Abstract
Acute lymphoblastic leukemia (ALL) is a very common pediatric malignancy with high survival rates. The course of treatment is modified according to the occurrence of central nervous system (CNS) disease. Aim: To relate serum and cerebrospinal fluid levels of five biomarkers (matrix metalloprotienase 9, CCL-2, sVCAM-1, IFN-γ and inducible protein 10) at diagnosis to the development of CNS infiltration. Methods: The present study was carried on 64 children with ALL and 20 controls. Multiplexed cytokines were measured by Luminex technology (Matrix metalloprotienase 9, CCL-2, sVCAM-1, IFN-γ and inducible protein 10). Results: Significantly higher sMMP-9 and lower sCCL2 were found in patients who developed CNS leukemia. Conclusion: Serum multiplexed parameters at diagnosis of childhood ALL may predict of development of CNS leukemia.
Keywords: : acute lymphoblastic leukemia, biomarkers, CNS infiltration
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy. In the past ALL was a fatal disease; however, survival rates have improved to be as high as 80–90%. This is attributed to improved supportive care, treatment modalities and prediction of relapse risk [1]. Central nervous system (CNS) involvement in childhood ALL is defined as five or more leukocytes/mm3 and blast cells in cerebrospinal fluid (CSF) or cranial nerve palsy [2]. CNS involvement is rarely detected at initial presentation, while CNS relapses occur frequently. All patients, therefore, receive intensive, CNS-directed chemotherapy, an approach associated with short- and long-term neurological toxicities. Still, CNS leukemia (CNSL) relapses occur and, despite high dose systemic and intrathecal chemotherapy [3], 20–40% of relapses continue to occur in the CNS as either isolated or combined disease [4].
CNS involvement is either diagnosed by cytological analysis, which can be limited by difficulty in low cell counts, or flow cytometry. Though more sensitive, flow cytometry is still limited by the low number of antibodies. Risk factors for CNS involvement include high peripheral blast counts, high risk genetic abnormalities, as well as T-lineage phenotype. It is still unclear how leukemic blasts penetrate CNS, how CNS relapse is related to systemic relapse and how blasts survive in the CSF microenvironment. This points to the role of different cytokines in CNS involvement. Early prediction of CNSL would also help adapt treatment protocols [5].
Many markers were studied in this context, some of these (sIL-2R, CCR7 and IL-15) were related to CNS infiltration in acute leukemia [6–8]. We aimed to multiplex several cytokines and cytokine inducible molecules in a Luminex (TX, USA)-based assay and test its efficiency in predicting CSF infiltration in childhood ALL. We chose our parameters based on their function in both tumor progression and breakdown of the blood–brain barrier (BBB) [9]. Monocyte chemotactic protein 1 (MCP-1/CCL2) is a chemokine secreted by fibroblasts, endothelial/epithelial cells and monocytes and plays a role in the migration of leukocytes in states of homeostasis, inflammation as well as tumor initiation and progression [10,11]. MCP1/CCL2 is also associated with BBB breakdown [12]. Vascular cell adhesion molecule-1 (VCAM-1) is a cytokine inducible molecule that promotes the cell adhesion and diapedesis of white blood cells, therefore, has a role in tumor growth and angiogenesis [13]. It was also shown to have a role in assisting other cytokines in T-cell trafficking across BBB endothelium [14].
Matrix metalloprotienase 9 (MMP-9) is a cytokine inducible enzyme that has been implicated in invasion, metastasis and angiogenesis in different tumors. Moreover, it has been thought to help enable leukemic cells to infiltrate the brain [15,16]. IFN-γ is a cytokine that plays a central role in innate and adaptive arms of host immune defense and is thus related to cancer immunology [17]. IFN-γ was shown in one research to safeguard the BBB during experimental autoimmune encephalomyelitis [18]. IFN-y-inducible protein 10 (IP-10) is a CXC chemokine, it is produced in response to INFs and is involved in chemotaxis, induction of apoptosis and regulation of cell growth. It is thought that IP-10 may be a distal mediator of the angiostatic effects of interferons and has been associated with many diseases including tumor development, metastasis and dissemination. It was also recently implicated in cell trafficking across the BBB [19,20].
Circulating biomarkers are easily accessible and measurable. With the feasibility of multiplexing, we were able to measure multiple parameters in one sample with high through output and sensitivity. This gives better monitoring of disease course, risk stratification and methods of evaluating the outcome of treatment.
The aim of the study was the detection of serum and CSF levels of multiplexed biomarkers (MMP-9, chemokine [C-C motif] ligand 2 [CCL-2], serum vascular cell adhesion molecule 1 [sVCAM-1], IFN-γ and IP-10) and their utility in predicting CSF infiltration in pediatric ALL.
Patients & methods
Patients
The present study was carried out in Alexandria University Children Hospital (Alexandria, Egypt) on 64 children with ALL and 20 controls. The control group included children who were suspected to have meningitis though were proven to be free after analysis. Sampling was done after approval of the local ethical committee and informed written consent was gained from a guardian of every patient included in the study.
Treatment protocol
The remission induction phase (4 weeks) included vincristine, daunomycin, prednisone, L-asparaginase, Cytarabine (ara C) and intrathecal methotrexate in cases of CSF infiltration. The consolidation phase (5 weeks) included intrathecal methotrexate, cyclophosphamide, ara C, mercaptopurine (6 MP) and prednisone as well as radiation in cases of CNS involvement. Eight weeks interim maintenance used methotrexate and 6 MP. The 7 weeks of delayed intensification utilized vincristine IV, doxorubicin IV, L-asparaginase, oral dexamethasone, cyclophosphamide IV (day 28), ara C and intrathecal methotrexate. The maintenance phase (an 84-day course) included vincristine IV, oral prednisone, oral methotrexate, 6 MP and intrathecal methotrexate.
