 | (1.22–4.15) | .010 | … | … | … |
Abbreviations: CI, confidence interval; HR, hazard ratio.
aAdjusted for missing data.
bIn the previous 3 or 6 months depending on cohort interview intervals.
In bivariate analysis, incident HCV infection was significantly higher among males exposed to OAT who did not complete high school (HR, 1.73 [95% CI, 1.24–2.42]; P = .001), reported recent unstable housing (HR, 3.99 [95% CI, 3.25–4.89]; P < .001), injected daily or more frequently (HR, 4.02 [95% CI, 2.27–7.09]; P < .001), and reported recent RSS (HR, 2.25 [95% CI, 1.22–4.15]; P = .010). Males who reported most frequently injecting opioids were significantly less likely to acquire HCV (HR, 0.60 [95% CI, .45–.82]; P = .001). In multivariable analysis, incident HCV infection was significantly higher among males exposed to OAT who were nonwhite (aHR, 1.40 [95% CI, 1.06–1.84]; P = .017) reported recent unstable housing (aHR, 3.29 [95% CI, 2.03–5.32]; P < .001), and injected daily or more frequently (aHR, 3.79 [95% CI, 3.43–4.18]; P < .001). Frequent injection of opioids remained independently associated with lower risk of HCV acquisition in males (aHR, 0.60 [95% CI, .48–.75]; P < .001).
DISCUSSION
Results indicate that among PWID exposed to OAT, females were almost twice as likely as males to acquire HCV infection. This finding is consistent with the meta-analysis by Platt et al, which observed a 59% reduction in the effectiveness of OAT with every 10% increase in female participants [22]. Previous research has documented sex-based differences in immune function [26]. Compared to males, females produce more vigorous cellular and humoral immune reactions and although females are more resistant to infections, they also experience higher prevalence of several autoimmune diseases [27], with increased severity and number of immune and inflammatory responses compared to males [26]. Sex differences in immunity have been linked to influences on the function of immune cells caused by the binding of sex steroids to specific receptors expressed in lymphoid tissue cells, macrophages, dendritic cells, and lymphocytes [26]. Some evidence also suggests sex-based differences in human brain μ- and κ-opioid receptor binding potential as measured by positron emission tomography [28, 29]. These studies merely suggest differences in the opioid pathways in the central nervous system that could lead to differential effects for opioid effects, including those relevant for reward, withdrawal, and addiction. This is merely to suggest that the effects of OAT on withdrawal and addiction may not be the same among females compared to males [29]. Fluctuations in female hormone levels (eg, during pregnancy) may also influence opioid binding and dosing requirements [30]. It is possible that sex differences observed in the current study could, in part, be related to differences in pharmacokinetics and drug metabolism of OAT among females compared to males. While methadone metabolism is clearly altered in pregnancy, there is an absence of literature to fully establish whether differences might also exist among nonpregnant women compared to men [31].
Among females in the current study, nonwhite race, recent unstable housing, daily or more frequent injection, and RSS were associated with an increased risk of incident HCV infection. Nonwhite females exposed to OAT were almost twice as likely as white females to acquire HCV infection. The attenuated protective effect of OAT observed in nonwhite females is consistent with previous work by our group, which found that HCV incidence among nonwhite females was twice that of white females [8]. The association between nonwhite race and the attenuated protective effect of OAT against incident HCV is significant, as race not only plays a role in the transmission of HCV [12], but is associated with an increased risk of disease progression. In a US study of 464 people living with chronic HCV, African American and Asian females were, respectively, 2.2 and 4.6 times more likely to develop hepatocellular cancer than white females [32]. Moreover, compared to white patients, African American and Hispanic patients in real-world settings are significantly less likely to achieve a sustained virological response when treated with HCV direct-acting antiviral therapies [33]. Further work is required to explore the relationship between race and incident HCV infection, including the attenuated protective efficacy of OAT, as well as HCV-related disease progression and treatment outcomes.
Recent unstable housing was significantly associated with incident HCV infection in both females and males. Females who acquired HCV were 4 times more likely to report unstable housing in the previous 6 months, compared with females who did not. Housing instability has been independently associated with both HIV [34, 35] and HCV acquisition [36, 37], and unstably housed PWID are more likely to engage in injection risk behavior [35], such as RSS [37]. Stable housing is associated with better health status [34, 36], including more frequent and timely utilization of health and social services [38]. Further work is needed to evaluate novel interventions to address housing instability among PWID, and to better integrate HCV prevention, testing, and linkage to care and treatment services into such services.
