Abstract
Simple Summary
MicroRNAs play pivotal roles in skeletal muscle development, but the molecular basis of their functions in fetal bovine skeletal muscle development is largely unknown. Here, we report a mechanistic study of bta-miR-24-3p, a key miRNA regulator of the myogenic differentiation of fetal bovine platelet-derived growth factor receptor alpha negative (PDGFRα-) progenitor cells. We isolated progenitor cells from the bovine fetal longissimus dorsi muscle and purified them with PDGFRα antibodies to remove fibro-adipogenic progenitors. We observed elevated bta-miR-24-3p expression during differentiation, and bta-miR-24-3p overexpression led to promoted myogenic differentiation but suppressed proliferation. Moreover, activin receptor type 1B (ACVR1B) was identified as a direct target of bta-miR-24-3p, and ACVR1B-silencing cells exhibited similar phenotypes to bta-miR-24-3p-overexpressing bovine PDGFRα- progenitor cells. These results extended our understanding on the roles of miRNA in fetal muscle development. The method of removing fibro-adipogenic progenitors in our study will also provide useful information for other investigators.
Abstract
MicroRNAs modulate a variety of cellular events, including skeletal muscle development, but the molecular basis of their functions in fetal bovine skeletal muscle development is poorly understood. In this study, we report that bta-miR-24-3p promotes the myogenic differentiation of fetal bovine PDGFRα- progenitor cells. The expression of bta-miR-24-3p increased during myogenic differentiation. Overexpression of bta-miR-24-3p significantly promoted myogenic differentiation, but inhibited proliferation. A dual-luciferase assay identified ACVR1B as a direct target of bta-miR-24-3p. Similarly, knocking down ACVR1B by RNA interference also significantly inhibited proliferation and promoted the differentiation of bovine PDGFRα- progenitor cells. Thus, our study provides a mechanism in which bta-miR-24-3p regulates myogenesis by inhibiting ACVR1B expression.
Keywords: bta-miR-24-3p, bovine, fetal skeletal muscle, proliferation, differentiation, ACVR1B
1. Introduction
The fetal stage is crucial for skeletal muscle development, because the majority of skeletal muscle fibers form during this stage [1]. Skeletal muscle growth and development involve a series of complex biological processes regulated by myogenic regulatory factors (MRFs) [2]. For example, myogenic factor 5 (Myf5) and myogenic differentiation 1 (MyoD) are critical for the commitment, proliferation, and survival of myoblasts, whereas myogenin (MyoG) and myogenic factor 6 (Myf6) play essential roles in terminal differentiation [3,4,5]. Myogenic differentiation is also regulated at the post-transcriptional level, such as with microRNAs (miRNAs).
MicroRNAs (miRNAs) are endogenous, non-coding RNAs (ncRNA) of 20–24 nucleotides, and suppress translation via binding to the 3′-untranslated region (3′-UTR) of their target mRNAs [6,7]. It has been reported that miRNAs regulate muscle development [8,9]. For example, miR-143 regulates bovine muscle satellite cell (MSCs) differentiation and proliferation by directly targeting insulin like growth factor binding protein 5 (IGFBP5) [10]. miR-148-3p could inhibit the proliferation and enhance the apoptosis of bovine muscle cells by targeting Kruppel like factor 6 (KLF6) [11]. bta-miR-378 could promote bovine skeletal muscle-derived satellite cell (bMDSC) differentiation by suppressing DNA polymerase alpha 2 (POLA2) expression [12]. These findings point to a role of miRNAs in bovine skeletal muscle development.
bta-miR-24-3p is 22 nt in length and is derived from its precursor miRNA bta-miR-24-2. miR-24 is upregulated during myogenesis and promotes myogenic differentiation through an unknown mechanism [13]. According to published data, miR-24-3p hampers skeletal muscle fibrosis by interacting with its target, Smad2, which is involved in transforming growth factor-β (TGFβ)/Smad signaling [14]. However, the regulatory role of bta-miR-24-3p in the prenatal development of bovine skeletal muscle is still not clear.
Activin receptor type 1B (ACVR1B, also known as ALK4) encodes a transducer of activin or activin-like ligand signals. It is known to participate in embryonic development, nervous system differentiation, germ cell development, tumor formation, and immunosuppression [15,16,17]. ACVR1B influences myogenesis and regulates the balance between protein synthesis and degradation by modulating the MSTN pathway to maintain muscle mass [18]. In addition, miR-210 regulates transforming growth factor-β (TGF-β)/activin signaling to promote osteoblastic differentiation by targeting ACVR1B [19]. A study with ACVR1B-knockout mice provided evidence for a specific and indispensable role of ACVR1B signaling in the cycling and differentiation of hair follicle and tooth morphogenesis [20]. However, the role of ACVR1B in fetal bovine myogenesis and proliferation, and whether it is regulated by miRNAs in the differentiation and proliferation of skeletal muscle, is still unknown.
In this study, we purified myogenic progenitor cells using antibodies of platelet-derived growth factor receptor alpha (PDGFRα), which is the cell surface marker of fibro/adipogenic lineages [21], and named the cells as PDGFRα- progenitor cells. This study investigates the underlying molecular basis of how miR-24-3p modulates the differentiation and proliferation of fetal bovine skeletal, muscle-derived progenitor cells. Moreover, we predicted the potential targets of bta-miR-24-3p and experimentally demonstrated its regulatory mechanism. The effect of ACVR1B on the differentiation and proliferation of fetal bovine skeletal muscle-derived progenitor cells was also explored. Our results demonstrate that bta-miR-24-3p inhibits bovine PDGFRα- progenitor cell proliferation and improves their differentiation by targeting ACVR1B. This research provides insight into the epigenetic regulation of skeletal muscle development during the fetal stage.
2. Materials and Methods
2.1. Ethics Statement
All animal experiments followed the guidelines of the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, 2004). All animal experimental protocols in this study were strictly monitored by the Animal Ethics Committee of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (IAS-CAAS). Ethical approval of animal survival was given by the animal ethics committee of IAS-CAAS (No. IAS2019-48). Pregnant cows were raised at the Inner Mongolia Aokesi Agriculture Co., Ltd. (Wulagai, China). Efforts were made to minimize the cows’ suffering.
