Abstract
Porcine circovirus 3 (PCV‐3) prevalence has been minimally investigated in wild boar; dynamics of infection and viral tissue distribution are currently unknown. In this study, serum samples from 518 wild boar (from years 2004 to 2018) were used to study frequency of infection. Also, serum samples from 19 boar captured and recaptured at least two times for a period of time from 1 month to 1 year were collected to determine PCV‐3 infection dynamics. Finally, to elucidate PCV‐3 DNA organic distribution, sera, different tissues and faeces were obtained from 35 additional wild boar. PCV‐3 DNA was extracted and amplified with a conventional PCR. For the PCV‐3 PCR‐positive sera from the longitudinally sampled and different tissue types, a quantitative PCR was performed. Genome sequence was obtained from a number of PCV‐3 PCR‐positive samples from different years, different time‐points of infection and tissues. Obtained results confirmed the susceptibility of wild boar to the virus, showing high frequency of PCV‐3 detection (221 out of 518, 42.66%) and demonstrating circulation at least since 2004. Compiled data indicate the possibility of long‐term infections, since 5 out of 10 PCV‐3 PCR‐positive boars longitudinally sampled showed positivity in samplings separated for more than 5 months. All tested tissue types’ harboured PCV‐3 genome, with the highest percentage of PCR positivity in submandibular lymph node, tonsil, lung, liver, spleen and kidney. The amount of DNA in all tested PCV‐3 PCR‐positive samples was moderate to low. All partial and complete PCV‐3 sequences obtained from wild boar displayed high nucleotide identity, higher than 98%. In conclusion, this study further confirms that wild boar is susceptible to PCV‐3 infection, showing high frequency of detection in this animal species. Furthermore, PCV‐3 can be found in different tissues of wild boar and is apparently able to cause persistent infection.
Keywords: infection dynamics, Porcine circovirus 3, prevalence, retrospective, tissue distribution, wild boar
1. INTRODUCTION
Porcine circovirus 3 (PCV‐3), a recently described virus belonging to the family Circoviridae, is the third member of genus Circovirus able to infect swine (International Committee for the Taxonomy of Viruses, ICTV, [Link]‐ https://talk.ictvonline.org/). Members of this family are icosahedral, single‐stranded DNA (ssDNA) viruses with a circular, ambisense genome that contains two major opening reading frames (ORFs). ORFs are oriented in the opposite strands encoding for specifically viral capsid and replicase proteins (Biagini et al., 2012; Ritchie et al., 1990).
Porcine circovirus 3 was firstly identified in the USA (Palinski et al., 2017; Phan et al., 2016) and the presence of viral genome was detected subsequently in different continents such as Asia (Ku et al., 2017; Kwon, Yoo, Park, & Lyoo, 2017; Shen et al., 2017), Europe (Faccini et al., 2017; Franzo, Legnardi, Hjulsager et al., 2018; Stadejek, Woźniak, Miłek, & Biernacka, 2017) and South America (Tochetto et al., 2017). This infectious agent has been found in serum and/or tissues of domestic pigs affected by several clinical–pathological presentations including respiratory disorders, reproductive failure, myocarditis, porcine dermatitis and nephropathy syndrome (PDNS) and congenital tremors (Chen et al., 2017; Ku et al., 2017; Palinski et al., 2017; Phan et al., 2016). Moreover, PCV‐3 genome has also been detected in asymptomatic animals (Franzo, Legnardi, Tucciarone et al., 2018; Zhai et al., 2017; Zheng et al., 2017). In addition, retrospective studies revealed the circulation of PCV‐3 at least since 1993 in Sweden (Ye, Berg, Fossum, Wallgren, & Blomström, 2018) and 1996 in both Spain (Klaumann et al., 2018) and China (Sun et al., 2018). Phylogenetic studies have proposed the existence of a most common ancestor dated around 50 years ago (Fu et al., 2017; Saraiva et al., 2018) and the available sequences revealed a high nucleotide identity between strains (Zheng et al., 2017). Nevertheless, phylogenetic analysis also revealed two main groups of PCV‐3 strains and several subclusters (Fux et al., 2018). Very recently, the genome has been also detected in sera from wild boar (Franzo, Tucciarone, et al., 2018).
Wild boar (Sus scrofa scrofa) are susceptible to several pathogens with potential for transmission to humans and animals (Meng, Lindsay, & Sriranganathan, 2009). In fact, many viral diseases present in domestic pigs can also affect boars and these animals may act as a disease reservoir (Ruiz‐Fons, Segalés, & Gortázar, 2008). In the last decades, many European countries have experienced an increase of the wild boar population in forested and urban areas as a result of the ability of the wild boar to adapt to different environments, the high prolificacy and increased contact with humans (Castillo‐Contreras et al., 2018; Fernández‐Aguilar et al., 2018). In consequence, the risk of potential disease transmission between wild boar population and domestic pigs is not negligible.
The present work had a threefold objective. First, the frequency of detection of PCV‐3 in a large wild boar population of Catalonia (Spain) was tested retrospectively from 2004 to 2018. The second aim consisted of exploring the long‐term dynamics of the virus in captured and recaptured wild boars. Finally, a set of captured, necropsied wild boar was used to study the tissue distribution of PCV‐3.
2. MATERIAL AND METHODS
2.1. Sampling designs
2.1.1. Retrospective study
Serum samples (n = 518) were collected from resident wild boars of 33 counties in Catalonia (Northeastern Spain) between 2004 and 2018 (Figure 1). Blood was obtained by heart puncture from animals hunted during the hunting season and within the framework of the official wildlife diseases surveillance scheme or captured and euthanized for management purposes. Blood samples were centrifuged at 1,500 g for 15 min and obtained sera were stored at −20°C until processing. The number of available sera obtained per year ranged from 3 to 18 between years 2007 and 2012, and from 30 to 88 between years 2013 and 2018 (Table 1). According to the age classification based on the tooth eruption patterns described by Buruaga, Lucio, and Purroy (2001), wild boar were classified as juveniles (less than 12 months), subadults (between 12 and 24 months) and adults (over 24 months). The gender of the animals was also recorded.
Figure 1.
