Abstract
Objective:
It is increasingly recognized that trauma victims, particularly Veterans, have co-occurring psychological and physical conditions that impact cognition, especially the domains of sustained attention and executive functioning. While previous work has generally attempted to isolate the unique cognitive effects of common combat-related co-morbidities, less work has been done to examine how these conditions co-occur, and whether unique cognitive signatures accompany certain clinical combinations.
Method:
To address this gap, we examined how a number of deployment-related conditions were associated with performance on a well-validated measure of sustained attention (gradual onset continuous performance task; gradCPT), as well as a battery of standard neuropsychological measures, in 123 Veterans from the Translational Research Center for TBI and Stress Disorders (TRACTS). Initially, a PCA was conducted to investigate how comorbid conditions grouped together.
Results:
A number of sustained attention measures from the gradCPT were differentially associated with four unique combinations of trauma-related pathology. Specifically, a component representing the combination of current pain, sleep disturbance, and mTBI (somatic) was associated with a higher rate of failures of attentional engagement. On the other hand, a comorbid PTSD and mood disorder component (mood-PTSD), as well as a substance use disorder component (SUD), were associated with higher rates of inhibitory control failures. Increased attentional instability was associated with mood-PTSD as well as an anxiety disorder component. In contrast, the cognitive effects of deployment-related trauma were not observed on standard neuropsychological measures.
Conclusion:
These findings suggest that unique combinations of trauma-related pathology have dissociable effects on sustained attentional control.
Keywords: PTSD, mTBI, sustained attention, gradCPT, Veterans
Introduction
It is increasingly recognized that trauma victims, particularly Veterans, have co-occurring psychological and physical conditions that may impact cognition, especially the domains of sustained attention and executive functioning. For example, our work and others have shown that posttraumatic stress disorder (PTSD) is associated with deficits in sustained attention and inhibitory control (Aupperle, Melrose, Stein, & Paulus, 2012; DeGutis et al., 2015; Esterman, DeGutis, et al., 2013; Scott et al., 2015; Swick, Honzel, Larsen, & Ashley, 2013; Swick, Honzel, Larsen, Ashley, & Justus, 2012; van Rooij et al., 2014), though other studies have failed to find such relationships (Golier et al., 1997; Jenkins, Langlais, Delis, & Cohen, 2000; Leskin & White, 2007). Such discrepancies may be related to the fact that very often PTSD does not occur in isolation, but co-occurs and interacts with other psychological symptoms thought to also influence these cognitive processes, such as depression (Bleich, Koslowsky, Dolev, & Lerer, 1997; Green, PD, Grace, & Leonard, 1992; Milliken, Auchterlonie, & Hoge, 2007; O’Donnell, Creamer, & Pattison, 2004; Shalev et al., 1998), substance abuse (Brown, Recupero, & Stout, 1995; Brown & Wolfe, 1994; Keane, Gerardi, Lyons, & Wolfe, 1987; McFall, Mackay, & Donovan, 1992; Ouimette & Brown, 2003), and other anxiety disorders (Green et al., 1992; Helzer, Robins, & McEvoy, 1987; Kar & Bastia, 2006; Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995; Zlotnick et al., 1999), which are often not taken into account. In addition, physical and somatic symptoms, like pain (Beckham et al., 1997; Lew, Tun, & Cifu, 2009; Norman, Stein, Dimsdale, & Hoyt, 2008; Walker, Clark, & Sanders, 2010), sleep disorders (DeGutis et al., 2016; Lavie, 2001), and for Veterans in particular, mild traumatic brain injuries (mTBI; Lippa et al., 2015; McGlinchey, Milberg, Fonda, & Fortier, 2017; Walker et al., 2010) are frequently co-morbid and are also thought to influence cognition. Critically, some of these studies (Amick et al., 2018; Lippa et al., 2015) have shown that specific empirically motivated configurations of common co-occurring diagnoses (e.g. PTSD, depression and mTBI) predict severe disability at levels not accounted for by any individual diagnostic category, or simply by burden of disease. These data imply that some syndromic clinical phenotypes may impact function in ways that are not intrinsic to standard psychiatric diagnostic categories. This raises the possibility that some combinations of clinical symptoms are associated with difficulties in cognitive processes that might not be measurable otherwise.
Sustained attention is one of the most fundamental cognitive operations, as it is necessary for the optimal engagement of a variety of other higher-level processes, such as learning, memory, and future planning, and plays an important role in modulating lower-level sensory processing functions (Barkley, 1997; Carrasco, 2011; Chun, 2011; Fortenbaugh, Robertson, & Esterman, 2017; Ling & Carrasco, 2006; Sarter, Givens, & Bruno, 2001; Silver & Feldman, 2005). Frequently, sustained attention ability is characterized with continuous performance tasks, in which participants respond to frequent non-target stimuli and withhold responses to rare targets events (not-X CPTs; for review/discussion see Fortenbaugh, DeGutis, & Esterman, 2017; Langner & Eickoff, 2013). While previous studies have shown general deficits in sustained attention in trauma-related disorders (Auerbach et al., 2014; DeGutis et al., 2015; Robertson, Manly, Andrade, Baddeley, & Yiend, 1997; Swick et al., 2013; Swick et al., 2012), sustained attention is a multifaceted process (Cheyne, Solman, Carriere, & Smilek, 2009) and studies have not yet fully characterized how sustained attention may be disrupted in these populations. One of the primary processes required to perform these not-X CPTs is sustained inhibitory control; thus errors of commission, or failures to inhibit responses to no-go targets, characterize one type of attentional lapse on these tasks. On the other hand, errors of omission, failing to respond to frequent go-trials, are considered failures to maintain constant attentional engagement or arousal. When examining more subtle measures of reaction time (RT), intra-individual variability of RTs has been associated with greater attentional fluctuations, or lack of attentional stability, whereas the mean reaction time reflects a combination of processing speed and strategic factors (Fortenbaugh et al., 2015; Seli, Jonker, Cheyne, & Smilek, 2013). Finally, post-error slowing of reaction time is a hallmark of these tasks, and reflects the degree to which participants make online performance-based adjustments to their strategy after an error (Dutilh et al., 2012). These different aspects of performance make not-X CPT tasks potentially sensitive and specific enough to detect and differentiate sustained attention impairments in trauma-related psychopathology.
Indeed, there is some evidence that sustained attention tasks are sensitive to different trauma-related psychopathology in this population. For example, PTSD has been associated with both deficits in sustained attention and inhibitory control, as reflected in greater RT variability and greater commission errors on go/no-go tasks (DeGutis et al., 2015; Jenkins et al., 2000; Vasterling, Brailey, Constans, & Sutker, 1998; Vasterling et al., 2002). This is consistent with the diagnostic symptoms of “difficulty concentrating” as well as hypervigilance, or dysregulated arousal, which is known to negatively impact sustained attention (Arnsten, 1998). Additionally, deficits in sustained attention have been found to be common in patients dealing with depression (Clark, Iversen, & Goodwin, 2002; Paelecke-Habermann, Pohl, & Leplow, 2005; Zakzanis, Leach, & Kaplan, 1998). Difficulty concentrating or inappropriate hypervigilance to mundane events is also a feature in persons with anxiety disorders (Akiskal, 1998; Forster, Nunez Elizalde, Castle, & Bishop, 2015), and recent work has shown a relationship between poorer sustained attention/greater distractibility and higher trait anxiety levels in sub-clinical populations (Forster et al., 2015; Moser, Becker, & Moran, 2012). Additionally, impairments in attention have been associated with sleep disturbances (Ayalon, Ancoli-Israel, Aka, McKenna, & Drummond, 2009; Doran, Van Dongen, & Dinges, 2001; Lim & Dinges, 2008) and chronic pain (Dick & Rashiq, 2007; Moriarty, McGuire, & Finn, 2011), two other issues commonly faced in Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans (Lippa et al., 2015). While reports of deficits or difficulties in sustained attention are highly prevalent in the clinical literature, whether the nature of these deficits is similar across various clinical disorders or in different combinations of disorders remains understudied. In part, this may be a limitation of the types of tasks or measures reported that are traditionally used to assess attention, which may attempt to summarize sustained attention performance into a single metric. It is thus possible that more recent developments of sustained attention assessments, which capture multiple dimensions and more subtle fluctuations in performance, may be more sensitive than tradition neuropsychological measures of executive function to detect variations in attentional dysfunction (DeGutis et al., 2015; Esterman, Noonan, Rosenberg, & DeGutis, 2013; Fortenbaugh, DeGutis, et al., 2017).
The present study examined the relationship between deployment-related trauma and cognitive function in Veterans in two innovative ways. First, based on Lippa et al. (2015), the current study used a PCA approach to create empirically-derived clinical components consisting of multiple psychiatric (PTSD, mood disorder, anxiety disorder, and substance use disorder) and somatic issues (mTBI, sleep disturbance and current pain) in a large sample of well-characterized OEF/OIF/OND Veterans. Lippa and colleagues discovered four independent deployment-related components: (a) depression, PTSD, and military mTBI (deployment trauma component); (b) pain and sleep (somatic component); (c) anxiety disorders, other than PTSD (anxiety component); and (d) substance abuse or dependence (substance use component). To validate this PCA structure, we sought to perform the identical analysis with a significantly larger sample of Veterans, hypothesizing that we would discover similar components. We next sought to characterize how the resulting clinical components related to performance on our sustained attention measure, the gradCPT, as well as traditional neuropsychological tests of attention and executive function in a sub-sample of these Veterans. Based on Lippa et al. as well as the literature and our prior results, we anticipated that a deployment trauma component (e.g., PTSD, depression) would be most associated with failures of sustained inhibitory control and increased variability (Aupperle et al., 2012; J. DeGutis et al., 2015; Swick et al., 2013; Swick et al., 2012), while somatic issues (e.g., pain, sleep dysfunction) would be most associated with failures of engagement and reduced error reactivity (Cheyne et al., 2009; O’Keeffe, Dockree, Moloney, Carton, & Robertson, 2007). We further predicted that the clinical components would be more weakly associated with the traditional neuropsychological tests, as they are not optimized to measure more subtle aspects of sustained attention.
