Abstract
Background
Clostridium difficile causes toxin-mediated nosocomial diarrhea and community-acquired infections; no preventive vaccine is licensed. In this phase 2 study, we explored safety, tolerability, and immunogenicity in older US adults of an investigational bivalent C. difficile vaccine that contains equal dosages of genetically and chemically detoxified toxins A and B.
Methods
Conducted from July 2015 through March 2017, 855 healthy adults aged 65–85 years from 15 US centers were randomized 3:3:1 to receive vaccine (100 or 200 μg) or placebo at 0, 1, and 6 months (month regimen) or 1, 8, and 30 days (day regimen). Serum toxin A– and B–specific neutralizing antibodies were measured. Participant-reported local reactions (LRs) and systemic events (SEs), adverse events (AEs), serious AEs, newly diagnosed chronic medical conditions, and immediate AEs were recorded.
Results
The 200-μg dose level elicited higher immune responses than the 100-µg dose level across regimens. Compared with the day regimen, the month regimen induced stronger and more persistent immune responses that remained elevated 12 months after dose 3. Responses peaked at month 7 (month regimen) and day 37 (day regimen). LRs (primarily injection site pain) were more frequent in vaccine recipients than controls; SE frequency was similar across groups. More related AEs were reported in the day regimen group than the month regimen group.
Conclusions
The C. difficile vaccine was safe, well tolerated, and immunogenic in healthy US adults aged 65–85 years. Immune responses were particularly robust in the 200-μg month regimen group. These results support continued vaccine development.
Clinical Trials Registration
NCT02561195.
Keywords: Clostridium difficile infection, toxoid vaccine, adults, United States, nosocomial diarrhea
Prevention of Clostridium difficile infection is a significant unmet medical need. Here, a 3-dose series of an investigational C. difficile vaccine provided robust immune responses in older US adults. The vaccine was generally well tolerated, supporting its continued clinical development.
Clostridium difficile is a gram-positive bacterium that is a major cause of nosocomial diarrhea worldwide [1–3]. In addition to healthcare-associated C. difficile infections (CDIs), community-associated infections have increased in prevalence in recent years [1, 2, 4]. Symptoms range from mild diarrhea to severe outcomes, including pseudomembranous colitis, toxic megacolon, intestinal perforation, and even death [2, 3, 5]. Clostridium difficile carriage may be asymptomatic or can progress to CDI [5]; asymptomatic carriage rates are 1.6% to 6.6% in the general population and 13% to 51% in healthcare settings [6–10].
Rates of CDI significantly increased in recent years, owing largely to the emergence of highly virulent, fluoroquinolone-resistant polymerase chain reaction–ribotype 027 strains [1, 2, 11, 12]. An estimated 453 000 US cases occurred in 2011; in Europe, an estimated 172 000 cases occurred annually in 2011/2012 [3, 11]. In 2011, the 30-day healthcare-associated US CDI mortality rate was 9.3% [3]. Major CDI risk factors are older age, exposure to healthcare settings, recent antibiotic use, and presence of certain comorbidities or multiple common comorbidities [1–3, 13–15].
Generally, CDI is treated with antibiotics (metronidazole, vancomycin, or fidaxomicin) [1, 16, 17]; however, CDI recurs in 13.5% (community associated) to 20.9% (healthcare associated) of initial cases [3]. Further antibiotic treatment is indicated for recurrent infections, but this approach is not always successful [1, 16, 17]. Surgical treatments for severe complications (eg, toxic megacolon) are associated with high mortality rates [1, 16]. Bezlotoxumab (Zinplava; Merck & Co; Whitehouse Station, NJ) is a recently approved neutralizing monoclonal antibody that targets C. difficile toxin B for preventing recurrent CDI [18–20]. Additionally, fecal microbiota transplantation (FMT; ie, transplanting healthy donor feces into the infected patient’s intestinal tract) has been used to treat recurrent CDI [17, 21, 22]. Although regulatory approval of FMT is complicated and remains unrealized, multiple stool banks in the United States and elsewhere generate FMT products [23, 24]. Current preventive strategies for primary CDI focus on infection control (eg, hand hygiene, surface decontamination, isolation) and antibiotic stewardship [16, 17]; no vaccine is yet available.
An investigational vaccine currently in development contains genetically and chemically detoxified toxins A and B, the principal virulence factors produced by C. difficile [25]. In a previous first-in-human study, the vaccine was immunogenic in adults aged 50–85 years when given at 0, 1, and 6 months and was overall safe and well tolerated, with comparatively decreased reactogenicity when administered together with aluminum hydroxide [26]. Immune responses were comparatively higher with the toxoid-alone formulation, but both formulations induced robust immune responses. In the current study, we evaluated the safety and immunogenicity of the aluminum hydroxide–formulated vaccine when given to older US adults at 0, 1, and 6 months or 1, 8, and 30 days at 2 different dose levels.
METHODS
Study Design and Population
This phase 2, placebo-controlled, randomized, observer-blinded study was conducted from July 2015 through March 2017 and enrolled participants across 15 US sites. Participants received doses at either months 0, 1, and 6 (month regimen) or days 1, 8, and 30 (day regimen). Within each regimen, participants were randomized using an interactive response technology system in a 3:3:1 ratio to receive 100 μg C. difficile vaccine, 200 μg C. difficile vaccine, or placebo. All site personnel and the sponsor were blinded, other than study staff who dispensed and administered the vaccine, select sponsor-affiliated individuals who ensured adherence to study protocol, and those who internally reviewed interim analyses and made associated recommendations. The sponsor and site personnel were unblinded once month 7 data were available for all continuing participants so as to identify those eligible for an extension stage. Laboratory personnel who performed the immunologic assays remained blinded throughout the study.