Samples
A CSF and serum sample were taken simultaneously from all patients at diagnosis. At the time of sampling, all patients’ CSF was free of infiltration. For the control group, CSF samples were taken for a diagnostic purpose and proven to be free. CSF was taken in duplicate tubes, cytological analysis from cytospin and immunophenotyping was done from one tube. The other sample was preserved, together with the serum sample, at a temperature of -80°C until the time of analysis. All patients were diagnosed as ALL by a workup of complete blood picture, bone marrow examination and immunophenotyping of the bone marrow sample to be further classified into T and B ALL as well as to detect any aberrant expression. Bone marrow aspirate with minimal residual disease (MRD) analysis was done by immunophenotyping on regular intervals, according to the treatment protocol. CSF analysis and immunophenotyping was done before intrathecal injections and in any patient showing a sign of CSF infiltration. The median follow-up period of patients was 1 year.
Levels of the tested biomarkers
CCL-2, MMP-9, sVCAM-1, IFN-γ and IP-10 were measured in CSF and serum samples using multiplexed polysterene beads, custom made and supplied by R and D. Color-coded beads, precoated with analyte-specific capture antibody allowed for multiple analytes to be simultaneously detected in the same sample. Analyte-specific antibodies captured the analyte of interest. Biotinylated detection antibodies that were specific to the analyte of interest were added and formed an antibody–antigen sandwich. Phycoerythrin-conjugated streptavidin is added. The beads were read on a dual-laser flow-based detection instrument, Luminex 200. One laser classified the bead and determined the analyte that was being detected. The second laser determined the magnitude of the phycoerythrin-derived signal, which is in direct proportion to the amount of bound analyte. Analysis of data was done using xPONENT 3.1 software (Luminex) and a p-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
The study was performed on 64 acute leukemia children and 20 healthy controls. The patients' ages ranged from 1.5 to 18 years, with mean of 4.68 ± 2.34 years. Of all ALL patients in the study, 46 (71.9%) were males and 18 (28.1%) were females. Fifty cases were B-ALL type (78.1 %) and 14 cases were T-ALL (21.9 %). Flow cytometer results showed aberrant expression in 14 cases with B-ALL with CD2, CD13 and CD33 expression. Only two cases showed aberrant expression of CD33 in T-ALL. The patients were followed up for a median of 1 year. During the follow-up period, 34 cases (53.1%) were MRD positive and 30 cases (46.9 %) were MRD negative. Of the 34 MRD positive cases, 26 cases were B-ALL (76.5%) and 8 cases were T-ALL (23.5%). Of the 34 MRD positive cases, eight were diagnosed with CNSL (23.5). Out of the 64 patients studied, cytogenetic analysis was successful performed in 40 cases; 18 cases showed hyperdiploidy, 14 cases showed t(12,21), four cases showed t(9,22), three cases showed t(1,19) and one case showed mixed lineage leukemia gene (MLL) rearrangement.
Comparison of serum levels of biomarkers in patients & controls