Methods
Participants
For the initial PCA we leveraged the full available sample of OEF/OIF/OND Veterans from the longitudinal cohort study of the Traumatic Brain Injury (TBI) National Network Research Center at the Translational Research Center for TBI and Stress Disorders at VA Boston Healthcare System (TRACTS, for a more in-depth description of recruitment and characteristics of this sample, see Amick et al., 2013; Fortier et al., 2014; McGlinchey et al., 2017). At the time of this study, the sample included 388 consecutively enrolled Veterans who had complete datasets and met the major inclusion criteria as outlined below. All participants recruited to TRACTS complete 8–10 hours of testing that includes a comprehensive psychiatric and neuropsychological assessment. For participants without any contraindications, this is followed by a MRI session during which structural and functional scans are collected. For a subset of participants (see below), this included completion of the gradCPT sustained attention task with concurrent fMRI (Fortenbaugh, Rothlein, McGlinchey, Degutis, & Esterman, 2018); however, this paper focuses on the behavioral performance of this task. General exclusion criteria for recruitment into the TRACTS cohort includes prior serious medical and/or neurological illness unrelated to TBI, active suicidal and/or homicidal ideation requiring intervention, or a current diagnosis of bipolar disorder or psychotic disorder (except psychosis not otherwise specified due to trauma-related hallucinations) according to the Diagnostic and Statistical Manual of Mental Disorders (5th editition, DSM-IV; American Psychiatric Association [APA], 2013). For the present study, data was available for 327 participants after additionally excluding participants who had not been deployed to a combat zone (n = 22), had a history of moderate/severe TBI (n = 15), or failed an assessment of symptom validity (n = 24, detailed below). Demographic information regarding the sample of 327 participants who met these inclusion criteria and were included in the following analyses is outlined in Table 1. While the TRACTS dataset employs a convenience sample, recent analyses (Lippa et al., 2015) have found that there is no significant difference between the TRACTS cohort and the OEF/OIF/OND veterans who utilize the VA Healthcare system.
Table 1.
Demographic, Current Psychiatric, and Behavioral Status for Total Sample and by gradCPT Participation
| Total (N = 327) |
gradCPT (n = 123) |
No gradCPT (n = 204) |
|||||
|---|---|---|---|---|---|---|---|
| Variable | n or M | % or SD | n or M | % or SD | n or M | % or SD | X2 or t |
| Age | 31.78 | 8.30 | 31.14 | 7.32 | 32.17 | 8.82 | 1.09 |
| Males | 295 | 90.2% | 116 | 94.3% | 179 | 87.7% | |
| Education (years) | 13.93 | 1.92 | 14.05 | 1.95 | 13.86 | 1.91 | −0.85 |
| Race | 3.63 | ||||||
| White | 244 | 74.6% | 89 | 72.4% | 155 | 76.0% | |
| Black | 24 | 7.3% | 12 | 9.8% | 12 | 5.9% | |
| Hispanic | 48 | 14.7% | 18 | 14.6% | 30 | 14.7% | |
| Other | 5 | 1.5% | 2 | 1.6% | 3 | 1.5% | |
| Unknown/missing | 6 | 1.8% | 2 | 1.6% | 4 | 2.0% | |
| Service Branch | 7.21 | ||||||
| Army | 80 | 24.5% | 19 | 15.4% | 61 | 29.9% | |
| Navy | 10 | 3.1% | 5 | 4.1% | 5 | 2.5% | |
| Marines | 62 | 19.0% | 27 | 22.0% | 35 | 17.2% | |
| Air Force | 14 | 4.3% | 4 | 3.3% | 10 | 4.9% | |
| National Guard | 119 | 36.4% | 45 | 36.6% | 74 | 36.3% | |
| Reserves | 42 | 12.8% | 23 | 18.7% | 19 | 9.3% | |
| Deployments | |||||||
| Number | 1.47 | 0.80 | 1.51 | 0.84 | 1.44 | 0.78 | −0.78 |
| Months | 14.53 | 8.75 | 14.59 | 8.93 | 14.45 | 8.66 | −0.22 |
| Months since last | 39.03 | 31.85 | 40.77 | 30.99 | 38.12 | 32.42 | −0.67 |
| Combat exposure | 17.10 | 12.00 | 17.71 | 12.32 | 16.73 | 11.81 | −0.72 |
| N of military TBIs | 0.78 | 1.45 | 0.80 | 1.70 | 0.77 | 1.27 | −0.16 |
| PTSD | 198 | 60.6% | 75 | 61.0% | 123 | 60.9% | 0.01 |
| PTSD Severity | 49.28 | 28.98 | 46.33 | 26.41 | 51.07 | 30.35 | 1.44 |
| Depressive disorders | 81 | 24.8% | 25 | 20.3% | 56 | 27.5% | 2.09 |
| Anxiety disorders | 72 | 22.0% | 25 | 20.3% | 47 | 23.0% | 0.33 |
| Substance use disorder | 51 | 15.6% | 21 | 17.1% | 30 | 14.7% | 0.33 |
| Current pain | 224 | 68.5% | 85 | 69.1% | 139 | 68.1% | 0.03 |
| 30-day average pain | 29.83 | 25.79 | 31.17 | 25.65 | 28.91 | 25.93 | −0.72 |
| Sleep disturbance | 254 | 77.7% | 95 | 77.2% | 159 | 77.9% | 0.02 |
| Sleep quality | 9.85 | 4.72 | 9.75 | 4.53 | 9.77 | 4.86 | −0.08 |
| # Psych/Behav conditions | 3.13 | 1.70 | 3.08 | 1.61 | 3.16 | 1.75 | 0.39 |
| 3+ comorbidities | 203 | 62.1% | 78 | 63.4% | 125 | 61.3% | 0.15 |
Note: all ps > 0.15
Of our sample of 327 deployed Veterans, 123 participants completed the gradCPT task and were included in this part of the analyses. Demographic information regarding this sub-sample is outlined in Table 1. We also conducted additional analyses that compared our subset of participants to the greater TRACTS sample to ensure that no systematic differences existed between those who completed the gradCPT and those who did not. The VA Boston Healthcare System Institutional Review Board approved the study protocol and all participants provided written informed consent. Participants were provided $210 for their time and travel costs.
Clinical Measures.
Demographics, Combat Exposure, and Symptom Validity.
During the initial clinical and neuropsychological assessment period, demographic and military experience information was determined using self-report questionnaires. Combat exposure was assessed using the Deployment Risk and Resilience Inventory (DRRI): Combat Experience Scale (King, King, Vogt, Knight, & Samper, 2006). To assess potential issues with effort/validity on our clinical measures, participants were administered Green’s verbal Medical Symptom Validity Test (v-MSTV; Green, 2004) and were excluded if they did not complete or scored under 85 on immediate recall, delayed recall, or consistency (n = 24 for the initial sample).
Traumatic Brain Injury.
The Boston Assessment of TBI-Lifetime (BAT-L; Fortier et al., 2014), was used to assess each participant’s history of mild, moderate, and severe TBI that occurred pre-, during, and post-deployment. This is a validated, semi-structured clinical interview administered by a doctoral-level psychologist. A history of military mTBI was defined as a period of self-reported loss of consciousness < 30 minutes, posttraumatic amnesia < 24 hours, and/or altered mental status < 24 hours following a credible injury mechanism that occurred during military service (U.S. Department of Veterans Affairs and U.S. Department of Defence, 2009). The BAT-L was reviewed in weekly diagnostic consensus meetings consisting of at least three doctoral psychologists and/or psychiatrists.
Psychiatric Disorders.
In order to assess the presence and/or history PTSD, a doctoral-level psychologist administered the Clinician Administered PTSD Scale for the DSM-IV (CAPS; Blake et al., 1990). Additionally, the psychologists administered the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First, Spitzer, Gibbon, & Williams, 1996; Lobbestael, Leurgans, & Arntz, 2011; Williams et al., 1992) non-patients edition to assess for mood disorders (e.g., depression), anxiety disorders, and substance use disorders. This assessment was also used to screen for psychotic disorders using in our exclusion criteria. The CAPS and SCID assessments were reviewed in weekly diagnostic consensus meetings consisting of at least three doctoral psychologists and/or psychiatrists.
Sleep Quality and Pain.
To assess current sleep quality, the Pittsburgh Sleep Quality Index (PSQI; Carpenter & Andrykowski, 1998) was administered. Following the methodology of previous studies (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989; Lippa et al., 2015), global cutoff scores of > 5 were used to define the presence of sleep disturbances in this sample. The Short Form McGill Pain Questionnaire (SFMPQ; Grafton, Foster, & Wright, 2005; Melzack, 1987) was used to assess pain. We classified participants as having current pain if the self-reported current overall level of pain was rated as mild or greater.
Sustained Attention Measures
Gradual-onset Continuous Performance Task (gradCPT).
The gradCPT was the primary cognitive focus of this investigation (see Figure 1). The gradCPT is a well-validated not-X CPT task used to measure sustained attention, in which participants are instructed to respond to frequently appearing non-targets and withhold responses to infrequently appearing targets (DeGutis et al., 2015; Esterman, Noonan, et al., 2013; Fortenbaugh et al., 2015). Over the course of an 8-minute run, scene images transition from one to the next over an 800 ms interval using linear interpolation, such that images continuously fade in from one to next. Images are randomly selected with the constraint that no image was repeated across two consecutive trials. Since stimuli are presented both rapidly and without discrete periods of transition, this task relies heavily upon an intrinsic ability to sustain attention (Esterman, Noonan, et al., 2013). The current version of the task consisted of 20 randomly displayed gray-scale city and mountain scenes. For every displayed city, which occurred on 90% of trials, participants were instructed to respond via pressing a button on the response box. For every mountain, occurring on 10% of trials, participants were instructed to inhibit this response. This paradigm measures multiple aspects of sustained attention behaviorally, including commission and omission error rates, reaction time (RT) speed, RT variability, and post-error slowing as described below. For additional information on this task, see Esterman, et al. (2013); and for overall behavioral and fMRI results in this Veteran population, see Fortenbaugh et al. (2018).
