Abstract
Renal transplant recipients (RTR) commonly suffer from vitamin B6 deficiency and its functional consequences add to an association with poor long-term outcome. It is unknown whether niacin status is affected in RTR and, if so, whether this affects clinical outcomes, as vitamin B6 is a cofactor in nicotinamide biosynthesis. We compared 24-h urinary excretion of N1-methylnicotinamide (N1-MN) as a biomarker of niacin status in RTR with that in healthy controls, in relation to dietary intake of tryptophan and niacin as well as vitamin B6 status, and investigated whether niacin status is associated with the risk of premature all-cause mortality in RTR. In a prospective cohort of 660 stable RTR with a median follow-up of 5.4 (4.7–6.1) years and 275 healthy kidney donors, 24-h urinary excretion of N1-MN was measured with liquid chromatography-tandem mass spectrometry LC-MS/MS. Dietary intake was assessed by food frequency questionnaires. Prospective associations of N1-MN excretion with mortality were investigated by Cox regression analyses. Median N1-MN excretion was 22.0 (15.8–31.8) μmol/day in RTR, compared to 41.1 (31.6–57.2) μmol/day in healthy kidney donors (p < 0.001). This difference was independent of dietary intake of tryptophan (1059 ± 271 and 1089 ± 308 mg/day; p = 0.19), niacin (17.9 ± 5.2 and 19.2 ± 6.2 mg/day; p < 0.001), plasma vitamin B6 (29.0 (17.5–49.5), and 42.0 (29.8–60.3) nmol/L; p < 0.001), respectively. N1-MN excretion was inversely associated with the risk of all-cause mortality in RTR (HR 0.57; 95% CI 0.45–0.71; p < 0.001), independent of potential confounders. RTR excrete less N1-MN in 24-h urine than healthy controls, and our data suggest that this difference cannot be attributed to lower dietary intake of tryptophan and niacin, nor vitamin B6 status. Importantly, lower 24-h urinary excretion of N1-MN is independently associated with a higher risk of premature all-cause mortality in RTR.
Keywords: urinary excretion of N1-methylnicotinamide, kidney transplantation, mortality, niacin status, dietary intake, tryptophan, vitamin B3
1. Introduction
Kidney transplantation is the preferred treatment for end-stage renal disease in terms of survival, quality of life and costs [1,2]. Advances in transplantation medicine have lifted the 1-year patient survival higher than 90% [3]. While short-term patient outcomes are continuing to improve, the long-term posttransplant survival has remained largely unchanged over the past few decades [4]. Compared with the general population, renal transplant recipients (RTR) are at highly increased risk of premature mortality [5]. Improving perspectives relies on the management of modifiable factors that impact long-term outcome in RTR, of which nutrition is increasingly acknowledged [6,7].
Recently, we found that RTR commonly suffer from vitamin B6 deficiency and its functional consequences that add to an association with poor long-term outcomes [8]. As vitamin B6 is an essential cofactor of key enzymes involved in de novo biosynthesis of nicotinamide from tryptophan [9], niacin deficiency might be lurking in these patients as well. Nicotinamide, nicotinic acid, and nicotinamide riboside are collectively referred to as niacin or vitamin B3, and are precursors of the metabolically active NAD+. Besides dietary intake of pre-formed niacin, the so-called tryptophan-nicotinamide pathway is critical to maintaining niacin status [10]. Ongoing NAD+ supply from its metabolic precursors, collectively referred to as “niacin equivalents”, is required to provide reducing equivalents for energy metabolism and substrates of NAD+ consuming enzymes [11]. NAD+ is catabolized to N1-methylnicotinamide (N1-MN) through methylation of nicotinamide in the liver, and the 24-h urinary excretion of N1-MN is considered the most reliable biomarker of niacin status [12,13,14].
It is unknown whether niacin status is affected in RTR and, if so, whether this affects clinical outcomes. Hence, this study aims to compare 24-h urinary excretion of N1-MN in RTR with that in healthy kidney donors, in relation to dietary intake of tryptophan and niacin as well as vitamin B6 status, and to investigate whether niacin status is associated with the risk of premature all-cause mortality in RTR.
2. Materials and Methods
2.1. Study Population
This prospective study was conducted in a well-characterized, single-center cohort of 707 RTR (aged ≥18 years) with a functioning graft for at least 1 year who visited the outpatient clinic of the University Medical Center Groningen, Groningen, the Netherlands, between 2008 and 2011 [15,16,17]. As a control group, 367 healthy kidney donors were included who participated in a screening program before kidney donation. Signed informed consent was obtained from all participating subjects and the study protocol was approved by the institutional review board (METc 2008/186) adhering to the Declaration of Helsinki. Exclusion of subjects with missing biomaterial or niacin supplementation use left 660 RTR and 275 kidney donors eligible for statistical analyses (Figure S1).
2.2. Data Collection
All baseline measurements were obtained during a morning visit to the outpatient clinic. Participants were instructed to collect a 24-h urine sample on the day before their visit, and to fast overnight for 8 to 12 h. Urine samples were collected under oil, and chlorhexidine was added as an antiseptic agent. Fasting blood samples were drawn after completion of the urine collection. Blood was collected in a series of evacuated tubes with different additives (Vacutainer®; BD, Franklin Lakes, NJ, USA) for preparation of plasma and serum. Body composition and hemodynamic parameters were measured according to a previously described, strict protocol [15]. Serum parameters, including lipid, inflammation, and glucose homeostasis variables were measured with spectrophotometric-based routine clinical laboratory methods (Roche Diagnostics, Rotkreuz, Switzerland). Diabetes was diagnosed if fasting plasma glucose was ≥7.0 mmol/L or antidiabetic medication was used [15]. Plasma vitamin B6 was determined as its principal, metabolically active form pyridoxal-5′-phosphate using a HPLC method (Waters Alliance, Milford, MA, USA) with fluorescence detection (JASCO, Inc., Easton, MD, USA) [8].
Renal function was assessed by estimation of the glomerular filtration rate (eGFR) and detection of proteinuria. The eGFR was calculated using the combined creatinine and cystatin C-based Chronic Kidney Disease Epidemiology Collaboration equation [18], which has been shown to be the most accurate equation in RTR [19]. Proteinuria was diagnosed if total urinary protein excretion was ≥0.5 g/day as measured by a biuret reaction-based assay (MEGA AU510; Merck Diagnostica, Darmstadt, Germany).
Dietary intake including tryptophan and niacin intakes was assessed with a validated semi-quantitative food frequency questionnaire (FFQ) [20,21,22]. The self-administered questionnaire was filled out at home and inquired about 177 food items during the last month, taking seasonal variations into account. During the visit to the outpatient clinic, the FFQ was checked for completeness by a trained researcher and inconsistent answers were verified with the participant. The FFQ was validated for RTR as previously reported [16]. Dietary data were converted into daily nutrient intake using the Dutch Food Composition Table of 2006 [23]. Alcohol consumption and smoking behavior were assessed with a separate questionnaire [6]. Additional data on medical history and use of medication and vitamin supplements were obtained from medical records [6].
2.3. Assessment of N1-MN Excretion
Measurement of N1-MN concentration was performed with a validated liquid chromatography (Luna HILIC column; Phenomenex, Torrance, CA, USA) isotope dilution-tandem mass spectrometry (LC-MS/MS) (Quattro Premier; Waters, Milford, MA, USA) method, as described previously [24]. The 24-h urinary excretion of N1-MN (μmol/day) was obtained after multiplying N1-MN concentration (μmol/L) by total urine volume calculated from weight (L/day). The reference range of N1-MN excretion in healthy individuals was previously established at 17.3–115 μmol/day [24].