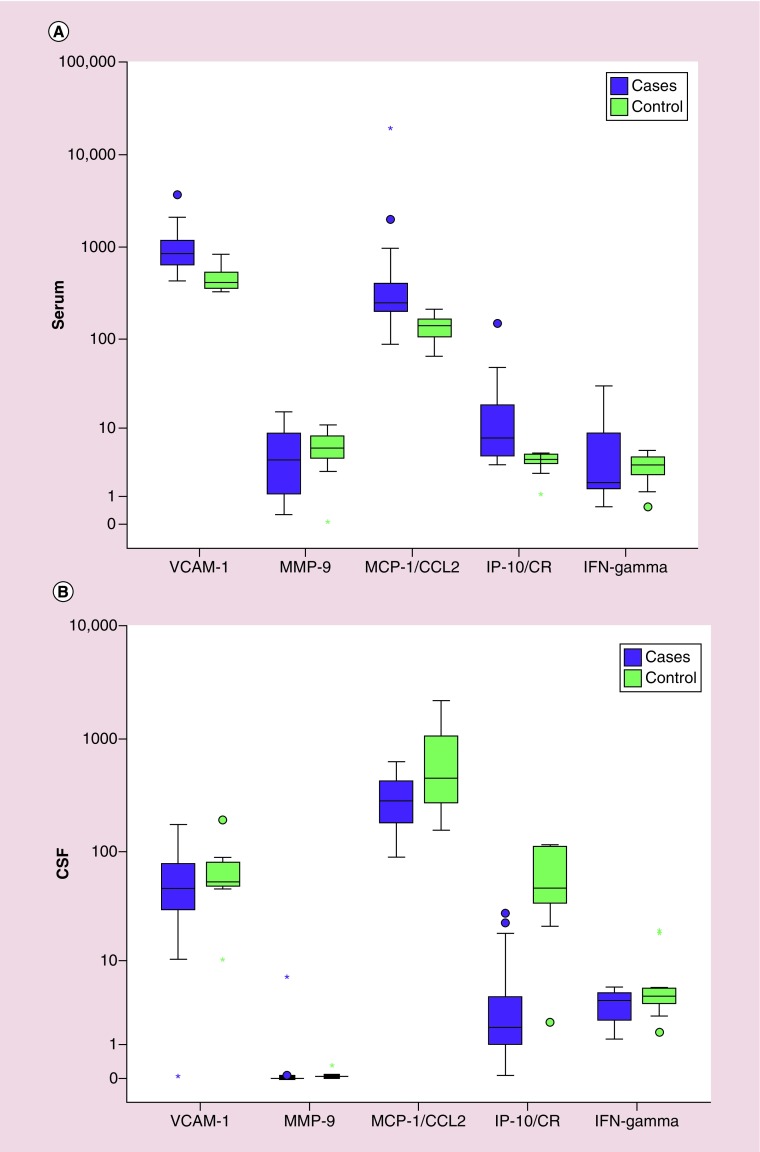
The mean of VCAM-1 in cases of CNSL (1080.0 ± 670.6 ng/ml) was significantly higher than controls (473.2 ± 156.0 ng/ml), with a significant difference between the two groups (p = < 0.001). The mean of MCP-1/CCL2 in cases (945.5 ± 3315.7 ng/ml) was higher than in the control group (137.0 ± 46.53 ng/ml), with significant difference between the two groups (p < 0.001). The mean of IP-10/CR in cases (16.75 ± 26.72 ng/ml) was higher than in the control group (3.84 ± 1.19 ng/ml), with significant difference between the two groups. (p = 0.001). The mean of MMP-9 in cases (4.85 ± 4.18 ng/ml) was lower than the control group (5.77 ± 3.14 ng/ml), with no significant difference between the two groups (p = 0.301). The mean of IFN-γ in cases (5.73 ± 7.52 ng/ml) was higher than control groups (3.25 ± 1.49 ng/ml) with no significant difference between the two groups. (p = 0.673; Figure 1A).
Figure 1. . Comparison of serum and cerebrospinal fluid biomarkers in cases and controls.
(A) Boxplot showing the comparison of the serum levels of different biomarkers in cases of central nervous system leukemia (purple) and controls (green), (B) boxplot showing the comparison of the cerebrospinal fluid levels of different biomarkers in cases of central nervous system leukemia (purple) and controls (green).
*Represents extreme values.
‘o’ represents outliers.
CSF: Cerebrospinal fluid; IP-10/CR: IFN-y-inducible protein 10; MCP-1/CCL2: Monocyte chemotactic protein 1; MMP-9: Matrix metalloprotienase 9; VCAM-1: Vascular cell adhesion molecule 1.
Comparison of CSF levels of biomarkers in patients & controls
Comparing patients and controls, MMP-9 was found to be significantly higher in the CSF of patients compared with the control group (0.24 ± 1.22 ng/ml vs 0.07 ± 0.09 ng/ml, p = 0.02). MCP-1/CCL2 and IP-10/CR were significantly lower in patients than controls. Values for MCP-1/CCL2 were 304.6 ± 140.6 ng/ml in patients compared with 728.4 ± 673.ng/ml in the control group while IP-10/CR values were 4.74 ± 6.72 ng/ml in patients compared with 61.38 ± 41.11 ng/ml in controls. (p = 0.039 and <0.001 respectively). VCAM-1 and IFN-y were found to have no significant different between CSF of both groups. Values for VCAM-1 were 57.37 ± 37.30 ng/ml in patients versus 68.66 ± 47.90 ng/ml in controls while IFN-y values were 3.51 ± 1.34 ng/ml in cases versus 6.86 ± 6.45 ng/ml in controls (p = 0.383 and 0.117, respectively; Figure 1B).
Comparison of biomarkers in B & T ALL patients
There was no significant difference between B and T ALL in all studied parameters. The mean of VCAM-1 in cases with B-ALL group (57.94 ± 37.43 ng/ml) was higher than T-ALL group (55.31 ± 39.7 ng/ml). The mean of MMP-9 in cases with B-ALL group (0.29 ± 1.38 ng/ml) was higher than T-ALL group (0.03 ± 0.02 ng/ml). The mean of MCP-1/CCL2 in cases with B-ALL group (290.1 ± 144.06 ng/ml) was lower than T-ALL group (356.4 ± 122.8 ng/ml). The mean of IP-10/CR in cases with B-ALL group (4.95 ± 7.29 ng/ml) was higher than T-ALL group (3.99 ± 4.45 ng/ml). The mean of IFN-y in cases with B-ALL group (3.34 ± 1.41 ng/ml) was lower than T-ALL type (4.13 ± 0.87 ng/ml).