Figure 1. The gradCPT.
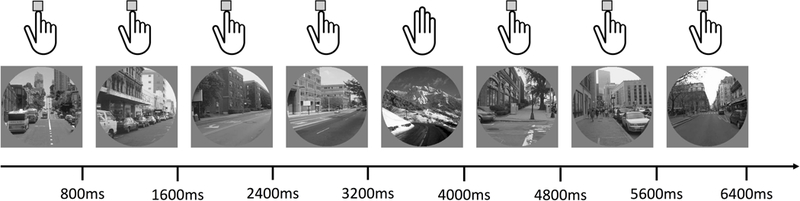
This figure illustrates the gradual-onset continuous performance (gradCPT) sustained attention task used in the study. In the gradCPT scene images transition from one to the next over an 800 ms interval using linear interpolation so that each image fades from one to the next. Participants are asked to press a button to each city image scene and withhold responses to each image of a mountain scene (illustrated with the hands at the top of the image). City images are shown on 90% of trials while rare mountain scenes are shown on the remaining 10% of trials.
Accuracy measures.
Accuracy was assessed by examining the two types of errors possible during this task. First, failure to withhold button response to a (rare) mountain scene was considered a commission error (CE), which constitutes an error of sustained inhibitory control. Second, failure to respond to a (frequent) city scene was considered an omission error (OE). While rarer than commission errors, omission errors likely represent more severe lapses of attention (Cheyne et al., 2009), wherein complete disengagement from the task is suspected. Omission errors may be a result of two potential patterns of disengagement: long periods of task disengagement or more brief, intermittent failures to stay on task. To capture these differences, omission errors were additionally broken down into two separate components. First, we assessed the number of discrete intervals where omission errors occurred, referred to as the number of omission lapses. Second, we determined the average number of trials on which failure to respond to a city image occurred during a consecutive periods of omissions, referred to as the average omission duration.
Reaction Time Measures.
RTs were calculated relative to the beginning of each image transition using an iterative algorithm (for details, see Esterman, Noonan, et al., 2013; Fortenbaugh et al., 2015), such that an RT of 800 ms indicated a button press at the moment the current trial’s scene image was 100% coherent and not mixed with other images. A shorter RT indicated that the current scene was still in the process of transitioning from the previous, and a longer RT indicated that the current scene was in the process of transitioning to the subsequent scene. Mean RT and RT variability (defined as the standard deviation of RT) were computed from RTs. Post-error slowing (PES) was computed by subtracting the mean RT on trials immediately following a CE from trials immediately preceding CEs (Fortenbaugh et al., 2015). Thus, positive values represent greater slowing. Participants who did not make any commission errors during the task (n = 2) had no data to calculate this measure and were thus not included in this analysis.
Neuropsychological Measures
Attention.
We used the Test of Variable Attention (TOVA; Leark, Greenberg, Kindschi, Dupuy, & Hughes, 2007) as another measure of sustained visual attention. The TOVA is a CPT task designed to capture attention and impulsivity. We included response time, response time variability, commission errors and omission errors as our dependent measures.
Task Switching.
We used the Cambridge Automated Neuropsychological Test Battery (CANTAB) Intra-Extra Dimensional Set Shift (I-EDSS; CANTAB, Cambridge Cognition, 2017, www.cantab.com) as a measure of task switching. The IED is a computerized analogue to the Wisconsin Card Sorting Task and is used to measure attentional flexibility. The dependent measures were the total errors adjusted score and the completed stage trials.
Inhibitory Control.
We used the Color-Word Interference Test (Stroop; Delis, Kaplan, & Kramer, 2001), the CANTAB Affective Go/No-Go (AGN; CANTAB, Cambridge Cognition, 2017, www.cantab.com), and TOVA response inhibition measure to assess inhibitory control. For the Stroop task, the dependent measure used was interference trial total time. The dependent measures for the AGN were the total positive and total negative commission errors. The dependent measure for the TOVA was the total commission errors.
Working Memory.
We used the Auditory Consonant Trigrams (ACT; Stuss et al., 1985) and Digit Span Sequencing (DSPSS; Wechsler, 2008) to measure working memory. The ACT dependent measure was the total number of correct responses and the dependent measure for the DSPSS was the sum of the forward, backward, and sequencing scores.
Multiple Executive Domains.
The Trail Making Test (Trails B) and Verbal Fluency Test from the Delis-Kaplan Executive Function System (DKEFS; www.pearsonclinical.com, Delis et al., 2001) were used to assess multiple subdomains of executive function. The Trails B assesses working memory and task switching by requiring participants to connect, sequentially, alternating numbers and letters. Our dependent measure for the Trails B was time to complete the task. Verbal Fluency measures both working memory and inhibition and the dependent measure was total score.
Statistical Analyses
Exploratory Principal Component Analysis.
While clinical diagnoses are often studied in isolation, it is well established that various symptoms co-occur frequently in veteran populations (McGlinchey et al., 2017). We examined the shared variance explained by empirically derived clinical components following the approach used by Lippa et al. (2015), where an exploratory PCA was conducted using diagnoses (yes/no) from the seven clinical areas outlined above (mTBI, PTSD, mood disorder, anxiety disorder, substance use disorder, sleep disturbance, and current pain). The clinical component scores were generated using a PCA and varimax rotation in the greater TRACTS sample (n = 327) and were applied to the current study sample. Every participant was assigned a component score, which is a standardized numerical value estimated from the PCA (M = 0, SD = 1), for each of the derived clinical components.
gradCPT/clinical relationships.
Clinical component scores were entered as independent variables into multiple linear regression models to predict gradCPT measures of sustained attention, including our three reaction time measures (mean RT, reaction time variability defined by the standard deviation of reaction times, and post-error slowing) as well as our four accuracy measures (commission error rate, omission error rate, number of omission lapses, and the average duration of individual omission lapse periods). Separate models were calculated for each of the seven dependent variables. As mean reaction time can significantly affect overall reaction time variability and post-error slowing values, mean reaction time was included as a covariate in these two models. Additionally, as the number of omission error lapses and the average duration of these lapses both contribute to overall omission error rates, each of these variables was included as a covariate in the regression model of the other.
Neuropsychological/clinical relationships.
Our eight neuropsychological measures of attention and executive functioning were also modeled with identical regression models to investigate if performance deficits could be predicted from our clinical components. Here, the clinical components were entered as the independent variables to predict the neuropsychological test outcomes. Because some of the neuropsychological tests had more than one dependent measure, a total of twelve separate multiple linear regressions were conducted.
Results
Participant Characteristics
Demographic and clinical characteristics of the sample population are described in Table 1. No significant differences were found between participants who did and did not complete gradCPT on any of the clinical or demographic variables (all ps > .15). This suggests that the 123 participants who completed the gradCPT task are representative of the larger TRACTS sample.
Exploratory Principal Component Analysis
We selected the four-component solution (eigenvalue > 0.85) as it produced clinically meaningful groupings of the diagnoses and accounted for at least 70% of the total variance, replicating our prior analysis with a smaller sample in the same cohort and cutoff criteria (Lippa et al., 2015). These components accounted for 72.1% of the total variance (see Table 2 for component loadings, eigenvalues, and variance explained). The first component, Somatic/TBI (somTBI), accounted for the highest percent of the variance (23%), followed by the second component, PTSD/mood (moodPTSD) disorder (19%). The third and fourth components, anxiety disorder (Anxiety) and substance use disorder (Substance), each accounted for 15% of the variance. These components were mostly consistent with the Lippa et al. (2015), although mTBI was more associated with somatic symptoms in the present, larger sample, as opposed to PTSD/mood in the Lippa analysis.
Table 2.
Phenotype Component Loading Scores
| Component 1 somTBI |
Component 2 moodPTSD |
Component 3 Anxiety |
Component 4 Substance |
|
|---|---|---|---|---|
| PTSD | 0.461 | 0.609 | 0.115 | 0.222 |
| Mood Disorders | 0.028 | 0.925 | 0.037 | −0.035 |
| mTBI | 0.738 | 0.104 | −0.261 | −0.010 |
| Pain | 0.720 | −0.013 | 0.305 | −0.137 |
| Sleep | 0.569 | 0.273 | 0.151 | 0.254 |
| Substance Use | ||||
| Disorders | 0.004 | 0.042 | 0.012 | 0.961 |
| Anxiety Disorders | 0.060 | 0.092 | 0.929 | 0.028 |
| Eigenvalue | 2.161 | 1.045 | .981 | .859 |
| % Variance | 22.907 | 18.896 | 15.176 | 15.101 |
Clinical Components and gradCPT
In order to examine how the four clinical component scores were able to predict performance, separate multiple linear regression models were calculated with each the seven measures of performance on the gradCPT as dependent variables. Tables 3 & 4 detail the resulting model fits for each of these regression models. Below we outline the main findings from these regression analyses.
Table 3.
Summary of multiple linear regression models predicting reaction time variables: mean reaction time (RT), reaction time variability (StDev RT), and post-error slowing (PES).
| RT | StDev RT | PES | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β |
| somTBI | .044 | .006 | .061 | .003 | .002 | .091 | −.02 | .010 | −.168* |
| moodPTSD | −.009 | .007 | −.115 | .006 | .003 | .146* | .017 | .011 | .126 |
| Anxiety | −.009 | .007 | −.118 | .008 | .003 | .190** | .029 | .011 | .225* |
| Substance | −.007 | .007 | −.094 | .003 | .003 | .083 | .007 | .010 | .059 |
| RT | .376 | .037 | .687** | .725 | .147 | .417** | |||
| R2 | .039 | .487 | .218 | ||||||
| F | 1.212 | 22.227** | 6.427** | ||||||
Note
p < 0.05,
p ≤ 0.01
Table 4.
