Abstract
The incidence of autoimmune type 1 diabetes (T1DM) is increasing worldwide and disease onset tends to occur at a younger age. Unfortunately, clinical trials aiming to detect predictive factors of disease, in individuals with a high risk of T1DM, reported negative results. Hence, actually there are no tools or strategies to prevent T1DM onset. The importance of the gut microbiome in autoimmune diseases is increasingly recognized and recent data suggest that intestinal dysbiosis has a pathogenic role in T1DM by affecting both intestinal immunostasis and the permeability of the gut barrier. An improved understanding of the mechanisms whereby dysbiosis in the gut favors T1DM development may help develop new intervention strategies to reduce both the incidence and burden of T1DM. This review summarizes available data on the associations between gut microbiota and T1DM in both experimental animals and humans and discusses future perspectives in this novel and exciting area of research.
Keywords: type 1 diabetes, microbiota, microbiome, auto-immunity, gut permeability
1. Introduction
In the last decade, the gut microbiota has gained increasing interest because of a major shift in our way of thinking: the microbiota is no longer considered a passive collection of microbes hosted in the gut, but an integral part of the body, providing important functions to the host. Landmark studies have significantly improved our understanding of microbiota function, dynamic, and interactions. Today, we know that the gut microbiota is involved in both the development and progression of many pathological conditions and targeting the microbiota has become a novel promising intervention strategy. Recent data suggest that an imbalance in gut microbiota (dysbiosis) could be involved in the pathogenesis of autoimmune type 1 diabetes (T1DM) and may contribute to explaining the global rise in T1DM incidence, particularly in childhood [1,2,3]. Indeed, a dysfunctional microbiota can favor the development of autoimmune diseases by altering the maturation of the immune system in early infancy and by increasing both intestinal permeability and inflammation. Despite major research effort, clinical trials in individuals with high T1DM risk reported negative results and there is no effective strategy for the primary prevention of T1DM. Hopefully, studying the role of the intestinal microbiota in T1DM may provide insights into developing new strategies to reduce T1DM incidence.
2. Type 1 Diabetes Mellitus
T1DM accounts for 5–10% of all diagnosed cases of diabetes and onset of the disease usually occurs during childhood or adolescence [4]. The International Diabetes Federation (IDF) estimated that more than a million subjects (<20 years) were affected by T1DM in 2017 and approximately 86,000 children develop T1DM every year; however, the incidence varies among countries and even in different areas within the same country [5]. In Europe, there is a north–south gradient, with Scandinavian countries exhibiting the highest incidence rate. The incidence is much lower in the Mediterranean area, though Sardinia has the second highest incidence in the world after Finland [6]. T1DM incidence has increased over the last 50–60 years in many countries, with an approximate 3% rise in incidence rate per year [6,7]. In some countries, the disease is also occurring at a much earlier age, with a marked increase in the 0–4 years age range [8], indicating a lowered threshold for T1DM development.
T1DM is characterized by absolute insulin deficiency due to pancreatic β cell loss. This process, occurring in genetically susceptible individuals, may be triggered by environmental factors, and progresses over many months or years, during which the subject is asymptomatic and euglycemic. Indeed, autoimmune β cell destruction by autoimmune T cells progresses slowly and hyperglycemia and symptomatic diabetes only develops when a critical mass of β cells (~80%) is lost. Therefore, clinical T1DM is preceded by a silent phase in which the presence of autoantibodies (insulin, glutamic acid decarboxylase, insulinoma-2-associated, and zinc transporter 8 autoantibodies), which are biomarkers of T1DM-associated autoimmunity, is the only detectable abnormality [9,10].
Clinical onset is characterized by polyuria, thirst, polydipsia, weight loss, hunger, polyphagia, and fatigue. Early recognition is important to prevent the development of diabetic ketoacidosis (DKA), a severe condition characterized by hyperglycemia, acidosis, ketonemia, and ketonuria. DKA occurs at the time of T1DM diagnosis in approximately 30% of children in the United States and the incidence rate in children with established diabetes is approximately 6–8% per year. DKA is the leading cause of morbidity and mortality in pediatric T1DM. Indeed, in children, cerebral injury occurs in 0.3 to 0.9 percent of DKA episodes and has a mortality of 20–25% [11].
In the absence of DKA, T1DM diagnosis is based on one of the following criteria: (1) random plasma glucose ≥ 200 mg/dL in a patient with classic symptoms of hyperglycemia, (2) fasting plasma glucose ≥ 126 mg/dL, (3) plasma glucose ≥ 200 mg/dL two hours after a glucose load of 1.75 g/kg (oGTT), and (4) glycated hemoglobin (HbA1C) ≥ 6.5%. Unless symptomatic hyperglycemia is present, the diagnosis should be confirmed by repeat testing [4]. Because of the rapid T1DM onset, HbA1C may not be markedly elevated; therefore, an HbA1C level below 6.5% does not exclude the diagnosis of diabetes in pediatric patients.
Once it is diagnosed, T1DM requires immediate treatment with insulin replacement therapy. Intensive insulin therapy combines the administration of long-acting basal insulin together with pre-meal boluses of rapid-acting insulin to mimic the physiological secretion of insulin by the β cells. This can be achieved by either Multiple Daily insulin Injections (MDI) or Continuous Subcutaneous Insulin Infusion (CSII). The general aim of T1DM therapy is to maintain glucose control as near to normal as possible without causing hypoglycemia. In addition, T1DM care should also include diabetes education, self-management training, patient-tailored plans on healthy nutrition and exercise, and psychosocial support [4].
To prevent the burden of T1DM care, the best approach would be the primary prevention of T1DM in subjects at risk of developing the disease [12]. However, clinical trials focusing on primary prevention failed to prove benefit so far and there is no effective tool to block the development of the disease [12]. This is, at least in part, due to our poor understanding of T1DM pathogenesis. HLA-DR and -DQ genes account for 40–50% of the susceptibility to the disease [13]. Moreover, recent studies suggest an important role of gene polymorphisms, such as Toll-like receptor exon polymorphisms and polymorphisms in genes critical for vitamin D metabolism [14,15,16,17]. However, genetics alone cannot explain T1DM onset, as less than 10% of the children, who are genetically predisposed, develop the disease. Thus, environmental factors that affect the immune response, changing the probability of an autoimmune reaction against β cells in genetically susceptible subjects, play a crucial role. Environmental influences implicated in the pathogenesis of T1DM include pregnancy-related and perinatal factors, viruses, ingestion of cow’s milk and cereals, and use of both antibiotics and probiotics [18]. These factors also affect the microbiota, adding to the hypothesis of a role of the gut microbiome in linking environment influences with the development of T1DM.
