Abstract
Multiple studies have demonstrated low vagally-mediated heart rate variability (HRV) being associated with a range of risk factors for heart disease and stroke, including inflammation, hyperglycemia, hyperlipidemia, and hypertension. Yet, no cut point exists that indicates elevated risk. In the present study we sought to identify a cut point-value for HRV that is associated with elevated risk across a range of known risk factors. Methods: A total of 9550 working adults from 19 study sites took part in a health assessment that included measures of inflammation, hyperglycemia, hyperlipidemia, and hypertension and vagally-mediated HRV (Root mean square of successive differences between normal heartbeats (RMSSD)). Multiple age and sex adjusted logistic regressions were calculated per risk factor (normal versus clinical range), with RMSSD being entered in binary at different cut points ranging from 15–39 msec with a 2 msec increment. Results: For daytime RMSSD, values below 25 ± 4 indicated elevated risk (odds ratios (OR) 1.5–3.5 across risk factors). For nighttime RMSSD, values below 29 ± 4 indicated elevated risk (OR 1.2–2.0). Conclusion: These results provide the first evidence that a single value of RMSSD may be associated with elevated risk across a range of established cardiovascular risk factors and may present an easy to assess novel marker of cardiovascular risk.
Keywords: heart rate variability, novel risk factor, CVD, prevention, risk stratification
1. Introduction
Research from the past decades has demonstrated that low vagally-mediated heart rate variability (HRV) is predictive of cardiac and all-cause mortality [1,2,3]. Furthermore, HRV is associated with a wide range of risk factors for heart disease and stroke, including elevated low-grade inflammation, hyperglycemia, hyperlipidemia, and hypertension [4,5,6,7,8,9,10]. Similarly, decreased HRV correlates with increased cardiovascular risk scores [11,12]. In the majority of other clinically health-related risk factor measures, such as blood pressure, heart rate, or glucose status, established clinical cut points are routinely applied to provide an indication of current health status and future risk. However, to date, clinicians in search of existing diagnostic reference levels (DRLs) in HRV that indicate elevated risk have had little guidance.
Some HRV cutoff values have been proposed but their generalizability is often limited because they were derived in specific subgroups, i.e., patients or children [13,14], where the chosen HRV parameter cutoff is unexplained, or based on short term recordings [14,15,16] (<15 min), ignoring apparent circadian rhythms [17,18] in HRV parameters.
Heart rate variability cutoff values may be useful in health care settings to determine at risk populations (i.e., risk stratification) prior to the development of certain clinical conditions. Decreased HRV values are known to be associated with a wide range of clinical conditions, such as hypertension, type 2 diabetes, obesity, or depression [1,4,19]. Therefore, HRV is often criticized as being non-specific. Yet, because of its non-specificity to any particular disease, it may serve as an easy to assess and cheap antecedent risk marker of exposure at an early etiology stage, for example, in the primary care setting of company physicians.
Clearly, there is a need to assess the value of novel markers of cardiovascular disease risk. Accurate assessment of cardiovascular risk is essential not only for clinical decision making, but also for primary prevention, because the benefits, risks, and costs of alternative management strategies must be weighed to choose the best prevention strategy or treatment for the individual.
Several statistical methods for the assessment of the usefulness of new biomarkers have been reviewed [20,21,22,23], and recommendations for the reporting of novel risk markers have been proposed [24]. Specifically, guidelines from the American Heart Association [22] suggest that the evaluation of novel biomarkers proceed in phases, with the first phase being proof of concept, in which it is shown that the novel biomarker differs between those with and without the relevant outcome. In addition, these guidelines give recommendations for the reporting of studies that evaluate novel biomarkers. The reporting recommendations include conducting the study in accordance with standards for observational studies, reporting levels of the standard risk factors, and reporting the odds ratio (OR) or related statistics, including confidence intervals (CI), associated with proposed risk factor. In the present study we sought to use these guidelines to identify a value for HRV for use in primary prevention that is associated with elevated cardiovascular disease risk factors across a range of established but noninvasive risk factors. Specifically, we report the results of an observational study in employees with standard risk factors using odds ratios associated with the discrimination between individuals in the normal range versus those in the clinical range, with a time domain measurement of HRV as an independent variable.
2. Material and Methods
2.1. Study Population
We utilized a large cross-sectional, secondary data set from the Mannheim Industrial Cohort Study (MICS). The data were collected as part of a voluntary health risk assessment that was offered to all employees during working hours. An agent independent from the employer conducted the health risk assessments and data collection (HealthVision Ltd., Berlingen, Switzerland). A total of 19,985 participants from 30 study sites (companies from the secondary and tertiary sectors; response rate 55% (ranging from 49%–60%)) across Germany were eligible for this study. Data were collected between 2010 and 2014.
In brief, participants were invited to take part in a “Work Health Check” and were offered a detailed individual report containing their health status as assessed by medical examination and self-reports. This sample encompassed the entire workforce between 18 and 65 years.
The Ethical Committee of the Mannheim Medical Faculty, Heidelberg University approved the secondary analysis of this data (2010-296E-MA). All participants gave written informed consent prior to examination.