Summary of multiple linear regression models predicting accuracy variables: commission error rate (CE), omission error rate (OE), average number of discrete omission error periods (# OE Lapses), and the average duration in trials of omission error lapse periods (Ave OE Duration).
| CE | OE | # OE Lapses | Ave OE Duration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β | B | SE B | β |
| somTBI | .013 | .012 | .091 | .010 | .006 | .150 | 6.43 | 2.57 | .223* | −.088 | .049 | −.163 |
| moodPTSD | .035 | .014 | .218* | − .011 |
.007 | −.135 | −4.11 | 2.93 | −.125 | .018 | .056 | .030 |
| Anxiety | .003 | .014 | .018 | − .011 |
.007 | −.012 | 1.44 | 2.81 | .046 | −.070 | .053 | −.121 |
| Substance | .029 | .013 | .199* | − .005 |
.007 | −.075 | −2.60 | 2.73 | −.086 | .030 | .052 | .052 |
| # OE Lapses | .004 | .002 | .197 | |||||||||
| Ave OE Duration | 10.21 | 4.78 | .190* | |||||||||
| R2 | .081 | .048 | .101 | .068 | ||||||||
| F | 2.591* | 1.499 | 2.63* | 1.703 | ||||||||
note
p < 0.05
Model 1: Mean RT.
The outcome for Model 1 was mean RT, a reflection of processing speed and decision criterion. No clinical components were found to have a significant association (overall model: F(4,118) = 1.212, p = 0.309, R2 = 0.039; see Table 3).
Model 2: RT Variability.
The outcome for Model 2 was RT variability, a measure of attentional fluctuations/stability (overall model: F(5,117) = 22.227,p < 0.001, R2 = 0.487). PTSD/Mood (β = 0.146, p = 0.033) and Anxiety (β= 0.190, p = 0.006) were positively and uniquely associated such that those with higher scores tended to be more variable (see Table 3 & Figure 2). Overall RT (β = 0.687, p < 0.001), which was included as a covariate, was also positively associated with the model such that slower participants on the task tended to have more variable reaction times.
Figure 2.
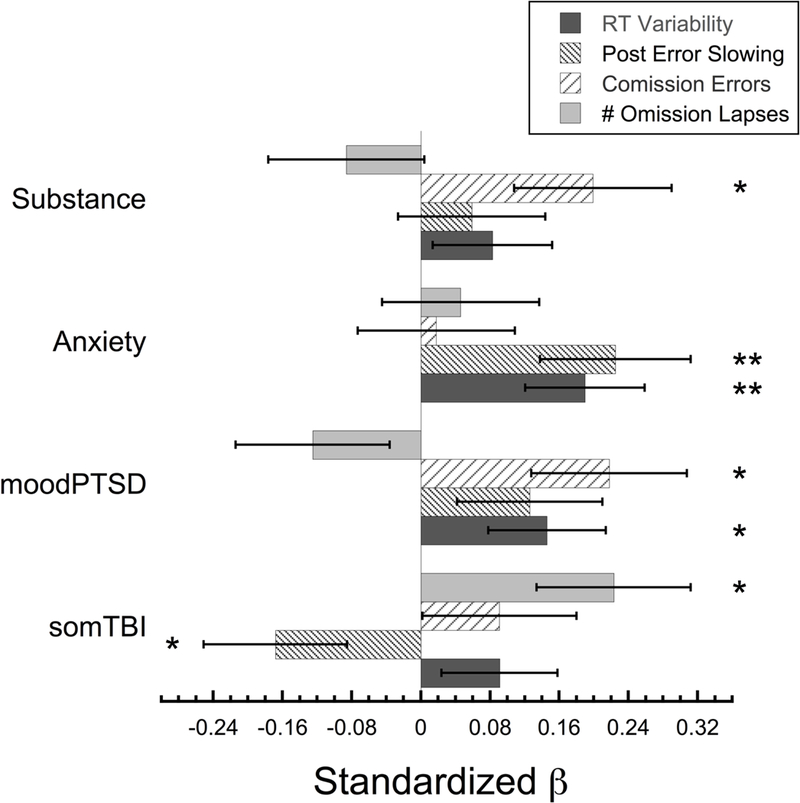
Bar graph showing the standardized beta-weights for each of the four clinical components used to predict performance on the gradCPT sustained attention task. For clarity, only the performance variables where the overall regression model was significant are shown. This includes reaction time variability (standard deviation of the reaction time), post-error slowing, commission error rate, and the number of omission lapses. Error bars show ±1 S.E.M. * = p < 0.05, ** p ≤ 0.01.
Model 3: Post-Error Slowing.
The outcome for Model 3 was post-error slowing, a measure of error reactivity (overall model: F(5,115) = 6.427,p < 0.001, R2 = 0.218). While Anxiety was associated with increased reactivity (β= 0.225, p = 0.010), Somatic/TBI was associated with decreased reactivity to errors (β= −0.168, p = 0.047). Overall reaction time (β = 0.417, p < 0.001), which was included as a covariate, was positively associated with the model such that participants who were slower on the task showed larger post-error slowing (see Table 3 & Figure 2).
Model 4: Inhibitory Control.
The outcome for Model 4 was commission error rate, reflecting inhibitory control failures (overall model: F(4,118) = 2.591, p = 0.04, R2 = 0.081). PTSD/Mood (β = 0.281, p = 0.016) and Substance Use Disorder (β = 0.199, p = 0.031) were significantly associated with CEs, such that those with higher loadings on these components tended to make more inhibitory errors by pressing to mountain/no-go targets (see Table 4 & Figure 2).
Model 5: Disengagement.
The outcome for Model 5 was overall omission error rate, a measure of disengagement from the task. No clinical components were found to be significantly associated with overall omission error rate (overall model: F(4,118) = 1.499, p = 0.207, R2 = 0.048; see Table 4). The final two models examined the prediction ability of the clinical components when omission errors were broken down into the number of discrete omission lapse periods and the average duration of individual lapse periods.
Model 6: Discrete Periods of Disengagement.
The outcome for Model 6 was the number of omission lapses across the experiment, including the average duration of each of these periods as a covariate (overall model: F(5,117) = 2.630, p = 0.027, R2 = 0.101). The model revealed that, only the Somatic/TBI component (β= 0.223, p = 0.014) was found to be positively associated, such that those with higher Somatic/TBI component scores had more discrete periods of disengagement defined by failing to press to a city image/go trial (see Table 4 & Figure 2). The additional covariate in this model, the average durations of each omission period, was also positively associated with the number of discrete omission lapse periods (β= 0.190, p = 0.035).
Model 7: Average Duration of Disengagement.
The outcome for Model 7 was the average duration in trials that participants disengaged in the task as measured by omission errors. The overall number of omission lapse periods was included as a covariate. In contrast with the number of omission error lapse periods, no clinical components were found to have a significant association with the average duration of individual disengagement periods (overall model: F(5,117) = 1.703, p = 0.139, R2 = 0.068; see Table 4).
Additional models.
We also explored whether discrete diagnoses themselves, rather than clinical phenotypes, predicted gradCPT performance (see Supplementary Materials for details). Although anxiety and substance use disorder diagnoses models were similar to the phenotype models, in no case did the PTSD/mood or Somatic/TBI component diagnoses explain unique variance in performance. Thus the association between these two components and attention were not captured by the individual diagnoses.
Neuropsychology Tests and Clinical Components
Models 8–19.
We modeled the relationship between 12 neuropsychological measures of attention/executive functioning and our clinical phenotypes. Each model measured different aspects of attention and executive function, including switching, working memory, and inhibition. No clinical components were found to have a significant association with any of our individual neuropsychological tests (overall model: ps > .05). Table 5 details the resulting model fits for each of these regression models.
Table 5.
Multiple Regression Models for Neuropsychology Tests.
| Total Model | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | B | SE B | β | B | SE B | β | B | SE B | β | B | SE B | β | R2 | F | P |
| Trails NL Total | −2.39 | 2.58 | −.087 | −1.33 | 3.00 | −.042 | 3.35 | 2.87 | .111 | −5.57 | 2.87 | .186 | .042 | 1.24 | .300 |
| Stroop Total | .130 | 1.17 | .011 | 1.50 | 1.32 | .109 | .047 | 1.27 | .004 | −.384 | 1.27 | −.029 | .014 | .383 | .821 |
| ACT Total | .153 | .680 | .021 | −.600 | .772 | −.074 | −.190 | .748 | −.025 | −.463 | .718 | −.063 | .010 | .280 | .890 |
| DSPSS | −.069 | .271 | −.024 | .050 | .312 | .015 | −.208 | .297 | −.066 | .119 | .290 | .039 | .006 | .177 | .950 |
| IED Error | .263 | .649 | .041 | .794 | .741 | .110 | −.400 | .717 | −.059 | .128 | .677 | .020 | .017 | .412 | .800 |
| IED Stage Trial | .404 | 1.47 | 1.50 | 1.47 | 1.50 | .100 | −.571 | 1.45 | −.038 | .278 | 1.37 | .021 | .012 | .298 | .879 |
| AGN CE + | −.203 | .296 | −.063 | .671 | .340 | .181 | −.007 | .324 | −.002 | .188 | .317 | .055 | .040 | 1.22 | .308 |
| AGN CE - | .255 | .275 | .083 | .859 | .317 | .245 | .215 | .302 | .065 | .002 | .295 | .001 | .065 | 2.05 | .091 |
| Fluency Total | −.503 | .954 | −.049 | .651 | 1.08 | .054 | -.472 | 1.04 | −.043 | .258 | 1.04 | .024 | .009 | .258 | .904 |
| TOVA RT | −26.86 | 16.08 | −.156 | −31.38 | 18.39 | −.161 | 16.41 | 17.84 | .087 | −19.28 | 17.18 | −.107 | .057 | 1.64 | .169 |
| TOVA Commission | −.254 | 1.35 | −.018 | −.573 | 1.55 | −.036 | −1.75 | 1.50 | −.114 | 1.08 | 1.44 | .073 | .017 | 0.65 | .761 |
| TOVA Omission | .320 | 1.76 | .011 | −3.57 | 2.01 | −.166 | 2.86 | 1.95 | .139 | −3.54 | 1.88 | −.179 | .033 | 1.95 | .107 |
Discussion
This study demonstrates that four distinct clinical phenotypes of deployment trauma-related pathology are associated with partially dissociable impairments in sustained attention. Thus, rather than a generalized cognitive deficit across any trauma-related disorder, this study indicates that particular combinations of disorders may lead to particular cognitive impairments. Specifically, we found that a clinical phenotype characterized by mood disorders and PTSD was associated with less attentional stability and worse sustained inhibitory control, as indexed by higher reaction time variability and commission errors, respectively. Similarly, a substance use disorders phenotype was also associated with more errors of inhibitory control. On the other hand, a phenotype characterized by current pain, sleep disturbance and mTBI (and to a lesser extent, PTSD) was associated with greater errors of disengagement and decreased reactivity to errors, as indexed by more intermittent errors of omission and decreased post-error slowing. Finally, an anxiety disorder phenotype was associated with less attention stability and greater error reactivity. In contrast to performance on the gradCPT, our measure of sustained attention and inhibitory control, performance on traditional neuropsychological tests of attention and executive function could not be predicted by clinical phenotypes.