Comparison of the studied biomarkers in patients with CNS infiltration & patients without CNS infiltration
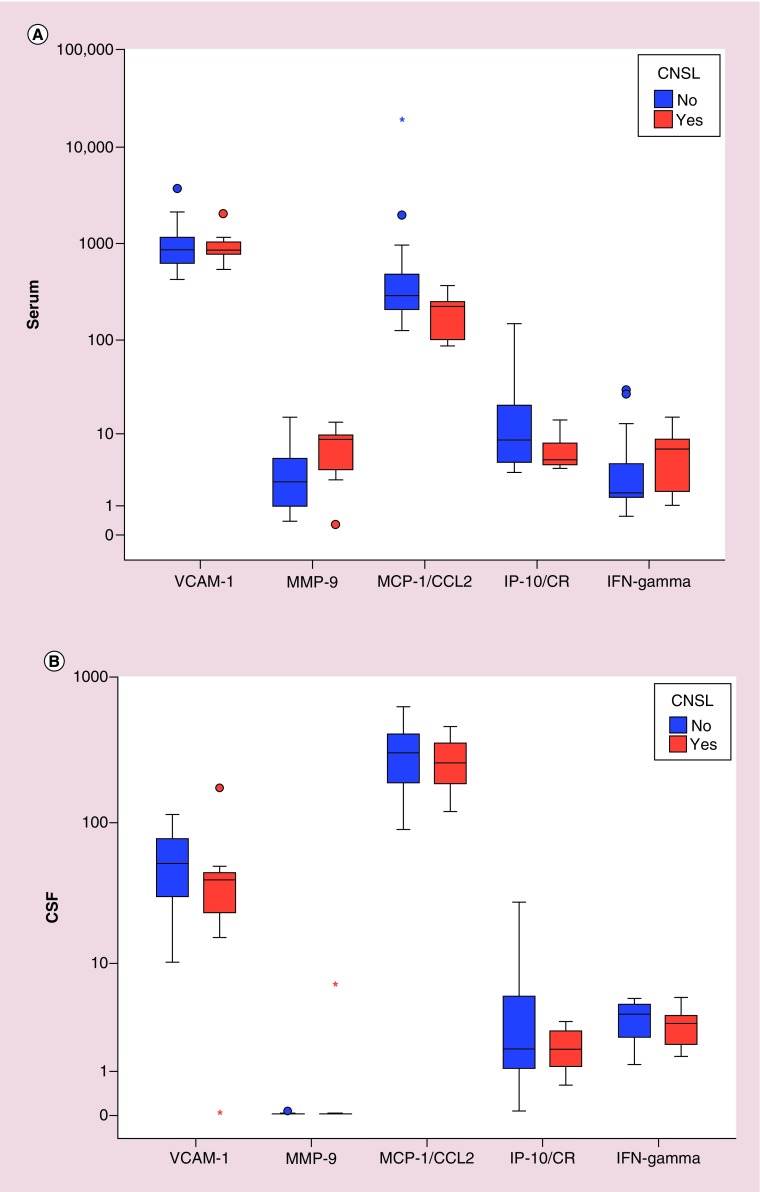
CSF infiltration occurred in 14 cases during follow-up period, while 50 cases were free of CNS infiltration. Comparing CSF parameters, there was no significant difference between the five studied parameters in patients with and without CNS infiltration. Comparing CSF levels of the studied cytokines, we observed that in the CSF of the CNSL group, VCAM, MCP-1/CCL-2, IP-10/CR and IFN-y were lower compared with non-CNSL group. Only the level of MMP-9 was higher in this group. All the differences were statistically insignificant.
There was no statistically significant difference between the five biomarkers measured in serum samples between the CNSL group and non-CNSL group. In the CNSL group, the mean of VCAM-1 was lower (1009.4 ± 488.1 vs 1099.8 ± 720.8; p = 0.802), the mean of MMP-9 was higher (7.30 ± 4.42 vs 4.16 ± 3.93; p = 0.096) while the mean of MCP-1/CCL-2 was lower (197.1 ± 104.0 vs 1155.1 ± 3740.1 ng/ml; p = 0.065). IP-10/CR in the CNSL group was lower (6.72 ± 3.70 vs 19.56 ± 29.69 ng/ml; p = 0.151) while IFN-y was higher (6.29 ± 5.31 vs 5.57 ± 8.12, p = 0.327; Figure 2A & B).
Figure 2. . Comparison of serum and cerebrospinal fluid biomarkers in cases with and without central nervous system (CNS) infiltration.
(A) Boxplot showing comparison of the serum biomarkers in patients with CNS leukemia infiltration (orange) and patients without CNS infiltration (blue), (B) boxplot showing comparison of the cerebrospinal fluid biomarkers in patients with CNS leukemia infiltration (orange) and patients without CNS infiltration (blue).
*Represents extreme values.
‘o’ represents outliers.
CNSL: CNS leukemia; CSF: Cerebrospinal fluid; IP-10/CR:IFN-y-inducible protein 10; MCP-1/CCL2: Monocyte chemotactic protein 1; MCP-1: Monocyte chemotactic protein 1; MMP-9: Matrix metalloprotienase 9; VCAM-1: Vascular cell adhesion molecule 1.
We summarized the pattern of change in serum and CSF cytokines in CNS and non-CNSL in Table 1. As shown, none of these results reached statistical significance, hence we tried combining all markers together and combining the two parameters with the lowest p-value (MMP-9 and MCP-1/CCL-2).
Table 1. . Pattern of change of serum and cerebrospinal fluid markers in CNS leukemia.
| Biomarker | Pattern of change in CNSL compared with non CNSL | p-value |
|---|---|---|
| VCAM-1 CSF | Lower | 0.227 |
| VCAM-1 serum | Lower | 0.802 |
| MMP-9 CSF | Higher | 0.909 |
| MMP-9 serum | Higher | 0.096 |
| MCP-1/CCL2 CSF | Lower | 0.632 |
| MCP-1/CCL2 serum | Lower | 0.065 |
| IFN-gamma CSF | Lower | 0.327 |
| IFN-gamma serum | Higher | 0.425 |
| IP-10/CR CSF | Lower | 0.412 |
| IP-10/CR serum | Lower | 0.151 |
CNSL: Central nervous system leukemia; CSF: Cerebrospinal fluid; IP-10: Inducible protein 10; MCP-1: Monocyte chemotactic protein 1; MMP-9: Matrix metalloprotienase 9; VCAM-1: Vascular cell adhesion molecule-1.