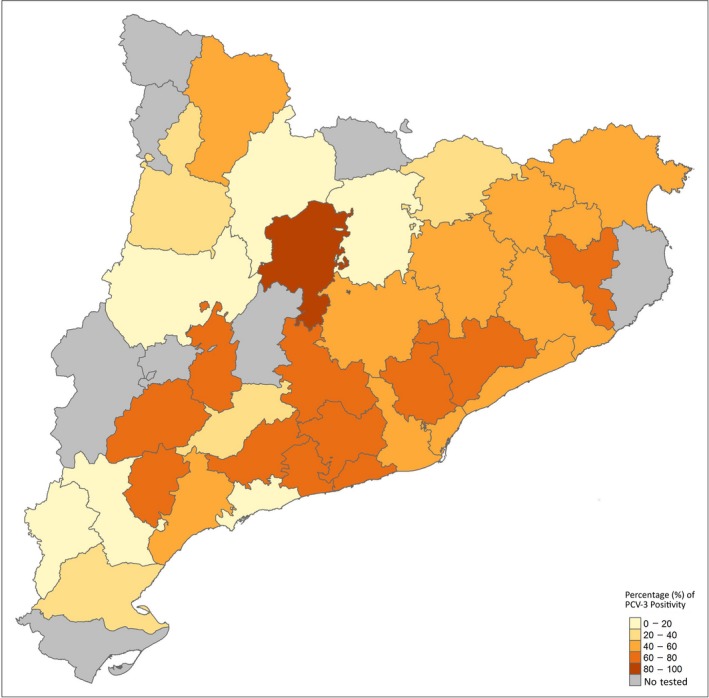
Distribution of the tested wild boar in Catalonia (Spain) of PCV‐3 PCR‐positive animals according to each county. The darker the colour intensity, the higher PCV‐3 frequency detection by PCR. PCV‐3: Porcine circovirus 3 [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Number of examined wild boar and those with Porcine circovirus 3 (PCV‐3) PCR‐positive results according to the tested year
| Year | Number of examined cases (n) | PCV‐3 PCR‐positive cases (n) | Percentage (%) |
|---|---|---|---|
| 2004 | 30 | 3 | 10.00 |
| 2005 | 50 | 2 | 4.00 |
| 2006 | 46 | 11 | 23.91 |
| 2007 | 18 | 6 | 33.33 |
| 2008 | 17 | 5 | 29.41 |
| 2009 | 4 | 2 | 50.00 |
| 2010 | 10 | 6 | 60.00 |
| 2011 | 12 | 7 | 58.33 |
| 2012 | 3 | 3 | 100.00 |
| 2013 | 40 | 31 | 77.50 |
| 2014 | 50 | 33 | 66.00 |
| 2015 | 50 | 28 | 56.00 |
| 2016 | 50 | 4 | 8.00 |
| 2017 | 88 | 63 | 71.59 |
| 2018 | 50 | 17 | 34.00 |
| Total | 518 | 221 | 42.66 |
2.1.2. Longitudinal study
Nineteen boars from the metropolitan area of Barcelona (Northeastern Spain) were captured and recaptured at least two times (maximum of six times) for a period varying from 1 month to 1 year (Table 2). Blood was collected from the cranial cava vein into sterile tubes, centrifuged at 1,500 g for 15 min and obtained serum stored at −20°C until further analysis. Age group and gender were also recorded.
Table 2.
Porcine circovirus 3 (PCV‐3) PCR results and their respective amount of viral DNA in log10/μl (in positive cases) in serum of wild boars longitudinally sampled according to the tested month and age group at first sampling (juvenile/subadult, Ju./Sa., <2 years; Adult, >2 years). Pos: qPCR positive but under quantification limit (<101 PCV‐3 DNA copies/μl); Neg: negative PCR result. In bold, those animals with a positive result (quantifiable or not). Underlined viral loads corresponded to those animals from which partial sequences were obtained
| Animal no. | Age group at first sampling | Gender | 2017 | 2018 | No total of PCV‐3 positive | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| April | May | June | July | Aug. | Sept. | Oct. | Nov. | Dec. | Jan. | Feb. | March | April | May | ||||
| 1 | Ju./Sa. | Female | Neg. | Neg. | Neg. | 10 1.3 | Pos. | Neg. | 2/6 | ||||||||
| 2 | Ju./Sa. | Male | 10 2.39 | 10 2.17 | 10 1.53 | 10 1.1 | 10 1.28 | 5/5 | |||||||||
| 3 | Ju./Sa. | Male | 10 3 | 10 1.3 | 10 1.3 | 3/3 | |||||||||||
| 4 | Ju./Sa. | Female | 10 3.06 | Neg. | 1/2 | ||||||||||||
| 5 | Adult | Male | Neg. | 10 1.3 | 1/2 | ||||||||||||
| 6 | Ju./Sa. | Female | 10 1.62 /10 2 a | 10 1.3 | 3/3 | ||||||||||||
| 7 | Adult | Female | Neg. | 10 1.3 | 1/2 | ||||||||||||
| 8 | Adult | Female | Neg. | Neg. | 0/2 | ||||||||||||
| 9 | Ju./Sa. | Male | Neg.b | 0/1 | |||||||||||||
| 10 | Adult | Female | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. | 0/6 | ||||||||
| 11 | Adult | Female | Neg. | Neg. | 0/2 | ||||||||||||
| 12 | Ju./Sa. | Female | 10 1.8 | 10 1.22 | Neg. | Neg. | 2/4 | ||||||||||
| 13 | Ju./Sa. | Female | Neg. | Neg. | Neg. | Neg. | Neg. | 0/5 | |||||||||
| 14 | Ju./Sa. | Male | 10 3.1 | 10 2,75 | 10 1.56 | 10 1.56 | 4/4 | ||||||||||
| 15 | Adult | Female | Neg. | Neg. | 0/2 | ||||||||||||
| 16 | Ju./Sa. | Female | Neg. | Neg. | Neg. | 0/3 | |||||||||||
| 17 | Ju./Sa. | Male | Neg. | Neg. | 0/2 | ||||||||||||
| 18 | Ju./Sa. | Female | Pos. | Pos. | 2/2 | ||||||||||||
| 19 | Ju./Sa. | Male | Neg. | Neg. | 0/2 | ||||||||||||
aAnimal longitudinally sampled twice in April, 2017. PCV‐3‐PCR was positive in both samplings. bAnimal longitudinally sampled twice in May, 2017. PCV‐3 PCR was negative in both tested samples.
2.1.3. Study on tissues, faeces and serum
Thirty‐five wild boar captured and euthanized for management purposes in Catalonia (Northeastern Spain) were selected for this study. Sera samples from 28 out of 35 selected animals were available as well as 33 faecal samples. Seven different tissue types were collected. Tonsil, liver, lung, spleen, kidney and brain were analysed from all boars selected for this study, while submandibular lymph nodes were only available from 30 wild boar (Table 3).
Table 3.
Number of tested samples (serum and tissues) and their PCV‐3 PCR result and percentage of positives
| Samples | No of tested samples (n) | No of PCV‐3 PCR‐positive samples | Percentage (%) |
|---|---|---|---|
| Sera | 28 | 5 | 17.86a |
| Faeces | 33 | 3 | 9.09a |
| Brain | 35 | 10 | 28.57a |
| Kidney | 35 | 10 | 28.57a |
| Liver | 35 | 19 | 54.29a |
| Lung | 35 | 20 | 57.14b |
| Submandibular lymph node | 30 | 9 | 30a |
| Spleen | 35 | 19 | 54.29b |
| Tonsil | 35 | 15 | 42.86b |
Different letters in superscript mean statistically significant differences (p < 0.05) among different sample types tested.