Our findings of differential patterns of sustained attention failures across clinical phenotypes align well with the three-state model of attentional lapses proposed by Cheyne and colleagues (2009). This model outlines three increasingly severe types of task disengagement: “occurrent” task inattention (brief or partial disengagement), “generic” task inattention (loss of sensitivity to task variation), and “response” disengagement (non-responding). These three states correspond to increasingly severe alterations in performance, namely 1) higher RT variability, 2) commission errors, and 3) omission errors. Our mood/PTSD phenotype was associated with both occurrent and generic task disengagement (higher reaction time variability and commission error rates), whereas the somatic/TBI phenotype was associated with the more severe response disengagement (higher omission error rates). Thus the mood/PTSD phenotype was associated with less devastating, though perhaps more consistent levels of disengagement as compared to the somatic/TBI phenotype. We additionally found that Veterans with anxiety disorders exhibited the least severe behavioral marker of attentional lapses, increased reaction time variability, with no concurrent increases in overall error rates. Interestingly, substance use disorders were associated with the more moderate lapses (commission errors), without the least severe behavioral marker, potentially suggesting a more “pure” inhibitory control deficit. With regard to post-error slowing, it has been suggested that those with TBI would have less post-error slowing, as their response disengagement would lead to less error awareness, while anxiety disorders would lead to ruminations regarding performance, and greater error reactivity (Cheyne et al., 2009). Our results are entirely consistent with these hypotheses. These theoretical models and empirical results indicate that the manifestations of attentional dysfunction are not equivalent and, as such, attempts to remediate attentional deficits in this polymorbid population may require different approaches.
The somatic/TBI phenotype, which loads most heavily on a history deployment-related mTBI, current pain, and to a lesser extent sleep disturbance, is associated with more catastrophic disengagement from the task, as reflected in omission errors, as well as less reactivity to, and potentially lack of awareness of errors. Individuals who experience mild, moderate, and severe TBI often experience chronic pain afterwards (Defrin, Riabinin, Feingold, Schreiber, & Pick, 2015; Sang & Sundararaman, 2017; Seal et al., 2017; Wu & Graham, 2016), and as such is it unsurprising that pain and (mild) TBI were included together in a component. Both pain and TBI have been associated with deficits in executive function particularly with sustained attention (Buhle & Wager, 2010; Moore, Eccleston, & Keogh, 2017; Weiss et al., 2017; Chan, 2005; Robertson et al., 1997; Oosterman, Derksen, van Wijck, Kessels, & Veldhuijzen, 2012; Kucyi, Salomons, & Davis, 2013). While these somatic/TBI-related errors may represent more severe lapses of attention, another distinction is that they are errors of “inaction” (omission) vs. errors of “action” (commission). Thus, differences in strategy, criterion, or impulsiveness, could also differentiate these phenotypes (Fortenbaugh et al., 2015). PTSD and disrupted sleep did contribute more modestly to the somatic/TBI component, and thus more work is needed to understand how these conditions may also contribute to these more severe failures of attention characterized by response disengagement. It may be that the effects of PTSD are heterogeneous and interact with comorbidities, such that PTSD can be associated with both the disengagement of sustained attention (with Somatic/TBI) and a loss of sustained inhibitory control (with mood/PTSD).
Our mood/PTSD phenotype, which loads most heavily on depression and PTSD was associated with small to moderate failures of sustained attention (Cheyne et al., 2009), which can also be thought of as deficits in inhibitory control and attentional stability (Esterman, Noonan, et al., 2013; Fortenbaugh et al., 2015; Robertson et al., 1997). Studies that have investigated sustained attention and other domains of executive function are generally consistent with our findings. Specifically, other studies that use CPTs, go/no-go tasks or visual/auditory oddball tasks have found that PTSD and depression are associated with increased errors of commission, indicating difficulty inhibiting responses, as well as greater reaction time variability (DeGutis et al., 2015; Esterman, DeGutis, et al., 2013; Scott et al., 2015; Shucard, McCabe, & Szymanski, 2008; Swick et al., 2013; Swick et al., 2012; van Rooij et al., 2014; Wu et al., 2010). This is less consistently observed in other subdomains of executive functioning, such as working memory or task switching (Aupperle et al., 2012; DeGutis et al., 2015). Together, our results and the literature more broadly demonstrate that mood/PTSD-related deficits in attentional control are not limited to trauma-related or negative emotional stimuli (Pineles, Shipherd, Mostoufi, Abramovitz, & Yovel, 2009; Pineles, Shipherd, Welch, & Yovel, 2007; Vasterling et al., 1998) (Ellenbogen & Schwartzman, 2009; Sanchez, Vazquez, Marker, LeMoult, & Joormann, 2013).
The anxiety disorders and substance use disorders phenotypes accounted for the least variance in our PCA but yielded distinct patterns of responding on the gradCPT. It has been well established that substance abuse is associated with deficits in inhibition and impulse control (Fillmore & Rush, 2002; Monterosso, Aron, Cordova, Xu, & London, 2005; Pope, Gruber, Hudson, Huestis, & Yurgelun-Todd, 2001). Therefore, our finding that the substance use disorder component was predictive of deficits in inhibition align with the larger body of evidence, and as such provide a good validity check for the relationship between our measure and clinical components. Similar to the mood/PTSD component, the Anxiety component was associated with more variable reaction time. However, anxiety was also associated with an increased reactivity to errors. Combined, these results are consistent with existing models of anxiety-related deficits in attention, which suggest these alterations stem from both internal factors (worry, rumination; Eysenck, 1979) as well as external factors (enhanced error monitoring/processing; Forster et al., 2015).
Interestingly, we did not find robust differences between the phenotypes on any of our neuropsychological tests. This may indicate that, as predicted, the gradCPT is more sensitive and is better at capturing subtle changes in attention/executive function associated with deployment-related pathologies (DeGutis et al., 2015). Similarly, it may be that the gradual and sustained attention demands of the gradCPT (and similar tasks) tap into a more fundamental aspect of attention/executive functioning. Interestingly, while performance on the TOVA, a CPT test of attention with some similar properties as the gradCPT, was not significantly predicted by the clinical phenotypes in this study, it did demonstrate a similar pattern of results. This suggests that the unique properties of the gradCPT increase its sensitivity, even when compared to other tests of sustained attention. The gradCPT, in smaller samples, has already revealed sustained attention deficits associated with PTSD symptom severity, depression symptom severity (DeGutis et al., 2015), the presence of early life trauma (Fortenbaugh, Corbo, et al., 2017), non-suicidal self-injury (Auerbach et al., 2014), and ADHD (Rosenberg et al., 2016), further indicating that this task is sensitive to a range of neuropsychiatric conditions. Future work comparing a broader range of computer-based tests of sustained attention and other aspects of executive function could help determine the extent to which the gradCPT is uniquely correlated with clinical symptoms, and whether other domains of cognition cluster with the gradCPT. Another possibility is that other clinical components would better explain the neuropsychological test results, thus the replicability of the current clinical phenotypes with different cohorts and analytic approaches will be important for future studies.
One potential mechanism for these attention deficits could be the presence of more frequent ruminations and mind wandering. In particular this has been implicated in PTSD (Michael, Halligan, Clark, & Ehlers, 2007; Speckens, Ehlers, Hackmann, Ruths, & Clark, 2007) and depression (Ehring, Frank, & Ehlers, 2008; Joormann, Levens, & Gotlib, 2011; Takano & Tanno, 2009). Increased mind wandering is known to impact sustained attention by increasing variability and increasing errors of commission (Seli, Cheyne, & Smilek, 2013; Kucyi, Esterman, Riley, & Valera, 2016). Future studies that include “thought probes” during sustained attention (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Kucyi, Esterman, Riley, & Valera, 2016) could help determine if individuals with these trauma related conditions are more distracted by task-unrelated thoughts, and whether these thoughts are disrupting or coopting attentional and inhibitory control mechanisms. Another possible mechanism for these deficits is anhedonia and lack of motivation and/or arousal. Several recent studies of healthy participants found that rewarding performance in a sustained attention task improved inhibitory control and reduced reaction time variability (Esterman et al., 2016; Esterman, Poole, Liu, & DeGutis, 2017; Esterman, Reagan, Liu, Turner, & DeGutis, 2014). A recent meta-analysis found that PTSD is characterized by deficits in multiple aspects of reward and motivation (Nawijn et al., 2015), suggesting this could be a potential reason for impaired attentional and inhibitory control. Further, substance use disorders are typically conceived as disorders of reward circuitry (Koob & Volkow, 2010). Future work should explore how these attentional impairments are modulated by motivation and reward. The degrees to which mind wandering and/or motivation impact these sustained attention deficits have important clinical implications (Bedard et al., 2013). For example, mindfulness interventions can reduce symptoms of depression, PTSD, and anxiety, and may do so in part by helping to increase awareness of and reduce mind wandering (for a review see-Fortenbaugh, DeGutis, & Esterman, 2017; Creswell, 2017). On the other hand, attention deficits due to anhedonia may benefit from interventions aimed at increasing internal motivation and self-efficacy, such as exercise (McAuley & Blissmer, 2000; Ryan & Deci, 2000). Similarly, neuroscience-based interventions such as TMS could differentially target networks associated with mind wandering vs. motivation (Esterman et al., 2017; Esterman, Poole, Liu, & Degutis, 2017; Kucyi, Esterman, Riley, & Valera, 2016).