2.4. Clinical Endpoints
The primary outcome of this study was all-cause mortality which was recorded until 30 September 2015 with no loss due to follow-up. RTR status was kept up-to-date through the continuous surveillance system of the outpatient program.
2.5. Statistical Analysis
Data are presented as the mean ± SD, median (IQR) and absolute number (percentage) for normally distributed, skewed, and nominal data, respectively. Assumptions for normality were checked by visual judgments of the corresponding frequency distribution and Q-Q plot.
Baseline characteristics of RTR and healthy kidney donors were compared by means of t, Mann-Whitney, and Chi-Square tests. Niacin status in RTR and healthy kidney donors was compared by linear regression analyses of 2-base log-transformed N1-MN excretion, with subsequent cumulative adjustment for age and sex (model 1), eGFR (model 2) and intake of energy, tryptophan, and niacin and plasma vitamin B6 (model 3).
RTR characteristics were divided into tertiles of N1-MN excretion stratified by sex (T1, T2, and T3) and compared by means of ANOVA, Kruskal-Wallis, and Chi-Square tests.
For prospective analyses, a Cox proportional hazards regression model for all-cause mortality outcome was fitted to N1-MN excretion as a sex-stratified tertile-based categorical variable, as well as a continuous variable adjusted for sex (model 1). Confounding was controlled for by including potential confounders as covariates in the regression model. Crude associations were adjusted cumulatively for age (model 2), smoking and body surface area (model 3) and, to prevent overfitting, additionally for intake of alcohol and energy and plasma vitamin B6 (model 4), kidney function (model 5), medication use (model 6), and high-sensitivity C-reactive protein (hs-CRP) (model 7). Variables that could lie in the causal pathway of N1-MN excretion and all-cause mortality were not adjusted for because this might obscure otherwise existing associations unintentionally. Assumptions of proportionality of the hazard functions and the linearity of log-hazards were checked by visual judgements of Kaplan Meier plots of the survival and log-survival function entering the sex-stratified N1-MN excretion tertile group variable.
In secondary analyses, effect modification was assessed by including the cross product term of each potential confounder and 2-base log-transformed N1-MN excretion in the Cox regression model adjusted for age and sex (model 2). Subsequent stratified analyses were performed for subgroups of significant effect modifiers on the association of N1-MN excretion with all-cause mortality.
For all statistical analyses, a two-sided p-value of less than 0.05 was considered to indicate statistical significance and SPSS Statistics version 23.0 (IBM, Armonk, NY, USA) was used as software.
3. Results
3.1. Baseline Characteristics and Comparison of N1-MN Excretion
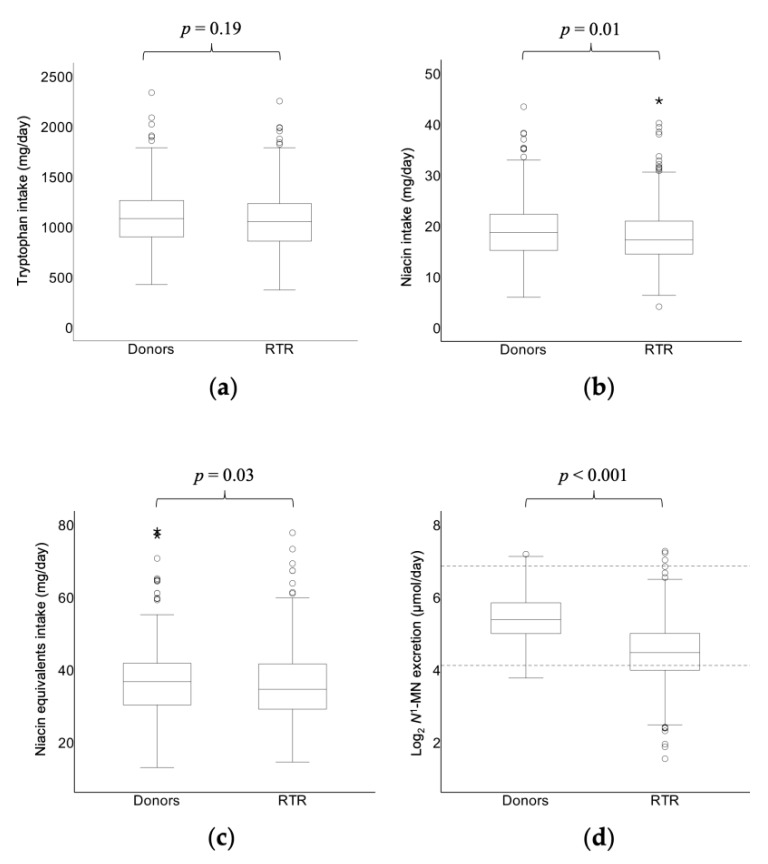
This study included 660 stable RTR (57% male; mean age 53.0 ± 12.7 years), at a median time of 5.6 (2.0–12.0) years after transplantation, and 275 healthy kidney donors (41% male; mean age 53.3 ± 10.7 years) (Table 1). Intake of tryptophan was similar in both groups (1059 ± 271 and 1089 ± 308 mg/day, respectively; p = 0.19), while intake of niacin was lower in RTR than in kidney donors (17.9 ± 5.2 and 19.2 ± 6.2 mg/day, respectively; p = 0.01). Taken together, intake of niacin equivalents was lower in RTR than in kidney donors (35.6 ± 9.2 mg/day and 37.4 ± 10.8, respectively; p = 0.03) (Figure 1). All RTR and kidney donors complied with the recommended daily intake that is set at a minimum of 6.6 niacin equivalents per 1000 kcal (≥ 9.6 and ≥ 11.7 mg/1000 kcal, respectively) [12]. As previously reported, RTR had significantly lower plasma vitamin B6 compared to kidney donors (29.0 (17.5–49.5) and 42.0 (29.8–60.3) nmol/L, respectively; p < 0.001). Median N1-MN excretion was 22.0 (15.8–31.8) μmol/day in RTR, compared to 41.1 (31.6–57.2) μmol/day in kidney donors (p < 0.001) (Figure 1). Furthermore, urinary excretion of N1-MN was below the reference limit of 17.3 μmol/day in 202 (31%) RTR, against 4 (2%) kidney donors. The difference in N1-MN excretion between RTR and kidney donors was independent of age, sex, eGFR, intake of energy, tryptophan, and niacin and plasma vitamin B6 (Table 2). Cyclosporine, azathioprine, and anticonvulsants were used by, respectively, 253 (38%), 112 (17%) of 19 (3%) of RTR, and none of the controls received drugs that are known to potentially affect niacin status.
Table 1.