2.2. Primary Cell Isolation and Cell Culture
The progenitor cells were enzymatically isolated from longissimus dorsi tissues obtained from three male bovine fetuses about 90–120 days after conception, as described previously [22]. Briefly, longissimus dorsi was minced into small fragments and then incubated with 0.1% type IV collagenase (Sigma, St. Louis, MO, USA) for 1 h. This enzymatic digestion was terminated by adding cell culture medium containing low-glucose DMEM (Dulbecco’s Modified Eagle medium; Gibco, Grand Island, NY, USA), supplemented with 10% FBS (fetal bovine serum; Gibco, Grand Island, NY, USA). The digested tissue suspension was passed through a nylon mesh (40 μm pore size), and the pellets were obtained by centrifuging and resuspending in ice-cold phosphate-buffered saline (PBS) buffer containing 2 mM EDTA and 0.5% bovine serum albumin. The cell suspension was incubated with anti-PDGFRα antibody (Cell Signaling Technology, Danvers, MA, USA) at 4 °C for 30 min. After washing and resuspending in PBS, the cells were incubated with Anti-Rabbit IgG MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) at 4 °C for 15 min. Subsequently, the cells were harvested and resuspended in the buffer. PDGFRα- cells were separated by a MACS column (Miltenyi Biotec) and magnetic MiniMACS Separator (Miltenyi Biotec). These cells were cultured in DMEM supplemented with 10% FBS and maintained at 37 °C with a 5% CO2 atmosphere. The cells from each fetus were maintained separately. The cells were passaged with 0.25% trypsin-EDTA (Gibco, Grand Island, NY, USA) at 70–80% confluence and induced in the differentiation medium (DM) containing Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) and 5% horse serum when reaching 100% confluence.
DMEM was used to culture HEK293, a human embryonic kidney cell line. The medium contains high glucose, 100 U/mL penicillin, 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA), and 100 μg/mL streptomycin at 37 °C and 5% CO2.
2.3. RNA Isolation, Reverse Transcription (RT), and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated from the cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA concentration and quality were assessed by a NanoPhotometer N50 (Implen, Munich, Germany) and 1.5% agarose gel electrophoresis. We synthesized first-strand cDNA by reverse-transcribing the isolated total RNA using PrimeScript RT Master Mix (Perfect Real Time) (TaKaRa, Kusatsu, Japan). For MicroRNA, the reverse transcripts were obtained with a miRcute Plus miRNA First-Stand cDNA Synthesis Kit (KR211, TIANGEN, Beijing, China). Then quantitative real-time PCR (qRT-PCR) was performed by the QuantStudio 7 Flex Real-Time PCR System (Life, Carlsbad, CA, USA), with the KAPA SYBR® FAST qPCR Kit (KAPABiosystems, Wilmington, MA, USA) and a miRcute Plus miRNA qPCR Kit (FP411, TIANGEN). All assays were performed following the manufacturer’s recommendations. Three technical replicates were used for all reactions. The sequences of miRNA-specific and qRT-PCR primers are summarized in Table 1.
Table 1.
Primers used for quantitative real-time PCR (qRT-PCR).
| Name | Forward (5’–3’) | Reverse (5’–3’) |
|---|---|---|
| MyoG | CAAATCCACTCCCTGAAA | GCATAGGAAGAGATGAACA |
| MYH1 | GGGAAACTGGCTTCTGCTGAT | TGGGTTGGTGGTGATTAGGAG |
| MYH2 | GTCAAAGGGACTATCCAGAGCAG | AGAAGAGGCCCGAGTAGGTGT |
| MYH4 | CTCCTAATCACCACCAACCCATA | TGTCAGCAACTTCAGTGCCATC |
| MYH7 | AAGACAGTGACCGTGAAGGAGG | GGTTGATGGTGACGCAGAAGA |
| ACVR1B | TGCCCTCTGACCCTTCCATC | CACTCCCGCATCATCTTCCC |
| bta-miR-24-3p | CAGTGGCTCAGTTCAGCAGGA | |
| 18s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| Cyclin D1 | GACGAGCTGCTGCACATGGA | TGCTTGTTCTCCTCGGCCAC |
| Cyclin B1 | TGGGAGAGACATAAACGG | TGGAAGCCAAGAGCAGTG |
| PCNA | GCTGTGTAGTAAAGATGCCT | ATCTCTATGGCAACAGCTTC |
| CDK2 | TCTTTGCTGAGATGGTGACCC | TAACTCCTGGCCAAACCACC |
| CDKN1A | AAACGGCGGCAGACC | GCCCAAGGCAAAAGG |
MyoG: myogenin; MYH: myosin heavy chain; PCNA: proliferating cell nuclear antigen; CDK2: cyclin-dependent kinase 2; CDKN1A: cyclin-dependent kinase inhibitor 1A.
2.4. Immunofluorescence
For immunofluorescence, the cells were cultured in 12-well plates, fixed in 4% paraformaldehyde for 15 min, and washed three times (five min each) with PBS. Next, we permeabilized the cells with 0.1% Triton X-100 for 10 min and blocked the cells with 1% albumin bovine serum (Beyotime, Shanghai, China) for 30 min. After incubation with the anti-myosin heavy chain (MHC) antibody (1:100, Developmental Studies Hybridoma Bank, Iowa, USA) overnight at 4 °C, FITC-labeled Goat Anti-Mouse IgG (H + L) (1:1000, Beyotime) was added, and the cells were incubated at room temperature for 1 h. The cell nuclei were stained with DAPI (Sigma-Aldrich, St. Louis, MO USA) for 5 min, and images were obtained with a confocal microscope (TCS SP8, Leica, Wetzlar, Germany).
2.5. Western Blot
The cells were lysed with proteinase inhibitors, and protein samples of equal amount were separated on 4–12% SurePAGE gels (GenScript, Nanjing, China). The proteins were transferred to nitrocellulose membranes (Pall, Mexico) after electrophoresis and blocked in 5% (w/v) non-fat milk. The membranes were incubated with the ACVR1B (1:3000, Proteintech, Chicago, IL, USA), MyoG (1:1000, Santa Cruz, Delaware Ave Santa Cruz, CA, USA), MHC (1:50, Developmental Studies Hybridoma Bank), and β-tubulin (1:2000, Proteintech) antibodies—all of which are primary antibodies—at 4 °C overnight, washed three times with saline/Tween (in Tris), and incubated with HRP-labeled Goat Anti-Mouse IgG (1:1000, Beyotime) or HRP-labeled Goat Anti-Rabbit IgG (1:1000, Beyotime) at room temperature for 1 h. ECL western blotting detection reagent (Beyotime) was used to visualize the protein bands.
2.6. 5-Ethynyl-2′-deoxyuridine (EdU) Assay
The cells were cultured until 50% density and transfected with the miR-24-3p mimic, the mimic NC, the ACVRIB siRNA (si-ACVR1B), and the siRNA negative control (si-NC). The cells were subjected to the 5-Ethynyl-2’-deoxyuridine (EdU) assay 24 h after transfection using an EdU Apollo In Vitro Imaging Kit (RiboBio, Guangzhou, China). We captured three randomly selected fields using a fluorescence microscope (TCS SP8, Leica) and determined the number of EdU-stained cells. ImageJ was used to determine the percentage of EdU-positive cells.
2.7. Cell Counting Kit-8 (CCK8) Assay
The cells were cultured in 96-well plates and transfected with the miR-24-3p mimic or the mimic NC. After 24, 48, 72, and 96 h of transfection, a 10 μL Cell Counting Kit-8 reagent (Beyotime) was added into each well and incubated for 1 h. Optical absorption at 450 nm (A450) was measured by an Imark Microplate Reader (Bio-Rad), and the average A450 values of six independent experiments were calculated.