Characterizing and rehabilitating attentional impairments has real world implications, as evidence demonstrates that difficulty with sustaining attention can cause trouble in an individual’s ability to successfully navigate daily life activities, such as education, driving, cooking, and taking care of offspring (Robertson et al., 1997; Steinmayr, Ziegler, & Träuble, 2010; Yanko & Spalek, 2014). The comorbidity of mTBI and PTSD was represented across two components that each had differential impairments in attention. This may explain why the combination of PTSD, depression, and mTBI leads to the most devastating functional impairments (Lippa et al., 2015). Essentially, these conditions affect most aspects of attention, potentially contributing to more pronounced functional impairment/disability. Given this, future research is needed to develop effective treatments for regulating attention in individuals with trauma-related pathologies. Dimensional approaches could be ideal, which focus on neurocognitive mechanisms, rather than traditional disorders and diagnoses. Neurocognitive-based interventions, such as attention training (DeGutis & Van Vleet, 2010) and non-invasive brain stimulation (Esterman, Thai, et al., 2017) to attention networks could lead to improved attention, the ability to get “in the zone” (Esterman, Noonan, et al., 2013), and greater treatment engagement. Given the important role of attention in navigating daily responsibilities, emphasis on the development of innovative and effective treatments for attentional deficits could have real-world implications for a range of neuropsychiatric and behavioral disorders.
Supplementary Material
Public Significance Statement:
Trauma victims have clinical comorbidities that can impair cognition. We find that dissociable impairments in the ability to sustain attention are associated with unique combinations of clinical conditions in a sample of returning Veterans. This has important implications, as attentional lapses have real world consequences and are one of the most common cognitive complaints in this population.
Acknowledgement:
This research was supported in part by the Department of Veterans Affairs by the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research and Development National Network Center for TBI Research (B9254-C). M.S.E. has a Career Development award from the Department of Veterans Affairs Clinical Sciences Research and Development (1IK2CX000706-01A2). F.C.F has a Career Development award from the Department of Veterans Affairs Rehabilitation Research and Development (1IK2RX002268-01A2). REM has a Merit Review Award from Department of Veterans Affairs Clinical Sciences Research and Development (CX001327). The contents within do not represent the views of the Department of Veterans Affairs or the United States government. W.M. is supported by NIH NCCIH R21 AT009430-01. M.E.P. is supported by the Stuart T. Hauser Clinical Research Training Program T32, NIMH (4T32MH016259-37).
References
- Akiskal H (1998). Toward a definition of generalized anxiety disorder as an anxious temperament type. Acta Psychiatrica Scandinavica, 98(s393), 66–73. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (Ed.) (2013). Diagnostic and statistical manual of mental disorders (5th ed.). [Google Scholar]
- Amick MM, Clark A, Fortier CB, Esterman M, Rasmusson AM, Kenna A, . . . McGlinchey R. (2013). PTSD modifies performance on a task of affective executive control among deployed OEF/OIF veterans with mild traumatic brain injury. Journal of the International Neuropsychological Society, 19(7), 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amick MM, Meterko M, Fortier CB, Fonda JR, Milberg WP, & McGlinchey RE (2018). The Deployment Trauma Phenotype and Employment Status in Veterans of the Wars in Iraq and Afghanistan. The Journal of head trauma rehabilitation, 33(2), E30–E40. doi: 10.1097/HTR.0000000000000308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF (1998). Catecholamine modulation of prefrontal cortical cognitive function. Trends in Cognitive Sciences, 2(11), 436–447. [DOI] [PubMed] [Google Scholar]
- Auerbach RP, Kim JC, Chango JM, Spiro WJ, Cha C, Gold J, . . . Nock MK. (2014). Adolescent nonsuicidal self-injury: Examining the role of child abuse, comorbidity, and disinhibition. Psychiatry Research, 220(1–2), 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, & Paulus MP (2012). Executive function and PTSD: disengaging from trauma. Neuropharmacology, 62(2), 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon L, Ancoli-Israel S, Aka AA, McKenna BS, & Drummond SP (2009). Relationship between obstructive sleep apnea severity and brain activation during a sustained attention task. Sleep, 32(3), 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin, 121(1), 65–94. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Crawford AL, Feldman ME, Kirby AC, Hertzberg MA, Davidson JRT, & Moore SD (1997). Chronic posttraumatic stress disorder and chronic pain in Vietnam combat veterans. Journal of Psychosomatic Research, 43(4), 379–389. doi: 10.1016/S0022-3999(97)00129-3 [DOI] [PubMed] [Google Scholar]
- Bedard M, Felteau M, Marshall S, Cullen N, Gibbons C, Dubois S, Maxwell H, Mazmanian D, Weaver B, Rees L, Gainer R, Klein R, & Moustgaard A (2013). Mindfulness-based cognitive therapy reduces symptoms of depression in people with a traumatic brain injury: results from a randomized controlled trial. Journal of Head Trauma Rehabilitation, 29(4), E13–22. doi: 10.1097/HTR.0b013e3182a615a0 [DOI] [PubMed] [Google Scholar]
- Blake D, Weathers F, Nagy L, Kaloupek D, Klauminzer G, Charney D, & Keane T (1990). A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. Behavior Therapist, 13, 187–188. doi: 10.1007/BF02105408 [DOI] [Google Scholar]
- Bleich A, Koslowsky M, Dolev A, & Lerer B (1997). Post-traumatic stress disorder and depression. An analysis of comorbidity. The British Journal of Psychiatry, 170(5), 479–482. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Recupero PR, & Stout R (1995). PTSD substance abuse comorbidity and treatment utilization. Addictive behaviors, 20(2), 251–254. [DOI] [PubMed] [Google Scholar]
- Brown PJ, & Wolfe J (1994). Substance abuse and post-traumatic stress disorder comorbidity. Drug and alcohol dependence, 35(1), 51–59. [DOI] [PubMed] [Google Scholar]
- Buhle J, & Wager TD (2010). Performance-dependent inhibition of pain by an executive working memory task. Pain, 149(1), 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, & Andrykowski MA (1998). Psychometric evaluation of the Pittsburgh sleep quality index. Journal of Psychosomatic Research, 45(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Carrasco M (2011). Visual attention: The past 25 years. Vision Research, 51(13), 1484–1525. doi: 10.1016/j.visres.2011.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RC (2005). Sustained attention in patients with mild traumatic brain injury. Clinical Rehabilitation, 19(2), 188–193. [DOI] [PubMed] [Google Scholar]
- Cheyne JA, Solman GJ, Carriere JS, & Smilek D (2009). Anatomy of an error: A bidirectional state model of task engagement/disengagement and attention-related errors. Cognition, 111(1), 98–113. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, & Schooler JW (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences, 106(21), 8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM (2011). Visual working memory as visual attention sustained internally over time. Neuropsychologia, 49(6), 1407–1409. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, & Goodwin GM (2002). Sustained attention deficit in bipolar disorder. The British Journal of Psychiatry, 180(4), 313–319. [DOI] [PubMed] [Google Scholar]
- Creswell JD (2017). Mindfulness Interventions. Annual Review of Psychology, 68, 491–516. doi : 10.1146/annurev-psych-042716-051139 [DOI] [PubMed] [Google Scholar]
- Defrin R, Riabinin M, Feingold Y, Schreiber S, & Pick CG (2015). Deficient Pain Modulatory Systems in Patients with Mild Traumatic Brain and Chronic Post-Traumatic Headache: Implications for its Mechanism, Journal of Neurotrauma(32), 1. doi: 10.1089/neu.2014.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGutis J, Chiu C, Thai M, Esterman M, Milberg W, & McGlinchey R (2016). Trauma sequelae are uniquely associated with components of self-reported sleep dysfunction in OEF/OIF/OND Veterans. Behavioral sleep medicine, 1–26. [DOI] [PubMed] [Google Scholar]
- DeGutis J, Esterman M, McCulloch B, Rosenblatt A, Milberg W, & McGlinchey R (2015). Posttraumatic Psychological Symptoms are Associated with Reduced Inhibitory Control, not General Executive Dysfunction. Journal of the International Neuropsychological Society, 21(05), 342–352. [DOI] [PubMed] [Google Scholar]
- DeGutis JM, & Van Vleet TM (2010). Tonic and phasic alertness training: A novel behavioral therapy to improve spatial and non-spatial attention in patients with hemispatial neglect. Frontiers in Human Neuroscience, 4, 60:117. doi: 10.3389/fnhum.2010.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kaplan E, & Kramer GL (2001). D-KEFS Examiner’s and Technical Manual. San Antonio, TX: Pearson Education. [Google Scholar]
- Dick BD, & Rashiq S (2007). Disruption of attention and working memory traces in individuals with chronic pain. Anesthesia & Analgesia, 104(5), 1223–1229. [DOI] [PubMed] [Google Scholar]
- Doran S, Van Dongen H, & Dinges DF (2001). Sustained attention performance during sleep deprivation: evidence of state instability. Archives italiennes de biologie, 139(3), 253–267. [PubMed] [Google Scholar]
- Dutilh G, Vandekerckhove J, Forstmann BU, Keuleers E, Brysbaert M, & Wagenmakers E-J (2012). Testing theories of post-error slowing. Attention, Perception, & Psychophysics, 74, 454–465. doi: 10.3758/s13414-011-0243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T, Frank S, & Ehlers A (2008). The role of rumination and reduced concreteness in the maintenance of posttraumatic stress disorder and depression following trauma. Cognitive therapy and research, 32(4), 488–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen MA, & Schwartzman AE (2009). Selective attention and avoidance on a pictorial cueing task during stress in clinically anxious and depressed participants. Behaviour research and therapy, 47(2), 128–138. [DOI] [PubMed] [Google Scholar]