The study was conducted in compliance with Good Clinical Practice, the Declaration of Helsinki, and the International Council for Harmonisation. An institutional review board and/or independent ethics committee at each study site reviewed and approved the final protocol, amendments, and informed consent documents. Each participant provided written informed consent before enrollment and performance of any study-related procedures.
Participants were healthy adults aged 65 to 85 years; those with demonstrably stable underlying comorbidities were eligible. Women could not be of childbearing potential, and men had to agree to use effective contraception. Participants had to be available for the study duration and able to be contacted by phone. The Supplementary Appendix lists exclusion criteria and reasons for temporary vaccination delay.
Investigational Product
The investigational product was administered by intramuscular injection into the upper deltoid muscle, preferably of the nondominant arm, with 0.5 mL study vaccine containing 100 or 200 µg (toxoids A and B combined) of the C. difficile vaccine candidate (reconstituted with 1 mg/mL aluminum hydroxide) or placebo (0.9% sodium chloride). The toxoids were produced by expressing genetically detoxified toxins in C. difficile and chemically treating the purified antigens to remove residual cytotoxicity [27].
Immunogenicity Assessments
The primary immunogenicity objective was to describe immunogenicity as measured by C. difficile toxin A– and toxin B–specific neutralizing antibody levels at month 7 (month regimen) and day 37 (day regimen). Vaccine-induced immune responses were evaluated using toxin neutralization assays (TNAs) for toxins A and B [26].
The primary immunogenicity analysis population was the evaluable immunogenicity population, which included all participants who were eligible at randomization, received all 3 randomized investigational product doses, had blood drawn at month 7 (month regimen) or day 37 (day regimen) within specified time frames following dose 3 (with this blood draw providing at least 1 valid and determinate assay result), and had no major protocol violations.
Primary and secondary immunogenicity evaluations at each sampling time point for each vaccine group included percentages of participants who met prespecified neutralization thresholds (details regarding threshold determination are provided in the Supplementary Appendix), geometric mean concentrations (GMCs), and geometric mean fold rises (GMFRs) from baseline of toxin A– and B–specific neutralizing antibody levels. Responses were evaluated through 12 months post-dose 3 for each regimen for the overall population and the subpopulations who were seronegative or seropositive for antibodies against each toxin at baseline.
Safety Assessments
The primary safety objective was to assess safety and tolerability of the C. difficile vaccine. Safety evaluations included acute reactions within 30 minutes after each dose, participant-reported electronic diary assessment of local reactions (LRs) and systemic events (SEs) occurring within 14 days after each dose (7 days post-dose 1 in the day regimen), adverse events (AEs) from informed consent through 1 month post-dose 3, and serious AEs (SAEs) and newly diagnosed chronic medical conditions (NDCMCs) from informed consent through 6 months post-dose 3. LRs and SEs (including fever) were graded using the US Food and Drug Administration Center for Biologics Evaluation and Research toxicity grading scale [28]. For all AEs, the study investigator determined causality with regard to the investigational product.
The safety population included all participants who received at least 1 investigational product dose.
Statistical Analyses
Safety and immunogenicity results were analyzed separately for each regimen.
For binary immunogenicity endpoints and safety endpoints (ie, yes/no outcomes), Clopper-Pearson exact 95% confidence intervals (CIs) were calculated. For continuous immunogenicity data (ie, GMCs and GMFRs), 95% CIs were calculated using a Student t distribution on log-transformed data; these values were then back-transformed to the antilog scale.
For immunogenicity analyses, any neutralizing antibody level below the TNA’s lower limit of quantitation (LLOQ; 158.0 and 249.5 neutralization units/mL for toxin A– and B–specific TNAs, respectively) was assigned a value of 0.5× LLOQ.
RESULTS
Study Participants
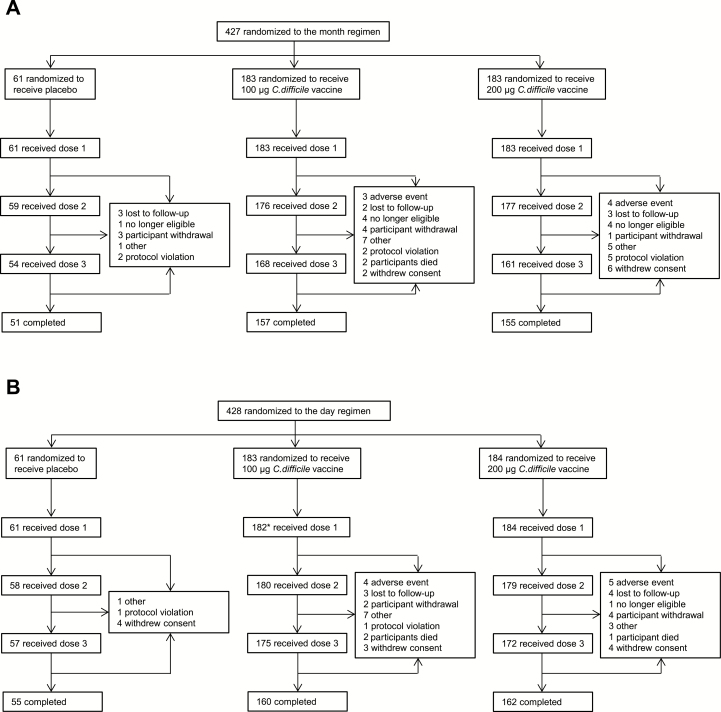
Overall, 855 participants were randomized (Figure 1); 85.0% to 88.1% of participants across both dosing regimens completed the study. Demographic characteristics were generally balanced across vaccine groups (Table 1); most participants were seronegative by TNA for both toxins A and B at baseline.