2.8. RNA Oligonucleotide Preparation and Cell Transfection
The bta-miR-24-3p mimics, the mimic NC, the siRNAs used to knockdown ACVR1B, and the non-specific siRNA negative control were designed and synthesized by RiboBio (Guangzhou, China). The si-ACVR1B sequence is 5’-CGCTGACAATAAAGATAAC-3’. Transfection was performed with the Lipofectamine RNAiMAX reagent (Invitrogen). All procedures were performed according to the manufacturers’ protocols.
2.9. Prediction of miRNA Target Genes
The miRNA target gene prediction was performed by TargetScanHuman 7.2 (http://www.targetscan.org/vert_72/).
2.10. Dual-Luciferase Reporter Assay
The binding site of bta-miR-24-3p in ACVR1B was amplified from bovine DNA and inserted into the psi-CHECK2 vector (Promega, Madison, WI, USA) via XhoI and NotI double digestion. Site-directed mutagenesis of the resulting construct was performed using the Fast Site-Directed Mutagenesis Kit (TIANGEN) to remove the potential binding site. Refer to Table 2 for details on primers used in plasmid construction and mutagenesis.
Table 2.
Primers used for vector construction.
| Name | Forward (5’–3’) | Reverse (5’–3’) |
|---|---|---|
| Psi-CHECK-ACVR1B-W | ACTCTCGAGAACAACCACACACACAAAA | ATAGCGGCCGCGGATGAGAAGGTGCAACTT |
| Psi-CHECK-ACVR1B-Mut | TATCTGTCCTTGTACTGCTCCCCCCCGCCC | GGGCGGGGGGGAGCAGTACAAGGACAGATA |
For luciferase reporter assays, HEK293T cells were co-transfected with bta-miR-24-3p mimics (or the mimic NC) and the wild-type or mutant psi-CHECK2 plasmid, using the Lipofectamine 3000 reagent (Invitrogen). Luciferase activity was determined by the Dual-Luciferase Reporter Assay System (Promega) 48 h after transfection.
2.11. Statistical Analysis
At least three technical replicates were used for statistical analysis. The Normality of the data was verified by Student’s t-test using GraphPad Prism (version 6.0, GraphPad Software, San Diego, CA, USA); p < 0.05 was considered statistically significant.
3. Results
3.1. Bta-miR-24-3p Is Up-Regulated During the Myogenic Differentiation of PDGFRα- Progenitor Cells
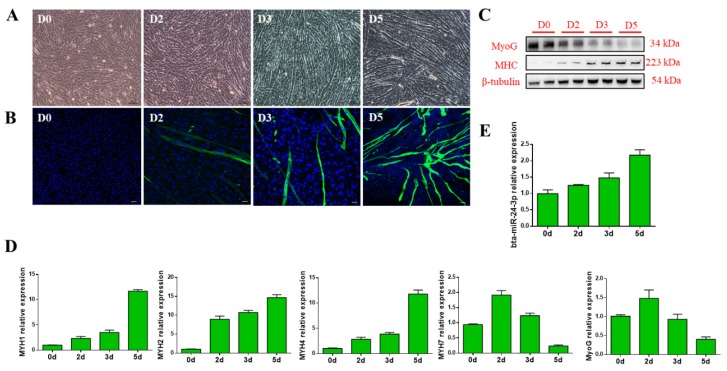
To investigate the expression of bta-miR-24-3p during myogenesis, PDGFRα- progenitor cells were isolated from the longissimus dorsi tissue of bovine fetus, according to a previous study [21], and then myogenic differentiation was induced in vitro. The PDGFRα- progenitor cells formed obvious myotubes two days after myogenic induction (Figure 1A,B). Moreover, immunostaining of muscle-specific protein showed that MyoG was downregulated during myogenic differentiation, whereas myosin heavy chain (MHC) was upregulated (Figure 1C). We then determined the transcript levels of the MYH genes during myogenic differentiation, and found that the MYH1, MYH2 and MYH4 expression increased, whereas that of MYH7 decreased two days after differentiation (Figure 1D). In addition, a gradual increase in bta-miR-24-3p expression was observed during myogenic differentiation (Figure 1E).
Figure 1.
bta-miR-24-3p expression during the myogenic differentiation of platelet-derived growth factor receptor alpha (PDGFRα-) progenitor cells. (A) Microscopic images of bovine PDGFRα- progenitor cells on days 0, 2, 3, and 5 (D0, D2, D3, and D5, respectively) of differentiation. Scale bars = 100 µm. (B) Myosin heavy chain (MHC)-positive cells (green) on D0, D2, D3, and D5 of myogenic differentiation, visualized by immunofluorescence; scale bars = 100 µm. (C) Western blot evaluating the protein levels of myogenin and MHCs in cells cultured, as described in A. (D) Transcript levels of myogenin and MHCs in cells cultured, as described in (A). (E) The transcript level of bta-miR-24-3p in cells cultured, as described in (A). All data are represented as mean ± standard deviation (SD), based on at least three independent experiments for each treatment.
3.2. Bta-miR-24-3p Promotes the Myogenic Differentiation of Bovine PDGFRα- Progenitor Cells
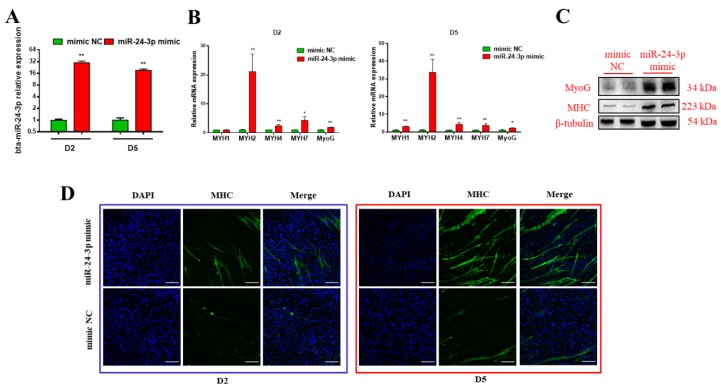
To investigate the potential roles of bta-miR-24-3p in bovine skeletal muscle myogenesis during the fetal period, we transfected bta-miR-24-3p mimics and the negative control (NC) into PDGFRα- progenitor cells. The levels of mature bta-miR-24-2 in the mimic group on day 2 and day 5 were 30- and 19-fold higher than those in the NC group, respectively (Figure 2A). bta-miR-24-3p accumulation led to a significant increase in the transcript levels of myogenic differentiation marker genes, including MyoG, MYH1, MYH2, MYH4, and MYH7 (Figure 2B). Consistent with the results of transcript analysis, significantly higher levels of MyoG and MHC proteins were observed in the mimic group than in the NC group (Figure 2C). The immunofluorescence assay showed that bta-miR-24-3p mimics significantly increased the total number of MHC-positive cells at the end of myogenic differentiation, as compared with the control group (Figure 2D). Taken together, these results point to a role of bta-miR-24-3p in promoting myogenic differentiation.