When taken continuously and at therapeutic doses, OAT is protective against incident HIV and HCV infection due to reduced frequency of injection and high-risk injecting practices [21, 22]. However, our results indicate that females exposed to OAT who acquired HCV were 1.5 times more likely to report daily or more frequent injection compared to females who did not. Similarly, males who acquired HCV were 2.1 times more likely to inject daily or more frequently compared to males who did not acquire HCV. These findings suggest that dosage and retention in OAT (not measured in the current study) may have a significant impact on the epidemiology of HCV transmission through their influence on injection frequency. Future research should examine this in order to fully assess sex differences in HCV acquisition among PWID.
Finally, among females exposed to OAT, those who reported recent RSS were 1.4 times more likely to have acquired HCV compared to those who did not. Parenteral transmission of HCV, through the sharing of needles and syringes, is the predominant route of exposure for HCV and HIV among PWID [5]. Several studies have concluded that female PWID have elevated rates of RSS compared to male PWID [9, 39]. Gender inequalities likely play a role, as females who experience intimate partner violence are more likely to engage in RSS [39], and females who experience violence are often reluctant to access health services [13]. Harm reduction services need to be sensitive to the specific needs of females, and how gender neutrality may inadvertently disadvantage female PWID [12, 13].
Our study has several limitations. Data were retrospectively merged from 7 cohort studies, and there were inconsistencies in data collected and biological measures. Variability between sites was addressed through use of a random-effects model for clustered data. Nonetheless, there was heterogeneity with regard to data availability for some variables of possible interest; for example, recent imprisonment, sexual relationships with other PWID, and utilization of needle syringe programs were excluded due to >50% missing data. However, we conducted sex-specific sensitivity analyses and results demonstrated complete alignment with the multivariable model for males and near-complete alignment for females (Supplementary Tables 1 and 2). Because detailed data on OAT were not available in the InC3 dataset, potentially unmeasured confounders such as duration, type, and dose of OAT, as well as sexual risk behavior, mental health, intimate partner violence, and pregnancy and/or breastfeeding among female participants, may have influenced our results.
CONCLUSIONS
Our study has shown that despite exposure to OAT, female PWID were twice as likely to acquire HCV than were their male counterparts. Our findings are consistent with those of Platt et al where a disparity in the protective effect of OAT was reported based on biological sex [22]. Factors independently associated with the attenuated effect of OAT in females included biosocial (nonwhite race), structural (unstable housing), and behavioral (frequent injecting and RSS) factors. Structural and behavioral interventions that target women, such as access to affordable housing and increasing safe injection self-efficacy to reduce RSS [40], are needed to bolster the efficacy of OAT in females to prevent HCV transmission. Additionally, further research regarding the dose and duration of OAT is required to better understand sex-related differences in immune function and opioid pharmacokinetic and pharmacodynamic parameters, as well as the impact of factors such as mental health, homelessness, injecting networks, and pregnancy and breastfeeding, on incident HCV infection among PWID exposed to OAT.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. L. M. and K. P. conceptualized and designed the study. L. G., H. W., and L. M. conducted the analysis. L. G., H. W., J. I., J. T., K. P., and L. M. interpreted the results. L. G. and L. M. drafted and revised the manuscript. J. I., H. W., A. E., J. T., M. H., G. D., J. G., P. M. D., J. B., M. P., M. D. M., N. H. S., A. L., A. Y. K., G. L., A. L. C., and K. P. contributed to interpretation of the analysis and revised the manuscript. All authors approved the final manuscript.
Acknowledgments. The authors thank the participants and study staff in each of the participating cohorts. The International Collaboration of Incident HIV and HCV in Injecting Cohorts (InC3) study group Steering Committee includes Kimberly Page (Chair, U-Find-Out [UFO] Study), Julie Bruneau and Naglaa H. Shoukry (St Luc Hepatitis Cohort [HEPCO]), Andrea L. Cox (Baltimore Before and After Acute Study in Hepatitis [BBAASH]), Gregory J. Dore and Jason Grebely (Australian Trial in Acute Hepatitis C [ATAHC]), Paul M. Dietze (SuperMIX), Margaret Hellard (Networks 2), Georg Lauer, Arthur Y. Kim, and Barbara H. McGovern (Boston Acute HCV Study: Transmission, Immunity, Outcomes Network [BAHSTION]), Andrew Lloyd (Hepatitis C Incidence and Transmission Study–prison [HITS-p]), Lisa Maher (Hepatitis C Incidence and Transmission Study–community [HITS-c] and Hepatitis C Virus Cohort Study [HCVC]), and Maria Prins (Amsterdam Cohort Study [ACS]). The coordinating center includes Meghan D. Morris (study coordinator), Judy Hahn (co-investigator), Stephen Shiboski (co-investigator), and Ali Mirzazadeh (data manager). Site data managers include Maryam Alavi (ATAHC), Brittany Wells (BBAASH), Rachel Bouchard (HEPCO), Jennifer Evans (UFO Study), Bart Grady (ACS), Jasneet Aneja (BAHSTION), Rachel Sacks-Davis (Networks 2), Bethany White (HITS-c), Suzy Teutsch (HITS-p), and G. Zang (HEPCO).