Figure 1.
CONSORT diagram with participant dispositions in the (A) month and (B) day regimens. Reasons for withdrawals after vaccination include all withdrawals from dose 1 onward. *One participant in the 100-μg dose level group in the day regimen withdrew after randomization before receiving any study vaccination.
Table 1.
Demographic Characteristics of Participants in the Safety Populations of the Month and Day Regimens
| Characteristic | Month Regimen Vaccine Group | Day Regimen Vaccine Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (na = 61) |
100 µg C. diff (na = 183) |
200 µg C. diff (na = 183) |
Total (Na = 427) |
Placebo (na = 61) |
100 µg C. diff (na = 182) |
200 µg C. diff (na = 184) |
Total (Na = 427) |
|
| Sex, nb (%) | ||||||||
| Female | 37 (60.7) | 93 (50.8) | 97 (53.0) | 227 (53.2) | 35 (57.4) | 93 (51.1) | 106 (57.6) | 234 (54.8) |
| Male | 24 (39.3) | 90 (49.2) | 86 (47.0) | 200 (46.8) | 26 (42.6) | 89 (48.9) | 78 (42.4) | 193 (45.2) |
| Race, nb (%) | ||||||||
| White | 56 (91.8) | 151 (82.5) | 157 (85.8) | 364 (85.2) | 59 (96.7) | 172 (94.5) | 168 (91.3) | 399 (93.4) |
| Black | 3 (4.9) | 16 (8.7) | 13 (7.1) | 32 (7.5) | 2 (3.3) | 6 (3.3) | 16 (8.7) | 24 (5.6) |
| Asian | 1 (1.6) | 13 (7.1) | 10 (5.5) | 24 (5.6) | 0 (0.0) | 2 (1.1) | 0 (0.0) | 2 (0.5) |
| Other | 1 (1.6) | 3 (1.6) | 3 (1.6) | 7 (1.6) | 0 (0.0) | 2 (1.1) | 0 (0.0) | 2 (0.5) |
| Ethnicity, nb (%) | ||||||||
| Non-Hispanic/non-Latino | 57 (93.4) | 168 (91.8) | 172 (94.0) | 397 (93.0) | 48 (78.7) | 127 (69.8) | 143 (77.7) | 318 (74.5) |
| Hispanic/Latino | 4 (6.6) | 15 (8.2) | 11 (6.0) | 30 (7.0) | 13 (21.3) | 55 (30.2) | 41 (22.3) | 109 (25.5) |
| Age at randomization, y | ||||||||
| Mean (standard deviation) | 70.4 (4.65) | 71.5 (4.96) | 71.3 (4.70) | 71.3 (4.81) | 71.9 (5.27) | 71.4 (4.89) | 71.2 (5.04) | 71.4 (5.01) |
| Median | 69.0 | 71.0 | 71.0 | 71.0 | 71.0 | 71.0 | 70.0 | 70.0 |
| Min, max | 65, 81 | 65, 85 | 65, 84 | 65, 85 | 65, 85 | 65, 84 | 65, 85 | 65, 85 |
| Baseline Clostridium difficile serostatus,c nb (%) | ||||||||
| Seronegative | ||||||||
| Toxin A−/toxin B− | 40 (65.6) | 135 (73.8) | 123 (67.2) | 298 (69.8) | 44 (72.1) | 141 (77.5) | 137 (74.5) | 322 (75.4) |
| Seropositive | ||||||||
| Toxin A+/toxin B− | 3 (4.9) | 16 (8.7) | 18 (9.8) | 37 (8.7) | 4 (6.6) | 6 (3.3) | 11 (6.0) | 21 (4.9) |
| Toxin A−/toxin B+ | 17 (27.9) | 28 (15.3) | 36 (19.7) | 81 (19.0) | 11 (18.0) | 31 (17.0) | 28 (15.2) | 70 (16.4) |
| Toxin A+/toxin B+ | 1 (1.6) | 4 (2.2) | 6 (3.3) | 11 (2.6) | 2 (3.3) | 4 (2.2) | 7 (3.8) | 13 (3.0) |
| Toxin A and/or toxin B not evaluated | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Toxin A not evaluated | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Toxin B not evaluated | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Toxin A and B not evaluated | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.2) |
Includes all participants who received at least 1 dose of an investigational product.
Abbreviation: C diff, Clostridium difficile vaccine.
an or N = total number of participants in the specified group.
bn = number of participants with the specified characteristic.
cBaseline serostatus (before dose 1 on day 1) defined based on lower limit of quantitation (LLOQ) value for toxin A– or toxin B–specific neutralizing antibody level, with “+” indicating neutralizing antibody level ≥LLOQ and “–“ indicating neutralizing antibody level <LLOQ.