2.2. Exclusion Criteria
Participants were excluded from all analysis based on their self-reported diagnosis by a physician: respiratory diseases (e.g., Asthma, chronic obstructive pulmonary disease (COPD)), angina pectoris, stroke, infarction, Coronary Heart Disease (CHD), depression, burnout, other chronic diseases, and cancer (12.84% of 19,985). Also, participants indicating a daily analgesic, hypnotic, or calmative intake were excluded (0.83%).
In addition, participants with incomplete psychobiological data (see Section 2.3: Measures) had to be excluded due to missing HRV data (42.56%) or other data (2.99%).
Participants in the preclinical range or in the second tertile (see Table 1) were excluded from this analysis to contrast normal versus clinical values of risk markers. The normal versus preclinical contrasting is beyond the scope of this paper.
Table 1.
Classification of biomarkers into (clinical) categories.
| Variable | Condition | Normal | Preclinic | Clinic | |
|---|---|---|---|---|---|
| Glycemic Status | FPG (mg/dL) | 80–100 | 100–126 | ≥126 | |
| HbA1c (%) | 4–6 | 6–6.5 | ≥6.5 | ||
| Inflammation Status | CRP (mg/L) | <1 | 1–3 | 3–5 | |
| WBC (μL) | <p33 | ≥p33–p66 | ≥p66 | ||
| Lipid Status | LDL (mg/dL) | <160 | ≥160 | ||
| HDL (mg/dL) | females | ≥65 | 45 – 65 | <45 | |
| HDL (mg/dL) | males | ≥55 | 35 – 55 | <35 | |
| TC (mg/dL) | age < 20 | <170 | - | ≥170 | |
| TC (mg/dL) | age 20–29 | <200 | - | ≥200 | |
| TC (mg/dL) | age 30–39 | <220 | - | ≥220 | |
| TC (mg/dL) | age 40+ | <240 | - | ≥240 | |
| TRIG (mg/dL) | <200 | - | ≥200 | ||
| Blood Pressure | Sys BP (mmHg) | In combination with Dia BP | <120 | ≥120–140 | ≥140 |
| Dia BP (mmHg) | In combination with Sys BP | <90 | <90 | ≥90 |
FPG: Fasting plasma glucose; HbA1c: Glycosylated hemoglobin (A1c); CRP: C-reactive protein (high sensitive); WBC: White blood count; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; TC: Total cholesterol; TRIG: Triglyceride; SysBP: Systolic blood pressure; Dia BP: Diastolic blood pressure; p33 / p66: 33. percentile or 66. percentile respectively.
2.3. Measures
Demographic, medical, and lifestyle variables were obtained from an online questionnaire. The questionnaire had to be completed prior to being able to schedule a medical examination. All participants were enrolled and examined between 10:00 and 17:00 on a typical workday (Monday to Friday) during work hours. Upon arrival, a medical examination was performed and the heart rate (HR)-recorder was attached. The next morning, between 7:00 and 9:00, a fasting blood sample was collected from all individuals. Samples were transported to a commercial laboratory (Synlab, Augsburg, Germany) within two hours of sample collection and analyzed within 24 h.
All measures were categorized according to standard clinical cutoffs (http://www.synlab.com/en/human/parameter-index/international-parameter-index/) (as far as existing) or categorized into tertiles (described in Table 1). During the course of this study the American College of Cardiology/American Heart Association guidelines for hypertension changed (ACC/AHA 2017). We used the most recent cut points in the current analyses, such that those classified in the clinical range corresponded to those with stage 2 hypertension in the new guidelines. Participants indicating the usage of medication such as anti-hypertensive, lipid lowering, or glucose lowering drugs or reporting corresponding physician diagnoses were re-assigned as cases on the respective biomarker.
2.3.1. Blood withdrawal
Inflammatory markers (high sensitivity C-reactive protein (CRP) (CardioPhase hsCRP, DADE BEHRING)), white blood count (WBC)), blood glucose (fasting plasma glucose (FPG) OSR6121, Olympus; glycosylated hemoglobin (HbA1c) “Tina-quant, Hemoglobin A1c Gen.2”, Roche Diagnostics [25]), and blood lipids (Total cholesterol (TC), triglyceride (TRI) OSR60118, Olympus), low density lipoprotein (LDL), high density lipoprotein (HDL) OSR6187, Olympus) were determined using routine laboratory analyzers (for details see [26]).
2.3.2. Blood Pressure
Using the oscillometric technique, blood pressure (BP) was recorded twice using the CRITIKON Dinamap Portable Adult/Pediatric and Neonatal Vital Signs Monitor (Model 8100). Measurements were taken from the dominant arm in the seated position after a five-minute rest period. A study physician repeated the reading using sphygmomanometry if one or more BP values exceeded 135 mmHg (systolic) or 90 mmHg (diastolic). The arithmetic mean of all two to three measurements was calculated.
2.3.3. Heart Rate Monitoring
Heart rate (HR) was recorded as beat-to-beat intervals (IBI) using a t6 Suunto Memory Belt® (SuuntoVantaa, Finland), sampling at a rate of 1000 Hz. The Suunto Memory Belt® is a reliable measure of electrocardiography (ECG) compared to a five lead ECG [27].
IBIs were determined as the interval between two successive R-spikes. After attaching the ambulatory HR recorder, participants commenced their routine work duties, followed by after work leisure and sleep activities. Participants were asked to record their sleep routine protocol and to return the HR recorder after a minimum of 22 h of wearing or in case of any difficulties.