Figure 2.
The expression of bta-miR-24-3p during the myogenic differentiation of bovine PDGFRα- progenitor cells. (A) bta-miR-24-3p expression 48 h after miR-24-3p mimics transfection, determined by qRT-PCR. (B) Transcript levels of myogenin and MHCs detected by qRT-PCR on D2 and D5. (C) Western blot evaluating the protein levels of myogenin and MHC 96 h following differentiation. (D) MHC-positive cells (green) on D2 and D5 of myogenic differentiation, visualized by immunofluorescence. Scale bars represent 100 µm. All data are represented as mean ± standard deviation (SD,) based on at least three independent experiments for each treatment. * p < 0.05 and ** p < 0.01.
3.3. Bta-miR-24-3p Inhibits Bovine PDGFRα- Progenitor Cell Proliferation
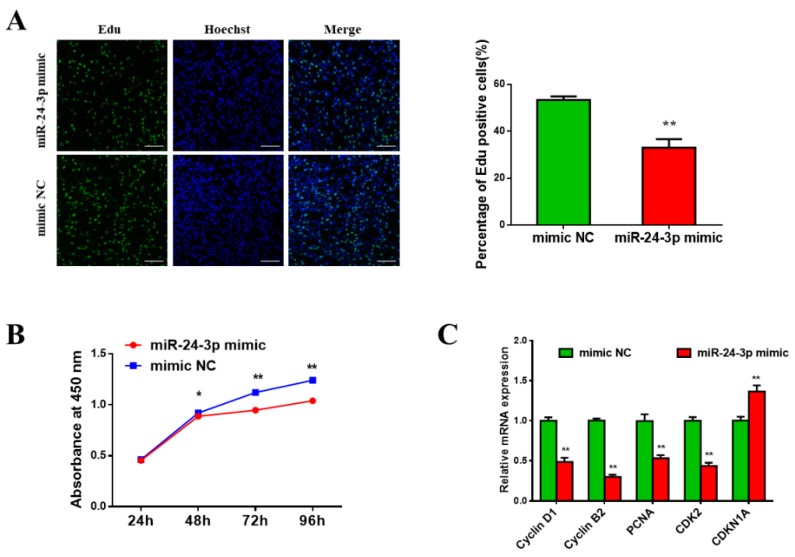
To further investigate the role of bta-miR-24-3p in bovine PDGFRα- progenitor cell proliferation, an EdU assay and CCK8 assay were used to analyze the proliferation of cells transfected by miR-24-3p mimics or NC. The EdU assays showed that miR-24-3p overexpression significantly decreased the proportion of EdU-positive cells (Figure 3A). The CCK8 assay revealed that miR-24-3p overexpression inhibited bovine PDGFRα- progenitor cell proliferation (Figure 3B). In addition, the transcript levels of cyclin D1, cyclin B1, proliferating cell nuclear antigen (PCNA), and cyclin-dependent kinase 2 (CDK2)—all of which are cell cycle-promoting genes—were decreased upon miR-24-3p overexpression. Conversely, miR-24-3p overexpression promotes the expression of the cell proliferation-inhibiting gene cyclin-dependent kinase inhibitor 1A (CDKN1A) (Figure 3C). These results demonstrate the inhibitory effect of bta-miR-24-3p on bovine PDGFRα- progenitor cell proliferation.
Figure 3.
The effect of miR-24-3p on bovine PDGFRα- progenitor cell proliferation. (A) EdU staining of the PDGFRα- progenitor cells after the transfection of miR-24-3p mimics and EdU-positive cell determination. (B) Cell Counting Kit-8 (CCK8) assay showing that miR-24-3p mimics inhibit PDGFRα- progenitor cell proliferation. (C) The relative transcript levels of cell cycle-related genes after transfection. Scale bars = 100 µm. All data are represented as mean ± standard deviation (SD), based on at least three independent experiments for each treatment. * p < 0.05 and ** p < 0.01.
3.4. ACVR1B Is a Direct Target of Bta-miR-24-3p
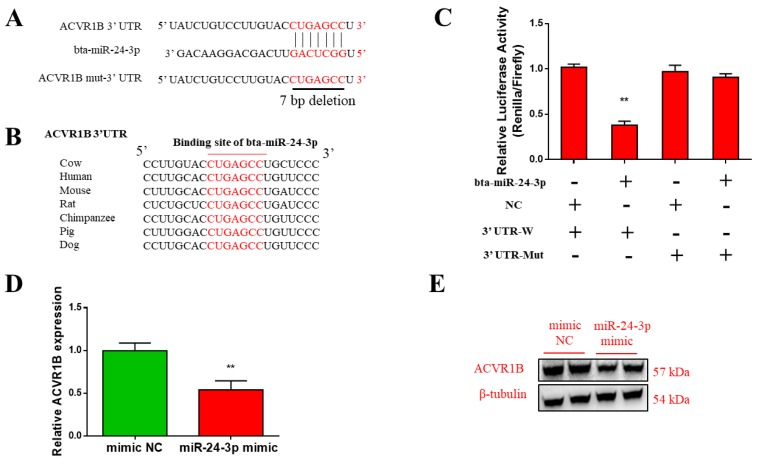
To further explore the molecular mechanism of how bta-miR-24-3p regulates bovine myogenesis, we predicted miR-24-3p targets using the TargetScanHuman 7.2 databases (http://www.targetscan.org/vert_72/). The seed sequence of bta-miR-24-3p perfectly matches the 3′ untranslated region (UTR) of ACVR1B, suggesting that ACVR1B is a potential target of bta-miR-24-3p (Figure 4A). Furthermore, we found that the potential binding site of bta-miR-24-3p was highly conserved among mammalian ACVR1B genes (Figure 4B).
Figure 4.
bta-miR-24-3p directly targets ACVR1B gene. (A) The predicted binding site of bta-miR-24-3p in ACVR1B 3’-UTR. The predict binding site (marked in red) was deleted in the mutated 3′-UTR reporter. (B) Sequence alignment of the mature bta-miR-24-3p binding sites in seven different species. (C) HEK293T cells were co-transfected with bta-miR-24-3p mimics, as well as the dual-luciferase reporters linked to the wild-type or mutant bta-miR-24-3p binding sites. Relative luciferase activity was analyzed 48 h after transfection (** p < 0.01). (D and E) The transcript and protein levels of ACVR1B in HEK293T cells transfected with bta-miR-24-3p mimics 48 h after transfection. All data are represented as mean ± standard deviation (SD), based on at least three independent experiments for each treatment. * p < 0.05 and ** p < 0.01.