Immunogenicity
Month Regimen
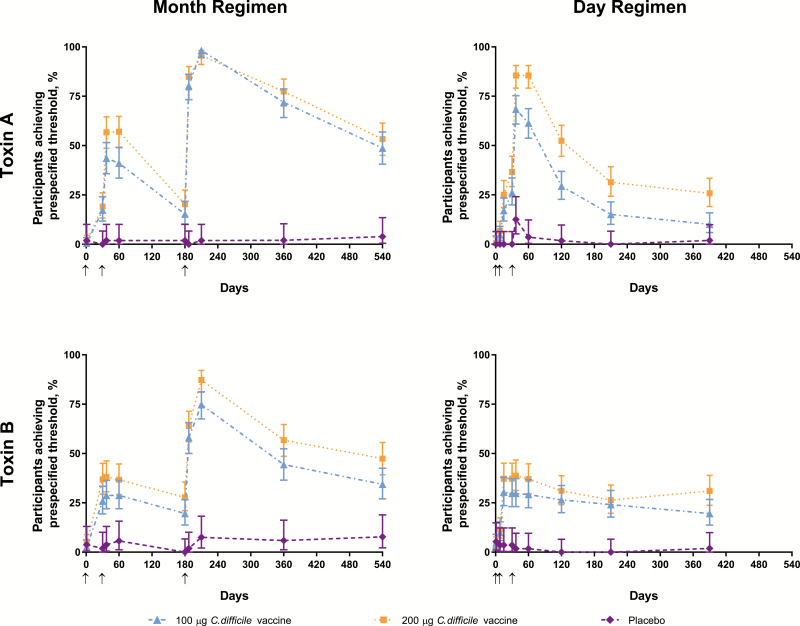
In the month regimen, 98.2%, 95.6%, and 1.9% of participants in the 100-μg, 200-μg, and placebo groups, respectively, achieved prespecified threshold levels of toxin A–neutralizing antibodies 1 month post-dose 3 (Figure 2, Supplementary Table S1). For toxin B, respective percentages were 74.8%, 87.3%, and 7.5%. Percentages for toxin B–neutralizing antibodies were consistently high after a single dose among participants who were seropositive at baseline (Figure 3). Based on percentages of participants who achieved threshold levels across all groups, a 200-µg dose level was selected for phase 3 investigation; presentation of the remaining immunogenicity endpoints therefore focuses on this dose level [29].
Figure 2.
Percentages of participants who achieved prespecified levels of toxin A– and toxin B–specific neutralizing antibodies in the month and day regimens. Arrows indicate days on which doses were administered. Abbreviation: C diff, Clostridium difficile vaccine.
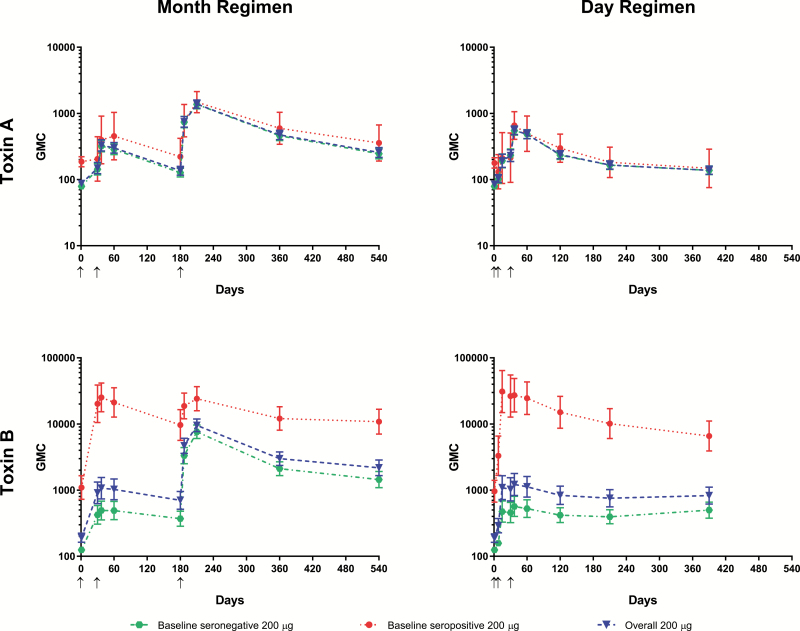
Figure 3.
Geometric mean concentrations of toxin A– and toxin B–specific neutralizing antibodies for baseline seronegative, baseline seropositive, and all participants in the 200-µg group in the month and day regimens. Arrows indicate days on which doses were administered. Abbreviation: GMC, geometric mean concentration.
The GMCs for toxin A– and B–specific neutralizing antibodies rose after dose 2 from baseline and further increased after dose 3 in the overall (combined baseline seronegative and seropositive) 200-µg group. This pattern was also observed in the baseline seronegative group for both toxins, but baseline seropositive individuals had consistently high toxin B–neutralizing antibody GMCs after 1 dose (Figure 3). At the 18-month time point, GMC fold change from baseline in the overall 200-μg group was 3.0 for toxin A–specific neutralizing antibodies and 11.4 for toxin B–specific neutralizing antibodies. Immune responses peaked at month 7 and remained elevated at month 18.