The root mean square of successive differences between normal heartbeats (RMSSD) was calculated from 24-h long-term HR monitoring (beat to beat) derived from 5.35-min averages recorded for day time and night time as indicators of vagal tone. This index uses what the econometrics literature calls first-differencing and acts like a high pass filter, thus removing long-term trends and slower-frequency variability from the signal. Because of the frequency characteristics of the autonomic influences on the heart, such that vagal influences cover the full frequency range and sympathetic influences are primarily restricted to the lower frequencies, RMSSD reflects primarily vagal influences [28,29]. In addition, whereas spectral-derived indices of HRV are common in the literature, the exact values obtained vary as a function of choice of algorithm, detrending methods, and other computational parameters [23,30]. RMSSD provides an index in a common metric (milliseconds) and is thus better suited for use as a biomarker in a clinical setting.
Researchers at the Center for Neuropsychological Research (University of Trier, 54296 Trier, Germany) analyzed the raw IBIs according to the Task Force Guidelines of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [30]. The ANS-Explorer Software [31] was used to calculate the RMSSD in all valid adjacent IBIs if the artifact rate of the respective 335 second (s) segment was below 5%. The artifact correction is part of the NEUROCOR® precisionHRV-Algorithm (NEUROCOR ltd. Trier, Germany). The algorithm marked all RR-intervals as artifacts if they deviated from a moving average of five RR-intervals by an age-dependent amount. Non-physiological RR-intervals, e.g., due to a too fast, too slow, or too quickly changing heart rate, were also marked as artifacts. In order to detect artefacts caused by arrhythmias in persons with pronounced variability, the RR-intervals were finally assessed with a statistical outlier test. The correction of the RR-intervals marked as artifacts was carried out without any violation of the phase relationship of the signal and a change in the total time. The affected areas were not cut out, but replaced by spline interpolated RR-intervals of the same length. For larger artifact phases, the distribution of the R-times was adapted to the R-time dynamics before and after the defect. The number of replaced RR-intervals was counted (i.e., artifact rate). This led to an exclusion of 5.40% segments from total of 2,551,965 segments (prior to any other exclusion of participants, e.g., due to missing questionnaire or medical information). To avoid the case of retaining an individual HRV dataset with only a few valid 335 s intervals, the number of valid 335 s segments had to be ≥30 for night time and ≥50 for daytime. This corresponded to a minimum valid recording time of ~2.5hours night time and ~4.5hours for daytime, leading to an exclusion of 7.4% of recordings (again, prior to any other exclusion of participants, e.g., due to missing questionnaire or medical information).
2.4. Statistical Methods
Logistic regression models were calculated to quantify odds ratios for various risk factors as dependent variables, comparing individuals in the normal versus clinical range by HRV (independent variable) during HRV nighttime and daytime recording. For example, the odds ratio was calculated as having blood pressure in the clinically elevated (≥140/90 mmHg) versus normal range (<120/80 mmHg) with an RMSSD during night above versus below 30 msec).
We calculated 24 models per risk factor: 12 for the day time period and 12 for the night time period, in three steps. The model included age, sex, and heart rate by using the heart period (i.e., IBI) as recommended by [32], as independent variables. In addition, the model included the binary RMSSD cutoff values from daytime (or nighttime) and increased the cutoff of by two milliseconds, starting with RMSSD < 15 ms, then RMSSD < 17 ms and so on until RMSSD < 39 was reached. As we were interested in the performance of HRV as an integrative marker for psychosomatic health, we abstained from adjusting the regression models for further known influencing factors on HRV, such as work stress or physical fitness.
All linear regression models were corrected for the nested structure of employees in companies using the Stata vce(cluster) option [33]. Here, standard errors were adjusted for intragroup correlation on the company level, relaxing the usual requirement of the observations (i.e., employees) to be independent of each other.
We compared all odds ratios (OR) and their respective 95% confidence intervals (CI) between all models using forest plots. We used the forest plot for blood pressure as a benchmark to get a first approximation of an HRV value to be examined across the various established biomarkers. We then used that value to examine the value of HRV associated with values in the clinical range of the other biomarkers. All analyses were conducted using Stata (v15.1 SE, College Station, TX, USA: StataCorp LP).
3. Results
A total of 9550 participants from 19 study sites (48%) out of the eligible 19,985 participants (30 study sites) had complete data on all variables of interest (n = 8425 for night time HRV; 42%). The mean age was 41.7 (Standard deviation (SD) 11.0) and 18.9% were female. The overall RMSSD mean was 27.8 msec (SD 12.2) during day time and 38.9 msec (SD 19.3) during nighttime. Further sample characteristics and prevalence of clinical cutoffs are depicted in Table 1 and Table 2. The primary analysis compared the normal versus clinical population. Therefore, all participants in the preclinical range of the risk marker were excluded from analysis, resulting in varying analysis samples (see Table 2). For example, the fasting glucose models included a total of 93.42% from 9550 participants, since 6.48% were in the preclinical range of FPG (100–126 mm/dL; see Table 1 and Table 2). In turn, the HbA1c models included a total of 75.90% from 9550 participants, since 24.10% were in the preclinical range of HbA1c (6–6.5%; see Table 1 and Table 2).
Table 2.