2.2. DNA extraction
DNA was extracted from 200 μl of serum using MagMAx™ Pathogen RNA/DNA Kit (Applied Biosystems®) according to the manufacturer's protocol. For faecal samples, DNA was extracted from 200 mg of faeces with QIAmp DNA Stool Mini Kit (QIAGEN®). Finally, approximately 1 cm3 of tissues (corresponding to 180–200 mg) were diluted in 1 ml of sterile phosphatase‐buffered saline (PBS, pH 7.4), and then homogenized with the TissueLyser II (QIAGEN®) for 30 min at 3,500 g, DNA from the homogenized tissue was extracted according to the same protocol described for serum samples.
2.3. Conventional and quantitative PCRs to detect PCV‐3
All primers and probes used in this study are included in Table 4.
Table 4.
Primers and probes implemented in the conventional PCR, quantitative PCR (qPCR), and PCRs for the partial/complete genome sequencing used in this study
| Primers and/or probes | Start position | Sequence 5′‐3′ | Assay | Reference | |
|---|---|---|---|---|---|
| PCV3233F | 233 | 5′‐AAAGCCCGAAACACAGGTGGTGT‐3′ | Conventional PCR | Franzo, Legnardi, Centelleghe et al. (2018) | |
| PCV3718R | 718 | 5′‐TTTTCCCGCATCCTGGAGGACCAAT‐3′ | |||
| PCV3353F | 353 | 5′‐TGACGGAGACGTCGGGAAAT‐3′ |

|
qPCR | Franzo, Legnardi, Centelleghe et al. (2018) |
| PCV3465R | 465 | 5′‐CGGTTTACCCAACCCCATCA‐3′ | |||
| Probe_qPCR | 418 | 5′‐FAM‐GGGCGGGGTTTGCGTGATTT‐BHQ1‐3′ | |||
| PCV3506F_IC | 506 | 5′‐TCCTGGGCAATAAGATGGAG‐3′ |

|
This manuscript | |
| PCV3661R_IC | 661 | 5′‐TGGGGGTATTCTGCTGGTAG‐3′ | |||
| Probe_IC | 528 | 5′‐VIC‐CCACTACAACGCCCATG‐MGBNFQ‐3′ | |||
| PCV74F | 74 | 5′‐CACCGTGTGAGTGGATATAC‐3′ | Conventional PCR‐ Partial and Complete genomes | Fux et al. (2018) | |
| PCV31444R | 1,444 | 5′‐CACCCCAACGCAATAATTGTA‐3′ | |||
| PCV31137F | 1,137 | 5′‐TTGGGGTGGGGGTATTTATT‐3′ | |||
| PCV31561R | 1,561 | 5′‐ACACAGCCGTTACTTCAC‐3′ | |||
| PCV31427F | 1,427 | 5′‐AGTGCTCCCCATTGAACG‐3′ | |||
| PCV3433R | 433 | 5′‐CGACCAAATCCGGGTAAGC‐3′ |
To detect the presence of PCV‐3 DNA in the tested samples, a conventional PCR assay was performed based on a previous protocol described by Franzo, Legnardi, Centelleghe et al. 2018, with slight modifications. Three microlitres of extracted DNA was added to a PCR mix and amplified using the described thermal protocol. The reaction was carried out in a final volume of 50 μl mixture containing 5× PCR buffer, 10 pmol of dNTPs, 10 pmol of each primer, 1 Units of DNA polymerase Platinum™ SuperFi™ (Invitrogen™) and water to bring the final volume up to 50 μl. The PCR products were checked on 1.2% TAE agarose gel.
A previously published quantitative PCR (qPCR) protocol (Franzo, Legnardi, Centelleghe et al., 2018 with slight modifications was performed on all positive samples tested by conventional PCR from different tissue types and animals longitudinally sampled. Reactions were carried out with an Applied Biosystems® 7500 Real‐Time machine. Briefly, 2 μl of extracted DNA was added to a standard mixture containing 1× Quantitect Probe PCR mix (QIAGEN®), 0.6 pmol of each primer and 0.3 pmol probe, 1 pg of the internal control (IC) plasmid vector pAcGFP1‐1 (Takara‐Clontech®), 0.4 and 0.2 pmol of IC primers and probe, respectively, and sterile water to bring the final volume up to 20 μl. The thermal protocol included 95°C for 15 min followed by 45 cycles of 95°C for 10 s and 60°C for 1 min. Viral concentrations were expressed as mean of log10 PCV‐3 genome copies/μl and the limit of quantification were considered at least 10 copies of DNA per μl.
2.4. PCV‐3 sequencing and phylogenetic studies
For genome sequencing, 3 μl of the extracted DNA was added to the PCR mixture described above for the conventional PCV‐3 PCR, using the thermal protocol and three specific primer pairs (Table 4) able to detect three amplicons described by Fux et al. (2018). The PCV‐3 PCR‐positive samples were purified using NucleoSpin® Gel and PCR Clean‐up (Macherey‐Nagel) according to the manufacturer's protocols and the quality and quantity of genomic DNA were analysed with BioDrop DUO (BioDrop Ltd).
The selected samples were submitted to Sanger sequencing, which was performed with BigDye® Terminator v3.1 Cycle Sequencing Kit, following the manufacturer's protocol at the Genomic and Bioinformatics Service of the Universitat Autònoma de Barcelona (Barcelona, Spain). The sequencing reactions were analysed using an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystem®).
Sequences and chromatograms were manually explored to trim bad‐quality bases with BioEdit 7.2 (Hall, 1999). The assembly of the consensus sequences extracted from different fragments was done with DNASTAR Lasergene software (Burland, 1999). Both complete and partial genomes obtained were aligned using Clustal Omega (Thompson, Gibson, Plewniak, Jeanmougin, & Higgis, 1997). A collection of reference sequences available at the GenBank were included in the analysis (Supporting Information Table S1). Pairwise identity matrices were obtained using BioEdit software. Phylogenetic analysis was performed with MEGA software vs 7 (Kumar, Stecher, & Tamura, 2016) with the maximum‐likelihood (ML) method based on the best predicted‐model (lowest BIC score), i.e. Tamura‐Nei substitution model (Tamura & Nei, 1993) using Robustness of the ML tree was evaluated by analysis with 1,000 bootstrap replicates.
In total, 24 samples were selected to obtain partial and complete PCV‐3 genome sequences. Twelve complete sequences were obtained from animals corresponding to different years of the retrospective study. Partial sequences were obtained from five animals longitudinally sampled in two and/or three different time‐points (n = 9) and from different tissue samples (n = 3) of one wild boar. The sequences obtained throughout this study are available at the GenBank (accession numbers MH579736‐MH579747 for complete sequences of the retrospective study, MH751283‐MH751287 and MH751293‐MH751296 for partial sequences of the longitudinal study, and MH751289‐ MH751291 for partial sequences from tissues).