3. The Microbiota
The microbiota is defined as the set of microorganisms living in symbiosis with their human/animal host [19]. The human microbiota consists of symbiotic microbial cells harbored on skin, respiratory and urogenital systems and gastrointestinal tract. In the latter, microbial populations reach the highest density and form a complex microbial community of ~4 × 1013 cells [19,20]. Although the gut microbiota includes different types of microorganisms, bacteria are the best studied. Sequencing the 16S rRNA is a key methodology to identify and classify these bacterial populations [21,22]. Furthermore, new metagenomic methods can precisely identify functional and strain-specific differences in the microbiome [23]. Healthy adults share most gut bacterial populations, which constitute a “core microbiota”. In fact, even if thousands of bacterial species have been isolated from human intestine, most of them belong to just four phyla: Bacteroidetes and Firmicutes are predominant (90%), while Actinobacteria and Proteobacteria are less abundant [24]. However, the concept of the “core microbiota” has been challenged by data showing a vast microbial diversity both over time and across human populations [25].
Colonization of the gut starts at birth, though recent studies questioned the dogma that the womb is a sterile environment, and is markedly affected by the mode of delivery. Vaginally delivered infants acquire microbial communities resembling maternal vaginal microbiota, predominantly Lactobacillus. In contrast, C-section infants harbor bacterial strains similar to those found on the skin, such as Staphylococcus and Clostridium genera [26,27]. Fecal microbiota resembles that of the mothers in 72% of vaginally delivered infants, while this percentage is reduced to 41% in babies delivered by C-section [26]. However, recent evidence shows that maternal skin and vaginal strains only transiently colonize the infant and that mother-to-infant transmission of gut strains, which occurs after birth via unclear mechanisms, is more relevant and persistent [28].
The intestinal microbiota of newborns is generally low in diversity and is dominated by two main phyla: Actinobacteria and Proteobacteria. Later, the gut microbiota becomes more diverse, with the predominance of Firmicutes and Bacteroidetes. By approximately 2–3 years of age, the composition, diversity and function of the microbiota are very similar to that of adult subjects.
Although the gut microbiota is relatively stable in adults compared to infants, it can undergo major changes in response to environmental influences, such as diet composition, the use of drugs, the lifestyle, environment hygiene standards and geographical location [29]. For instance, diet composition can modulate gut bacteria composition since childhood [26,30]. In breastfed infants, the gut microbiota is characterized by higher levels of probiotic genera, such as Lactobacillus and Bifidobacterium, and shows a lower microbial diversity compared to that of formula-fed babies [26,30]. Moreover, the consumption of animal proteins is positively associated with the presence of Bacteroides and Alistipes genera, while fiber intake is linked to increased Bifidobacterium and Lactobacillium genera, both in childhood and adulthood [31,32]. These genera are also enhanced by supplementation with probiotics [31,33]. Other environmental factors that greatly influence the gut microbiota composition are the use of drugs, particularly antibiotics, and geographical location [25]. Although it is well established that antibiotic therapy can reshape the gut microbiota, it remains unclear whether the microbial community can fully recover following antibiotic treatment [34,35].
The gut microbiota becomes unstable and less diverse with aging and the centenarian microbiota shows an increase in facultative anaerobe species, such as Escherichia coli, and a rearrangement of the profile of butyrate producers, such as a decrease in Faecalibacterium prausnitzii [36]. These events are closely associated with both an age-related decline in immunocompetence and coexisting illnesses [36].
4. Importance of the Microbiota to the Host
The human gut microbiota plays a multitude of important functions. It provides protection against pathogen overgrowth, preventing their colonization via the inhibition of adherence, production of bacteriocins, and both site and nutrient competition. It metabolizes specific drugs and eliminates exogenous toxins, thus playing an important detoxifying role. It is involved in the synthesis of essential vitamins, such as B1, B2, B5, B6, B12, K, folic acid, and performs biliary acid deconjugation. In addition, fermentation of indigestible carbohydrates by intestinal bacteria allows their use as a source of energy and provides 5–10% of daily energy requirement [37,38]. Furthermore, in the normal colon, the gut microbiota contributes to intestinal repair by promoting cellular proliferation and differentiation and it is of paramount importance in maintaining the integrity of the gut barrier [37]. Growing evidence points to a key role of the gut microbiota in both maturation and continued education of the host immune system [39]. Indeed, the immune system learns to discriminate between commensal and pathogenic bacteria and, following education, commensal bacteria triggers an anti-inflammatory response, while pathogenic bacteria a pro-inflammatory reaction. Moreover, the gut microbiota modulates both the migration and function of neutrophils and affects T cell differentiation, favoring both the differentiation and expansion of regulatory T cells (Tregs), which are key mediators of immune tolerance. An important mechanism by which the gut microbiota controls the immune system is through the formation of short-chain fatty acids (SCFAs), such as butyrate, acetate, and propionate [38,40]. SCFAs are mainly generated by bacterial fermentation of non-digestible carbohydrates, such as dietary fiber [40]. In T lymphocytes, SCFAs activate G protein-coupled receptors (GPR41/GPR43) signaling pathways, inhibit histone deacetylases, and induce metabolic alterations by enhancing the activity of the mammalian target of rapamycin (mTOR) complex. This results in the inhibition of inflammatory cascades, the expansion of mucosal Tregs, and the decreased production of inflammatory cytokines, such as interleukin-10 (IL-10) and interferon-γ (IFN-γ) [40].
Given the key role of the gut microbiota in maintaining the host health, it is not surprising that alterations in the microbiota (dysbiosis) could be implicated in the pathogenesis of a growing number of extraintestinal diseases including T1DM [41,42].
The term “dysbiosis” refers to an imbalance of the gut microbiota due to the loss of beneficial microbial organisms, the expansion of potentially harmful microorganisms and/or lower microbial diversity [43,44]. Although this term has been widely used in the field of microbiota research, the concept of gut microbiota dysbiosis, which is predominantly based on early microbiome taxonomic studies, is poorly defined and has been recently questioned [45]. Given the complexity and great variability of the human microbiota, changes in the microbiota may be irrelevant to the host health and there is an increasing quest for more rigorous criteria, such as modified Koch criteria, to identify abnormalities pertinent to the microbiota that can be of clinical relevance.