Classification of biomarkers into (clinical) categories.
| Cutoff (%) | |||||||
|---|---|---|---|---|---|---|---|
| Variable | n | Mean | SD | Normal | Preclinical | Clinical | |
| Glycemic Status | FPG (mg/dL) | 9550 | 87.1 | 12 | 92.50% | 6.48% | 1.02% |
| HbA1c (%) | 9550 | 5.4 | 0.5 | 74.36% | 24.10% | 1.54% | |
| Inflammation Status | CRP (mg/L) | 9550 | 1.6 | 3.4 | 61.91% | 31.12% | 6.97% |
| WBC (μL) | 9550 | 6.3 | 1.7 | 34.34% | 35.69% | 29.98% | |
| Lipid Status | LDL (mg/dL) | 9550 | 127.9 | 34 | 82.62% | 17.38% | |
| HDL (mg/dL) | 9550 | 56.0 | 14.1 | 43.51% | 51.91% | 4.59% | |
| TC (mg/dL) | 9550 | 209 | 40.2 | 69.49% | 30.51% | ||
| TRIG (mg/dL) | 9550 | 121.2 | 72.6 | 88.31% | 11.69% | ||
| Blood Pressure | Sys BP (mmHg) | 9550 | 136.6 | 13.8 | 10.71% | 75.24% | 14.06% |
| Dia BP (mmHg) | 9550 | 78.6 | 11.3 | ||||
| Demographics | Age | 9550 | 41.7 | 11 | - | - | - |
| Male (n; %) | 9550 | 7745 | 81% | - | - | ||
| HRV | RMSSD Daytime (msec) | 9550 | 27.8 | 12 | - | - | - |
| RMSSD nighttime (msec) | 8425 | 38.9 | 19 | - | - | - | |
Scatterplots containing the predicted values from linear regression models adjusted for age and sex indicated a relationship between the several biomarkers and RMSSD values. A fractional polynomial function represented the average predicted value stratified by sex and included the 95% confidence interval (CI; shaded area; see supplementary material figures Figure S1–Figure S20). In summary, the associations between RMSSD and the risk markers (continuous) showed in a curvilinear fashion with inflection points, with values towards the clinically relevant areas being associated with lower levels of RMSSD in both men and women. In addition, the unadjusted plain associations are shown in scatterplots in the supplementary material figures (Figure S21–Figure S40), stratified by sex and again with a fractional polynomial function.
Multiple Adjusted Logistic Regression Models
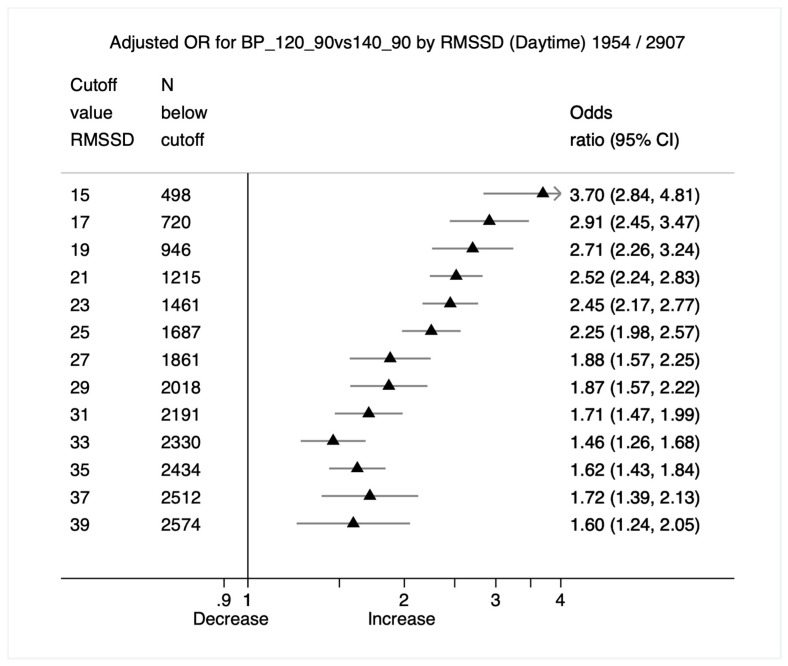
Graphical analysis using 18 forest plots showing adjusted odds ratio and corresponding 95% confidence intervals (CI) per risk factor by the cutoff value range of RMSSD were designed to aid decision making. Here, a clear dose response relationship for both white blood count and low-grade inflammation, as indicated by CRP, could be observed. An example for blood pressure is included (see Figure 1), the other figures are enclosed in the online supplementary files (Figure S41–S57).
Figure 1.
Forest plot: prediction of root mean square of successive differences between normal heartbeats (RMSSD) for hypertension.
For daytime RMSSD a value of 25 ± 4 msec and below indicated elevated risk, as indexed by clinical cut points for CRP, WBC, FPG, HbA1c, LDL, HDL, TRI, and BP (OR range from 1.35 to 3.45). For nighttime RMSSD a value of 29 ± 4 and below indicated elevated risk, as indexed by the clinical cut points for CRP, WBC, FPG, HbA1c, LDL, and BP (OR range 1.16–2.00), but not for HDL and TRI. Overall, daytime values appeared to be stronger predictors of clinical outcomes than nighttime values.
The forest plot shows the odds ratios with according 95%CI estimates per RMSSD cutoff (msec).