2.5. Statistical analyses
Statistical analyses were performed using the R software (http://www.r-project.org/). Shapiro Wilk's test was used to evaluate the normality of the distribution of the quantitative variables.
Differences over the years were analysed by the Pearson's Chi‐squared test (χ2) in the retrospective study; for such comparison, a subdivision of five groups containing three tested years each one was created. To assess the association between age groups, the same test was implemented. To test differences between gender and the PCV‐3 PCR positivity between the tested counties, the Fisher's exact test was performed.
For the purpose to test the differences between the PCV‐3 PCR positivity frequency in tissues, the Pearson's Chi‐squared test (χ2) was carried out. p‐Values lower than 0.05 were considered to be statistically significant.
3. RESULTS
3.1. PCV‐3 detection by PCR and quantification by qPCR
3.1.1. Retrospective study
Porcine circovirus 3 was found in wild boar of all counties studied (Figure 1). No significant association was found between county abundance of wild boar and frequency of PCV‐3 detection (Figure 2).
Figure 2.
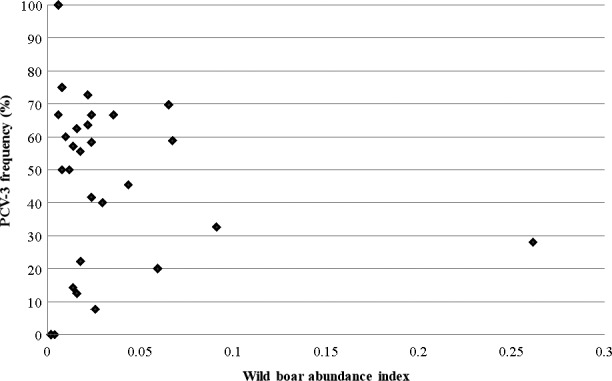
Wild boar abundance in the different counties according to PCV‐3 frequency of detection by PCR. The wild boar abundance is based in the relative density index (RDI). RDI is calculated by dividing the number of wild boars hunted between the geographical surface of hunting and the number of hunting beaters. PCV‐3: Porcine circovirus 3
The first PCV‐3 PCR‐positive sample was detected in the first year of testing (2004) and subsequently PCV‐3 genome was found in all examined years (Table 1). In total, 221 out of 518 (42.66%) serum samples were PCR‐positive for PCV‐3 and the percentage of PCV‐3 positivity ranged from 4% (2 out of 50) in 2005 to 100% in 2012 (in which only three samples were tested). Significant differences were observed across the tested year triads, with higher frequencies of PCV‐3 PCR positivity found in both periods 2013–15 and 2016–18 (p < 0.05) compared to the previous ones. The frequency of PCV‐3 genome detection in wild boar was significantly higher in adults than in was subadults or juveniles (adults 47.5%, 152 out of 320; subadults 25.27%, 23 out of 91; juveniles 8.69%, 2 out of 23) (p < 0.05). PCV‐3 positivity was found in 111 out of 253 tested females and in 90 out of 212 males; no significant difference of PCV‐3 frequency was found between genders.
3.1.2. Longitudinal study
PCR and qPCR results are summarized in Table 2.
Porcine circovirus 3 PCR positivity was found in 10 out of 19 longitudinally tested wild boar (52.63%). Five of these animals (i.e., No. 2, 3, 6, 14 and 18) were PCV‐3 PCR‐positive at all samplings performed throughout the study period. Globally, three animals were positive for PCV‐3 in only one sampling (No. 4, 5 and 7), while the rest of wild boar were positive in at least two samplings separated by 2 (n = 2, No. 6 and 12), 5 (n = 2, No. 3 and 18), 6 (n = 1, No. 14) or 7 months (n = 2, No. 1 and 2).
The amount of PCV‐3 DNA obtained through qPCR was low to moderate, ranging from 101.1 to 103.1 copies of DNA/μl serum. Three out of 24 qPCR‐positive samples (from wild boar No. 1 and 18) were not within the limits of quantification (less than 10 copies of DNA/μl). PCV‐3 PCR positivity was found in 8 out of 13 (61. 54%) tested juveniles/subadults and in 2 out of 6 (33.33%) adults. PCV‐3 was found in 50% (6 out of 12) of tested females and in 57. 14% (4 out of 7) of males. No significant differences were detected in PCV‐3 PCR positivity between the tested age groups and genders.
3.1.3. Study on tissues, faeces and serum
The frequency of PCV‐3 DNA detection in tissues, serum and faeces is displayed in Table 3.
Porcine circovirus 3 DNA was found in all tested sample types. In total, 32 out of 35 (91.43%) wild boar were positive for PCV‐3 in at least one tested sample. The median of the amount of DNA per sample type per μg ranged from 101.8 to 103.7 copies of DNA/μl in tonsil and submandibular lymph node, respectively. Only 30 samples (out of 111 positives by PCR) were quantifiable, with more than 10 copies of DNA/μl. Figure 3 shows the viral load found in the different tested tissues from wild boar. Significant differences in PCV‐3 frequency were detected in tonsil (p = 0.0334), lung (p = 0.0017), liver (p = 0.0039) and spleen (p = 0.0039) compared to the other tissue types.
Figure 3.
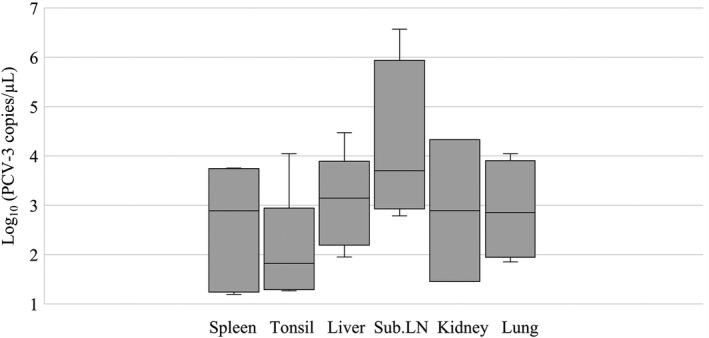
Boxplot reporting the viral load found in the different tested tissues from wild boar; line within the boxplot represents de median of viral load. Samples with more than 10 copies of DNA/μl were considered quantifiable (Franzo, Legnardi, Centelleghe et al., 2018). Sub.LN: submandibular lymph node
3.2. Phylogenetic analysis
The PCV‐3 complete nucleotide sequence of 12 animals from the retrospective study were obtained and compared through phylogenetic analysis. Different complete sequences available at GenBank both from Europe, including one from Spain, and worldwide were used for comparison. The full‐genome sequences from wild boar from different years clustered in the same group together with few strains of domestic pigs from China and Germany (Figure 4). The pairwise distance analysis showed a minimum of 98% of identity among the samples from wild boar and between the PCV‐3 full‐genome sequences from domestic pigs. Wild boar partial cap sequences available from Genbank (Franzo, Tucciarone, et al., 2018) were translated and used for comparison with obtained full sequences, showing as well >98% nucleotide identity and clustering together.