- Esterman M, DeGutis J, Mercado R, Rosenblatt A, Vasterling JJ, Milberg W, & McGlinchey R (2013). Stress-related psychological symptoms are associated with increased attentional capture by visually salient distractors. Journal of the International Neuropsychological Society, 19(07), 835–840. [DOI] [PubMed] [Google Scholar]
- Esterman M, Grosso M, Liu G, Mitko A, Morris R, & DeGutis J (2016). Anticipation of monetary reward can attenuate the vigilance decrement. PLoS One, 11(7), e0159741; 0159741–0159719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Noonan SK, Rosenberg MD, & DeGutis J (2013). In the Zone or Zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cerebral Cortex, 23(11), 2712–2723. doi: 10.1093/cercor/bhs261 [DOI] [PubMed] [Google Scholar]
- Esterman M, Poole V, Liu G, & DeGutis J (2017). Modulating Reward Induces Differential Neurocognitive Approaches to Sustained Attention. Cerebral Cortex, 27(8), 4022–4032. doi: 10.1093/cercor/bhw214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Reagan A, Liu G, Turner C, & DeGutis J (2014). Reward reveals dissociable aspects of sustained attention. Journal of Experimental Psychology: General, 143(6), 2287–2295. doi: 10.1037/xge0000019 [DOI] [PubMed] [Google Scholar]
- Esterman M, Thai M, Okabe H, DeGutis J, Saad E, Laganiere SE, & Halko MA (2017). Network-targeted cerebellar transcranial magnetic stimulation improves attentional control. NeuroImage, 156, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW (1979). Anxiety, learning, and memory: A reconceptualization. Journal of research in personality, 13(4), 363–385. [Google Scholar]
- Fillmore MT, & Rush CR (2002). Impaired inhibitory control of behavior in chronic cocaine users. Drug & Alcohol Dependence, 66(3), 265–273. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (1996). Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press. [Google Scholar]
- Forster S, Nunez Elizalde A. O., Castle E, & Bishop SJ (2015). Unraveling the Anxious Mind: Anxiety, Worry, and Frontal Engagement in Sustained Attention Versus Off-Task Processing. Cerebral Cortex, 25(3), 609–618. doi: 10.1093/cercor/bht248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh FC, Corbo V, Poole V, McGlinchey R, Milberg W, Salat D, . . . Esterman M. (2017). Interpersonal early-life trauma alters amygdala connectivity and sustained attention performance. Brain and Behavior, 7(5), e00684, 00681–00616. doi: 10.1002/brb3.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh FC, DeGutis J, & Esterman M (2017). Recent theoretical, neural, and clinical advances in sustained attention research. Annals of the New York Academy of Sciences, 1396, 70–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh FC, DeGutis J, Germine L, Wilmer JB, Grosso M, Russo K, & Esterman M (2015). Sustained Attention Across the Life Span in a Sample of 10,000: Dissociating Ability and Strategy. Psychological Science, 26(9), 1497–1510. doi: 10.1177/0956797615594896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh FC, Robertson LC, & Esterman M (2017). Changes in the distribution of sustained attention alter the perceived structure of visual space. Vision Research, 131, 26–36. doi: 10.1016/j.visres.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh FC, Rothlein D, McGlinchey R, Degutis J, & Esterman M (2018). Tracking behavioral and neural fluctuations during sustained attention: a robust replication and extension. Neuroimage, 171, 148–164. doi: 10.1016/j.neuroimage.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Amick MM, Grande L, McGlynn S, Kenna A, Morra L, . . . McGlinchey RE. (2014). The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) semistructured interview: evidence of research utility and validity. The Journal of head trauma rehabilitation, 29(1), 89–98. doi: 10.1097/HTR.0b013e3182865859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golier J, Yehuda R, Cornblatt B, Harvey P, Gerber D, & Levengood R (1997). Sustained attention in combat-related posttraumatic stress disorder. Integrative Physiological and Behavioral Science, 32(1), 52–61. [DOI] [PubMed] [Google Scholar]
- Grafton KV, Foster NE, & Wright CC (2005). Test-retest reliability of the Short-Form McGill Pain Questionnaire: assessment of intraclass correlation coefficients and limits of agreement in patients with osteoarthritis. The Clinical journal of pain, 21(1), 73–82. doi: 10.1097/00002508-200501000-00009 [DOI] [PubMed] [Google Scholar]
- Green BL, PD JDL, Grace MC, & Leonard AC (1992). Diagnostic Comorbidity in a Disaster Sample. Journal of Nervous and Mental Disease, 180, 760–766. [DOI] [PubMed] [Google Scholar]
- Green P (2004). Green’s Medical Symptom Validity Test (MSVT) for Windows: User’s Manual: Edmonton: Green’s Publishing. [Google Scholar]
- Helzer JE, Robins LN, & McEvoy L (1987). Post-traumatic stress disorder in the general population. New England Journal of Medicine, 317(26), 1630–1634. [DOI] [PubMed] [Google Scholar]
- Jenkins MA, Langlais PJ, Delis D, & Cohen RA (2000). Attentional dysfunction associated with posttraumatic stress disorder among rape survivors. The Clinical Neuropsychologist, 14(1), 7–12. [DOI] [PubMed] [Google Scholar]
- Joormann J, Levens SM, & Gotlib IH (2011). Sticky thoughts: depression and rumination are associated with difficulties manipulating emotional material in working memory. Psychological Science, 22(8), 979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar N, & Bastia BK (2006). Post-traumatic stress disorder, depression and generalized anxiety disorder in adolescents after a natural disaster: a study of comorbidity. Clinical Practice and Epidemiology in Mental Health, 2(1), 17. doi: 10.1186/1745-0179-2-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Gerardi RJ, Lyons JA, & Wolfe J (1987). The interrelationship of substance abuse and posttraumatic stress disorder. Epidemiological and clinical considerations. Recent developments in alcoholism: an official publication of the American Medical Society on Alcoholism, the Research Society on Alcoholism, and the National Council on Alcoholism, 6, 27–48. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, & Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry, 52(12), 1048–1060. [DOI] [PubMed] [Google Scholar]
- King LA, King DW, Vogt DS, Knight J, & Samper RE (2006). Deployment Risk and Resilience Inventory: a collection of measures for studying deployment-related experiences of military personnel and veterans. Military Psychology, 18(2), 89–120. doi: 10.1207/s15327876mp1802_1 [DOI] [Google Scholar]
- Koob GF & Volkow ND (2010). Neurocircuity of Addiction. Neuropsychopharmacology, 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Esterman M, Riley CS, & Valera EM (2016). Spontaneous default network activity reflects behavioral variability independent of mind-wandering. Proceedings of the National Academy of Sciences, 113(48), 13899–13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Salomons TV, & Davis KD (2013). Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proceedings of the National Academy of Sciences, 110(46), 18692–18697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, & Eickoff SB (2013). Sustaining attention to simple tasks: A meta-analytic review of the neural mechanisms of vigilant attention. Psychological Bulletin, 139(4), 870–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie P (2001). Sleep disturbances in the wake of traumatic events. New England Journal of Medicine, 345(25), 1825–1832. [DOI] [PubMed] [Google Scholar]
- Leark R, Greenberg L, Kindschi C, Dupuy T, & Hughes S (2007). Test of variables of attention: Clinical manual. Los Alamitos: The TOVA Company. [Google Scholar]
- Leskin LP, & White PM (2007). Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychology, 21(3), 275–284. doi: 10.1037/0894-4105.21.3.275 [DOI] [PubMed] [Google Scholar]
- Lew HL, Tun C, & Cifu DX (2009). Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. Journal of rehabilitation research and development, 46(6), 697–702. [DOI] [PubMed] [Google Scholar]
- Lim J, & Dinges DF (2008). Sleep deprivation and vigilant attention. Annals of the New York Academy of Sciences, 1129(1), 305–322. [DOI] [PubMed] [Google Scholar]
- Ling S, & Carrasco M (2006). When sustained attention impairs perception. Nature Neuroscience, 9(10), 1243–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa SM, Fonda JR, Fortier CB, M.A., A., A., K., Milberg WP., & McGlinchey R.(2015). Deployment-Related Psychiatric and Behavioral Conditions and Their Association with Functional Disability in OEF/OIF/OND Veterans. Journal of Traumatic Stress, 28, 155–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobbestael J, Leurgans M, & Arntz A (2011). Inter - rater reliability of the Structured Clinical Interview for DSM - IV Axis I disorders (SCID I) and Axis II disorders (SCID II). Clinical psychology & psychotherapy, 18(1), 75–79. doi: 10.1002/cpp.693 [DOI] [PubMed] [Google Scholar]
- McAuley E & Blissmer B (2000). Self-efficacy determinants and consequences of physical activity. Exercise and Sports Sciences Reviews, 28(2), 85–88. [PubMed] [Google Scholar]
- McFall ME, Mackay PW, & Donovan DM (1992). Combat-related posttraumatic stress disorder and severity of substance abuse in Vietnam veterans. Journal of studies on alcohol, 53(4), 357–363. [DOI] [PubMed] [Google Scholar]
- McGlinchey RE, Milberg WP, Fonda JR, & Fortier CB (2017). A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: The TRACTS longitudinal prospective cohort study. International journal of methods in psychiatric research, e1556, 1–15. doi: 10.1002/mpr.1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R (1987). The short-form McGill pain questionnaire. Pain, 30(2), 191–197.doi : 10.1016/0304-3959(87)91074-8 [DOI] [PubMed] [Google Scholar]
- Michael T, Halligan SL, Clark DM, & Ehlers A (2007). Rumination in posttraumatic stress disorder. Depression and Anxiety, 24(5), 307–317. [DOI] [PubMed] [Google Scholar]