5. Microbiota and Type 1 Diabetes Mellitus
5.1. The Microbiota in Experimental T1DM
The possible role of the gut microbiota in the pathogenesis of autoimmune diabetes was first suggested in the 1980s, but scientific interest on this topic has dramatically increased in the last two decades. Experimental studies have been predominantly performed in animal models of autoimmune diabetes such as Non-Obese Diabetic (NOD) mice and BioBreeding diabetes-prone rats. Consistent with the hypothesis that dysbiosis occurs in T1DM, most of the studies in experimental autoimmune diabetes reported changes in both diversity and composition of gut microbiota. Moreover, fecal transplantation from NOD to Non-Obese Diabetes-Resistant (NOR) mice caused insulitis, indicating a diabetogenic effect of the NOD microbiota [46]. Cross fostering is another effective mean to induce a sustained microbial shift. Cross fostering of NOD mice by NOR mice was capable to protect from diabetes. Specifically, no development of diabetes was observed in NOD male mice nursed by a NOR mother, compared to an 80% incidence of T1DM in male NOD mice nursed by NOD mothers [47].
The link between microbiota-induced innate immunity alterations and T1DM risk was highlighted by studies performed in animals lacking Myeloid differentiation primary response 88 (MyD88), an adaptor protein for the toll-like receptors that recognize microbial patterns and regulate the innate immune response [48]. NOD mice lacking MyD88 were protected from T1DM, indicating that MyD88 is important in T1DM development [48]. However, MyD88 deletion in germ-free NOD mice increased the risk of T1DM [48]. Therefore, the protective effect of MyD88 deficiency is microbiota-dependent, confirming that interactions between the microbiota and innate immunity are important in the development of the disease. In addition, inflammatory lymphocytes, such as T helper 17 and type 3 innate lymphoid cells, are increased in the intestinal lamina propria of NOD mice, while tolerogenic dendritic cells and Treg are reduced in the lymph nodes draining the gut [49]. These changes in adaptive immunity may favor the activation of auto-reactive T cells against β cell antigens and thus T1DM development.
Dysbiosis can also affect the integrity of the gut barrier, resulting in enhanced permeability and the transit of antigens. Tight junctions, sealing the gap between adjacent intestinal epithelial cells, control gut permeability, allowing nutrient absorption, while preventing the transit of dietary, bacterial, and viral antigens. The mucus layer covering the epithelium represents another important barrier and controls the type of commensal bacteria residing in the epithelium [50]. In early experimental autoimmune diabetes prior to the development of insulitis, dysbiosis and altered immunostasis are paralleled by abnormalities of the gut barrier, such as decreased numbers of goblet cells and diminished mucus production [49], leading to enhanced intestinal permeability [51,52,53]. Cross fostering of NOD mice by NOR mothers corrected the defect in mucus production, indicating a key role of NOD microbiota in the dysfunction of the gut barrier [49].
How the loss of gut barrier integrity triggers β cell autoimmunity remains unclear. It has been proposed that the entrance of antigenic bacterial components into the systemic circulation can directly induce β cell damage and/or activate β cell autoimmunity in the pancreatic lymph nodes. Consistent with this hypothesis, translocation of the microbiota to the pancreatic lymph nodes has been observed in the NOD model [49]. Alternatively, T cells recognizing β cell antigens can be activated by bacterial products in the gut and then travel to the pancreatic lymph nodes to induce β cell injury [54]. In line with this, the induction of chronic colitis, which alters both gut barrier integrity and mucus layer composition, triggered the activation of islet-reactive T cells in the gut mucosa and led to autoimmune diabetes in BDC2.5XNOD mice, an animal model mimicking subjects at high risk of T1DM. Moreover, treatment of these mice with antibiotics prevented T1DM, confirming that the microbiota was required to induce β cell autoimmunity [55]
Taken together, results from experimental animals support the hypothesis that the microbiota may play a role in the pathogenesis of T1DM by affecting both the immune response and the gut barrier integrity. However, the mechanisms leading to β cell autoimmunity are still unclear and require further investigations.
5.2. Intervention Studies in Experimental Autoimmune Diabetes
Several studies in susceptible experimental animals have explored whether intervention strategies affecting the microbiota can modulate the risk of developing T1DM. Probiotic supplementation [56,57,58], prebiotic assumption [59], antibiotic therapy [46,60,61,62,63,64], and SCFA supplementation [65,66] has been shown to reduce the risk of T1DM onset. NOD pups born to mothers treated with broad-spectrum antibiotics showed increased T1DM incidence together with changes in the microbiota and inflammatory alterations in the intestinal mucosa [67]. Similarly, NOD mice intermittently treated with a macrolide in early life showed increased insulitis, enhanced T1DM incidence, and abnormalities in both innate immunity and T cell differentiation [63]. More recently, a study has shown that a single early-life antibiotic course accelerated T1DM development in male NOD mice and this was paralleled by persistent changes in the gut microbiome and altered the expression of genes controlling both innate and adaptive immunity [68]. On the contrary, supplementation with VSL#3, a probiotic preparation containing eight different strains, decreased T1DM incidence in NOD mice [56]. Similarly, high doses of butyrate in the diet significantly reduced T1DM incidence in NOD mice through the expansion of Treg cells [65]. Recently, supplementation with low-methoxyl pectin (LMP) dietary fiber has been shown to reduce T1DM incidence and fasting glucose levels in NOD mice. This was paralleled by the enrichment of bacterial species producing SCFAs, reduced inflammation, the amelioration of gut barrier integrity and an increase in Treg cells. Antibiotic treatment worsened T1DM autoimmunity in this model, but the transfer of feces from LMP-fed mice reversed the effect of antibiotics [69], confirming the key role of the microbiome in mediating the beneficial effects of LMP. Treatment of NOD mice with the probiotic bacteria Clostridium butyricum increased Tregs in both mesenteric and pancreatic lymph nodes, enhanced expression of microbial genes important in butyrate production, and delayed the onset of T1DM [70].