Combined serum parameters & CNSL
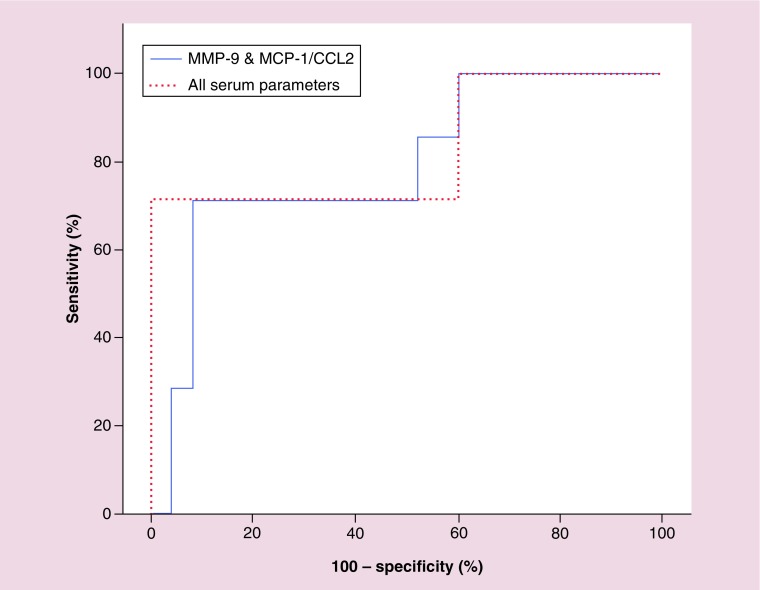
We combined the results of all measured parameters and correlated it with CNS infiltration during follow-up. Using the five parameters altogether, the area under the curve was 0.829, 95% CI: 0.618–1.039 with p-value 0.009, the sensitivity and specificity were 57 and 100%, respectively. The positive and negative predictive values were 100 and 89, respectively. We combined the two parameters with lowest p-value (MCP-1/CCL2 and MMP-9). For these two parameters, area under the curve was 0.794, 95% CI: 0.600–0.989 with a p-value of 0.019. The sensitivity and specificity were 42.8 and 92%, respectively, while the positive and negative predictive values were 60 and 85.19 at a cutoff of >5.64 for MMP-9 and ≤224.6 for MCP-1/CCL2 (Figure 3).
Figure 3. . Receiver operating characteristic curve showing combined biomarkers in cerebrospinal fluid leukemia.
MCP-1/CCL2: Monocyte chemotactic protein 1; MMP-9: Matrix metalloprotienase 9.
Discussion
ALL is the most common childhood malignancy with high incidence of CSF involvement. The significance of cytokines and chemokines in early diagnostics is gaining attention, especially when relating to malignancies. As MMP-9, CCL2, VCAM-1, IFN-y and IP-10 are closely related to tumor metastasis as well as cell trafficking across BBB, we customized a luminex assay multiplexed with these biomarkers.
CNS infiltration occurred throughout the course of treatment in 14 out of 64 ALL patients (21.8%). This percentage is higher than that reported in literature, which does not exceed 10% in most published data [2,3]. Our cases were selected as a consecutive cohort with no predilection to cases who are more prone to develop CNS disease. Eight of the 14 CNSL patients were associated with positive MRD, denoting systemic relapse. It was suggested in a study by Alsadeq et al. that ALL cells that are refractory to therapy or particularly receptive to microenvironment-derived protective signals are able to survive in the CNS niche for prolonged periods of time as extramedullary MRD [21].
The first of our multiplexed biomarkers was sVCAM-1, which was significantly higher in patients’ serum compared with that of the control group, with no difference in CSF and no role in differentiating leukemic infiltration. Horacek et al. similarly found a significant increase in sVCAM-1 in newly diagnosed ALL and acute myeloid leukemia (AML) patients [22,23]. We found no role for serum and CSF VCAM in differentiating leukemic infiltration, while Si et al. [24] found that serum VCAM-1 could differentiate patients with CNSL with an insignificant increase in CSF of this group. The study by Si measured the VCAM1 levels in patients with CNSL, this was in contrast to our study where we aimed to measure the level and take it as a predictor to later development of infiltration. The short half-life of VCAM is probably a reason for insignificance of this marker, which would hence make it unsuitable for prediction of later occurrence of CNSL [25]. In a study similar to our own, Mengya et al. showed no role for VCAM in leukemia CNS metastasis and reported VEGF-A and MMPs as the main factors resulting in the degradation of the BBB and inducing the migration of leukemia cells to the CNS [26].
Regarding CCL2, serum levels were significantly higher while CSF levels were lower in leukemic patients compared with control group. CSF levels could not differentiate CSF leukemia patients while serum levels tended to be lower in this group. All the previous studies definitely reported increase in serum CCL2 level in leukemia patients compared with controls, a finding also demonstrated in our study [22–24,27–29]. The elevated serum levels of this cytokine is closely linked to leukemogenesis and it’s elevated level has even been implicated in thymus atrophy in leukemic patients [30,31]. However, the CSF level of this cytokine is debatable. Regarding CSF CCL2 levels, Ahmed et al. [28] found that a significant increase in the CSF level of CCL2 could be observed in acute leukemia patients compared with the control group, same as Si et al. [24]. The lower levels of CCL2 in the CSF of leukemia patients compared with controls could be explained by a hypothesis stated by Mahad et al., who stated that CCL2 levels are decreased in the CSF of patients with chronic neuroinflammatory conditions, despite abundant expression within lesional tissues [32]. This study used an in vitro BBB model to test the hypothesis that CCL2 is removed from the extracellular fluid by CCR2-positive migrating cells as they cross the BBB, resulting in decreased CSF CCL2 levels. We suggest this as an explanation to the lower levels of CCL2 in CSF, as it has been shown that CCR2 protein is upgraded in leukemia cells [32,33].