Day Regimen
In the day regimen, 68.4%, 85.5%, and 12.5% of participants achieved prespecified levels of toxin A–neutralizing antibodies in the 100-μg, 200-μg, and placebo groups, respectively, at 7 days post-dose 3 (Figure 2, Supplementary Table S1). For toxin B, respective percentages were 29.8%, 38.8%, and 1.8%.
The GMCs rose from baseline in the overall 200-µg group by day 8 and peaked at day 37 (Figure 3). At month 13, GMC fold changes from baseline in the overall 200-µg group were 1.6 for toxin A–specific neutralizing antibodies and 4.4 for toxin B–specific neutralizing antibodies. GMCs in the day regimen groups were generally lower than in the corresponding groups in the month regimen. Consistent with other observations, GMCs for toxin B–neutralizing antibodies were substantially higher among participants seropositive at baseline.
Safety and Tolerability
Month Regimen
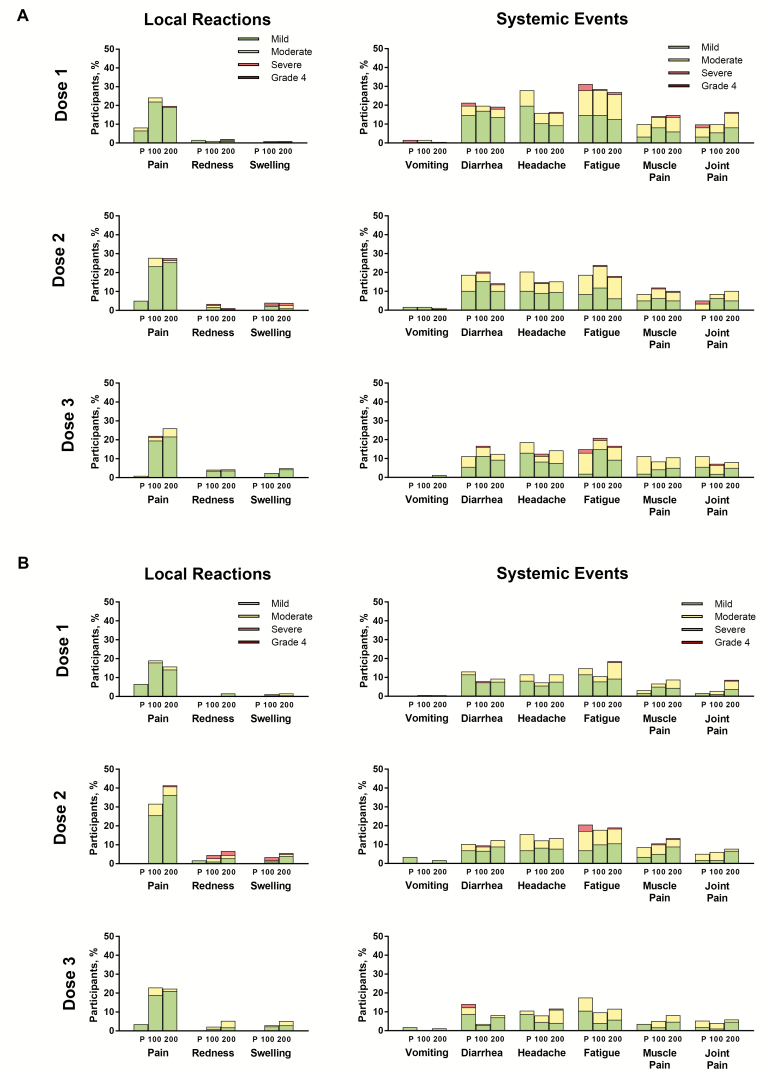
For the month regimen, LRs were more common in the vaccine groups compared with placebo (Figure 4A). Injection site pain was the most common LR across groups (19.8% to 27.8% of participants per dose in the C. difficile groups). Most LRs were mild or moderate in severity. Seven participants (3 and 4 in the 100-μg and 200-μg groups, respectively, mostly post-dose 2) experienced severe LRs; none were grade 4. For both C. difficile groups, LR frequency and severity increased after dose 2 compared with dose 1, but further increases after dose 3 were not apparent. LRs in the C. difficile groups lasted a median of 1.0 (multiple types of LRs, doses, and dose levels) to 6.0 (200-μg; swelling post-dose 1) days.
Figure 4.
Local reactions and systemic events by dose in the (A) month and (B) day regimens. Abbreviations: 100 = 100 μg Clostridium difficile vaccine; 200 = 200 μg Clostridium difficile vaccine; P, placebo.
Systemic events were observed with similar frequencies in the C. difficile vaccine and placebo groups alike (Figure 4A). Fatigue, headache, and diarrhea were the most commonly reported SEs; most cases were mild or moderate in severity. All 7 fever reports (all in the C. difficile groups) were 38.0°C to 38.4°C. SEs in the C. difficile groups had a median duration of 1.0 (multiple types of SEs, doses, and dose levels) to 6.5 (200-μg; headache post-dose 1) days.
AEs were reported by 62.3% to 63.9% of participants across groups (Table 2); infections and infestations was the most common system organ class among the AEs reported. The most common related AEs were injection site nodule, diarrhea, and injection site swelling; other AEs were each reported by only 1 or 2 study participants. The 3 related severe AEs were atrial fibrillation, sinus node dysfunction (both 100-μg), and dizziness (200-μg). One of 2 immediate AEs reported (injection site pain, 200-μg) was considered vaccine related. No SAEs, NDCMCs, AEs leading to withdrawal, or deaths were considered related (Table 2). The 2 unrelated deaths that occurred in the month regimen (both 100-μg) were attributed to a malignant lung neoplasm and myocardial infarction.