Figure 4.
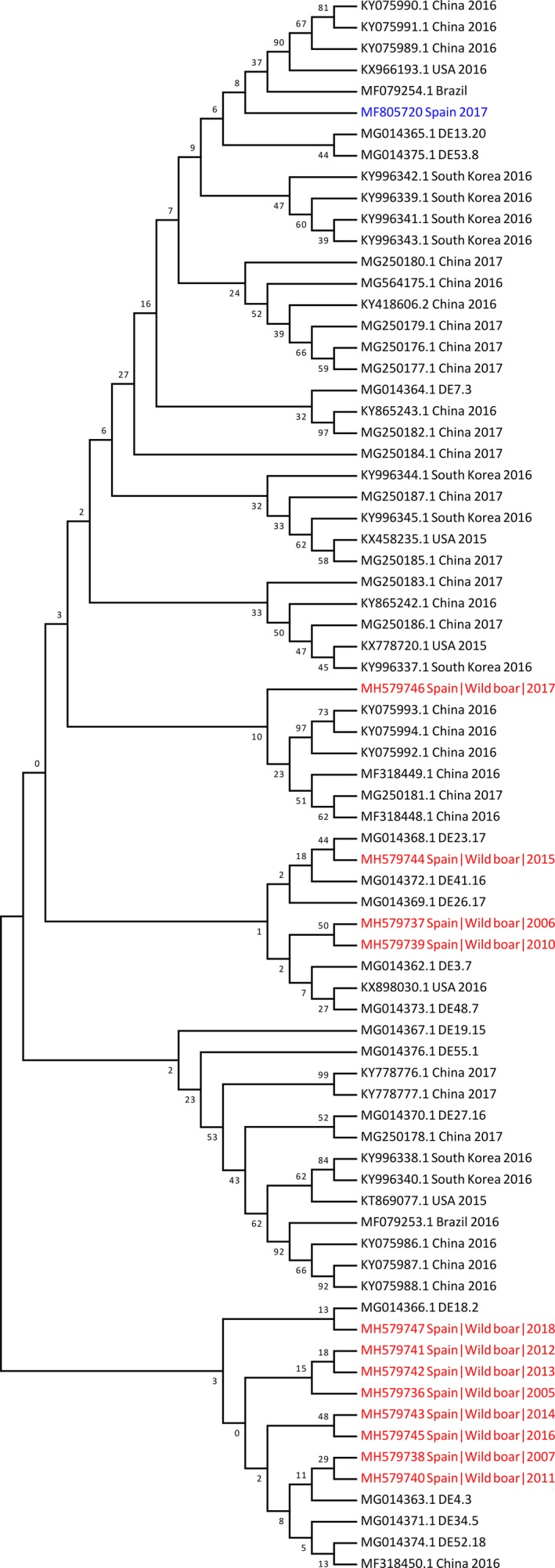
Phylogenetic tree based on the complete genomes of PCV‐3 Spanish strains from wild boar and PCV‐3 freely available sequences at Genbank. The phylogenetic tree was constructed using the maximum‐likelihood algorithm of MEGA 7 software with 1,000 bootstrap replicates. Spanish sequences obtained in the present study and the Spanish sequence from domestic pigs have been coloured in red and blue respectively. PCV‐3: Porcine circovirus 3 [Colour figure can be viewed at http://wileyonlinelibrary.com]
The similarity of partial nucleotide sequences obtained from the other two studies was compared to determine potential coinfection with different strains in the same animal (tissue/faeces/serum study) or infection with different strains at different time‐points (longitudinal study). When partial sequences obtained from different tissues of the same wild boar were compared, they demonstrated to be highly similar (>98.4%) supporting the idea of being likely the same strain. Globally, high similarity was also found between sequences of the longitudinal study (>96.6%); however, when comparing those coming from the same animal, nucleotide identity was >99%, suggesting that animals were infected by the same strain for a long period of time.
4. DISCUSSION
This study represents the first report evaluating the PCV‐3 frequency in retrospective serum samples from wild boars. Moreover, the assessment of the dynamics of the virus in captured and recaptured boars and the tissue distribution of PCV‐3 genome in this species was also investigated for the first time. Importantly, all three independent studies confirmed that wild boar are susceptible to PCV‐3 infection as previously indicated (Franzo, Tucciarone, et al., 2018). Such susceptibility was probably expected since several reports indicates that most of the pathogens infecting domestic pigs are also present in the wild boar population (Ruiz‐Fons et al., 2008). This scenario is paralleled with another circovirus species infecting swine, Porcine Circovirus 2 (PCV‐2), which has been shown to be ubiquitous in the wild boar population worldwide (Ruiz‐Fons et al., 2008). In the particular case of PCV‐2, it has also been demonstrated that wild boar can develop the PCV‐2 systemic disease, characterized by weight loss, wasting, diarrhoea, weakness, jaundice, lymphadenopathy and respiratory problems without response to antibiotic treatments (Lipej et al., 2007). No evidence of disease caused by PCV‐3 in wild boar is so far available.
According to the present study, PCV‐3 has been circulating in the Spanish wild boar population at least since 2004, the earliest evidence of infection found in this animal species. This result is in line with recently published reports confirming the circulation of PCV‐3 in domestic pigs since 1990s (Ye et al., 2018). Although the PCV‐3 frequency reported in pigs vary greatly, ranging from 10% to 75% in serum samples, the overall results obtained in this study suggest that, apparently, the PCV‐3 frequency in boars is higher than that found in domestic pigs from European countries like Spain (11.46%), Italy (18.18%), Poland (25%) and Denmark (30%) (Faccini et al., 2017; Franzo, Legnardi, Hjulsager et al., 2018; Klaumann et al., 2018; Stadejek et al., 2017). Therefore, obtained data may suggest a potential reservoir role of the wild boar in respect PCV‐3 infection.
Noteworthy, the frequency of infected wild boar between years 2013 and 2018 (53.66%) was higher than that between 2004 and 2012 (23.68%). Although it may be speculated that this was due to infection dissemination into a potential naïve population of animals, it cannot be ruled out the simple effect of highly efficient contacts between susceptible and infected wild boar due to the significant increase of wild boar densities in the studied geographical area during last decade (Massei et al., 2015).
Porcine circovirus 3 evidence of infection was observed over a large period of time in few animals (5–7 months). On the one hand, this may suggest a persistent or long‐lasting viral infection, similar to what has been described for hepatitis E virus (HEV) and PCV‐2 in the wild boar (Boadella et al., 2011). On the other hand, an infection with subsequent reinfection could also be a possibility. However, the highly similar nucleotide identity among PCV‐3 sequences available in this study and the GenBank database (for both wild boar and domestic pigs) prevents the confirmation of this latter hypothesis. For HEV, the longer the period of viremia, the higher the likelihood for the role of wild boar as reservoir (Schlosser, Vina‐Rodriguez, Fast, Groschup, & Eiden, 2015). For example, such role as reservoir for persistently infecting viruses like bovine viral diarrhoea, Aujeszky's disease virus and classical swine fever virus is well known in the wild boar (Ruiz‐Fons et al., 2008). In consequence, the apparent long‐lasting infection described in the present study for PCV‐3 would also reinforce the notion of wild species as potential reservoir for the domestic pig. However, it is still too early to confirm such reservoir status for PCV‐3, and further studies will be needed to elucidate the epidemiological role of this virus infection in wild boar.