To verify the interaction between bta-miR-24-3p and ACVR1B, we inserted the fragment harboring either the wild-type or mutated bta-miR-24-3p binding sequence in ACVR1B into a dual-luciferase reporter vector. Each of the resulting constructs was separately introduced into 293T cells, along with bta-miR-24-3p mimics, and luciferase activity was detected 48 h after transfection. We detected significantly decreased luciferase activity in 293T cells expressing the wild-type ACVR1B 3’ UTR fragment, whereas that of 293T cells expressing the mutated fragment was not changed (Figure 4C). This result suggests that the analyzed 3’ UTR sequence of ACVR1B is involved in bta-miR-24-3p recognition. More importantly, compared with the control, we observed a significant reduction in the ACVR1B transcript level in bovine PDGFRα- progenitor cells overexpressing bta-miR-24-3p (Figure 4D). The protein level of ACVR1B in PDGFRα- progenitor cells was also decreased upon overexpressing miR-24-2 mimics (Figure 4E). Collectively, these results demonstrate that ACVR1B is a direct target of bta-miR-24-3p in bovine PDGFRα- progenitor cells.
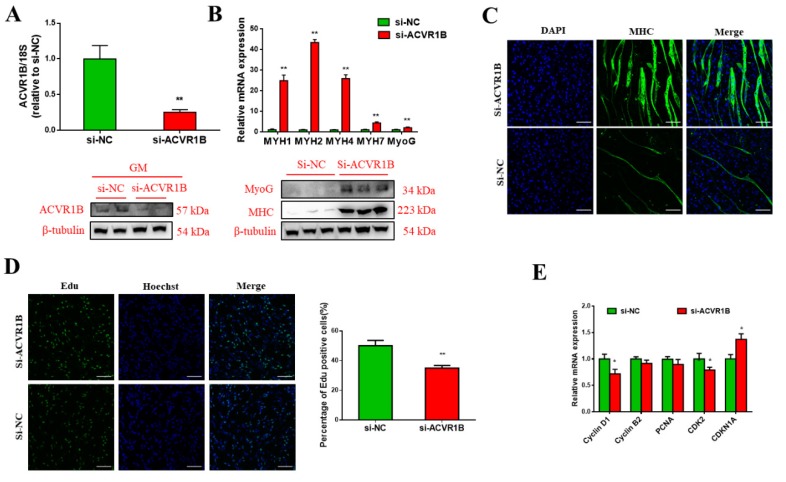
3.5. ACVR1B Silencing Promotes Myogenic Differentiation But Inhibits Proliferation
To examine the role of ACVR1B in myogenic differentiation, we silenced the endogenous ACVR1B in bovine PDGFRα- progenitor cells using small interfering RNA (siRNA). The interference efficiency was verified, and the siRNA against ACVR1B (si-ACVR1B) significantly decreased the transcript and protein levels of ACVR1B (Figure 5A). Moreover, a similar result was obtained on day 5 of myogenic differentiation, when si-ACVR1B significantly upregulated the transcript levels of myogenesis marker genes, MyoG, MYH1, MYH2, MYH4, and MYH7 (Figure 5B). Consistently, the protein levels of MyoG and MHC in the myogenic differentiation increased upon ACVR1B knockdown (Figure 5B). Furthermore, more multinuclear myotubes were observed in the knockdown group (Figure 5C). The EdU assay revealed decreased number of EdU-positive cells in the si-ACVR1B group compared with the control (Figure 5D), along with reduced Cyclin D1 and CDK2 but increased CDKN1A mRNA levels (Figure 5E). Together, these findings suggest that knocking down ACVR1B significantly promotes myogenic differentiation, but represses the proliferation of bovine PDGFRα- progenitor cells.
Figure 5.
ACVR1B knockdown promotes myogenic differentiation, but inhibits proliferation. (A) The transcript and protein levels of ACVR1B in PDGFRα- progenitor cells transfected with si-ACVR1B or si-NC. (B) The transcript and protein levels of MHC and MyoG on D5 of differentiation in bovine PDGFRα- progenitor cells transfected with si-ACVR1B or si-NC. (C) MHC-positive cells on D5 of myogenic differentiation in PDGFRα- progenitor cells transfected with si-ACVR1B and si-NC, as visualized by immunofluorescence. (D) EdU staining assay of PDGFRα- progenitor cells transfected with si-ACVR1B or si-NC, and the determination of EdU-positive cells. (E) Relative transcript levels of cell cycle-related genes in PDGFRα- progenitor cells transfected with si-ACVR1B or si-NC. Scale bars = 100 µm. All data are represented as mean ± standard deviation (SD), based on at least three independent experiments for each treatment. * p < 0.05 and ** p < 0.01.
4. Discussion
miR-24-2 is transcribed and processed from miR-23a/27a/24-2 clusters. It is suggested that miR-24-2 is involved in many physiological and pathological processes, including cell differentiation, proliferation, apoptosis, and metabolism, as well as disease occurrence [23,24,25,26]. miR-24-3p suppresses apoptosis by targeting the Keap1-Nrf2 Pathway [27]. In lung cancer cells, miR-24-3p could promote cell proliferation and migration; it also accelerates tumor growth in xenograft mice by directly binding to SRY-box transcription factor 7 (SOX7) [28]. MiR-24 also participates in erythropoiesis [29]. miR-24-3p controls osteogenic differentiation via regulating SMAD family member 5 (Smad5) in human periodontal ligament stem cells (hPDLSCs) [30]. Previous studies have demonstrated that miR-24-3p can promote the proliferation of several types of cancer cells [25,31,32,33,34,35], which contradicts our results. However, some studies have demonstrated that miR-24 could target high mobility group box 1 (HMGB1) and inhibit the proliferation and migration of vascular smooth muscle cells [36]. These conflicting results may be mainly due to the differences in the cell types used. Also, miR-24 was found to inhibit proliferation by prohibiting or delaying the G1-to-S transition [37]. Although the effect of miR-24-3p on proliferation and myogenic differentiation has been previously reported, its target remains unclear. Here, we identified ACVR1B as a direct target of bta-miR-24-3p using an in vitro model of fetal bovine myogenesis, and illustrated that bta-miR-24-3p inhibits proliferation and promotes myogenic differentiation of progenitor cells by targeting ACVR1B.
ACVR1B is a member of the TGF-β pathway, which transduces extracellular signaling into the cytoplasm [38]. TGF-β inhibits myogenesis through inhibiting the expression and function of MRFs. It has been shown that TGF-β reduces the expression of myogenin and myocyte enhancer factor 2D (MEF2D), as well as myotube formation in the C2C12 model via the Smad pathway, in a cell density-dependent manner [39]. In addition, TGF-β induces MEF2 translocation from the nucleus to the cytoplasm, thereby inhibiting MEF2 transcription [40]. It has also been demonstrated that TGF-β inhibits myogenic differentiation via intracellular effector Smad3 in C3H10T1/2 and C2C12 cell lines by repressing the activity of MyoD family members [41]. Moreover, TGF-β treatment inhibits the transcriptional upregulation of p21 and muscle creatinine kinase (MCK) in C2C12 cells, which are associated with growth arrest at the G1 phase and representative cell-lineage-specific expression during myogenesis [42]. Furthermore, TGF-β could induce miRNAs, such as miR-206 and miR-29, to control myogenic differentiation by regulating the expression of HDAC4 [43].