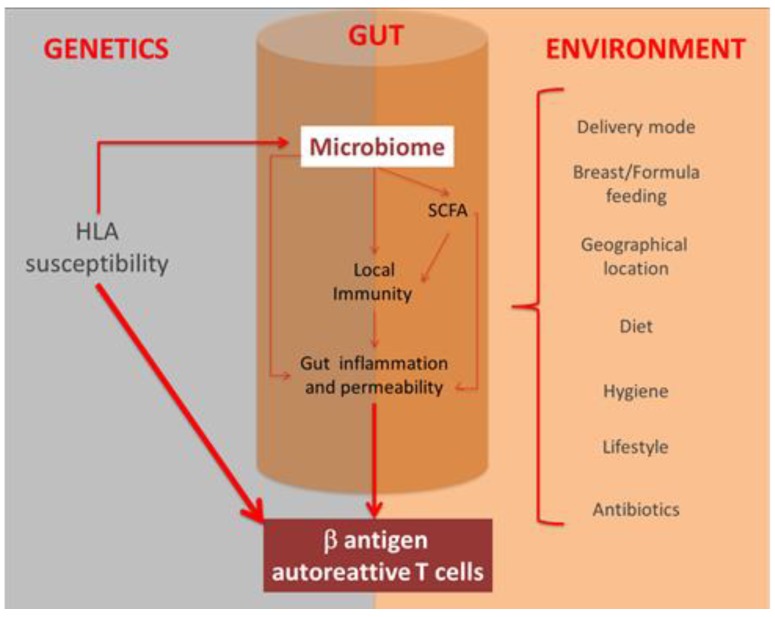
Taken together, these findings support the hypothesis of a causal link between changes in the microbiota composition and the risk of T1DM onset and they also suggest potential therapeutic strategies to restore a healthy microbiome. However, inbred mouse strains are genetically homogenous, and thus they cannot capture the inherent genetic variations of humans. Moreover, a number of factors (i.e., mode of delivery, diet, social activity) that shape human microbiota are absent in mice. Therefore, results from animal models are not easily translatable to humans and conclusions in experimental models should be taken with caution (Figure 1).
Figure 1.
Interplay between genetics, environment, and gut microbiota in T1DM pathogenesis. Specific HLA allele can increase susceptibility to the development of type 1 diabetes and influence the composition of the gut microbiota. Many environmental factors can also shape the gut microbiota. Abnormalities in the gut microbiome are believed to favor autoimmunity against pancreatic b-cells by modulating the gut immune/inflammatory response and by enhancing the gut barrier permeability both directly and indirectly through reduced production of short-chain fatty acids (SCFAs).
5.3. The Microbiota in Human T1DM
Table 1 summarizes both the design and results of a selection of recent studies performed in humans [1]. Studies exploring the role of the microbiota in human T1DM have been performed in different stages of the disease, ranging from genetic predisposition to clinical diabetes [71,72,73,74,75,76,77,78,79,80,81,82]. To support the hypothesis of a microbiota contribution to T1DM pathogenesis, studies carried out in the preclinical stage of the disease, identified by the appearance of one or more T1DM-specific autoantibodies in the circulation (seroconversion), are of particular relevance [71,72,73,76,77,78,79,80,82]. Longitudinal studies in large series of children are also of paramount importance as they can prove that microbiome abnormalities precede seroconversion and/or clinical T1DM onset [71,73,76,77,78,80,82].
Table 1.
Type 1 Diabetes and the Microbiota: Human Studies.
| Study Design | Study Sample | Main Findings | Ref. |
|---|---|---|---|
| Prospective | In total, 33 genetically predisposed infants | Microbial diversity in T1DM progressors in the time window between seroconversion and T1DM onset and inflammation-favoring organisms | [71] |
| Case Control | In total, 18 Ab (+) vs. 18 Ab (-) children matched for HLA | Ab-positive children: microbial diversity, Bacteroidetes (Bacteroides and Prevotella), Bifidobacterium species, short-chain fatty acid (SCFA)-producing bacteria | [72] |
| Case Control (longitudinal data) | In total, four HLA-matched case (Ab+) –control (Ab-) pairs (three time points) | Bacteroidetes (Bacteroidales) and Firmicutes (Clostriales) in cases vs. controls at all time points. Children progressing T1DM: microbial diversity | [73] |
| Case Control | In total, 28 newly diagnosed type 1 diabetes (T1DM) (average duration 4.8 years) vs. 27 age-matched control children | In children < 3 years: Bacteroidetes in cases and Clostridium clusters IV and XIVa in controls | [74] |
| Case Control | In total, 16 T1DM vs. 16 healthy children | Cases: Bacteroidetes, Firmicutes and Actinobacteria | [75] |
| Case Control (longitudinal data) | In total, 29 Ab (+) cases vs. 47 Ab (-) healthy controls | B. dorei and B. vulgatus in cases prior to seroconversion | [76] |
| Prospective | In total, 783 genetically predisposed children | SCFA-producing bacteria genes in children who seroconverted or developed T1DM | [77] |
| Prospective | In total, 19 Ab (+) and 21 Ab (-) children | Bacteroides Akkermansia SCFA-producing bacteria genes. A functional association between diet (early introduction of non-milk diet), gut microbiome (Bacteroides), metagenomic changes (genes for the production of butyrate) and development of islet Ab | [78] |
| Case Control | In total, 33 recent-onset T1DM, 17 Ab (+), 29 Ab (-), and 22 healthy subjects | T1DM: microbial taxa associated with host proteins involved in maintaining the function of the mucous barrier, microvilli adhesion, and exocrine pancreas | [79] |
| Cohort Study | In total, 403 children (age = 1 year) | Genetic risk for developing T1DM autoimmunity is associated with distinct changes in the gut microbiome | [80] |
| RCT | T1DM children (8–17 years) randomized to prebiotic oligofructose-enriched inulin (n = 17) or placebo (n = 21) for 12 weeks | At 3 months, C-peptide was significantly higher in the group that received prebiotics | [81] |
| Prospective Cohort | In total, 7473 children (4–10 years) | Early probiotic supplementation (at the age of 0–27 days) associated with a decreased risk of islet autoimmunity in children with the HLADR3/4 genotype | [82] |
T1DM: type 1 diabetes, RCT: randomized control trial, HLA: human leukocyte antigen, Ab (+): islet auto-antibodies positive, and Ab (-): islet auto-antibodies negative.
However, establishing a causal relationship between microbiome alterations and T1DM in humans is very challenging because of the complexity of the microbiota immune system cross talk. It is also difficult to identify microbiota abnormalities consistently associated with diabetes, because seroconversion occurs in early life when the microbiota is unstable and it undergoes substantial changes over time [25,26]. Furthermore, factors as delivery mode, diet, geographical location, and lifestyle dramatically affect the microbiota and, besides being potential causes of dysbiosis, they can also act as relevant confounders [27,29,32]. Although causality between dysbiosis and T1DM has not yet been proven in humans, as there are no placebo-controlled trials showing that long-term changes in the intestinal microbiota modify the risk of T1DM, there are data suggesting a role of the gut microbiota in the development of human T1DM.