Baseline characteristics of stable RTR compared to that in healthy kidney donors 1.
| Variable | Donors n = 275 |
RTR n = 660 |
p-Value 2 |
|---|---|---|---|
| Age, years | 53.3 ± 10.7 | 53.0 ± 12.7 | 0.68 |
| Male, n (%) | 112 (41) | 379 (57) | 0.001 |
| Body surface area, m2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.90 |
| Current smoker, n (%) | 39 (14) | 78 (12) | <0.001 |
| Alcohol intake, g/day | 6.7 (1.1–16.4) | 3.1 (0.1–11.9) | <0.001 |
| Energy intake, kcal/day | 2295 ± 746 | 2182 ± 642 | 0.04 |
| Niacin equivalents intake, mg/day 3 | 37.4 ± 10.8 | 35.6 ± 9.2 | 0.03 |
| Tryptophan intake, mg/day | 1089 ± 308 | 1059 ± 271 | 0.19 |
| Niacin intake, mg/day | 19.2 ± 6.2 | 17.9 ± 5.2 | 0.01 |
| N1-MN excretion, μmol/day | 41.4 (31.6–57.2) | 22.0 (15.8–31.8) | <0.001 |
| <17.3 μmol/day, n (%) | 4 (2) | 202 (31) | 0.03 |
| Plasma vitamin B6 (nmol/L) | 42.0 (29.8–60.3) | 29.0 (17.5–49.5) | <0.001 |
| Systolic blood pressure, mmHg | 125.1 ± 13.9 | 135.8 ± 17.3 | <0.001 |
| Diastolic blood pressure, mmHg | 75.6 ± 9.1 | 82.5 ± 11.0 | <0.001 |
| Triglycerides, mmol/L | 1.2 (0.9–1.7) | 1.7 (1.2–2.3) | <0.001 |
| HbA1c, (%) | 5.6 (5.4–5.8) | 5.8 (5.5–6.2) | <0.001 |
| eGFR, ml/min/1.73 m2 | 91.0 ± 14.2 | 53.0 ± 20.0 | <0.001 |
| Acetylsalicylic acid, n (%) | 4 (2) | 127 (19) | <0.001 |
| Proton pump inhibitor, n (%) | 5 (2) | 326 (49) | <0.001 |
| Diuretic, n (%) | 9 (3) | 261 (40) | <0.001 |
1 Data are presented as mean ± SD, median (IQR) and absolute number (percentage) for normally distributed, skewed and nominal data, respectively. 2 p-value for difference was tested by t and Mann-Whitney tests for normally and skewed distributed continuous variables, respectively, and Chi-Square tests for nominal variables. 3 Intake of niacin equivalents was calculated by adding up niacin and one-sixtieth of tryptophan intake. Subjects who were using niacin supplementation were excluded. eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; N1-MN, N1-methylnicotinamide; RTR, renal transplant recipients.
Figure 1.
Box plots of dietary intake of (a) tryptophan, (b) niacin and (c) niacin equivalents and (d) log2 24-h urinary excretion of N1-MN in RTR compared to that in healthy kidney donors. Boxes, bars and whiskers represent IQRs, medians and values <1.5 × IQR, respectively, whereas outliers (1.5–3 × IQR) are indicated by circles and extreme outliers (>3 × IQR) by asterisks. Log2 of the lower and upper bound of the reference range of N1-MN excretion in healthy individuals (17.3–115.0) μmol/day [24] are indicated with dotted lines (d). p-value for difference between RTR and donors was tested by t and Mann-Whitney tests for normally and skewed distributed continuous variables, respectively. Intake of niacin equivalents was calculated by adding up niacin and one-sixtieth of tryptophan intake. N1-MN, N1-methylnicotinamide; RTR, renal transplant recipients.
Table 2.
Association of RTR and healthy kidney donors grouping with N1-MN excretion 1.
| Variable | Model 1 2 | Model 2 3 | Model 3 4 | Model 4 5 | ||||
|---|---|---|---|---|---|---|---|---|
| Std.β | p-Value | Std.β | p-Value | Std.β | p-Value | Std.β | p-Value | |
| Grouping | −0.42 | <0.001 | −0.44 | <0.001 | −0.25 | <0.001 | −0.21 | <0.001 |
| Sex | - | - | −0.15 | <0.001 | −0.14 | <0.001 | −0.10 | 0.002 |
| Age, years | - | - | −0.16 | <0.001 | −0.11 | <0.001 | −0.07 | 0.02 |
| eGFR, ml/min/1.73 m2 | - | - | - | - | 0.31 | <0.001 | 0.29 | <0.001 |
| Energy intake, kcal/day | - | - | - | - | - | - | −0.10 | 0.08 |
| Tryptophan intake, mg/day | - | - | - | - | - | - | 0.007 | 0.91 |
| Niacin intake, mg/day | - | - | - | - | - | - | 0.25 | <0.001 |
| Plasma vitamin B6, nmol/L | - | - | - | - | - | - | 0.23 | <0.001 |
| R2 | 0.18 | 0.23 | 0.28 | 0.37 | ||||
1 Linear regression analyses were performed to investigate the association of RTR and healthy kidney donors as grouping variable with N1-MN excretion, with adjustment for potential confounders. 2 Model 1: crude model. 3 Model 2: adjusted for age and sex. 4 Model 3: adjusted as for model 2 and for eGFR. 5 Model 4: adjusted as for model 3 and for intake of energy, tryptophan and niacin and plasma vitamin B6. eGFR, estimated glomerular filtration rate; N1-MN, N1-methylnicotinamide; RTR, renal transplant recipients; std.β, standardized beta coefficient.
RTR characteristics across tertiles of sex-stratified N1-MN excretion (M: <19.2, 19.2–28.8, >28.8 μmol/day; F: <16.1, 16.1–25.6, >25.6 μmol/day in T1, T2, and T3, respectively) are shown in Table 3. Age and the presence of acetylsalicylic acid, proton pump inhibitors, diuretics and post mortem donors were lower with increasing tertiles of N1-MN excretion, while intake of alcohol, energy, tryptophan and niacin, plasma vitamin B6, kidney function and the presence of proliferation inhibitors and primary glomerular disease were higher with increasing tertiles of N1-MN excretion.
Table 3.
Baseline characteristics of RTR across tertiles of N1-MN excretion stratified by sex 1.