ACVR1B regulates the pluripotency and differentiation of stem cells in osteoclastogenesis, myogenesis, and adipogenesis [44,45,46]. The activated type I receptor phosphorylates and thereby activates specific Smads in the canonical signaling pathway, which enter into the nucleus to control the gene transcriptional cascades [38,47,48]. Although several types of ligands share common receptors or downstream Smads, they could fulfill their essential functions in vivo without interference from the many other proteins of the superfamily. The ligands structure evolution enables distinct modes of receptor binding, and engenders the TGF-βs with high specificity for their receptors [49]. The SMAD complexes have weak affinity for DNA and limited specificity, and need cooperate with other site-specific transcription factors [48]. The differences in the expression of these transcription factors may contribute to the diverse effects of ACVR1B activation in different cell types.
In skeletal muscle, ACVR1B mediates the myostatin signaling, whose dysregulation is associated with increased muscle mass in mammals [50,51,52]. Myostatin prefers ACVR1B in myoblasts, whereas it utilizes ALK5 in non-myogenic cells [53]. In previous studies, myostatin has been demonstrated to inhibit the myogenic differentiation of C2C12 cells [54,55]. ACVR1B inhibition promoted myogenetic differentiation in C2C12 in vitro, and reduced muscle mass and muscle fiber size in ACVR1B-silencing mice [18]. It is logical that miR-24-2 promotes differentiation by inhibiting ACVR1B expression to block myostatin signaling. However, some contradictory results have been reported in previous research studying the effect of myostatin on myogenic cell proliferation. Some studies have reported that myostatin inhibits cell proliferation [55,56,57,58]. Another study reported that myostatin stimulates C2C12 myoblast proliferation [59]. Such a contradiction may be a result of the alteration in gene expression caused by the immortalization process. Our study using cells freshly isolated from the fetal bovine skeletal muscle very likely revealed the actual function of ACVR1B in myogenic cell proliferation [60].
Other members of the miR-23a/27a/24-2 cluster also regulate muscle development. For example, miR-27a enhances myoblast differentiation, but the mechanism remains unclear [61]. Meanwhile, some studies have found that miRNA-23a/27a inhibits muscle atrophy [62,63]. The similar functions observed for miR-23a/27a/24-2 cluster members in myogenesis corroborate previous findings that miRNAs from the same cluster evolved to regulate functionally-related genes in a coordinated manner [64]. In this study, we demonstrate that bta-miR-24-3p accumulates and functions as a positive regulator during myogenic differentiation of fetal bovine PDGFRα- progenitor cells.
5. Conclusions
Taken together, our findings reveal that bta-miR-24-3p promotes myogenic differentiation by directly targeting and suppressing ACVR1B expression. Therefore, our findings on bta-miR-24-3p and its target gene ACVR1B provide a mechanistic basis for related future research, and have great potential in improving bovine skeletal muscle differentiation.
Acknowledgments
The authors would like to thank all of the staff at the cattle experimental unit in Beijing for sharing their insights.
Author Contributions
Conceptualization, X.H. and L.Z.; formal analysis, X.H.; funding acquisition, J.L. and L.Z.; investigation, X.H., Y.X., L.R., Y.W., Q.L., L.X., and L.W.; supervision, L.Z.; writing—original draft, X.H.; writing—review and editing, X.F. and Q.Y.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31672384), the Project of College Innovation Improvement under Beijing Municipality (PXM2016_014207_000012), and the Cattle Breeding Innovative Research Team (ASTIPIAS03).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Du M., Tong J., Zhao J., Underwood K.R., Zhu M., Ford S.P., Nathanielsz P.W. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010;88:E51–E60. doi: 10.2527/jas.2009-2311. [DOI] [PubMed] [Google Scholar]
- 2.Sabourin L.A., Rudnicki M.A. The molecular regulation of myogenesis. Clin. Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- 3.Lassar A.B., Skapek S.X., Novitch B. Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr. Opin. Cell Biol. 1994;6:788–794. doi: 10.1016/0955-0674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 4.Pownall M.E., Gustafsson M.K., Emerson C.P., Jr. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 2002;18:747–783. doi: 10.1146/annurev.cellbio.18.012502.105758. [DOI] [PubMed] [Google Scholar]
- 5.Yun K., Wold B. Skeletal muscle determination and differentiation: Story of a core regulatory network and its context. Curr. Opin. Cell Biol. 1996;8:877–889. doi: 10.1016/S0955-0674(96)80091-3. [DOI] [PubMed] [Google Scholar]
- 6.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Wahid F., Shehzad A., Khan T., Kim Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. BBA Mol. Cell Res. 2010;1803:1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Xie S.J., Li J.H., Chen H.F., Tan Y.Y., Liu S.R., Zhang Y., Xu H., Yang J.H., Liu S., Zheng L.L., et al. Inhibition of the JNK/MAPK signaling pathway by myogenesis-associated miRNAs is required for skeletal muscle development. Cell Death Differ. 2018;25:1581–1597. doi: 10.1038/s41418-018-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Yan H., Zhou P., Zhang Z., Liu J., Zhang H. MicroRNA-152 Promotes Slow-Twitch Myofiber Formation via Targeting Uncoupling Protein-3 Gene. Animals. 2019;9 doi: 10.3390/ani9090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W.R., Zhang H.N., Wang Y.M., Dai Y., Liu X.F., Li X., Ding X.B., Guo H. miR-143 regulates proliferation and differentiation of bovine skeletal muscle satellite cells by targeting IGFBP5. In Vitro Cell Dev. Biol. Anim. 2017;53:265–271. doi: 10.1007/s11626-016-0109-y. [DOI] [PubMed] [Google Scholar]
- 11.Song C., Yang J., Jiang R., Yang Z., Li H., Huang Y., Lan X., Lei C., Ma Y., Qi X., et al. miR-148a-3p regulates proliferation and apoptosis of bovine muscle cells by targeting KLF6. J. Cell. Physiol. 2019;234 doi: 10.1002/jcp.28232. [DOI] [PubMed] [Google Scholar]
- 12.Tong H., Jiang R., Liu T., Wei Y., Li S., Yan Y. bta-miR-378 promote the differentiation of bovine skeletal muscle-derived satellite cells. Gene. 2018;668:246–251. doi: 10.1016/j.gene.2018.03.102. [DOI] [PubMed] [Google Scholar]