Children diagnosed with T1DM have reduced gut microbiota diversity in fecal samples. A lower microbial diversity was also shown in children with at least two positive disease-associated autoantibodies compared to autoantibody-negative children. Moreover, longitudinal studies in children at risk of T1DM have shown that the decrease in microbial diversity occurs after seroconversion, but prior to T1DM onset [71,72,73].
Several studies on the microbiota composition in T1DM reported an increase in Bacteroidetes (Gram negative) and a decrease in Firmicutes (Gram positive) [72,73,74,75]. The prevalent species from the Firmicutes phylum produce protective SCFAs, including butyrate [83], suggesting that the reduction in Firmicutes seen in T1DM may be deleterious because of diminished butyrate production. On the other hand, the Bacteroidetes phylum is dominated by Bacteroides and Prevotella [19]. In T1DM, the quantity of Bacteroides is significantly higher, whereas that of Prevotella is significantly lower than in healthy controls and this is of relevance, as Bacteroides have been associated with gastrointestinal inflammation and increased intestinal permeability, while Prevotella appear protective. In a cross-sectional study an increased abundance of Bacteroides characterized the microbiota of children who seroconverted compared with non-converters [72]. Similarly, a study performed in 76 Finnish children found increased Bacteroides dorei and Bacteroides vulgatus species in seroconverted T1DM patients. Of interest, longitudinal data from this study showed that the increase in Bacteroides dorei peaked at 7.6 months of age in autoantibody-positive children and preceded the appearance of the first anti-islet autoantibodies, suggesting that early dysbiosis may predict T1DM in genetically predisposal subjects [76]. The increased abundance of Bacteroides species in Finnish and Estonian compared to Russian children has been even proposed as a possible explanation for their higher incidence of T1DM [84]. Bifidobacterium species were also reduced in autoantibody-positive compared to autoantibodies negative children [72] and this may be of functional relevance as Bifidobacteria contribute to the production of butyrate and inhibit bacterial translocation through the gut barrier.
However, recent metagenomic studies suggest that changes in microbiome function are more relevant and consistently associated with the risk of seroconversion and/or T1DM onset than changes in specific taxa. The Environmental Determinants of Diabetes in the Young (TEDDY) study did not find important taxonomic differences in the gut microbiome of children who seroconverted or developed T1DM. However, metagenomic analysis revealed that the microbiome of these children contained significantly lower numbers of genes involved in the production of SCFAs [77]. This finding is in line with a previous study by de Goffau et al., reporting that β cell autoimmunity prior to T1DM onset is associated with a reduction in butyrate-producing species [72]. Dietary factors may induce changes in the composition of the gut microbiome, leading to both reduced butyrate production and enhanced risk of autoantibodies associated to the development of T1DM [78]. A recent metaproteomic analysis has also shown that microbial taxa associated with host proteins involved in maintaining function of the mucous barrier and microvilli adhesion were depleted in patients with new-onset T1DM [79]. Moreover, intestinal permeability was increased in children at risk of developing T1DM and correlated with microbiota alterations [85,86]. These findings support the notion that increased gut permeability is important in linking the microbiome to T1DM in humans as previously demonstrated in animal model of autoimmune diabetes.
Adding a further layer of complexity to the field, a recent study has shown that HLA can influence the composition of the human gut microbiome [80]. Indeed, genetic risk for developing T1DM autoimmunity was associated with distinct changes in the gut microbiome. Therefore, in T1DM genetic susceptibility can not only synergically interact with, but also contribute to shape the microbiome [80]. This finding may also be relevant for the design of future clinical studies as recruitment of children at high genetic risk for T1DM is less desirable than we previously thought because it can mask the effect of HLA on the microbiome.
Overall data in humans are more conflicting than in experimental animals. Most of the available data in humans come from underpowered case-control studies and this may partially explain inconsistencies. Moreover, human studies mainly prove associations between dysbiosis and T1DM rather than a causal relationship and they explored taxonomic rather than functional changes in the microbiota. New approaches such as metagenomics and metaproteomics applied to large cohort of patients and intervention studies may provide better insights on this topic in the future and move this area of research from a description of abnormalities to translational applications.
6. Future perspective
Research on the microbiome in T1DM has expanded dramatically in recent years; however, a causal relationship between dysbiosis and the risk of T1DM has not yet been established in humans. Even though intervention studies in susceptible animals have provided evidence that strategies ameliorating/reversing dysbiosis reduce the incidence of T1DM, this evidence is still lacking in humans. However, tailored changes in the intestinal microbiome may represent a novel therapeutic approach for the primary prevention of T1DM and various strategies have been proposed to reshape the gut microbiota in children at high risk of T1DM. Infants delivered by C-section can be exposed to maternal vaginal fluids at birth to make their microbiota composition comparable to that of vaginally born babies [87]. Supplementation with LMP dietary fiber may be beneficial in humans as recently proven in mice. Prebiotics, such as inulin, lactulose, and in particular human milk oligosaccharides, can promote the growth of Bifidobacteria and a recent pilot study showed that treatment for 3 months with the prebiotic oligofructose-enriched inulin in children with T1DM significantly increased C-peptide levels [81]. Clinical trials are currently testing the efficacy of supplementation with probiotics in human T1DM [88]. Notably, a recent study demonstrated that the administration of probiotics in the first month of life was associated with a 60% reduction in the autoimmunity risk in children with the high-risk HLA-DR3/4 genotype [82]. Moreover, a clinical trial with the probiotics Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12L is currently ongoing in children with new-onset T1DM [89]. In the era of precision medicine, personalized diets, tailored in accordance with both the host’s genetic background and individual microbiome may hopefully correct dysbiosis and reduce the risk of developing T1DM.
Acknowledgments
We apologize to all the investigators whose important works have not been cited due to space restrictions.
Author Contributions
M.D. designed the review and critically revised the work; A.F. performed the research; M.D. and A.F. wrote the paper; G.G. revised the text.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gülden E., Wong F.S., Wen L. The gut microbiota and type 1 diabetes. Clin. Immunol. 2015;159:143–153. doi: 10.1016/j.clim.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paun A., Yau C., Danska J.S. The influence of the microbiome on type 1 diabetes. J. Immunol. 2017;198:590–595. doi: 10.4049/jimmunol.1601519. [DOI] [PubMed] [Google Scholar]
- 3.Hu C., Wong F.S., Wen L. Type 1 diabetes and gut microbiota: Friend or foe? Pharmacol. Res. 2015;98:9–15. doi: 10.1016/j.phrs.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Classification and diagnosis of diabetes: Standards of medical care in diabetes. Diabetes Care. 2019;4:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 5.International Diabetes Federation [(accessed on 1 November 2019)];IDF Diabetes Atlas. (8th ed.). 2017 Available online: http://www.diabetesatlas.org.