- Milliken CS, Auchterlonie JL, & Hoge CW (2007). Longitudinal assessment of mental health problems among active and reserve component soldiers returning from the Iraq war. JAMA, 298(18), 2141–2148. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, & London ED (2005). Deficits in response inhibition associated with chronic methamphetamine abuse. Drug & Alcohol Dependence, 79(2), 273–277. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Eccleston C, & Keogh E (2017). Cognitive load selectively influences the interruptive effect of pain on attention. Pain, 158(10), 2035–2041. [DOI] [PubMed] [Google Scholar]
- Moriarty O, McGuire BE, & Finn DP (2011). The effect of pain on cognitive function: a review of clinical and preclinical research. Progress in neurobiology, 93(3), 385–404. [DOI] [PubMed] [Google Scholar]
- Mortier P, Kiekens G, Auerbach R, Green J, Kessler R, Nock M, . . . Bruffaerts R(2016). Comorbid psychopathological symptoms and suicidality among college students. Paper presented at the WPA Section on Epidemiology Public Health, Munich. [Google Scholar]
- Moser JS, Becker MW, & Moran TP (2012). Enhanced attentional capture in trait anxiety. Emotion, 12(2), 213–216. [DOI] [PubMed] [Google Scholar]
- Nawijn L, van Zuiden M, Frijling JL, Koch SB, Veltman DJ, & Olff M (2015). Reward functioning in PTSD: A systematic review exploring the mechanisms underlying anhedonia. Neuroscience & Biobehavioral Reviews, 51, 189–204. doi: 10.1016/j.neubiorev.2015.01.019 [DOI] [PubMed] [Google Scholar]
- Norman SB, Stein MB, Dimsdale J, & Hoyt DB (2008). Pain in the aftermath of trauma is a risk factor for post-traumatic stress disorder. Psychological medicine, 38(4), 533–542. [DOI] [PubMed] [Google Scholar]
- O’Donnell ML, Creamer M, & Pattison P (2004). Posttraumatic stress disorder and depression following trauma: understanding comorbidity. American Journal of Psychiatry, 161(8), 1390–1396. [DOI] [PubMed] [Google Scholar]
- O’Keeffe F, Dockree P, Moloney P, Carton S, & Robertson I (2007). Characterising error-awareness of attentional lapses and inhibitory control failures in patients with traumatic brain injury. Experimental Brain Research, 180(1), 59–67. [DOI] [PubMed] [Google Scholar]
- Oosterman JM, Derksen LC, van Wijck AJ, Kessels RP, & Veldhuijzen DS (2012). Executive and attentional functions in chronic pain: Does performance decrease with increasing task load? Pain Research and Management, 17(3), 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimette PE, & Brown PJ (2003). Trauma and substance abuse: Causes, consequences, and treatment of comorbid disorders: American Psychological Association. [Google Scholar]
- Paelecke-Habermann Y, Pohl J, & Leplow B (2005). Attention and executive functions in remitted major depression patients. Journal of Affective Disorders, 89(1), 125–135. [DOI] [PubMed] [Google Scholar]
- Pineles SL, Shipherd JC, Mostoufi SM, Abramovitz SM, & Yovel I (2009). Attentional biases in PTSD: More evidence for interference. Behaviour research and therapy, 47(12), 1050–1057. [DOI] [PubMed] [Google Scholar]
- Pineles SL, Shipherd JC, Welch LP, & Yovel I (2007). The role of attentional biases in PTSD: Is it interference or facilitation? Behaviour research and therapy, 45(8), 1903–1913. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, & Yurgelun-Todd D (2001). Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry, 58(10), 909–915. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, & Yiend J (1997). ‘Oops!’: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia, 35(6), 747–758. doi: 10.1016/S0028-3932(97)00015-8 [DOI] [PubMed] [Google Scholar]
- Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT, & Chun MM (2016). A neuromarker of sustained attention from whole-brain functional connectivity. Nature Neuroscience, 19(1), 165–171. doi: 10.1038/nn.4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM & Deci EL (2000). Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist, 55(1), 67–78. doi: 10.1037/0003-066X.55.1.68 [DOI] [PubMed] [Google Scholar]
- Sanchez A, Vazquez C, Marker C, LeMoult J, & Joormann J (2013). Attentional disengagement predicts stress recovery in depression: An eye-tracking study. Journal of Abnormal Psychology, 122(2), 303–313. [DOI] [PubMed] [Google Scholar]
- Sang CN, & Sundararaman L (2017). Chronic Pain Following Concussion. Current pain and headache reports, 21(1), 1. doi: 10.1007/s11916-016-0601-9 [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, & Bruno JP (2001). The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Research Reviews, 35, 146–160. [DOI] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, . . . Schweinsburg BC. (2015). A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin, 141(1), 105–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Bertenthal D, Barnes DE, Byers AL, Strigo I, & Yaffe K (2017). Association of traumatic brain injury with chronic pain in Iraq and Afghanistan veterans: effect of comorbid mental health conditions. Archives of physical medicine and rehabilitation, 98(8), 1636–1645. [DOI] [PubMed] [Google Scholar]
- Seli P, Cheyne JA, & Smilek D (2013). Wandering minds and wavering rhythms: Linking mind wandering and behavioral variability. Journal of Experimental Psychology: Human Perception and Performance, 39(1), 1–5. [DOI] [PubMed] [Google Scholar]
- Seli P, Jonker TR, Cheyne JA, & Smilek D (2013). Enhancing SART validity by statistically controlling speed-accuracy trade-offs. Frontiers in Psychology, 4, 265: 261–268. doi: 10.3389/fpsyg.2013.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, & Pitman RK (1998). Prospective study of posttraumatic stress disorder and depression following trauma. American Journal of Psychiatry, 155(5), 630–637. [DOI] [PubMed] [Google Scholar]
- Shucard JL, McCabe DC, & Szymanski H (2008). An event-related potential study of attention deficits in posttraumatic stress disorder during auditory and visual Go/NoGo continuous performance tasks. Biological Psychology, 79(2), 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver H, & Feldman P (2005). Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. The Journal of Neuropsychiatry and Clinical Neurosciences, 17(3), 391–398. doi: 10.1176/jnp.17.3.391 [DOI] [PubMed] [Google Scholar]
- Speckens AE, Ehlers A, Hackmann A, Ruths FA, & Clark DM (2007). Intrusive memories and rumination in patients with post-traumatic stress disorder: A phenomenological comparison. Memory, 15(3), 249–257. [DOI] [PubMed] [Google Scholar]
- Steinmayr R, Ziegler M, & Trauble B (2010). Do intelligence and sustained attention interact in predicting academic achievement? Learning and Individual Differences, 20(1), 14–18. [Google Scholar]
- Stuss DT, Ely P, Hugenholtz H, Richard MT, LaRochelle S, Poirier CA, & Bell I (1985). Subtle neuropsychological deficits in patients with good recovery after closed head injury. Neurosurgery, 17(1), 41–47. [DOI] [PubMed] [Google Scholar]
- Swick D, Honzel N, Larsen J, & Ashley V (2013). Increased response variability as a marker of executive dysfunction in veterans with post-traumatic stress disorder. Neuropsychologia, 51(14), 3033–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Honzel N, Larsen J, Ashley V, & Justus T (2012). Impaired response inhibition in veterans with post-traumatic stress disorder and mild traumatic brain injury. Journal of the International Neuropsychological Society, 18(05), 917–926. [DOI] [PubMed] [Google Scholar]
- Takano K, & Tanno Y (2009). Self-rumination, self-reflection, and depression: Self-rumination counteracts the adaptive effect of self-reflection. Behaviour research and therapy, 47(3), 260–264. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs and U.S. Department of Defence. (2009). VA/DOD clinical practice guidelines for the management of concussion/mild traumatic brain injury. Retrieved from http://www.healthquality.va.gov/mtbi/concussionmtbifull10.pdf
- van Rooij SJ, Rademaker AR, Kennis M, Vink M, Kahn RS, & Geuze E (2014). Impaired right inferior frontal gyrus response to contextual cues in male veterans with PTSD during response inhibition. Journal of psychiatry & neuroscience, 39(5), 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, & Sutker PB (1998). Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology, 12(1), 125–133. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN Jr, & Sutker PB (2002). Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology, 16(1), 5–14. [DOI] [PubMed] [Google Scholar]
- Walker RL, Clark ME, & Sanders SH (2010). The “Postdeployment multi-symptom disorder”: an emerging syndrome in need of a new treatment paradigm. Psychological Services, 7(3), 136–147. [Google Scholar]
- Wechsler D (2008). Wechsler Adult Intelligence Scale (Manual) (4th ed.). New York: Psychological Corporation. [Google Scholar]
- Weiss KE, Harbeck-Weber C, Zaccariello MJ, Kimondo JN, Harrison TE, & Bruce BK (2017). Executive Functioning in Pediatric Chronic Pain: Do Deficits Exist? Pain Medicine, 19(1), 60–67. [DOI] [PubMed] [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, . . . Wittchen HU. (1992). The structured clinical interview for DSM-III-R (SCID): II. Multisite test-retest reliability. Archives of General Psychiatry, 49(8), 630–636. [DOI] [PubMed] [Google Scholar]
- Wu E, & Graham DP (2016). Association of chronic pain and community integration of returning veterans with and without traumatic brain injury. The Journal of head trauma rehabilitation, 31(1), E1–E12. [DOI] [PubMed] [Google Scholar]
- Wu J, Ge Y, Shi Z, Duan X, Wang L, Sun X, & Zhang K (2010). Response inhibition in adolescent earthquake survivors with and without posttraumatic stress disorder: A combined behavioral and ERP study. Neuroscience Letters, 486(3), 117–121. [DOI] [PubMed] [Google Scholar]
- Yanko MR, & Spalek TM (2014). Driving With the Wandering Mind: The Effect That Mind-Wandering Has on Driving Performance. Human Factors: The Journal of the Human Factors and Ergonomics Society, 56(2), 260–269. doi: 10.1177/0018720813495280 [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, & Kaplan E (1998). On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry, Neuropsychology, & Behavioral Neurology, 11(3), 111–119. [PubMed] [Google Scholar]
- Zlotnick C, Warshaw M, Shea MT, Allsworth J, Pearlstein T, & Keller MB (1999). Chronicity in posttraumatic stress disorder (PTSD) and predictors of course of comorbid PTSD in patients with anxiety disorders. Journal of Traumatic Stress, 12(1), 89–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