| Variable | Tertiles of Sex-Stratified N1-MN Excretion | p-Value 2 | ||
|---|---|---|---|---|
| T1 n = 219 |
T2 n = 221 |
T3 n = 220 |
||
| Males, μmol/day | <19.2 | 19.2–28.8 | >28.8 | |
| Females, μmol/day | <16.1 | 16.1–25.6 | >25.6 | |
| Male, n (%) | 126 (58) | 127 (58) | 126 (57) | - |
| Age, years | 54.6 ± 12.7 | 53.7 ± 13.1 | 50.7 ± 12.1 | 0.004 |
| BMI, kg/m2 | 25.8 (22.7–29.4) | 26.1 (23.3–29.0) | 26.0 (23.6–29.6) | 0.41 |
| Body surface area, m2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 | 0.13 |
| Lifestyle | ||||
| Current smoker, n (%) | 21 (10) | 25 (11) | 32 (15) | 0.26 |
| Alcohol consumption, g/day | 0.5 (0.0–7.0) | 3.2 (0.1–11.3) | 6.7 (0.8–20.9) | <0.001 |
| Vegetarian, n (%) | 7 (3) | 2 (1) | 3 (1) | 0.16 |
| Dietary intake | ||||
| Energy, kcal/day | 2065 ± 586 | 2197 ± 675 | 2285 ± 647 | 0.002 |
| Tryptophan, mg/day | 1001 ± 253 | 1063 ± 273 | 1112 ± 274 | <0.001 |
| Niacin, mg/day | 16.6 ± 4.9 | 17.6 ± 4.8 | 19.5 ± 5.5 | <0.001 |
| Plasma vitamin B6, nmol/L | 20.3 (14.0–39.0) | 29.5 (19.0–47.0) | 39.0 (22.0–65.0) | <0.001 |
| Hemodynamic | ||||
| Systolic blood pressure, mmHg | 139 ± 18 | 134 ± 18 | 135 ± 16 | 0.01 |
| Diastolic blood pressure, mmHg | 83 ± 11 | 82 ± 12 | 83 ± 11 | 0.20 |
| Mean arterial pressure, mmHg | 109 ± 15 | 106 ± 15 | 106 ± 14 | 0.07 |
| Heart rate, beats per minute | 69 ± 11 | 68 ± 12 | 68 ± 12 | 0.52 |
| Antihypertensive use, n (%) | 199 (91) | 193 (87) | 189 (86) | 0.26 |
| Lipids | ||||
| Total cholesterol, mmol/L | 5.1 ± 1.2 | 5.2 ± 1.1 | 5.0 ± 1.1 | 0.36 |
| HDL, mmol/L | 1.3 (1.0–1.6) | 1.3 (1.1–1.6) | 1.3 (1.1–1.7) | 0.06 |
| LDL, mmol/L | 3.0 ± 0.9 | 3.1 ± 0.9 | 2.9 ± 0.9 | 0.31 |
| Triglycerides, mmol/L | 1.7 (1.3–2.3) | 1.7 (1.3–2.3) | 1.6 (1.1–2.2) | 0.03 |
| Statin, n (%) | 122 (56) | 115 (52) | 112 (51) | 0.55 |
| Glucose homeostasis | ||||
| Glucose, mmol/L | 5.3 (4.8–6.0) | 5.3 (4.8–5.9) | 5.2 (4.7–6.2) | 0.58 |
| HbA1c, (%) | 5.8 (5.5–6.3) | 5.9 (5.6–6.1) | 5.7 (5.4–6.1) | 0.05 |
| Diabetes, n (%) | 58 (27) | 44 (20) | 50 (23) | 0.26 |
| Antidiabetic, n (%) | 41 (19) | 28 (13) | 27 (12) | 0.10 |
| Other serum parameters | ||||
| Hs-CRP, mg/L | 1.7 (0.8–5.3) | 1.6 (0.6–3.8) | 1.4 (0.7–4.6) | 0.42 |
| Phosphate, mmol/L | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.2 | 0.01 |
| Immunosuppressant medication | ||||
| Prednisolon dose, mg/day | 10 (7.5–10) | 10 (7.5–10) | 10 (7.5–10) | 0.18 |
| Calcineurin inhibitor, n (%) | 136 (62) | 125 (57) | 112 (51) | 0.06 |
| Cyclosporine, n (%) | 87 (40) | 82 (37) | 84 (38) | 0.85 |
| Azathioprine, n (%) | 35 (16) | 36 (16) | 41 (19) | 0.72 |
| Proliferation inhibitor, n (%) | 171 (78) | 186 (84) | 191 (87) | 0.04 |
| Other medication | ||||
| Acetylsalicylic acid, n (%) | 55 (25) | 47 (21) | 25 (11) | 0.001 |
| Anticonvulsant, n (%) | 7 (3) | 5 (2) | 7 (3) | 0.80 |
| Proton pump inhibitor, n (%) | 127 (58) | 107 (48) | 92 (42) | 0.003 |
| Diuretic, n (%) | 104 (48) | 79 (36) | 78 (36) | 0.01 |
| Kidney function | ||||
| Serum creatinine, μmol/L | 138 (104–189) | 122 (101–153) | 114 (94–140) | <0.001 |
| Cystatin C, mg/L | 2.0 (1.4–2.8) | 1.6 (1.3–2.1) | 1.4 (1.2–1.9) | <0.001 |
| eGFR, ml/min/1.73 m2 | 39.0 ± 18.7 | 45.8 ± 16.9 | 52.7 ± 18.0 | <0.001 |
| Proteinuria ≥ 0.5 g/day, n (%) | 55 (25) | 39 (18) | 38 (17) | 0.07 |
| Kidney transplantation | ||||
| Time since transplantation, years | 5.6 (1.7–12.9) | 5.0 (1.5–11.0) | 6.5 (2.9–12.3) | 0.16 |
| Donor | ||||
| Age, years | 46 (33–54) | 47 (29–57) | 43 (29–53) | 0.22 |
| Male, n (%) | 104 (48) | 110 (50) | 112 (51) | 0.60 |
| Post mortem status, n (%) | 161 (74) | 143 (65) | 121 (55) | <0.001 |
| Primary kidney disease | ||||
| Primary glomerular disease, n (%) | 48 (22) | 67 (30) | 71 (32) | 0.04 |
| Glomerulonephritis, n (%) | 12 (6) | 17 (8) | 21 (10) | 0.27 |
| Tubulointerstitial disease, n (%) | 27 (12) | 30 (14) | 20 (9) | 0.32 |
| Polycystic renal disease, n (%) | 52 (24) | 45 (20) | 40 (18) | 0.35 |
| Dysplasia and hypoplasia, n (%) | 9 (4) | 10 (5) | 9 (4) | 0.97 |
| Renovascular disease, n (%) | 17 (8) | 8 (4) | 11 (5) | 0.15 |
| Diabetic nephropathy, n (%) | 15 (7) | 7 (3) | 13 (6) | 0.20 |
| Other or unknown cause, n (%) | 39 (18) | 36 (16) | 35 (16) | 0.85 |
1 Data are presented as mean ± SD, median (IQR) and absolute number (percentage) for normally distributed, skewed and nominal data, respectively. 2 p-value for difference was tested by ANOVA and Kruskal-Wallis tests for normally and skewed distributed continuous variables, respectively, and Chi-Square tests for nominal variables. eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; hs-CRP, high-sensitivity C-reactive protein; N1-MN, N1-methylnicotinamide; RTR, renal transplant recipients.
3.2. N1-MN Excretion and Mortality
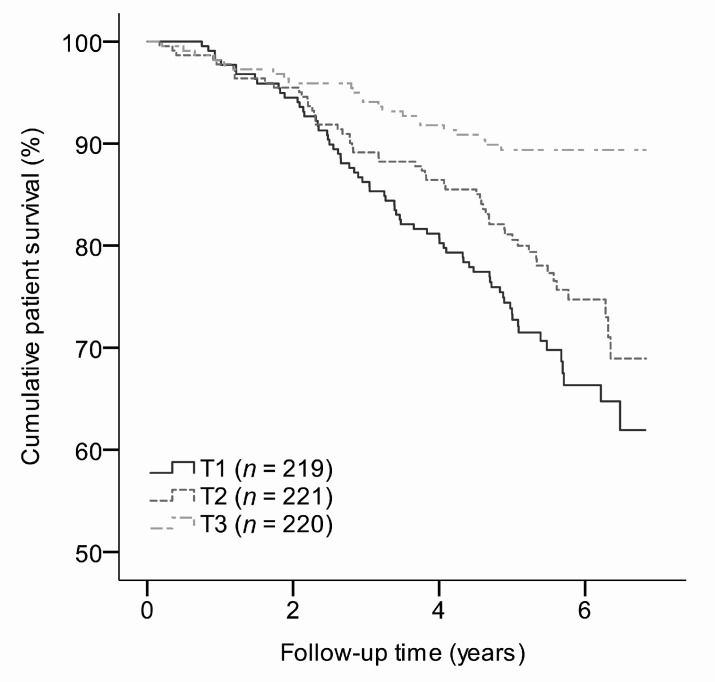
During a median follow-up time of 5.4 (4.7–6.1) years, 143 (22%) RTR died. The risk of all-cause mortality increased with lower tertiles of N1-MN excretion, as depicted by Kaplan-Meier curves (Figure 2). Cox regression analyses revealed an inverse association of N1-MN excretion with all-cause mortality (Model 2: HR 0.57; 95% CI 0.45–0.71; p < 0.001), independent of potential confounders (Table 4). The same held for analyses across tertiles of sex-stratified N1-MN excretion (Table 4). RTR in the lowest and middle tertiles were at higher risk of all-cause mortality compared to those in the highest tertile as reference (Model 2: HR 2.68; 95% CI 1.67–4.33; p < 0.001 and HR 2.04; 95% CI 1.25–3.34; p = 0.004, respectively), independent of potential confounders (Table 4).