Financial support. InC3 was funded by the National Institute on Drug Abuse (NIDA) (grant number R01DA031056). Research support for the InC3 cohorts includes the following: Netherlands National Institute for Public Health and the Environment (support to the ACS); National Institutes of Health (NIH) to BBAASH (grant number U19 AI088791); the National Institute of Allergy and Infectious Diseases (NIH) to BAHSTION (grant number U19 U19 AI066345); the NIDA to the ATAHC (grant number RO1 DA 15999-01); the National Health and Medical Research Council (Australia) (NHMRC) to HITS-p (project number 222887, partnership number 1016351, program numbers 510488 and 1053206); the University of New South Wales Hepatitis C Vaccine Initiative and NHMRC to HITS-c (project grant number 630483); the NHMRC (project grant numbers 331312 and 545891); the Victorian Operational Infrastructure Support Programme (Department of Health, Victoria, Australia) to Networks/SuperMIX; the Canadian Institutes of Health Research to HepCo (grant numbers MOP-103138 and MOP-19106468); Réseau SIDA Maladies Infectieuses, Rése ctieuses, Fonds de la Recherche du Québec-Santé supports the HepCo cohort (grant number Octroi 52905); the NHMRC to HCVC (project grant number 991357); and the NIH to UFO (grant number R01 DA016017). J. I., M. H., P. D., A. L., G. D., J. G., and L. M. receive fellowship support from the Australian NHMRC. N. S. receives support from the Fonds de la Recherche du Québec-Santé, Canada. In addition to grant support for the InC3 research, K. P. receives support from The Australian Government Research Training Program Scholarship is a program which funds PhDs in Australia (grant numbers 3R01 DA016017, 1ULTR001449, and U54GM104944). L. G. is supported through an Australian Government Research Training Program Scholarship. P. D. received grant funding from the Colonial Foundation Trust.
Potential conflicts of interest. J. B., P. D., and M. H. have received funding from Gilead Sciences for work on HCV treatment. P. D. has received funding from Reckitt Benckiser, the Ramsay Foundation, and Indivior. J. G. is a consultant/advisor and reports grants from AbbVie and Cepheid, and grants and personal fees from Gilead Sciences and Merck. M. H. has received funding from AbbVie and Bristol-Meyers Squibb for investigator-initiated HCV research. G. D. is a consultant/advisor and has received research grants from AbbVie, Bristol-Myers Squibb, Cepheid, Gilead, Merck, Janssen, and Roche. J. B. has received personal fees from Gilead Sciences and Merck Canada (Merke Sharp Dohme). A. L. has received grants from Gilead Sciences and AbbVie. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Blach S, Zeuzem S, Manns M, et al. . Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2:161–76. [DOI] [PubMed] [Google Scholar]
- 2. Terrault NA, Dodge JL, Murphy EL, et al. . Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV Partners Study. Hepatology 2013; 57:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS 2015; 29:2335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morris MD, Shiboski S, Bruneau J, et al. . Geographic differences in temporal incidence trends of hepatitis C virus infection among people who inject drugs: the InC3 collaboration. Clin Infect Dis 2017; 64:860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol 2007; 13:2436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Degenhardt L, Peacock A, Colledge S, et al. . Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5:e1192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345:41–52. [DOI] [PubMed] [Google Scholar]
- 8. Esmaeili A, Mirzazadeh A, Morris MD, et al. . The effect of female sex on hepatitis C incidence among people who inject drugs: results from the international multicohort InC3 collaborative. Clin Infect Dis 2017; 66:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iversen J, Wand H, Topp L, Kaldor J, Maher L. Reduction in HCV incidence among injection drug users attending needle and syringe programs in Australia: a linkage study. Am J Public Health 2013; 103:1436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maher L, Jalaludin B, Chant KG, et al. . Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction 2006; 101:1499–508. [DOI] [PubMed] [Google Scholar]
- 11. Hagan H, Thiede H, Weiss NS, Hopkins SG, Duchin JS, Alexander ER. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health 2001; 91:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maher L, Li J, Jalaludin B, Chant KG, Kaldor JM. High hepatitis C incidence in new injecting drug users: a policy failure? Aust N Z J Public Health 2007; 31:30–5. [DOI] [PubMed] [Google Scholar]