Regarding CNSL, De Vasconcellos et al. [27] found, similar to our study, lower levels of bone marrow plasma CCL2 in CNSL patients while Si et al. [24] found that serum and CSF CCL2 concentrations in CNSL patients were higher than those in non-CNSL patients.
Eisenkraft et al. [34] analyzed CSF samples from pediatric patients with ALL at different stages of therapy. They found that patients with CNS disease show an increase of CCL2 levels as the CSF returns to normal. This gives the impression that CNS infiltration is associated with lower levels of CCL2, which increases as the CNS disease resolves. These conflicting results would urge us to study CCL2 more in CNS infiltration with larger cohorts and different time points to help detect its diagnostic value.
MMP-9 was significantly higher in serum and CSF of patients than controls. In addition, both levels were higher in CNSL patients. Similarly, Schneider et al. [35] analyzed the level of secreted MMPs by ELISA in culture of supernatants of leukemic cells and found that MMP-9 was significantly higher in patients with peripheral infiltrations than in patients without peripheral infiltrations. The results of Si et al. [24] were conflicting as he found that the lowest concentrations of MMP-9 were in the CNSL group, followed by non-CNSL group and the control group while he found that CSF level of MMP-9 tended to be higher in CNSL than non-CNSL or the control group. An observation of Klein et al. [36] supports our results where MMP-9 expression in lymphoblastic cell lines was found to be important for invasion and metastasis.
Regarding serum and CSF IFN-y, this marker did not show any significant difference between either patients and controls or CNSL and non-CNSL. This came in agreement with Horacek et al. [23] who evaluated serum IFN in AML patients and a control group. They found that there was no significant difference between AML patients or the control group. Still, another study by Khloussi et al. showed increased levels of IFN-y in ALL children compared with controls while Kupsa et al. found that AML patients had lower levels of this cytokine [37,38]. The publications regarding IFN-y in childhood ALL is scarce and further evaluation of its utility has to be issued.
Finally, serum IP-10 concentrations were significantly higher in cases of CNSL where as CSF levels were lower with no significance in detecting CNSL. A study by Khandany et al. stated increased levels of serum IP-10 in patients with ALL with persistent increase after bone marrow transplantation [39]. However, we could not provide an explanation to the lower levels of IP-10 in patients’ CSF. IP-10 facilitates the trafficking of activated type 1 helper T cells and natural killer (NK) cells expressing the CXCR3 receptor across the BBB, and can regulate their recruitment to sites of inflammation, as stated by Shimizu et al. [40].
We combined the data collected for serum MMP-9 and serum CCL2 (the parameters showing the lowest p-values) in order to increase the sensitivity and specificity of differentiating between CNSL patients versus non-CNSL patients. The combination showed a statistically significant difference between CNSL and non-CNSL. Combination of biomarkers to improve sensitivity and specificity, especially in the context of cancer, has been tried before. An example of this is a study by Gang et al. who tested for nine serum biomarkers in breast cancer patients, where each one singly did not show significance but altogether had good discriminative value [41]. Another example is a study by Likov et al. who tested value of multiplexed biomarkers in differentiation of benign and malignant thyroid disease, making different combinations for better sensitivity [42].
Our study is limited by the small number of cases; therefore, we recommended a similar study be implemented it on larger cohort of patients. Our samples were taken at a single time It could also add to the study if samples were taken at different time points so that patients with CNSL would have levels of cytokines compared before and during actual CNS infiltration.
We therefore hypothesize that the serum biomarkers we studied may predict CNSL metastasis. We hope to extend this hypothesis to future studies with larger number of patients, comparing levels at different time points during treatment. The potential of adding other biomarkers to improve sensitivity of the assay should also be considered.
Summary points.
Central nervous system (CNS) infiltration is a relatively common event that has an impact on prognosis of childhood acute lymphoblastic leukemia.
Cerebrospinal fluid (CSF) and serum biomarkers may be multiplexed to help diagnosis and prediction of CNS leukemia (CNSL).
Serum vascular cell adhesion molecule-1, IP10 and MCP1/CCL2 were significantly higher in acute lymphoblastic leukemia patients compared with controls.
Matrix metalloprotienase 9 (MMP-9) was significantly higher in the CSF of patients while monocyte chemotactic protein 1 (MCP-1)/CCL2 and inducible protein 10 (IP-10)/CR were significantly lower in patients’ CSF than control.
In the CNSL group, CSF VCAM, MCP-1/CCL-2, IP-10/CR and IFN-γ were lower compared with the non-CNSL group. Level of MMP-9 only was higher in this group.
In the CNSL group, serum vascular cell adhesion molecule-1, MCP-1/CCL-2 and IP-10/CR were lower in the CNSL group while IFN-γ and MMP-9 were higher.
Combining all measured parameters, CNS infiltration can be detected with sensitivity of 57% and specificity of 100%.