Figure 2.
Survival curves for all-cause mortality in RTR according to tertiles of sex-stratified N1-MN excretion. N1-MN excretion was <19.2, 19.2–28.8, and >28.8 μmol/day for males, and <16.1, 16.1–25.6 and >25.6 μmol/day for females in T1, T2, and T3, respectively. N1-MN, N1-methylnicotinamide; RTR, renal transplant recipients.
Table 4.
Association of N1-MN excretion with risk of all-cause mortality in RTR 1.
| Model | N1-MN Excretion (log2) As Continuous Variable n = 660 | Tertiles of Sex-Stratified N1-MN Excretion 2 | |||||
|---|---|---|---|---|---|---|---|
| T1 n = 219 |
T2 n = 221 |
T3 n = 220 |
|||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | Reference HR | |
| 1 3 | 0.53 (0.43–0.65) | <0.001 | 3.28 (2.04–5.26) | <0.001 | 2.41 (1.48–3.93) | <0.001 | 1.00 |
| 2 4 | 0.57 (0.45–0.71) | <0.001 | 2.68 (1.67–4.33) | <0.001 | 2.04 (1.25–3.34) | 0.004 | 1.00 |
| 3 5 | 0.59 (0.47–0.74) | <0.001 | 2.65 (1.60–4.39) | <0.001 | 2.10 (1.25–3.52) | 0.005 | 1.00 |
| 4 6 | 0.69 (0.53–0.90) | 0.005 | 2.10 (1.17–3.78) | 0.01 | 2.04 (1.15–3.63 | 0.02 | 1.00 |
| 5 7 | 0.75 (0.58–0.96) | 0.02 | 1.86 (1.07–3.25) | 0.02 | 1.80 (1.04–3.13) | 0.04 | 1.00 |
| 6 8 | 0.65 (0.51–0.82) | <0.001 | 2.25 (1.35–3.75) | 0.002 | 2.06 (1.23–3.46) | 0.006 | 1.00 |
| 7 9 | 0.60 (0.48–0.76) | <0.001 | 2.59 (1.54–4.35) | <0.001 | 2.13 (1.26–3.61) | 0.005 | 1.00 |
| Events (n) | 143 | 67 | 53 | 23 | |||
1 Cox regression analyses were performed to investigate the association of N1-MN excretion with risk of all-cause mortality in RTR, with adjustment for potential confounders. 2 N1-MN excretion was <19.2, 19. 2–28.8, and >28.8 μmol/day for males, and <16.1, 16.1–25.6, and >25.6 μmol/day for females in T1, T2, and T3, respectively. 3 Model 1: not adjusted in tertiles of sex-stratified N1-MN excretion, adjusted for sex in continuous analyses. 4 Model 2: adjusted as for model 1 and for age. 5 Model 3: adjusted as for model 2 and for smoking and body surface area. 6 Model 4: adjusted as for model 3 and for intake of alcohol and energy and plasma vitamin B6. 7 Model 5: adjusted as for model 3 and for eGFR, proteinuria, donor status and primary glomerular disease. 8 Model 6: adjusted as for model 3 and for use of proliferation inhibitors, acetylsalicylic acid, proton pump inhibitors and diuretics. 9 Model 7: adjusted as for model 3 and for hs-CRP. eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; N1-MN, N1-methylnicotinamide; RTR, renal transplant recipients.
Secondary analyses exposed significant effect modification of hs-CRP on the association of N1-MN excretion with all-cause mortality (p = 0.05), independent of age and sex. The inverse association of N1-MN excretion with all-cause mortality was stronger for individuals in the subgroup with serum hs-CRP <2.4 mg/L (HR 0.47; 95% CI 0.35–0.64; p < 0.001), than in the subgroup with serum hs-CRP ≥2.4 mg/L (HR 0.70; 95% CI 0.50–0.96; p = 0.03) according to subsequent stratified analysis.
4. Discussion
In this large prospective cohort study, we showed that RTR excrete less N1-MN in 24-h urine than healthy controls and our data suggest that this difference cannot be attributed to lower dietary intake of tryptophan and niacin, nor vitamin B6 status. Furthermore, lower 24-h urinary excretion of N1-MN as a biomarker of niacin status was independently associated with a higher risk of premature all-cause mortality in RTR.
To the best of our knowledge, niacin status has not been studied within the context of kidney transplantation and its concomitant long-term implications yet. In fact, prospective data on the urinary excretion of N1-MN have been limited to one previous study in patients recovering from leukemia treatment [25]. Studies on niacin nutrition in relation to prospective outcomes are likewise scarce, as the prevailing intake of niacin equivalents is suggested to be not sufficiently low to compromise survival. Presumed health benefits of niacin are pharmacological rather than physiological [26,27,28,29], although higher survival with higher niacin intake in elderly has been reported previously [30] in congruence with our findings.
Niacin is considered the least critical vitamin to meet the recommendations through dietary intake in western societies [31], as niacin equivalents are found in a wide range of foods [12]. In line with this, dietary intake of niacin equivalents was sufficient according to WHO guidelines in all RTR and healthy kidney donors, while we found that urinary excretion of N1-MN was commonly below the established reference bound in RTR. The observed disparity of N1-MN excretion between RTR and healthy kidney donors could moreover not be explained by lower dietary intake of niacin equivalents in RTR in the present study.
The fact that we found a positive association of plasma vitamin B6 concentration with N1-MN excretion strengthens our hypothesis that inadequacies of this cofactor might affect niacin status in RTR. Adjustment for plasma vitamin B6, however, neither did alter the discrepancy of N1-MN excretion between RTR and healthy kidney donors. Therefore, one should consider other factors that could interfere with N1-MN excretion as a biomarker of niacin status, and add to poor long-term outcome in RTR.
Whereas secondary dietary inadequacies may interrupt niacin metabolism, this also holds for certain medications including specific antituberculosis, anticonvulsant and antiproliferative drugs, as well as cyclosporine and azathioprine [32,33,34], which are common immunosuppressant drugs in RTR, although in our population those did not appear to affect N1-MN excretion.
We can furthermore speculate on the presence of enhanced consumption of tryptophan for protein biosynthesis at the cost of niacin status in RTR. Interestingly, tryptophan is argued to be quantitatively the most important NAD+ precursor, as it is more effective in elevating liver NAD+ and urinary excretion of N1-MN than the salvageable precursors [35,36,37,38]. The tryptophan-nicotinamide pathway is, however, mainly regulated by tryptophan intake rather than niacin status, since the generally accepted conversion ratio of 60:1 falls when dietary tryptophan is limiting [39]. Indeed, tryptophan is used primarily for protein biosynthesis and only after nitrogen balance has been achieved for the nicotinamide pathway [40]. This allows us to speculate on protein catabolism and negative protein balance as part of protein-energy wasting in RTR, engendered by metabolic derangement, systemic inflammation, acidemia, and the use of immunosuppressive drugs, to induce tryptophan consumption for protein synthesis in this population [41,42]. However, as our study was not designed to assess protein-energy wasting, we cannot conclusively address such an effect on N1-MN excretion in RTR.