Interestingly, some significant differences in the PCV‐3 frequency were observed by age; compiled data in the retrospective study showed that adult animals were more often PCV‐3 PCR‐positive than juveniles or subadults. However, this difference was not observed between age groups in the longitudinal study, probably due to a much lower sample size. If data from both studies are taken together, adult wild boar seem to be viremic to a higher frequency. This is in line with the so far only published article on PCV‐3 in wild boar, where a lower prevalence in juveniles was also detected (Franzo, Tucciarone, et al., 2018). Such age‐group comparison was also performed for PCV‐2 in wild boar by means of serology, but again no significant differences were observed (Vicente et al., 2004). Similarly, available data on domestic pigs do not point out to a potential higher frequency of PCV‐3 detection in any specific age group (Klaumann et al., 2018; Kwon et al., 2017; Stadejek et al., 2017).
The pathogenic role of PCV‐3 infection is still unclear (Franzo, Legnardi, Centelleghe et al., 2018; Li, Qiao, Sun, & Tian, 2018a). Detection of viral genome in serum indicates a systemic infection, but there are still no clues on the main target organs or cell tropism. As an exploratory approach, different tissue samples and faeces in addition of serum were tested in a subset of wild boar. PCV‐3 genome was detected in all tested tissue types as well as in faeces to a higher frequency and viral load when compared with serum. In consequence, it seems evident that detection of PCV‐3 in serum underestimates significantly the percentage of infected wild boar, fact that also happens to a number of ssDNA viruses in domestic pig (Calsamiglia, Segalés, Quintana, Rosell, & Domingo, 2002; Nieto, Kekarainen, Aramouni, & Segalés, 2013). Although obtained results are preliminary, percentage of truly infected wild boar surpassed 90%, further suggesting potential long‐lasting infections and putative defective immune responses (not able to neutralize and clear the virus from the organism). Also, significant differences in the PCV‐3 frequency between the tested tissues showed that the most useful tissues for PCV‐3 detection were tonsil, liver, spleen and lung, which may account as target organs for PCV‐3 replication. Moreover, although positivity was lower than in other tissues, the submandibular lymph node offered the highest viral loads. Different reports have been shown the presence of the virus in different lymphoid tissues with a higher frequency than in lung and other tissues, but others found similar amounts (Fan et al., 2017; Fu et al., 2017; Li, Zhang, Qiao, et al., 2018b). In all cases, and although it implies underestimating the real frequency of infection, serum is still the most appropriate sample for epidemiological studies. The amount of PCV‐3 DNA in tissues was considered low to moderate, in agreement with several studies which detected low viral load in the analysed samples of domestic pigs (Fux et al., 2018; Stadejek et al., 2017; Zhai et al., 2017). In all cases, these low viral loads suggest that wild boar, similar to domestic pigs, might be subclinically infected with this virus. At least for PCV‐2, an association between the viral load and the severity of lesions has been described, suggesting that high amount of DNA is a major feature of pigs affected by PCV‐2 systemic disease (Olvera, Sibila, Calsamiglia, Segalés, & Domingo, 2004). Moreover, in the few described cases of PCV‐2 systemic disease in wild boar, high loads of PCV‐2 were also found in tissues (Ellis et al., 2003; Vicente et al., 2004).
A total of 24 partial or complete sequences of PCV‐3 corresponding to the three different studies were obtained. The phylogenetic analysis of these sequences indicated a close distance between them and with other PCV‐3 genomes available at the GenBank from both domestic pig and wild boar. The Spanish wild boar sequences were located mainly in the same clusters that others from domestic pigs from Germany and, only one of them, close to Chinese sequences. Interestingly, the only complete genome so far available from domestic pigs from Spain was located relatively away from the wild boar sequences obtained in the present study (although the overall nucleotide identity of these PCV‐3 sequences available was >98%). These data also reinforce the notion that PCV‐3 does not show independent molecular evolution in the particular areas of the world where it has been detected to date (Klaumann et al., 2018).
In conclusion, the present study further demonstrates that wild boar are susceptible to PCV‐3 infection and confirms the virus circulation at least since 2004 with a relatively high frequency. According to the results, PCV‐3 can be detected over a long period of time, suggesting long‐lasting infections do occur in wild boar. In addition, PCV‐3 was detected in all tested tissue sample types and faeces, being the most frequently positive tissues tonsil, lung, liver and spleen. Globally, high nucleotide identity was found in all PCV‐3 sequences obtained from wild boar.
CONFLICT OF INTEREST
All authors have declared no conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
The authors acknowledge the funding of the E‐RTA2017‐00007‐00‐00 project, from the Instituto Nacional de Investigación y Tecnologia Agraria y Alimentaria (Spanish Government). We also thank Dr. Francesc Closa‐Sebastià (Vets & Wildlife, http://www.senglaring.com) for his assistance in the capture and management of wild boars of the longitudinal study. The funding from CERCA Programme/Generalitat de Catalunya to IRTA is also acknowledged.