An odds ratio larger than 1 indicates an increased risk for hypertension. Estimates from logistic regression models were adjusted for age, sex, and heart period (IBI). The number in the heading of the graphs indicates the total number of cases (e.g., n = 1954) where participants fell into the criteria of clinically relevant elevated blood pressure versus normal. A total of 2907 participants were included in the regression model. Each line represents the logistic regression model with the corresponding binary RMSSD cut off in milliseconds.
4. Discussion
These results provide the first evidence that a single value of RMSSD may be associated with elevated risk across a range of established cardiovascular risk factors, including inflammatory markers, blood lipids, and glucose, as well as blood pressure. Consistent with the American Heart Association guidelines for the identification of novel biomarkers, we report the results of a large observational study in which levels of standard risk factors were assessed and the odds of various values of HRV in predicting those with and without established clinical levels of a range of risk factors were evaluated [22]. Thus, the present study provides a proof of concept study (the first phase) in the progressive development of HRV as a low-cost, noninvasive biomarker for clinical use.
Consistent with prior research from our group and others, all linear regression models showed HRV to be significantly associated with each established risk factor [2,4,5,7,9]. For example, our group has shown that HRV was independently associated with fasting glucose, HbA1c, white blood count, and C-reactive protein (CRP), even after accounting for activity of the sympathetic nervous system as indexed by overnight urinary norepinephrine [34,35]. Relatedly, we have shown that HRV was more significantly associated with a measure of self-rated health than a range of other biomarkers, including measures of glycemic status, inflammation, lipids, and blood pressure (BP) [10]. Thus, when a clinician asks a patient about their self-rated health, this information is more closely related to their level of HRV than their level of other common biomarkers.
Whereas all linear regression models were significant, in most cases a curvilinear model provided the best fit. This suggests that there may be a critical value beyond which the risk associated with a given value of HRV increases. This is a crucial point in the quest for clinical cut-points for HRV. Therefore, we next used adjusted logistic regression models and the associated forest plots of the odds ratios at various levels of HRV to aid our decision making.
Given the shared autonomic regulation of both blood pressure and HRV, we used BP as a benchmark against which HRV was examined to evaluate other biomarkers. Examination of the forest plot for the odds ratios of different values of HRV suggested a value of HRV at which the risk of blood pressure in the hypertensive range increased (see Figure 1). Using this value, we then examined the forest plots associated with the other risk factors to search for a value, or more precisely a narrow range of values, for which the odds of having values of that risk factor in the clinical range increased.
For some risk factors, such as blood pressure and HBA1c, there was a clear inflection point indicating a value of HRV beyond which the odds ratios increased greatly. However, for other risk factors, such as C-reactive protein (CRP) or fibrinogen there appeared to be a linear dose response relationship between values of HRV and the odds ratios associated with the risk factor. Despite this linear relationship for some biomarkers, clinical cut points are often suggested. For example, we have shown that the association between fasting glucose and HRV is linear across the clinical cut points for glycemic status, including across the normal range [26]. This is also consistent with recommendations that for some biomarkers, lower values even within the “normal” range are associated with reduced risk [36,37].
We investigated HRV values during both the daytime and the nighttime. In general, the daytime values were stronger predictors of clinical values than nighttime measures. However, the amount of variance accounted for was similar for daytime and nighttime values of HRV and ranged from approximately 1% for total cholesterol (TC) to approximately 30% for BP.
Limitations
There are several limitations of the present study. First, the percentage of females in the sample was less than 20%. Nonetheless, there were nearly 2000 females (18%) in the study. However, studies with a larger percentage of females would be useful, especially to evaluate if evidence appears suggesting sex-specific values for HRV as a clinical biomarker. Second, the sample was primarily Caucasian. Thus, future research is needed to ascertain if the present results hold for other ethnic and racial groups. In particular, in a recent meta-analysis we showed that despite having greater cardiovascular disease risk, African Americans on average have been shown to have greater HRV [38]. While it is been repeatedly shown that not all risk factors have the same association with risk in differing populations, the importance of identifying the boundary conditions of all risk factors cannot be emphasized enough. Third, the current study was cross-sectional in nature.
Future work is needed to establish the prospective value of a cut point for RMSSD in relation to cardiovascular disease risk. We and others have shown that low HRV often precedes elevated risk factors. For example, we have shown that low HRV predicted higher C-reactive protein (CRP) four years into the future [8]. This type of prospective association is the second phase of the evaluation of novel biomarkers as stated by the American Heart Association guidelines and should follow the proof of concept in which the biomarker is shown to differ between those with and without the outcome, as demonstrated in the present study [22]. Further studies may evaluate the optimal recording length that incorporates the most prognostic value (e.g., resting short term recordings, night or daytime or long-term (i.e., 24h) recordings).
5. Conclusions
This is the first study to attempt to identify a value of HRV that might be used in a clinical setting to identify persons at risk for across a range of cardiovascular disease risk factors. The present proof of concept study demonstrated that a measure of HRV can be used to discriminate between persons with and without clinical values on a broad range of established risk factors. Clearly, however, additional research is needed, consistent with the AHA guidelines, to further validate clinical cut-off values for HRV.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/8/11/1940/s1.