- 6.Soltesz G., Patterson C.C., Dahlquist G., EURODIAB Study Group Worldwide childhood type 1 diabetes incidence–what can we learn from epidemiology? Pediatric Diabetes. 2007;8:6–14. doi: 10.1111/j.1399-5448.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 7.Patterson C.C., Gyürüs E., Rosenbauer J., Cinek O., Neu A., Schober E., Parslow R.C., Joner G., Svensson J., Castell C. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: Evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55:2142–2147. doi: 10.1007/s00125-012-2571-8. [DOI] [PubMed] [Google Scholar]
- 8.Harjutsalo V., Sjöberg L., Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: A cohort study. Lancet. 2008;371:1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 9.Knip M., Korhonen S., Kulmala P., Veijola R., Reunanen A., Raitakari O.T., Viikari J., Åkerblom H.K. Prediction of type 1 diabetes in the general population. Diabetes Care. 2010;33:1206–1212. doi: 10.2337/dc09-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regnell S.E., Lernmark Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia. 2017;60:1370–1381. doi: 10.1007/s00125-017-4308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfsdorf J., Glaser N., Sperling M.A. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes care. 2006;29:1150–1159. doi: 10.2337/dc06-9909. [DOI] [PubMed] [Google Scholar]
- 12.Dayan C.M., Korah M., Tatovic D., Bundy B.N., Herold K.C. Changing the landscape for type 1 diabetes: The first step to prevention. Lancet. 2019 doi: 10.1016/S0140-6736(19)32127-0. [DOI] [PubMed] [Google Scholar]
- 13.Pociot F., Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet. 2016;387:2331–2339. doi: 10.1016/S0140-6736(16)30582-7. [DOI] [PubMed] [Google Scholar]
- 14.Sun C., Zhi D., Shen S., Luo F., Sanjeevi C.B. SNPs in the exons of Toll-like receptors are associated with susceptibility to type 1 diabetes in Chinese population. Hum. Immunol. 2014;75:1084–1088. doi: 10.1016/j.humimm.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Infante M., Ricordi C., Sanchez J., Clare-Salzler M.J., Padilla N., Fuenmayor V., Chavez C., Alvarez A., Baidal D., Alejandro R., et al. Influence of Vitamin D on Islet Autoimmunity and Beta-Cell Function in Type 1 Diabetes. Nutrients. 2019;11:2185. doi: 10.3390/nu11092185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam H.K., Rhie Y.J., Lee K.H. Vitamin D level and gene polymorphisms in Korean children with type 1 diabetes. Pediatric Diabetes. 2019;20:750–758. doi: 10.1111/pedi.12878. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed A.E.A., Sakhr H.M., Hassan M.H., El-Amir M.I., Ameen H.H. Vitamin D receptor rs7975232, rs731236 and rs1544410 single nucleotide polymorphisms, and 25-hydroxyvitamin D levels in Egyptian children with type 1 diabetes mellitus: Effect of vitamin D co-therapy. Diabetes Metab. Syndr. Obes. 2019;12:703. doi: 10.2147/DMSO.S201525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rewers M., Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–2348. doi: 10.1016/S0140-6736(16)30507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bäckhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 20.Sender R., Fuchs S., Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Fuks G., Elgart M., Amir A., Zeisel A., Turnbaugh P.J., Soen Y., Shental N. Combining 16S rRNA gene variable regions enables high-resolution microbial community profiling. Microbiome. 2018;6:17. doi: 10.1186/s40168-017-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poretsky R., Rodriguez-R L.M., Luo C., Tsementzi D., Konstantinidis K.T. Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS ONE. 2014;9:e93827. doi: 10.1371/journal.pone.0093827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bäckhed F., Roswall J., Peng Y., Feng Q., Jia H., Kovatcheva-Datchary P., Li Y., Xia Y., Xie H., Zhong H., et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferretti P., Pasolli E., Tett A., Asnicar F., Gorfer V., Fedi S., Armanini F., Truong D.T., Manara S., Zolfo M., et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. 2018;24:133–145. doi: 10.1016/j.chom.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Praveen P., Jordan F., Priami C., Morine M.J. The role of breast-feeding in infant immune system: A systems perspective on the intestinal microbiome. Microbiome. 2015;3:41. doi: 10.1186/s40168-015-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh R.K., Chang H.W., Yan D., Lee K.M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T.H., et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., Collini S., Pieraccini G., Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkataraman R., Juwal J., Princy J. The effect of probiotics on glycemic index. Panminerva Med. 2018;60:234–235. doi: 10.23736/S0031-0808.18.03454-7. [DOI] [PubMed] [Google Scholar]
- 34.Fjalstad J.W., Esaiassen E., Juvet L.K., van den Anker J.N., Klingenberg C. Antibiotic therapy in neonates and impact on gut microbiota and antibiotic resistance development: A systematic review. J. Antimicrob. Chemother. 2017;73:569–580. doi: 10.1093/jac/dkx426. [DOI] [PubMed] [Google Scholar]
- 35.Antonopoulos D.A., Huse S.M., Morrison H.G., Schmidt T.M., Sogin M.L., Young V.B. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect. Immun. 2009;77:2367–2375. doi: 10.1128/IAI.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claesson M.J., Jeffery I.B., Conde S., Power S.E., O’connor E.M., Cusack S., Harris H.M., Coakley M., Lakshminarayanan B., O’Sullivan O., et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 37.Krishnan S., Alden N., Lee K. Pathways and functions of gut microbiota metabolism impacting host physiology. Curr. Opin. Biotechnol. 2015;36:137–145. doi: 10.1016/j.copbio.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LeBlanc J.G., Chain F., Martín R., Bermúdez-Humarán L.G., Courau S., Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017;16:79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535:65. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 40.Luu M., Visekruna A. Short-Chain fatty acids: Bacterial messengers modulating the immunometabolism of T cells. Eur. J. Immunol. 2019;49:842–848. doi: 10.1002/eji.201848009. [DOI] [PubMed] [Google Scholar]