Klaumann F, Dias‐Alves A, Cabezón O, et al. Porcine circovirus 3 is highly prevalent in serum and tissues and may persistently infect wild boar (Sus scrofa scrofa). Transbound Emerg Dis. 2019;66:91–101. 10.1111/tbed.12988
REFERENCES
- Biagini, P. , Bendinelli, M. , Hino, S. , Kakkola, L. , Mankertz, A. , Niel, C. , … Todd, D. (2012). Family Circoviridae In King A. M. Q., Adams M. J., Carstens E. B., & Lefkowitz E. J. (Eds.), Virus taxonomy: Classification and nomenclature of viruses: Ninth report of the International Committee on Taxonomy of Viruses (pp. 343–349). Waltham, MA: Academic Press. [Google Scholar]
- Boadella, M. , Ruiz‐Fonz, J. F. , Vicente, J. , Martín, M. , Segalés, J. , & Gortazar, C. (2011). Seroprevalence evolution of selected pathogens in Iberian Wild Boar. Transboundary and Emerging Diseases, 59, 395–404. 10.1111/j.1865-1682.2011.01285.x [DOI] [PubMed] [Google Scholar]
- Burland, T. G. (1999). DNASTAR's lasergene sequence analysis software. Bioinformatics Methods and Protocols, 132, 71–91. 10.1385/1-59259-192-2:71 [DOI] [PubMed] [Google Scholar]
- Buruaga, M. S. , Lucio, A. J. , & Purroy, F. J. (2001). Reconocimiento de sexo y edad en espécies cinegéticas In Ediciones Leonesas, 1st ed 128p. ISBN: 8480123710 [Google Scholar]
- Calsamiglia, M. , Segalés, J. , Quintana, J. , Rosell, C. , & Domingo, M. (2002). Detection of porcine circovirus types 1 and 2 in serum and tissue samples of pigs with and without postweaning multisystemic wasting syndrome. Journal of Clinical Microbiology, 40, 1848–1850. 10.1128/JCM.40.5.1848-1850.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo‐Contreras, R. , Carvalho, J. , Serrano, E. , Mentaberre, G. , Fernández‐Aguilar, X. , Colom, A. , … López‐Olvera, J. (2018). Urban wild boars prefer fragmented areas with food resources near natural corridors. Science of the Total Environment, 615, 282–288. 10.1016/j.scitotenv.2017.09.277 [DOI] [PubMed] [Google Scholar]
- Chen, G. H. , Mai, K. J. , Zhou, L. , Wu, R. T. , Tang, X. Y. , Wu, J. L. , … Ma, J. Y. (2017). Detection and genome sequencing of porcine circovirus 3 in neonatal pigs with congenital tremors in South China. Transboundary and Emerging Diseases, 64, 1650–1654. 10.1111/tbed.12702 [DOI] [PubMed] [Google Scholar]
- Ellis, J. , Spinato, M. , Yong, C. , West, K. , McNelly, F. , Meehan, B. , … Allan, G. (2003). Porcine circovirus 2‐associated disease in Eurasian wild boar. Journal of Veterinary Diagnostic Investigation, 15(4), 364–368. 10.1177/104063870301500411 [DOI] [PubMed] [Google Scholar]
- Faccini, S. , Barbieri, I. , Gilioli, A. , Sala, G. , Gibelli, L. R. , Moreno, A. , … Nigrelli, A. (2017). Detection and genetic characterization of Porcine circovirus type 3 in Italy. Transboundary and Emerging Diseases, 64, 1661–1664. 10.1111/tbed.12714 [DOI] [PubMed] [Google Scholar]
- Fan, S. , Ku, X. , Chen, F. , Wang, Y. , Yu, X. , & He, Q. (2017). Complete genome sequence of a novel porcine circovirus type 3 strain, PCV3/CN/Hubei‐618/2016, isolated from China. Genome Announcements, 5, 2 10.1128/genomeA.00100-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Aguilar, X. , Gottschalk, M. , Aragon, V. , Càmara, J. , Ardanuy, C. , Velarde, R. , … Cabezón, O. (2018). Urban wild boars and risk for zoonotic Streptococcus suis . Spain. Emerging Infectious Diseases Journal, 24, 1083 10.3201/eid2406.171271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzo, G. , Legnardi, M. , Centelleghe, C. , Tucciarone, C. M. , Cecchinato, M. , Cortey, M. , … Drigo, M. (2018). Development and validation of direct PCR and quantitative PCR assays for the rapid, sensitive, and economical detection of porcine circovirus 3. Journal of Veterinary Diagnostic Investigation, 30, 538–544. 10.1177/1040638718770495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzo, G. , Legnardi, M. , Hjulsager, C. K. , Klaumann, F. , Larsen, L. E. , Segales, J. , & Drigo, M. (2018). Full‐genome sequencing of porcine circovirus 3 field strains from Denmark, Italy and Spain demonstrates a high within‐Europe genetic heterogeneity. Transboundary and Emerging Diseases, 65, 602–606. 10.1111/tbed.12836 [DOI] [PubMed] [Google Scholar]
- Franzo, G. , Legnardi, M. , Tucciarone, C. M. , Drigo, M. , Klaumann, F. , Sohrmann, M. , & SegalÉs, J. (2018). Porcine circovirus type 3: A threat to the pig industry? The Veterinary Record, 182(3), 83 10.1136/vr.k91 [DOI] [PubMed] [Google Scholar]
- Franzo, G. , Tucciarone, C. M. , Drigo, M. , Cecchinato, M. , Martini, M. , & Menandro, M. L. (2018). First report of wild boar susceptibility to Porcine circovirus type 3: High prevalence in the Colli Euganei Regional Park (Italy) in the absence of clinical signs. Transboundary and Emerging Diseases, 65, 957–962. 10.1111/tbed.12905 [DOI] [PubMed] [Google Scholar]
- Fu, X. , Fang, B. , Ma, J. , Liu, Y. , Bu, D. , Zhou, P. , … Zhang, G. (2017). Insights into the epidemic characteristics and evolutionary history of the novel porcine circovirus type 3 in southern China. Transboundary and Emerging Diseases, 65, e296–e303. 10.1111/tbed.12752 [DOI] [PubMed] [Google Scholar]
- Fux, R. , Söckler, C. , Link, E. K. , Renken, C. , Krejci, R. , Sutter, G. , … Eddicks, M. (2018). Full genome characterization of porcine circovirus type 3 isolates reveals the existence of two distinct groups of virus strains. Virology Journal, 15, 25 10.1186/s12985-018-0929-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, T. A. (1999). BioEdit: A user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98. [Google Scholar]
- International Committee on Taxonomy of Viruses – ICVT . Retrieved from http://www.ncbi.nlm.nih.gov/ICTVdb/
- Klaumann, F. , Franzo, G. , Sohrmann, M. , Florencia, C.‐F. , Drigo, M. , Núñez, J. I. , … Segalés, J. (2018). Retrospective detection of Porcine circovirus 3 (PCV‐3) in pig serum samples from Spain. Transboundary and Emerging Diseases, 2018(00), 1–7. 10.1111/tbed.12876 [DOI] [PubMed] [Google Scholar]
- Ku, X. , Chen, F. , Li, P. , Wang, Y. , Yu, X. , Fan, S. , … He, Q. (2017). Identification and genetic characterization of porcine circovirus type 3 in China. Transboundary and Emerging Diseases, 64, 703–708. 10.1111/tbed.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology Evolution, 33, 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, T. , Yoo, S. J. , Park, C.‐K. , & Lyoo, Y. S. (2017). Prevalence of novel porcine circovirus 3 in Korean pig populations. Veterinary Microbiology, 207, 178–180. 10.1016/j.vetmic.2017.06.013 [DOI] [PubMed] [Google Scholar]