On the contrary, the tryptophan-nicotinamide pathway is implicated in disease states in which systemic inflammation is present, by the enhanced action of indoleamine 2,3-dioxygenase in response to inflammatory cytokines and mediators. This upregulation of tryptophan degradation towards nicotinamide is known to yield relativity large amounts of quinolinic acid to fuel NAD+-consuming poly (ADP-ribose) polymerase (PARP) reaction in response to immune-related (oxidative) damage [35]. Although we observed lower serum hs-CRP levels as a low-grade inflammation biomarker with higher tertiles of N1-MN excretion, this difference did not reach significance.
Finally, the renal clearance of N1-MN itself can also be affected by several factors and not in the least by impaired kidney function. In fact, N1-MN is eliminated almost exclusively by the kidneys, being partly excreted partly by glomerular filtration and partly by tubular secretion with negligible and saturable tubular reabsorption [43]. Whereas renal clearance of N1-MN has been investigated as a model of renal secretory function [43] and to predict renal clearance of cationic drugs in renal insufficiency [44], plasma concentrations are suggested to be less sensitive to kidney function because of the contribution of aldehyde oxidase to N1-MN clearance, yielding N1-methyl-2-pyridone-5-carboxamide (2Py) [45]. Although our findings appeared independent of kidney function, future studies are warranted to rule out enhanced oxidative metabolism, causing a shift towards urinary excretion of 2Py in this population.
Regarding potential mechanisms for the association of N1-MN excretion with mortality, NAD+ homeostasis has been linked to increased resistance against a range of pathophysiological processes that are predominant and impact poor long-term outcome in RTR, including cardiovascular, inflammatory, malignant and metabolic disorders [46]. The availability of NAD+ is determined by its production from niacin equivalents, as well as its degradation in NAD+ consuming activities [47]. NAD+ levels remain constant when used as a coenzyme, being recycled back and forth between its oxidized and reduced forms [11], but are depleted by three distinct classes of enzymes that consume NAD+ as a substrate: PARP, cyclic ADP ribose synthases (CD38 and CD157), and sirtuins [48]. Excessive activation of PARP and CD38 is induced by stresses such as inflammation, oxidative stress and DNA damage that are predominant in in RTR [48,49]. As a result, NAD+ availability might become limiting for beneficial sirtuin activities; in particular with lower niacin status. These beneficial effects of sirtuins have been described more specifically for renal diseases, including renoprotective effects by inhibition of renal cell apoptosis, inflammation, and fibrosis and regulation of mitochondrial function and glucose, lipid, and energy metabolism [50,51,52,53].
Whereas we did not find an association of N1-MN excretion with hs-CRP, this low-grade inflammation biomarker appeared to affect the magnitude of the inverse association of N1-MN excretion with all-cause mortality. Although we can only speculate on the underlying mechanism, earlier mentioned inflammation-related overconsumption of NAD+ limiting its downstream beneficial activities might at least partly explain the lower protective effect of niacin status on mortality in the subgroup with higher serum hs-CRP levels.
The current study should be interpreted within its strengths and limitations. First, its observational nature prohibits causal inferences, but it also did not allow us to draw conclusions on underlying mechanisms of lower N1-MN excretion in RTR and its contribution to worse survival. Second, the generalizability of our findings might be compromised by overrepresentation of Caucasian individuals from a single center, despite being controlled for by the inclusion of a large, representative control group. Third, the reliability of FFQ data is subject to sources of measurement error, including recall and social desirability biases and limitations in food composition databases [54]. Higher similarity in dietary sources could be achieved by including spouses as a control group. Finally, the present study is confined to the 24-h urinary excretion of N1-MN as the recommended biomarker of niacin nutritional status by authorities, including the WHO and the European Food Safety Authority [12,13,14]. Future studies are, however, encouraged to elaborate on plasma concentrations of niacin and its metabolites, or NAD+ and the ratio of NAD+ to NADP+ in erythrocytes as additional indices of niacin status. Although observational evidence is inherent to limitations, prospective cohort studies provide a strong design to address nutritional status and health outcome associations over a long period of time. Strengths of our study include its large sample size, with a sufficient number of incident cases and no loss to follow-up, and therefore minimizing the risk of bias in the assessment of outcome. The extensive characterization of participants, moreover, allowed us to control for confounding and effect modification in estimates of the effect.
5. Conclusions
In conclusion, 24-h urinary excretion of N1-MN as a biomarker of niacin status is lower in RTR than in healthy controls, and other factors than dietary intake of niacin equivalents and vitamin B6 status appear to reinforce this discrepancy. Importantly, 24-h urinary excretion of N1-MN is inversely associated with a higher risk of premature all-cause mortality in RTR and niacin status is therefore revealed as a potential target for nutritional strategies to improve long-term outcome after kidney transplantation. However, further research is warranted to unravel underlying mechanisms that potentially interfere with N1-MN excretion in RTR, and to strengthen causal inferences for health outcomes to support dietary recommendation.
Acknowledgments
Supported by FrieslandCampina and Danone Nutricia Research. The cohort on which the study was based is registered at clinicaltrials.gov as “TransplantLines Food and Nutrition Biobank and Cohort Study (TxL-FN)” with number NCT02811835.
Supplementary Materials
The following is available online at https://www.mdpi.com/2077-0383/8/11/1948/s1, Figure S1: Flow of participants through study protocol.
Author Contributions
The authors’ responsibilities were as follows—S.J.L.B. and I.P.K.: designed research; M.v.F., A.W.G.-N., J.M.G. and K.J.B.-v.d.B.: provided essential materials; C.P.J.D. and A.v.d.V.: analyzed data; C.P.J.D. and S.J.L.B.: wrote paper and had primary responsibility for final content; A.v.d.V., M.v.F., I.M., A.W.G.-N. and J.M.G.: critically revised the manuscript for important intellectual content; and all authors: read and approved the final manuscript.