Figures S1–S20
Scatter plots showing the predicted values from simple linear regression models adjusted for age and sex (y-axis) and each RMSSD value of daytime or nighttime (x-axis). Grey x indicates measurements from females, black dots indicate measurements from male. A fractional polynomial function represents the average predicted value stratified by sex (dashed black line = males; solid grey line = women) and includes the 95% confidence interval (grey band). The red vertical line aids orientation for the 30 msec RMSSD value.
| Item Number | Filename | Description |
| Figure S1 | chol_predicted_day.png | Daytime |
| Figure S2 | chol_predicted_night.png | Nighttime |
| Figure S3 | crps_predicted_day.png | Daytime |
| Figure S4 | crps_predicted_night.png | Nighttime |
| Figure S5 | glucn_predicted_day.png | Daytime |
| Figure S6 | glucn_predicted_night.png | Nighttime |
| Figure S7 | hba1c_predicted_day.png | Daytime |
| Figure S8 | hba1c_predicted_night.png | Nighttime |
| Figure S9 | hdl_predicted_day.png | Daytime |
| Figure S10 | hdl_predicted_night.png | Nighttime |
| Figure S11 | ldllg_predicted_day.png | Daytime |
| Figure S12 | ldllg_predicted_night.png | Nighttime |
| Figure S13 | leuk_predicted_day.png | Daytime |
| Figure S14 | leuk_predicted_night.png | Nighttime |
| Figure S15 | rrdiam_predicted_day.png | Daytime |
| Figure S16 | rrdiam_predicted_night.png | Nighttime |
| Figure S17 | rrsysm_predicted_day.png | Daytime |
| Figure S18 | rrsysm_predicted_night.png | Nighttime |
| Figure S19 | trig_predicted_day.png | Daytime |
| Figure S20 | trig_predicted_night.png | Nighttime |
Scatter plots showing the actual values of each measure (y-axis) and each RMSSD value of daytime or nighttime (x-axis). Grey x indicates measurements from females, black dots indicate measurements from male. A fractional polynomial function represents the average predicted value stratified by sex (dashed black line = males; solid grey line = women) and includes the 95% confidence interval (grey band). The red vertical line aids orientation for the 30 msec RMSSD value.
Figures S21–S40
| Item Number | Filename | Description |
| Figure S21 | chol_actual_day.png | Daytime |
| Figure S22 | chol_actual_night.png | Nighttime |
| Figure S23 | crps_actual_day.png | Daytime |
| Figure S24 | crps_actual_night.png | Nighttime |
| Figure S25 | glucn_actual_day.png | Daytime |
| Figure S26 | glucn_actual_night.png | Nighttime |
| Figure S27 | hba1c_actual_day.png | Daytime |
| Figure S28 | hba1c_actual_night.png | Nighttime |
| Figure S29 | hdl_actual_day.png | Daytime |
| Figure S30 | hdl_actual_night.png | Nighttime |
| Figure S31 | ldllg_actual_day.png | Daytime |
| Figure S32 | ldllg_actual_night.png | Nighttime |
| Figure S33 | leuk_actual_day.png | Daytime |
| Figure S34 | leuk_actual_night.png | Nighttime |
| Figure S35 | rrdiam_actual_day.png | Daytime |
| Figure S36 | rrdiam_actual_night.png | Nighttime |
| Figure S37 | rrsysm_actual_day.png | Daytime |
| Figure S38 | rrsysm_actual_night.png | Nighttime |
| Figure S39 | trig_actual_day.png | Daytime |
| Figure S40 | trig_actual_night.png | Nighttime |
Figures S41–S57
Forest plots showing the odds ratios with according 95% confidence interval estimates for per RMSSD cut off (per 2 msec increment) for each risk factor. Odds ratios larger than 1 indicates an increased risk, e.g., for hypertension (HvsC_night_BP_120_90vs140_90.png). Estimates from logistic regression models adjusted for age, sex, and heart period (IBI). The number in the heading of the graphs indicate the total number of cases (e.g., in HvsC_night_BP_120_90vs140_90.png n = 1954 fell into the criteria of clinically relevant elevated blood pressure versus normal). A total of n = 2907 participants went into the regression model. Each line represents the logistic regression model with the according binary RMSSD cut off in milliseconds.
| Item Number | Filename |
| Figure 1 | HvsC_day_BP_120_90vs140_90.png |
| Figure S41 | HvsC_day_CHOL_170plus_agedep.png |
| Figure S42 | HvsC_day_CRPS_1vs3.png |
| Figure S43 | HvsC_day_GLUC_100vs126.png |
| Figure S44 | HvsC_day_HBA1C_6vs6point5.png |
| Figure S45 | HvsC_day_HDL_55_65vs45_35.png |
| Figure S46 | HvsC_day_LDL_160plus.png |
| Figure S47 | HvsC_day_LEUK_33pc_vs66pc.png |
| Figure S48 | HvsC_day_TRIG_200plus.png |
| Figure S49 | HvsC_night_BP_120_90vs140_90.png |
| Figure S50 | HvsC_night_CHOL_170plus_agedep.png |
| Figure S51 | HvsC_night_CRPS_1vs3.png |
| Figure S52 | HvsC_night_GLUC_100vs126.png |
| Figure S53 | HvsC_night_HBA1C_6vs6point5.png |
| Figure S54 | HvsC_night_HDL_55_65vs45_35.png |
| Figure S55 | HvsC_night_LDL_160plus.png |
| Figure S56 | HvsC_night_LEUK_33pc_vs66pc.png |
| Figure S57 | HvsC_night_TRIG_200plus.png |
Author Contributions
Conceptualization, J.F.T., M.N.J.; Methodology, M.N.J.; Software, M.N.J., A.W. (HRV calculation); Formal Analysis, M.N.J.; Resources, J.E.F.; Writing–Original Draft Preparation, M.N.J., J.K.; Writing–Review & Editing, M.N.J., J.K., A.W., J.E.F., & J.F.T.; Visualization, M.N.J., J.K.; Supervision, J.F.T.