- 13.Sun Q., Zhang Y., Yang G., Chen X., Zhang Y., Cao G., Wang J., Sun Y., Zhang P., Fan M., et al. Transforming growth factor-beta-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res. 2008;36:2690–2699. doi: 10.1093/nar/gkn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y., Wang H., Li Y., Liu S., Chen J., Ying H. miR-24 and miR-122 Negatively Regulate the Transforming Growth Factor-beta/Smad Signaling Pathway in Skeletal Muscle Fibrosis. Mol. Nucleic Acids. 2018;11:528–537. doi: 10.1016/j.omtn.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman C.M., Mathews L.S. Activin receptors: Cellular signalling by receptor serine kinases. Biochem. Soc. Symp. 1996;62:25–38. [PubMed] [Google Scholar]
- 16.Reissmann E., Jornvall H., Blokzijl A., Andersson O., Chang C., Minchiotti G., Persico M.G., Ibanez C.F., Brivanlou A.H. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–2022. doi: 10.1101/gad.201801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsdell A.F., Bernanke J.M., Trusk T.C. Left-right lineage analysis of the embryonic Xenopus heart reveals a novel framework linking congenital cardiac defects and laterality disease. Development. 2006;133:1399–1410. doi: 10.1242/dev.02292. [DOI] [PubMed] [Google Scholar]
- 18.Pasteuning-Vuhman S., Boertje-van der Meulen J.W., van Putten M., Overzier M., Ten Dijke P., Kielbasa S.M., Arindrarto W., Wolterbeek R., Lezhnina K.V., Ozerov I.V., et al. New function of the myostatin/activin type I receptor (ALK4) as a mediator of muscle atrophy and muscle regeneration. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017;31:238–255. doi: 10.1096/fj.201600675R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuno Y., Tokuzawa Y., Ninomiya Y., Yagi K., Yatsuka-Kanesaki Y., Suda T., Fukuda T., Katagiri T., Kondoh Y., Amemiya T., et al. miR-210 promotes osteoblastic differentiation through inhibition of AcvR1b. FEBS Lett. 2009;583:2263–2268. doi: 10.1016/j.febslet.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Qiu W., Li X., Tang H., Huang A.S., Panteleyev A.A., Owens D.M., Su G.H. Conditional activin receptor type 1B (Acvr1b) knockout mice reveal hair loss abnormality. J. Investig. Dermatol. 2011;131:1067–1076. doi: 10.1038/jid.2010.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uezumi A., Fukada S., Yamamoto N., Takeda S., Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 22.Guan L., Hu X., Liu L., Xing Y.S., Zhou Z.K., Liang X.W., Yang Q.Y., Jin S.Y., Bao J.S., Gao H.J., et al. bta-miR-23a involves in adipogenesis of progenitor cells derived from fetal bovine skeletal muscle. Sci. Rep. UK. 2017;7 doi: 10.1038/srep43716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu F., Pang G., Zhao G. ANRIL acts as onco-lncRNA by regulation of microRNA-24/c-Myc, MEK/ERK and Wnt/beta-catenin pathway in retinoblastoma. Int. J. Biol. Macromol. 2019;128:583–592. doi: 10.1016/j.ijbiomac.2019.01.157. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y., Sun Y., Zhou Y., Zhang Y., Zhang T., Li Y., You W., Chang X., Yuan L., Han X. MicroRNA-24 promotes pancreatic beta cells toward dedifferentiation to avoid endoplasmic reticulum stress-induced apoptosis. J. Mol. Cell Biol. 2019 doi: 10.1093/jmcb/mjz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J., Yin K., Lv X., Yang Q., Shao M., Liu X., Sun H. MicroRNA-24-3p regulates Hodgkin’s lymphoma cell proliferation, migration and invasion by targeting DEDD. Oncol. Lett. 2019;17:365–371. doi: 10.3892/ol.2018.9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Y., Yang Y. MiR-24 inhibits inflammatory responses in LPS-induced acute lung injury of neonatal rats through targeting NLRP3. Pathol. Res. Pract. 2019;215:683–688. doi: 10.1016/j.prp.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Xiao X., Lu Z., Lin V., May A., Shaw D.H., Wang Z., Che B., Tran K., Du H., Shaw P.X. MicroRNA miR-24-3p Reduces Apoptosis and Regulates Keap1-Nrf2 Pathway in Mouse Cardiomyocytes Responding to Ischemia/Reperfusion Injury. Oxidative Med. Cell. Longev. 2018;2018:7042105. doi: 10.1155/2018/7042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan L., Ma J., Zhu Y., Zan J., Wang Z., Ling L., Li Q., Lv J., Qi S., Cao Y., et al. miR-24-3p promotes cell migration and proliferation in lung cancer by targeting SOX7. J. Cell. Biochem. 2018;119:3989–3998. doi: 10.1002/jcb.26553. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q., Huang Z., Xue H., Jin C., Ju X.L., Han J.D., Chen Y.G. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood. 2008;111:588–595. doi: 10.1182/blood-2007-05-092718. [DOI] [PubMed] [Google Scholar]
- 30.Li Z., Sun Y., Cao S., Zhang J., Wei J. Downregulation of miR-24-3p promotes osteogenic differentiation of human periodontal ligament stem cells by targeting SMAD family member 5. J. Cell. Physiol. 2019;234:7411–7419. doi: 10.1002/jcp.27499. [DOI] [PubMed] [Google Scholar]
- 31.Jin L., Li Y., Nie L., He T., Hu J., Liu J., Chen M., Shi M., Jiang Z., Gui Y., et al. MicroRNA242 is associated with cell proliferation, invasion, migration and apoptosis in renal cell carcinoma. Mol. Med. Rep. 2017;16:9157–9164. doi: 10.3892/mmr.2017.7705. [DOI] [PubMed] [Google Scholar]
- 32.Hua K., Chen Y.T., Chen C.F., Tang Y.S., Huang T.T., Lin Y.C., Yeh T.S., Huang K.H., Lee H.C., Hsu M.T., et al. MicroRNA-23a/27a/24-2 cluster promotes gastric cancer cell proliferation synergistically. Oncol. Lett. 2018;16:2319–2325. doi: 10.3892/ol.2018.8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou N., Yan H.L. MiR-24 promotes the proliferation and apoptosis of lung carcinoma via targeting MAPK7. Eur. Rev. Med. Pharmacol. Sci. 2018;22:6845–6852. doi: 10.26355/eurrev_201810_16153. [DOI] [PubMed] [Google Scholar]
- 34.Liu L., Pan J., Wang H., Ma Z., Yin J., Yuan F., Yuan Q., Zhou L., Liu X., Zhang Y., et al. von Willebrand factor rescued by miR-24 inhibition facilitates the proliferation and migration of osteosarcoma cells in vitro. Biosci. Rep. 2018;38 doi: 10.1042/BSR20180372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu G., Jia Z., Dou Z. miR-24-3p regulates bladder cancer cell proliferation, migration, invasion and autophagy by targeting DEDD. Oncol. Rep. 2017;37:1123–1131. doi: 10.3892/or.2016.5326. [DOI] [PubMed] [Google Scholar]