Funding
This research was funded by Top Institute Food and Nutrition, grant numbers A-1003 and 16NH01.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Garcia G.G., Harden P., Chapman J., World Kidney Day Steering Committee 2012 The Global Role of Kidney Transplantation. Nephrol. Dial. Transplant. 2013;28:e1–e5. doi: 10.1093/ndt/gfs013. [DOI] [PubMed] [Google Scholar]
- 2.Nerini E., Bruno F., Citterio F., Schena F.P. Nonadherence to Immunosuppressive Therapy in Kidney Transplant Recipients: Can Technology Help? J. Nephrol. 2016;29:627–636. doi: 10.1007/s40620-016-0273-x. [DOI] [PubMed] [Google Scholar]
- 3.Nankivell B.J., Kuypers D.R. Diagnosis and Prevention of Chronic Kidney Allograft Loss. Lancet. 2011;378:1428–1437. doi: 10.1016/S0140-6736(11)60699-5. [DOI] [PubMed] [Google Scholar]
- 4.Tong A., Budde K., Gill J., Josephson M.A., Marson L., Pruett T.L., Reese P.P., Rosenbloom D., Rostaing L., Warrens A.N., et al. Standardized Outcomes in Nephrology-Transplantation: A Global Initiative to Develop a Core Outcome Set for Trials in Kidney Transplantation. Transplant. Direct. 2016;2:e79. doi: 10.1097/TXD.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuberger J.M., Bechstein W.O., Kuypers D.R., Burra P., Citterio F., De Geest S., Duvoux C., Jardine A.G., Kamar N., Kramer B.K., et al. Practical Recommendations for Long-Term Management of Modifiable Risks in Kidney and Liver Transplant Recipients: A Guidance Report and Clinical Checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation. 2017;101:S1–S56. doi: 10.1097/TP.0000000000001651. [DOI] [PubMed] [Google Scholar]
- 6.Eisenga M.F., Kieneker L.M., Soedamah-Muthu S.S., van den Berg E., Deetman P.E., Navis G.J., Gans R.O., Gaillard C.A., Bakker S.J., Joosten M.M. Urinary Potassium Excretion, Renal Ammoniagenesis, and Risk of Graft Failure and Mortality in Renal Transplant Recipients. Am. J. Clin. Nutr. 2016;104:1703–1711. doi: 10.3945/ajcn.116.134056. [DOI] [PubMed] [Google Scholar]
- 7.Sotomayor C.G., Gomes-Neto A.W., Eisenga M.F., Nolte I.M., Anderson J.L.C., de Borst M.H., Oste M.C.J., Rodrigo R., Gans R.O.B., Berger S.P., et al. Consumption of Fruits and Vegetables and Cardiovascular Mortality in Renal Transplant Recipients: A Prospective Cohort Study. Nephrol. Dial. Transplant. 2018:1–9. doi: 10.1093/ndt/gfy248. [DOI] [PubMed] [Google Scholar]
- 8.Minovic I., van der Veen A., van Faassen M., Riphagen I.J., van den Berg E., van der Ley C., Gomes-Neto A.W., Geleijnse J.M., Eggersdorfer M., Navis G.J., et al. Functional Vitamin B-6 Status and Long-Term Mortality in Renal Transplant Recipients. Am. J. Clin. Nutr. 2017;106:1366–1374. doi: 10.3945/ajcn.117.164012. [DOI] [PubMed] [Google Scholar]
- 9.Combs G.F., McClungh J.P. The Vitamins. 5th ed. Academic Press; Cambridge, MA, USA: 2017. Vitamin B6; pp. 350–371. [Google Scholar]
- 10.Fukuwatari T., Shibata K. Nutritional Aspect of Tryptophan Metabolism. Int. J. Tryptophan Res. 2013;6:3–8. doi: 10.4137/IJTR.S11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogan K.L., Brenner C. Nicotinic Acid, Nicotinamide, and Nicotinamide Riboside: A Molecular Evaluation of NAD+ Precursor Vitamins in Human Nutrition. Annu. Rev. Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization and United Nations High Commissions for Refugees . Pellagra and Its Prevention and Control in Major Emergencies. [(accessed on 26 March 2018)]. WHO/NHD/00.10.2000. Available online: https://www.who.int/nutrition/publications/emergencies/WHO_NHD_00.10/en/ [Google Scholar]
- 13.Menon R.M., Gonzalez M.A., Adams M.H., Tolbert D.S., Leu J.H., Cefali E.A. Effect of the Rate of Niacin Administration on the Plasma and Urine Pharmacokinetics of Niacin and its Metabolites. J. Clin. Pharmacol. 2007;47:681–688. doi: 10.1177/0091270007300264. [DOI] [PubMed] [Google Scholar]
- 14.EFSA NDA Panel Scientific Opinion on Dietary Reference Values for Niacin. EFSA J. 2014;12:3759. doi: 10.2903/j.efsa.2014.3759. [DOI] [Google Scholar]
- 15.Van den Berg E., Engberink M.F., Brink E.J., van Baak M.A., Joosten M.M., Gans R.O., Navis G., Bakker S.J. Dietary Acid Load and Metabolic Acidosis in Renal Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2012;7:1811–1818. doi: 10.2215/CJN.04590512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van den Berg E., Engberink M.F., Brink E.J., van Baak M.A., Gans R.O., Navis G., Bakker S.J. Dietary Protein, Blood Pressure and Renal Function in Renal Transplant Recipients. Br. J. Nutr. 2013;109:1463–1470. doi: 10.1017/S0007114512003455. [DOI] [PubMed] [Google Scholar]
- 17.Van den Berg E., Pasch A., Westendorp W.H., Navis G., Brink E.J., Gans R.O., van Goor H., Bakker S.J. Urinary Sulfur Metabolites Associate with a Favorable Cardiovascular Risk Profile and Survival Benefit in Renal Transplant Recipients. J. Am. Soc. Nephrol. 2014;25:1303–1312. doi: 10.1681/ASN.2013050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inker L.A., Schmid C.H., Tighiouart H., Eckfeldt J.H., Feldman H.I., Greene T., Kusek J.W., Manzi J., Van Lente F., Zhang Y.L., et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvador C.L., Hartmann A., Asberg A., Bergan S., Rowe A.D., Morkrid L. Estimating Glomerular Filtration Rate in Kidney Transplant Recipients: Comparing a Novel Equation with Commonly used Equations in this Population. Transplant. Direct. 2017;3:e332. doi: 10.1097/TXD.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feunekes G.I., Van Staveren W.A., De Vries J.H., Burema J., Hautvast J.G. Relative and Biomarker-Based Validity of a Food-Frequency Questionnaire Estimating Intake of Fats and Cholesterol. Am. J. Clin. Nutr. 1993;58:489–496. doi: 10.1093/ajcn/58.4.489. [DOI] [PubMed] [Google Scholar]
- 21.Feunekes I.J., Van Staveren W.A., Graveland F., De Vos J., Burema J. Reproducibility of a Semiquantitative Food Frequency Questionnaire to Assess the Intake of Fats and Cholesterol in the Netherlands. Int. J. Food Sci. Nutr. 1995;46:117–123. doi: 10.3109/09637489509012539. [DOI] [PubMed] [Google Scholar]
- 22.Verkleij-Hagoort A.C., de Vries J.H., Ursem N.T., de Jonge R., Hop W.C., Steegers-Theunissen R.P. Dietary Intake of B-Vitamins in Mothers Born a Child with a Congenital Heart Defect. Eur. J. Nutr. 2006;45:478–486. doi: 10.1007/s00394-006-0622-y. [DOI] [PubMed] [Google Scholar]
- 23.Dutch Nutrient Databank . NEVO Table 2006. Voorlichtingsbureau Voor de Voeding; The Hague, The Netherlands: 2006. [Google Scholar]
- 24.Bouma G., van Faassen M., Kats-Ugurlu G., de Vries E.G., Kema I.P., Walenkamp A.M. Niacin (Vitamin B3) Supplementation in Patients with Serotonin-Producing Neuroendocrine Tumor. Neuroendocrinology. 2016;103:489–494. doi: 10.1159/000440621. [DOI] [PubMed] [Google Scholar]