- 13. Iversen J, Page K, Madden A, Maher L. HIV, HCV and health-related harms among women who inject drugs: implications for prevention and treatment. J Acquir Immune Defic Syndr 2015; 69:S176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grebely J, Page K, Sacks-Davis R, et al. . InC3 Study Group The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014; 59:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of “perfectovir.” Clin Infect Dis 2015; 60:1829–36. [DOI] [PubMed] [Google Scholar]
- 16. Lin M, Kramer J, White D, et al. . Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther 2017; 46:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis 2013; 57(Suppl 2):S32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nolan S, Dias Lima V, Fairbairn N, et al. . The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction 2014; 109:2053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med 2014; 174:1974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White B, Dore GJ, Lloyd AR, Rawlinson WD, Maher L. Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS-c study. Med J Aust 2014; 201:326–9. [DOI] [PubMed] [Google Scholar]
- 21. Gowing L, Farrell M, Bornemann R, Sullivan L, Ali R. Substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev 2008; 2:CD004145. [DOI] [PubMed] [Google Scholar]
- 22. Platt L, Minozzi S, Reed J, et al. . Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane review and meta-analysis. Addiction 2018; 113:545–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grebely J, Morris MD, Rice TM, et al. . InC Study Group Cohort profile: the International Collaboration of Incident HIV and Hepatitis C in Injecting Cohorts (InC3) study. Int J Epidemiol 2013; 42:1649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health 2001; 25:464–9. [PubMed] [Google Scholar]
- 25. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999; 8:3–15. [DOI] [PubMed] [Google Scholar]
- 26. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis 2010; 10:338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update 2005; 11:411–23. [DOI] [PubMed] [Google Scholar]
- 28. Vijay A, Wang S, Worhunsky P, et al. . PET imaging reveals sex differences in kappa opioid receptor availability in humans, in vivo. Am J Nucl Med Mol Imaging 2016; 6:205–14. [PMC free article] [PubMed] [Google Scholar]
- 29. Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry 1999; 156:842–8. [DOI] [PubMed] [Google Scholar]
- 30. Unger A, Jung E, Winklbaur B, Fischer G. Gender issues in the pharmacotherapy of opioid-addicted women: buprenorphine. J Addict Dis 2010; 29:217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Graziani M, Nisticò R. Gender differences in pharmacokinetics and pharmacodynamics of methadone substitution therapy. Front Pharmacol 2015; 6:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen MH, Whittemore AS, Garcia RT, et al. . Role of ethnicity in risk for hepatocellular carcinoma in patients with chronic hepatitis C and cirrhosis. Clin Gastroenterol Hepatol 2004; 2:820–4. [DOI] [PubMed] [Google Scholar]
- 33. Su F, Green PK, Berry K, Ioannou GN. The association between race/ethnicity and the effectiveness of direct antiviral agents for hepatitis C virus infection. Hepatology 2017; 65:426–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robertson MJ, Clark RA, Charlebois ED, et al. . HIV seroprevalence among homeless and marginally housed adults in San Francisco. Am J Public Health 2004; 94:1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Milloy MJ, Marshall BD, Montaner J, Wood E. Housing status and the health of people living with HIV/AIDS. Curr HIV/AIDS Rep 2012; 9:364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hickman M, Hope V, Brady T, et al. . Hepatitis C virus (HCV) prevalence, and injecting risk behaviour in multiple sites in England in 2004. J Viral Hepat 2007; 14:645–52. [DOI] [PubMed] [Google Scholar]
- 37. Kim C, Kerr T, Li K, et al. . Unstable housing and hepatitis C incidence among injection drug users in a Canadian setting. BMC Public Health 2009; 9:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nickasch B, Marnocha SK. Healthcare experiences of the homeless. J Am Acad Nurse Pract 2009; 21:39–46. [DOI] [PubMed] [Google Scholar]
- 39. Azim T, Bontell I, Strathdee SA. Women, drugs and HIV. Int J Drug Policy 2015; 26(Suppl 1):S16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pitpitan EV, Patterson TL, Abramovitz D, et al. . Policing behaviors, safe injection self-efficacy, and intervening on injection risks: moderated mediation results from a randomized trial. Health Psychol 2016; 35:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.