Table 2.
Adverse Events Among Participants in the Month and Day Regimens
| Month Regimen Vaccine Group | Day Regimen Vaccine Group | |||||
|---|---|---|---|---|---|---|
| Category of AE Relationship | Placebo (na = 61), nb (%) |
100 µg C. diff (na = 183), nb (%) |
200 µg C. diff (na = 183), nb (%) |
Placebo (na = 61), nb (%) |
100 µg C. diff (na = 182), nb (%) |
200 µg C. diff (na = 184), nb (%) |
| AE | 39 (63.9) | 114 (62.3) | 117 (63.9) | 20 (32.8) | 57 (31.3) | 76 (41.3) |
| Related | 2 (3.3) | 15 (8.2) | 11 (6.0) | 2 (3.3) | 16 (8.8) | 25 (13.6) |
| Serious AE | 2 (3.3) | 19 (10.4) | 22 (12.0) | 0 (0.0) | 11 (6.0) | 6 (3.3) |
| Related | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe or life-threatening AE | 5 (8.2) | 22 (12.0) | 20 (10.9) | 0 (0.0) | 9 (4.9) | 10 (5.4) |
| Related | 0 (0.0) | 2 (1.1) | 1 (0.5) | 0 (0.0) | 1 (0.5) | 3 (1.7) |
| Immediate AEc | 0 (0.0) | 1 (0.5) | 1 (0.5) | 0 (0.0) | 3d (1.6) | 2 (1.1) |
| Related | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 2d (1.1) | 2 (1.1) |
| Newly diagnosed chronic medical conditione | 2 (3.7) | 3 (1.8) | 5 (3.1) | 3 (5.3) | 5 (2.9) | 3 (1.7) |
| Related | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AE leading to withdrawal | 0 (0.0) | 5f (2.7) | 4 (2.2) | 0 (0.0) | 5 (2.7)g | 5 (2.7) |
| Related | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 5 (2.7) |
| Deaths | 0 (0.0) | 2 (1.1)h | 0 (0.0) | 0 (0.0) | 2 (1.1)i | 1(0.5)j |
| Related | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Abbreviations: AE, adverse event; C. diff, Clostridium difficile vaccine.
an = total number of participants in the specified group.
bn = number of participants reporting at least 1 specified event.
cOccurring within 30 minutes following vaccination.
dOne participant in the 100-μg group experienced immediate, related AEs after doses 2 and 3 (1 event after each dose); these events are considered 2 separate AEs belonging to 1 participant.
eFrom 1–6 months after dose 3.
fIncludes 2 participants whose AEs (malignant lung neoplasm and myocardial infarction) eventually resulted in death; these participants are also counted under “deaths” and noted in Figure 1 as having withdrawn because of death.
gIncludes 1 participant whose AE (pancreatic carcinoma) eventually resulted in death; this participant is also counted under “deaths” and noted in Figure 1 as having withdrawn because of death.
hOne attributed to malignant lung neoplasm and 1 attributed to myocardial infarction.
iOne attributed to brain neoplasm and 1 attributed to pancreatic carcinoma.
jAttributed to cardiac arrest.
Day Regimen
Similar to the month regimen, LRs in the day regimen were more common in the C. difficile groups compared with placebo (Figure 4B), with injection site pain being the most common. Most LRs were mild in severity, although 13 (7.2%) and 11 (6.1%) participants in the 100-μg and 200-μg groups, respectively, reported moderate LRs post-dose 2. Nine participants (3 and 6 in the 100-μg and 200-μg groups, respectively, all post-dose 2) experienced severe LRs; none were grade 4. Similar to the month regimen, LR frequency and severity increased after dose 2, but further increases after dose 3 were not apparent. LRs in the C. difficile groups lasted a median of 1.0 (multiple types of LRs, doses, and dose levels) to 9.0 (100-μg; swelling post-dose 1) days.
Observations regarding SEs were similar to those for the month regimen (Figure 4B). Most events were mild or moderate in severity; 13 participants across groups and doses reported severe SEs. Six fevers were reported across doses (all in the C. difficile groups); 5 were 38.0°C to 38.4°C (mostly 200-μg and mostly post-dose 3), and 1 was 39.0°C (100-μg post-dose 2). SEs in the C. difficile groups had a median duration of 1.0 (multiple types of SEs, doses, and dose levels) to 4.0 (100-μg; joint pain post-dose 2) days.
Adverse events were reported by 31.3% to 41.3% of participants across groups (Table 2); general disorders and administration site conditions was the most common system organ class among the AEs reported. The most common related AEs were injection site hemorrhage, injection site pain, injection site erythema, and injection site pruritus, with remaining related AEs each reported by only 1 or 2 study participants. The 4 related severe AEs were rash (100-μg), injection site macule, injection site rash, and injection site swelling (all 200-μg). Related immediate AEs were reported by 1.1% to 1.6% of participants in the C. difficile groups; these included chest pain, dyspnea, injection site pain (3 events), and injection site bruising. The 6 related AEs that led to withdrawal included 1 case of rash (100-μg; the severe AE noted previously) and 1 case each of injection site rash, injection site swelling, injection site macule (all severe AEs noted previously), dyspnea (the immediate AE noted previously), and pruritic rash (all 200-μg). There were no related SAEs, NDCMCs, or deaths (Table 2). The 3 unrelated deaths that occurred in the day regimen were attributed to cardiac arrest (200-μg), brain neoplasm (100-μg), and pancreatic carcinoma (100-μg).