Funding
The work was supported by internal funds of the Mannheim Institute of Public Health, Social and Preventive Medicine, Mannheim Medical Faculty, Heidelberg University, Mannheim, Germany. Julian F. Thayer was partly funded by a Humboldt Award. Marc N. Jarczok’s position and Julian Koenig´s Position were partly funded by the Physician Scientist Program from the Ruprechts-Karls University Heidelberg, Germany.
Conflicts of Interest
The authors of this study report the following relevant conflict of interest: Prof. Dr. Joachim E. Fischer was the major shareholder of HealthVision Ltd. Until December 2012. HealthVision Ltd. was responsible for data collection and field work. The company had no role in the design of the study; analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The major work was funded by internal resources of the first authors home institution at the time of writing (MNJ; Mannheim Institute of Public Health). All authors are responsible for the content and writing of the paper. The other authors declare no conflict of interest.
References
- 1.Kamphuis M.H., Geerlings M.I., Dekker J.M., Giampaoli S., Nissinen A., Grobbee D.E., Kromhout D. Autonomic dysfunction: A link between depression and cardiovascular mortality? The FINE Study. Eur. J. Prev. Cardiol. 2007;14:796–802. doi: 10.1097/HJR.0b013e32829c7d0c. [DOI] [PubMed] [Google Scholar]
- 2.Dekker J.M., Schouten E.G., Klootwijk P., Pool J., Swenne C.A., Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. Am. J. Epidemiol. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- 3.Samsudin M.I., Liu N., Prabhakar S.M., Chong S.L., Kit Lye W., Koh Z.X., Guo D., Rajesh R., Ho A.F.W., Ong M.E.H. A novel heart rate variability based risk prediction model for septic patients presenting to the emergency department. Medicine. 2018;97:e10866. doi: 10.1097/MD.0000000000010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thayer J.F., Yamamoto S.S., Brosschot J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 5.Stuckey M.I., Tulppo M.P., Kiviniemi A.M., Petrella R.J. Heart rate variability and the metabolic syndrome: A systematic review of the literature. Diabetes Metab. Res. Rev. 2014;30:784–793. doi: 10.1002/dmrr.2555. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg E.M. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thayer J.F., Lane R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Jarczok M.N., Koenig J., Mauss D., Fischer J.E., Thayer J.F. Lower heart rate variability predicts increased level of C-reactive protein 4 years later in healthy, nonsmoking adults. J. Intern. Med. 2014;276:667–671. doi: 10.1111/joim.12295. [DOI] [PubMed] [Google Scholar]
- 9.Williams D.P., Koenig J., Carnevali L., Sgoifo A., Jarczok M.N., Sternberg E.M., Thayer J.F. Heart rate variability and inflammation: A meta-analysis of human studies. Brain. Behav. Immun. 2019;80:219–226. doi: 10.1016/j.bbi.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Jarczok M.N., Kleber M.E., Koenig J., Loerbroks A., Herr R.M., Hoffmann K., Fischer J.E., Benyamini Y., Thayer J.F. Investigating the mechanisms of self-rated health: Heart rate variability is more strongly associated than other frequently used biomarkers in a cross sectional occupational sample. PLoS ONE. 2015;10:1–19. doi: 10.1371/journal.pone.0117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster A.K., Fischer J.E., Thayer J.F., Mauss D., Jarczok M.N. Decreased heart rate variability correlates to increased cardiovascular risk. Int. J. Cardiol. 2016;203:728–730. doi: 10.1016/j.ijcard.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Wulsin L., Herman J., Thayer J.F. Stress, autonomic imbalance, and the prediction of metabolic risk: A model and a proposal for research. Neurosci. Biobehav. Rev. 2018;86:12–20. doi: 10.1016/j.neubiorev.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Michels N., Clays E., De Buyzere M., Huybrechts I., Marild S., Vanaelst B., De Henauw S., Sioen I. Determinants and reference values of short-term heart rate variability in children. Eur. J. Appl. Physiol. 2013;113:1477–1488. doi: 10.1007/s00421-012-2572-9. [DOI] [PubMed] [Google Scholar]
- 14.Seppälä S., Laitinen T., Tarvainen M.P., Tompuri T., Veijalainen A., Savonen K., Lakka T. Normal values for heart rate variability parameters in children 6-8 years of age: The PANIC Study. Clin. Physiol. Funct. Imaging. 2014;34:290–296. doi: 10.1111/cpf.12096. [DOI] [PubMed] [Google Scholar]
- 15.Voss A., Heitmann A., Schroeder R., Peters A., Perz S. Short-term heart rate variability—age dependence in healthy subjects. Physiol. Meas. 2012;33:1289–1311. doi: 10.1088/0967-3334/33/8/1289. [DOI] [PubMed] [Google Scholar]
- 16.Hemingway H., Shipley M., Brunner E., Britton A., Malik M., Marmot M. Does autonomic function link social position to coronary risk? The Whitehall II study. Circulation. 2005;111:3071–3077. doi: 10.1161/CIRCULATIONAHA.104.497347. [DOI] [PubMed] [Google Scholar]