Combining the two parameters with the lowest p-value (MCP-1/CCL2 and MMP-9), CNS infiltration can be detected with sensitivity of 42.8% and specificity of 92%.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/ijh-2019-0008
Financial & competing interests disclosures
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Kato M, Manabe A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr. Int. 60(1), 4–12 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Scrideli CA. Involvement of the cerebrospinal fluid cells in children with acute lymphoblastic leukemia: prognostic implications. Rev. Bras. Hematol. Hemoter. 34(6), 408–409 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gossai NP, Gordon PM. The role of the central nervous system microenvironment in pediatric acute lymphoblastic leukemia. Front. Pediatr. 5, 90 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Explains experimental approaches to study kinetics of CNS leukemia (CNSL) and states different genes and pathways involved in trafficking of leukemia cells to the brain. It summarizes previous published data involving CNSL.
- 4.Masurekar AN, Parker CA, Shanyinde M. et al. Outcome of central nervous system relapses in childhood acute lymphoblastic leukaemia – prospective open cohort analyses of the ALLR3 trial. PLoS ONE 9(10), e108107 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frishman-Levy L, Izraeli S. Advances in understanding the pathogenesis of CNS acute lymphoblastic leukaemia and potential for therapy. Br. J. Heamatol. 176(2), 157–167 (2017). [DOI] [PubMed] [Google Scholar]; • Addresses some of the major research questions regarding pathogenesis of CNSL. The article also studies the interaction of leukemic cells with the CNS microenvironment, which promotes their survival.
- 6.Alsadeq A, Lenk L, Vadakumchery A. et al. IL7R is associated with CNS infiltration and relapse in pediatric B-cell precursor acute lymphoblastic leukemia. Blood 132(15), 1614–1617 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buonamici S, Trimarchi T, Ruocco MG. et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 459(7249), 1000–1004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee W, Kim SJ, Lee S. et al. Significance of cerebrospinal fluid sil-2r level as a marker of CNS involvement in acute lymphoblastic leukemia. Ann. Clin. Lab. Sci. 35(4), 407–412 (2005). [PubMed] [Google Scholar]
- 9.Jonart LM, Ebadi M, Basile P, Johnson K, Makori J, Gordon PM. Disrupting the leukemia niche in the central nervous system attenuates leukemia chemoresistance. Haematologica (2019) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshmane SL, Kremlev S, Amini S, SawayaBE Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29(6), 313–326 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Knight DA, Snyder LA, Smyth MJ, Stewart TJ. A role for CCL2 in both tumor progression and immunosurveillance. Oncoimmunology 2(7), e25474 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y, Tsirka SE. Monocyte chemoattractant protein-1 and blood-brain barrier. Cell. Mol. Life Sci. 71(4), 683–697 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong DH, Kim YK, Kim MR, Jang JH, Lee S. Emerging roles of vascular cell adhesion molecule-1 (vcam-1) in immunological disorders and cancer. Int. J. Mol. Sci. 19(4), 1057 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steiner O, Coisne C, Cecchelli R. et al. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood–brain barrier endothelium. J. Immunol. 185(8), 4846–4855 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Huang H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors (Basel) 18(10), E3249 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng S, Cen J, Huang Y. et al. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS ONE 6(8), e20599 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mojic M, Takeda K, Hayakawa Y. The dark side of IFN-γ: its role in promoting cancer immunoevasion. Int. J. Mol. Sci. 19(1), 89 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni C, Wang C, Zhang J. et al. Interferon- γ safeguards blood-brain barrier during experimental autoimmune encephalomyelitis. Am. J. Pathol. 184(12), 3308–3320 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Liu M, Guo S, Stiles JK. The emerging role of CXCL10 in cancer. Oncol. Lett. 2(4), 583–589 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K, Wang H, Lou W. et al. IP-10 promotes blood–brain barrier damage by inducing tumor necrosis factor alpha production in Japanese encephalitis. Front. Immunol. 9, 1148 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsadeq A, Schewe DM. Acute lymphoblastic leukemia of the central nervous system: on the role of PBX1. Haematologica 102(4), 611–613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horacek JM, Kupsa T, Vasatova M, Jebavy L, Zak P. Evaluation of serum levels of multiple cytokines and adhesion molecules in patients with newly diagnosed acute lymphoblastic leukemia using biochip array technology. Exp. Oncol. 35(3), 229–230 (2013). [PubMed] [Google Scholar]; • Evaluates serum levels of 17 cytokines and five adhesion molecules in acute lymphoblastic leukemia (ALL) patients and showed alterations reflecting disease activity. This gives an idea about cytokines as a prognostic indicator for ALL.
- 23.Horacek JM, Kupsa T, Vasatova M, Jebavy L, Zak P. Biochip array technology and evaluation of serum levels of multiple cytokines and adhesion molecules in patients with newly diagnosed acute myeloid leukemia. Exp. Oncol. 36(1), 50–51 (2014). [PubMed] [Google Scholar]
- 24.Si MY, Fan ZC, Li YZ, Chang XL, Xie QD, Jiao XY. The prognostic significance of serum and cerebrospinal fluid MMP-9, CCL2 and SVCAM-1 in leukemia CNS metastasis. J. Neurooncol. 122(2), 229–244 (2015). [DOI] [PubMed] [Google Scholar]; •• Describes different cytokines (matrix metalloprotienase 9, CCL2 and sVCAM-1) measured in serum and cerebrospinal fluid and their use in leukemia metastatic CNS and the outcome of CNSL patients. It is the research with the design nearest to our work.