DISCUSSION
Incidence of CDI has increased recently alongside associated morbidity and mortality, resulting in increased economic burden in the United States and elsewhere [1, 2, 12]. A preventive CDI vaccine or other preventive strategies are necessary to address this ongoing and significant unmet medical challenge, particularly following the recent withdrawal of a late-stage toxoid-based vaccine from phase 3 studies [29] and the delayed development of a recombinant fusion protein vaccine candidate [30].
The candidate vaccine tested in this study was designed to induce high levels of C. difficile toxin neutralizing antibodies and has demonstrably reduced disease in preclinical assessments [27]. The vaccine was immunogenic when administered to healthy US adults aged 65 to 85 years at either 100- or 200-μg dose levels at 0, 1, and 6 months or 1, 8, and 30 days. The month regimen, particularly at the 200-μg dose level, induced immune responses that were more robust and persistent compared with the day regimen. Immune responses as measured by toxin A– or B–neutralizing antibodies peaked at month 7 and day 37 for the month and day regimens, respectively. Compared with the day regimen, immune responses for both dose levels in the month regimen were much higher post-dose 3, particularly for toxin B, and remained elevated above baseline through 12 months post-dose 3 at both dose levels. Baseline seropositivity was associated with greater immune responses, particularly for toxin B.
The vaccine was well tolerated, with low rates of severe LRs and SEs; the observed safety profile supported advancement to a large phase 3 efficacy trial (NCT03090191) [31]. In both regimens, LRs were more common in the C. difficile groups and were dominated by injection site pain. Most LRs were mild or moderate in severity; none were grade 4. LRs increased in frequency after dose 2 in both regimens, particularly for the day regimen 200-μg group; dose 3 was not associated with further increases. SEs were mostly mild to moderate in severity and had similar frequencies across all groups.
There were more related AEs in the C. difficile groups compared with placebo. Although AEs were reported more frequently in the month regimen compared with the day regimen due to the former having 5 additional months of follow-up time, there were more related AEs in the day regimen than in the month regimen. Several AEs that led to withdrawal, some severe, were considered vaccine related; these mostly concerned LRs and were all in day regimen participants. Related immediate AEs similarly occurred mostly in the day regimen and were largely injection site reactions. There were no related SAEs, NDCMCs, or deaths.
Several ongoing and recently completed studies will further evaluate immune persistence, response to a booster, and inform use of this vaccine in Asian populations. Specifically, a study similar to but smaller than the current study (NCT02725437) in older Japanese adults was recently completed [32]. Additionally, an ongoing extension phase of the current study will evaluate immunogenicity of a fourth dose approximately 1 year post-dose 3 and antibody persistence for up to 4 years post-dose 3. An ongoing phase 3 study in >15 000 adults aged ≥50 years worldwide (NCT03090191) will assess vaccine efficacy to prevent primary CDI.
Although there may be practical logistical advantages to the day regimen, the month regimen led to a higher level of sustained neutralizing antibodies and may be more capable of providing sustained protection in adults, whose CDI risk increases with age [3]. This schedule may also facilitate coadministration with other vaccines (eg, zoster) [33], although this has not yet been studied. One study limitation is the lack of information regarding immune responses to delayed doses, as might occur in a real-world situation.
Overall, in this study, we demonstrated encouraging tolerability and immunogenicity, supporting the continued development of this C. difficile vaccine for CDI prevention in older adults. The 200-μg dose level was selected for development in ongoing phase 3 studies [29].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments.The authors thank all of the study participants and study site staff at the participating institutions for their contributions to patient enrollment and data collection. Statistical support was provided by Kevin Yi (Pfizer US) and Wayne Drews (Syneos). Clinical scientist support was provided by Ruth Bailey, Sarah Mirza, and Harpreet Seehra of Pfizer UK.
Data sharing statement.Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Financial support.This work was supported by Pfizer Inc. Medical writing support was provided by Judith Kandel, PhD, of Complete Healthcare Communications, LLC (North Wales, PA), a Complete Healthcare Communications Group company, and was funded by Pfizer Inc. Pfizer was involved in study design, data collection, data analysis, data interpretation, writing of the study report, and the decision to submit the paper for publication.