- 17.Jarczok M.N., Guendel H., McGrath J.J., Balint E.M. Circadian Rhythms of the Autonomic Nervous System: Scientific Implication and Practical Implementation. In: Pavol S., editor. Chronobiology - Te Science of Biological Time Structure. IntechOpen; London, UK: 2019. [Google Scholar]
- 18.Li X., Shaffer M.L., Rodríguez-Colón S.M., He F., Bixler E.O., Vgontzas A.N., Wolbrette D.L., Wu C., Ball R.W., Liao D. Systemic inflammation and circadian rhythm of cardiac autonomic modulation. Auton. Neurosci. 2011;162:72–76. doi: 10.1016/j.autneu.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemp A.H., Quintana D.S. The relationship between mental and physical health: Insights from the study of heart rate variability. Int. J. Psychophysiol. 2013;89:288–296. doi: 10.1016/j.ijpsycho.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Pencina M.J., D’Agostino R.B., Vasan R.S. Statistical methods for assessment of added usefulness of new biomarkers. Clin. Chem. Lab. Med. 2010;48:1703–1711. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pencina M.J., D’Agostino R.B., Vasan R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 22.Hlatky M.A., Greenland P., Arnett D.K., Ballantyne C.M., Criqui M.H., Elkind M.S.V., Go A.S., Harrell F.E., Hong Y., Howard B.V., et al. American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragland D.R. Dichotomizing continuous outcome variables: Dependence of the magnitude of association and statistical power on the cutpoint. Epidemiology. 1992;3:434–440. doi: 10.1097/00001648-199209000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Vasan R.S. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 25.Abadie J.M., Koelsch A.A. Performance of the Roche second generation hemoglobin A1c immunoassay in the presence of HB-S or HB-C traits. Ann. Clin. Lab. Sci. 2008;38:31–36. [PubMed] [Google Scholar]
- 26.Jarczok M.N., Li J., Mauss D., Fischer J.E., Thayer J.F. Heart rate variability is associated with glycemic status after controlling for components of the metabolic syndrome. Int. J. Cardiol. 2013;167:855–861. doi: 10.1016/j.ijcard.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Weippert M., Kumar M., Kreuzfeld S., Arndt D., Rieger A., Stoll R. Comparison of three mobile devices for measuring R-R intervals and heart rate variability: Polar S810i, Suunto t6 and an ambulatory ECG system. Eur. J. Appl. Physiol. 2010;109:779–786. doi: 10.1007/s00421-010-1415-9. [DOI] [PubMed] [Google Scholar]
- 28.Saul J.P. Beat-To-Beat Variations of Heart Rate Reflect Beat-to-Beat Variations of Heart Rate Reflect Modulation of Cardiac Autonomic Outflow. Physiology. 1990;5:32–37. doi: 10.1152/physiologyonline.1990.5.1.32. [DOI] [Google Scholar]
- 29.Shaffer F., Ginsberg J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camm A., Malik M., Bigger J., Breithardt G., Cerutti S., Cohen R., Coumel P., Fallen E., Kennedy H., Kleiger R. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart. J. 1996;17:354–381. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 31.Wittling W., Wittling R.A. Herzschlagvariabilität: Frühwarnsystem, Stress- und Fitnessindikator. 1st ed. Eichsfeld; Heilbad Heiligenstadt, Germany: 2012. [Google Scholar]
- 32.de Geus E.J.C., Gianaros P.J., Brindle R.C., Jennings J.R., Berntson G.G. Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology. 2019;56:1–26. doi: 10.1111/psyp.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers W.H. Regression standard errors in clustered samples. Stata Tech. Bull. 1993;13:19–23. [Google Scholar]
- 34.Thayer J.F., Fischer J.E. Heart rate variability, overnight urinary norepinephrine and C-reactive protein: Evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J. Intern. Med. 2009;265:439–447. doi: 10.1111/j.1365-2796.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- 35.Jarczok M.N., Koenig J., Schuster A.K., Thayer J.F., Fischer J.E. Nighttime heart rate variability, overnight urinary norepinephrine, and glycemic status in apparently healthy human adults. Int. J. Cardiol. 2013;168:3025–3026. doi: 10.1016/j.ijcard.2013.04.147. [DOI] [PubMed] [Google Scholar]
- 36.Pearson T.A., Mensah G.A., Alexander R.W., Anderson J.L., Cannon R.O., Criqui M., Fadl Y.Y., Fortmann S.P., Hong Y., Myers G.L., et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 37.Xu J., Knowler W.C., Devereux R.B., Yeh J., Umans J.G., Begum M., Fabsitz R.R., Lee E.T. Albuminuria within the “normal” range and risk of cardiovascular disease and death in American Indians: The Strong Heart Study. Am. J. Kidney Dis. 2007;49:208–216. doi: 10.1053/j.ajkd.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Hill L.K., Hu D.D., Koenig J., Sollers J.J., Kapuku G., Wang X., Snieder H., Thayer J.F. Ethnic differences in resting heart rate variability: A systematic review and meta-analysis. Psychosom. Med. 2015;77:16–25. doi: 10.1097/PSY.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.