- 41.Lazar V., Ditu L., Pircalabioru G., Gheorghe I., Curutiu C., Holban A.M., Chifiriuc C.M. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology and cancer. Front. Immunol. 2018;9:1830. doi: 10.3389/fimmu.2018.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skonieczna-Żydecka K., Marlicz W., Misera A., Koulaouzidis A., Łoniewski I. Microbiome—The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. J. Clin. Med. 2018;7:521. doi: 10.3390/jcm7120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen C., Round J.L. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16:1024–1033. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vangay P., Ward T., Gerber J.S., Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe. 2015;17:553–564. doi: 10.1016/j.chom.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brüssow H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2019:1–12. doi: 10.1111/1751-7915.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown K., Godovannyi A., Ma C., Zhang Y., Ahmadi-Vand Z., Dai C., Gorzelak M.A., Chan Y., Chan J.M., Lochner A., et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 2016;10:321. doi: 10.1038/ismej.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daft J.G., Ptacek T., Kumar R., Morrow C., Lorenz R.G. Cross-fostering immediately after birth induces a permanent microbiota shift that is shaped by the nursing mother. Microbiome. 2015;3:17. doi: 10.1186/s40168-015-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen L., Ley R.E., Volchkov P.Y., Stranges P.B., Avanesyan L., Stonebraker A.C., Hu C., Wong F.S., Szot G.L., Bluestone J.A., et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miranda M.C.G., Oliveira R.P., Torres L., Aguiar S.L.F., Pinheiro Rosa N., Lemos L., Guimarães M.A., Reis D., Silveira T., Ferreira Ê., et al. Frontline Science: Abnormalities in the gut mucosa of non-obese diabetic mice precede the onset of type 1 diabetes. J. Leukoc. Biol. 2019;106:513–529. doi: 10.1002/JLB.3HI0119-024RR. [DOI] [PubMed] [Google Scholar]
- 50.Caviglia G.P., Rosso C., Ribaldone D.G., Dughera F., Fagoonee S., Astegiano M., Pellicano R. Physiopathology of intestinal barrier and the role of zonulin. Minerva Biotecnol. 2019;31:83–92. doi: 10.23736/S1120-4826.19.02554-0. [DOI] [Google Scholar]
- 51.Neu J., Reverte C.M., Mackey A.D., Liboni K., Tuhacek-Tenace L.M., Hatch M., Li N., Caicedo R.A., Schatz D.A., Atkinson M. Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J. Pediatric Gastroenterol. Nutr. 2005;40:589–595. doi: 10.1097/01.MPG.0000159636.19346.C1. [DOI] [PubMed] [Google Scholar]
- 52.Vaarala O., Atkinson M.A., Neu J. The “perfect storm” for type 1 diabetes: The complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee A.S., Gibson D.L., Zhang Y., Sham H.P., Vallance B.A., Dutz J.P. Gut barrier disruption by an enteric bacterial pathogen accelerates insulitis in NOD mice. Diabetologia. 2010;53:741–748. doi: 10.1007/s00125-009-1626-y. [DOI] [PubMed] [Google Scholar]
- 54.Turley S.J., Lee J.W., Dutton-Swain N., Mathis D., Benoist C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc. Natl. Acad. Sci. USA. 2005;102:17729–17733. doi: 10.1073/pnas.0509006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sorini C., Cosorich I., Conte M.L., De Giorgi L., Facciotti F., Lucianò R., Rocchi M., Ferrarese R., Sanvito F., Canducci F., et al. Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 2019;116:15140–15149. doi: 10.1073/pnas.1814558116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calcinaro F., Dionisi S., Marinaro M., Candeloro P., Bonato V., Marzotti S., Corneli R.B., Ferretti E., Gulino A., Grasso F., et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48:1565–1575. doi: 10.1007/s00125-005-1831-2. [DOI] [PubMed] [Google Scholar]
- 57.Dolpady J., Sorini C., Di Pietro C., Cosorich I., Ferrarese R., Saita D., Clementi M., Canducci F., Falcone M. Oral probiotic VSL# 3 prevents autoimmune diabetes by modulating microbiota and promoting indoleamine 2, 3-dioxygenase-enriched tolerogenic intestinal environment. J. Diabetes Res. 2016;2016:7569431. doi: 10.1155/2016/7569431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valladares R., Sankar D., Li N., Williams E., Lai K.K., Abdelgeliel A.S., Gonzalez C.F., Wasserfall C.H., Larkin J., Schatz D., et al. Lactobacillus johnsonii N6. 2 mitigates the development of type 1 diabetes in BB-DP rats. PLoS ONE. 2010;5:e10507. doi: 10.1371/journal.pone.0010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen K., Chen H., Faas M.M., de Haan B.J., Li J., Xiao P., Zhang H., Diana J., de Vos P., Sun J. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol. Nutr. Food Res. 2017;61:1601006. doi: 10.1002/mnfr.201601006. [DOI] [PubMed] [Google Scholar]
- 60.Hansen C.H.F., Krych L., Nielsen D.S., Vogensen F.K., Hansen L.H., Sørensen S.J., Buschard K., Hansen A.K. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55:2285–2294. doi: 10.1007/s00125-012-2564-7. [DOI] [PubMed] [Google Scholar]
- 61.Brugman S., Klatter F.A., Visser J.T.J., Wildeboer-Veloo A.C.M., Harmsen H.J.M., Rozing J., Bos N.A. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 62.Hu Y., Jin P., Peng J., Zhang X., Wong F.S., Wen L. Different immunological responses to early-life antibiotic exposure affecting autoimmune diabetes development in NOD mice. J. Autoimmun. 2016;72:47–56. doi: 10.1016/j.jaut.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Livanos A.E., Greiner T.U., Vangay P., Pathmasiri W., Stewart D., McRitchie S., Li H., Chung J., Sohn J., Kim S., et al. Antibiotic-Mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat. Microbiol. 2016;1:16140. doi: 10.1038/nmicrobiol.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hara N., Alkanani A.K., Ir D., Robertson C.E., Wagner B.D., Frank D.N., Zipris D. Prevention of virus-induced type 1 diabetes with antibiotic therapy. J. Immunol. 2012;189:3805–3814. doi: 10.4049/jimmunol.1201257. [DOI] [PubMed] [Google Scholar]
- 65.Mariño E., Richards J.L., McLeod K.H., Stanley D., Yap Y.A., Knight J., McKenzie C., Kranich J., Oliveira A.C., Rossello F.J., et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017;18:552. doi: 10.1038/ni.3713. [DOI] [PubMed] [Google Scholar]