- 25.Tamulevicius P., Streffer C. N-Methylnicotinamide as a Possible Prognostic Indicator of Recovery from Leukaemia in Patients Treated with Total-Body Irradiation and Bone Marrow Transplants. Strahlentherapie. 1984;160:249–254. [PubMed] [Google Scholar]
- 26.Canner P.L., Berge K.G., Wenger N.K., Stamler J., Friedman L., Prineas R.J., Friedewald W. Fifteen Year Mortality in Coronary Drug Project Patients: Long-Term Benefit with Niacin. J. Am. Coll. Cardiol. 1986;8:1245–1255. doi: 10.1016/S0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 27.Qiao Y.L., Dawsey S.M., Kamangar F., Fan J.H., Abnet C.C., Sun X.D., Johnson L.L., Gail M.H., Dong Z.W., Yu B., et al. Total and Cancer Mortality After Supplementation with Vitamins and Minerals: Follow-Up of the Linxian General Population Nutrition Intervention Trial. J. Natl. Cancer Inst. 2009;101:507–518. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duggal J.K., Singh M., Attri N., Singh P.P., Ahmed N., Pahwa S., Molnar J., Singh S., Khosla S., Arora R. Effect of Niacin Therapy on Cardiovascular Outcomes in Patients with Coronary Artery Disease. J. Cardiovasc. Pharmacol. Ther. 2010;15:158–166. doi: 10.1177/1074248410361337. [DOI] [PubMed] [Google Scholar]
- 29.Schandelmaier S., Briel M., Saccilotto R., Olu K.K., Arpagaus A., Hemkens L.G., Nordmann A.J. Niacin for Primary and Secondary Prevention of Cardiovascular Events. Cochrane Database Syst. Rev. 2017;6:CD009744. doi: 10.1002/14651858.CD009744.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y.C., Lee M.S., Wahlqvist M.L. Prediction of all-Cause Mortality by B Group Vitamin Status in the Elderly. Clin. Nutr. 2012;31:191–198. doi: 10.1016/j.clnu.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Troesch B., Hoeft B., McBurney M., Eggersdorfer M., Weber P. Dietary Surveys Indicate Vitamin Intakes below Recommendations are Common in Representative Western Countries. Br. J. Nutr. 2012;108:692–698. doi: 10.1017/S0007114512001808. [DOI] [PubMed] [Google Scholar]
- 32.Hegyi J., Schwartz R.A., Hegyi V. Pellagra: Dermatitis, Dementia, and Diarrhea. Int. J. Dermatol. 2004;43:1–5. doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- 33.Li R., Yu K., Wang Q., Wang L., Mao J., Qian J. Pellagra Secondary to Medication and Alcoholism: A Case Report and Review of the Literature. Nutr. Clin. Pract. 2016;31:785–789. doi: 10.1177/0884533616660991. [DOI] [PubMed] [Google Scholar]
- 34.Muller F., Sharma A., Konig J., Fromm M.F. Biomarkers for in Vivo Assessment of Transporter Function. Pharmacol. Rev. 2018;70:246–277. doi: 10.1124/pr.116.013326. [DOI] [PubMed] [Google Scholar]
- 35.Badawy A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017;10:1178646917691938. doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bender D.A., Magboul B.I., Wynick D. Probable Mechanisms of Regulation of the Utilization of Dietary Tryptophan, Nicotinamide and Nicotinic Acid as Precursors of Nicotinamide Nucleotides in the Rat. Br. J. Nutr. 1982;48:119–127. doi: 10.1079/BJN19820094. [DOI] [PubMed] [Google Scholar]
- 37.McCreanor G.M., Bender D.A. The Metabolism of High Intakes of Tryptophan, Nicotinamide and Nicotinic Acid in the Rat. Br. J. Nutr. 1986;56:577–586. doi: 10.1079/BJN19860138. [DOI] [PubMed] [Google Scholar]
- 38.Williams J.N., Jr., Feigelson P., Elvehjem C.A. Relation of Tryptophan and Niacin to Pyridine Nucleotides of Tissue. J. Biol. Chem. 1950;187:597–604. [PubMed] [Google Scholar]
- 39.Kirkland J.B. Niacin Status, NAD Distribution and ADP-Ribose Metabolism. Curr. Pharm. Des. 2009;15:3–11. doi: 10.2174/138161209787185823. [DOI] [PubMed] [Google Scholar]
- 40.Shibata K., Matsuo H. Effect of Dietary Tryptophan Levels on the Urinary Excretion of Nicotinamide and its Metabolites in Rats Fed a Niacin-Free Diet Or a Constant Total Protein Level. J. Nutr. 1990;120:1191–1197. doi: 10.1093/jn/120.10.1191. [DOI] [PubMed] [Google Scholar]
- 41.Teplan V., Valkovsky I., Teplan V., Jr., Stollova M., Vyhnanek F., Andel M. Nutritional Consequences of Renal Transplantation. J. Ren. Nutr. 2009;19:95–100. doi: 10.1053/j.jrn.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 42.Ter Wee P.M. Protein Energy Wasting and Transplantation. J. Ren. Nutr. 2013;23:246–249. doi: 10.1053/j.jrn.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Nasseri K., Daley-Yates P.T. A Comparison of N-1-Methylnicotinamide Clearance with 5 Other Markers of Renal Function in Models of Acute and Chronic Renal Failure. Toxicol. Lett. 1990;53:243–245. doi: 10.1016/0378-4274(90)90138-C. [DOI] [PubMed] [Google Scholar]
- 44.Maiza A., Daley-Yates P.T. Estimation of the Renal Clearance of Drugs using Endogenous N-1-Methylnicotinamide. Toxicol. Lett. 1990;53:231–235. doi: 10.1016/0378-4274(90)90135-9. [DOI] [PubMed] [Google Scholar]
- 45.Maiza A., Waldek S., Ballardie F.W., Daley-Yates P.T. Estimation of Renal Tubular Secretion in Man, in Health and Disease, using Endogenous N-1-Methylnicotinamide. Nephron. 1992;60:12–16. doi: 10.1159/000186698. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y., Sauve A.A. NAD (+) Metabolism: Bioenergetics, Signaling and Manipulation for Therapy. Biochim. Biophys. Acta. 2016;1864:1787–1800. doi: 10.1016/j.bbapap.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein L.R., Imai S. The Dynamic Regulation of NAD Metabolism in Mitochondria. Trends Endocrinol. Metab. 2012;23:420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chini C.C.S., Tarrago M.G., Chini E.N. NAD and the Aging Process: Role in Life, Death and Everything in Between. Mol. Cell. Endocrinol. 2017;455:62–74. doi: 10.1016/j.mce.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canto C., Menzies K.J., Auwerx J. NAD (+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell. Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao C.M., Haase V.H. Sirtuins and their Relevance to the Kidney. J. Am. Soc. Nephrol. 2010;21:1620–1627. doi: 10.1681/ASN.2010010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitada M., Kume S., Takeda-Watanabe A., Kanasaki K., Koya D. Sirtuins and Renal Diseases: Relationship with Aging and Diabetic Nephropathy. Clin. Sci. 2013;124:153–164. doi: 10.1042/CS20120190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wakino S., Hasegawa K., Itoh H. Sirtuin and Metabolic Kidney Disease. Kidney Int. 2015;88:691–698. doi: 10.1038/ki.2015.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong Y.J., Liu N., Xiao Z., Sun T., Wu S.H., Sun W.X., Xu Z.G., Yuan H. Renal Protective Effect of Sirtuin 1. J. Diabetes Res. 2014;2014:843786. doi: 10.1155/2014/843786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naska A., Lagiou A., Lagiou P. Dietary Assessment Methods in Epidemiological Research: Current State of the Art and Future Prospects. F1000Research. 2017;6:926. doi: 10.12688/f1000research.10703.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.