- 36.Yang J., Chen L., Ding J., Fan Z., Li S., Wu H., Zhang J., Yang C., Wang H., Zeng P., et al. MicroRNA-24 inhibits high glucose-induced vascular smooth muscle cell proliferation and migration by targeting HMGB1. Gene. 2016;586:268–273. doi: 10.1016/j.gene.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 37.Lal A., Navarro F., Maher C.A., Maliszewski L.E., Yan N., O’Day E., Chowdhury D., Dykxhoorn D.M., Tsai P., Hofmann O., et al. miR-24 Inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3’UTR microRNA recognition elements. Mol. Cell. 2009;35:610–625. doi: 10.1016/j.molcel.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng X.H., Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 39.Song C., Fan B., Xiao Z. Overexpression of ALK4 inhibits cell proliferation and migration through the inactivation of JAK/STAT3 signaling pathway in glioma. Biomed. Pharmacother. 2018;98:440–445. doi: 10.1016/j.biopha.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 40.Zhu S., Goldschmidt-Clermont P.J., Dong C. Transforming growth factor-beta-induced inhibition of myogenesis is mediated through Smad pathway and is modulated by microtubule dynamic stability. Circ. Res. 2004;94:617–625. doi: 10.1161/01.RES.0000118599.25944.D5. [DOI] [PubMed] [Google Scholar]
- 41.De Angelis L., Borghi S., Melchionna R., Berghella L., Baccarani-Contri M., Parise F., Ferrari S., Cossu G. Inhibition of myogenesis by transforming growth factor beta is density-dependent and related to the translocation of transcription factor MEF2 to the cytoplasm. Proc. Natl. Acad. Sci. USA. 1998;95:12358–12363. doi: 10.1073/pnas.95.21.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu D., Black B.L., Derynck R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001;15:2950–2966. doi: 10.1101/gad.925901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murakami M., Ohkuma M., Nakamura M. Molecular mechanism of transforming growth factor-beta-mediated inhibition of growth arrest and differentiation in a myoblast cell line. Dev. Growth Differ. 2008;50:121–130. doi: 10.1111/j.1440-169X.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto Y., Otsuka F., Hino J., Miyoshi T., Takano M., Miyazato M., Makino H., Kangawa K. Bone morphogenetic protein-3b (BMP-3b) inhibits osteoblast differentiation via Smad2/3 pathway by counteracting Smad1/5/8 signaling. Mol. Cell. Endocrinol. 2012;350:78–86. doi: 10.1016/j.mce.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 45.Rebbapragada A., Benchabane H., Wrana J.L., Celeste A.J., Attisano L. Myostatin Signals through a Transforming Growth Factor β-Like Signaling Pathway To Block Adipogenesis. Mol. Cell. Biol. 2003;23:7230–7242. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine A.J., Brivanlou A.H. GDF3 at the crossroads of TGF-beta signaling. Cell Cycle. 2006;5:1069–1073. doi: 10.4161/cc.5.10.2771. [DOI] [PubMed] [Google Scholar]
- 47.Hata A., Chen Y.G. TGF-beta Signaling from Receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016;8 doi: 10.1101/cshperspect.a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill C.S. Transcriptional Control by the SMADs. Cold Spring Harb. Perspect. Biol. 2016;8 doi: 10.1101/cshperspect.a022079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinck A.P. Structural studies of the TGF-betas and their receptors - insights into evolution of the TGF-beta superfamily. FEBS Lett. 2012;586:1860–1870. doi: 10.1016/j.febslet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 50.Clop A., Marcq F., Takeda H., Pirottin D., Tordoir X., Bibe B., Bouix J., Caiment F., Elsen J.M., Eychenne F., et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat. Genet. 2006;38:813–818. doi: 10.1038/ng1810. [DOI] [PubMed] [Google Scholar]
- 51.McPherron A.C., Lawler A.M., Lee S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 52.McPherron A.C., Lee S.J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kemaladewi D.U., de Gorter D.J., Aartsma-Rus A., van Ommen G.J., ten Dijke P., ’t Hoen P.A., Hoogaars W.M. Cell-type specific regulation of myostatin signaling. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012;26:1462–1472. doi: 10.1096/fj.11-191189. [DOI] [PubMed] [Google Scholar]
- 54.Langley B., Thomas M., Bishop A., Sharma M., Gilmour S., Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 2002;277:49831–49840. doi: 10.1074/jbc.M204291200. [DOI] [PubMed] [Google Scholar]
- 55.Joulia D., Bernardi H., Garandel V., Rabenoelina F., Vernus B., Cabello G. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp. Cell Res. 2003;286:263–275. doi: 10.1016/S0014-4827(03)00074-0. [DOI] [PubMed] [Google Scholar]
- 56.Taylor W.E., Bhasin S., Artaza J., Byhower F., Azam M., Willard D.H., Jr., Kull F.C., Jr., Gonzalez-Cadavid N. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am. J. Physiol. Endocrinol. Metab. 2001;280:E221–E228. doi: 10.1152/ajpendo.2001.280.2.E221. [DOI] [PubMed] [Google Scholar]
- 57.Thomas M., Langley B., Berry C., Sharma M., Kirk S., Bass J., Kambadur R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J. Biol. Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 58.Garikipati D.K., Rodgers B.D. Myostatin inhibits myosatellite cell proliferation and consequently activates differentiation: Evidence for endocrine-regulated transcript processing. J. Endocrinol. 2012;215:177–187. doi: 10.1530/JOE-12-0260. [DOI] [PubMed] [Google Scholar]
- 59.Rodgers B.D., Wiedeback B.D., Hoversten K.E., Jackson M.F., Walker R.G., Thompson T.B. Myostatin stimulates, not inihibits, C2C12 myoblast proliferation. Endocrinology. 2014;155:670–675. doi: 10.1210/en.2013-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manceau M., Gros J., Savage K., Thome V., McPherron A., Paterson B., Marcelle C. Myostatin promotes the terminal differentiation of embryonic muscle progenitors. Genes Dev. 2008;22:668–681. doi: 10.1101/gad.454408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X., Huang Z., Chen D., Yang T., Liu G. Role of microRNA-27a in myoblast differentiation. Cell Biol. Int. 2014;38:266–271. doi: 10.1002/cbin.10192. [DOI] [PubMed] [Google Scholar]
- 62.Zhang A., Li M., Wang B., Klein J.D., Price S.R., Wang X.H. miRNA-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. J. Cachexia Sarcopenia Muscle. 2018;9:755–770. doi: 10.1002/jcsm.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang B., Zhang C., Zhang A., Cai H., Price S.R., Wang X.H. MicroRNA-23a and MicroRNA-27a Mimic Exercise by Ameliorating CKD-Induced Muscle Atrophy. J. Am. Soc. Nephrol. JASN. 2017;28:2631–2640. doi: 10.1681/ASN.2016111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y., Luo J., Zhang H., Lu J. microRNAs in the Same Clusters Evolve to Coordinately Regulate Functionally Related Genes. Mol. Biol. Evol. 2016;33:2232–2247. doi: 10.1093/molbev/msw089. [DOI] [PMC free article] [PubMed] [Google Scholar]