- 66.Needell J.C., Ir D., Robertson C.E., Kroehl M.E., Frank D.N., Zipris D. Maternal treatment with short-chain fatty acids modulates the intestinal microbiota and immunity and ameliorates type 1 diabetes in the offspring. PLoS ONE. 2017;12:e0183786. doi: 10.1371/journal.pone.0183786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Candon S., Perez-Arroyo A., Marquet C., Valette F., Foray A.P., Pelletier B., Milani C., Ventura M., Bach J.F., Chatenoud L. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE. 2015;10:e0125448. doi: 10.1371/journal.pone.0125448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X.S., Li J., Krautkramer K.A., Badri M., Battaglia T., Borbet T.C., Koh H., Ng S., Sibley R.A., Li Y., et al. Antibiotic-induced acceleration of type 1 diabetes alters maturation of innate intestinal immunity. Elife. 2018;7:e37816. doi: 10.7554/eLife.37816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu C., Pan L.L., Niu W., Fang X., Liang W., Li J., Li H., Pan X., Chen W., Zhang H., et al. Modulation of gut microbiota by low methoxyl pectin attenuates type 1 diabetes in non-obese diabetic mice. Front. Immunol. 2019;10:1733. doi: 10.3389/fimmu.2019.01733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jia L., Shan K., Pan L.L., Feng N., Lv Z., Sun Y., Li J., Wu C., Zhang H., Chen W., et al. Clostridium butyricum CGMCC0313.1 protects against autoimmune diabetes by modulating intestinal immune homeostasis and inducing pancreatic regulatory T cells. Front. Immunol. 2017;8:1345. doi: 10.3389/fimmu.2017.01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kostic A.D., Gevers D., Siljander H., Vatanen T., Hyötyläinen T., Hämäläinen A.M., Peet A., Tillmann V., Pöhö P., Mattila I., et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Goffau M.C., Luopajärvi K., Knip M., Ilonen J., Ruohtula T., Härkönen T., Orivuori L., Hakala S., Welling G.W., Harmsen H.J., et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes. 2012;62:1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giongo A., Gano K.A., Crabb D.B., Mukherjee N., Novelo L.L., Casella G., Drew J.C., Ilonen J., Knip M., Hyöty H., et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5:82. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Goffau M.C., Fuentes S., van den Bogert B., Honkanen H., de Vos W.M., Welling G.W., Hyöty H., Harmsen H.J. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia. 2014;57:1569–1577. doi: 10.1007/s00125-014-3274-0. [DOI] [PubMed] [Google Scholar]
- 75.Murri M., Leiva I., Gomez-Zumaquero J.M., Tinahones F.J., Cardona F., Soriguer F., Queipo-Ortuño M.I. Gut microbiota in children with type 1 diabetes differs from that in healthy children: A case-control study. BMC Med. 2013;11:46. doi: 10.1186/1741-7015-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davis-Richardson A.G., Ardissone A.N., Dias R., Simell V., Leonard M.T., Kemppainen K.M., Drew J.C., Schatz D., Atkinson M.A., Kolaczkowski B., et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front. Microbiol. 2014;5:678. doi: 10.3389/fmicb.2014.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vatanen T., Franzosa E.A., Schwager R., Tripathi S., Arthur T.D., Vehik K., Lernmark Å., Hagopian W.A., Rewers M.J., She J.X., et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562:589. doi: 10.1038/s41586-018-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Endesfelder D., Engel M., Davis-Richardson A.G., Ardissone A.N., Achenbach P., Hummel S., Winkler C., Atkinson M., Schatz D., Triplett E., et al. Towards a functional hypothesis relating anti-islet cell autoimmunity to the dietary impact on microbial communities and butyrate production. Microbiome. 2016;4:17. doi: 10.1186/s40168-016-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gavin P.G., Mullaney J.A., Loo D., Lê Cao K.A., Gottlieb P.A., Hill M.M., Zipris D., Hamilton-Williams E.E. Intestinal metaproteomics reveals host-microbiota interactions in subjects at risk for type 1 diabetes. Diabetes Care. 2018;41:2178–2186. doi: 10.2337/dc18-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Russell J.T., Roesch L.F., Ördberg M., Ilonen J., Atkinson M.A., Schatz D.A., Triplett E.W., Ludvigsson J. Genetic risk for autoimmunity is associated with distinct changes in the human gut microbiome. Nat. Commun. 2019;10:1–12. doi: 10.1038/s41467-019-11460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ho J., Nicolucci A.C., Virtanen H., Schick A., Meddings J., Reimer R.A., Huang C. Effect of prebiotic on microbiota, intestinal permeability and glycemic control in children with type 1 diabetes. J. Clin. Endocrinol. Metab. 2019;104:4427–4440. doi: 10.1210/jc.2019-00481. [DOI] [PubMed] [Google Scholar]
- 82.Uusitalo U., Liu X., Yang J., Aronsson C.A., Hummel S., Butterworth M., Lernmark Å., Rewers M., Hagopian W., She J.X., et al. Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatric. 2016;170:20–28. doi: 10.1001/jamapediatrics.2015.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Canani R.B., Di Costanzo M., Leone L., Pedata M., Meli R., Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011;17:1519. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vatanen T., Kostic A.D., d’Hennezel E., Siljander H., Franzosa E.A., Yassour M., Kolde R., Vlamakis H., Arthur T.D., Hämäläinen A.M., et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165:842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maffeis C., Martina A., Corradi M., Quarella S., Nori N., Torriani S., Plebani M., Contreas G., Felis G.E. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. Diabetes Metab. Res. Rev. 2016;32:700–709. doi: 10.1002/dmrr.2790. [DOI] [PubMed] [Google Scholar]
- 86.Harbison J.E., Roth-Schulze A.J., Giles L.C., Tran C.D., Ngui K.M., Penno M.A., Thomson R.L., Wentworth J.M., Colman P.G., Craig M.E., et al. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: A prospective cohort study. Pediatric Diabetes. 2019;20:574–583. doi: 10.1111/pedi.12865. [DOI] [PubMed] [Google Scholar]
- 87.Dominguez-Bello M.G., De Jesus-Laboy K.M., Shen N., Cox L.M., Amir A., Gonzalez A., Bokulich N.A., Song S.J., Hoashi M., Rivera-Vinas J.I., et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016;22:250. doi: 10.1038/nm.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mishra S., Wang S., Nagpal R., Miller B., Singh R., Taraphder S., Yadav H. Probiotics and prebiotics for the amelioration of type 1 diabetes: Present and future perspectives. Microorganisms. 2019;7:67. doi: 10.3390/microorganisms7030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Groele L., Szajewska H., Szypowska A. Effects of Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 on beta-cell function in children with newly diagnosed type 1 diabetes: Protocol of a randomised controlled trial. BMJ Open. 2017;7:e017178. doi: 10.1136/bmjopen-2017-017178. [DOI] [PMC free article] [PubMed] [Google Scholar]