- 25.Kraus J, Gerriets T, Leis S, Stolz E, Oschmann P, Heckmann JG. Time course of VCAM-1 and ICAM-1 in CSF in patients with basal ganglia haemorrhage. Acta Neurol. Scand. 116(1), 49–55 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Si M, Jiao X, Li Y, Chen H, He P, Jiang F. The role of cytokines and chemokines in the microenvironment of the blood–brain barrier in leukemia central nervous system metastasis. Cancer Manag. Res. 10, 305–313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Designs an analog blood–brain barrier by coculturing human brain microvascular endothelial cells and leukemia cells (U937 and IL-60), as well as human brain microvascular endothelial cells and sera from leukemia patients, therefore studying in vitro cytokines affecting permeability of the blood–brain barrier and dynamics of leukemia cell migration.
- 27.De Vasconcellos JF, Laranjeira AB, Zanchin NI. et al. Increased CCL2 and IL-8 in the bone marrow microenvironment in acute lymphoblastic leukemia. Pediatr. Blood Cancer 56(4), 568–577 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Ahmed MI, Nafeb DA, Kamalc MY. Cerebrospinal fluid and serum levels of monocyte chemoattractant protein 1 in acute leukemia patients: correlation to other prognostic factors. Egypt. J. Haematol. 39(4), 183–189 (2014). [Google Scholar]
- 29.Mazur G, Wróbel T, Butrym A, Kapelko-Słowik K, Poreba R, Kuliczkowski K. Increased monocyte chemoattractant protein 1 (MCP1/CCL-2) serum level in acute myeloid leukemia. Neoplasma 54(4), 285–289 (2007). [PubMed] [Google Scholar]
- 30.Wu SY, Yang J, Hong D. et al. HU Suppressed CCL2 expression inhibits the proliferation of leukemia cells via the cell cycle protein Cyclin D1: preliminary in vitro data. Eur. Rev. Med. Pharmacol. Sci. 22(17), 5588–5596 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Driss V, Quesnel B, Brinster C. Monocyte chemoattractant protein 1 (MCP-1/CCL2) contributes to thymus atrophy in acute myeloid leukemia. Eur. J. Immunol. 45(2), 396–406 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Mahad D, Callahan MK, Williams KA, Ubogu EE, Kivisäkk P, Tucky B. Modulating CCR2 and CCL2 at the blood–brain barrier: relevance for multiple sclerosis pathogenesis. Brain 129(Pt 1), 212–223 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Macanas-Pirard P, Quezada T, Navarrete L. et al. The CCL2/CCR2 axis affects transmigration and proliferation but not resistance to chemotherapy of acute myeloid leukemia cells. PLoS ONE 12(1), e0168888 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenkraft A, Keidan I, Bielorai B, Keller N, Toren A, Paret G. MCP-1 in the cerebrospinal fluid of children with acute lymphoblastic leukemia. Leuk. Res. 30(10), 1259–1261 (2006). [DOI] [PubMed] [Google Scholar]; • Shows that CNS involvement in ALL is associated with significantly higher levels of MCP1 during therapy. They measured monocyte chemotactic protein 1 levels in cerebrospinal fluid at different stages of treatment in childhood ALL.
- 35.Schneider P, Costa O, Legrand E. et al. In vitro secretion of matrix metalloproteinases 9 is a prognostic marker in childhood acute lymphoblastic leukemia. Leuk. Res. 34(1), 24–31 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit. Rev. Oncol. Hematol. 50(2), 87–100 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Kholoussi SM, Bayoumi FS, El-Nady H. Estimation of serum interferon-gamma level in childhood acute lymphoblastic leukemia patients. J. Med. Sci 8, 68–72 (2008). [Google Scholar]
- 38.Kupsa T, Vasatova M, Karesova I, Zak P, Horacek JM. Baseline serum levels of multiple cytokines and adhesion molecules in patients with acute myeloid leukemia: results of a pivotal trial. Exp. Oncol. 36(4), 252–257 (2014). [PubMed] [Google Scholar]
- 39.Khandany BK, Hassanshahi G, Khorramdelazad H. et al. Evaluation of circulating concentrations of CXCL1 (Gro-alpha), CXCL10 (IP-10) and CXCL12 (SDF-1) in ALL patients prior and post bone marrow transplantation. Pathol. Res. Pract. 208(10), 615–619 (2012). [DOI] [PubMed] [Google Scholar]; •• Highlights a role for IP10, one of the cytokines studied in our research, in ALL. There are not much data about this role for IP10.
- 40.Shimizu F, Nishihara H, Sano Y. et al. Markedly increased IP-10 production by blood brain barrier in neuromyelitits optica. PLoS ONE 10(3), e0122000 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He G, Holcroft CA, Beauchamp MC. et al. Combination of serum biomarkers to differentiate malignant from benign ovarian tumours. J. Obstet. Gynaecol. Can. 34(6), 567–574 (2012). [DOI] [PubMed] [Google Scholar]; • This is an example of a study using different multiplexed biomarkers and calculating their cutoffs alone and combined in differentiating malignant and non-malignant masses.
- 42.Linkov F, Ferris RL, Yurkovetsky Z. et al. Multiplex analysis of cytokines as biomarkers that differentiate benign and malignant thyroid diseases. Proteomics Clin. Appl. 2(12), 1575–1585 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.