Potential conflicts of interest.J. P. reports being compensated by Pfizer as a study investigator. N. K., S. A. R., Y. P., W. C. G., K. U. J., M. W. P., A. S. A., C. K., and C. W. are employees of Pfizer Inc and may hold stock and/or stock options. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372:1539–48. [DOI] [PubMed] [Google Scholar]
- 2. Bauer MP, Notermans DW, van Benthem BH, et al. ; ECDIS Study Group Clostridium difficile infection in Europe: a hospital-based survey. Lancet 2011; 377:63–73. [DOI] [PubMed] [Google Scholar]
- 3. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta A, Khanna S. Community-acquired Clostridium difficile infection: an increasing public health threat. Infect Drug Resist 2014; 7:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med 1994; 330:257–62. [DOI] [PubMed] [Google Scholar]
- 6. Alasmari F, Seiler SM, Hink T, Burnham CA, Dubberke ER. Prevalence and risk factors for asymptomatic Clostridium difficile carriage. Clin Infect Dis 2014; 59:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galdys AL, Nelson JS, Shutt KA, et al. Prevalence and duration of asymptomatic Clostridium difficile carriage among healthy subjects in Pittsburgh, Pennsylvania. J Clin Microbiol 2014; 52:2406–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis 1990; 162:678–84. [DOI] [PubMed] [Google Scholar]
- 9. Rea MC, O’Sullivan O, Shanahan F, et al. Clostridium difficile carriage in elderly subjects and associated changes in the intestinal microbiota. J Clin Microbiol 2012; 50:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis 2007; 45:992–8. [DOI] [PubMed] [Google Scholar]
- 11. Barbut F, Cornely O, Fitzpatrick F, et al. Clostridium difficile infection in Europe: a CDI Europe report Available at: http://www.multivu.com/assets/60637/documents/60637-CDI-HCP-Report-original.pdf. Accessed 30 April 2018.
- 12. Choi HY, Park SY, Kim YA, et al. The epidemiology and economic burden of Clostridium difficile infection in Korea. Biomed Res Int 2015; 2015:510386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McFarland LV, Clarridge JE, Beneda HW, Raugi GJ. Fluoroquinolone use and risk factors for Clostridium difficile-associated disease within a Veterans Administration health care system. Clin Infect Dis 2007; 45:1141–51. [DOI] [PubMed] [Google Scholar]
- 14. Furuya-Kanamori L, Stone JC, Clark J, et al. Comorbidities, exposure to medications, and the risk of community-acquired Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2015; 36:132–41. [DOI] [PubMed] [Google Scholar]
- 15. Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis 2014; 11:E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–98; quiz 499. [DOI] [PubMed] [Google Scholar]
- 17. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:987–94. [DOI] [PubMed] [Google Scholar]
- 18. Zinplava™ (bezlotoxumab) injection, for intravenous use. Full Prescribing Information, Whitehouse Station, NJ: Merck & Co., Inc., 2016. [Google Scholar]
- 19. KEGG Drug Database. New drug approvals in the USA, Europe and Japan Available at: http://www.genome.jp/kegg/drug/br08328.html. Accessed 2 February 2018.
- 20. European Medicines Agency. EPAR summary for the public: Zinplava Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/004136/WC500222645.pdf. Accessed 3 May 2018.
- 21. Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142:490–6. [DOI] [PubMed] [Google Scholar]
- 22. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368:407–15. [DOI] [PubMed] [Google Scholar]
- 23. Terveer EM, van Beurden YH, Goorhuis A, et al. How to: establish and run a stool bank. Clin Microbiol Infect 2017; 23:924–30. [DOI] [PubMed] [Google Scholar]
- 24. US Food and Drug Administration, Department of Health and Human Services. Enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies: draft guidance for industry Available at: https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm488223.pdf. Accessed 30 April 2018.
- 25. Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature 2010; 467: 711–3. [DOI] [PubMed] [Google Scholar]
- 26. Sheldon E, Kitchin N, Peng Y, et al. A phase 1, placebo-controlled, randomized study of the safety, tolerability, and immunogenicity of a Clostridium difficile vaccine administered with or without aluminum hydroxide in healthy adults. Vaccine 2016; 34:2082–91. [DOI] [PubMed] [Google Scholar]
- 27. Donald RG, Flint M, Kalyan N, et al. A novel approach to generate a recombinant toxoid vaccine against Clostridium difficile. Microbiology 2013; 159:1254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. US Food and Drug Administration. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/ucm091977. Accessed 18 April 2018.
- 29. Kitchin N, Remich SA, Pride M, Anderson AS, Knirsch C, Webber C. A phase 2, placebo-controlled, randomized, observer-blinded study to evaluate the safety, tolerability, and immunogenicity of two 3 dose regimens of a Clostridium difficile vaccine in healthy adults aged 65 to 85 years, through 12 months post dose 3. In: European Congress of Clinical Microbiology and Infectious Diseases; 21–24 April 2018; Madrid, Spain. [Google Scholar]
- 30. Sanofi. Sanofi ends development of Clostridium difficile vaccine Available at: http://mediaroom.sanofi.com/sanofi-ends-development-of-clostridium-difficile-vaccine/. Accessed 19 April 2018.
- 31. Pfizer Inc. Pfizer announces positive top-line results from phase 2 study of investigational Clostridium difficile vaccine for the prevention of C. difficile infection Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_announces_positive_top_line_results_from_phase_2_study_of_investigational_clostridium_difficile_vaccine_for_the_prevention_of_c_difficile_infection. Accessed 3 May 2018.
- 32. Inoue M, Yonemura T, de Solom R, et al. Safety and immunogenicity of two 3-dose regimens of Clostridium difficile vaccine at 2 antigen dose levels in healthy older Japanese adults: results from a phase 1 study. In: 28th European Congress of Clinical Microbiology and Infectious Diseases; 21–24 April 2018; Madrid, Spain. [Google Scholar]
- 33. US Centers for Disease Control and Prevention. Recommended immunization schedule for adults aged 19 years or older, United States, 2018 Available at: https://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed 13 June 2018.
- 34. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010; 62:197–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.