- Li, X. , Qiao, M. , Sun, M. , & Tian, K. (2018a). A Duplex Real‐Time PCR Assay for the Simultaneous Detection of Porcine Circovirus 2 and Circovirus 3. Virologica Sinica, 33, 181–186. 10.1007/s12250-018-0025-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Zhang, C. , Qiao, M. , Guo, J. , Xing, G. , Jin, C. , … Tian, K. (2018b). Molecular Epidemiology of Porcine Circovirus Type 3 Infection in Swine Herds in China. Virologica Sinica, 1–5, 10.1007/s12250-018-0041-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipej, Z. , Segalés, J. , Jemeršić, L. , Olvera, A. , Roić, B. , Novosel, D. , … Manojlović, L. (2007). First description of postweaning multisystemic wasting syndrome (PMWS) in wild boar (Sus scrofa) in Croatia and phylogenetic analysis of partial PCV2 sequences. Acta Veterinaria Hungarica, 55, 389–404. 10.1556/AVet.55.2007.3.13 [DOI] [PubMed] [Google Scholar]
- Massei, G. , Kindberg, J. , Licoppe, A. , Gačić, D. , Šprem, N. , Kamler, J. , … Náhlik, A. (2015). Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Management Science, 71(4), 492–500. 10.1002/ps.3965 [DOI] [PubMed] [Google Scholar]
- Meng, X. J. , Lindsay, D. S. , & Sriranganathan, N. (2009). Wild boars as sources for infectious diseases in livestock and humans. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 2697–2707. 10.1098/rstb.2009.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto, D. , Kekarainen, T. , Aramouni, M. , & Segalés, J. (2013). Torque teno sus virus 1 and 2 distribution in tissues of porcine circovirus type 2‐systemic disease affected and age‐matched healthy pigs. Veterinary Microbiology, 163, 364–367. 10.1016/j.vetmic.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Olvera, A. , Sibila, M. , Calsamiglia, M. , Segalés, J. , & Domingo, M. (2004). Comparison of porcine circovirus type 2 load in serum quantified by a real time PCR in postweaning multisystemic wasting syndrome and porcine dermatitis and nephropathy syndrome naturally affected pigs. Journal of Virological Methods, 117, 75–80. 10.1016/j.jviromet.2003.12.007 [DOI] [PubMed] [Google Scholar]
- Palinski, R. , Piñeyro, P. , Shang, P. , Yuan, F. , Guo, R. , Fang, E. , … Hause, B. M. (2017). A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. Journal of Virology, 91(1), e01879–16. 10.1128/JVI.01879-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan, T. G. , Giannitti, F. , Rossow, S. , Marthaler, D. , Knutson, T. , Li, L. , … Delwart, E. (2016). Detection of a novel circovirus PCV3 in pigs with cardiac and multi‐systemic inflammation. Virology Journal, 13(1), 1–8. 10.1186/s12985-016-0642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, B. W. , Niagro, F. D. , Latimer, K. S. , Lukert, P. D. , Steffens, W. L. , Rakich, P. M. , & Pritchard, N. (1990). Ultrastructural, protein composition, and antigenic comparison of psittacine beak and feather disease virus purified from four genera of psittacine birds. Journal of Wildlife Research, 26, 196–203. 10.7589/0090-3558-26.2.196 [DOI] [PubMed] [Google Scholar]
- Ruiz‐Fons, F. , Segalés, J. , & Gortázar, C. (2008). A review of viral diseases of the European wild boar: Effects of population dynamics and reservoir role. Veterinary Journal, 176, 158–169. 10.1016/j.tvjl.2007.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva, G. L. , Vidigal, P. M. P. , Fietto, J. L. R. , Bressan, G. C. , Silva Júnior, A. , & de Almeida, M. R. (2018). Evolutionary analysis of Porcine circovirus 3 (PCV3) indicates an ancient origin for its current strains and a worldwide dispersion. Virus Genes, 54, 376–384. 10.1007/s11262-018-1545-4 [DOI] [PubMed] [Google Scholar]
- Schlosser, J. , Vina‐Rodriguez, A. , Fast, C. , Groschup, M. H. , & Eiden, M. (2015). Chronically infected wild boar can transmit genotype 3 hepatitis E virus to domestic pigs. Veterinary Microbiology, 180, 15–21. 10.1016/j.vetmic.2015.08.022 [DOI] [PubMed] [Google Scholar]
- Shen, H. , Liu, X. , Zhang, P. , Wang, L. , Liu, Y. , Zhang, L. , … Song, C. (2017). Genome characterization of a porcine circovirus type 3 in South China. Transboundary and Emerging Diseases, 65, 264–266. 10.1111/tbed.12639 [DOI] [PubMed] [Google Scholar]
- Stadejek, T. , Woźniak, A. , Miłek, D. , & Biernacka, K. (2017). First detection of porcine circovirus type 3 on commercial pig farms in Poland. Transboundary and Emerging Diseases, 64, 1350–1353. 10.1111/tbed.12672 [DOI] [PubMed] [Google Scholar]
- Sun, J. , Wei, L. , Lu, Z. , Mi, S. , Bao, F. , Guo, H. , … Gong, C. (2018). Retrospective study of porcine circovirus 3 infection in China. Transboundary and Emerging Diseases, 65, 607–613. 10.1111/tbed.12853 [DOI] [PubMed] [Google Scholar]
- Tamura, K. , & Nei, M. (1993). Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Molecular Biology and Evolution, 10, 512–526. 10.1093/oxfordjournals.molbev.a040023 [DOI] [PubMed] [Google Scholar]
- Thompson, J. D. , Gibson, T. J. , Plewniak, F. , Jeanmougin, F. , & Higgis, D. G. (1997). The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25(24), 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochetto, C. , Lima, D. A. , Varela, A. P. M. , Loiko, M. R. , Paim, W. P. , Scheffer, C. M. , … Roehe, P. M. (2017). Full‐Genome Sequence of Porcine Circovirus type 3 recovered from serum of sows with stillbirths in Brazil. Transboundary and Emerging Diseases, 65(1), 5–9. 10.1111/tbed.12735 [DOI] [PubMed] [Google Scholar]
- Vicente, J. , Segalés, J. , Hofle, U. , Balasch, M. , Plana‐Duran, J. , Domingo, M. , & Gortazar, C. (2004). Epidemiological study on porcine circovirus type 2 (PCV2) infection in the European wild boar (Sus scrofa). Veterinary Research, 35, 243–253. 10.1051/vetres:2004008 [DOI] [PubMed] [Google Scholar]
- Ye, X. , Berg, M. , Fossum, C. , Wallgren, P. , & Blomström, A.‐L. (2018). Detection and genetic characterisation of porcine circovirus 3 from pigs in Sweden. Virus Genes, 54, 466–469. 10.1007/s11262-018-1553-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, S.‐L. , Zhou, X. , Zhang, H. , Hause, B. M. , Lin, T. , Liu, R. , … Wang, D. (2017). Comparative epidemiology of porcine circovirus type 3 in pigs with different clinical presentations. Virology Journal, 14, 222 10.1186/s12985-017-0892-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, S. , Wu, X. , Zhang, L. , Xin, C. , Liu, Y. , Shi, J. , … Wang, J. (2017). The occurrence of porcine circovirus 3 without clinical infection signs in Shandong Province. Transboundary and Emerging Diseases, 64, 1337–1341. 10.1111/tbed.12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


